Abstract
Biodegradable mulches are considered a promising alternative to polyethylene-based, nonbiodegradable mulch for sustainable agriculture. In the present study, a bioactive 2-methyl-4- cholorophenoxyacetic acid/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (MCPA-PHBV) conjugate blended with biodegradable poly(butylene adipate-co-terephthalate/polylactide (PBAT/PLA) was developed and used as mulch under controlled condition greenhouse pot experiment with fava bean (Vicia faba) as the nontarget crop species. The objectives were to examine the effectiveness of sustained-release of MCPA herbicide from biodegradable mulch for broadleaf weed suppression and to assess any adverse effects of the herbicide on the nontarget species (fava bean). The energy-dispersive X-ray spectroscopy analysis (EDS) suggests that a substantial quantity of the herbicide was released from the biodegradable mulch which effectively killed the broadleaf weed species even at 1% MCPA concentration. However, the higher concentrations of the herbicide adversely affected several physiological parameters of fava bean growth and development. Stomatal conductance decreased, while leaf temperature subsequently rose (at MCPA concentrations 5, 7.5, and 10%). The quantum yield of the Photosystem II (PSII) indicates that the photosynthetic efficiency was also restricted at concentrations 7.5% and 10%. Evidently, this slow-release herbicide system worked efficiently for broadleaf weed control but at higher concentrations, resulted in adverse physiological effects on the nontarget crop species. This study has demonstrated that biodegradable mulches containing MCPA herbicide are able to effectively inhibit the growth of broad leaf weed species and may be of potential importance in a wide variety of horticultural and agricultural applications.
Keywords: Herbicide MCPA-PHBV conjugate, Biodegradable PBAT/PLA blend, Bioactive mulch film, Weed suppression, Fava bean, Vicia faba
Short abstract
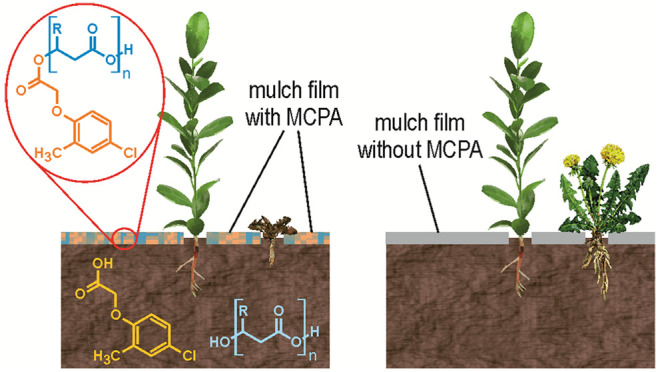
The image shows the integrated use of mulch film with herbicide which provides more effective and sustainable weed control.
Introduction
Agricultural mulches are used worldwide to control weeds, alter soil temperature, and conserve soil moisture, which subsequently improves both the yield and quality of food crops.1−3 Moreover, mulching material helps to protect delicate crop species from unfavorable biotic and abiotic stress conditions, resulting from extreme weather, insects, and weeds. Therefore, mulching in field crops is becoming increasingly popular in modern agriculture. Historically, the commercial use of plastic mulches, mainly for vegetable production, started in the early 1960s.4 These plastic products included polyethylene (PE), polyvinyl chloride, and ethylene vinyl acetate, Most of which have been produced from petroleum-based plastics, usually PE polymers, which are nonbiodegradable.4
The global application of plastic films for use in greenhouses and mulching was expected to grow by 69% from 4.4 million tons in 2012 to 7.4 million tons in 2019.5 Unfortunately, the use of PE film in agriculture often entails environmental problems, because it comprises high molecular weight molecules with hydrophobic properties. Furthermore, the degradation of polyethylene mulch in the soil may lead to the formation of environmentally harmful chemical products, such as aldehydes and ketones.6 Due to high chemical stability, polyethylene requires about 100 years for its complete decomposition.1
To rectify this problem, an “ideal” plastic mulch should have biodegradable properties and undergo full mineralization at the end of growing season.7 The innovation in formulations to make mulch films more biodegradable began several decades ago and still remains a significant focus of research and development.8 The use of biodegradable plastic mulch was suggested as a viable alternative to polyethylene plastic mulch in crop production.9 Interest in biodegradable films has strongly increased in recent years, due to consumer demand, agricultural needs, and the current prominence of environmental concerns. Conventional plastic processing technology has been adapted to manufacture biodegradable plastic mulch.10 A number of biopolymers such as polylactic acid (PLA) and polyhydroxyalkanoates (PHA) play an important role in manufacturing biodegradable plastic mulch. Biodegradable polymers can be made by processes that use additives to improve the mechanical and physical properties of the resultant film.10
Studies have demonstrated that mineral nutrients and herbicides can be embedded in the bioactive films to further improve crop yield.11 For example, the application of nitrogen combined with biodegradable film significantly improved nitrogen uptake and subsequently enhanced crop yield in rice. This improved fertilization efficiency was enabled by the use of biodegradable film as mulch in rice.10
Among food crops, legumes inherently have the ability of symbiotic nitrogen fixation, leading to a major advantage for agricultural and environmental sustainability worldwide.12 Therefore, food security and soil fertility could be significantly improved by increasing the cultivated area and production of legume crops.12 Fava bean (Vicia faba L.) is one of the most important food legumes and is regarded as the main source of affordable protein for many societies.13,14 However, successful production of fava bean in the presence of biotic factors such as weeds depends upon timely application of appropriate chemical treatments.15 The increasing global interest in cropping systems has renewed scientific interest in the development of innovative methods for weed management in food legumes.16 To improve the yield of such species, the main principal of conservation agriculture is to cover the soil with mulch, particularly when grown as a vegetable crop for green pods.17
Weed infestation is the main biotic constraint in agriculture production systems, leading to poor establishment of crops which heavily constrains their production.18 Hence, the integration of herbicide with other crop management strategies such as mulch could result in greater yield advantage over the application of herbicide alone. For example, an integrated use of mulch and herbicide provided more effective and sustainable weed control which subsequently increased crop yield in the seeded rice systems.19
Many of the advances made in the agricultural sector have been due to the development of agrochemicals, including herbicides.20 One of the innovative approaches investigated recently is the introduction of controlled release systems for weed suppression to enhance productivity of food crops.21 Furthermore, advances made in the area of biopolymers have stimulated research in the development of carrier systems for sustained release of biologically active compounds for agricultural applications. These controlled release carrier systems have the potential to reduce adverse environmental impacts, while ensuring high crop productivity. For example, researchers modified the formulation and preparation for sustained release of the herbicide chloridazone using biodegradable polymers such as lignin and ethylcellulose.22 The use of such biopolymers in controlled release formulations helped to increase efficiency of the delivery of herbicide. Similarly, the release profile of the herbicide amertyn was improved by its encapsulation in the form of microparticles using the polymers poly(3-hydroxybutyrate), PHB, and poly(3-hydroxybutyrate-co-3-hydroxyvalerate), PHBV, with delivery being slower and more prolonged compared to free ametryn application. The successful encapsulation of ametryn in polymeric microparticles indicated that such systems could be useful in reducing the adverse environmental effects of herbicide.22 Preliminary research on release kinetics using microspheres composed of poly(3-hydroxybutyrate-co-3-hydroxyvalerate), PHBV, loaded with the herbicide atrazine demonstrated that PHBV is a promising carrier for atrazine, though it is suggested that more work is required to determine its toxicity and the mechanism of action.23 Another more recent study demonstrated the feasibility of using blends of biodegradable polylactide (PLA) and poly(ethylene glycol), PEG, as an environment friendly controlled-release system for soil-applied herbicide. This study demonstrated the potential for using immobilized herbicides blended with slowly biodegradable PLA and water-soluble PEG to release herbicide for up to six months from application for control of broad-leaf weed species.24
Recently, a study on biodegradable light converting agricultural films derived from the blend of PLA (35%) with PBAT (65%) and contained rare earth complexes was conducted.25 It was concluded that PLA/PBAT/EuTT films could emit red fluorescence (617 nm) under ultraviolet light (365 nm) and were very promising for applications related to agricultural mulching films due to their light conversion ability and biodegradable properties. These studies indicate that MCPA-PHBV conjugates may have potential for application in agricultural systems to provide relatively longer duration of contact of selected herbicide with the weed species. This could help target weeds with greater precision, while at the same time reducing impacts on the wider environment and nontarget crop plants. Hence, the use of biofilm biodegradable mulch formulations based on the modified release of active ingredients of herbicides can help to reduce contamination of the environment, since smaller quantities of the agrochemicals are required compared to traditional applications to achieve the desired result.
In this regard, the results of our preliminary study on the application of biodegradable mulch for the control of broad leaf weed species indicated that MCPA-PHBV conjugate suppressed weed growth.26 However, the main limitation of this pilot study was the relatively large range of MCPA levels tested, against limited broadleaf weed species, which germinated as a result of natural soil infestation. The current study is a follow-up to our previous investigation and has been meticulously designed to examine a narrower range of herbicide MCPA levels embedded within the biodegradable bioactive films, against a wider variety of broadleaf species, using artificial weed infestation of soil media.
The aim of the current study was to test the biodegradable mulch as a modified release system for herbicide MCPA conjugated with poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) using the melt transesterification route. The main purpose of conjugating MCPA with PHBV was to produce a sustained release system that could enable the herbicide to be used more safely in the agricultural system, thus minimizing the adverse environmental impact. Therefore, the feasibility of the biodegradable mulch containing herbicide MCPA was investigated for controlling broad leaf weeds in fava bean (nontarget food legume crop). The hypothesis is that the herbicide incorporated in the mulch can act on weed species of a diverse range with greater specificity, without adverse effect on nontarget species (fava bean).
Material and Methods
The experiment was designed as a completely randomized design having four replications. The study was conducted during the summer season (June–August) of 2017 in the roof-top greenhouse facility, based at the University of Wolverhampton, United Kingdom. Fava bean plants were grown in special cylindrical plastic pots (10 × 40 cm) filled with 3400 g of loamy sand soil. Each pot (2/3rd based on volume) was first filled with 2600 g of common soil then the upper 15 cm was topped up with 800 g of weed-infested soil to allow weed seeds to germinate.
Soil used for this study was collected from Hilton Research Station, Shropshire, U.K. This location is situated 15 km west of Wolverhampton 52°33′05.7″ N, 2°19′18.3″W (U.K. National Grid Reference S0778952). The region experiences a temperate climate with a mean annual precipitation of 620 mm, and soil is naturally infested with weed flora found in such ecological conditions. Soil texture at the experimental site is loamy sand contained soil organic matter content of 1.9%, sand of 79.8% (2000–60 μm), silt of 14.8% (60–2 μm), and clay of 5.4% (<2 μm).27
The mulch film comprising herbicide 2-methyl-4-chlorophenoxyacetic acid (MCPA) conjugated with poly(3-hydroxybutyrate-co-3-hydroxyvalerate), PHBV was prepared via a melt transesterification route. The resultant bioactive oligomer was then mixed with a blend of 30 mol % of polylactide (PLA) and 70 mol % of poly(butylene adipate-co-terephthalate), PBAT, with different loadings of MCPA-PHBV conjugate to manufacture the films to be used as bioactive, biodegradable mulch in fava bean to slowly release herbicide for weed suppression.26 The molar mass of plain PHBV (contained below 5% of HV units) was Mw = 316000 g/mol, and the molar masses of plain PLA and PBTA were Mw = 91200 g/mol and Mw = 30000 g/mol, respectively.
Fava bean (Vicia faba) inbred line Melodie (spring type variety) was used as a nontarget crop species in this experiment. Fava bean seeds were germinated on moist Whatman filter paper at 25 °C for 5 days in an incubator. Two radicle-emerged seeds were sown at 3 cm depth in each pot. After 7 days, plants were thinned out to one plant per pot. Weeds were allowed to freely grow in pots for the first 3 weeks during the experiment.
When fava bean plants were 21 days old and all pots were showing vigorous weed growth, numbers of weed plants were counted and weed species were photographed for further identification. Bioactive films (BioFlex) containing different concentrations of herbicide MCPA (Table 1) were placed over the soil surface (10 cm diameter in each pot) in mulch film treatments, while no film was used in control pots. The ambient greenhouse temperature was maintained at 25 °C ± 2 °C and a relative humidity of 60% ± 5%. Phytolux Attis7 LED growth lights were used for supplemental lighting to maintain Photosynthetic Photon Flux Density (PPFD) of approximately 200 μmol m2 s–1 at the canopy level during day time.
Table 1. Bioactive Mulch Films Containing Following Concentrations of Herbicide MCPA, Tested in the Pot Experiment.
| No. | Treatments |
|---|---|
| 1 | Control (No film) |
| 2 | Bio Flex V-008 MCPA 0% |
| 3 | BioFlex V-008 MCPA 1.0% |
| 4 | BioFlex V-008 MCPA 2.5% |
| 5 | BioFlex V-008 MCPA 5.0% |
| 6 | BioFlex V-008 MCPA 7.5% |
| 7 | BioFlex V-008 MCPA 10% |
Data collection on physiological parameters related to plant health were started when fava bean plants were four-week-old. Stomatal conductance was determined using a leaf porometer (SC1-Decagon, USA) on the abaxial surface of the youngest fully expanded leaf (third/fourth from the top) between 11:00 am to 13:00 pm. Chlorophyll content index was assessed from a fully lit youngest expanded leaf with a SPAD meter (SPAD-502 plus, Konica Minolta, Japan) while photosynthetic efficiency as quantum yield of photosystem II (Yield-II) was determined by a chlorophyll fluorometer (Mini-PAM II Photosynthesis Yield Analyzer, WALZ, Germany). The leaf temperature was measured using a dual laser infrared thermometer (Extech, USA). Light intensity and greenhouse temperatures were noted regularly when measurements on stomatal conductance and chlorophyll fluorescence were recorded to avoid any confounding effect on these sensitive physiological parameters.
In order to obtain an elemental distribution scan for the elements present on the surface of the bioactive mulch film, both before and after the greenhouse pot experiment, a sample of each bioactive mulch film (approximately 10 × 10 mm) was mounted on to a scanning electron microscope (SEM) stub using double-sided carbon sticky tabs (Agar Scientific G3348N). The sample was then coated with ∼12 nm of Pt in an Emscope Sc 500 sputter coater and the samples were examined in a Hitachi TM3030 SEM at 15 kV. The energy-dispersive X-ray spectroscopy (EDS) was performed using an Oxford Instruments SwiftED3000 module attached to the SEM. The objective of this analysis was to determine the distribution of chlorine (a signature element present in herbicide MCPA but not in the PHBV polymer matrix) both before and after the greenhouse pot experiments to monitor the release of the MCPA over the course of the experiment.
The molar mass and the molar mass distribution of the mulch films, collected before and after the greenhouse experiment, were analyzed using a TOSOH EcoSec HLC/GPC 8320 system equipped with a RI detector, operating at a temperature of 40 °C. The column used was TSKgel HZM-N calibrated against polystyrene standards with low dispersity ranging from 560 to 70000 Da. The UV detector was set at a wavelength of 254 nm. Chloroform was used as the eluent at a flow rate of 0.25 mL/min. A sample size of 2 μL was injected into the system using an autosampler. Additionally, FTIR analysis was run for mulch films, before and after use, on a Bruker alpha ATR for a small sample of the film, which was cleaned using deionized water and then dried in an oven at 70 °C overnight. The infrared spectra were obtained by averaging the 16 scans from 600 to 4000 cm–1 with a resolution of 4 cm–1.
The data recorded during the course of experiment were subjected to analysis of variance using statistical software IBM SPSS 24 and means were compared using least significant differences (LSD) at 5% level of probability (P < 0.05).
Results and Discussion
Weed Infestation and Suppression
Data on the weed population recorded 3 weeks after the planting of the fava beans indicated that the soil used in the greenhouse pot experiment was heavily infested predominantly with seeds of broadleaf weeds (Table 2). The weed population counted at the time of bioactive mulch film application demonstrated nonsignificant statistical differences in weed numbers between the experimental treatments, which is an important point to determine the validity of the present study on weed infestation and suppression caused by the MCPA-PHBV bioactive film (Table 2). As expected, the weed species flourished in the negative controls, i.e., when no mulch film was applied and also when the mulch film containing zero MCPA was used (Figure 1).
Table 2. Weed Population (Number of Weeds/Pot) Counted at the Time of Bioactive Mulch Film Application When Faba Bean Plants Were 3 Weeks Old.
| Treatments | Weed count/pot |
|---|---|
| Control–no mulch | 12.5 |
| Bioflex V-008 0% conc | 11.5 |
| Bioflex V-008 1.0% conc | 13.0 |
| BioFlex V-008 2.5% conc. | 12.0 |
| BioFlex V-008 5.0% conc. | 10.5 |
| BioFlex V-008 7.5% conc. | 16.5 |
| BioFlex V-008 10.0% conc. | 16.0 |
| LSD (P ≤ 0.05) | |
| Standard Error | 1.36a |
Nonsignificant
Figure 1.
Complete weed suppression in pots having MCPA-bioactive mulch film while weeds can be seen growing in control treatments.
The results showed that MCPA effectively killed all broadleaf weed species commonly grown under temperate climatic conditions of England (Table 3). Weed infestation was clearly controlled when bioactive film containing MCPA was applied as postemergence mulch. More specifically, no weed growth was observed even when the bioactive film containing the lowest concentration of MCPA (1%) was used. These observations clearly indicate that the herbicide (MCPA) embedded into the bioactive film was very effective at controlling broadleaf weed growth at all concentrations tested. A study on rice showed that an integrated use of herbicide with other weed management strategies resulted in an effective weed control and increased grain yield in the seeded rice-system which was not possible with herbicide alone to provide season-long weed control.28 Similarly, it was reported that the combined use of herbicide and mulch provided more effective and sustainable weed control in dry-seeded rice systems.19 Our results are in agreement with the findings that a combination of herbicide and mulch suppresses weed growth more effectively than the mulch with no herbicide.
Table 3. List of the Broadleaf Weed Species Identified from the Pot Experiment When Bioactive Mulch Film Was Applieda.
| Botanical name | Common name | Image available at: |
|---|---|---|
| Artemisia vulgaris | Mugwort | https://depositphotos.com/stockphotos/arom.html; https://www.123rf.com/profile_photographieundmehr |
| Chenopodium album | Fat-hen | https://keyserver.lucidcentral.org/weeds/data/media/Html/chenopodium_album.pdf |
| Picris hieracioides | Hawkweed oxtongue | http://luirig.altervista.org/pics/index5.php?recn=193920&page=1 |
| Silene dioica | Red campion | http://science.halleyhosting.com/nature/gorge/5petal/pink/silene/rcamp.htm |
| Sonchus arvensis | Sow-thistle | http://thaoduocquyhcm.com/tanphat/cay-thuoc-viet-nam/diep-dai/ |
| Urtica dioica | Common nettle | http://kepek.4ever.eu/termeszet/novenyek/csalan-230387 |
Weed composition show a prevalence of common broadleaf weed species grown under temperate climatic condition.
Release of Herbicide (MCPA) from Bioactive Mulch Films
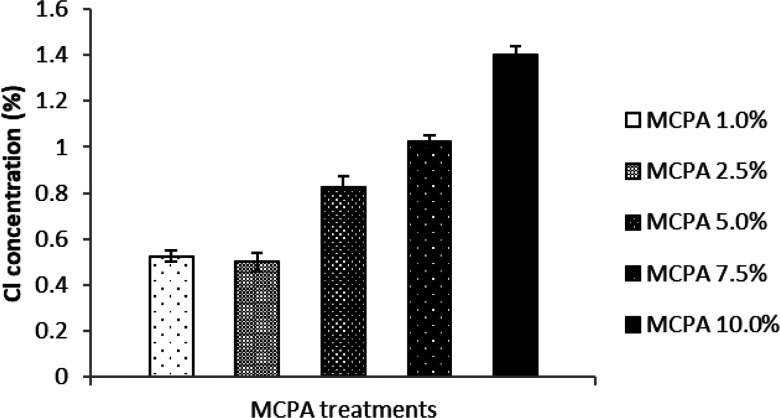
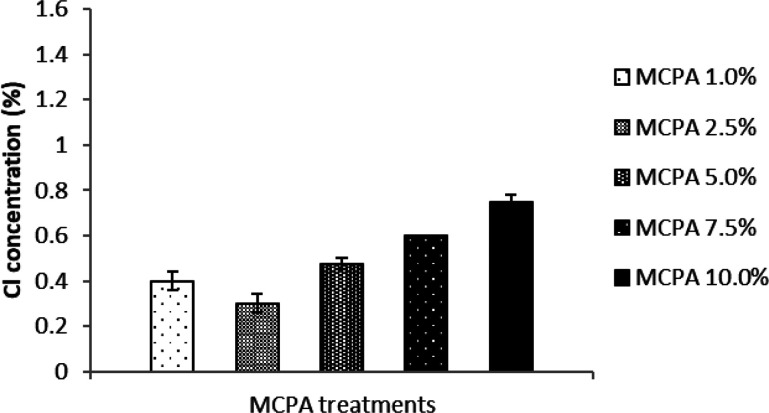
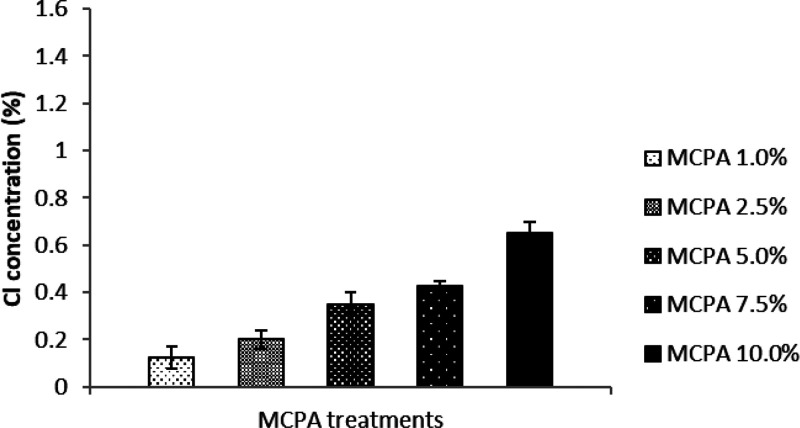
It can be seen from the EDS data depicted in Figure 2 that the quantity of the signature element Cl correlates well with the concentration of MCPA used to coat the bioactive mulch film and that it is absent in the negative control, as previously reported.26 Furthermore, the results from the EDS analysis performed after the greenhouse experiments indicate that the quantity of signature element Cl has decreased substantially in all the mulch film samples coated with MCPA. Overall, the trend in the results correlates with the initial concentration of MCPA present on the bioactive films (Figure 3). The data demonstrate that MCPA has been released from the bioactive mulch films over the course of the experiment. The release of the herbicide seems more pronounced in the higher level loadings (MCPA 5%–10%) than in the lower levels (MCPA 1%–2.5%) and some residual herbicide is still present on the films after the experiment is completed, which further supports the hypothesis that these bioactive mulch films provide a sustained release of the herbicide, over time (Figure 4).
Figure 2.
Concentration of signature element Cl indicative of the MCPA concentrations present in the bioactive mulch film before use in the greenhouse pot experiment. The vertical error bars represent standard error based on four replications.
Figure 3.
Concentration of signature element Cl indicative of the MCPA concentration present in the bioactive mulch film after use in the greenhouse pot experiment. The vertical error bars represent standard error based on four replications.
Figure 4.
Release of herbicide (MCPA) from the bioactive mulch film over the course of the greenhouse pot experiment as shown by the difference in the Cl concentration recorded in the mulch film before and after the greenhouse experiment. The vertical error bars represent standard error based on four replications.
The results of GPC analysis of the bioactive films with various concentrations of MCPA-PHBV oligomer (0%–10%) before and after the pot experiment are shown in Table 4. The molar masses estimated are only apparent and do not reflect the changes for individual blend components. However, with an increase of MCPA concentration, the original apparent molar mass of the sample decreased gradually, as shown in the Table 4. It may be caused by some degradation during mulch films preparation using a hot pressing method. A similar effect was recently observed for the PLA component, after being extruded by a double screw extruder of PLA and PBAT with rare earth complexes.25 After the pot experiment, the apparent molar mass of the mulch film that does not contain MCPA-PHBV oligomer (the first entry in Table 4) has some decrease (both Mn and Mw), indicating that the polymer blend component had a degradation. However, the changes of molar mass dispersity (Mw/Mn factor), observed for the other entries in the Table 4, may indicate on the MCPA-PHBV oligomers release from the biodegradable mulches into the soil as to what prevented the growth of the weeds. This phenomenon is consistent with our previous study.26 Despite the fact that PLA/PBAT blends are compostable (according to ASTM G160–98) they do not reach total degradation in a 12-month period in soil.29 The blend has low wettability and lower sorption of water which most probably affects the fragmentation, mineralization, and subsequent assimilation of the bioactive film by soil microorganisms.30
Table 4. Molar Mass of the Bioactive Films before and after Use As Mulch, Measured by GPCa.
| Sample | Mn | Mw | Mw/Mn |
|---|---|---|---|
| 0%-before | 7700 | 83400 | 10.8 |
| 0%-after | 7200 | 78600 | 10.9 |
| 1%-before | 6700 | 68200 | 10.2 |
| 1%-after | 7000 | 65900 | 9.4 |
| 2.5%-before | 4700 | 60700 | 12.9 |
| 2.5%-after | 5700 | 59600 | 10.4 |
| 5%-before | 3600 | 41200 | 11.4 |
| 5-after | 5700 | 53800 | 9.4 |
| 7.5%-before | 4100 | 39300 | 9.5 |
| 7.5%-after | 3600 | 43300 | 12.0 |
| 10%-before | 2700 | 34000 | 12.5 |
| 10%-after | 3500 | 36500 | 10.3 |
Mn is the number averaged molar mass, and Mw is the weight averaged molar mass. The Mw/Mn is a measure of the dispersity of the molar mass distribution. The percentage indicates the MCPA-PHBV oligomer concentration in the films.
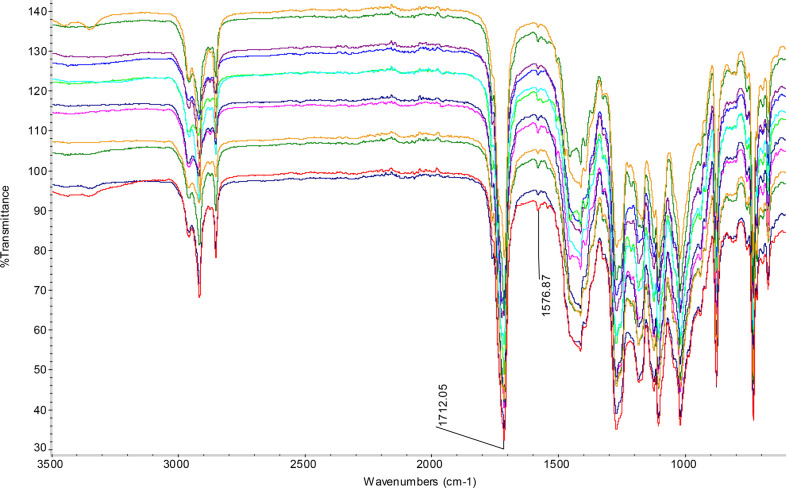
The FTIR spectra of the mulch films before and after the use are shown in Figure 5. All the spectra are quite similar before and after the experiment. The band at 1712 cm–1 is attributed to all C=O group absorption of the ester bonds, which includes both aliphatic ester from both PBAT and PLA and PHBV, and aromatic ester from only PBAT. The band at 1576 cm–1 is attributed to the C=C bond of aromatic ring, which is mainly from the aromatic ester in the PBAT. As evident from Figure 5, the intensity of the band for the benzene ring increases after the test. In order to focus on this increase, the areas of both the bands at 1712 cm–1 and at 1576 cm–1 are integrated and the ratios are presented in the Table 5. This indicates a substantial increase in benzene ring content after the pot experiment because some of the aliphatic part left the film by degradation and diffusion into the soil clearly showing degradation of the MCPA-PHBV oligomer.
Figure 5.
FTIR spectra of the bioactive films before and after use as mulch. From the top to the bottom, the spectra are for 0-b, 0-a, 1-b, 1-a, 2.5-b, 2.5-a, 5-b, 5-a, 7.5-b, 7.5-a, 10-b, 10-a. The ‘b’ represents before test and ‘a’ represents after application while the number before the letter indicates the MCPA-PHBV oligomer concentration in the film.
Table 5. FTIR Band Area (in Arbitrary Units) at 1712 and 1576 cm–1a.
| Sample | A1712 [a.u.] | A1576 [a.u.] | A1581/A1712 [a.u.] × 102 |
|---|---|---|---|
| 0%-before | 18.24 | 0.038 | 0.21 |
| 0%-after | 22.052 | 0.084 | 0.38 |
| 1%-before | 21.47 | 0.039 | 0.18 |
| 1%-after | 19.255 | 0.066 | 0.34 |
| 2.5%-before | 21.253 | 0.035 | 0.16 |
| 2.5%-after | 23.504 | 0.116 | 0.49 |
| 5%-before | 23.87 | 0.04 | 0.17 |
| 5%-after | 23.629 | 0.173 | 0.73 |
| 7.5%-before | 21.123 | 0.037 | 0.18 |
| 7.5%-after | 19.496 | 0.122 | 0.63 |
| 10%-before | 19.921 | 0.047 | 0.24 |
| 10%-after | 18.587 | 0.177 | 0.95 |
The 1712 cm–1 is for C=O bond absorption due to the presence of ester, and 1576 cm–1 is for benzene ring absorption of the aromatic ester. The percentage indicates the MCPA-PHBV oligomer concentration in the films.
Plant Growth in Nontarget Plants, Fava Bean
Plant Height
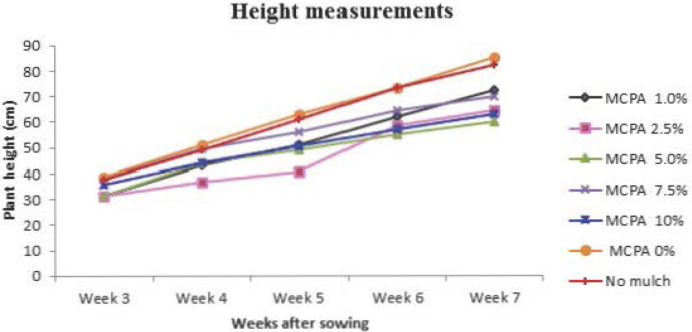
Among plant health parameters, plant height is considered a primary indicator of plant growth rate. Therefore, plant height was measured to detect if there were any adverse effects of the MCPA concentrations on growth of fava bean plants. Data were recorded weekly from the early vegetative stage when fava bean plants were at the three leaf growth stage. Plants treated with high concentrations of MCPA showed reductions in plant height in comparison with plants exposed to bioactive mulch film without MCPA and no film (Figures 6 and 7). It seems that stunted growth is probably related to the mode of action of the herbicide restricting metabolic activities. Results of an herbicide study on winter wheat showed that plant height was decreased substantially when the fluoroxypyr plus MCPA ester was applied at a relatively high rate.31 It has also been reported that herbicidal treatments of metribuzin and isoproturon + diflufenican produced stunted plants which may be due to the phytotoxic effect of these herbicides on wheat.32 These observations support the results of present study that the toxicity of MCPA adversely affects plant growth in fava bean and that higher concentrations of the herbicide further exacerbate the phytotoxic effect on the crop plants.
Figure 6.
Plant image showing reduction in plant growth particularly at higher concentrations of MCPA in the bioactive mulch films in six-week-old fava bean plants. MCPA concentrations from 10 to 0% (left to right) in comparison with control treatment (extreme right).
Figure 7.
Differences in growth evident from plant height measurements of fava bean recorded periodically during the experiment.
Leaf Area
The leaf area of the youngest fully expanded leaf was determined when the plants were 53 days old. As mentioned above, the symptoms of the phytotoxic effect of the MCPA on the plants, such as stunted growth and chlorotic leaves, were visible mainly at higher concentrations. Statistical analysis of the data showed highly significant differences in the leaf area between bioactive mulch film treatments. Plants treated with higher concentrations of MCPA showed smaller leaf area when compared with that of plants grown under no herbicide or no film conditions (Table 6). Highest leaf area (35.2 cm2) was recorded in Bioflex 0% MCPA treatment, followed by no-biodegradable mulch control, while leaf area at higher concentrations (2.5–10% MCPA) exhibited as much as 50% reduction. The smaller leaf area clearly indicates that when MCPA is applied at concentrations higher than 1%, it induced a phytotoxic effect in fava bean, most probably due to crop’s broadleaf morphology. Hence, there is a need to standardize the MCPA concentration which can effectively suppress weeds without having any adverse effect on the crop.
Table 6. Leaf Area (cm2) of the Youngest Fully Expanded Trifoliate Leaf of Faba Bean Recorded after 53 Days in Different MCPA Treatments and Controls with Zero MCPA and No Mulch Film.
| Treatments | Leaf area (cm2) |
|---|---|
| Control–no mulch | 31.2 |
| Bioflex V-008 0% conc | 35.0 |
| Bioflex V-008 1.0% conc | 27.3 |
| BioFlex V-008 2.5% conc. | 13.9 |
| BioFlex V-008 5.0% conc. | 16.5 |
| BioFlex V-008 7.5% conc. | 19.2 |
| BioFlex V-008 10.0% conc. | 17.2 |
| LSD (P ≤ 0.05) | |
| Standard Error | 1.98a |
Highly significant.
Physiological Parameters
Stomatal Conductance (gs)
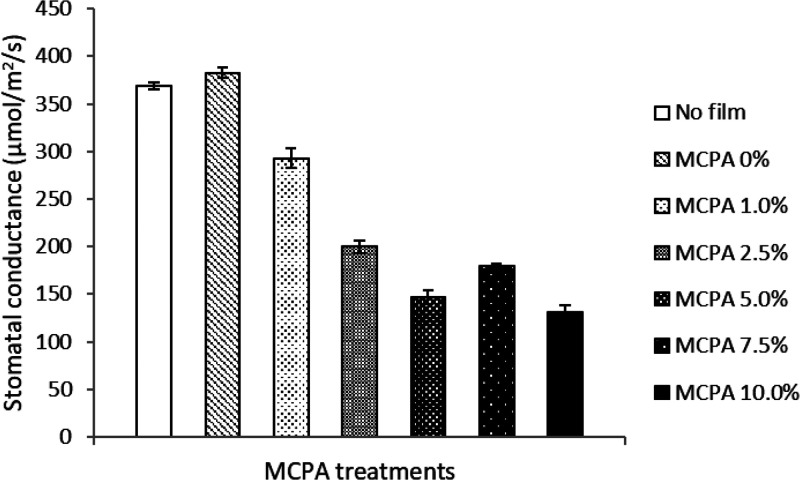
Stomatal conductance mainly drives the plant’s relations with the environment and is regarded as a physiological determinant for photosynthetic rate and metabolic profile, which finally governs crop productivity. The stomata influence plant responses to abiotic and biotic stresses, regulation of water fluxes, and nutrient uptake in plants.33 The data on stomatal conductance (gs) in the present study showed a decreasing trend with an increase in MCPA concentrations from 0 to 10% in the bioactive mulch film (Figure 8). Plants treated with 0% MCPA showed maximum stomatal conductance (383 mmol/m2/s) while minimum stomatal conductance (131 mmol/m2/s) was recorded in the 10% MCPA treatment (Figure 8). Thus, higher stomatal conductance was exhibited at lower concentrations of MCPA in the bioactive mulch film treatments. These results indicate that stomatal conductance was significantly lower in stunted plants probably due to the phytotoxic effect of MCPA at higher concentrations. In an earlier study, the application of herbicide iodosulfuron mixed with mesosulfuron-methyl at higher concentrations reportedly caused a reduction in stomatal conductance and subsequently reduced photosynthetic rate in wheat plants.34 The physiological role of stomata is to act as a gateway for efficient gas exchange and water movement. Hence, stomatal conductance regulates plant water potential through balancing water supply from the roots with the transpirational demand of plants.35
Figure 8.
Stomatal conductance (μmol/m2/s) recorded in the experimental treatments. The vertical bars represent standard errors based on four replications.
Leaf Temperature
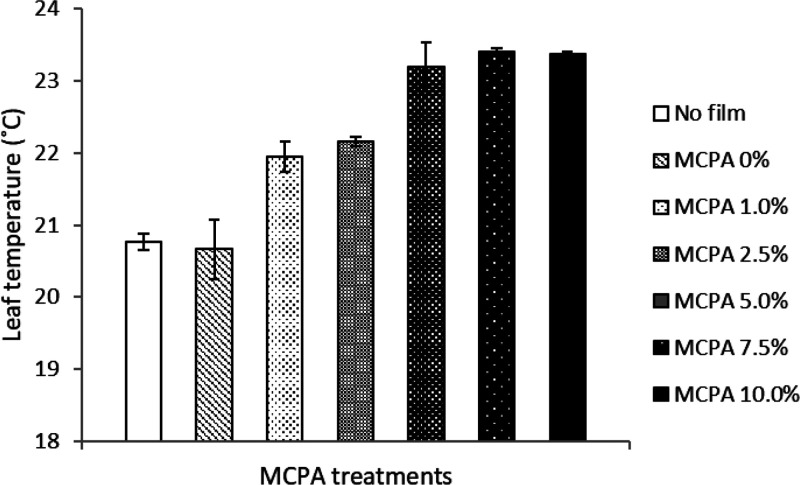
Canopy temperature data can provide important physiological information for plant health. Leaf surface temperature measurements from the present study showed an increase with increase in the herbicide concentration (Figure 9). Plants in bioactive film embedded treatments with higher concentrations of MCPA (5.0, 7.5, and 10.0%) used as mulch showed significantly higher leaf temperatures when compared with those of the control treatments. The possible explanation is that the plants displayed a higher leaf surface temperature because they were experiencing restricted transpiration due to stomatal closure as depicted in the stomatal conductance data, which in turn reduced the cooling capacity of the leaves. A recent study revealed the adverse effect of elevated leaf temperature on vegetative growth and grain yield of maize because higher leaf temperatures induced early leaf senescence which subsequently reduced the ability of maize to continue normal growth.36
Figure 9.
Variation in leaf temperature of fava bean in response to MCPA concentrations in the bioactive mulch films. The vertical bars represent standard error based on four replications.
Photosynthetic Efficiency: Quantum Yield of Photosystem II (PSII)
Fluorescence emission measurement is a modern, noninvasive, and widely used technique for understanding changes in the photosynthetic capacity of plants. Therefore, chlorophyll fluorescence data are frequently used to determine the state of energy distribution in thylakoid membrane and to assess quantum efficiency of the photosystem II (PSII).37 In the present study, data recorded on the quantum yield of PSII (Yield-II) indicate that higher concentrations of MCPA (particularly 7.5% and 10%) were responsible for restricting the photosynthetic efficiency of fava bean plants, as in these treatments, the values for Yield-II were significantly lower (0.733- 0.735). Plants with no bioactive mulch film were more efficient in performing photosynthesis as reflected in their Yield-II (0.747). Interestingly, an even higher photosynthetic rate (Yield-II 0.751) was recorded when bioactive film with zero MCPA was applied as mulch (Table 7). Hence, this study demonstrates that fava bean plants are sensitive to higher levels of herbicide MCPA because the high concentrations treatments resulted in reduced quantum yield of photosystem II (Yield-II) restricting photosynthetic efficiency in fava bean plants.
Table 7. Influence of MCPA Application on Quantum Yield of Photosystem II (Yield-II) in Faba Bean Plants Measured by a Chlorophyll Fluorometer (Mini-PAM 03).
| Treatments | Quantum yield of photosystem II |
|---|---|
| No film | 0.747 |
| MCPA 0% | 0.751 |
| MCPA 1.0% | 0.738 |
| MCPA 2.5% | 0.737 |
| MCPA 5.0% | 0.742 |
| MCPA 7.5% | 0.735 |
| MCPA 10% | 0.733 |
| LSD (P ≤ 0.05) | |
| Standard Error | 0.001a |
Highly significant
The observations reported from maize seedlings support our results where higher doses of the herbicide Imazapic lowered chlorophyll contents and caused a reduction in net photosynthetic rate.38 In addition, the results of the chlorophyll fluorescence evaluation in radish indicated that plants treated with herbicide glyphosate had lower Yield-II values compared to the untreated control. Glyphosate treatment resulted in substantial decrease in the Fv/Fm ratio, clearly an adverse effect of higher concentrations. Furthermore, the analysis of chloroplast pigment additionally revealed that the content of both chlorophyll a and b decreased with increase in glyphosate concentrations.39
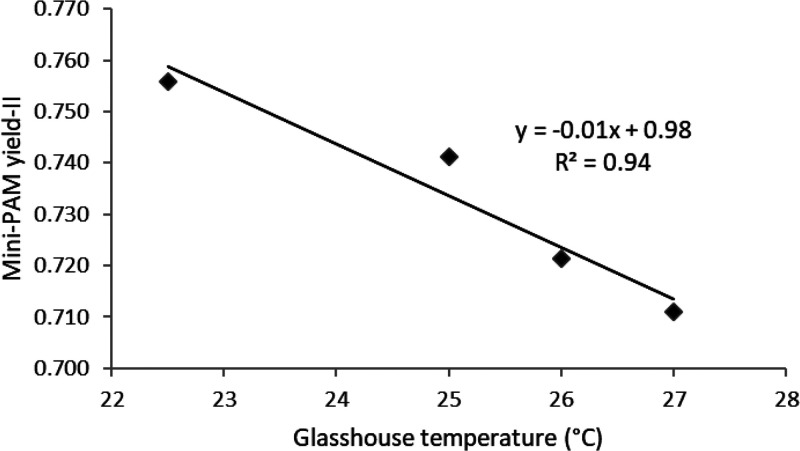
Chlorophyll fluorescence is a convenient means of measuring the function of photosystem-II (PS-II) because this technique has great potential in the diagnosis of stress effects in plants. However, our results show that the attributes associated with Photosystem II are highly sensitive to temperature variation. It is evident from the chlorophyll fluorescence data that exposure of fava bean plants to higher greenhouse temperatures led to a significant (P < 0.05) decline in the quantum yield of photosystem II regardless of MCPA concentrations (Figure 10). Apparently, exposure to relatively higher temperatures decreased the operating quantum efficiency of PS-II, which characterizes the functional activity of the photosynthetic apparatus. These results provide a better understanding of the high temperature effect on the photosynthetic process and its underlying reactions, notably photochemistry.
Figure 10.
Influence of temperature variation on measurements of quantum yield of photosystem II (Yield II). Mean value shown in the graph is based on 52 readings across experimental treatments. Data for treatments were pooled to show the effect of temperature variations on Yield II in greenhouse environment.
One probable explanation is that exposure of the plants to high temperature caused an inactivation of the PS-II apparatus, particularly the maximum photochemical efficiency and the quantum yield of PS-II.40 The high temperature exposure studies on plants suggest that hot temperature damaged the light harvesting process and lead to the blockage of PS-II.41 In light of these observations, it would be wise to conduct future chlorophyll fluorescence investigations in temperature-controlled environments to avoid any adverse effect of high temperature on responses of photosystem-II.
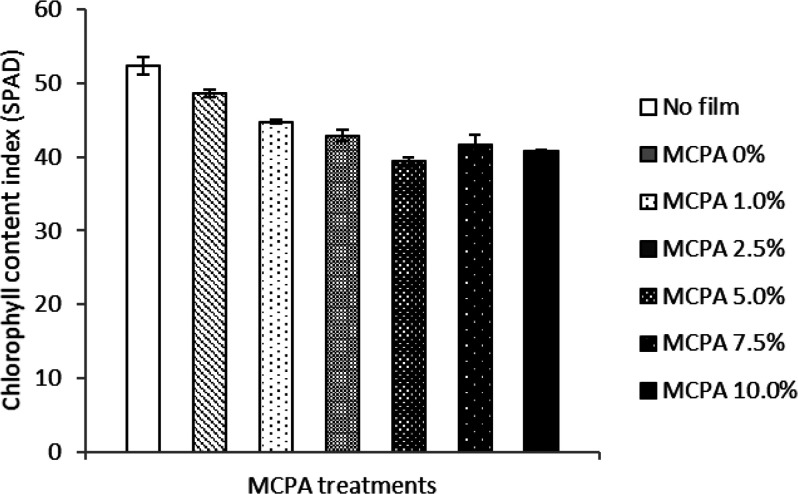
Chlorophyll Content Index (SPAD)
The higher concentrations of the herbicide MCPA in the bioactive film altered the chlorophyll content index (SPAD) in fava bean plants (Figure 11). The data show that the SPAD index significantly decreased with increasing the concentration of MCPA in bioactive film while the index was recorded higher in both control treatments (0% MCPA and control without film). The plants treated with MCPA concentrations 5 and 10% displayed significantly lower values (39.4 and 40.8) indicating a considerable reduction in chlorophyll contents of leaves, while plants grown without mulch cover, or those treated with bioactive film containing no MCPA, looked healthier and also showed higher values of SPAD index (52.4 and 48.7, respectively).
Figure 11.
Difference in chlorophyll content index (SPAD) in leaves of fava bean plants treated with different MCPA concentrations in bioactive mulch film. The vertical bars represent standard errors based on four replications.
Similar results were reported from an herbicide study on creeping bentgrass. The plants experienced substantial decline in chlorophyll content in the same period of growth when MCPA concentration increased.42 In short, our results suggest that the higher concentration of MCPA in the bioactive mulch films caused a reduction in the chlorophyll content of fava bean leaves when compared with controls.
Conclusions
In conclusion, it is evident from the results obtained in the current study that the innovative, slow-release MCPA-PHBV conjugate worked efficiently. The MCPA herbicide embedded in the biodegradable mulches and used as postemergence mulch effectively killed all broadleaf weed species that commonly grow under temperate climatic conditions. It is also important to note that the lowest concentration (1%) of MCPA tested was equally effective for weed suppression as the higher concentrations (2.5, 5.0, 7.5 and 10%), but higher concentrations adversely affected the growth of fava bean plants. The common toxicity symptoms observed included restricted plant height, chlorotic leaves, lesser stomatal conductance, higher leaf temperature, and a substantial reduction in photosynthetic efficiency. Therefore, further studies are warranted to optimize a safe concentration of the herbicide MCPA (probably less than 1%) to use in bioactive mulch films, for effective broadleaf weed control, while causing no adverse effect on agricultural crops.
Acknowledgments
The authors would like to thank Dr. Kristof Molnar from Department of Food, Agricultural and Biological Engineering, The Ohio State University, USA for helpful discussion. This work was funded by the Research Investment Fund, University of Wolverhampton (Wolverhampton, UK). This paper is partly supported by the European Regional Development Fund Project EnTRESS No 01R16P00718 and European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 872152, project GREEN-MAP.
The authors declare no competing financial interest.
References
- Bilck A. D.; Grossmann M. V. E.; Yamashita F. Biodegradable mulch films for strawberry production. Polym. Test. 2010, 29, 471–476. 10.1016/j.polymertesting.2010.02.007. [DOI] [Google Scholar]
- Waterer D. Evaluation of biodegradable mulches for production of warm-season vegetable crops. Can. J. Plant Sci. 2010, 90, 737–743. 10.4141/CJPS10031. [DOI] [Google Scholar]
- Miles C.; DeVetter L.; Ghimire S.; Hayes D. Suitability of biodegradable plastic mulches for organic and sustainable agricultural production systems. HortScience 2017, 52, 10–15. 10.21273/HORTSCI11249-16. [DOI] [Google Scholar]
- Hussain I.; Hamid H.. Plastics in Agriculture. In: Andrady A. L., Ed., Plastic and the Environment. John Wiley & Sons: NJ, 2003; pp 185–206, 10.1002/0471721557. [DOI] [Google Scholar]
- Sintim H. Y.; Flury M. Is biodegradable plastic mulch the solution to agriculture’s plastic problem?. Environ. Sci. Technol. 2017, 51, 1068–1069. 10.1021/acs.est.6b06042. [DOI] [PubMed] [Google Scholar]
- Hakkarainen M.; Albertsson A. C. Environmental degradation of polyethylene. Adv. Polym. Sci. 2004, 169, 177–200. 10.1007/b13523. [DOI] [Google Scholar]
- Hayes D. G.; Dharmalingam S.; Wadsworth L. C.; Leonas K. K.; Miles C.; Inglis D. A.. Biodegradable agricultural mulches derived from biopolymers. In Degradable Polymers and Materials: Principles and Practices, 2nd ed.; Khemani K.; Carmin Scholz C., Eds., American Chemical Society: Washington, D.C., 2012; pp 201–223, 10.1021/bk-2012-1114.ch013. [DOI] [Google Scholar]
- Brodhagen M.; Peyron M.; Miles C.; Inglis D. A. Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl. Microbiol. Biotechnol. 2015, 99, 1039–1056. 10.1007/s00253-014-6267-5. [DOI] [PubMed] [Google Scholar]
- Kasirajan S.; Ngouajio M. Polyethylene and biodegradable mulches for agricultural applications: a review. Agron. Sustainable Dev. 2012, 33, 443–443. 10.1007/s13593-011-0068-3. [DOI] [Google Scholar]
- Zhang Y.; Liu M.; Dannenmann M.; Tao Y.; Yao Z.; Jing R.; Zheng X.; Butterbach-Bahl K.; Lin S. Benefit of using biodegradable film on rice grain yield and N use efficiency in ground cover rice production system. Field Crop Res. 2017, 201, 52–59. 10.1016/j.fcr.2016.10.022. [DOI] [Google Scholar]
- Minuto G.; Pisi L.; Tinivella F.; Bruzzone C.; Guerrini S.; Versari M.; Pini S.; Capurro M. Weed control with biodegradable mulch in vegetable crops. Acta Hortic. 2008, 801, 291–298. 10.17660/ActaHortic.2008.801.29. [DOI] [Google Scholar]
- Foyer C.; Lam H.; Nguyen H.; Siddique K.; Varshney R.; Colmer T.; Cowling W.; Bramley H.; Mori T.; Hodgson J.; Cooper J.; Miller A.; Kunert K.; Vorster J.; Cullis C.; Ozga J.; Wahlqvist M.; Liang Y.; Shou H.; Shi K.; Yu J.; Fodor N.; Kaiser B.; Wong F.; Valliyodan B.; Considine M. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 2. 10.1038/nplants.2016.112. [DOI] [PubMed] [Google Scholar]
- Khan H. R.; Paull J. G.; Siddique K. H. M.; Stoddard F. L. Faba bean breeding for drought tolerance: a physiological and agronomic perspective. Field Crop Res. 2010, 115, 279–286. 10.1016/j.fcr.2009.09.003. [DOI] [Google Scholar]
- Maalouf F.; Hu J., O’Sullivan D., Zong X.; Hamwieh A.; Kumar S.; Baum M.. Breeding and genomics status in faba bean (Vicia faba L.). Plant Breed. 2019, 138465. 10.1111/pbr.12644. [DOI] [Google Scholar]
- Stoddard F.; Nicholas A.; Rubiales D.; Thomas J.; Villegas-Fernández A. Integrated pest management in faba bean. Field Crop Res. 2010, 115, 308–318. 10.1016/j.fcr.2009.07.002. [DOI] [Google Scholar]
- Avola G.; Tuttobene R.; Gresta F.; Abbate V. Weed control strategies for grain legumes. Agron. Sustainable Dev. 2008, 28, 389–395. 10.1051/agro:2008019. [DOI] [Google Scholar]
- Kumar B. R. M.; Angadi S. S. Effect of tillage, mulching and weed management practices on the performance and economics of chickpea. Legume Res. 2016, 39, 786–791. 10.18805/lr.v0iOF.3552. [DOI] [Google Scholar]
- Gasim S.; Hamad S. A. A; Abdelmula A.; Ahmed I. A. M. Yield and quality attributes of faba bean inbred lines grown under marginal environmental conditions of Sudan. Food Sci. Nutr. 2015, 3, 539–547. 10.1002/fsn3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan B. S.; Abugho S. B. Integrated use of herbicide and crop mulch in suppressing weed growth in a dry-seeded rice system. Am. J. Plant Sci. 2013, 4, 1611–1616. 10.4236/ajps.2013.48195. [DOI] [Google Scholar]
- Zimdahl R.Six Chemicals That Changed Agriculture. First ed.. Academic Press, USA, 2015; pp 216. [Google Scholar]
- Campos E. V. R.; Oliveira J. L.; Fraceto L. F. Applications of controlled release systems for fungicides, herbicides, acaricides, nutrients, and plant growth hormones: A review. Adv. Sci., Eng. Med. 2014, 6, 1–15. 10.1166/asem.2014.1538. [DOI] [Google Scholar]
- Grillo R.; Pereira A. E. S.; de Melo N. F. S.; Porto R. M.; Feitosa L. O.; Tonello P. S.; Filho N. D.; Rosa A. H.; Lima R.; Fraceto L. F. Controlled release system for ametryn using polymer microspheres: Preparation, characterization and release kinetics in water. J. Hazard. Mater. 2011, 186, 1645–1651. 10.1016/j.jhazmat.2010.12.044. [DOI] [PubMed] [Google Scholar]
- Lobo F.; de Aguirre C.; Silva M.; Grillo R.; de Melo N.; de Oliveira L.; de Morais L.; Campos V.; Rosa A.; Fraceto L. Poly(hydroxybutyrate-co-hydroxyvalerate) microspheres loaded with atrazine herbicide: screening of conditions for preparation, physico-chemical characterization, and in vitro release studies. Polym. Bull. 2011, 67, 479–495. 10.1007/s00289-011-0447-6. [DOI] [Google Scholar]
- Rychter P.; Lewicka K.; Rogacz D. Environmental usefulness of PLA/PEG blends for controlled release systems of soil applied herbicides. J. Appl. Polym. Sci. 2019, 136, 47856.DOI: 10.1002/app.47856 10.1002/app.47856. [DOI] [Google Scholar]
- Wang D.; Yu Y.; Ai X.; Pan H.; Zhang H.; Dong L.. Polylactide/poly(butylene adipate-co-terephthalate)/rare earth complexes as biodegradable light conversion agricultural films. Polym. Adv. Technol. 2019, 30203. 10.1002/pat.4459. [DOI] [PubMed] [Google Scholar]
- Kwiecien I.; Adamus G.; Jiang G.; Radecka I.; Baldwin T.; Khan H.; Johnston B.; Pennetta V.; Hill D.; Bretz I.; Kowalczuk M. Biodegradable PBAT/PLA blend with bioactive MCPA-PHBV conjugate suppresses weed growth. Biomacromolecules 2018, 19, 511–520. 10.1021/acs.biomac.7b01636. [DOI] [PubMed] [Google Scholar]
- Fullen M. A.Technical report - Summary of teaching and research activities on the Hilton Experimental Site, East Shropshire (1976–2013). University of Wolverhampton. 2013; pp 38. [Google Scholar]
- Chauhan B. Weed ecology and weed management strategies for dry-seeded rice in asia. Weed Technol. 2012, 26, 1–13. 10.1614/WT-D-11-00105.1. [DOI] [Google Scholar]
- Rychter P.; Kawalec M.; Sobota M.; Kurcok P.; Kowalczuk M. Study of aliphatic-aromatic copolyester degradation in sandy soil and its ecotoxicological impact. Biomacromolecules 2010, 11, 839–847. 10.1021/bm901331t. [DOI] [PubMed] [Google Scholar]
- Harada J.; de Souza A.; de Macedo J.; Rosa D. Soil culture: Influence of different natural fillers incorporated in biodegradable mulching film. J. Mol. Liq. 2019, 273, 33–36. 10.1016/j.molliq.2018.09.109. [DOI] [Google Scholar]
- Brown L. R.; Sikkema P. H. Tolerance of three types of winter wheat (Triticum aestivum L.) to fluroxypyr plus MCPA ester. Can. J. Plant Sci. 2010, 90, 785–789. 10.4141/cjps10042. [DOI] [Google Scholar]
- Hussain S.; Sabir M. U. D.; Ali M.; Shah S. A. U. H. Efficacy and economics of different herbicides against narrow leaved weeds in wheat. Int. J. Agric. Biol. 2004, 6, 647–51. [Google Scholar]
- Costa R.; Saraiva A.; Carvalho L.; Duarte E. The use of biodegradable mulch films on strawberry crop in Portugal. Sci. Hortic. 2014, 173, 65–70. 10.1016/j.scienta.2014.04.020. [DOI] [Google Scholar]
- Guo X.; Guo P. Y.; Wang X. Y.; Zhao R.; Nan Y.; Wang W. Effects of Sigma Broad on photosynthesis and stomata conductance of winter wheat. Agric. Technol. Serv. 2009, 26, 71–72. (In Chinese). [Google Scholar]
- Ariff E. A. R. E.; Suratman M. N.; Abdullah S. Height-related changes in stomatal conductance, chlorophyll content index and diameter of rubber tree (Hevea brasiliensis) sapling. Proceedings of the IEEE Symposium on Bus., Engin. Ind. Appl. 2012, 318–321. 10.1109/ISBEIA.2012.6422894. [DOI] [Google Scholar]
- Hatfield J. L.; Prueger J. H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extremes. 2015, 10, 4–10. 10.1016/j.wace.2015.08.001. [DOI] [Google Scholar]
- Ibrahim M. H.; Jaafar H. Z. E. Photosynthetic capacity, photochemical efficiency and chlorophyll content of three varieties of Labisia pumila Benth. exposed to open field and greenhouse growing conditions. Acta Physiol. Plant. 2011, 33, 2179–2185. 10.1007/s11738-011-0757-1. [DOI] [Google Scholar]
- Su W. C.; Sun L. L.; Wu R. H.; Ma Y. H.; Wang H. L.; Xu H. L.; Yan Z. L.; Lu C. T. Effect of imazapic residues on photosynthetic traits and chlorophyll fluorescence of maize seedlings. Photosynthetica 2017, 55, 294–300. 10.1007/s11099-016-0641-3. [DOI] [Google Scholar]
- Silva F. B.; Costa A. C.; Alves R. R. P.; Megguer C. A. Chlorophyll fluorescence as an indicator of cellular damage by glyphosate herbicide in Raphanus sativus L. plants. Am. J. Plant Sci. 2014, 5, 2509–2519. 10.4236/ajps.2014.516265. [DOI] [Google Scholar]
- Greer D. Temperature-dependent responses of the photosynthetic and chlorophyll fluorescence attributes of apple (Malus domestica) leaves during a sustained high temperature event. Plant Physiol. Biochem. 2015, 97, 139–146. 10.1016/j.plaphy.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Georgieva K.; Lichtenthaler H. K. Photosynthetic activity and acclimation ability of pea plants to low and high temperature treatment as studied by means of chlorophyll fluorescence. J. Plant Physiol. 1999, 155, 416–423. 10.1016/S0176-1617(99)80125-4. [DOI] [Google Scholar]
- Gao Y. F.; Shi M. W.; Wang J. H. The influence of chlorophenoxy herbicides MCPA on creeping bentgrass physiological index. Adv. Mater. Res. 2011, 356–360, 2763–2766. 2012 10.4028/www.scientific.net/AMR.356-360.2763. [DOI] [Google Scholar]