Abstract
The use of electronic nicotine delivery systems has increased in popularity dramatically over the past decade. Although lung diseases caused by vaping have been reported since the modern invention of the electronic cigarette, in the summer of 2019, patients began to present to health care centers at epidemic levels with an acute respiratory illness relating to vaping, which the Center for Disease Control termed E-cigarette or vaping product use-associated lung injury (EVALI). This review discusses electronic nicotine delivery systems as well as the etiology, clinical presentation, imaging findings, pathologic features, treatment, and long-term consequences of EVALI. We conclude with the practical impact EVALI has had on the practice of pathology.
Keywords: Electronic nicotine delivery systems, Vaping, E-cigarette or vaping product use-associated lung injury, Pathology
Introduction
In the summer of 2019, an acute, mysterious, and deadly respiratory illness related to vaping emerged, primarily in young patients, in the USA. Cases increased dramatically and peaked in late September 2019. The Center for Disease Control and Prevention (CDC) termed the disease causing this epidemic E-cigarette or vaping product use-associated lung injury (EVALI). Prior to EVALI, vaping had been associated with a variety of different pulmonary presentations ranging from lipoid pneumonia to diffuse alveolar hemorrhage, but at low numbers. In this review, we discuss electronic nicotine delivery systems (ENDS) as well as the etiology, clinical presentation, imaging findings, pathologic features, treatment, and long-term consequences of EVALI. We conclude with a discussion on the practical impact EVALI has had on the practice of pathology.
Electronic nicotine delivery systems
ENDS, also known as E-cigarettes and vaping devices, were originally developed as a replacement device for conventional tobacco cigarette smokers [1]. However, their success in the arena of smoking cessation has been very limited, and they remain unapproved as cessation tools due to a lack of data demonstrating efficacy relative to currently approved nicotine replacement therapies [2]. The aerosols produced by E-cigarettes are known to cause a variety of deleterious health effects, although more research and long-term studies are still needed [2]. E-devices have rapidly evolved since entering the international market in 2013, with vape pens, box mods, and pod-based devices being the most commonly used vaping devices in 2020 [3]. Although E-cigarettes are used in conjunction with conventional tobacco by many cigarette smokers (dual users), their sole use in young adults and adolescents has skyrocketed [4]. This is concerning as use of tobacco products had been declining worldwide for over 50 years, and now, a new generation of nicotine addicts is being created through these novel vaping devices through the use of appealing flavors and packaging [5]. Even more concerning is that children and teenagers who use E-cigarettes are more likely to smoke conventional tobacco [6].
E-cigarette or vaping product use-associated lung injury
In mid to late 2019, vaping caused a novel disease in the USA which rapidly reached epidemic levels, termed EVALI. Thousands of E-cigarette users, predominantly males aged 13–34, developed respiratory, gastrointestinal, and systemic symptoms after vaping [7]. Testing of both e-liquids vaped and samples from the airways of those affected found vitamin E acetate (VEA) [8]. VEA is a clear viscous solution that was used as a cutting agent to increase tetrahydrocannabinol (THC) dealer profits. Mixtures of VEA and THC oils were used in the production of black market and gray market vaping devices and cartridges in the spring of 2019 [9]. When VEA is heated to the typical temperatures of an ENDS, it decomposes into the highly toxic ketene gas [10]. When tested in animals, VEA caused acute lung injury when inhaled through E-cigarette aerosols, confirming it as the probable chemical responsible for EVALI [11, 12]. While there is substantial evidence associating VEA with many cases of EVALI, there are other adulterants that likely are responsible in a subset of cases.
Clinical presentation
In patients presenting with EVALI, the main symptoms include shortness of breath, cough, chest pain, diarrhea, abdominal pain, fever, and fatigue [13, 14]. Symptoms occur anywhere from hours to weeks prior to presentation. Laboratory tests commonly reveal an elevated erythrocyte sedimentation rate and c-reactive protein level, transaminitis, and leukocytosis [13]. To meet the CDC criteria for a “confirmed” EVALI case, patients must have vaped within 90 days before symptom onset, have bilateral infiltrates on chest imaging, have a negative evaluation for infection, and have no other plausible alternative diagnoses. Cases of “Probable” EVALI share similar criteria, except that infection may be present, but the clinical team caring for the patient has high confidence that infection is not the primary cause for the patient’s respiratory condition.
Imaging findings
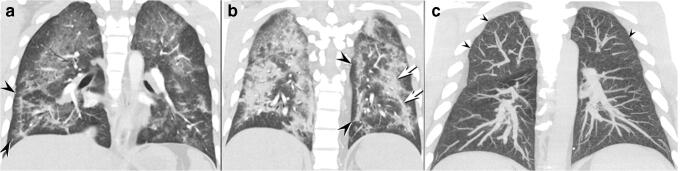
A wide arrange of imaging abnormalities have been described in the setting of EVALI, most commonly acute lung injury (ALI) and organizing pneumonia, as well as imaging patterns resembling non-fibrotic hypersensitivity pneumonitis (HP) and acute eosinophilic pneumonia (AEP) [15–18]. These features include multifocal or diffuse ground-glass opacities, often with areas of organizing consolidation (Fig. 1a and b). The ground-glass opacities often show predilection to the central regions and show a variable cephalocaudad distribution. Imaging manifestations resembling lipoid pneumonia, diffuse alveolar hemorrhage (DAH), and respiratory bronchiolitis-interstitial lung disease (RB-ILD) have also been suggested as manifestations of EVALI, but these patterns overlap with the more commonly reported EVALI patterns, particularly ALI and HP [15–18]. Correlating the pathologic features with the patterns of imaging abnormalities suggests that the majority of imaging findings are related to varying degrees of ALI, sometimes airway centered and upper lobe predominant due to the inhaled nature of the injury, with or without features of organization. Subpleural sparring is frequently encountered but is a non-specific finding.
Fig. 1.
a–c Coronal enhanced CT images through the anterior a and posterior b lungs show multifocal ground-glass opacity in the bilateral anterior upper lobes; note conspicuous subpleural sparing (arrowheads) affecting the peripheral right lower and medial left basal lung. The posterior lungs b show more pronounced consolidation with developing organization, evidenced by the mild architectural distortion and the band-like nature of the consolidation (arrows) distributed along the bronchi. Coronal maximum intensity projected unenhanced computed tomography c in a different EVALI patient shows diffusely distributed, small, centrilobular ground-glass opacity nodules (arrowheads) typical of a non-fibrotic hypersensitivity pneumonitis pattern
The imaging findings of EVALI can closely resemble HP, leading to an incorrect presumptive diagnosis based on imaging alone (Fig. 1c). In these patients, high-resolution computed tomography (HRCT) shows upper lobe predominant of diffuse, poorly defined centrilobular ground-glass opacity nodules and/or opacities which have been shown to correspond to acute and organizing lung injury pathologically.
Despite the published reports of lipoid pneumonia as a mechanism of injury in EVALI, no well-characterized radiologic and pathologic cases have been published. Most of the clinicopathologic diagnoses of lipoid pneumonia have been based on the finding of lipid-laden macrophages in BAL cytology specimens (discussed in detail below). To date, there have been no HRCT features of classic exogenous lipoid pneumonia, specifically the demonstration of macroscopic fat on HRCT, described in the literature.
Pathologic findings
Cases of EVALI present to pathology in a variety of ways, including cytologic preparations of bronchoalveolar lavage (BAL) specimens, transbronchial biopsies, cryobiopsies, and surgical lung biopsies. The primary consideration in the clinical differential is often infection, and thus, many cases have associated microbiology specimens.
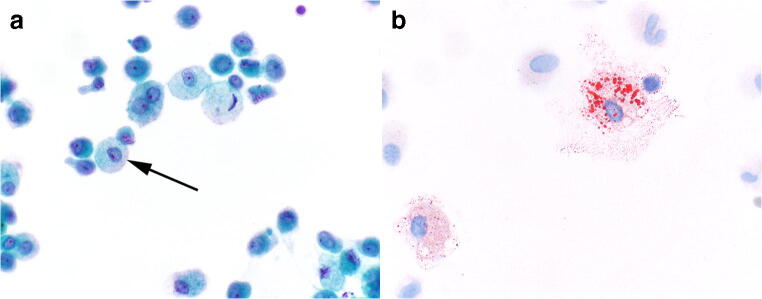
BAL specimens from EVALI patients are mostly inflammatory with the majority of inflammatory cells being macrophages. Rare neutrophils, lymphocytes, and eosinophils may be encountered. The macrophages are unique in that their cytoplasm is often distended with mostly fine cytoplasmic vacuoles of similar size (Fig. 2a) [19]. Enlarged cytoplasmic vacuoles and variably sized vacuoles, features seen in exogenous lipoid pneumonia, are encountered much less frequently. Oil red-O staining highlights the vacuoles to be composed of lipid material (Fig. 2b). The lipid-laden macrophage that was first feature associated with E-cigarette use in 2012 based only on clinical presentation, imaging findings, presence of lipid-laden macrophages, and a history of E-cigarette use [20]. No confirmatory biopsy was performed in this initial report, and the patient had many risk factors for pulmonary injury other than vaping. Perhaps due to the precedent set in the literature, the finding of lipid-laden macrophages in BAL specimens in the early stage of the EVALI epidemic in 2019 led investigators to conclude that the lung injury was related to a form of exogenous lipoid pneumonia related to vaping [17, 21, 22].
Fig. 2.
a, b Bronchoalveolar lavage (BAL) specimens in electronic cigarette or vaping-associated lung injury. BAL specimens are often quite cellular a, mostly inflammatory in nature and contain numerous macrophages. A subset of the macrophages show distended cytoplasm with numerous fine vacuoles (arrow), Papanicolaou stain stain, × 600. While Oil red-O staining is not a useful diagnostic stain, it does highlight the cytoplasmic lipid vacuoles b to be bright red, Oil red-O, × 600
The fine cytoplasmic vacuoles encountered in the macrophages actually represent the accumulation of endogenous cellular lipid material from epithelial injury, a process referred to as endogenous lipoid pneumonia. Endogenous lipoid pneumonia is seen in a variety of settings including acute lung injury, obstructed airways, and infections [23–25].
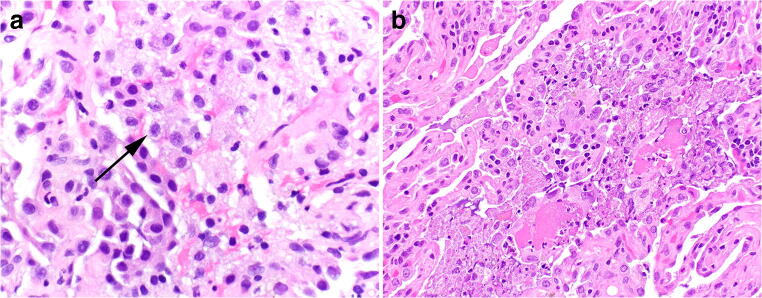
Transbronchial, cryo-, and surgical wedge biopsies all show similar features in the setting of EVALI. The predominant pathologic pattern falls along the spectrum of acute lung injury [26, 27], the natural progression of which has been well described [28]. In very early stages of acute lung injury, the only visible histologic finding may be edema and slightly enlarged pneumocytes. This is followed by the formation of hyaline membranes and fibrin deposition in the exudative phase. The proliferative, or organizing, phase shows the infiltration of inflammatory cells into the injurious process with subsequent polyps of organizing immature fibroblastic tissue. From a pathologic perspective, EVALI cases may show features of any of the patterns of acute lung injury, including diffuse alveolar damage, acute fibrinous and organizing pneumonia (AFOP), and organizing pneumonia (Fig. 3). Some cases show distinctly airway-centered pathology, while others appear as more of a diffuse process (Fig. 4). The relative presence of exudative and organizing features, along with the distribution of injury likely depends on (a) the temporal relationship between the toxic exposure (or repetitive toxic exposure) and the biopsy and (b) the severity/dose of the exposure. Cases of diffuse alveolar damage in the organizing phase may mimic cellular non-specific interstitial pneumonia (Fig. 4c).
Fig. 3.
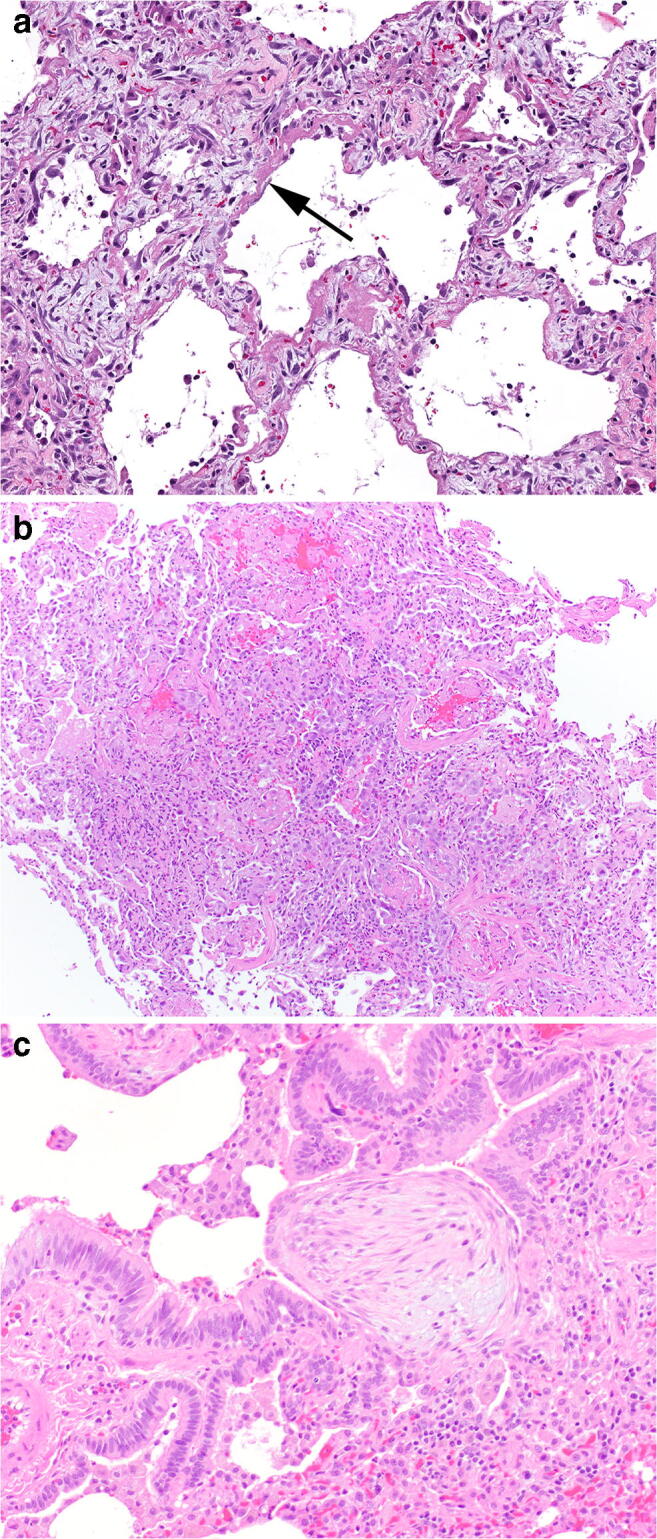
a–c The major pathologic acute lung injury patterns seen in the setting of electronic cigarette or vaping-associated lung injury. Hyaline membranes of diffuse alveolar damage a with significant background interstitial edema, H&E, × 200. Acute fibrinous and organizing pneumonia characterized by the deposition of abundant fibrin in airspaces b, exceeding the number of polyps of organizing pneumonia, H&E, × 100. A polyp of immature fibroblastic tissue representing organizing pneumonia c in the terminal bronchiole, H&E, × 200
Fig. 4.
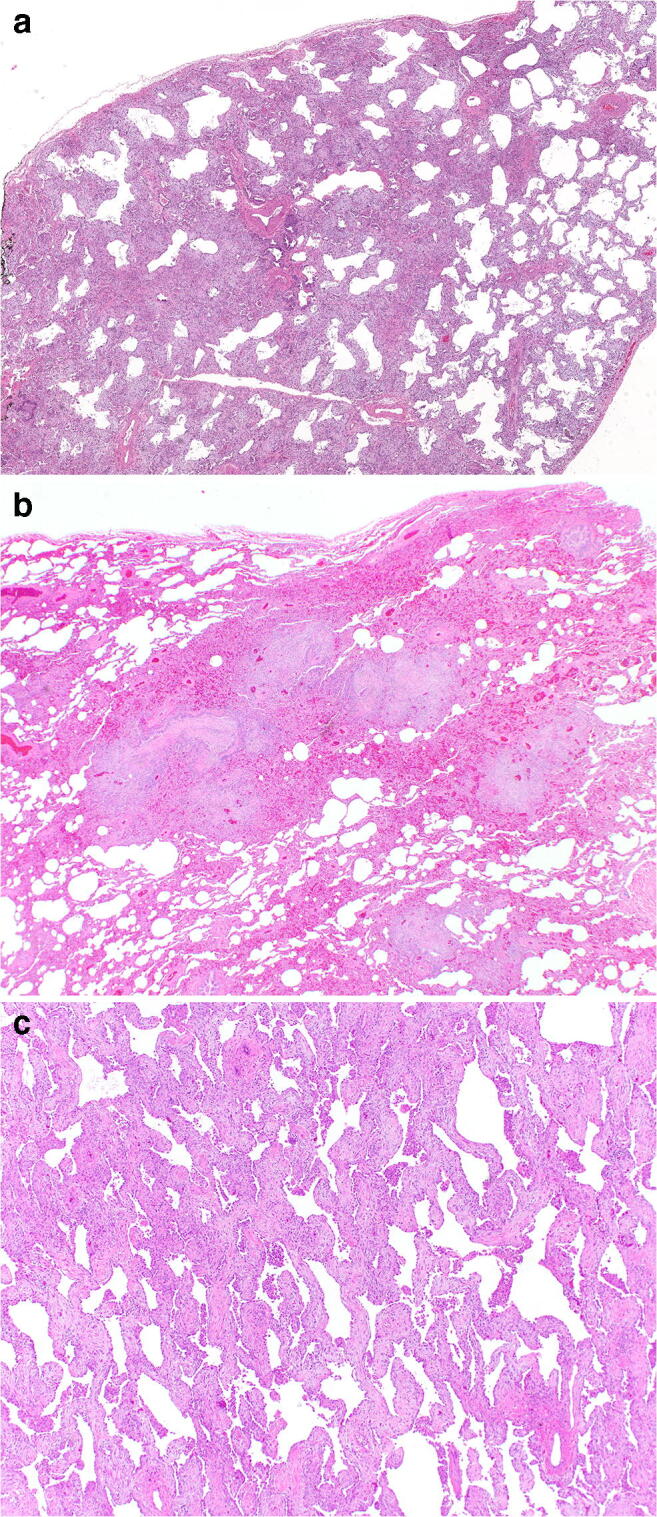
a–c Distribution of acute lung injury seen in electronic cigarette or vaping-associated lung injury. Diffuse involvement of the surgical lung biopsy by acute and organizing diffuse alveolar damage a. The diffuse nature of injury precludes any suggestion of airway centricity, H&E, × 20. Distinctly centrilobular distribution b of acute and organizing pneumonia, H&E, × 20. Following a more diffuse injurious insult, the healing phase of diffuse alveolar damage may mimic non-specific interstitial pneumonia c, H&E, × 100
Intra-alveolar, or airspace, macrophages are described as a relatively consistent finding, although this feature is not always prominent [26, 27]. When prominent, the histologic features mirror what has been described in the BAL findings of EVALI. The macrophages show finely vacuolated cytoplasm, occasionally with pigment or pigmented debris (Fig. 5).
Fig. 5.
a, b Airspace macrophages seen in biopsy specimens from patients with electronic cigarette or vaping-associated lung injury. Marked accumulation of macrophage with distended and foamy cytoplasm is frequently encountered (arrow) a, H&E, × 400. Some of the macrophages may have pigment or pigmented material in the cytoplasm b, H&E, × 200
Rare descriptions of additional patterns of lung injury have been described, including giant cell interstitial pneumonia that is a form of pneumoconiosis related to hard metal exposure and shows the accumulation of numerous multinucleated giant cells within the air spaces.
In one case report, the presence of giant cell interstitial pneumonia on lung biopsy was hypothesized to be from the combustion and aerosolization of metal elements of the vaping apparatus, which was supported by investigation with inductively coupled plasma mass spectrometry [29]. Interestingly, energy-dispersive X-ray spectroscopy on the actual tissue samples failed to reveal evidence of tungsten or cobalt.
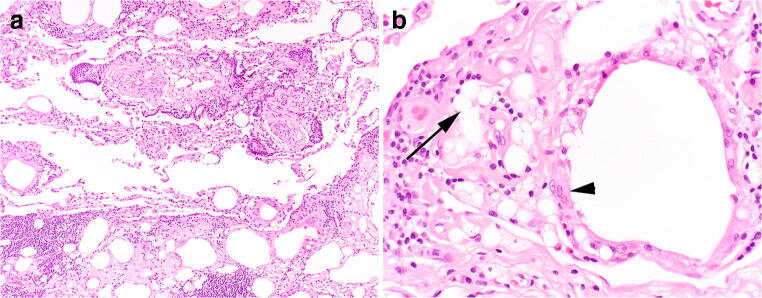
To date, no cases of histologic confirmed exogenous lipoid pneumonia have been described in association with vaping [26, 27]. The histology of exogenous lipoid pneumonia includes the deposition of variably sized lipid droplets into the interstitium with an associated foreign body giant cell response and often a component of fibrosis (Fig. 6). In comparison to EVALI, the lipid droplets of exogenous lipoid pneumonia are typically much larger and have more significant variability in size.
Fig. 6.
a, b The histologic distinction between exogenous lipoid pneumonia and electronic cigarette or vaping-associated lung injury (EVALI) is dramatic and distinctive. Exogenous lipoid pneumonia shows numerous lipid vacuoles a, most of which are much larger than individual cells. There is associated fibrosis in which many of the droplets are embedded. Occasional macrophages contain lipid droplets within their cytoplasm. However, the droplets are much larger and more variable (arrow) compared to EVALI, H&E, × 100. Larger lipid vacuoles are surrounded by several multinucleated giant cells and a foreign body giant cell reaction (arrowhead) b, a feature not seen in EVALI, H&E, × 200
Similarly, despite the radiologic features in some cases that seem to suggest subacute hypersensitivity pneumonitis, no cases of histologically confirmed hypersensitivity pneumonitis have been described in the literature in association with vaping. Subacute HP is classically described as a centrilobular alveolitis with airway centered, poorly formed interstitial granulomas. HP results from exposure to inhaled organic antigens with an associated hypersensitivity response. In considering the inhalational mechanism of injury between subacute HP and EVALI, it is not surprising that the radiologic features overlap. However, the histologic distinction between EVALI and HP is typically straightforward.
All cases of ALI, regardless of the pathologic pattern, should include testing for infectious organisms with acid-fast bacilli and Grocott’s methenamine silver (GMS) stains at a minimum. If there is significant necrosis and neutrophilic inflammation, viral immunohistochemistry may also be considered (cytomegalovirus, herpes virus, adenovirus). Oil red-O does not have a clear role in the histologic assessment and work-up of lung specimens, especially considering that fresh, unprocessed tissue must be used for effective Oil red-O. Despite the low sensitivity and specificity of Oil red-O, it remains on many clinical algorithms [30] for the pathologic work-up suspected EVALI. However, given its lack of utility, Oil red-O is not necessary for diagnostic purposes in suspected EVALI cases, cytology or surgical pathology specimens.
The spectrum of histologic acute lung injury produces a long differential diagnosis, including infection, connective tissue disease-associated ILD, adverse drug/toxin reaction, acute eosinophilic pneumonia, diffuse alveolar hemorrhage, foreign material, acute exacerbation of underlying ILD, subacute hypersensitivity pneumonitis, and idiopathic disease. This requires detailed histologic inspection in an attempt to identify histologic clues to an etiology and close clinical correlation. Acute lung injury pattern with distinct airway centricity, prominent foamy macrophages, pigment, and an absence of other histology to suggest alternatives should be enough to at least consider the possibility of EVALI. However, the diagnosis can only be made confidently following correlation with a history of exposure to ENDS.
Treatment
Given the novelty of EVALI, treatment has not been studied outside of observational studies and case series, and therefore, the optimal treatment is not known. The vast majority of the documented patients with EVALI required hospitalization (~ 95%), although this likely represents significant bias towards patients with more severe disease [7]. In a large series of 98 EVALI patients, 76% required supplemental oxygen, 22% required non-invasive ventilation, and 26% required intubation and mechanical ventilation, with extracorporeal membrane oxygenation (ECMO) rarely being needed [17]. Systemic glucocorticoid use was reported in the majority of patients with EVALI [17, 21]. However, the studies investigating the efficacy of systemic glucocorticoids were observational, and studies formally investigating their efficacy in a controlled prospective manner have not been conducted. Although over sixty deaths have been documented, many patients with EVALI may have resolution of symptoms upon cessation of vaping [31].
Prognosis and long-term consequences
According to the CDC as of February 18, 2020, 2807 patients have been hospitalized with EVALI in the USA with 68 confirmed EVALI-associated deaths [32]. EVALI is a serious respiratory illness that, in its most severe form, manifests as acute respiratory distress syndrome [7, 17]. Studies investigating imaging abnormalities in ARDS survivors have shown that the disease can resolve completely. The degree of resolution reported among different studies varies widely, with between 25 and 85% of ARDS survivors reported to have residual fibrotic changes on chest imaging [33, 34]. Therefore, it is hypothesized that a fraction of EVALI survivors may develop similar chronic fibrotic changes, but long-term studies will be needed to investigate this hypothesis. Clinical and imaging follow-up will be critical to ensure resolution of the pathologic process. Case reports have suggested that there may be residual lung dysfunction, mainly diffusion abnormalities, present for at least up to 2 months after discharge [35, 36]. However, follow-up studies must be conducted to determine whether these abnormalities persist long term.
It is also well-known that many of the chronic pathologic sequelae linked to tobacco cigarette smoking, such as emphysema, smoking-related interstitial fibrosis, and lung cancer, were not characterized until many years, and even decades, after the habit became popularized, [37]. It may take a similar amount of time before the long-term pathologic sequelae of E-cigarette use become apparent.
Practical impact of EVALI on pathology practice
The introduction of EVALI as a new diagnostic consideration when patients present with acute or subacute respiratory symptoms has had multiple practical impacts for practicing pathologists. First, while the general histologic findings lack specificity for EVALI, it is critical that pathologists consider and report EVALI as a possible etiology when confronted with a lung biopsy showing acute lung injury, especially in the younger demographic. Many patients are reluctant to admit to vaping, and many do not equate vaping with smoking, so the standard social history questions regarding smoking may miss potential vaping and dabbing exposures. A note in the pathology report specifically suggesting vape-related lung injury as a possible etiology may be the impetus for the clinician to question further regarding inhalational exposures. Like many cases of interstitial lung disease, clinicopathologic correlation is essential.
Second, pathologists must be aware that the clinical and imaging presentation of some patients with EVALI can strongly mimic subacute hypersensitivity pneumonitis [38], where both disorders can present with poorly defined, ground-glass attenuation centrilobular nodules or multifocal ground-glass opacity. In this setting, lung biopsy can be very helpful in arriving at the correct diagnosis, as the pathologic pattern seen in HP and EVALI are quite distinct. Rather than the cellular interstitial infiltrates with lymphocytes and/or vague poorly formed granulomas typical of subacute HP, the pathologic pattern of EVALI shows features of acute lung injury without the presence of granulomatous inflammation.
Finally, the recent coronavirus disease of 2019 (COVID-19) pandemic has resulted in further diagnostic challenges related to EVALI. There is a tremendous amount of overlap between the imaging findings of COVID-19 and EVALI [14]. However, the movement towards widespread pre-procedural testing for COVID has made it less likely for pulmonary specimens to be obtained from unrecognized COVID-19-positive patients. Additionally, the societal changes associated with the pandemic, including stay-at-home orders, isolation, and untoward economic impacts, have resulted in increased anxiety. The impact of COVID-19 on smoking and vaping habits remains unknown. The University of Utah group has identified the first rise in EVALI cases since the fall of 2019 [39]. These data support the ongoing nature of the EVALI epidemic with new cases still being reported. However, the CDC stopped tracking new cases in February of 2020 making it difficult to know the true current prevalence.
Conclusion
EVALI is a new consideration for patients presenting with acute and subacute respiratory illnesses. The imaging appearance is diverse, ranging from bilateral opacities similar to DAD to centrilobular nodules resembling HP. The pathologic features primarily include the spectrum of acute lung injury, including DAD, AFOP, and organizing pneumonia. Some cases may be accentuated in the centrilobular regions, similar to the imaging findings. Prior to EVALI, vaping had been associated with a wide variety of pulmonary presentations including lipoid pneumonia, acute respiratory distress syndrome, and diffuse alveolar hemorrhage. However, the majority of the cases of EVALI are likely related to vaping VEA. It remains possible that other forms of pulmonary illness related to vaping of other chemical compounds will contribute to be identified, and they may not fall into the spectrum of what has currently been described for EVALI. While the overall prevalence has decreased dramatically since the peak in September, 2019, the health care system must remain vigilant in screening for potentially harmful pathologic sequelae associated with the practice of vaping.
Authors’ contributions
Drs. Hariri, Gotway, Crotty Alexander, and Smith: drafting and critical review of the manuscript and reading and approving the final version.
Data availability
Data derived from currently existing medical literature and authors’ experience.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have read the final version and consent to publication.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Crotty Alexander LE, et al. Electronic cigarettes: the new face of nicotine delivery and addiction. J Thorac Dis. 2015;7(8):E248–E251. doi: 10.3978/j.issn.2072-1439.2015.07.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozier J, et al.,( 2020) The evolving landscape of electronic cigarettes: a systematic review of recent evidence. Chest [DOI] [PubMed]
- 3.Huang J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control. 2019;28(2):146–151. doi: 10.1136/tobaccocontrol-2018-054382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrazola RA, et al. Tobacco use among middle and high school students - United States, 2011-2014. MMWR Morb Mortal Wkly Rep. 2015;64(14):381–385. [PMC free article] [PubMed] [Google Scholar]
- 5.Leventhal AM, et al. Flavored E-cigarette use and progression of vaping in adolescents. Pediatrics. 2019;144(5):e20190789. doi: 10.1542/peds.2019-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaffee BW, Watkins SL, Glantz SA. Electronic cigarette use and progression from experimentation to established smoking. Pediatrics. 2018;141(4):e20173594. doi: 10.1542/peds.2017-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatham-Stephens K, et al. Characteristics of hospitalized and nonhospitalized patients in a nationwide outbreak of E-cigarette, or vaping, product use-associated lung injury - United States, November 2019. MMWR Morb Mortal Wkly Rep. 2019;68(46):1076–1080. doi: 10.15585/mmwr.mm6846e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blount, B.C., et al., Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI N Engl J Med, 2020. 382(8): p. 697–705 [DOI] [PMC free article] [PubMed]
- 9.Downs, D. Vape cart additive makers pull products as others go dark. 2019 [cited 2020 July 10th, 2020]; Available from: https://www.leafly.com/news/health/some-vape-cart-additive-makers-pull-products-others-go-dark. Accessed 19 Aug 2020
- 10.Wu D, O'Shea DF. Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. Proc Natl Acad Sci U S A. 2020;117(12):6349–6355. doi: 10.1073/pnas.1920925117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat TA et al (2020) An animal model of inhaled vitamin E acetate and EVALI-like lung injury. N Engl J Med [DOI] [PMC free article] [PubMed]
- 12.Alexander LEC, Bellinghausen AL, Eakin MN. What are the mechanisms underlying vaping-induced lung injury? J Clin Invest. 2020;130(6):2754–2756. doi: 10.1172/JCI138644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crotty Alexander LE, et al., (2020) NIH workshop report: E-cigarette or vaping product use associated lung injury (EVALI): developing a research agenda. Am J Respir Crit Care Med [DOI] [PMC free article] [PubMed]
- 14.Kligerman S, et al. Radiologic, pathologic, clinical, and physiologic findings of electronic cigarette or vaping product use-associated lung injury (EVALI): evolving knowledge and remaining questions. Radiology. 2020;294(3):491–505. doi: 10.1148/radiol.2020192585. [DOI] [PubMed] [Google Scholar]
- 15.Henry TS, Kanne JP, Kligerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. 2019;381(15):1486–1487. doi: 10.1056/NEJMc1911995. [DOI] [PubMed] [Google Scholar]
- 16.Henry TS, et al. Imaging findings of vaping-associated lung injury. AJR Am J Roentgenol. 2020;214(3):498–505. doi: 10.2214/AJR.19.22251. [DOI] [PubMed] [Google Scholar]
- 17.Layden JE, et al. Pulmonary illness related to E-cigarette use in Illinois and Wisconsin - final report. N Engl J Med. 2020;382(10):903–916. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 18.Viswam D et al (2018) Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep:2018 [DOI] [PMC free article] [PubMed]
- 19.Saqi A, et al. E-cigarette or vaping product use-associated lung injury: what is the role of cytologic assessment? Cancer Cytopathol. 2020;128(6):371–380. doi: 10.1002/cncy.22237. [DOI] [PubMed] [Google Scholar]
- 20.McCauley L, Markin C, Hosmer D. An unexpected consequence of electronic cigarette use. Chest. 2012;141(4):1110–1113. doi: 10.1378/chest.11-1334. [DOI] [PubMed] [Google Scholar]
- 21.Davidson K, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia - North Carolina, July-august 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36):784–786. doi: 10.15585/mmwr.mm6836e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddock SD, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019;381(15):1488–1489. doi: 10.1056/NEJMc1912038. [DOI] [PubMed] [Google Scholar]
- 23.Basset-Leobon C, et al. Cut-off values and significance of Oil Red O-positive cells in bronchoalveolar lavage fluid. Cytopathology. 2010;21(4):245–250. doi: 10.1111/j.1365-2303.2009.00677.x. [DOI] [PubMed] [Google Scholar]
- 24.Corwin RW, Irwin RS. The lipid-laden alveolar macrophage as a marker of aspiration in parenchymal lung disease. Am Rev Respir Dis. 1985;132(3):576–581. doi: 10.1164/arrd.1985.132.3.576. [DOI] [PubMed] [Google Scholar]
- 25.Pambuccian SE. Testing for lipid-laden macrophages in bronchoalveolar lavage fluid to diagnose vaping-associated pulmonary injury. Are we there yet? J Am Soc Cytopathol. 2020;9(1):1–8. doi: 10.1016/j.jasc.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Butt YM, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381(18):1780–1781. doi: 10.1056/NEJMc1913069. [DOI] [PubMed] [Google Scholar]
- 27.Mukhopadhyay S, et al. Lung biopsy findings in severe pulmonary illness associated with E-cigarette use (vaping) Am J Clin Pathol. 2020;153(1):30–39. doi: 10.1093/ajcp/aqz182. [DOI] [PubMed] [Google Scholar]
- 28.Beasley MB. The pathologist’s approach to acute lung injury. Arch Pathol Lab Med. 2010;134(5):719–727. doi: 10.5858/134.5.719. [DOI] [PubMed] [Google Scholar]
- 29.Fels Elliott DR et al (2019) Giant cell interstitial pneumonia secondary to cobalt exposure from e-cigarette use. Eur Respir J 54(6) [DOI] [PubMed]
- 30.Kalininskiy A, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017–1026. doi: 10.1016/S2213-2600(19)30415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander LEC, Perez MF (2019) Identifying, tracking, and treating lung injury associated with e-cigarettes or vaping. Lancet [DOI] [PubMed]
- 32.Centers for Disease Control and Prevention (CDC). Outbreak of lung injury associated with the use of E-cigarette, or vaping, products.; Available from: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#latest-information. Accessed 19 Aug 2020
- 33.Desai SR, et al. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 1999;210(1):29–35. doi: 10.1148/radiology.210.1.r99ja2629. [DOI] [PubMed] [Google Scholar]
- 34.Sheard S, Rao P, Devaraj A. Imaging of acute respiratory distress syndrome. Respir Care. 2012;57(4):607–612. doi: 10.4187/respcare.01731. [DOI] [PubMed] [Google Scholar]
- 35.Carroll BJ, et al. Impaired lung function following e-cigarette or vaping product use associated lung injury in the first cohort of hospitalized adolescents. Pediatr Pulmonol. 2020;55(7):1712–1718. doi: 10.1002/ppul.24787. [DOI] [PubMed] [Google Scholar]
- 36.Corcoran A, Carl JC, Rezaee F. The importance of anti-vaping vigilance-EVALI in seven adolescent pediatric patients in Northeast Ohio. Pediatr Pulmonol. 2020;55(7):1719–1724. doi: 10.1002/ppul.24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC). Health effects of cigarette smoking. . August 24, 2020]; Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm. Accessed 19 Aug 2020
- 38.Cecchini, M.J., et al., (2020) E-cigarette or vaping product use-associated lung injury: a review for pathologists. Arch Pathol Lab Med [DOI] [PubMed]
- 39.Callahan, S.J., et al., (2020) Diagnosing EVALI in the time of COVID-19. Chest [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data derived from currently existing medical literature and authors’ experience.