Abstract
Background:
Immunological cross-reactivity between common cold coronaviruses (CCC) and SARS-CoV-2 might account for the reduced incidence of COVID-19 in children. Evidence to support speculation includes in vitro evidence for humoral and cellular cross-reactivity with SARS-CoV-2 in specimens obtained before the pandemic started.
Method:
We used retrospective health insurance enrollment records, claims, and laboratory results to assemble a cohort of 869,236 insured individuals who had a PCR test for SARS-CoV-2. We estimated the effects of having clinical encounters for various diagnostic categories in the year preceding the study period on the risk of a positive test result.
Findings:
After adjusting for age, gender and care seeking behavior, we identified that individuals with diagnoses for common cold symptoms, including acute sinusitis, bronchitis, or pharyngitis in the preceding year had a lower risk of testing positive for SARS-CoV-2 (OR=0.76, 95%CI=0.75, 0.77). No reduction in the odds of a positive test for SARS-CoV-2 was seen in individuals under 18 years. The reduction in odds in adults remained stable for four years but was strongest in those with recent common cold symptoms.
Interpretation:
While this study cannot attribute this association to cross-immunity resulting from a prior CCC infection, it is one potential explanation. Regardless of the cause, the reduction in the odds of being infected by SARS-CoV-2 among those with a recent diagnosis of common cold symptoms may have a role in shifting future COVD-19 infection patterns from endemic to episodic.
Keywords: Covid-19, SARS-Cov-2, Common cold, Cross reactive
Introduction
Early in the COVID-19 epidemic, public health experts noted that coronavirus (CoV) infections were “More Than Just the Common Cold”.1 SARS-Cov-2, which causes COVID-19, is a member of the beta coronavirus genus which includes the SARS-CoV-1 and MERS (Middle East respiratory syndrome) virus. The coronavirus family also includes four viruses (229E, NL63, OC43, and HKU1), collectively referred to as common cold coronaviruses (CCC), which account for 10–30% of upper respiratory infections in adults.2 , 3 More than 90% of the human population is seropositive for at least three of the CCCs, indicating that they have been previously exposed.4 The prevalence of infection with these four CCCs follows multi-year cycles and varies across the United States.5 , 6 These infections may be a potential source of partial immunity for SARS-CoV-2, as noted for SARS-CoV-1.7, 8, 9 Altman and Byten recently summarized the emerging evidence related to SARS-CoV-2 T-cell immunity.10 Additional evidence continues to emerge.11 Ng et al. provide evidence of CCC and SARS-CoV-2 humoral cross-reactivity.12 In one study of the effect of antibodies to SARS-CoV-2 were not protective in cell cultures.13 However, the authors suggested that “Future studies need to investigate whether these non-neutralizing antibody responses can confer in vivo protection despite the lack of in vitro neutralization activity.”
The rate of infection with SARS-CoV-2 appears lower in children than adults, which may be due to many potential explanations.14 , 15 In one study, Henry and Oliveira reported that the reduced susceptibility among pediatric patients is still unknown.16 In addition to underreporting, a combination of epidemiologic and biologic factors may account for the lower rate of infection. Children have a less mature form of the ACE-2 receptor which the SARS-CoV-2 virus’ S protein binds which might decrease their susceptibility.17 Alternatively, Lee et al. reported that fewer outdoor activities and less international travel could account for decreased incidence.18 The immune systems of children and adults are different, both in their composition and functional responsiveness.19 Their more active immune response and generally healthier respiratory tracts may make them less susceptible to SARS-CoV-2 infection.20 Finally, children have a higher rate of recent CCC related infections than adults and, if there is any immunological cross-reactivity, they might be expected to have a lower rate of COVID-19 as a result.
To evaluate whether cross-reactive immune memory from past common cold coronaviruses may protect against infection by SARS-CoV-2, we undertook an analysis of the effect of prior diagnoses of conditions with a likely viral etiology on a positive PCR test for SARS-CoV-2 associate.
Methods
Study design
We used enrollment, claims, and clinical test results data from individuals enrolled with a large private US insurer with commercial or Medicare Advantage coverage. The cohort comprised of insured patients who underwent outpatient PCR tests for SARS-CoV-2 during the study period from March 1, 2020 to July 22, 2020 and for whom the insurer received the clinical results and who had at least 12 months of enrollment in the health plan before being tested. The insurer received clinical results from testing performed at either of two large national laboratories but not public health or health system laboratories.
We identified clinical diagnoses based on diagnoses provided on all types of administrative claims.
Statistical analysis
We retrieved all ICD-9 and ICD-10 diagnosis codes for the cohort between March 1, 2019, and February 29, 2020, mapped the ICD-9 codes to ICD-10 and created 308 diagnostic categories based on the phenotype definitions created by Kuan et al.21 We included in the logistic regression analysis the fifty-one categories that were present in at least 1% of the cohort. For each of these 51 diagnostic categories individually, we estimated the odds of a positive PCR test result based on the presence or absence of a diagnosis in the disease category during the preceding year. Age and gender were entered into the model as covariates. We also performed a stratified analysis by age of the risk of having a positive PCR test for SARS-CoV-2 associated with prior diagnoses of conditions with an underlying viral etiology.
After identifying that diagnostic categories likely associated with CCC infection were associated with a decreased likelihood of SARS-CoV-2 test positivity, we analyzed the 15 individual ICD-10 codes (out of 113) that comprise the ‘Ear and upper respiratory tract infection’ and ‘Other or unspecified infectious organisms’ that were present in at least 0.5% of the cohort along with age and gender for a positive PCR test.
To test care seeking behavior differences, we counted the number of distinct days with diagnoses in the study period and used this number as an additional covariate in the regression analyses. Probabilistic sensitivity analysis for exposure misclassification was performed using the episensr R package (version 0.9.6). We assumed non-differential misclassification bias, and applied the probsens function, with 1 million replications of uniform distribution of the whole range of possible sensitives and specificities that do not lead to negative cells in the adjusted 2 × 2 table.
To test for temporal effects, we performed a 30-days rolling window of the time of last CCC-related symptoms starting from January 1st, 2016.
Results
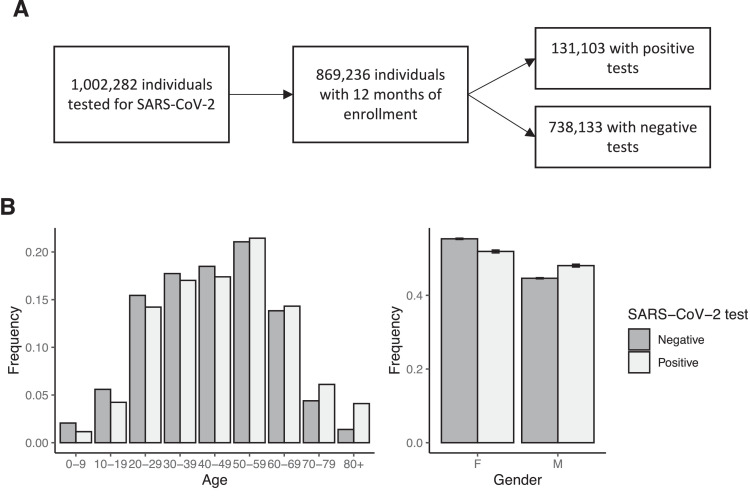
Our cohort consisted of 869,236 tested individuals, of whom 131,103 (15.1%) had a positive COVID-19 result. It included individuals from all age groups, and almost all US states. Age and gender distributions were similar for individuals whose SARS-CoV-2 test was positive or negative (Fig. 1 A and B).
Fig. 1.
Study cohort. A) Individuals that were not enrolled for 12 months prior the SARS-CoV-2 test were excluded. B) The age (left) and gender (right) distributions of individuals with positive and negative PCR tests for SARS-CoV-2 were similar. 15.1% of the individuals in the cohort were SARS-CoV-2 positive.
Risk of positive SARS-CoV-2 PCR test
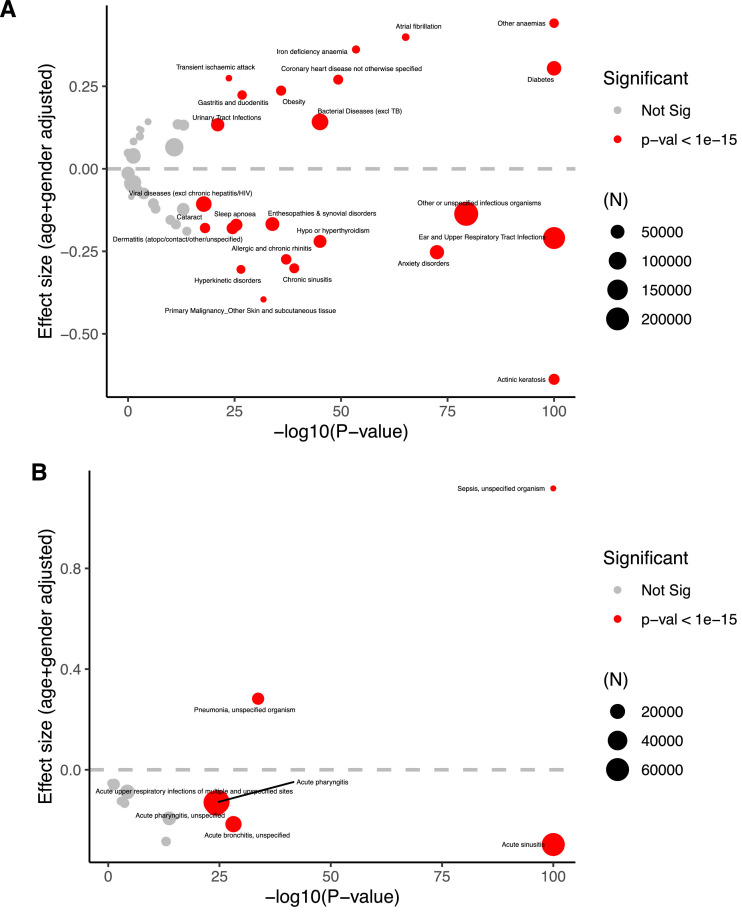
To identify diagnostic categories associated with COVID-19 infection, we analyzed 51 diagnostic categories (Fig. 2 A and Supplementary Table 1). Two diagnostic categories associated with viral etiologies were associated with a reduced risk of a positive PCR test for SARS-CoV-2: ‘Ear and upper respiratory tract infection’ (Odds ratio = 0.81, 95% confidence intervals (CI) = 0.80, 0.82, p-value < 1e-16) and ‘Other or unspecified infectious organisms’ (Odds ratio = 0.87, 95%CI=0.86, 0.88, p-value < 1e-16).
Fig. 2.
Phenotypes and diagnoses associated with a positive SARS-CoV-2 test. A) A volcano plot of the effect size of prevalent diagnostic categories on SARS-CoV-2 PCR test positivity and significance (log scale). The size of the circle is proportional to the number of individuals with the phenotype, and red circles indicate significance (p < 1e-20). -log10(p-values are capped at 100. B) A volcano plot of the effect size of prevalent ICD-10 diagnosis codes on SARS-CoV-2 PCR test positivity and significance (log scale). The size of the circle is proportional to the number of individuals with the phenotype, and red circles indicate significance (p < 1e-15).
To identify individual diagnoses within the diagnostic categories associated with a reduced SARS-CoV-2 positivity rate, we performed a similar analysis on the specific codes that comprised the significantly associated groups (Fig. 2B and Supplementary Table 2). With this analysis, we identified the codes for acute sinusitis (J01), and acute bronchitis from an unspecified source (J20.9) and acute pharyngitis from an unspecified source (J02.8 and J02.9) to be strongly associated with the reduced positivity rate. All three of these symptoms can be caused by CCC.
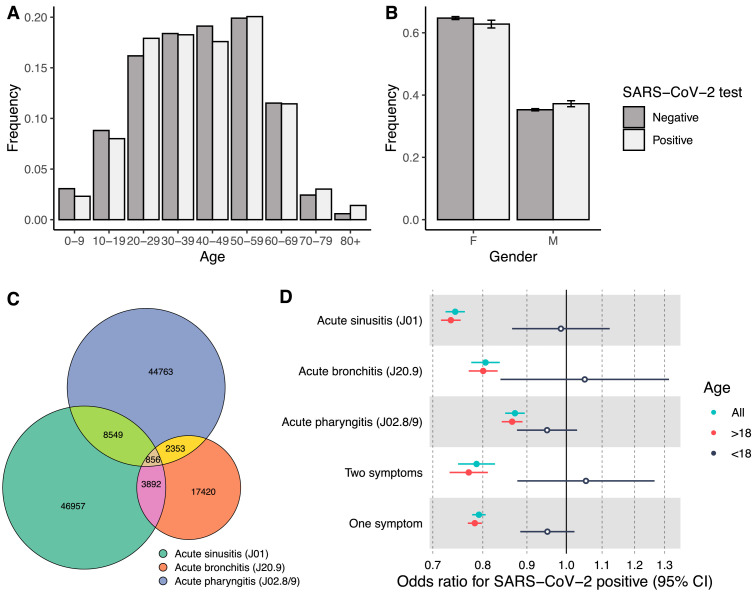
We identified 60,254 (6.9% of the entire cohort) affected individuals with acute sinusitis, 24,521 (2.8%) with acute bronchitis of an unspecified source, and 56,521 (6.5%) individuals with acute pharyngitis of an unspecified source in the year before the study period between March 1, 2019, and February 29, 2020. There were 126,998 (14.6%) individuals had at least one of those diagnoses, and 15,650 (1.8%) had two of the diagnoses in the year before the study period. The age distribution of individuals with these specific diagnoses was skewed to younger ages than the distribution of the overall cohort of tested individuals (41.4 years vs. 44.8 years, t-test p < 1e-16) (Fig. 3 A–C). Females accounted for a more significant proportion (64.5% of individuals vs. 54.8%, 1-sample proportion test p = 5e-6) (Fig. 3B). Of those with a diagnosis for one of the symptoms, 15,481 individuals had a positive SARS-CoV-2 test (12.2%) compared to our baseline of 15.1% for individuals without any of the diagnoses. Adjusted to age and gender, this translates to reduced odds for infection by 26.3% (OR=0.79, 95%CI=0.78, 0.81, p-value < 1e-16) (Fig. 3D).
Fig. 3.
Individuals with common cold symptom diagnoses. Age (A) and gender (B) distributions of 126,998 patients with acute sinusitis, acute bronchitis, or acute pharyngitis ICD-10 codes were similar in individuals with positive and negative SARS-CoV-2 PCR tests. C) The overlap of ICD-10 diagnosis codes, percentage from the cohort, and delta SARS-CoV-2 positive rate for each of the symptoms and combined, stratified by age. (D) Odds ratios for having a positive PCR test for SARS-CoV-2, stratified by age.
Age stratification
In the age-stratified analysis, we found that the risk of a positive SARS-CoV-2 PCR test varied by age.
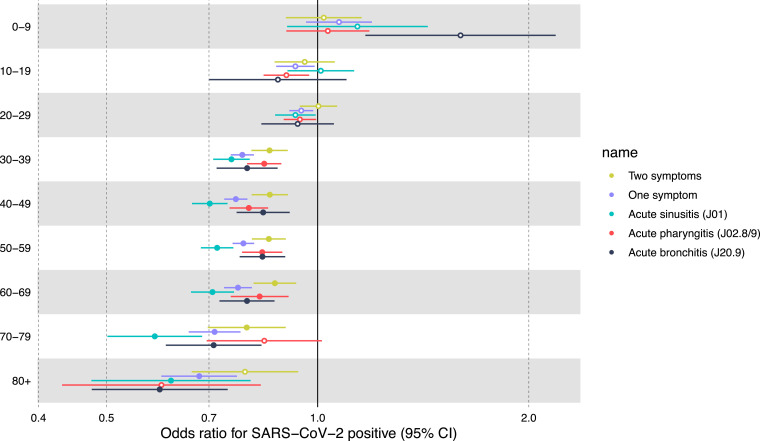
Contrary to adults, in individuals under 18, the odds of SARS-Cov-2 PCR test positivity did not differentiate between similar in those with and without prior visits for symptoms (OR in >18 years=0.78, 95%CI=0.77, 0.8. OR in <18 years=0.95, 95%CI=0.88, 1.02) (Fig. 3D). We further noticed that the odds gradually increased with age: while there is no effect in individuals under ten years old, and mild protective effect in individuals under 30 years old, the odds ratio gradually decreases to 0.61 (95%CI = 0.52, 0.71) (Extended Fig. 1 ).
Extended Figure 1.
Age stratification. Odds ratios for having a positive PCR test for SARS-CoV-2, stratified by age groups.
Care-seeking behavior
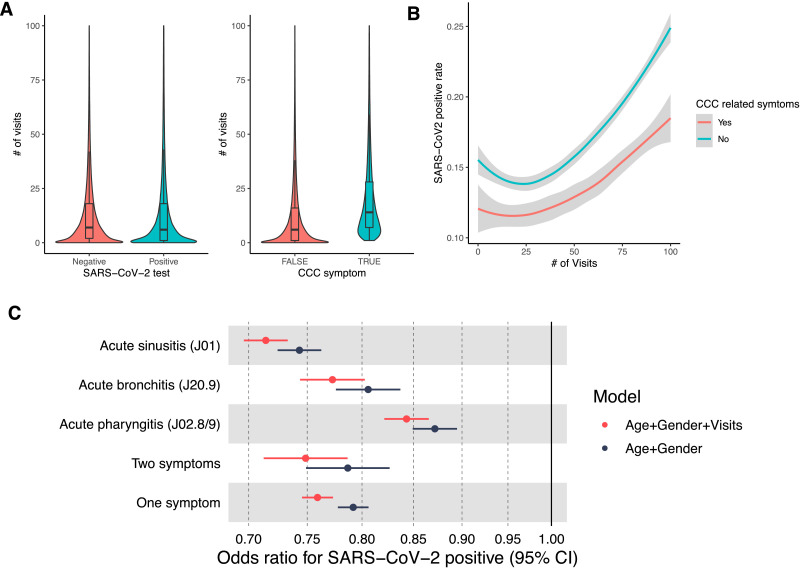
We further tested whether this reduced risk for a positive SARS-CoV-2 PCR test can be explained by differential care-seeking behaviors. For each individual, we counted the number of distinct days with a clinical visit with any diagnosis code during the year preceding the study period. The median number of days with a diagnosis in the overall cohort was seven days. Individuals with a CCC-related symptom, on the other hand, had a median of 15 days with symptoms in the year preceding the study period (Wilcoxon rank-sum p-value < 1e-16), which might suggest greater care-seeking among individuals with CCC symptoms (Extended Fig. 2A). However, in our data, SARS-CoV-2 positive test rates are also associated with the number of days with a diagnosis, and the reduced positivity rate observed in individuals with CCC symptoms is lower across the whole range of the number of visits (Extended Fig. 2B). Accordingly, adding this feature as a covariate to the risk analysis reduces the odds ratio from 0.79 without adjusted to number of visits to 0.76 with the adjustment (Extended Fig. 2C).
Extended Figure 2.
Extended Fig. 2. Care seeking behavior. A) Violin plot of the number of visits for individuals stratified by SARS-CoV-2 test results (right) and exposure to CCC symptoms (left). Median, upper and lower quartiles are shown. B) Smoothed lines of the SARS-CoV-2 positive rate across a range of the number of visits. C) Odds ratios for SARS-CoV-2 positive tests for the age and gender-adjusted model vs the age, gender, and the number of visits model.
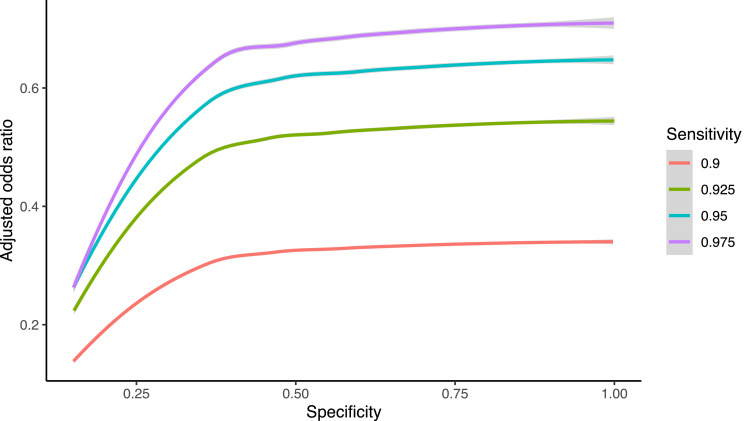
Given the difference in care-seeking behavior in individuals whose SARS-CoV-2 PCR tests were negative from those with positive tests and knowing that many individuals with symptoms of the common cold don't seek care from a physician, we performed a probabilistic sensitivity analysis for exposure misclassification using the whole range of possible sensitivities and specificities.22 Based on this analysis, the misclassification bias-corrected odds ratio decreased to 0.55 (95%CI = 0.06, 0.73). Importantly, in all the range of possible sensitivities and specificities, we observed that the misclassification bias is in the direction of increasing the negative association between CCC symptoms and SARS-CoV-2 PCR tests, up to an effect of 20-fold reduced risk for individuals with CCC symptoms in the preceding year (Extended Fig. 3).
Extended Figure 3.
Extended Fig. 3. Misclassification bias analysis. Plot shows the misclassification bias adjusted odds ratios across the whole range of specificity, and for sensitivities of 0.9, 0.925, 0.95 and 0.975.
Time interval between diagnosis and testing
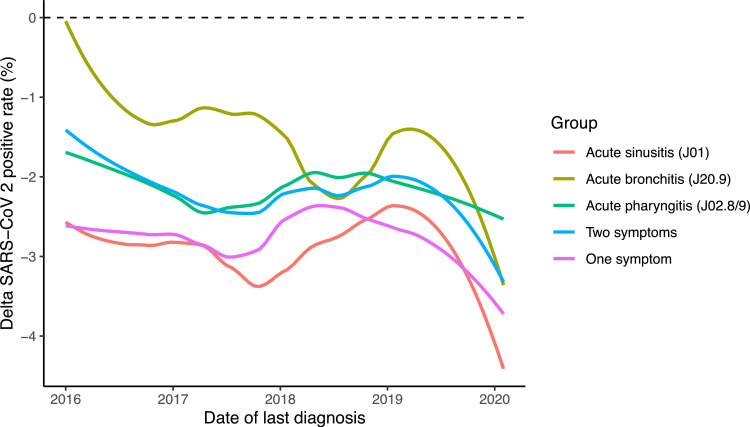
To test whether this association is short term or long term, we extracted information for the three CCC-related diagnoses from 2016 through 2020. Focusing on adults (age > 18 years) in our cohort, we identified 258,657 (31.4%) with at least one of the diagnoses since 2016. The SARS-CoV-2 positive rate for this group was 12.9% compared to 15.3% in all adults (Odds ratio adjusted for age and gender = 0.78, 95%CI = 0.76, 0.80), p-value < 1e-16). Interestingly, the reduction in risk was similar across the years 2016 through 2019 (Fig. 4 ). However, we observed marked potential immunity in individuals with symptoms in the first two months of 2020. Of the 19,902 individuals with the common cold symptoms in January and February 2020, only 11.8% had a positive test result.
Fig. 4.
Protective effect of common cold symptoms over time. Thirty-day moving average of the temporal association between the last date the ICD-10 diagnosis code was recorded and the PCR test for SARS-CoV-2 in 2020.
Discussion
Encounters for CCC-related symptoms are associated with a significantly decreased odds of a positive PCR test for SARS-CoV-2 in adults but not children. Cross-reactivity between CCC and SARS-CoV-2 is a potential explanation for this decrease.
Presumably, there should not be an association between most clinical diagnoses and having a positive test for SARS-CoV-2 due to immunological factors. For example, immunologically, we wouldn't expect an individual with type 2 diabetes to be more susceptible to infection. However, since the population tested for COVID-19 is likely to consist of individuals with symptoms, diagnostic categories that complicate COVD-19 would be more likely to get tested and confirmed as infected. In accordance, our analysis identified several diagnostic categories that are significantly positively associated with a positive SARS-CoV-2 PCR test result, including diabetes, heart disease, obesity, and anemia, all of which others have also found to be associated with increased risk of COVID-19 hospitalizations (Fig. 2A and Supplementary Table 1).23 Relevant to our hypothesis, we observed several diagnostic categories negatively associated with positive SARS-CoV-2 PCR test results, including actinic keratosis, ear and upper respiratory tract infection, and anxiety disorders (Fig. 2A). When we analyzed specific ICD-10 codes, we found that conditions sometimes caused by CCC, including acute sinusitis and acute bronchitis and acute pharyngitis, were most significant (Fig. 2B and Supplementary Table 2). Our analysis of the effect on SARS-CoV-2 PCR positivity based on how long before the test the relevant diagnosis was made suggests that the effect may be short lived.
While our measured risk factor of interest in our cohort was receiving a diagnosis related to common cold symptoms, CCCs account for 10–30% of colds and presumably of the office visits for common cold symptoms. The rest of these diagnoses are caused by a variety of other viruses, so our data don't provide direct evidence that prior infection with CCC is the cause of the reduced risk of a positive SARS-CoV-2 test. However, our data suggest that combined with the immunological evidence for cross-reactivity between SARS-CoV-2 and CCCs infection due to CCC seems a possible explanation for the difference in test positivity rate that we observe.
Children, who have four or five times as many common colds as adults each year presumably partly attributable to more CCC exposure, have been seen to have a lower incidence and less severe cases of COVID-19.24 One recent study, however, suggested that there is no difference in the prevalence of CCC antibodies in children with COVID-19 and control subjects.25 Our analysis revealed similar findings, where children had no difference in the COVID-19 test positivity rate, whether they had a diagnosis of a common cold-related symptom previously or not (Fig. 3D and Extended Fig. 1). One possible explanation is that essentially all children have had CCC exposures in the previous year whether they sought medical attention and received a diagnosis or not.
Given the high prevalence of CCC antibodies in the adult population, the size of the protective effect we observe on SARS-CoV-2 infection might seem small. Several potential factors may account for the effect size. First, while upper respiratory infections account for a significant number of healthcare encounters, individuals manage many episodes with over the counter medications and self-care. Using misclassification bias analysis of exposure to CCC, we estimated that CCC infection might reduce the risk for COVID-19 by up to 20-fold, and average by 81%. It is hard to estimate the true sensitivity and specificity parameters. However, we observed strong effects for a wide range of parameters, suggesting that our 26% reduced risk may be a conservative estimate or the real potential effect. A counter argument is that individuals who do not seek care might have had milder symptoms, and this may result in a less robust immune response than would be seen in a more severe infection.
Another potential factor that may increase the effect is study time period. Our primary estimate of the odds ratio is based exclusively on diagnoses during the year preceding the study period and didn't include diagnoses in previous years, which, as the time series analysis shows, may be relevant (Fig. 4).
As a non-randomized study, our findings are susceptible to bias such as unmeasured confounding. The demographics of the individuals in our study are fairly uniform, as supported by the demographic descriptions and the fact that they are all insured with commercial insurance or Medicare Advantage. The groups also had similar access to testing recognizing the access was likely widely different across the country. One potential bias might be differences in care-seeking behaviors between the two groups. Those with a diagnosis of infections sometimes caused by CCCs may be more prone to seek care and could have been tested for SARS-CoV-2 more often, resulting in a detection bias. Indeed, our analysis confirmed that this is the case (Extended Figure 2). However, further analysis showed that this confounder does not explain the reduced risk effect, and supposedly increases the protective effect. An explanation for this counter-intuitive result is that individuals with more office visits tend to be sicker, and those tend to have more severe symptoms COVID-19 and thus are less diluted with asymptomatic individuals.
There are several potential implications of our findings. Prior diagnosis of an infection that may be caused by CCC may reduce the severity of COVID-19. The severity of symptoms in patients infected with SARS-CoV-2 varies widely from asymptomatic to fatal. Others have reported factors such as age and comorbidities to be risk factors for severity, but the immunological response could be a risk. It is plausible that people with a high number of pre-existing memory CD4+ T cells that recognize SARS-CoV-2 could mount a faster and more robust immune response upon exposure to SARS-CoV-2 and thereby limit disease severity. Memory T follicular helper (TFH) CD4+ T cells could potentially facilitate an increased and more rapid neutralizing antibody response against SARS-CoV-2. Memory CD4+ and CD8+ T cells might also promote direct antiviral immunity in the lungs and nasopharynx early after exposure.
There may also be significant implications for the course of the pandemic as a result of introducing variability in the susceptibility of individuals, which could lower the threshold for herd immunity.26 Given that production capacity for COVID-19 vaccines, when they are ready, may be limited, cross-immunity from prior infection may help bridge the gap to herd immunity. In another study examining whether COVID-19 will become a cyclically recurring epidemic, burn itself out or follow another course, the investigators found that even weak cross-immunity to SARS-CoV-2 (approximately 30 percent) could substantially delay future outbreaks.27
There are several limitations to our study. The data is enriched with individuals with more severe COVID-19 cases as patients with more severe symptoms may have been more likely to be tested early in the pandemic when test availability was limited. In addition, our dataset does not include laboratory results for all patients. Finally, we are only able to demonstrate an association, not causality, and other confounding factors associated with these individuals may account for the differences in the odds of a positive SARS-CoV-2 test.
The decreased odds for a positive PCR test for SARS-CoV-2 among those with a prior CCC-related diagnosis is an important observation that warrants replication and consideration as stakeholders make the complex policy and clinical decisions about how to manage the COVID-19 pandemic.
Declaration of Competing Interest
Drs. Overhage, Beachler and Lanes are employees of HealthCore Inc., a subsidiary of Anthem Inc. Dr. Aran is a consutlant for Anthem Innovation Israel. None of the authors have any competing interests.
Acknowledgments
Ethical Declaration
This study was designed as an analysis based on medical claims data, and there was no active enrollment or active follow‐up of study subjects, and no data were collected directly from individuals. The study was not required to obtain additional IRB approval, as the HIPAA Privacy Rule permits protected health information (PHI) in a limited data set to be used or disclosed for research, without individual authorization, if certain criteria are met
Author Contributions
Dr. Aran contributed to the conception, design, data acquisition, analysis, interpretation, drafting and revision of the manuscript. Drs. Beachler and Lanes contributed to the analysis and substantially revised the manuscript. Dr. Overhage contributed to the conception, design, analysis, interpretation, drafting, and revision of the manuscript.
Additional Information
Correspondence and requests for materials should be addressed to D.A.
Reprints and permissions information is available at www.nature.com/reprint.
Footnotes
Funding: All authors are employees or consultants for Anthem Inc. or HealthCore Inc. There was no specific funding for this study. D. Aran is supported by the Azrieli Faculty Fellowship.
References
- 1.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. JAMA. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 2.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Roles of host gene and non-coding RNA expression in virus infection. Springer; 2017. Host factors in coronavirus replication; pp. 1–42. [Google Scholar]
- 3.De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorse G.J., Patel G.B., Vitale J.N., O'Connor T.Z. Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clin Vaccine Immunol. 2010;17(12):1875–1880. doi: 10.1128/CVI.00278-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Killerby M.E., Biggs H.M., Haynes A., Dahl R.M., Mustaquim D., Gerber S.I. Human coronavirus circulation in the United States 2014–2017. J Clin Virol. 2018;101:52–56. doi: 10.1016/j.jcv.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldridge R.W., Lewer D., Beale S., Johnson A.M., Zambon M., Hayward A.C. Seasonality and immunity to laboratory-confirmed seasonal coronaviruses (HCoV-NL63, HCoV-OC43, and HCoV-229E): results from the Flu Watch cohort study. Wellcome Open Res. 2020;5(52):52. doi: 10.12688/wellcomeopenres.15812.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer B., Drosten C., Müller M.A. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;194:175–183. doi: 10.1016/j.virusres.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia M. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. Published online. [DOI] [PubMed] [Google Scholar]
- 9.Patrick D.M., Petric M., Skowronski D.M., Guasparini R., Booth T.F., Krajden M. An outbreak of human coronavirus OC43 infection and serological cross-reactivity with SARS coronavirus. Can J Infect Dis Med Microbiol. 2006;17:330–336. doi: 10.1155/2006/152612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altmann D.M., Boyton R.J. SARS-CoV-2 T cell immunity: specificity, function, durability, and role in protection. Sci Immunol. 2020;5(49):eabd6160. doi: 10.1126/sciimmunol.abd6160. [DOI] [PubMed] [Google Scholar]
- 11.Mateus J., Grifoni A., Tarke A., Sidney J., Ramirez S.I., Dan J.M. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370(6512):89–94. doi: 10.1126/science.abd3871. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng KW, Faulkner N, Cornish GH, Rosa A, Harvey R, Hussain S, et al. Pre-existing and de novo humoral immunity to SARS-CoV-2 in humans. BioRxiv. Published online 2020. [DOI] [PMC free article] [PubMed]
- 13.Lv H., Wu N.C., Tsang O.T., Yuan M., Perera R.A.P.M., Leung W.S. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. Cell Rep. 2020;31(9) doi: 10.1016/j.celrep.2020.107725. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizumoto K., Omori R., Nishiura H. Age specificity of cases and attack rate of novel coronavirus disease (COVID-19). medRxiv. Published online 2020. [DOI] [PMC free article] [PubMed]
- 15.Coronavirus disease 2019 in children—United States. Morb Mortal Wkly Rep. 2020;69(14):422. doi: 10.15585/mmwr.mm6914e4. February 12–April 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry B.M., Oliveira M.H.S. Preliminary epidemiological analysis on children and adolescents with novel coronavirus disease 2019 outside Hubei Province, China: an observational study utilizing crowdsourced data. medRxiv. Published online 2020.
- 17.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee P.-.I., Hu Y.-.L., Chen P.-.Y., Huang Y.-.C., Hsueh P.-.R. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53(3):371–372. doi: 10.1016/j.jmii.2020.02.011. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon A.K., Hollander G.A., McMichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B Biol Sci. 2015;282(1821) doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai C.-.C., Shih T.-.P., Ko W.-.C., Tang H.-.J., Hsueh P.-.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuan V., Denaxas S., Gonzalez-Izquierdo A., Direk K., Bhatti O., Husain S. A chronological map of 308 physical and mental health conditions from 4 million individuals in the English National Health Service. Lancet Digit Health. 2019;1(2):e63–e77. doi: 10.1016/S2589-7500(19)30012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lash T.L., Fox M.P., Fink A.K. Springer Science & Business Media; 2011. Applying quantitative bias analysis to epidemiologic data. [Google Scholar]
- 23.de Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30371-6. Published onlinehttps://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sermet-Gaudelus I, Temmam S, Huon C, Behillil S, Gajdos V, Bigot T, et al. Prior infection by seasonal coronaviruses does not prevent SARS-CoV-2 infection and associated Multisystem Inflammatory Syndrome in children. medRxiv. Published online 2020.
- 26.Gomes MGM, Corder RM, King JG, Langwig KE, Souto-Maior C, Carneiro J, et al. Individual variation in susceptibility or exposure to SARS-CoV-2 lowers the herd immunity threshold. medRxiv. Published online 2020. [DOI] [PMC free article] [PubMed]
- 27.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]