Highlights
-
•
The Abbott IgM assay against SARS-CoV-2 is detected slightly earlier compared to IgG.
-
•
Both Abbott IgM and IgG assays exhibit excellent sensitivity and specificity.
-
•
Positive rate of IgM drops to 30.8 % after 3 months of symptoms, in contrast to IgG which is sustained in 92.3 % patients 3–6 months post symptom onset.
-
•
In symptomatic patients who test negative by PCR for a SARS-CoV-2 infection, assessing IgM and IgG antibodies can aid in supporting a diagnosis of COVID-19.
Keywords: Coronavirus, SARS-CoV-2 IgM, SARS-CoV-2 IgG, Abbott
Abstract
Background
Antibody testing has recently emerged as an option to assist with determining exposure to SARS-CoV-2, the causative agent of COVID-19. Elucidation of the kinetics and duration of the humoral response is important for clinical management and interpreting results from serological surveys.
Objectives
Here we evaluated the clinical performance of Abbott SARS-CoV-2 IgM and IgG assays, as well as the longitudinal dynamics of the antibody response in symptomatic COVID-19 patients.
Study design and results
The diagnostic specificity was 100 % for IgM and 99.67 % for IgG using 300 pre-COVID-19 serum specimens. Using 1349 sequential serum samples collected up to 168 days post symptom onset from 427 PCR-confirmed individuals, clinical test sensitivity of the SARS-CoV-2 IgM assay was 24.6 % at ≤7 days, 75.3 % at 8−14 days, 95.0 % at 15−21 days, and 96.0 % at 4−5 weeks (peak test sensitivity). The median duration of time for IgM seroconversion was 10 days. IgM levels declined steadily 4−5 weeks after symptom onset, and the positive rate dropped to 30.8 % at >3 months. The diagnostic sensitivity for the SARS-CoV-2 IgG assay post symptom onset was 23.2 % at ≤7 days, 69.5 % at 8−14 days, 93.6 % at 15−21 days, and 99.6 % at 4−5 weeks (peak test sensitivity). The median duration of time for IgG seroconversion was 11.5 days. During the convalescent phase of the infection, a decline in the IgG level was observed in patients who were followed for >100 days. Despite that decline, 92.3 % of the patient cohort remained IgG positive 3–6 months following symptom onset.
Conclusions
This study demonstrates the Abbott IgM assay against SARS-CoV-2 is detected slightly earlier compared to IgG, with both tests exhibiting excellent overall sensitivity and specificity. In symptomatic patients who test negative by PCR for a SARS-CoV-2 infection, assessing IgM and IgG antibodies can aid in supporting a diagnosis of COVID-19.
1. Introduction
Serological testing has recently emerged as an option to assist with determining exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19). Almost all patients with COVID-19 develop detectable IgG and IgM antibodies within several weeks of symptom onset [[1], [2], [3]], making serologic tests important tools for epidemiologic studies and aiding diagnosis at later time points following infection [4]. However, there are still gaps in our knowledge regarding the duration of time antibodies are present post-infection. Research on the SARS coronavirus responsible for the epidemic in 2003 (SARS-CoV) suggest the IgG humoral response is sustained for at least 1–2 years [5,6]. In contrast, early studies in small cohorts of asymptomatic individuals or patients with mild COVID-19 showed that 13–40 % of them became seronegative in the early convalescent phase [2,7]. Understanding the kinetics and duration of the humoral response is essential towards determining how to best utilize antibody testing in clinical practice, how to interpret results obtained from serological surveys and to aid in determining risk for re-infection in previously exposed individuals. In addition, there have been limited reports that describe the kinetics and possible utility of tests that measure IgM to the novel coronavirus. This could in part be due to the small number of SARS-CoV-2 IgM assays that demonstrate adequate test performance characteristics. At the time of writing, of the 15 SARS-CoV-2 IgM assays that have received emergency use authorization (EUA) from the FDA, only 4 are based on enzyme-linked immunosorbent assay (ELISA) or chemiluminescent immunoassay (CLIA) methods, whereas 11 are based on lateral flow immunoassay (LFIA) methodology. LFIAs are solely intended for determining the absence or presence of antibodies and therefore have limited use towards examining antibody kinetics and persistence [8]. It has also been shown that several LFIAs exhibit sub-optimal test performance characteristics including low sensitivity and specificity [9]. Lastly, only 3 IgM assays based on ELISA or CLIA methods have received CE certification from the European Union [10].
To address the knowledge gaps mentioned above, we established clinical performance characteristics for the Abbott SARS-CoV-2 IgM (prototype) and IgG (EUA) immunoassays. Furthermore, we evaluated the longitudinal dynamics of the antibody response against SARS-CoV-2.
2. Materials and methods
2.1. Patient cohorts
The investigation was approved by the Institutional Review Board at Beaumont Health (#2020−233). Residual peripheral blood samples collected from patients for standard of care purposes were utilized. Specimens derived from 427 patients who tested positive for SARS-CoV-2 by RT-PCR between March and August 2020 were used to assess test sensitivity (n = 1349). Specimens to assess test specificity included 427 patients who were symptomatic but PCR negative for SARS-CoV-2. Furthermore, a cohort of 300 archived samples collected between 2010 and 2015 and stored at −80 °C was included to assess test specificity. Archived samples from individuals with known conditions or treatments associated with immune impairment, such as cancer and chemotherapy, were excluded.
Duration from symptom onset was determined by review of the electronic medical record and inferred from physician encounter notes. Criteria for determining disease severity during the hospital admission were: Mild, no need for oxygen; Moderate, >0 and ≤6 L O2; Severe, >6 L O2 but not intubated; Critical, intubated or hypoxic despite maximal non-invasive oxygen.
2.2. Instrumentation and analysis
Specimens were analyzed on the Abbott Architect platform (Abbott Park, IL, USA) using the Abbott SARS-CoV-2 IgG (EUA) and IgM (prototype) assays according to the manufacturer’s instructions. The Abbott SARS-CoV-2 IgG and IgM assays are chemiluminescent microparticle immunoassays for the qualitative detection of antibody against the nucleocapsid protein and spike protein receptor binding domain (RBD), respectively. Both assays report an index value based on the ratio of specimen absorbance to the absorbance of an assay-specific calibrator. The manufacturer’s recommended index value cutoff of 1.40 and 1.00 was used for IgG and IgM, respectively.
Detection of SARS-CoV-2 viral RNA was performed by RT-PCR in nasopharyngeal swabs (main specimen type), and oropharyngeal swabs at Beaumont Health with assays validated for clinical use. Due to reagent shortages, three PCR platforms were used: Cepheid Xpert Xpress SARS-CoV-2 (limit of detection 100–200 copies/mL), Luminex NxTAG®CoV Extended Panel (limit of detection 500 copies/mL), and the CDC 2019-nCoV RT-PCR diagnostic panel (limit of detection 500 copies/mL).
2.3. Assay validation and precision
For precision studies, QC materials obtained from Abbott and patient pools with antibody index values close to the positive cutoff for each assay were analyzed. Reproducibility was assessed by analyzing 10 replicates on one day. Total precision was assessed in 8 replicates over 3 days.
2.4. Statistical analysis
Difference of positive rates between IgM and IgG was determined by Chi-square test for association [11]. Significant difference was determined as p < 0.05. All data visualization was performed with GraphPad Prism 8.
3. Results
3.1. Clinical specificity and sensitivity of the Abbott SARS-CoV-2 IgM and IgG assay
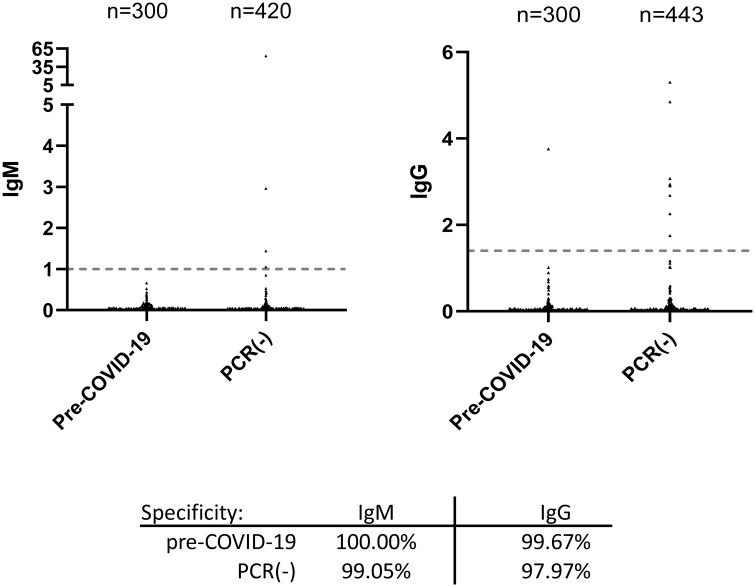
To assess test specificity, 300 archived specimens collected from healthy individuals between 2010 and 2015 were assayed. All specimens tested negative for IgM, yielding an assay specificity of 100 % (Fig. 1 ). One serum specimen from this set tested positive for IgG with an index value of 3.76, resulting in an assay specificity of 99.67 % for IgG from the pre-COVID-19 cohort (Fig. 1).
Fig. 1.
Clinical specificity of the Abbott SARS-COV-2 IgM and IgG assays was determined using 300 archived pre-COVID-19 specimens collected between 2010 and 2015. In addition, specimens from patients presenting with symptoms suspicious for a respiratory test infection but tested negative for COVID-19 by PCR were similarly assayed for IgM and IgG.
We subsequently tested an additional 443 specimens from 217 patients who presented to Beaumont Hospital (Royal Oak, Michigan) between March and April 2020 with symptoms consistent with a respiratory tract infection but tested negative by PCR for COVID-19 (Fig. 1). Four specimens from 4 patients in the cohort tested positive for IgM, resulting in an assay specificity of 99.05 % (95 % CI: 98.12–99.98) (Fig. 1 and patient 1–4 in Supplemental Fig. 1). Nine specimens from 5 patients tested positive for IgG, yielding a diagnostic specificity of 97.97 % (95 % CI: 96.65–99.28). Since we also validated the SARS-CoV-2 IgG assay on the EUROIMMUN (EI) platform, we tested those specimens that generated discordant results with the EI IgG assay (Supplemental Fig. 1). Six out of these twelve specimens tested positive on the EI platform. If these six specimens were excluded from analysis, the diagnostic specificity would be 99.52 % and 99.10 % for the Abbott IgM and IgG assays, respectively.
To determine assay sensitivity, we used 1349 specimens from 427 patients who tested positive for SARS-CoV-2 by PCR. Most of these patients were admitted to Beaumont Hospital between March and August 2020, which corresponds to the surge in COVID-19 hospitalization in Michigan. The age distribution by decade of life was: 10−19: 0.2 %, 20−29: 2.1 %, 30−39: 6.0 %, 40−49: 9.5 %, 50−59: 16.7 %, 60−69: 24.1 %, 70−79: 22.0 %, 80−89: 14.8 %, ≥90: 4.4 %.
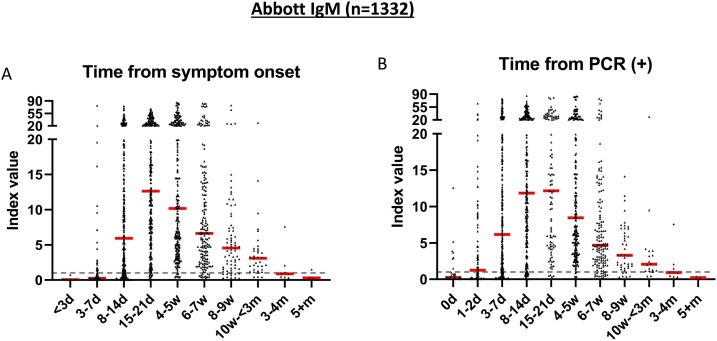
The sensitivity of the Abbott SARS-CoV-2 IgM assay relative to date of symptom onset was 24.6 % at ≤7 days (95 %CI: 16.8−32.3), 75.3 % at 8−14 days (95 %CI: 70.8−79.7), and 95.0 % at 15−21 days (95 %CI: 92.5−97.4) (Fig. 2 and Table 1 ). The sensitivity of IgM from the date of first PCR positivity was 44.5 % at <3 days (95 %CI: 36.5–52.6), 75.1 % at 3–7 days (95 %CI: 70.6−79.5), and 91.4 % at 8−14 days (95 %CI: 88.2–94.5). The proportion of patients that tested positive for IgM reached a peak of 96.0 % at 4−5 weeks after symptom onset (Table 1). There were five patients who had not mounted an immune response for IgM by week 4. Two of the 5 patients were on synthetic corticosteroid treatment, and the other three had no noted medications or disorders that may impair the immune system.
Fig. 2.
Seropositivity of Abbott SARS-CoV-2 IgM immunoassay. A) Seropositivity in 1332 specimens from 421 patients with positive PCR results relative to days from symptom onset and B) relative to days from testing positive by PCR. Dotted line represents the cutoff for positivity (index value ≥1.0). Red line represents the median of antibody index value for each time period.
Table 1.
Positive rate of Abbott SARS-CoV-2 IgM and IgG assays. Values in parentheses represent 95 % confidence interval.
| Time after symptom onset | IgM | n | IgG | n |
|---|---|---|---|---|
| 0−7d | 24.6 % (16.8−32.3) | 132 | 23.2 % (16.1−30.2) | 138 |
| 8−14d | 75.3 % (70.8−79.7) | 360 | 69.5 % (64.8−74.2) | 367 |
| 15−21d | 95.0 % (92.5−97.4) | 297 | 93.6 % (90.8−96.4) | 297 |
| 4−5w | 96.0 % (93.5−98.4) | 247 | 99.6 % (98.8−100.0) | 248 |
| 6−7w* | 92.7 % (88.8−96.5) | 177 | 99.4% (98.3−100.0) | 179 |
| 8−9w* | 84.5 % (76.1−92.5) | 71 | 97.2% (93.3−100.0) | 71 |
| 10w-<3m | 91.4 % (82.2−100.0) | 35 | 97.1% (91.4−100.0) | 34 |
| 3+m* | 30.8 % (5.7−55.9) | 13 | 92.3 % (77.8−100.0) | 13 |
N: number of specimens for each time period.
Significant difference was determined as p < 0.05 by Chi-square test for association.
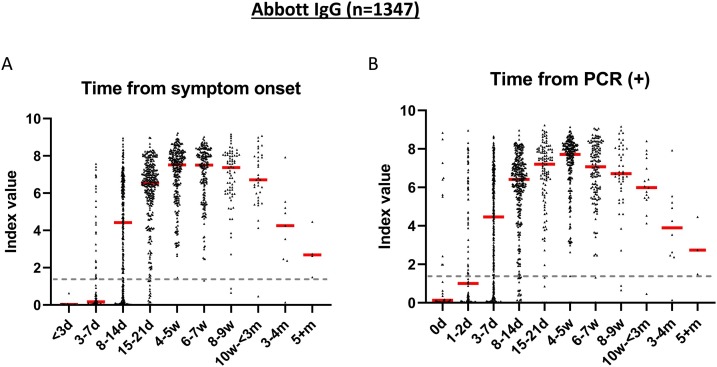
The sensitivity of the Abbott SARS-CoV-2 IgG assay relative to date of symptom onset was 23.2 % at ≤7 days (95 %CI: 16.1−30.2), 69.5 % at 8−14 days (95 %CI: 64.8−74.2), and 93.6 % at 15−21 days (95 %CI: 90.8−96.4) (Fig. 3 and Table 1). The sensitivity from the date of first PCR positivity was 43.0 % at <3 days (95 %CI: 35.0–50.9), 69.8 % at 3–7 days (95 %CI: 65.1–74.5), and 92.7 % at 8−14 days (95 %CI: 89.8−95.6). The seropositivity of IgG reached a peak of 99.6 % at 4−5 weeks post symptom onset. Whereas all patients had IgG seroconverted by the end of week 3, there was one whose IgG declined and became seronegative 5 weeks post symptom onset. The patient had no noted immunodeficiency nor received medications that may cause immune impairment.
Fig. 3.
Seropositivity of Abbott SARS-CoV-2 IgG immunoassay. A) Seropositivity in 1347 specimens from 427 patients with positive PCR results relative to days from symptom onset and B) relative to days from testing positive by PCR. Dotted line represents the cutoff for positivity (index value ≥1.4). Red line represents the median of antibody index value for each time period.
Assay imprecision was assessed using positive control material and a patient pool prepared to yield an index value close to the assay cutoff. For IgG, the coefficient of variation (%CV), which is a reflection of assay variability, was 1.2 % and 1.6 % for the positive control and patient pool, respectively (Supplemental Fig. 2). For IgM, the %CV for the positive control and patient pool was 2.0 % and 2.1 %, respectively. Total imprecision for IgG and IgM positive QC was 2.0 % and 2.3 %, respectively.
3.2. Longitudinal dynamics of IgG and IgM in SARS-CoV-2 infected patients
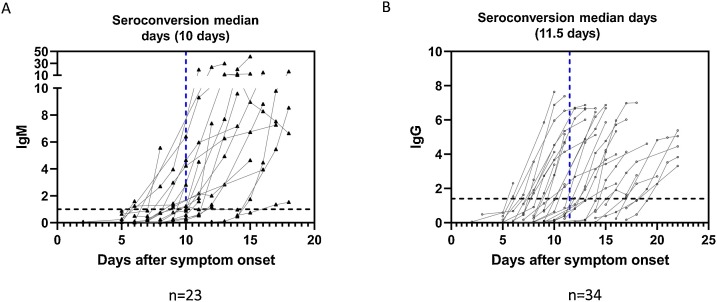
Serological status of 427 patients was followed using sequential serum samples collected up to 168 days post onset of symptom. Among these patients, we observed seroconversion of IgM in 23 patients, and the median day was 10 days following symptom onset (range: 6–15 days) (Fig. 4 ). Based on the analysis of 34 patients, the median day of seroconversion for IgG was 11.5 days post symptom onset (range: 6–20 days). Three different patterns of seroconversion were observed early in infection. Of the 163 patients who seroconverted for IgM and/or IgG <14 days post symptom onset, 72 % (n = 118) of individuals showed synchronous seroconversion, while 20 % (n = 32) of patients became IgM positive first and 8% (n = 13) became IgG positive first.
Fig. 4.
Serological courses of (A) IgM and (B) IgG for patients who were initially seronegative and then underwent seroconversion during the observation period. Blue dashed lines represent the median seroconversion days.
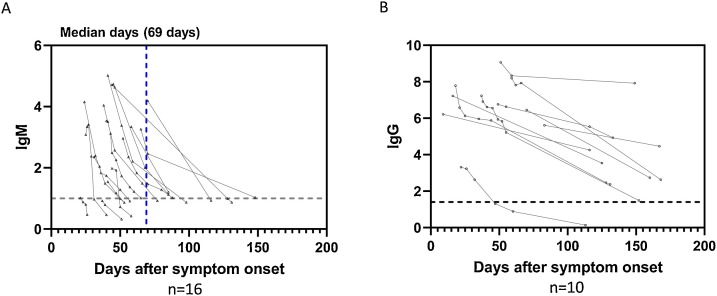
A steady decline was observed in the IgM index value starting from 4−5 weeks following symptom onset (Fig. 2). In 16 patients that were followed, the IgM level declined from above to at or below the positive assay cutoff during the observation period (Fig. 5 ). The median day of the IgM conversion was 69 days after symptom onset (range: 21–150 days). A decline in the IgG index value was also observed in patients who we followed for >100 days (Fig. 5). However, only two out of 427 patients became seronegative for IgG during the observation period (Supplemental Fig. 3). One of the patients received synthetic corticosteroid treatment, and the other had no noted immunodeficiency and was not on medication that may cause immune impairment. To be noted, IgM remained positive in one of these patients during the period that IgG became negative (47–113 days post symptom onset).
Fig. 5.
Serological courses and decline of antibody response in patients with COVID-19. (A) IgM index value in 16 patients who were initially seropositive for IgM and then antibody level declined to at or below the cutoff (index value 1.0) during the observation period. (B) Serological courses of IgG in 10 patients who were followed for >100 days. The first result of each patient was the peak level captured during the observation period.
3.3. Correlation of disease severity and antibody level
To investigate whether IgG and/or IgM levels correlate with disease severity, we assessed disease severity in a subset of patients based on their oxygen requirement and whether the patients were intubated. There was no apparent correlation between the IgG index value and the severity of COVID-19 (Supplemental Fig. 4). However, in patients with severe disease or in critical state, IgM level seemed to be higher 3–4 weeks post symptom onset compared to patients with mild to moderate disease (IgM median index value of 21.1 vs. 8.5), although the difference is not statistically significant due to limited number of specimens.
4. Discussion
Here we report the performance characteristics of the Abbott SARS-CoV-2 IgM assay that recently became available and compared its performance with the Abbott IgG assay. We report a diagnostic specificity of 100 % for IgM and 99.67 % for IgG using 300 pre−COVID-19 serum specimens. The IgM assay showed a slight increase in test sensitivity during the first three weeks following symptom onset relative to the IgG assay. IgM level declined steadily 4−5 weeks after symptom onset but remained positive in most patients in the first two months. The positive rate of IgG on the other hand remained at 92.3 % 3–6 months following symptom onset. To our knowledge, this is the first study that characterized the clinical performance of Abbott SARS-CoV-2 IgM assay using a large patient cohort with follow-up out to 168 days post onset of symptoms.
In the pre−COVID-19 cohort, the specificity of the Abbott IgM assay was demonstrated to be high (100 %), which is essential for achieving a high positive predictive value [4,12]. We found that the clinical specificity is higher for the Abbott IgM assay, which detects antibody against RBD of the spike protein, when compared to the IgG assay, which detects antibody against the nucleocapsid antigen. This could be because nucleocapsid protein is more conserved between SARS-CoV-2 and other pathogenic human coronaviruses compared with RBD of the spike protein [13].
The Infectious Diseases Society of America (IDSA) recommends that serological testing may be used to diagnose symptomatic patients with a high clinical suspicion and repeatedly negative molecular test results [4]. In this study, 8 out of 217 patients (3.7 %) were symptomatic and PCR negative but positive for IgM, IgG, or both assays. When samples were analyzed using an orthogonal IgG test from EI, five patients tested positive. It should be noted that our PCR negative specimens were collected early in the pandemic (between March and April 2020), making it unlikely that these patients experienced symptoms for a prolonged period. As SARS-CoV-2 RNA starts to decline 7–10 days after symptom onset and becomes undetectable in most patients around 20 days [1,14], the percentage of such PCR negative and serology positive patients could be higher in a cohort comprised of specimens collected at later time points. Our observation indicates that although it could be a low likelihood event, the Abbott IgM and IgG could serve as adjunctive diagnostic tools for symptomatic patients with negative PCR results.
From our analysis of 1347 specimens, the sensitivity of IgG assay increased weekly to reach 99.6 % at 4−5 weeks after symptom onset. The observed sensitivity of 93.6 % at 15−21 days following symptom onset is consistent with previous studies [15,16], but lower than what is claimed in the manufacturer’s package insert. The difference may be attributed in part to the patient population used for this study, in which the majority was hospitalized with multiple comorbidities. Furthermore, a large proportion of patients were elderly individuals who may have decreased immune responsiveness [17].
We followed the serological status of patients and observed steady decline of IgM response starting from 4−5 weeks post symptom onset, which is consistent with an earlier study [18]. Positive rate of IgM drops to 30.8 % after 3 months of symptoms, in contrast to IgG which is sustained in most patients (92.3 %) during that time frame. However, the kinetics for IgM serological reversion varies considerably amongst individuals. Our results suggest that IgM positivity usually indicates infection within the past 2–3 months. However, IgM can persist in a small proportion of individuals beyond 3 months.
Our study was limited by using specimens from patients who mostly required hospitalization. There could be differences in the humoral response in patients that require admission to a hospital compared to those that are asymptomatic or only experience mild symptoms, as the IgG response decays more rapidly in the latter population [2,7]. In our cohort, IgG response was sustained for 3–6 months in most patients. These findings need to be further corroborated by future studies.
In conclusion, the Abbott SARS-CoV-2 IgM assay offers high test specificity and sensitivity. The combined use of IgM and IgG testing is useful to support a diagnosis of COVID-19 most notably in symptomatic individuals who test negative by molecular detection methods.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The authors would like to thank the clinical laboratory staff at Beaumont Health Royal Oak for reserving remnant samples from COVID-19 positive patients and completing the testing for the study. A special thanks to Jillian Trueman and Kelly L Kerry.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2020.104663.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.To K.K. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long Q.X. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhao J. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanson K.E. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19:serologic testing. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo P.C. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin. Diagn. Lab. Immunol. 2004;11(4):665–668. doi: 10.1128/CDLI.11.4.665-668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mo H. Longitudinal profile of antibodies against SARS-coronavirus in SARS patients and their clinical significance. Respirology. 2006;11(1):49–53. doi: 10.1111/j.1440-1843.2006.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibarrondo F.J. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N. Engl. J. Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA . 2020. Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations for Medical Devices. [Google Scholar]
- 9.Adams E.R. Antibody testing for COVID-19: a report from the national COVID scientific advisory panel. medRxiv. 2020 doi: 10.12688/wellcomeopenres.15927.1. p. 2020.04.15.20066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.2020. Coronavirus Test Tracker: Commercially Available COVID-19 Diagnostic Tests. [Google Scholar]
- 11.Bewick V., Cheek L., Ball J. Statistics review 8: qualitative data - tests of association. Crit Care. 2004;8(1):46–53. doi: 10.1186/cc2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prevention, U.C.f.D.C.a . 2020. Interim Guidelines for COVID-19 Antibody Testing. [Google Scholar]
- 13.Premkumar L. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020;5(48) doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 15.Tang M.S. Clinical performance of two SARS-CoV-2 serologic assays. Clin. Chem. 2020;66(8):1055–1062. doi: 10.1093/clinchem/hvaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng D. SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood from the San Francisco Bay Area. medRxiv. 2020 doi: 10.1038/s41467-020-18468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montecino-Rodriguez E., Berent-Maoz B., Dorshkind K. Causes, consequences, and reversal of immune system aging. J. Clin. Invest. 2013;123(3):958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long Q.-x. Antibody responses to SARS-CoV-2 in COVID-19 patients: the perspective application of serological tests in clinical practice. medRxiv. 2020 p. 2020.03.18.20038018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.