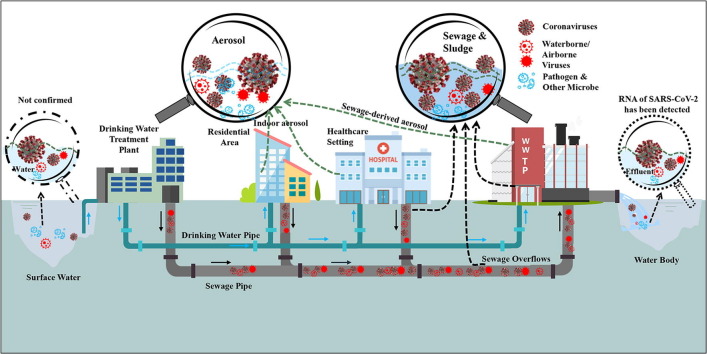
Graphical abstract
Keywords: Water and wastewater treatment, Aerosol, Coronavirus, Prevention and control strategies, Transmission, Water-related viruses
Abstract
By 17 October 2020, the severe acute respiratory syndrome coronavirus (SARS-CoV-2) has caused confirmed infection of more than 39,000,000 people in 217 countries and territories globally and still continues to grow. As environmental professionals, understanding how SARS-CoV-2 can be transmitted via water and air environment is a concern. We have to be ready for focusing our attention to the prompt diagnosis and potential infection control procedures of the virus in integrated water and air system. This paper reviews the state-of-the-art information from available sources of published papers, newsletters and large number of scientific websites aimed to provide a comprehensive profile on the transmission characteristics of the coronaviruses in water, sludge, and air environment, especially the water and wastewater treatment systems. The review also focused on proposing the possible curb strategies to monitor and eventually cut off the coronaviruses under the authors’ knowledge and understanding.
1. Introduction
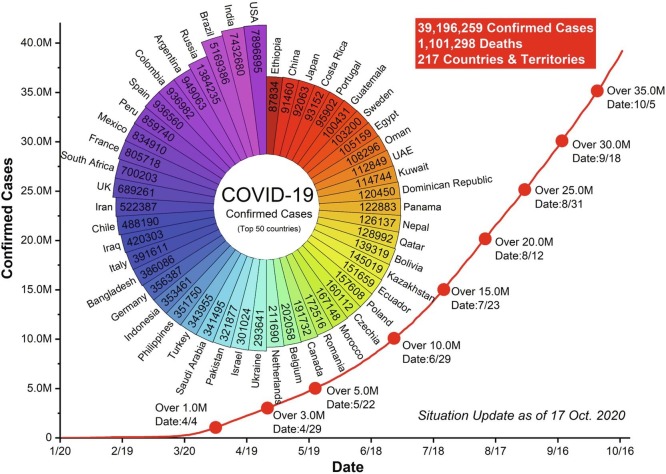
The new coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus (SARS-CoV-2) is the latest complex coronavirus disease and an ongoing pandemic in the world. According to the World Health Organization (WHO) report, there have been 39,196,259 confirmed cases including 1,101,298 deaths worldwide from January 17 to October 17, 2020 [1] (Fig. 1 ). This situation is as profoundly alarming with enormous consequences worldwide, and had a significant effect on global public healthcare systems and restructured society and economics [2], [3], [4]. The United Nations (UN) estimated global trade to fall between 13 and 32 per cent this year [5]. It goes far beyond any recent challenge that we have experienced. The most vital fact is that the SARS-CoV-2 can spread from person to person by several routes. The respiratory droplet and direct contact transmission have been proved to transmit this virus [6], [7], [8]. On the other hand, it is also possible that a person can be infected by airborne, fomite, fecal-oral, bloodborne, mother-to-child, and animal-to-human transmission [9]. Similarly, a review of severe acute respiratory syndrome coronavirus (SARS) and Middle East respiratory syndrome coronavirus (MERS) outbreaks suggested three possible routes to transmit: close person-to-person contact; aerosol transmission; and fecal-oral transmission [10]. We are very aware of the fast spread speed of the COVID-19 (Fig. 1), while the steps have been taking at national and international levels to curb it. Travel bans, social distancing, ‘lockdown’ and closed borders are becoming norm across the globe.
Fig. 1.
Confirmed cases of top 50 countries in SARS-CoV-2 (Source: World Health Organization).
Since only in the 21st century, the world has been repeatedly challenged by respiratory diseases caused by the emerging coronavirus, such as the SARS-CoV outbreak in China in 2002–2003 [11], [12], and MERS-CoV in Middle Eastern countries in 2012 [13], [14]. The coronavirus is a kind of enveloped, positive-sense and single-stranded RNA viruses that can be divided into alphacoronavirus(αCoV), betacoronavirus(βCoV), gammacoronavirus(γCoV), and deltacoronavirus(δCoV) [15]. The SARS-CoV-2 is closely related to SARS-CoV and MERS-CoV, they are genetic clusters within βCoV [16], [17]. Moreover, the coronaviruses can infect more than one host species, including humans, birds, pigs, bats, camels, and other wildlife [18], [19], meaning that both SARS and MERS belong to zoonotic viruses. More importantly, 10 out of the 11 diseases at high risk for severe outbreaks designated by WHO in 2015 have zoonotic diseases or transmission vectors [20]. Moreover, the monitoring on epidemiology and evolution of species jumps study showed that about 70% of the novel human infectious diseases were original from livestock or/and wildlife [21].
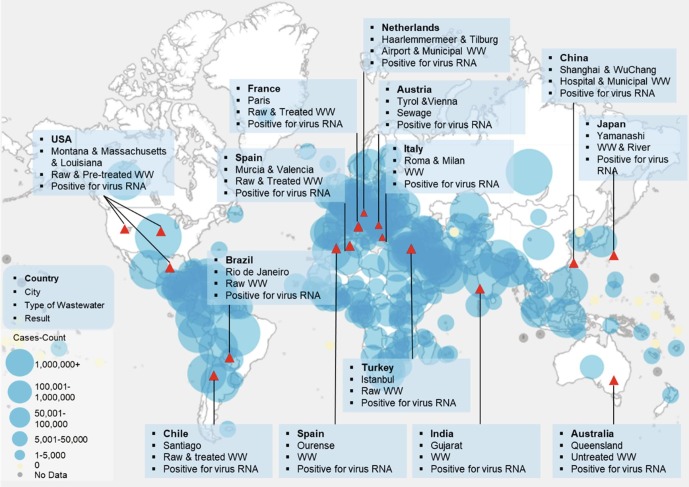
Looking past, the publications about coronavirus are mainly concentrated on veterinary medicine, human medicine, infectious disease, and public health field [22], [23], [24], while the natural environment vectors (water, air, soil) and social network are important vital mediators of zoonotic disease’s evolution into epidemics and have relatively rarely been explored and investigated. Recently, large numbers of investigations have reported that the RNA of SARS-CoV-2 has been detected in raw sewage, secondary treated wastewater (normally biological wastewater treatment process being applied), and even the non-potable water used for urban irrigation (Fig. 2 ) [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]. More importantly, the infectious virus has also been detected in feces and urine of patients [45], [46], [47], which triggered the public’s high concern about fecal-oral transmission and/or wastewater transmission. The virus that has been found can remain stable for up to a few days at 20 °C in wastewater [48]. On the other hand, a recent study reported that COVID-19 patients could exhale millions of SARS-CoV-2 RNA copies into the air per hour during the earlier disease stages [49], while most of the particles in the exhaled breath were smaller than PM 2.5 [50]. The limited but significant potential evidence supported that SARS-CoV-2 could survive in aerosols [51], [52], [53], [54], [55]. The presence of coronaviruses in the environment raises concerns about other possible transmission routes besides respiratory droplets and direct contact.
Fig. 2.
Confirmed cases of RNA of SARS-CoV-2 detection in global aqueous environment.
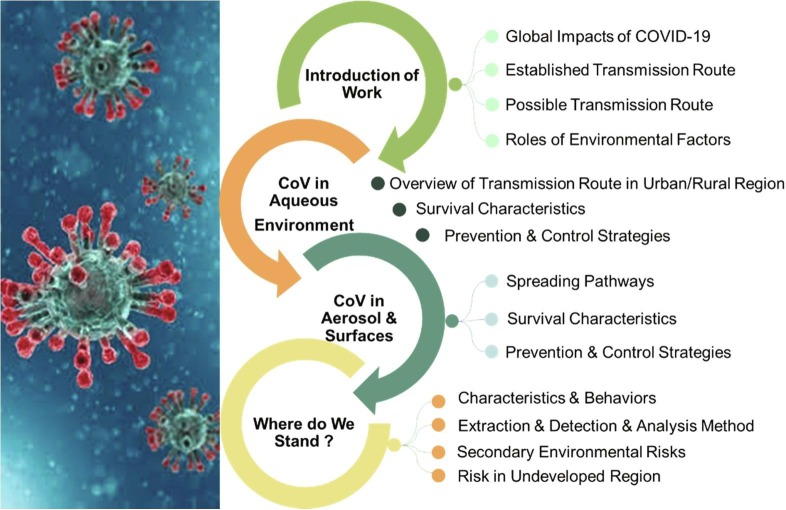
As such, wastewater and aerosols may contain some viable and infective coronaviruses, there is an urgent need to review the current knowledge for helping to understand the exposed and transmitted pathways of the SARS-CoV-2 in these environment vectors, and exploring the use of environmental engineering approaches to monitor and control it. The purpose of this review is to provide systematic while updated information based on various studies of SARS-CoV-2 from water, air, and environmental science and technology perspectives. Special emphasis is given to the occurrence, transmission, stability and persistence of SARS-CoV-2 for water and aerosol. Discussion on the possible prevention and control strategies of the SARS-CoV-2 is also presented. It is important to note that this review is not focused on COVID-19 itself, but it is the review related with the water and wastewater and their treatment processes under the effect of COVID-19. Fig. 3 presents the framework for this study.
Fig. 3.
The framework of this study.
2. Characteristics of coronaviruses in aqueous environment
2.1. Overview of transmission via water-related pathways
The discovery of SARS-CoV, SARS-CoV-2, and MERS-CoV in feces, urine, and vomit has widely been reported [45], [56], [57], [58], [59], [60], [61], [62], [63]. Although there is currently no evidence of fecal-oral transmission of SARS-CoV-2, some studies supported the possibility of this transmission route, for example, the virus has been isolated from infected individuals’ feces [64], [65]; virus RNA was recovered from untreated wastewater and secondary-treated wastewater [30], [31], [33], [37], [38]; and virus could highly be stable under certain conditions (at 20 °C in wastewater and tap water) [48]. It is also worth noting that saliva and various respiratory secretions from an infected individual may also be released into the collection system in wastewater [66], [67]. This implies that the viruses would spread with human or/and animal waste in sewage and hospital wastewater and then transfer into the aqueous environment [18], [68], [69], [70].
2.1.1. Urban/centralized water system
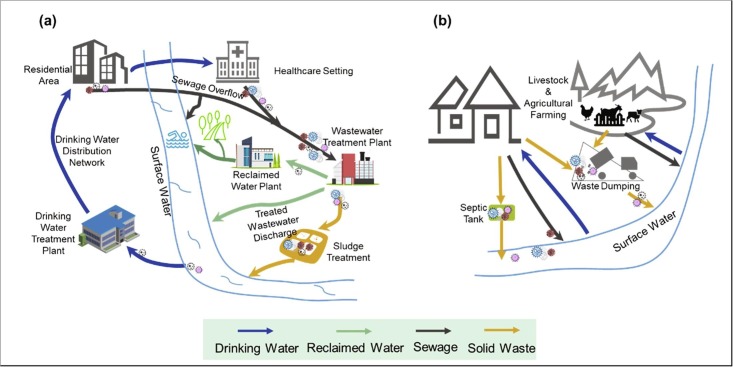
Global urbanization is accelerating with over half of people living in urban areas. However, the centralized living also creates a favorable environment for the rapid spread of the virus. The coronaviruses-containing waste has various routes to enter the aqueous environment and cause risks to public health (Fig. 4 a). The foremost among pathways are: 1) inadequate collection and treatment of wastewater; 2) untreated sewage flowing directly into receiving aquatic bodies; and 3) even contaminated water production of industry and agriculture [35], [71], [72], [73]. It is well accepted that the major exposure of human to water-related viruses is through unsafe water treatment [74], [75]. If wastewater treatment plants (WWTPs) is insufficient to inactivate/remove coronaviruses, or combined sewer overflows/bypasses are operational during heavy rainfalls, the viruses may enter the natural water body [76], [77]. A report has claimed that the infectious COVID-19 virus was found in the sewage overflow of a private rainwater/sewage pipe and caused community transmission in Guangzhou, China [78]. Similarly, burst sewer networks may be another mode of transmission [79]. In addition, due to the direct discharge of wastewater, the virus presence in river was detected in Ecuador with low sanitation [43]. In some cities, the non-potable water is directly from the river and canal to clear streets and to water the greenery. Clearly, this action is closely related to the public. Notably, the new coronavirus has been found in Paris’s non-potable water [80].
Fig. 4.
Overview spreading route of / water-related coronaviruses in urban region (a) and rural area (b).
On the other hand, an inadequate plumbing system in a residential building likely contributed to coronaviruses transmission. The coronavirus-containing water droplets have overflow from sewage pipe, causing virus transmission. For example, Hung [81] demonstrated in a survey of 321 SARS patients from Amoy Gardens in Hong Kong, two-thirds of SARS infected patients had diarrhea. A large amount of virus loads have been discharged into the sewage pipe in the block, and the water droplets containing coronaviruses spilled from the water closets, basins, bathtubs, and the bathroom floor drains which caused the spread of the virus in the community.
2.1.2. Rural/decentralized water system
Different from the city’s centralized water treatment, which builds a series of grey or technology-based infrastructure, the rural water supply and treatment are mostly decentralized and untreated (Fig. 4b), especially in developing and less developed areas [82]. In these regions, however, agricultural farming and animal husbandry are one of the main sources of potential virus. In these regions, agricultural farming and animal husbandry is one of the main sources of the potential virus. The livestock and/or poultry waste normally has not been effectively managed and disposed of, which may contain the zoonotic virus. It is worth mentioning that animal feces, during precipitation events, can be carried into surface water bodies with runoff. Although there is no report on the new coronavirus being detected in agricultural farming, caution should be taken on it as cats, ferrets and dogs [83], [84], and tiger [85] have been reported to be infected by the SARS-CoV-2. The more serious information is that the coronavirus has been detected at eight mink farms in the Netherlands, and at least two workers were infected, which could be the first known cases of animal-to-human transmission [86]. Likewise, around ninety minks and seven workers have also been tested positive for coronavirus in northern Spain [87]. Some studies have reported that livestock animal waste significantly associated with drinking water contamination in low-and middle-income countries [88], [89]. 67% of households sampled had faecal-contaminated drinking water in peri-urban communities of Kisumu, Kenya [89]. Contaminated water by humans and animals is one of the important ways of indirect transmission of virus [90]. Therefore, it should be urgently assessed about coronaviruses transmission via water in low sanitation regions.
In addition, the lack of wastewater treatment or improper management of wastewater disposal, leakage of septic tanks, and mismanagement of animal waste disposal are also possible pathways to cause contaminated aqueous environment [76]. The wastewater treatment ponds are most applied in rural and developing countries, which need more than two weeks to get reduction one log of viruses [91]. Therefore, if the wastewater and waste from these underdeveloped regions was not treated properly, it would result in snowballing transmission of COVID-19 in wastewater, as seen in other viral diseases previously [92], [93].
2.2. Survival characteristics of coronaviruses in the environment through water-related pathways
The survival and persistence of coronaviruses released into the aqueous environment determine the magnitude of human health risks [94]. Many factors affect the survival or infection of coronaviruses in the water/wastewater environment. The main effects are viral load and environmental conditions, especially water temperature and wastewater characteristics, such as type of medium, the presence of organic and inorganic matters [58], [68], [69], [95], [96], [97]. To date, there is limited scientific evidence for the persistence of coronaviruses in water and wastewater. The latest study estimated the times for 90% reduction (T90) of viable infectious SARS-CoV-2 with a high titer in wastewater and tap water by measuring tissue culture infectious dose per milliliter (TCID50 mL−1). The result has shown that T90 was 1.6 days in wastewater and 2.0 days in tap water under room temperature (20 °C), while it will be decreased to only 15 and 2.2 min in wastewater at 50 °C and 70 °C, respectively [48]. Another study about the stability of SARS-CoV-2 in different environmental conditions reported that the virus is highly stable at 4 °C, but very sensitive to temperature, and can be inactivated in 5 min at 70 °C [95]. Likewise, the SARS-CoV-2 can remain stable and infectious for up to 25 days at 5 °C in water, but rapidly inactivated at 30 ℃ [73] (not an experimentally determined value, it was estimated in vitro study data). In addition, SARS-CoV-2 can be stable at pH 3–10 at room temperature [95].
During the SARS epidemic outbreak in China, the Xiaotangshan hospital and the 309th hospital in Beijing were each equipped with sewage treatment site. The behavior of the SARS-CoV in hospital sewage has been investigated and claimed that the SARS-CoV could remain infectious for 2 days in sewage, while the RNA could be detected for 8 days [58]. The survival characteristics of the isolated SARS-CoV in different media and temperatures showed that the SARS-CoV could survive for 14 days in saline and 17 days in urine at 20 ℃, while in domestic sewage and dechlorinated tap water only survive for 2 days at the same temperature. While at 4 ℃, the virus was found to remain infectious for over two weeks in all mediums [96]. MERS-CoV was similar to SARS-CoV, and was more favorable for survival at low temperatures and humidity [98].
It should be noted that unlike other waterborne viruses, the membrane of coronaviruses is a lipid layer, thus highly unstable [99]. Disrupting the lipid envelope can eliminate risk. For example, SARS-CoV was more unstable in wastewater containing disinfectants compared to non-enveloped viruses [96]. On the other hand, some other surrogate coronaviruses survival characteristics were also investigated. For example, the persistence of human CoV 229E, enteric feline CoV (ATCC-990), transmissible gastroenteritis virus (TGEV), mouse heptitis virus (MHV), and Pseudomonas phage Φ6 in water and wastewater had been evaluated. The variable result showed that water temperature, co-existing pollutants (organic matter, suspended solids, etc.), test media, and biological activity were together determining the survival characteristics of the coronaviruses [68], [69], [100].
Overall, the stability of coronaviruses in aqueous was highly variable, while increased temperature or adding some strong oxidants like free chlorine could disrupt the lipid envelope to inactivate coronaviruses. At this point, we can deduce that SARS-CoV-2 is also highly dependent on water temperature and easily inactivated by disinfectants. In other words, however, the lower temperature will be more suitable for virus survival in aqueous. The special attention is required in winter to prevent a possible “second wave” of outbreaks.
2.3. Possible prevention and control strategies
As mentioned above, the RNA of SARS-CoV-2 has been widely detected in wastewater. However, most scientists believe that wastewater is currently not a significant transmission route [30], [94], [101], while WHO also stated that there is no evidence about the survival of SARS-CoV-2 in wastewater or drinking water. Indeed, some possible prevention and control strategies for coronaviruses have been adopted in the water cycle, but it is still a need to understand the characteristics of possible curb strategies for coronaviruses in water-related pathways and grasp some technologies for inactivation in daily life.
2.3.1. Wastewater treatment
To our knowledge, there are few studies on the fate and removal efficiency of coronaviruses in actual WWTPs, especially to monitor the change of coronaviruses infectivity (Table 1 ). It is because several barriers prevent investigating viable coronaviruses in wastewater treatment, from detection technology to the special working environment. Thus, most scientific efforts were placed on tracing for coronaviruses RNA in WWTPs. Randazzo et al. [33] investigated the occurrence of SARS-CoV-2 RNA in different WWTPs in Spain. They collected influent, secondary, and tertiary effluent water samples to analyze SARS-CoV-2 RNA, the result showed that 83% (35 out of 42) influent samples, and 11% (2 out of 18) secondary treated water samples were tested positive for SARS-CoV-2, while none was tested positive in the tertiary effluent samples (0 out of 12). This agrees with data reported by Rimoldi et al. [35] in Italy. Likewise, although the presence of the viral genome has been confirmed in untreated wastewater at higher ambient temperature (above 40 °C), the negative results for the presence of viral RNA in the effluent from the WWTPs were reported in India [36]. The conventional wastewater treatment process (normally the activated sludge process) should be good enough to inactivate SARS-CoV-2. In contrast, some studies reported that the positive results for viral RNA in effluent could still be discovered even after WWTP treatment [25], [44]. The possible reasons to explain these contradictory results are the lack of a standardized procedure of SARS-CoV-2 RNA detection in wastewater, and the various treatment techniques adopted in different WWTPs at reality environmental conditions. Therefore, further studies seem desirable to face the situation.
Table 1.
Summary of detection and quantification of SARS-CoV-2 in wastewater and sludge in WWTPs.
| Location | Period of examination | Sample condition (type, volume, temperature) | Method of RNA extraction, detection and quantification | RNA concentration of influent (copies/L or samples positive) | Wastewater treatment technologies | RNA concentration of effluent (copies/L or samples positive) | Reference |
|---|---|---|---|---|---|---|---|
| Spain, Region of Murcia | March 12 to April 14, 2020 | Municipal WW;Grab sampling 200 mL; 4 °C | NucleoSpin RNA virus Kit; TaqMan real-time RT-PCR; gc | 5.1 ± 0.3, 5.5 ± 0.2, and 5.5 ± 0.3 log10 gc/L of N1, N2 and N3 primer/probe mixes | CAS, Coagulation, Flocculation, SF, Disinfection, UV, NaClO | Secondary effluent: 5.4 log10 gc/L of N2 primer/probe mixes; Tertiary effluent: No detection | [33] |
| India, Ahmedabad | May 8 and 27, 2020 | Hospital treating WW; 4 °C | NucleoSpin® RNA virus Kit; TaqPathTM Covid-19 RT-PCR Kit; Ct value | 8 May: 100% of positive rate (5.6 × 10(copies/L)); 27 May:100% of positive rate (3.5 × 102(copies/L)) | UASB | 8 May: 0% of positive rate 27 May: 0% of positive rate | [41] |
| Itlay, Milano and Monza e Brianza | April 14 and 22, 2020 | Municipal WW; Grab sampling Separate stainless steel buckets | QIAMP Viral RNA mini Kit; 2019-nCoV real-time RT-PCR kit panel; Ct values | April 14: 75% of positive rate(3/4); April 22: 25% of positive rate(1/4) | CAS + peracetic acid or high intensity UV | No detection | [35] |
| India, Jaipur | May 4 to June 14,2020 | Municipal WW and Hospital WW; Sterile bottles | Allplex™ 2019- nCoV Assay Kit; TaqPath™ COVID-19 Combo Kit; Ct values | Positive | MBBR/SBR + Cl/UV | Negative | [36] |
| Chile, Santiago | May 20 to June 16,2020 | Sewage; 24 h composite sample; Sterile propylene bottles | QIAamp® Viral RNA Mini Kit; TaqMan 2019-nCoV Assay Kit v1; Ct values | 20 May: 354–628(copies/L); 16 June: 2304–4805(copies/L) | n.a. | 20 May: 10–20(copies/L); 16 June: 0–167(copies/L) | [44] |
| France, Paris | March 5 to April7, 2020 | Municipal WW; 11 mL | PowerFecal Pro kit on a QIAsymphony extractor,QIAGEN; PCR Inhibitor removal resin; Ct values | 100% of positive rate (over 106(copies/L)) | n.a. | 75% of positive rate (6/8); (near 105(copies/L)) | [25] |
| China, Wuchang | February 5 to March 10, 2020 | Municipal WW; Grab sampling; 2.0 L; 4 °C | EZ1 virus Mini Kit; AgPath-ID™ One-Step RT-PCR Kit; Ct values | Non-detected (After primary disinfection tank before septic tank) | Preliminary disinfection tank + Septic tank | (7.5 ± 2.8) × 103 to (14.7 ± 2.2) × 103 copies/L in the effluents of septic tanks | [71] |
| Spain, Ourense | April 6 to April 21,2020 | Municipal WW; 250 mL; 24 h composite samples; 4 °C | STARMag 96 × 4 Universal Cartridge Kit; RT-qPCR Allplex system™ 2019-nCoV; Ct values | 100% of positive rate (5/5); | Grit and sand separator, primary settler, SBR, Microfiltration | Secondary effluent: 25% of positive rate (1/4); Finally effluent: No detection | [34] |
| Japan, Kofu | March 17 and May 7, 2020 | Municipal WW; Grab sampling; 1.0 L; | RNeasy PowerWater Kit; High-Capacity cDNA Reverse Transcription Kit; Ct values | 4.0 × 103 to 8.2 × 104 copies/L | CAS: primary sedimentation, aeration, final sedimentation, chlorination | Secondary effluent: 1.4 × 102 to 2.5 × 103 copies/L | [39] |
| USA, Louisiana | January to April 2020 | Municipal WW; 24 h composite samples; −80 °C | ZR Viral RNA Kit; High Capacity cDNA Reverse Transcription Kit; Ct values | 13% of positive rate(2/15) | n.a. | None of the secondary treated and final effluent samples | [40] |
| Spain, Ourense | April 6 to April 21, 2020 | Sludge; 250 mL; 4 °C | STARMag 96 × 4 Universal Cartridge Kit; RT-qPCR Allplex system™ 2019-nCoV; Ct values | 41% of positive rate(14/34) in primary sludge, biologic sludge, and thickened sludge | Gravity thickening and centrifuge, thermal hydrolysis, anaerobic digestion | 0% of positive rate(0/5) in digested sludge | [34] |
| USA, New Haven | March 19 to May 1, 2020 | Sludge; −80 °C | RNeasey PowerSoil Total RNA Kit; Bio-Rad iTaq Universal Probes One-Step Kit; Ct values | 100% of positive rate; 1.7 × 103 to 4.6 × 105 copies/mL | Settler | n.a. | [27] |
| Turkey, Istanbul | May 7, 2020 | Sludge; Grab sample | Roche MagNA pure LC total nucleic acid isolation Kit; Ct values | 100% of positive rate in primary sludge, 1.25 × 104 to 2.33 × 104 copies/L | CAS | 100% of positive rate in waste activated sludge, 1.17 × 104 to 4.02 × 104 copies/L | [26] |
Note: n.a.: not available.
CAS: conventional activated sludge; SF: Sand filtration; UASB: upflow anaerobic sludge blanket reactor; MBBR: moving bed biofilm reactor; SBR: sequencing batch reactor.
As the size of the coronavirus ranges from 60 to 220 nm [69], the conventional primary treatment (screens, grit chamber, primary clarifier) may be insufficient to remove coronaviruses by physical processes. One study reported that up to 26% of enveloped viruses were removed by solid settling [100]. However, other studies pointed out that organic matters and suspended solids could help protect and survive for coronaviruses [69], [71]. A study stated that SARS-CoV-2 RNA was still detected in the wastewater after the primary settler process [34]. The non-enveloped human has been found to be more persistent in water for long periods of time than enveloped viruses [69], [76], [99], [102]. Therefore, the fate of coronaviruses in the secondary treatment process (activated sludge process or/and biofilm process) in WWTPs can be predicted by referring to the non-enveloped viruses. The enteric viruses, norovirus, and adenoviruses are commonly waterborne non-enveloped viruses and are normally used as surrogates for treatment performance evaluations in the aquatic environment [103], [104], [105], [106]. Based on the collective data, the level of virus removal in secondary treatment has a variable feature between less than 1 log unit to greater than 4 log units, depending on the treatment process used and the virus types [107], [108], [109], [110]. The mechanism of remove viruses in activated sludge is mainly attributed to sludge flocs adsorption and subsequent separation in secondary clarifiers [108]. The membrane bioreactor (MBR) shows a higher removal effect because it owns better solid separation than the conventional activated sludge technologies [109], [111]. In a recent report, no SARS-CoV-2 genetic material in effluent wastewater was detected in either sequencing batch reactor (SBR) or moving bed biofilm reactor (MBBR) [36].
As the last step of treatment process, disinfection is a vital process for inactivating the viruses. The ultraviolet (UV) light and/or chemical disinfection are the main disinfection processes in actual WWTPs. The chemical oxidants include chlorine, chloramines, ozone, and chlorine dioxide, etc. Regardless of the process applied, disinfection highly depends on wastewater physicochemical properties and operational conditions, especially the product of disinfectant concentration and contact time (CT value). A number of studies have been carried out and have demonstrated that the reduction in non-enveloped viruses, such as human adenovirus (HAdV), enterovirus (EV), in effluent treated with UV or chemical disinfection is more effective, with 0.1 to 5 log units, and point out coronaviruses are more sensitive to UV than non-enveloped viruses [107], [109], [112], [113]. For the inactivation of coronaviruses in wastewater, a study to explore the inactivation of SARS virus in municipal wastewater by chlorine and chlorine dioxide showed that the chlorine dosage of 20 mg L-1, a contact time of over 1 min, and free chlorine residual of >0.4 mg L−1 were found to complete coronaviruses inactivation, while chlorine dioxide needs to be using a dose of 40 mg L−1 with a required contact time of >5 min to achieve the same effect [58]. However, the co-existing high concentration of organic compounds and suspended solids in wastewater would interfere with disinfection for the virus. During the COVID-19 outbreak in Wuhan, China, the treatment of medical wastewater containing SARS-CoV-2 viral RNA located at Wuchang Fangcang hospital, China, in which the wastewater was firstly pumped from toilets and showers into the preliminary disinfection tank, while adding 800 mg L−1 of sodium hypochlorite for disinfecting with contacting time of 1.5 h, then the SARS-CoV-2 viral RNA was not fully detected after this kind of preliminary disinfection process [71]. Thereafter, the wastewater was pumped into the septic tank. It was surprising that the SARS-CoV-2 viral RNA was detected from the effluent sample of the septic tank. It is believed due to the suspended solids in the septic tank and thus noting that a small size of particles can shield viruses from disinfection [71]. It is noteworthy that chemical oxidants would mainly damage viral capsid proteins for disinfection, while the UV would damage the viral genome and proteins [114], [115]. A separate study demonstrated that UV disinfection would reduce single-stranded RNA virus levels below detection limits [116]. Ye, et al. [117] explored the inactivation mechanism of enveloped viruses in UV and free chlorine, showing that the molecular features of an enveloped virus were susceptible to chemical oxidants or UV radiation. As the coronaviruses are enveloped viruses, it should be considered that a combination of UV and chemical oxidation disinfection is suggested to better inactivate coronaviruses, which can achieve at least 5 log units reduction of the waterborne virus [118]. Similar to chemical oxidation disinfection, suspended solids would also affect UV disinfection efficacy. Overall, the regulation running of urban WWTPs would satisfactory to remove coronaviruses in wastewater.
For decentralized rural wastewater treatment, there have been a variety of technologies existed, ranged from the simple individual septic tank to comprehensive ecological engineering systems, such as constructed wetlands, soil infiltration treatment systems, and biofilter technologies. In general, these treatment facilities have a good performance on removing COD and considerable removal of nutrients, but research on the removal of waterborne viruses is still in its infancy [119], [120]. More importantly, because small decentralized wastewater treatment systems may be lack of disinfection approach, and lack of public’s poor risk and threat perception of water, caution should be taken to drainage system when infected patients live in the “catchment area”. For asymptomatic individuals or mild patients, when they are quarantined at home, it is recommended to put disinfected chlorine tablets in the toilet tank, which can effectively inactivate coronaviruses and reduce the wastewater loading of viruses and secondary transmission. The septic tank system should preferably be located at a certain horizontal separation distance (least 30 m) from the water source to avoid contaminating the water supply [121].
Overall, the adsorption with solids or sludge, and filtration with sand and/or membrane could remove coronaviruses from wastewater, but the inactivation process is highly dependent on chemical oxidation disinfection and UV disinfection for wastewater treatment.
2.3.2. Drinking water and reclaimed water treatment
Urban drinking water system is composed of four important parts, which are source water, waterworks, distribution network as well as point-of-use. Management and control of each part need to pay attention to prevent and control the water-related virus spread. We must point out that, so far, SARS-CoV-2 has not been detected in drinking water, and the drinking water is safe.
The rigorous pollution control in source water is the first step in the control of the drinking water quality. Due to SARS and MERS are zoonotic viruses, the management of animal farming near source water needs to pay attention. Waterworks or drinking water treatment plants (DWTPs) are the most critical part of controlling water-related viruses in the urban water cycle. The water-related virus can be removed by physical separation and inactivation in DWTPs. In a typical conventional drinking water treatment process, chemical coagulation could achieve 0.1–2.4 log unit viruses removal range with iron or aluminum coagulants [122]. The mechanism may be attributed to the physical removal of inclusions in the floc and inactivation of chemical oxidation [123]. The effect of precipitation and filtration on virus removal highly depends on the filtration technologies. The effect of membrane filtration on virus elimination is better than trickle filtration and sand filtration [124], [125]. The reverse osmosis (RO) process should be the best in theory for virus removal, but in practice, more ultrafiltration (UF) and nanofiltration (NF) membranes are adopted due to more cost-effective reason and similar removal level to be obtained (over 4 log units reduction for norovirus) [125], [126]. Disinfection, as the last checkpoint of DWTPs, is the controlling point for virus inactivation. Chlorine disinfection is a common method of drinking water, the concentration of free chlorine ≥ 0.4 mg L−1 could completely inactivate SARS-CoV [58]. For effective centralized disinfection, the WHO has suggested free chlorine ≥ 0.5 mg L−1 after at least 30 min of contact time at pH < 8.0. However, the performance of this concentration of free chlorine on SARS-CoV-2 real medical wastewater is unsatisfactory [71]. On the other hand, with the high dose of disinfectant, toxic disinfection by-products (DBPs) (such as chloramine, chlorite, bromate, etc.) will also significantly increase which will deteriorate the water quality [114]. Thus, it is important to ensure that the lower concentration of influent turbidity and nutrient enter the disinfection process, which would significantly influence the level of DBPs and disinfection efficiency. Therefore, more research on the efficacy of various disinfection options is needed on coronaviruses in water and wastewater. It is highly necessary to: 1) ensure the safety of the water distribution network system; 2) reduce the network accident and cross-contamination as much as possible; and 3) ensure the residual chlorine and/or monochloramine at the terminal water supply [114].
In the rural and/or underdeveloped regions where there is no centralized water supply, people may have a higher risk to directly drink unsanitary surface water. In this situation, the prolonged boiling and/or adding bleaching powder or chlorine tablets are possible ways to inactivate coronaviruses in water based on the survival characteristics of coronavirus. SARS-CoV-2 could be efficiently inactivated by heating at 92 °C for 15 min (>6 log units removal) [127].
As the advanced treatment process, reclaimed water is similar to that of ordinary DWTPs, the possible prevention and control strategies for the water-related virus in DWTPs are suitable for reclaimed water management [107], [128]. Based on the data during the outbreak of SARS and MERS, most DWTPs systems that meet the virus removal/inactivation regulations are effective for coronaviruses control. Furthermore, some combined advanced oxidation processes have begun to be applied in the treatment of reclaimed water and advanced drinking water, which include UV-based advanced oxidation processes (UV/AOPs) and ozone/biologically activated carbon (O3/BAC). Indeed, these will further enhance the ability to inactivate the water-related virus.
2.3.3. Sludge treatment
Like most pollutants are removed in traditional activated sludge process, virus removal can be seen as just a transfer from the aqueous phase to the solids phase, and then further into the sludge. Although the limited literature reported about the coronaviruses in sludge (Table 1), the risk of virus transmission in sludge disposal should not be ignored [26], [27], [34], [129]. The hydrophobicity of enveloped viruses makes coronaviruses more prone to adhere to solids [69]. Therefore, coronaviruses (RNA filament or its fragments) are generally concentrated in suspended solids [100]. Once they are not inactivated in time and exposed to the open environment, the virus or virus RNA adsorbed by the sludge may be leached out when spread in agriculture [129]. The SARS-CoV-2 RNA was not detected in the effluent from WWTPs, but the primary and thickened sludge showed higher and steadier concentrations [34]. The virus RNA presented in primary and waste activated sludges in WWTPs during the COVID-19 outbreak in Turkey has been reported [26]. In another study, it was found that the SARS-CoV-2 RNA concentrations in the primary sludge showed a strong correlation with the number of confirmed cases in local communication [27]. In 2013, a survey of 10 sewage sludge samples from five WWTPs throughout the continental USA has shown that contained genes from coronaviruses were found in more than 80% of the samples by metagenome analysis [130]. Another study also pointed out that the viruses could persist for several months in the sludge particles under a suitable environment [131]. It is important to note that the survival and fate of the virus in sludge would be difficult to exact interpreter and predict due to the various compounds mixed in sludge. Based on the currently available data, the coronaviruses transmission by sludge was not studied, especially when the viral load in the sludge is high.
Current research shows that coronaviruses can be hidden in fine particles and escape from disinfection [69], [71]. If sludge is proved to host coronaviruses with infective capacity then coronaviruses removal in sludge should focus on inactivation. It is difficult to inactivate the virus by physical methods such as sludge thickening, dewatering, and drying [132]. Strong oxidative chemicals (e.g. ozone and chlorine) can effectively inactivate viruses, but disposal costs and possible carcinogens (THMs, trichloromethane) limit their practical application. Using lime to condition sludge to stabilize it is an economical and reliable method. It has been reported that a sufficient amount of lime addition to increase temperature and make the pH of the sludge higher than 12 can effectively inhibit the growth of the microorganisms [95], [133]. When pH is adjusted to around 13, the SARS-CoV can be inactivated immediately and efficiently (average reduction of 3.6 log units) [134]. Although there is currently no study on the stabilizing effect of lime on inactivating SARS-CoV-2, it could be a foreseeable approach to use the lime for inactivation.
Sludge heating treatment processes, such as ultra/high-temperature aerobic fermentation, temperature-phased/thermophilic anaerobic digestion, and sludge incineration may effectively inactivate coronavirus. The mesophilic anaerobic digestion (MAD) process showed limited inactivation effectiveness for the pathogen (mean reduction of 1 log units) [132], but a study reported that no SARS-CoV-2 RNA was detected in the digested sludge, probably due to the result of the combined effect of thermal hydrolysis and long residence time [34]. Notably, sludge generated during the treatment of wastewater from hospitals and centralized quarantine centers must be disposed of in strict accordance with the guidelines of hazardous waste, and incineration is accordingly recommended.
2.3.4. Water users and water industrial workers
Until more evidence is available, the drinking water is safe. More attention should be paid to other daily water activities, such as hand washing, toilet flushing, etc. Household bleach can be used to clean items that may carry the virus [95]. When flushing the toilet, adding chlorine tablets to the water tank is an effective way to inactivate virus from fecal and urine, and should check floor drain for water seal protection to avoid spreading through sewage pipe. Moreover, before and after using the toilet, hands must be washed with soap.
Workers who handle drinking water, wastewater, and sludge face huge risks of direct exposure to diseases that may contain viruses. The risk is not only from water and/or sludge which may involve viruses or virus RNA, but also from the emission of airborne aerosols, which are derived from sewage-derived aerosol routes (Section 3.1). The possible risks can be reduced in two aspects. The first is to reduce unnecessary staff in WWTPs and DWTPs by minimizing manual sampling and monitoring water quality using automatic detection instruments. The second is that workers must operate in accordance with the guideline to reduce health risks. Of course, the workers should be provided with proper personal protective equipment (PPE), such as protective clothing and glasses, masks, and gloves, when working in a workplace where water droplets may be generated.
3. Characteristics of coronaviruses in aerosol and inanimate surfaces
The facts of COVID-19 human-to-human transmission and SARS-CoV-2 viral RNA being detected on different inanimate surfaces and droplets in air in healthcare and patient's family settings have indicated the virus may be airborne transmission [52], [55], [135], [136], [137], [138]. It is widely accepted that the respiratory droplets are the main transmission way when a person has close contact with an infected person [6], [139]. More and more scientists believe that the virus transmission via airborne is the dominant route for the spread of COVID-19 [7], [49], [52], [53], [55], [140], [141]. A WHO recent report pointed out that short-range aerosol transmission may occasion in crowded and inadequately ventilated spaces [142]. There is little scientific information about the infectivity and load of SARS-CoV-2 viruses in the air, causing some controversy regarding the airborne transmission. The following discussion of indirect evidence may help people to realize the high risk of the airborne transmission of coronavirus and the need to take corresponding prevention and control strategies to curb it.
3.1. Spreading pathways
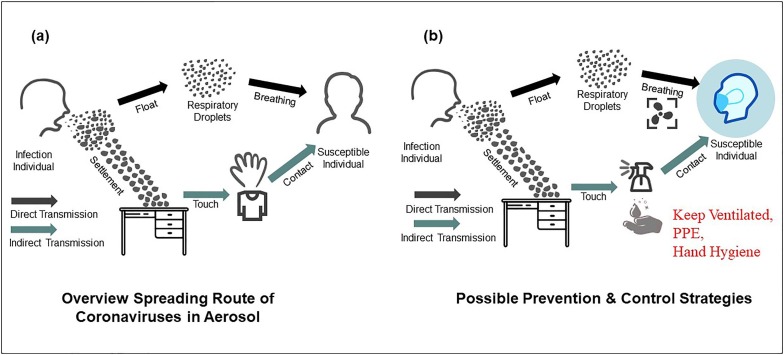
The possible routes of exposure to coronaviruses can be divided into direct and indirect contact transmission (Fig. 5 ). The direct pathway is via respiratory microdroplets, which is the dominant route of transmission. The tiny droplets from coughing, sneezing, singing, or talking/speech of infected individuals through their nose and mouth result in the spread of the virus [8], [52], [141], [143]. It has been reported that microscopic droplets can remain aloft in air and even propagate 2.5 m away by gas cloud entrainment dynamics [144], [145], [146], [147]. The airborne transmission of SARS-CoV proved to be the main mode of transmission in a relatively closed space [148], [149]. A study about the aerodynamic nature of SARS-CoV-2 in different areas of two Wuhan hospitals has shown that the SARS-CoV-2 RNA in aerosols has been detected in some relatively unventilated areas, such as patients’ toilet and medical staff areas [137]. Similarly, Guo et al. [150] tested air samples from an intensive care unit (ICU) and a general COVID-19 ward in a hospital, the results show that SARS-CoV-2 was widely distributed in the air, and distribution characteristics of SARS-CoV-2 indicated that the transmission distance can reach 4 m. Respiratory droplets, however, generally are greater than 5–10 µm in diameter which only remains in the air for a short time and travels only a short distance (within 1 m). A recent study on the exhaled breath samples from COVID-19 infected individual has shown that the breath emission was estimated to be from 1.03 × 105 to 2.25 × 107 viruses per hour [49]. SARS-CoV-2 was detected in surface swabs and air samples, indicating that expiratory breath emissions play an important role in the emission of SARS-CoV-2 into the air [49]. Although this study was occurring in medical site, some relatively unventilated spaces, such as chorale [53], restaurant [151], fitness [152], have also reported the possibility of aerosol transmission. On the outdoor, the SARS-CoV-2 RNA has also been found on particulate matter in northern Italy [42].
Fig. 5.
Overview spreading route of coronaviruses in aerosol (a) and possible prevention and control strategies (b).
The indirect contact transmission refers to the transmission of coronaviruses from contaminated dry inanimate surfaces to humans, initiate self-inoculation of mucous membranes in the nose, eyes, or mouth by contact with hands. Furthermore, the virus RNA in the table, chair, floor, and fan surfaces has been detected in the patient’s room, which may create a condition to transfer virus [153]. A number of studies have shown that coronaviruses could persist (a few hours to days) on inanimate surfaces [10], [51], [95], [135], [154].
Suspended water droplets in the air are also an important mode of viral transmission [155]. It is well known that wastewater could form virus bioaerosols after atomization, such as mechanically agitated, mixed, and aerated [156]. In oxidation ditch (OD) and anaerobic-anoxic–oxic (A2/O) process, for example, there are greatly produced aerosols with different particle size, microbial and chemical compositions [157], [158]. More importantly, the aerosols with sizes under 3.3 µm have been detected which are considered to be inhalable, called comparable respirable fraction [157]. Like other common viral and bacterial pathogens found in wastewater, the SARS-CoV-2 could also spread and survive in wastewater aerosols [94]. It is worth noting that the emission of airborne aerosols was dependent on the type of reactor and aeration mode [157], [159], [160]. Thus, it should be borne in mind that the transmission via sewage-derived aerosol in WWTP cannot be ruled out if aerosols retain the sufficient concentration of infectious virus [161]. Another overlooked transmission pathway is fecal bioaerosol transmission [162]. The toilet flushing also produces droplets that are enough small to become aerosol [163], while virus has been isolated from infected individuals’ feces.
3.2. Survival characteristics
3.2.1. Survival on aerosols
In general, the virus’s survival in the air depends on various factors, including biological stresses, light, and meteorological factors, such as ambient temperature, wind speed and direction, and relative humidity (RH) [164]. Previous experimental reports on MERS-CoV showed that it had a strong ability to survive in aerosols, and it could still maintain infectivity after 60 min of atomization at 25 °C and 79% RH [165]. Researchers using a 3-jet Collison nebulizer to generate aerosols containing the SARS-CoV-2 found that the virus can remain viable up to 3 h in aerosols, and concluded that the stability of SARS-CoV-2 was similar to that of SARS-CoV under laboratory conditions [51].
The effect of meteorological conditions (temperature, RH) on the viability of coronaviruses in aerosols is being debated. The stability of MERS-CoV during aerosolization has shown that the MERS-CoV was extremely stable at low temperatures (20 °C) and humidity (40% RH) [166], while more significant inactivation has occurred during experiments undertaken at 38 °C and 24% RH [164]. Researchers have proposed that viruses (bacteriophages) survived well at RHs lower than 33% and at 100%, whereas their viability was significantly decayed at 55% and 75% RHs [167]. However, SARS-CoV-2 may be resistant to high temperatures and intermediate RH [168]. Found in a cluster-spreading event investigation, a patient with SARS-CoV-2 may have transmitted the virus even with temperatures from 25 to 41 °C and humidity of approximately 60% [169]. Likewise, Fears et al. [170] also found that SARS-CoV-2 virus RNA was detected and aerosol suspension stability experiment for up to 16 h in laboratory conditions was 23 ± 2 °C and 53 ± 11% RH. In general, the higher temperatures would benefit to curb SARS-CoV-2 spread, whereas lower temperatures might increase its transmission. In terms of macro data statistics, however, this relationship between temperature and the number of COVID-19 patients is also controversial. Xie and Zhu [171]] did not find evidence to support that decrease in infection rate with temperature rise, while lower temperatures may increase the number of diagnosed cases [172], [173].
3.2.2. Survival on surfaces
Recently, a review about the persistence of coronaviruses on inanimate surfaces reported that SARS-CoV, MERS-CoV, and endemic human coronaviruses (HCoV) can remain infectious times from 2 h to up to 9 days on the different material surface [154]. A survey about virus stability during different environmental conditions has shown that SARS-CoV-2 was very stable on some inanimate surface (plastic, stainless steel, copper, and cardboard) for a few hours at 21 to 23 °C [51]. Another study investigated the stability of SARS-Cov-2 on different surfaces showed that the virus activity was dependent on material surfaces, more stable on smooth surfaces [95]. Under typical air-conditioned environments (temperature of 22–25 °C and RH of 40–50%), SARS-CoV retained viability for over 5 days on smooth surfaces, and the time is negatively proportional to temperature and relative humidity [174].
Overall, the characteristics of coronaviruses’ survival on aerosols and surfaces are similar to those in water, which can survive in a favorable environment. Therefore, surface disinfection and protective measures are particularly important.
3.3. Possible prevention and control strategies
Airborne/aerosol transmission of SARS-CoV-2 has played a potentially important role in relatively unventilated spaces [8], [49], [150], [151], [152]. Based on the mode of viral transmission and survival characteristics in aerosols and surfaces, it can be prevented and inactivated from several approaches. From the airborne transmission perspective, some engineering controls should be recommended in poorly ventilated spaces. These measures include increasing the ventilation rates in existing building vent, adopting air cleaning and disinfection devices for particle filtration and air disinfection, and avoiding air-recirculation as well as avoiding close contact and crowds, which have reached a consensus in the scientific communities to minimize viral transmission in semi-closed spaces [55], [137], [169], [175], [176], [177]. On the other hand, to avoid human hand plays a major vector in the viral transmission route, some personal precautions also need to be emphasized: (1) Proper PPE: personal protection must be observed when coming into contact with possible patients, especially when taking care of infected individuals in healthcare settings or working in water and wastewater treatment places; (2) Inactivating in time: commonly biocidal agents and disinfectants could effectively inactivate coronaviruses by surface disinfection procedures. With 67–71% ethanol, 0.5% hydrogen peroxide, 7.5% povidone-iodine, 0.1% sodium hypochlorite, and benzalkonium chloride, etc., coronaviruses could be inactivated within a few minutes [95], [154]; (3) Hand hygiene: unclean hands contact is the main way for indirect virus spread, such as hand-oral, hand-mucosae and food-oral transmission route. Thus, unnecessary touches must be avoided, and washing hands with soap should be done immediately when touching items that may contain viruses [95]. Overall, the evidence is emerging indicating that airborne SARS-CoV-2 may be the dominant reason contributing to the ongoing COVID-19 pandemic. The enhanced ventilation, and personal precautions are essential to minimize the risk.
4. Where do we stand?
It is quite plausible that COVID-19 will raise interest among water and wastewater industries, and atmospheric science. Given what little is known so far about how the new coronavirus might be transmitted in integrated water and wastewater systems or aerosol, additional research is desperately needed. No doubt, multidisciplinary while international collaboration will accelerate the fighting against the virus to establish control and preventing strategies from a scientific point of view. One Health approach flags the correct direction for us to work together. Indeed, there are lots of works ahead towards fully understanding the SARS-CoV-2, while environmental science in the water cycle and airborne study plays a pivotal role in virus tracing, transmission, health exposure, etc.
The first need is to have a more comprehensive understanding of the characteristics of the virus in the environmental medium, especially the survival characteristics and possible inactivation approaches. For example, so far, we do not know whether the viral particles of the SARS-CoV-2 can be aerosolized from water or suspended into air after settling and remaining infective. While such routes can occur for other coronaviruses, currently only limited direct pieces of evidence are to support the airborne transmission of the SARS-CoV-2. A precautionary approach should be taken until studies to eliminate other routes of transmission, especially in relatively unventilated areas. If the fecal-oral transmission is finally demonstrated with COVID-19, we have to design protocols to minimize risk in areas where open defecation is a fact, especially in underprivileged regions. It seems reasonable to minimize such virus load in toilet by adding chlorine tablets for disinfection. Furthermore, the safety of the drainage piping system in extra- and inter-home should also be emphasized. The sealing performance should be strengthened, especially to ensure that the toilets and kitchens are equipped with functioning U-bends. The epidemic is likely to continue into the rainstorm season. If the rainwater and sewage are mixed in the drainage pipeline, it will easily cause the sewage containing the virus to overflow into the environmental water bodies.
Secondarily, intensive studies should be done towards understanding the SARS-CoV-2 behavior in water and wastewater treatment to help developing the control strategies and to provide solid while enhanced technical solutions, which is also a key issue to personal safety who work for water and wastewater systems. So far, the available information supports that the current route wastewater treatment processes can provide satisfied treatment efficiency including eliminating SARS-CoV-2. However, detailed studies are desirable to confirm it regardless of the technology used for treating sewage. In activated sludge process, the wastewater-derived aerosols and virus-laden sludge may be occurred, which will also pose significant potential risks associated with wastewater utility personnel, and scientists. For safety reasons, it is wiser to adopt the enhanced/advanced wastewater treatment measure and highlight the importance of wearing PPE during the outbreak period.
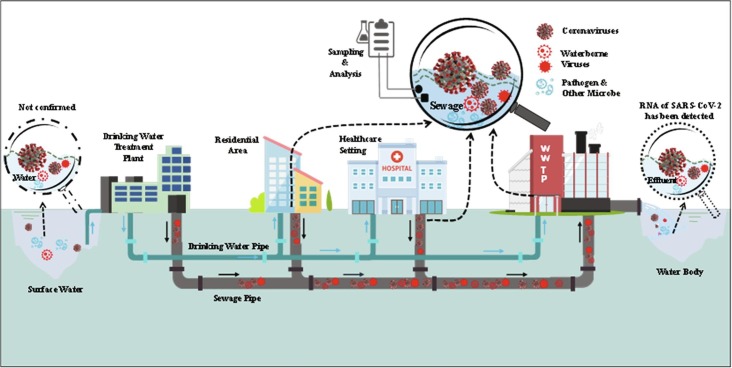
Thirdly, the important premise, for now, is to establish a safe and reliable analysis and monitoring method for SARS-CoV-2 in water, sludge and air. So far, there is no evidence to show that the SARS-CoV-2 was detected in drinking water. Therefore, drinking water is safe unless it was polluted during the network distribution. However, more than a dozen research groups worldwide have found the traces of the SARS-CoV-2 in wastewater and sludge. These include the groups in China, The Netherlands, Switzerland, the United States, UK, Australia, Spain and Sweden, Japan, India, etc. More significantly, these studies have led to a proposal that wastewater testing could be used as an early-warning sign if the virus returns to reveal the true scale of the outbreak and help estimate the total number of infections [178]. Especially, when the manual investigation is difficult for logistical, ethical, or economic reasons, wastewater investigation may provide an alternative method [25]. As wastewater goes through the drainage system to a treatment facility, analyzing wastewater could track infectious diseases that are excreted in urine or feces (Fig. 6 ), such as SARS-CoV-2. Sampling and monitoring sewage in sewer network and WWTP could provide better and quicker estimates for how widespread the coronavirus is than testing individual person, because wastewater surveillance accounts for those who have not been tested and have only mild or no symptoms and thus reflects the true situation. To accurately achieve this, investigating the concentration and viable viral RNA in feces and urine from an infected person, quantifying the viral load from wastewater samples to count of infection in the population, and selecting the representative sampling sites in sewer collection network are vital. The methods for detecting the virus in air also present a challenge. So far, there is also a lack of data related to the sampling, storage, and processing of sewage sludge used to detect SARS-CoV-2 [52], [179].
Fig. 6.
Schematic profile of water-related coronaviruses in the water cycle.
Fourthly, the optimization of extraction and detection methods for coronavirus nucleic acid in sewage is also worth discussing. The RT-qPCR amplification and sequencing are the main methods of confirming SARS-CoV-2 RNA in wastewater [25], [31], [32], but different assays and virus concentration methods may produce qualitative inconsistent RNA results (positive and negative) [32]. Furthermore, most publications focus on the qualitative and quantitative detection of viral RNA to assess the risk of viral transmission [35]. It should be noted that the detection of virus RNA by RT-qPCR provides no indication that the virus is infectious. Meanwhile, the virus infectious detection approaches face many challenges, which are also the key issues for scientists on whether acceptance of airborne and waterborne transmission of SARS-CoV-2. The factors influencing virus infectivity include the environment, host, virus properties, and transmission [180]. To further identify the infectivity of enveloped viruses, RT-qPCR should be carried out in combined with cell culture assays and transfection assays. Obviously, more research is highly desirable. In addition, fast monitoring technique and detecting the viral RNA at low levels are most important. In that context, researchers at Cranfield University (UK), and Chinese Academy of Sciences, are working on developing new paper-based rapid testing kits, which could be an effective and rapid way to be used on-site at sewer collecting point or WWTPs to trace sources and determine whether there are potential COVID-19 carriers in local areas [181]. Another issue worth exploring is that the viral RNA in the environment may be persistent than infectious viral [48], which may not reflect the actual outbreak by measuring the RNA in sewage. It requires more long term monitoring and in-depth research.
Fifthly, it is worth noting to prevent secondary environmental risks due to the overuse measures to control coronaviruses, such as the cumulative toxicity of DBPs under overdosed with disinfectant. Zhang et al. [71] pointed out that free chlorine more than 0.5 mg/L after disinfection might be not enough to completely remove SARS-CoV-2 viral RNA. On the other hand, the drugs residue and metabolite derived from the massive and unprecedented use of antiviral and anti-inflammatory drugs during pandemic events, no doubt, will eventually discharged into the environment [182]. The high concentration of by-product residuals would bring ecological risks that need special attention. Equally, the sludge generated in water and wastewater treatment needs safe disposal.
Last but not the least, in low- and middle-income countries and remote rural areas, due to the lack of adequate water and wastewater treatment facilities, the risks are more acute [72], [79]. Therefore, the development of decentralized water and wastewater treatment facilities with cost-effective approaches for UV and chemically disinfecting the coronaviruses should be strengthened. Regarding secondary treatments, it is probably useful to reduce the use of aerated systems like activated sludge where aeration-based aerosols can be formed. Alternatively, systems, where wastewater is not air-exposed, like a subsurface constructed wetland, seem to be a reasonable strategy for decentralized treatments.
5. Summary and conclusions
Based on the updated published papers, newsletters, and scientific websites, it appears that the wastewater, sludge, aerosol are potentially environmental transmission of coronavirus. Although there is lack of direct evidence to prove fecal-oral transmission during the COVID-19 outbreak, the presence and persistence of SARS-CoV-2 in water and wastewater, and the insufficient wastewater collection and treatment system of undeveloped regions/countries are drawing attention to exist virus spread via water-related pathways. There are some risks of virus transmission under the urban and rural water cycle if several possible prevention and control strategies are not adopted. Under the urban water cycle, the coronaviruses shed in the feces and urine of infected individuals can enter drainage system, and then WWTPs. So far, a few standard systems for treating wastewater have been tested and they seem to be enough for removing the presence of SARS-CoV-2. However, the diversity in existing methodologies for treating sewage suggests the need for further studies where air-exposition, temperature, hydraulic retention time, and sludge production can be key parameters to explore. It should be remembered that after understanding the fate of viruses and viral infections, in wastewater treatment, drinking water, reclaimed water, and sludge, water users and workers need to adopt targeted prevention and control strategies. According to the limited information of the virus transmission and survival characteristics, it seems that the processes in already existing DWTPs are able to reduce the total waterborne virus over 4 log units depending on the filtration removal and disinfection inactivation process. There are, however, increasing pieces of evidence that airborne SARS-CoV-2 may be the main cause of the ongoing COVID-19 pandemic. Strengthening building ventilation systems and personal protective measures are essential to minimize the risks.
To curb the coronaviruses especially the SARS-CoV-2 in integrated water and sludge, as well as airborne systems, multidisciplinary while international collaborative research is desperately needed under the novel concept of “One Health”. Indeed, a more comprehensive understanding of virus transmission and survival characteristics is a basis to establish reliable controlling strategies. Intensive studies to explore the SARS-CoV-2 behavior in water and wastewater treatment with fast while accurate virus monitoring tools are vital. If so, wastewater should be a strategic tool for following the epidemy evolution in communities, neighborhoods or even public buildings in real-time. The presence of a virus in wastewater should not be considered a real risk but a powerful ally to report its evolution. Merging accurate sensor tools in wastewater with machine learning strategies will provide a valuable scenario for making predictions way before symptoms appear in a certain community. Additional environmental risks associated with the enhanced treatment measures should be taken into account, while the development of cost-effective decentralized water and wastewater treatment facilities for low- and middle-income countries and remote rural areas for disinfecting the coronaviruses should be strengthened.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to Xi’an University of Technology for financial support for this study. We would also like to acknowledge financial support from the Special Fund for Science and Technology Innovation Strategy of Guangdong Province, China (2019A050505005). We sincerely thank the highly valuable comments from several anonymous reviewers for improving the overall quality of this review.
References
- 1.WHO, (2020). WHO Coronavirus Disease (COVID-19) Dashboard, https://covid19.who.int/ (accessed 17 October 2020).
- 2.Madurai Elavarasan R., Pugazhendhi R. Restructured society and environment: a review on potential technological strategies to control the COVID-19 pandemic. Sci. Total Environ. 2020;725:138858. doi: 10.1016/j.scitotenv.2020.138858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., Agha M., Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int. J. Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumaravel S.K., Subramani R.K., Jayaraj Sivakumar T.K., Madurai Elavarasan R., Manavalanagar Vetrichelvan A., Annam A., Subramaniam U. Investigation on the impacts of COVID-19 quarantine on society and environment: Preventive measures and supportive technologies. 3 Biotech. 2020;10(9) doi: 10.1007/s13205-020-02382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UN, (2020). Note to Correspondents on debt vulnerability and COVID-19, https://www.un.org/sg/en/content/sg/note-correspondents/2020-05-06/note-correspondents-debt-vulnerability-and-covid-19. (accessed 12 October 2020).
- 6.Chan J.F.W., Yuan S.F., Kok K.H., To K.K.W., Chu H., Yang J., Xing F.F., Liu J.L., Yip C.C.Y., Poon R.W.S., Tsoi H.W., Lo S.K.F., Chan K.H., Poon V.K.M., Chan W.M., Ip J.D., Cai J.P., Cheng V.C.C., Chen H.L., Hui C.K.M., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K., Lim X.F., Lim A.S., Sutjipto S., Lee P.H., Son T.T., Young B.E., Milton D.K., Gray G.C., Schuster S., Barkham T., De P.P., Vasoo S., Chan M., Ang B.S.P., Tan B.H., Leo Y.S., Ng O.T., Wong M.S.Y., Marimuthu K. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meselson M. Droplets and aerosols in the transmission of SARS-CoV-2. N. Engl. J. Med. 2020;382:2063. doi: 10.1056/NEJMc2009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UN, (2020). How COVID-19 Spreads, https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html. (accessed 12 October 2020).
- 10.Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J. Hosp. Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao W., Fang L., Xiao D. What we have learnt from the SARS epdemics in mainland China? Global Health J. 2019;3:55–59. doi: 10.1016/j.glohj.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Guan Y., Zheng B., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J., Butt K.M., Wong K.L., Chan K.W., Lim W., Shortridge K.F., Yuen K.Y., Peiris J.S., Poon L.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Sci. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 13.Hemida M.G., Chu D.K.W., Poon L.L.M., Perera R.A.P.M., Alhammadi M.A., Ng H., Siu L.Y., Guan Y., Alnaeem A., Peiris M. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg. Infect. Dis. 2014;20(7) doi: 10.3201/eid2007.140571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 15.Chan J.F.W., To K.K.W., Tse H., Jin D.Y., Yuen K.Y. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21(10):544–555. doi: 10.1016/j.tim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan J.F.W., Kok K.H., Zhu Z., Chu H., To K.K.W., Yuan S.F., Yuen K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X.T., Chen P., Wang J.F., Feng J.N., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien E., Xagoraraki I. A water-focused one-health approach for early detection and prevention of viral outbreaks. One Health. 2019;7:100094. doi: 10.1016/j.onehlt.2019.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corman V.M., Muth D., Niemeyer D., Drosten C. In: Adv. Kielian M., Mettenleiter T.C., Roossinck M.J., editors. Academic Press; Virus Res.: 2018. Chapter Eight – Hosts and Sources of Endemic Human Coronaviruses; pp. 163–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO, (2015). WHO publishes list of top emerging diseases likely to cause major epidemics, https://www.who.int/medicines/ebola-treatment/WHO-list-of-top-emerging-diseases/en/. (accessed 12 October 2020).
- 21.Woolhouse M.E.J., Haydon D.T., Antia R. Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol. Evol. 2005;20(5):238–244. doi: 10.1016/j.tree.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong M.L., Ahmed M.A., Sulaiman W.Y.W., Manin B.O., Leong C.S., Quan F.S., Chua T.H., Drakeley C., Snounou G., Vythilingam I. Genetic diversity of zoonotic malaria parasites from mosquito vector and vertebrate hosts. Infect. Genet. Evol. 2019;73:26–32. doi: 10.1016/j.meegid.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z.Z., Behloul N., Baha S., Wei W.J., Shi R.H., Meng J.H. Design and immunogenicity analysis of the combined vaccine against zoonotic hepatitis E and foot-and-mouth disease. Vaccine. 2019;37(46):6922–6930. doi: 10.1016/j.vaccine.2019.09.036. [DOI] [PubMed] [Google Scholar]
- 24.Ganter M. Zoonotic risks from small ruminants. Vet. Microbiol. 2015;181(1-2):53–65. doi: 10.1016/j.vetmic.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 25.S. Wurtzer, V. Marechal, J.-M. Mouchel, L. Moulin, Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases, medRxiv (2020). https://doi.org/10.1101/2020.04.12.20062679.
- 26.Alpaslan Kocamemi B., Kurt H., Sait A., Sarac F., Saatci A.M. SARS-CoV-2 Detection in istanbul wastewater treatment plant sludges. medRxiv. 2020 doi: 10.1101/2020.05.12.20099358. [DOI] [Google Scholar]
- 27.Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Omer S.B. SARS-CoV-2 RNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. medRxiv. 2020 doi: 10.1101/2020.05.19.20105999. [DOI] [Google Scholar]
- 28.Wu F.Q., Xiao A., Zhang J.B., Gu X.Q., Lin W.L., Kauffman K., Hanage W., Matus M., Newsha G., Endo N., Duvallet C., Moniz K., Erickson T., Chai P., Thompson J. Eric Alm, SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv. 2020 doi: 10.1101/2020.04.05.20051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nemudryi A., Nemudraia A., Surya K., Wiegand T., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft. B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Reports Medicine. 2020;1:100098. doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357.s001. [DOI] [PubMed] [Google Scholar]
- 31.Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J.Y., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J.L., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balboa S., Mauricio-Iglesias M., Rodríguez S., Martínez-Lamas L., Vasallo F.J., Regueiro B. The fate of SARS-CoV-2 in wastewater treatment plants points out the sludge line as a suitable spot for incidence monitoring. medRxiv. 2020 doi: 10.1101/2020.05.25.20112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arora S., Nag A., Sethi J., Rajvanshi J., Saxena S., Shrivastava S.K., Gupta A.B. Sewage surveillance for the presence of SARS-CoV-2 genome as a useful wastewater based epidemiology (WBE) tracking tool in India. medRxiv. 2020 doi: 10.1101/2020.06.18.20135277. [DOI] [PubMed] [Google Scholar]
- 37.La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collivignarelli M.C., Collivignarelli C., Carnevale Miino M., Abbà A., Pedrazzani R., Bertanza G. SARS-CoV-2 in sewer systems and connected facilities. Process Saf. Environ. Prot. 2020;143:196–203. doi: 10.1016/j.psep.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Borelli M., Palmisani J., Di Gilio A., Torboli V., Fontana F., Clemente L., Pallavicini A., Ruscio M., Piscitelli P., Miani A. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: first evidence. Environ. Res. 2020;188:109754. doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: Implications in low sanitation countries. Sci. Total Environ. 2020;743:140832. doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ampuero M., Valenzuela S., Valiente-Echeverria F., Soto-Rifo R., Barriga G.P., Chnaiderman J., Rojas C., Guajardo-Leiva S., Diez B., Gaggero A. SARS-CoV-2 Detection in sewage in Santiago, Chile - Preliminary results. medRxiv. 2020 doi: 10.1101/2020.07.02.20145177. [DOI] [Google Scholar]
- 45.Xiao F., Tang M.W., Zheng X.B., Liu Y., Li X.F., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun J., Zhu A.R., Li H.Y., Zheng K., Zhuang Z., Chen Z., Shi Y.X., Zhang Z.Y., Chen S.B., Liu X.S., Dai J., Li X.B., Huang S.X., Huang X.F., Luo L., Wen L.Y., Zhuo J.F., Li Y.M., Wang Y.Q., Zhang L., Zhang Y.J., Li F., Feng L.Q., Chen X.W., Zhong N.S., Yang Z.F., Huang J.C., Zhao J.C., Li Y.M. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg. Microbes Infect. 2020;9(1):991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeong H.W., Kim S.M., Kim H.S., Kim Y., Kim J.H., Cho J.Y., Kim S.H., Kang H., Kim S.G., Park S.J., Kim E.H., Choi Y.K. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bivins A., Greaves J., Fischer R., Yinda K.C., Ahmed W., Kitajima M., Munster V.J., Bibby K. Persistence of SARS-CoV-2 in water and wastewater. Environ. Sci. Techn. Lett. 2020 doi: 10.1021/acs.estlett.0c00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma Jianxin, Qi Xiao, Chen Haoxuan, Li Xinyue, Zhang Zheng, Wang Haibin, Sun Lingli, Zhang Lu, Guo Jiazhen, Morawska Lidia, SergeyGrinshpun A., Pratim Biswas, RichardFlagan C., Yao Maosheng. COVID-19 patients in earlier stages exhaled millions of SARS-CoV-2 per hour. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fabian P., Brain J., Andres Houseman E., Gern J., Milton D.K. Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. J. Aerosol. Med. Pulmonary Drug Delivery. 2011;24(3):137–147. doi: 10.1089/jamp.2010.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morawska L., Cao J.J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller S.L., Nazaroff W.W., Jimenez J.L., Boerstra A., Buonanno G., Dancer S.J., Kurnitski J., Marr L.C., Morawska L., Noakes C. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air. 2020 doi: 10.1111/ina.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buonanno G., Stabile L., Morawska L. Estimation of airborne viral emission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020;141:105794. doi: 10.1016/j.envint.2020.105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morawska L., Milton D.K. It is time to address airborne transmission of COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng L., Liu J., Xu W.X., Luo Q.M., Chen D.B., Lei Z.Y., Huang Z.L., Li X.J., Deng K.J., Lin B.L., Gao Z.L. SARS‐CoV‐2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J. Med. Virol. 2020;92(9):1676–1680. doi: 10.1002/jmv.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stalin Raj V., Farag E.A.B.A., Reusken C.B.E.M., Lamers M.M., Pas S.D., Voermans J., Smits S.L., Osterhaus A.D.M.E., Al-Mawlawi N., Al-Romaihi H.E., AlHajri M.M., El-Sayed A.M., Mohran K.A., Ghobashy H., Alhajri F., Al-Thani M., Al-Marri S.A., El-Maghraby M.M., Koopmans M.P.G., Haagmans B.L. Isolation of MERS coronavirus from a dromedary camel, Qatar, 2014. Emerg. Infect. Dis. 2014;20(8) doi: 10.3201/eid2008.140663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X.W., Li J.S., Guo T.K., Zhen B., Kong Q.X., Yi B., Li Z., Song N., Jin M., Xiao W.J., Zhu X.M., Gu C.Q., Yin J., Wei W., Yao W., Liu C., Li J.F., Ou G.R., Wang M.N., Fang T.Y., Wang G.J., Qiu Yao-Hui, Wu H.H., Chao F.H., Li J.W. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital. J. Virol. Methods. 2005;128(1–2):156–161. doi: 10.1016/j.jviromet.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Y.J., Guo C., Tang L.T., Hong Z.S., Zhou Ji.H., Dong X., Yin H., Xiao Q., Tang Y.P., Qu X.J., Kuang L.J., Fang X.M., Mishra N., Lu J.H., Shan H., Jiang G.M., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 61.Tang A., Tong Z.D., Wang H.L., Dai Y.X., Li K.F., Liu J.N., Wu W.J., Yuan C., Yu M.L., Li P., Yan J.B. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis. 2020;26(6):1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S.X., Lu X.Y., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y.F., Chen L.J., Deng Q.L., Zhang G.Q., Wu K.S., Ni L., Yang Y.B., Liu B., Wang W., Wei C.J., Yang J., Ye G.M., Cheng Z.S. The presence of SARS‐CoV‐2 RNA in the feces of COVID‐19 patients. J Med Virol. 2020;92(7):833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y., Chen C., Zhu S.L., Shu C., Wang D.Y., Song J.D., Song Y., Zhen W., Feng Z.J., Wu G.Z., Xu J., Xu W.B. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2:123–124. [PMC free article] [PubMed] [Google Scholar]
- 65.Wang W.L., Xu Y.L., Gao R.Q., Lu R.J., Han K., Wu G.Z., Tan W.J. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.To K.K.-W., Tsang O.T.-Y., Yip C.C.-Y., Chan K.-H., Wu T.-C., Chan J.M., Leung W.S, Chik T.S., Choi C.Y., Kandamby D.H., Lung D.C., Tam A.R., Poon R.W., Fung A.Y., Hung I.F., Cheng V.C., Chan J.F., Yuen K.Y. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W., Du R.H., Li B., Zheng X.S., Yang X.L., Hu B., Wang Y.Y., Xiao G.F., Yan B., Shi Z.L., Zhou P. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerging Microbes Infect. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ Virol. 2009;1(1) doi: 10.1007/s12560-008-9001-6. [DOI] [Google Scholar]
- 70.Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang D., Ling H.B., Huang X., Li J., Li W.W., Yi C., Zhang T., Jiang Y.Z., He Y.N., Deng S.Q., Zhang X., Wang X.Z., Liu Y., Li G.H., Qu J.H. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020;741:140445. doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Usman M., Farooq M., Hanna K. Existence of SARS-CoV-2 in wastewater: implications for its environmental transmission in developing communities. Environ. Sci. Technol. 2020;54(13):7758–7759. doi: 10.1021/acs.est.0c02777. [DOI] [PubMed] [Google Scholar]
- 73.Shutler J., Zaraska K., Holding T.M., Machnik M., Uppuluri K., Ashton I., Migdal L., Dahiya R. Risk of SARS-CoV-2 infection from contaminated water systems. medRxiv. 2020 doi: 10.1101/2020.06.17.20133504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.R.G. Sinclair, E.L. Jones, C.P. Gerba, Viruses in recreational water-borne disease outbreaks: a review, 107(2009) 1769-1780. https://doi.org/10.1111/j.1365-2672.2009.04367.x. [DOI] [PubMed]
- 75.Xagoraraki I., Yin Z.Q., Svambayev Z. Fate of Viruses in Water Systems. J. Environ. Eng. 2014;140(7):04014020. doi: 10.1061/(ASCE)EE.1943-7870.0000827. [DOI] [Google Scholar]
- 76.Reynolds K.A., Mena K.D., Gerba C.P. Risk of waterborne illness via drinking water in the United States. Rev. Environ. Contam. Toxicol. 2008;192:117–158. doi: 10.1007/978-0-387-71724-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han J., He S.S. Urban flooding events pose risks of virus spread during the novel coronavirus (COVID-19) pandemic. Sci. Total Environ. 2021;755:142491. doi: 10.1016/j.scitotenv.2020.142491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.P. Li, H. Bin, (2020). “Solving a case” of COVID-19 outbreak in a village-in-the-city community in Guangzhou: Sewage contaminated surrounding environments caused virus infections in residents, http://news.southcn.com/gd/content/2020-06/12/content_191016386.htm. (accessed 12 October 2020).
- 79.Amoah I., Kumari S., Bux F. Coronaviruses in wastewater processes: source, fate and potential risks. Environ. Int. 2020;143:105962. doi: 10.1016/j.envint.2020.105962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.AFP, (2020). ‘Minuscule traces’ of virus in non-potable Paris water found, https://www.dawn.com/news/1550639. (accessed 12 October 2020).
- 81.Hung L.S. The SARS Epidemic in Hong Kong: what lessons have we Learned? J. R. Soc. Med. 2003;96(8):374–378. doi: 10.1177/014107680309600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhowmick G.D., Dhar D., Nath D., Ghangrekar M.M., Banerjee R., Das S., Chatterjee J. Coronavirus disease 2019 (COVID-19) outbreak: some serious consequences with urban and rural water cycle. npj Clean Water. 2020;3(1) doi: 10.1038/s41545-020-0079-1. [DOI] [Google Scholar]
- 83.Shi J.Z., Wen Z.Y., Zhong G.X., Yang H.L., Wang C., Huang B.Y., Liu R.Q., He X.J., Shuai L., Sun Z.R., Zhao Y.B., Liu P.P., Liang L.B., Cui P.F., Wang J.L., Zhang X.F., Guan Y.T., Tan W.J., Wu G.Z., Chen H.L., Bu Z.G. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science. 2020;368(6494):1016–1020. doi: 10.1126/science:abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stout A.E., André N.M., Jaimes J.A., Millet J.K., Whittaker G.R. Coronaviruses in cats and other companion animals: Where does SARS-CoV-2/COVID-19 fit? Vet. Microbiol. 2020;247:108777. doi: 10.1016/j.vetmic.2020.108777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.J. Gomez, (2020). Bronx Zoo tiger develops dry cough, tests positive for coronavirus, https://www.northjersey.com/story/news/2020/04/05/bronx-zoo-tiger-tests-positive-covid-19/2951842001/. (accessed 12 October 2020).
- 86.D.F. Maron, (2020). Did a mink just give the coronavirus to a human? Here’s what we know., https://www.nationalgeographic.com/animals/2020/05/coronavirus-from-mink-to-human-cvd/. (accessed 12 October 2020).
- 87.S. Shenoy, (2020). Spain to kill over 90,000 mink after 87% of them test positive for coronavirus, https://curlytales.com/spain-to-kill-mink-after-they-test-positive-for-coronavirus/. (accessed 12 October 2020).
- 88.Delahoy M.J., Wodnik B., McAliley L., Penakalapati G., Swarthout J., Freeman M.C., Levy K. Pathogens transmitted in animal feces in low- and middle-income countries. Int. J. Hyg. Environ. Health. 2018;221(4):661–676. doi: 10.1016/j.ijheh.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.A.N. Barnes, J. Anderson, J. Mumma, Z.H. Mahmud, O. Cumming, The association between domestic animal presence and ownership and household drinking water contamination among peri-urban communities of Kisumu, Kenya, PLoS One 13 (2018). https://doi.org/10.1371/journal.pone.0197587. [DOI] [PMC free article] [PubMed]
- 90.S. Matsui, Protecting human and ecological health under viral threats in Asia, Water Sci. Technol. 51 (2005) 91-97. https://doi.org/10.2166/wst.2005.0234. [PubMed]
- 91.Verbyla M.E., Mihelcic J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015;71:107–124. doi: 10.1016/j.watres.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 92.Arslan M., Xu B., Gamal El-Din M. Transmission of SARS-CoV-2 via fecal-oral and aerosols–borne routes: Environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743:140709. doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adelodun B., Ajibade F.O., Ibrahim R.G., Bakare H.O., Choi K.S. Snowballing transmission of COVID-19 (SARS-CoV-2) through wastewater: any sustainable preventive measures to curtail the scourge in low-income countries? Sci. Total Environ. 2020;742:140680. doi: 10.1016/j.scitotenv.2020.140680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1):e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang X.W., Li J.S., Jin M., Zhen B., Kong Q.X., Song N., Xiao W.J., Yin J., Wei W., Wang G.J., Si B.Y., Guo B.Z., Liu C., Ou G.R., Wang M.N., Fang T.Y., Chao F.H., Li J.W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126(1–2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carducci A., Federigi I., Liu D., Thompson J.R, Verani M. Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179:115907. doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Doremalen N.V., Bushmaker T., Munster V. Stability of middle east respiratory Syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18:1263–1264. doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- 99.Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ. Sci. Water Res. Technol. 2015;1(6):735–746. doi: 10.1039/C5EW00125K. [DOI] [Google Scholar]
- 100.Ye Y.Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50(10):5077–5085. doi: 10.1021/acs.est.6b00876.s001. [DOI] [PubMed] [Google Scholar]
- 101.Naddeo V., Liu H.Z. Editorial Perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ. Sci. Water Res. Technol. 2020;6(5):1213–1216. doi: 10.1039/D0EW90015J. [DOI] [Google Scholar]
- 102.Casanova L.M., Weaver S.R. Inactivation of an enveloped surrogate virus in human sewage. Environ. Sci. Technol. Lett. 2015;2(3):76–78. doi: 10.1021/acs.estlett.5b00029. [DOI] [Google Scholar]
- 103.Hewitt J., Greening G.E., Leonard M., Lewis G.D. Evaluation of human adenovirus and human polyomavirus as indicators of human sewage contamination in the aquatic environment. Water Res. 2013;47(17):6750–6761. doi: 10.1016/j.watres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Bonadonna L., La Rosa G. A review and update on waterborne viral diseases associated with swimming pools. Int. J. Environ. Res. Public Health. 2019;16:166. doi: 10.3390/ijerph16020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.N.A. Moreira, M. Bondelind, Safe drinking water and waterborne outbreaks, J. Water Health 15 (2017) 83-96. https://doi.org/10.2166/wh.2016.103. [DOI] [PubMed]
- 106.G. La Rosa, M. Fratini, S.D. Libera, M. Iaconelli, M. Muscillo, Emerging and potentially emerging viruses in water environments, Ann. Ist. Super. Sanita 48 (2012) 397-406. [DOI] [PubMed]
- 107.Prado T., de Castro Bruni A., Barbosa M.R.F., Garcia S.C., de Jesus Melo A.M., Sato M.I.Z. Performance of wastewater reclamation systems in enteric virus removal. Sci. Total Environ. 2019;678:33–42. doi: 10.1016/j.scitotenv.2019.04.435. [DOI] [PubMed] [Google Scholar]
- 108.Sidhu J.P.S., Sena K., Hodgers L., Palmer A., Toze S. Comparative enteric viruses and coliphage removal during wastewater treatment processes in a sub-tropical environment. Sci. Total Environ. 2018;616-617:669–677. doi: 10.1016/j.scitotenv.2017.10.265. [DOI] [PubMed] [Google Scholar]
- 109.Simmons F.J., Xagoraraki I. Release of infectious human enteric viruses by full-scale wastewater utilities. Water Res. 2011;45(12):3590–3598. doi: 10.1016/j.watres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 110.Nordgren J., Matussek A., Mattsson A., Svensson L., Lindgren P.E. Prevalence of norovirus and factors influencing virus concentrations during one year in a full-scale wastewater treatment plant. Water Res. 2009;43(4):1117–1125. doi: 10.1016/j.watres.2008.11.053. [DOI] [PubMed] [Google Scholar]
- 111.Chaudhry R.M., Nelson K.L., Drewes J.E. Mechanisms of pathogenic virus removal in a full-scale membrane bioreactor. Environ. Sci. Technol. 2015;49:2815–2822. doi: 10.1016/j10.1021/es505332n. [DOI] [PubMed] [Google Scholar]
- 112.Carvajal G., Roser D.J., Sisson S.A., Keegan A., Khan S.J. Bayesian belief network modelling of chlorine disinfection for human pathogenic viruses in municipal wastewater. Water Res. 2017;109:144–154. doi: 10.1016/j.watres.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 113.Pinon A., Vialette M. Survival of viruses in water. Intervirology. 2019;61(5):214–222. doi: 10.1159/000484899. [DOI] [PubMed] [Google Scholar]
- 114.Gall A.M., Mariñas B.J., Lu Y., Shisler J.L. Waterborne Viruses: A barrier to safe drinking water. Plos Pathogens. 2015;11 doi: 10.1371/journal.ppat.1004867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wigginton K.R., Pecson B.M., Sigstam T., Bosshard F., Kohn T. Virus inactivation mechanisms: impact of disinfectants on virus function and structural integrity. Environ. Sci. Technol. 2012;46(21):12069–12078. doi: 10.1021/es3029473. [DOI] [PubMed] [Google Scholar]
- 116.Tort L.F.L., Garcia M., Gillman L., Alberti A., Leite J.P.G., Miagostovich M.P., Pou S.A., Cagiao A., Razsap A., Huertas J., Berois M., Victoria M., Colina R. Human enteric viruses in a wastewater treatment plant: evaluation of activated sludge combined with UV disinfection process reveals different removal performances for viruses with different features. Lett. Appl. Microbiol. 2018;66:215–221. doi: 10.1111/lam.12839. [DOI] [PubMed] [Google Scholar]
- 117.Ye Y.Y., Chang P.H., Hartert J., Wigginton K.R. Reactivity of enveloped virus genome, proteins, and lipids with free chlorine and UV 254. Environ. Sci. Technol. 2018;52(14):7698–7708. doi: 10.1021/acs.est.8b00824.s001. [DOI] [PubMed] [Google Scholar]
- 118.Sano D., Amarasiri M., Hata A., Watanabe T., Katayama H. Risk management of viral infectious diseases in wastewater reclamation and reuse: Review. Environ. Int. 2016;91:220–229. doi: 10.1016/j.envint.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rachmadi A.T., Kitajima M., Pepper I.L., Gerba C.P. Enteric and indicator virus removal by surface flow wetlands. Sci. Total Environ. 2016;542:976–982. doi: 10.1016/j.scitotenv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 120.Otter P., Hertel S., Ansari J., Lara E., Cano R., Arias C., Gregersen P., Grischek T., Benz F., Goldmaier A., Alvarez J.A. Disinfection for decentralized wastewater reuse in rural areas through wetlands and solar driven onsite chlorination. Sci. Total Environ. 2020;721:137595. doi: 10.1016/j.scitotenv.2020.137595. [DOI] [PubMed] [Google Scholar]
- 121.Graham J.P., Polizzotto M.L. Pit latrines and their impacts on groundwater quality: a systematic review. Environ. Health Perspect. 2013;121(5):521–530. doi: 10.1289/ehp.1206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shirasaki N., Matsushita T., Matsui Y., Marubayashi T., Murai K. Investigation of enteric adenovirus and poliovirus removal by coagulation processes and suitability of bacteriophages MS2 and φX174 as surrogates for those viruses. Sci. Total Environ. 2016;563-564:29–39. doi: 10.1016/j.scitotenv.2016.04.090. [DOI] [PubMed] [Google Scholar]
- 123.Heffron J., McDermid B., Maher E., McNamara P.J., Mayer B.K. Mechanisms of virus mitigation and suitability of bacteriophages as surrogates in drinking water treatment by iron electrocoagulation. Water Res. 2019;163:114877. doi: 10.1016/j.watres.2019.114877. [DOI] [PubMed] [Google Scholar]
- 124.Shirasaki N., Matsushita T., Matsui Y., Yamashita R. Evaluation of the suitability of a plant virus, pepper mild mottle virus, as a surrogate of human enteric viruses for assessment of the efficacy of coagulation–rapid sand filtration to remove those viruses. Water Res. 2018;129:460–469. doi: 10.1016/j.watres.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 125.Matsushita T., Shirasaki N., Tatsuki Y., Matsui Y. Investigating norovirus removal by microfiltration, ultrafiltration, and precoagulation–microfiltration processes using recombinant norovirus virus-like particles and real-time immuno-PCR. Water Res. 2013;47(15):5819–5827. doi: 10.1016/j.watres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 126.Wlodarczyk R., Kwarciak-Kozlowska A. Waterborne Pathogens. Elsevier; 2020. pp. 57–80. [Google Scholar]
- 127.Pastorino B., Touret F., Gilles M., Luciani L., de Lamballerie X., Charrel R.N. Evaluation of chemical protocols for inactivating SARS-CoV-2 infectious samples. Viruses. 2020;12 doi: 10.3390/v12060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deng S.X., Yan X.T., Zhu Q.Q., Liao C.Y. The utilization of reclaimed water: Possible risks arising from waterborne contaminants. Environ. Pollut. 2019;254:113020. doi: 10.1016/j.envpol.2019.113020. [DOI] [PubMed] [Google Scholar]
- 129.Yang W., Cai C., Dai X.H. The potential exposure and transmission risk of SARS-CoV-2 through sludge treatment and disposal. Resour. Conserv. Recycl. 2020;162:105043. doi: 10.1016/j.resconrec.2020.105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bibby K., Peccia J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ. Sci. Technol. 2013;47(4):1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.A.D. Schlindwein, C. Rigotto, C.M.O. Simoes, C.R.M. Barardi, Detection of enteric viruses in sewage sludge and treated wastewater effluent, Water Sci. Technol. 61 (2010) 537-544. [DOI] [PubMed]
- 132.Viau E., Bibby K., Paez-Rubio T., Peccia J. Toward a consensus view on the infectious risks associated with land application of sewage sludge. Environ. Sci. Technol. 2011;45(13):5459–5469. doi: 10.1021/es200566f. [DOI] [PubMed] [Google Scholar]
- 133.Liu X., Shi J., Zhao Y., Li Z.F., Zhang J. Experimental research on lime drying process of mechanical dewatered sludge from a wastewater treatment plant in beijing. Procedia Environ. Sci. 2012;16:335–339. doi: 10.1016/j.proenv.2012.10.047. [DOI] [Google Scholar]
- 134.Pagat A.M., Seux-Goepfert R., Lutsch C., Lecouturier V., Saluzzo J.F., Kusters I.C. Evaluation of SARS-coronavirus decontamination procedures. Appl Biosaf. 2007;12(2):100–108. doi: 10.1177/153567600701200206. [DOI] [Google Scholar]
- 135.Ye G.M., Lin H.L., Chen S., Wang S.C., Zeng Z.K., Wang W., Zhang S.Y., Rebmann T., Li Y.R., Pan Z.Y., Yang Z.H., Wang Y., Wang F.B., Qian Z.M., Wang X.H. Environmental contamination of SARS-CoV-2 in healthcare premises. J. Infect. 2020;81(2):e1–e5. doi: 10.1016/j.jinf.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jiang Y., Wang H., Chen Y., He J., Chen L., Liu Y., Hu X., Li A., Liu S., Zhang P., Zou H., Hua S. Clinical data on hospital environmental hygiene monitoring and medical staff protection during the coronavirus disease 2019 outbreak. medRxiv. 2020 doi: 10.1101/2020.02.25.20028043. [DOI] [Google Scholar]
- 137.Liu Y., Ning Z., Chen Y., Guo M., Liu Y.L., Gali N.K., Sun L., Duan Y.S., Cai J., Westerdahl D., Liu X.J., Xu K., Ho K.F., Kan H.D., Fu Q.Y., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 138.Hu X.W., Xing Y.H., Ni W., Zhang F., Lu S.Y., Wang Z.G., Gao R.Q., Jiang F.C. Environmental contamination by SARS-CoV-2 of an imported case during incubation period. Sci. Total Environ. 2020;742:140620. doi: 10.1016/j.scitotenv.2020.140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Richard M., Kok A., de Meulder D., Bestebroer T.M., Lamers M.M., Okba N.M.A., Fentener van Vlissingen M., Rockx B., Haagmans B.L., Koopmans M.P.G., Fouchier R.A.M., Herfst S. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-17367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang R.Y., Li Y.X., Zhang A.L., Wang Y., Molina M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. U.S.A. 2020;117(26):14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.J. Allen, L. Marr, Re-thinking the Potential for Airborne Transmission of SARS-CoV-2, (2020). https://doi.org/10.20944/preprints202005.0126.v1.
- 142.WHO, (2020). Transmission of SARS-CoV-2: implications for infection prevention precautions, https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions. (accessed 12 October 2020).
- 143.Stadnytskyi V., Bax C.E., Bax A., Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. U.S.A. 2020;117(22):11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bourouiba L., Dehandschoewercker E., Bush J.W.M. Violent expiratory events: on coughing and sneezing. J. Fluid Mech. 2014;745:537–563. doi: 10.1017/jfm.2014.88. [DOI] [Google Scholar]
- 145.Yan J., Grantham M., Pantelic J., Jacob Bueno de Mesquita P., Albert B., Liu F.J., Ehrman S., Milton D.K. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. U.S.A. 2018;115(5):1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Chao C.Y.H., Li Y., Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol. Sci. 2009;40(3):256–269. doi: 10.1016/j.jaerosci.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xie X., Li Y., Chwang A.T.Y., Ho P.L., Seto W.H. How far droplets can move in indoor environments: revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 148.Li Y., Huang X., Yu I.T.S., Wong T.W., Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15(2):83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- 149.Olsen S.J., Chang H.L., Cheung T.Y.Y., Tang A.F.Y., Fisk T.L., Ooi S.P.L., Kuo H.W., Jiang D.D.S., Chen K.T., Lando Ji., Hsu K.H., Chen T.J., Dowell S.F. Transmission of the severe acute respiratory syndrome on aircraft. N. Engl. J. Med. 2003;349(25):2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- 150.Guo Z.D., Wang Z.Y., Zhang S.F., Li X., Li L., Li C., Cui Y., Fu R.B., Dong Y.Z., Chi X.Y., Zhang M.Y., Liu K., Cao C., Liu B., Zhang K., Gao Y.W., Lu B., Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020;26(7):1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lu J.Y., Gu J.N., Li K.B., Xu C.H., Su W.Z., Lai Z.S., Zhou D.Q., Yu C., Xu B., Yang Z.C. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 2020;26(7):1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jang S., Han S.H., Rhee J.Y. Cluster of coronavirus disease associated with fitness dance classes, South Korea. Emerg. Infect. Dis. 2020;26(8):1917–1920. doi: 10.3201/eid2608.200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Van Leuken J.P.G., Swart A.N., Havelaar A.H., Van Pul A., Van der Hoek W., Heederik D. Atmospheric dispersion modelling of bioaerosols that are pathogenic to humans and livestock – A review to inform risk assessment studies. Microbial Risk Anal. 2016;1:19–39. doi: 10.1016/j.mran.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brisebois E., Veillette M., Dion-Dupont V., Lavoie J., Corbeil J., Culley A., Duchaine C. Human viral pathogens are pervasive in wastewater treatment center aerosols. J. Environ. Sci. 2018;67:45–53. doi: 10.1016/j.jes.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Han Y.P., Yang T., Yan X., Li L., Liu J.X. Effect of aeration mode on aerosol characteristics from the same wastewater treatment plant. Water Res. 2020;170:115324. doi: 10.1016/j.watres.2019.115324. [DOI] [PubMed] [Google Scholar]
- 158.Yang T., Han Y.P., Liu J.X., Li L. Aerosols from a wastewater treatment plant using oxidation ditch process: characteristics, source apportionment, and exposure risks. Environ. Pollut. 2019;250:627–638. doi: 10.1016/j.envpol.2019.04.071. [DOI] [PubMed] [Google Scholar]
- 159.Bauer H., Fuerhacker M., Zibuschka F., Schmid H., Puxbaum H. Bacteria and fungi in aerosols generated by two different types of wastewater treatment plants. Water Res. 2002;36(16):3965–3970. doi: 10.1016/S0043-1354(02)00121-5. [DOI] [PubMed] [Google Scholar]
- 160.Elsamadony M., Fujii M., Miura T., Watanabe T. Possible transmission of viruses from contaminated human feces and sewage: Implications for SARS-CoV-2. Sci. Total Environ. 2021;755:142575. doi: 10.1016/j.scitotenv.2020.142575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.LeChevallier M.W., Mansfield T.J., Gibson J.M. Protecting wastewater workers from disease risks: personal protective equipment guidelines. Water Environ. Res. 2020;92(4):524–533. doi: 10.1002/wer.1249. [DOI] [PubMed] [Google Scholar]
- 162.McDermott C.V., Alicic R.Z., Harden N., Cox E.J., Scanlan J.M. Put a lid on it: are faecal bio-aerosols a route of transmission for SARS-CoV-2? J. Hosp. Infect. 2020;105(3):397–398. doi: 10.1016/j.jhin.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Johnson D., Lynch R., Marshall C., Mead K., Hirst D. Aerosol generation by modern flush toilets. Aerosol. Sci. Technol. 2013;47(9):1047–1057. doi: 10.1080/02786826.2013.814911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang R.Y., Wang G.H., Guo S., Zamora M.L., Ying Q., Lin Y., Wang W.G., Hu M., Wang Y. Formation of urban fine particulate matter. Chem. Rev. 2015;115(10):3803–3855. doi: 10.1021/acs.chemrev.5b00067. [DOI] [PubMed] [Google Scholar]
- 165.Pyankov O.V., Bodnev S.A., Pyankova O.G., Agranovski I.E. Survival of aerosolized coronavirus in the ambient air. J. Aerosol Sci. 2018;115:158–163. doi: 10.1016/j.jaerosci.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Van Doremalen N., Bushmaker T., Munster V.J. Stability of middle east respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18:20590. doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- 167.Lin K., Marr L.C. Humidity-dependent decay of viruses, but not bacteria, in aerosols and droplets follows disinfection kinetics. Environ. Sci. Technol. 2020;54(2):1024–1032. doi: 10.1021/acs.est.9b04959.s001. [DOI] [PubMed] [Google Scholar]
- 168.Lin J., Huang W.H., Wen M.C., Li D.H., Ma S.Y., Hua J.W., Hu H., Yin S., Qian Y.J, Chen P.L., Zhang Q., Yuan N.B., Sun S.L. Containing the spread of coronavirus disease 2019 (COVID-19): meteorological factors and control strategies. Sci. Total Environ. 2020;744:140935. doi: 10.1016/j.scitotenv.2020.140935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Luo C., Yao L., Zhang L., Yao M.C., Chen X.F., Wang Q.L, Shen H.B. Possible transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a public bath center in Huai’an, Jiangsu Province, China. JAMA Netw Open. 2020;3(3):e204583. doi: 10.1001/jamanetworkopen.2020.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fears A.C., Klimstra W.B., Duprex P., Hartman A., Weaver S.C., Plante K.S., Mirchandani D., Plante J., Aguilar P.V., Fernandez D., Nalca A., Allison T., Dyer D., Brian K., Lackemeyer M., Bohannon K.J., Johnson R., Garry R.F., Reed D.S., Roy C.J. Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions. medRxiv. 2020 doi: 10.1101/2020.04.13.20063784. [DOI] [Google Scholar]
- 171.Xie J.G., Zhu Y.J. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724:138201. doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tobías A., Molina T. Is temperature reducing the transmission of COVID-19 ? Environ. Res. 2020;186:109553. doi: 10.1016/j.envres.2020.109553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Prata D.N., Rodrigues W., Bermejo P.H. Temperature significantly changes COVID-19 transmission in (sub)tropical cities of Brazil. Sci. Total Environ. 2020;729:138862. doi: 10.1016/j.scitotenv.2020.138862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chan K.H., Malik Peiris J.S., Lam S.Y., Poon L.L.M., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011;2011:1–7. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang D.X. SARS-CoV-2: air/aerosols and surfaces in laboratory and clinical settings. J. Hosp. Infect. 2020;105(3):577–579. doi: 10.1016/j.jhin.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Somsen G.A., van Rijn C., Kooij S., Bem R.A., Bonn D. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respirat. Med. 2020;8(7):658–659. doi: 10.1016/S2213-2600(20)30245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., Cao J.J., Dancer S., Floto A., Franchimon F., Haworth C., Hogeling J., Isaxon C., Jimenez J.L., Kurnitski J., Li Y.G., Loomans M., Marks G., Marr L.C., Mazzarella L., Melikov A.K., Miller S., Milton D.K., Nazaroff W., Nielsen P.V., Noakes C., Peccia J., Querol X., Sekhar C., Seppänen O., Tanabe S., Tellier R., Tham K.W., Wargocki P., Wierzbicka A., Yao M.S. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Thompson J.R, Nancharaiah Y.V, Gu X.Q., Lee W.L., Rajal V.B., Haines M.B., Girones R., Ng L.C., Alm E.J., Wuertz S. Making waves: wastewater surveillance of SARS-CoV-2 for population-based health management. Water Res. 2020;184:116181. doi: 10.1016/j.watres.2020.116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Michael-Kordatou I., Karaolia P., Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. J. Environ. Chem. Eng. 2020;8(5):104306. doi: 10.1016/j.jece:2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rodríguez-Lázaro D., Cook N., Ruggeri F.M., Sellwood J., Nasser A., Nascimento M.S.J., D’Agostino M., Santos R., Saiz J.C., Rzeżutka A., Bosch A., Gironés R., Carducci A., Muscillo M., Kovač K., Diez-Valcarce M., Vantarakis A., von Bonsdorff C.H., de Roda Husman A.M., Hernández M., van der Poel W.H.M. Virus hazards from food, water and other contaminated environments. FEMS Microbiol. Rev. 2012;36(4):786–814. doi: 10.1111/j.1574-6976.2011.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mao K., Zhang H., Yang Z.G. Can a paper-based device trace COVID-19 sources with wastewater-based epidemiology? Environ. Sci. Technol. 2020;54(7):3733–3735. doi: 10.1021/acs.est.0c01174. [DOI] [PubMed] [Google Scholar]
- 182.Kumar M., Mazumder P., Mohapatra S., Kumar Thakur A., Dhangar K., Taki K., Mukherjee S., Kumar Patel A., Bhattacharya P., Mohapatra P., Rinklebe J., Kitajima M., Hai F.I., Khursheed A., Furumai H., Sonne C., Kuroda K. A chronicle of SARS-CoV-2: Seasonality, environmental fate, transport, inactivation, and antiviral drug resistance. J. Hazard. Mater. 2020:124043. doi: 10.1016/j.jhazmat.2020.124043. [DOI] [PMC free article] [PubMed] [Google Scholar]