Abstract
Neonatal maternal separation alters adult HPA axis responsiveness to stress, adult emotionality, and glucocorticoid receptor (GR) concentrations in forebrain regions such as hippocampus. To investigate effects of neonatal maternal separation on emotion regulation and its neural substrates, we assessed acquisition and extinction of conditioned fear in adult rats that underwent neonatal maternal separation. Corticolimbic structures including basolateral amygdala and medial prefrontal cortex are critical for acquisition and extinction of conditioned fear, and such learning is N-methylD-aspartic acid (NMDA) receptor-dependent. Thus, we used immunohistochemistry to assess expression of the GR and the NR1 subunit of the NMDA receptor in basolateral amygdala and medial prefrontal cortex. On postnatal days 2–14, pups underwent control rearing or maternal separation for 15 min per day. Fear conditioning and extinction in adulthood were then assessed in male rats. Rats received five tone-alone habituation trials, then seven tone/footshock pairings. After 1 h, rats received tone-alone extinction trials to criterion, and 15 recall of extinction trials the next day. Brains were processed for immunohistochemical labeling of GR and NR1, and staining was quantified. Brief maternal separation did not alter acquisition or initial extinction, but impaired extinction recall. Brief maternal separation did not alter GR or NR1 expression in basolateral amygdala. However, brief maternal separation increased GR and decreased NR1 expression specifically in the infralimbic region of medial prefrontal cortex, consistent with work implicating this area in extinction recall. Thus, brief maternal separation impaired extinction recall and altered GR and NR1 expression in its neural substrate in adults.
Keywords: immunohistochemistry, fear conditioning, maternal separation, glucocorticoid receptors, NMDA receptor
INTRODUCTION
Neonatal maternal separation alters adult HPA axis responsiveness to stress, emotionality (McIntosh et al., 1999; Wigger and Neumann, 1999; Kalinichev et al., 2002; De Jongh et al., 2005), and glucocorticoid receptor concentrations in forebrain regions such as the hippocampus (Meaney et al., 1985; Ladd et al., 2004). Neonatal maternal separation also produces changes in several forms of adult learning and memory, including spatial learning and memory (Huot et al., 2002; Pryce et al., 2003; Gibb and Kolb, 2005; Uysal et al., 2005; Aisa et al., 2007), eyeblink conditioning (Wilber et al., 2007), inhibitory avoidance (Kosten et al., 2007), and object recognition (Kosten et al., 2007). Although acquisition of fear conditioning for context and tone is not affected by either brief or prolonged maternal separation (Pryce et al., 2003; Kosten et al., 2005, 2006), adult recall of conditioned fear may be reduced following maternal separation(e.g., Meerlo et al., 1999; but see Kosten et al., 2005). However, to our knowledge, the effects of maternal separation on learning and unlearning of conditioned fear and the corticolimbic structures mediating these behaviors have not been assessed.
The neural substrates for acquisition of conditioned fear and recall of extinction are well characterized, though the circuitry underlying initial extinction is less clear (Falls et al., 1992; Barad et al., 2006; Bouton et al., 2006). Electrophysiological data from basolateral amygdala indicate both a precise convergence of conditioned and unconditioned stimuli (Romanski et al., 1993; Bordi and LeDoux, 1994) and patterns of neuronal activity that model the conditioned response (McKernan and Shinnick-Gallagher, 1997; Quirk et al., 1997; Collins and Pare, 2000; Repa et al., 2001). Lesions of the basolateral amygdala impair acquisition and expression of conditioned fear (Blanchard and Blanchard, 1972; Kim et al., 1993), and temporary inactivation prevents normal conditioning (Muller et al., 1997; Wilensky et al., 1999; Calandreau et al., 2005). The central nucleus of the amygdala projects to brain areas that control the expression of the fear response (Price and Amaral, 1981; Schwaber et al., 1982; Cassell et al., 1986; Iwata et al., 1986; LeDoux et al., 1988; Van de Kar et al., 1991; Kim et al., 1993; Oliveira et al., 2004), and seems to be important for expression of conditioned fear. For instance, stimulation of the central nucleus of the amygdala results in expression of fear responses similar to those seen following fear conditioning (Applegate et al., 1983).
Although the amygdala is a key site of plasticity for acquisition of fear conditioning, medial prefrontal cortex is critical for recall of extinction. Lesions of medial prefrontal cortex (mPFC) impair extinction learning (Morgan and LeDoux, 1995; Quirk et al., 2000); in addition, electrophysiological data have demonstrated that firing patterns of neurons in ventral mPFC are correlated with memory for fear extinction (Milad and Quirk, 2002; Burgos-Robles et al., 2007). Lesion and stimulation studies have implicated the infralimbic (IL) region of the mPFC, in particular, as a critical structure for recall of extinction (Morgan and LeDoux, 1995; Milad and Quirk, 2002; Milad et al., 2004; Garcia et al., 2006; Corcoran and Quirk, 2007). Further, consolidation of extinction is impaired by inhibition of protein synthesis in ventral mPFC (Santini et al., 2004) or blockade of N-methylD-aspartic acid (NMDA) receptors in IL and prelimbic (PL) mPFC (Baker and Azorlosa, 1996; BurgosRobles et al., 2007).
NMDA receptors are important for both acquisition and extinction of fear conditioning. Blockade of NMDA receptors in the basolateral, but not the central amygdala, before fear conditioning prevents the acquisition of conditioned fear, and infusion of an NMDA receptor blocker into the amygdala after training prevents expression of the conditioned fear response (Miserendino et al., 1990; Fanselow and Kim, 1994; Lee and Kim, 1998; Lee et al., 2001; Rodrigues et al., 2001). Likewise, blockade of NMDA receptors in mPFC impairs consolidation of extinction (Baker and Azorlosa, 1996; Burgos-Robles et al., 2007).
Similarly, glucocorticoid receptors (GR) are present in the amygdala and prefrontal cortex and are altered in forebrain regions such as the hippocampus (Meaney et al., 1985; Ladd et al., 2004) and in the cerebellum by prolonged or brief neonatal maternal separation (Wilber et al., 2007). Additionally, glucocorticoids modulate adult learning and memory, with facilitated or impaired memory performance depending on the timing and duration of glucocorticoid exposure (Roozendaal et al., 1996; Kim et al., 2006). Thus, the effects of neonatal maternal separation on learning and memory could be mediated in part by GRs in the central nervous system. Additionally, glucocorticoids modulate NMDA receptor-mediated Ca2+ influx in cultured hippocampal neurons (Takahashi et al., 2002) and NMDA-dependent LTP (Shors et al., 1989), and these effects are mediated by glucocorticoid receptors (Xu et al., 1998; Yang et al., 2004). Many adult stress effects are mediated by NMDA-GR interactions (Magarinos and McEwen, 1995; Kim et al., 1996; Shors and Mathew, 1998; Roozendaal et al., 2003). Thus, neonatal maternal separation could influence fear conditioning and extinction via alterations in either glucocorticoid or NMDA receptors, or both.
Therefore, we examined the effect of brief neonatal maternal separation and handling on adult fear conditioning, extinction, and recall of extinction. To begin to assess potential changes underlying separation-induced effects on adult fear conditioning and extinction, we used immunohistochemistry to assess potential changes in NMDA NR1 subunit and GR expression in the amygdala and medial prefrontal cortex regions thought to be critical for fear conditioning acquisition and extinction.
METHOD
Animals
Untimed pregnant Long-Evans Blue Spruce rats (Harlan Indianapolis, IN, N =10) arrived 1 week before giving birth. Dams were housed individually in standard laboratory cages (48 cm x 20 cm x 26 cm), with food and water available ad libitum and a 12:12 h light/dark cycle (lights on at 0700 h). The day of birth was considered postnatal day (PND) 0. On PND two pups were culled to litters of 9–11 while maintaining a male:female ratio as close to 1:1 as possible. All experimental procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Bloomington Institutional Animal Care and Use Committee.
Maternal Separation and Handling
Male rats from each litter were randomly assigned to one of three groups: standard animal facilities rearing (control; n 11 from 4 litters), gentle handling for 15 min (n = 5 from 3 litters), or maternal separation for 15 min (n = 6 from 3 litters). Maternal separation and handling were carried out using procedures described previously (Wilber et al., 2007), and similar to those of Huot et al. (2002). All manipulations were initiated between 0700 and 0900 h each day. Dams were removed from the home cage and placed in an adjacent container. Pups in both groups were then removed from the home cage and placed in a Plexiglas cage (28 cm x 17 cm x 12 cm) lined with clean bedding and dams were returned to the home cage for the duration of the separation period. Pups in the brief maternal separation group were taken to a nearby room and placed in an incubator (Ambient Room Temperature Incubator; Avey Incubator; Evergreen, CO) maintained at room temperature (22.5 ± 0.5 deg C) for the duration of the separation period. Pups subjected to gentle handling were taken to a nearby thermostat-controlled room maintained at ~23 deg C for the duration of the handling period. Pups in both groups were permitted to huddle with littermates during the separation period. For the briefly handled group, only the pup that was currently being handled was prevented from huddling. On average, each pup received 90 s of handling per session. We have previously shown that both of these manipulations produce significant elevations of plasma corticosterone concentrations measured 12 h after the onset of separation (Wilber et al., 2007). On PND 28 all animals were weaned and housed in same sex/same litter groups of four or fewer until fear conditioning.
Fear Conditioning
Bar Press Training.
When rats had reached a minimum of 3 months of age, behavioral training and testing commenced. To obtain a baseline level of activity against which to measure freezing, rats were trained to bar press for food reinforcement (see Quirk et al., 2000; Miracle et al., 2006). Each rat was placed in an operant chamber within a sound-attenuating cabinet (Med Associates, St. Albans, VT). The chamber contained one operant lever on either side of a food receptacle, a house light on the opposite wall, a cue light over each lever, and a floor consisting of metal rods. The house light and the cue light over the reinforced lever were illuminated throughout each session. Rats were shaped to press the left lever for a food pellet reinforcer (BioServ pellets, Holton Industries, Frenchtown, NJ); shaping lasted 1–2 sessions, after which the reinforcement schedule was gradually reduced over 4 days from FR-1 to VI-60. During all subsequent phases of training and testing, rats were allowed to bar press for pellets on a VI-60 schedule. Computer-based operant software (MedPC-IV; Med Associates, St. Albans, VT) controlled pellet delivery.
Fear Conditioning and Extinction.
Fear conditioning and extinction took place over the following 2 days using a procedure similar to that of Quirk et al. (2000). On Day 1, rats were placed in the operant chambers and underwent fear conditioning. After a 3min acclimation period, rats received five habituation trials consisting of presentation of a 30-s tone (4.5kHz, 80 db). Rats then underwent fear conditioning, consisting of seven pairings of the tone conditioned stimulus (CS) with a footshock US (500-ms, 0.5 mA) coterminating with the tone CS. Rats were then returned to their home cages for 1 h, after which they were returned to the chambers and given extinction trials consisting of tone alone. To ensure comparable levels of extinction learning across both groups, on Day 1 extinction trials continued until the rat exhibited less than 10% (3 s) freezing on four consecutive trials (i.e., criterion). The following day, rats were given another 15 extinction trials. For all phases of conditioning and extinction, variable intertrial intervals averaged 4 min and computer-based operant software (MedPC-IV; Med Associates, St. Albans, VT) controlled the delivery of tones and shocks. For all trials, the duration of freezing (defined as the absence of any visible movements except that due to breathing) during the tone was measured with a digital stopwatch by an observer blind to experimental conditions. Percent freezing (seconds spent freezing/ 30 s) during habituation, fear conditioning, extinction on Day 1, and extinction on Day 2 (recall of extinction) were calculated and compared across groups. For statistical analyses, extinction Day 1 and Day 2 data were averaged into bins of three trials. Datawere analyzed using 2-way repeated measures ANOVAs (group 3 trial); when appropriate followup planned comparisons were conducted consisting of two-group t-tests done within the context of the overall ANOVA (Hays, 1994; Maxwell and Delaney, 2003).
Percent freezing did not differ between handled versus separated animals during fear conditioning (main effect of group; F(1,9) =1.21, p =0.30; group by trial interaction; F(6,54) =1.28, p =0.28), extinction of fear conditioning (main effect of group; F(1,9) =2.39, p =0.16; group by trial interaction; F(7,63) =0.21, p =0.98), or extinction recall (main effect of group; F(1,9) =0.42, p =0.53; group by trial interaction; F(4,36) = 0.46, p = 0.77). Therefore, these ani-mals were collapsed into a single group (n 11).
Finally, trials to criterion on extinction Days 1 and 2 were calculated and compared across groups using t-tests (unpaired).
NMDA NR1 and GR Immunohistochemistry and Quantification of Expression
Brains from rats that had undergone fear conditioning and extinction were processed using immunohistochemistry for the NR1 subunit of the NMDA receptor and three brains were eliminated due to histological processing errors (final n’s 6 control, 8 separated). Because the procedures for NR1 and GR immunohistochemistry are not compatible, a separate set of control and separated rats were processed for GR immunohistochemistry (control n = 7 from 3 litters and separated n = 7 from 3 litters). All rats were deeply anesthetized with urethane and transcardially perfused with cold 0.1M phosphate buffered saline (pH 7.4), followed by 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). Immediately following transcardial perfusion, the brain was removed, postfixed for 2 h (NR1) or 24 h (GR), and cryoprotected in 30% sucrose in 0.1M phosphate buffer (pH 7.4). Frozen sections were cut coronally at 40 μm on a sliding microtome and collected in 0.01M phosphate buffered saline (PBS, pH 7.4). For each brain, two series of adjacent sections were collected through the entire amygdala and prefrontal cortex. Each series contained equally spaced sections (~400 μm apart for the prefrontal cortex and ~600 μm apart for the amygdala). One series was processed free-floating for immunohistochemistry, while the second series was processed for thionin staining (see below).
NMDA NR1 Immunohistochemistry.
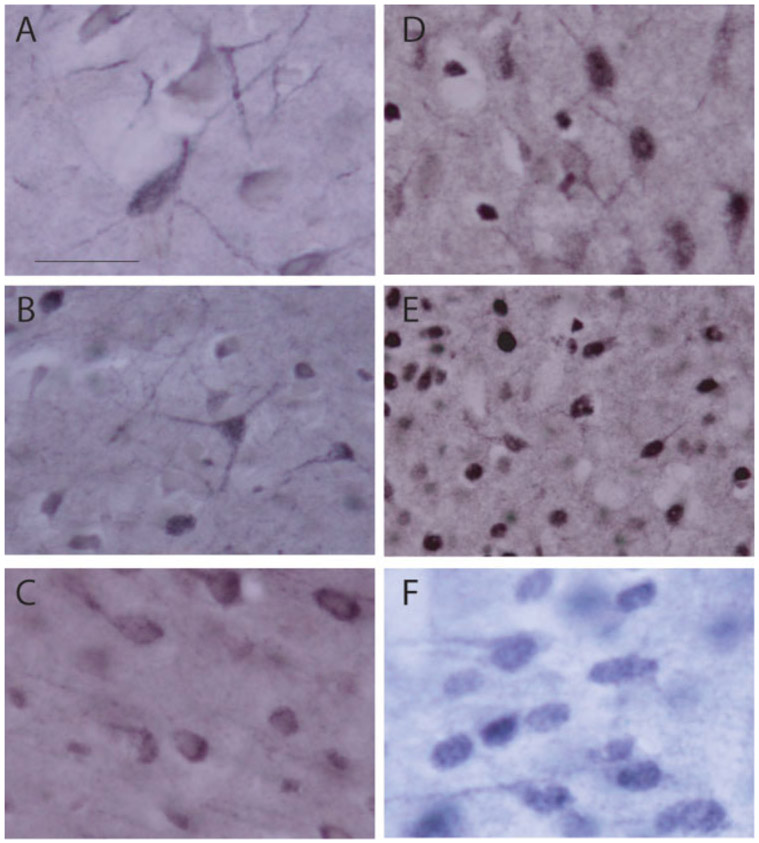
For immunohistochemical labeling of the NMDA NR1 subunit, sections were incubated for 30 min in 50% ethanol. Sections were then incubated for 30 min in 0.2% H2O2 in blocking solution (PBS, 1% normal goat serum and 0.1% Triton-X) followed by 1 h in blocking solution alone. Sections were incubated overnight at 48C in PBS containing 3% normal goat serum (NGS), 0.1% Triton-X, and a polyclonal antibody to the rat GR (1:500; Chemicon, Temecula, CA). After rinsing in PBS, sections were incubated for 30 min in PBS containing 2% NGS and biotinylated goat anti-rabbit IgG (1:200; Vector Laboratories; Burlingame, CA). After rinsing in 0.3% triton X-100 in 0.01M phosphate buffered saline (PBST), sections were incubated for 1 h in PBST with ABC Complex (Vector Laboratories). Staining was visualized using a nickelintensified DAB reaction [Fig. 1(A-C)]. After rinsing, sections were mounted on gelatin-subbed slides, dehydrated, cleared, and cover slipped. Control sections incubated without the primary antibody were generated and demonstrated no staining.
Figure 1.
(A–C) Digital light micrographs of NMDA NR1-immunopositive neurons in the basolateral amygdala (A), central amygdala (B), and medial prefrontal cortex (C). (D–F) GR-immunopositive neurons in the basolateral amygdala (D), central amygdala (E), and medial prefrontal cortex (F). Scale bar 25 lm.
GR Immunohistochemistry.
For immunohistochemical labeling of GR, sections were incubated for 1 h in PBS containing 3% normal goat serum and 0.1% Triton-X to block nonspecific binding. Sections were then incubated for 1 h in 0.2% H2O2 in 50% methanol. After rinsing in PBS, sections were incubated overnight at 48C in PBS containing 3% normal goat serum, 0.1% Triton-X, and a polyclonal antibody to the rat GR (1:3000; Santa Cruz Biotechnology; Santa Cruz, CA). After rinsing in PBS, sections were incubated for 30 min in PBS containing 5% NGS and biotinylated goat anti-rabbit IgG (1:200; Vector Laboratories; Burlingame, CA). After rinsing in 0.3% triton X-100 in 0.01M phosphate buffered saline (PBST), sections were incubated for 1 h in PBST with ABC Complex (Vector Laboratories). Staining was visualized using a nickel-intensified DAB reaction [Fig. 1(D-F)]. After rinsing, sections were mounted on gelatin-subbed slides, dehydrated, cleared, and cover slipped. Control sections incubated without the primary antibody were generated and demonstrated no staining. NR1 and GR expression were quantified in the central (CE) and basolateral (BLA) amygdala, and layers II-III, V, and VI of the anterior cingulate (AC), PL and IL regions of medial prefrontal cortex (see Fig. 2). All data were collected with the experimenter blind to condition.
Figure 2.
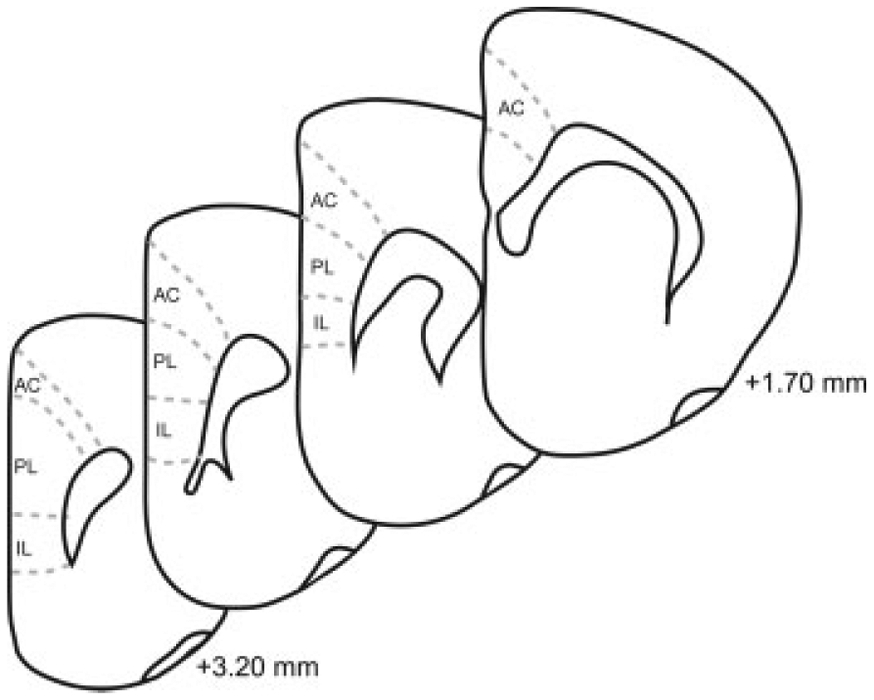
Schematic diagram of coronal sections through prefrontal cortex. The cortical regions (anterior cingulate, AC; prelimbic; PL; infralimbic, IL) from which samples were taken are shown. Coordinates indicate position relative to bregma (Paxinos and Watson, 1998).
Defining Medial Prefrontal Cortex Regions.
To accurately identify laminar and areal borders within medial prefrontal cortex, a series of sections adjacent to the immunohistochemically processed sections was stained with thionin. The thionin-stained sections were used to create templates of the laminar and areal boundaries using the Neurolucida system (Neurolucida; MBF Bioscience; Williston, VT) interfaced with a microscope (Nikon Eclipse 80i; Nikon Instruments; Melville, NY) via a video camera (Microfire; Optronics; Santa Barbara, CA). This system was used for the prefrontal cortex analyses to facilitate identification of cortical and laminar boundaries on immunohistochemically stained sections. These boundaries are readily identifiable in thionin-stained sections using standard cytoarchitectural criteria such as cell packing densities and thicknesses of layers. Templates were then aligned with the adjacent immunohistochemically processed section (NR1 or GR) to ensure sampling took place within the appropriate region.
Densitometry.
NR1 and GR staining in medial prefrontal cortex and the amygdala were quantified using a computer-based image analysis system interfaced with a microscope via a video camera (for medial prefrontal cortex, system: Neurolucida; MBF Bioscience; Willston, VT; microscope: Nikon Eclipse 80i; Nikon Instruments; Melville, NY; camera: Microfire; Optronics; Santa Barbara, CA; for amygdala, system: MCID; Imaging Research; St. Catharines, ON, Canada; microscope: Nikon Eclipse E600; Nikon Instruments; Melville, NY; camera: Sony XC-ST70; Sony; Park Ridge, NJ) (see Wilber et al., 2007). This method has been shown to be reliable for categorizing neurons by immunostaining intensity for subsequent frequency analyses and is sensitive to differences in pro tein expression assessed immunohistochemically (Osborne et al., 2007; Wilber et al., 2007). Data were compared across groups using repeated measures ANOVAs (Hays, 1994; Maxwell and Delaney, 2003).
RESULTS
Brief Maternal Separation Impairs Recall of Extinction in Adults
Brief maternal separation did not significantly alter baseline freezing to the tone alone (Fig. 3, Habituation). There was no main effect of group on freezing (F(1,20) = 2.13, ns) and no group by trial interaction (F(4,80) = 1.42, ns). Similarly, acquisition of the conditioned fear response was not altered by brief maternal separation (Fig. 3, Conditioning). Both groups acquired the conditioned fear response, with a significant increase in freezing across trials (F(6,120) = 29.49, p < 0.001). There was no group effect (F(1,20) = 3.29, ns); however, there was a difference in the rate at which briefly separated rats acquired the conditioned fear response, with a significant group by trial interaction (F(6,120) = 2.95, p < 0.05). To further explore this difference, acquisition data were further analyzed by performing separate analyses on Trials 1–4 (before control rats reached asymptotic performance) and Trials 5–7 (after control rats reached asymptotic performance). No significant effect of brief separation was present during either initial or asymptotic trials (for initial trials, F(1,20) = 2.56, ns; for asymptotic trials, F(1,20) = 2.58, ns). There was an interaction of brief separation and trial for initial trials (F(3,60) = 7.49, p < 0.001), in which briefly separated rats showed increased freezing on Trial 4 (Trial 4, t(20) = 3.20, p < 0.01; Trials 1–3, t(20) < 0.91, ns); however, there was no interaction for asymptotic trials (F(2,40) = 2.11, ns). Thus, though separated rats may have acquired the fear response slightly more rapidly, by the last acquisition trial the two groups showed equivalent learning. During initial extinction training, freezing diminished across trials (Fig. 3, Extinction; main effect of trial, F(7,140) = 20.31, p < 0.001), and brief maternal separation did not significantly alter the rate of extinction (for main effect of group, F(1,20) = 3.28, ns; for group 3 trial interaction, F(7,140) = 0.39, ns). Furthermore, trials to criterion (t(20) < 1.00, ns) did not differ significantly across groups. Therefore, brief maternal separation did not significantly alter initial extinction learning.
Figure 3.
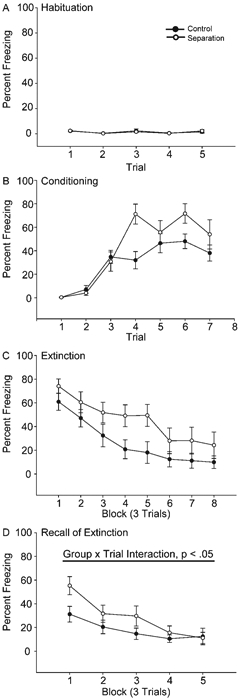
Mean percent freezing to tone in rats that underwent control (black circles) versus maternal separation for 15 min (Separated) per day on PND 2–14 (white circles) across habituation (A), conditioning (B), extinction (Day 1, C), and recall of extinction (extinction Day 2, D) trials. Vertical bars represent SEMs.
However, brief maternal separation significantly altered extinction recall assessed 1 day later. Although both groups showed a decrease in freezing across trials (main effect of trial, F(4,80) = 17.56, p < 0.001; main effect of group, F(1,20) = 2.36, ns), separated animals showed impaired extinction recall, with 77% more freezing than controls during the first three trials (group by trial interaction, F(4,80) = 2.54, p < 0.05). Planned comparisons indicated that briefly separated animals had significantly increased freezing during Block 1 trials (t(20) = 2.39, p < 0.05); however, consistent with performance on initial extinction, freezing was not significantly different between groups on subsequent trials (ts(20) = 1.54, ns). Likewise, the average number of trials required to reach criterion on extinction Day 2 was not affected by brief maternal separation (t(20) < 0.81, ns).
Brief Maternal Separation Decreases Adult NMDA NR1 Immunostaining in Medial Prefrontal Cortex But Not Amygdala
Overall, mean (6SEM) luminosity of the white matter samples in the amygdala were 211.19 6 0.59, and in the prefrontal cortex were 192.77 6 1.32. Mean white matter luminosity did not differ between the groups for the amygdala (group main effect, F(1,12) =0.96, ns) or the prefrontal cortex (group main effect, F(1,12) =0.90, ns). The average number of neurons measured per region was 44.10 6 9.03 for the amygdala and 26.14 6 0.93 for the prefrontal cortex. As with white matter values, the mean number of neurons per animal did not differ between the groups for the amygdala (group main effect, F(1,12) =0.01, ns) or prefrontal cortex (group main effect, F(1,12) =0.21, ns). Approximately two sections were sampled per animal for each region. Amygdala. Brief maternal separation did not alter NMDA NR1 expression in the basolateral or central amygdala, consistent with a lack of group differences in acquisition of fear conditioning (see Fig. 4). The distribution of staining luminosity in basolateral amygdala was not different from control in the separated group (group by bin interaction, F(9,108) =0.72, ns). Similarly, the distribution of staining luminosity in central amygdala did not differ from control (group by bin interaction, F(9,108) =0.10, ns). Medial Prefrontal Cortex. Brief maternal separation specifically and significantly decreased NMDA NR1 subunit staining in layer VI of infralimbic cortex (see Fig. 5). The distribution of staining luminosity in IL VI of separated rats was significantly shifted to the right, reflecting less NR1 expression (Fig. 5, group by bin interaction, F(9,108) =2.31, p < 0.05). For instance, in control animals, only 36% of neurons fell into the four most lightly-stained categories, whereas for separated animals, 60% of neurons fell into these categories. No significant group by bin interaction for NR1 staining luminosities was present in any layer of AC (Fs(9,108) < 1.64, ns) or PL (Fs(9,108) < 0.95, ns), or the more superficial layers of IL (Fig. 5; Fs(9,108) < 1.16, ns) of mPFC.
Figure 4.
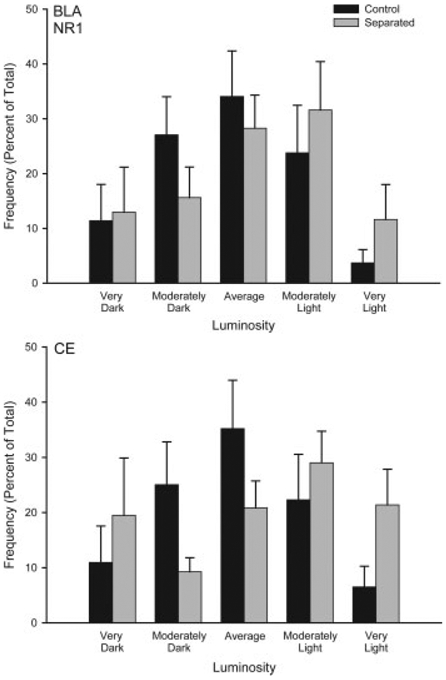
Histograms of the mean number of NMDA NR1 positive neurons (expressed as percent of total) in basolateral amygdala (BLA) and central amygdala (CE) categorized as having relative luminosities varying from two standard deviations below the mean of controls (darkest) to greater than two standard deviations above the mean for controls (lightest) for animals that underwent either standard animal facilities rearing (Control) or maternal separation for 15 min (Separated). For graphic purposes, data have been collapsed into five bins (very dark, greater than 1.5 standard deviations (SD) below the mean; moderately dark, 0.5 to 1.5 SD below the mean; average 60.5 SD from the mean; moderately light, 0.5 to 1.5 SD above the mean; very light, greater than 1.5 SD above the mean). Vertical bars represent SEMs.
Figure 5.
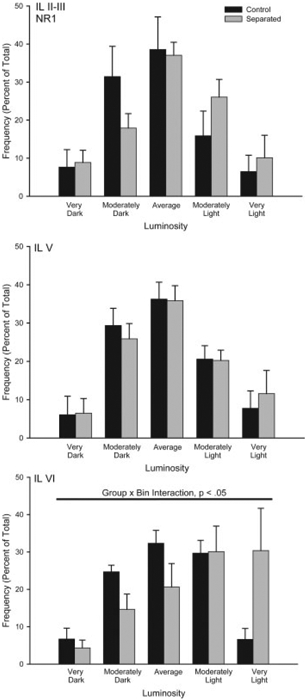
Histograms of the mean number of NMDA NR1 positive neurons (expressed as percent of total) in infralimbic (IL) cortex categorized as having relative luminosities varying from two standard deviations below the mean of controls (darkest) to greater than two standard deviations greater than the mean for controls (lightest) for animals that underwent either standard animal facilities rearing (Control) or maternal separation for 15 min (Separated). For graphic purposes, data have been collapsed into five bins (very dark, greater than 1.5 standard deviations (SD) below the mean; moderately dark, 0.5 to 1.5 SD below the mean; average 60.5 SD from the mean; moderately light, 0.5 to 1.5 SD above the mean; very light, greater than 1.5 SD above the mean). Vertical bars represent SEMs.
Brief Maternal Separation Increases Adult GR Immunostaining in Medial Prefrontal Cortex But Not Amygdala
Overall, mean (6SEM) luminosity of the white matter samples in the amygdala were 211.92 6 0.58, and in the prefrontal cortex were 192.77 6 1.32. Mean white matter luminosity did not differ between the groups for the amygdala (group main effect, F(1,12) =0.72, ns) or the prefrontal cortex (group main effect, F(1,10) =0.60, ns). The average number of neurons measured per region was 45.93 6 1.50 for the amygdala and 27.26 6 1.88 for the prefrontal cortex. As with white matter values, the mean number of neurons per animal did not differ between the groups for the amygdala (group main effect, F(1,12) =0.39, ns) or prefrontal cortex (group main effect, F(1,10) =3.56, ns). Amygdala. Brief maternal separation did not alter GR expression in the basolateral or central amygdala, consistent with a lack of group differences in acquisition of fear conditioning (see Fig. 6). The distribution of staining luminosity in basolateral amygdala was not different from control following brief maternal separation (group by bin interaction, F(9,108) =0.14, ns). Similarly, the distribution of staining luminosity in central amygdala did not differ from control (group by bin interaction, F(9,108) =0.20, ns). Medial Prefrontal Cortex. Prefrontal cortex data from two separated animals was eliminated due to histological processing error (thus, for prefrontal cortex n =7 control, and n =5 separated). Brief maternal separation increased GR subunit expression, and again this effect was regionand layerspecific (Figs. 6 and 7). The distribution of staining luminosity in layer VI in both PL and IL in separated rats was significantly shifted to the left, reflecting more GR expression (Fig. 7; PL VI, group by bin interaction, F(9,90) =2.08, p < 0.05 and Fig. 8; IL VI, group by bin interaction, F(9,90) =2.28, p < 0.05). For instance, in IL VI only 43% of control neurons fell into the four most darkly-stained categories, whereas for separated animals, 78% of neurons fell into these categories. Similarly, in PL VI only 37% of control neurons fell into the four most darkly-stained categories, whereas for separated animals, 62% of neurons fell into these categories. No significant group by bin interaction for GR staining intensities was present for any layer of AC (Fs(9,90) < 1.86, ns) or the more superficial layers of PL (Fig. 7; Fs(9,90) < 1.04, ns) and IL (Fig. 8; Fs(9,108) < 1.50, ns) of mPFC.
Figure 6.
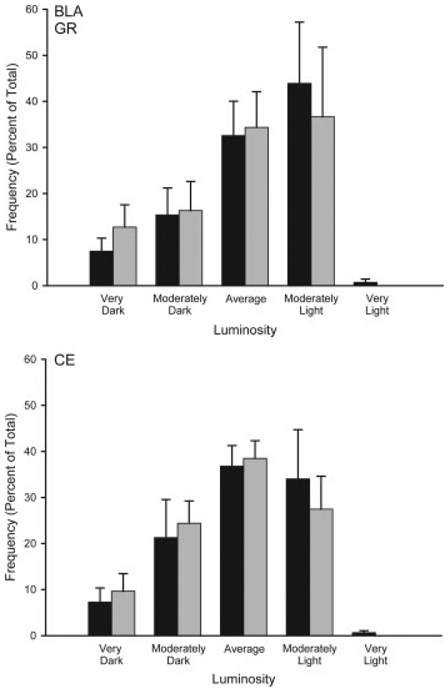
Histograms of the mean number of GR positive neurons (expressed as percent of total) in basolateral amygdala (BLA) and central amygdala (CE) categorized as having relative luminosities varying from two standard deviations below the mean of controls (darkest) to greater than two standard deviations above the mean for controls (lightest) for animals that underwent either standard animal facilities rearing (Control) or maternal separation for 15 min (Separated). For graphic purposes, data have been collapsed into five bins (very dark, greater than 1.5 standard deviations (SD) below the mean; moderately dark, 0.5 to 1.5 SD below the mean; average 60.5 SD from the mean; moderately light, 0.5 to 1.5 SD above the mean; very light, greater than 1.5 SD above the mean). Vertical bars represent SEMs.
Figure 7.
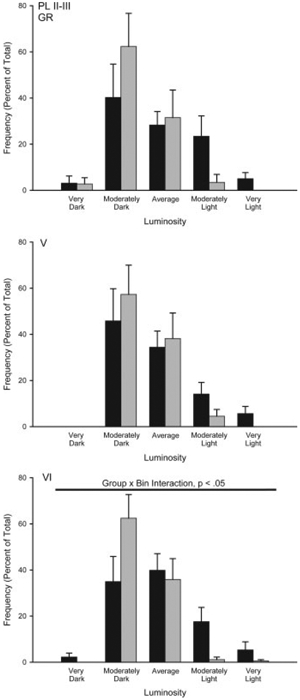
Histograms of the mean number of GR positive neurons (expressed as percent of total) in prelimbic (PL) cortex categorized as having relative luminosities varying from two standard deviations below the mean of controls (darkest) to greater than two standard deviations greater than the mean for controls (lightest) for animals that underwent either standard animal facilities rearing (Control) or maternal separation for 15 min (Separated). For graphic purposes, data have been collapsed into five bins (very dark, greater than 1.5 standard deviations (SD) below the mean; moderately dark, 0.5 to 1.5 SD below the mean; average 60.5 SD from the mean; moderately light, 0.5 to 1.5 SD above the mean; very light, greater than 1.5 SD above the mean). Vertical bars represent SEMs.
Figure 8.
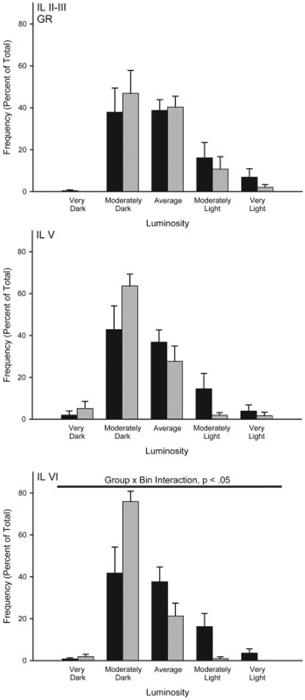
Histograms of the mean number of GR positive neurons (expressed as percent of total) in infralimbic (IL) cortex categorized as having relative luminosities varying from two standard deviations below the mean of controls (darkest) to greater than two standard deviations greater than the mean for controls (lightest) for animals that underwent either standard animal facilities rearing (Control) or maternal separation for 15 min (Separated). For graphic purposes, data have been collapsed into five bins (very dark, greater than 1.5 standard deviations (SD) below the mean; moderately dark, 0.5 to 1.5 SD below the mean; average 60.5 SD from the mean; moderately light, 0.5 to 1.5 SD above the mean; very light, greater than 1.5 SD above the mean). Vertical bars represent SEMs.
DISCUSSION
The present study demonstrates a long-lasting effect of brief (15 min) neonatal maternal separation on extinction of conditioned fear and a key component of its neural substrate, medial prefrontal cortex, in adult male rats. It is important to note that the 15 min of maternal separation used in the present study is comparable to the neonatal manipulation that is often referred to as handling (Meaney et al., 1985; O’Donnell et al., 1994). Brief maternal separation did not significantly alter acquisition of the conditioned fear response or its initial extinction, but impaired recall of extinction. Furthermore, brief maternal separation did not significantly alter immunohistochemical staining of either glucocorticoid receptors or the NR1 subunit of the NMDA receptor in either basolateral or central amygdala. However, brief maternal separation resulted in an increase in expression of glucocorticoid receptors in layer VI of prelimbic and infralimbic cortex and a decrease in expression of the NR1 subunit of the NMDA receptor in layer VI of infralimbic cortex. Previously, we demonstrated that the same brief maternal separation manipulation used in the present study marginally increases neonatal plasma corticosterone on PND 2 and significantly increases concentrations by PND 12 (Wilber et al., 2007), suggesting that the manipulation is stressful. Thus, the stress of brief neonatal maternal separation may have been responsible for the alterations in GR expression, NR1 expression, and recall of extinction.
Effects of Maternal Separation on Learning and Memory
The present study found that brief neonatal maternal separation impaired extinction of conditioned fear in adults. Our finding of a specific impairment of extinction recall is similar to the effect of adult stress, in which conditioning and initial extinction are also spared, but extinction recall is impaired (Miracle et al., 2006). Impaired extinction following brief maternal separation is consistent with other studies showing brief separation-induced deficits in learning and memory tasks such as fear conditioning and eyeblink conditioning in adults (Meerlo et al., 1999; Kosten et al., 2007; Wilber et al., 2007). On the other hand, others have failed to find effects of brief maternal separation on learning and memory tasks (Gibb and Kolb, 2005). Similarly, we previously found an effect of brief maternal separation but not handling on delay eyeblink conditioning (Wilber et al., 2007). Additionally, our finding that brief maternal separation specifically impaired recall of extinction with minimal effects on acquisition of the conditioned fear response is fairly consistent with previous studies that found no effect of brief separation on acquisition of cued conditioned fear (Pryce et al., 2003; Kosten et al., 2006) or retention of the conditioned fear response (Kosten et al., 2005). Thus, brief maternal separation differentially affects some types of learning and memory while sparing others, possibly dependent on how brief maternal separation differentially affects the neural substrates for various types of learning and memory.
Effects of Maternal Separation on NR1 Expression
In addition to the deficit in recall of extinction, briefly separated animals had reduced NR1 expression in layer VI of IL, whereas NR1 staining was unaltered in AC and PL, as well as in basolateral and central amygdala. Similarly, though maternal separation and isolation similarly failed to alter NMDA receptor binding in the amygdala of the 2 week old degus, it increased NMDA receptor binding in the mPFC in this precocial species (Ziabreva et al., 2000). Furthermore, our effect is consistent with recent findings that early postnatal shock exposure alters extinction of contextual fear conditioning in adults, and this effect is ameliorated by administration of an NMDA receptor facilitator during extinction (Matsumoto et al., 2008). Taken together, these results suggest that NMDA receptors in prefrontal cortex may be especially sensitive to early experience.
We found that NR1 expression is decreased in medial prefrontal cortex of adult rats that experience neonatal maternal separation. However, the NMDA receptor is a tetramer composed of pairs of NR1 subunits along with pairs of NR2A, B, or C subunits, which confer unique functional properties to the receptors. Thus, future studies should explore exactly how the NMDA receptor is changing by assessing other subunits and potential changes in the function of the receptor.
Effects of Maternal Separation on GR Expression
Brief maternal separation also altered GR expression, with increased expression in layer VI of PL and IL. This increase is consistent with previous demonstrations of increased GR expression in cerebellum and forebrain regions including the frontal cortex following brief neonatal maternal separation (Meaney et al., 1985; Ladd et al., 2004; Wilber et al., 2007). Additionally, GR’s have been reported to modulate learning and memory (Roozendaal et al., 1996; Kim et al., 2006).
The alterations in GR and NR1 expression in our study were specific to layer VI of mPFC. The specificity of this effect is particularly interesting because IL is particularly important for recall of extinguished fear (Morgan and LeDoux, 1995; Quirk et al., 2000; Milad and Quirk, 2002; Milad et al., 2004; Garcia et al., 2006; Burgos-Robles et al., 2007; Corcoran and Quirk, 2007). The reduced NR1 expression in IL in adulthood might contribute to the impaired recall of extinction, as infusion of an NMDA receptor antagonist into IL and PL mPFC also impairs recall of extinction (Burgos-Robles et al., 2007). Additionally, the alterations to mPFC were confined to layer VI. Layer VI is a major output layer, and IL sends more projections from layer VI to the amygdala than does either AC or PL (Vertes, 2002; Gabbott et al., 2005; Reynolds and Zahm, 2005), again suggesting a relationship between separation-induced changes in NMDA receptors in IL and deficits in recall of extinction.
Reduced Stress Responsiveness
The effect of brief maternal separation on recall of extinction is likely an effect on learning and not due to altered stress responsiveness modulating recall of extinction. However, given that brief maternal separation in adult male Long-Evans rats can decrease stress responsiveness and anxiety behavior, the extinction deficits we have found could be secondary to altered stress/anxiety behavior (Caldji et al., 2000). However, others have reported no effect of brief maternal separation on somatic response to footshock in male Sprague-Dawley rats (Kosten et al., 2005). Further, alteration in stress responsiveness would likely affect all phases of fear conditioning and extinction, whereas, we found that brief maternal separation only impaired recall of extinction, sparing fear conditioning, and initial extinction. Thus, it is unlikely that differences in stress responsiveness explain the deficit in recall of extinction of conditioned fear.
Possible Mechanisms
Given that our manipulation increases neonatal corticosterone, and perinatal corticosterone exposure inhibits neuronal growth and differentiation (Balazs and Cotterrell, 1972; Ardelenu and Sterescu, 1978; De Kloet et al., 1988), it is possible that the effects of brief maternal separation on adult learning and memory are mediated by neonatal increases in corticosterone. Additionally, increased GR expression in the prefrontal cortex could be present neonatally and thus may simulate long-term increased glucocorticoid exposure, which has been shown to impair prefrontally mediated behaviors (e.g., Lyons et al., 2000; Miracle et al., 2006). Thus, neonatal glucocorticoid exposure may be responsible for the alteration in GR and NR1 expression we observed, which may ultimately alter extinction of conditioned fear.
Alternatively, the observed effects may be mediated by maternal interactions. For example, brief maternal separation results in increased maternal licking and grooming (Lee and Williams, 1975; Lee and Williams, 1977; Liu et al., 1997; Pryce et al., 2001), and pups that received high, naturally occurring levels of maternal care show facilitated spatial learning and memory as adults (Liu et al., 2000). Thus, alterations in maternal care that result from brief separation may contribute to the changes in prefrontal cortex and extinction that we have observed.
Finally, brief maternal separation alters hippocampal GR expression (Meaney et al., 1985). The hippocampus provides direct inputs to prefrontal cortex (Swanson, 1981) and plays a modulatory role in some forms of fear conditioning (Selden et al., 1991; Anagnostaras et al., 2001). Thus, separation-induced changes in hippocampal inputs to the circuit may also play a role in the deficits in recall of extinction and alterations in prefrontal receptors that we have documented.
Regardless of the precise mechanism, we have shown that brief neonatal maternal separation affects extinction of conditioned fear, GR expression, and NMDA NR1 expression in layer VI of IL. This is the first demonstration that brief neonatal maternal separation affects recall of extinction and the structure that mediates this behavior. The specific impairment of extinction recall seen following brief neonatal separation is similar to the effect of adult stress on recall of extinction. Therefore, daily neonatal maternal separation, even when brief, can have quite selective and lasting effects on both brain and behavior.
ACKNOWLEDGEMENTS
The authors thank Samantha Singler for assistance with data collection.
REFERENCES
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. 2007. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology 32:256–266. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. 2001. Hippocampus and contextual fear conditioning: Recent controversies and advances.[see comment]. Hippocampus 11:8–17. [DOI] [PubMed] [Google Scholar]
- Applegate CD, Kapp BS, Underwood MD, McNall CL. 1983. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Behav 31:353–360. [DOI] [PubMed] [Google Scholar]
- Ardelenu A, Sterescu N. 1978. RNa and DNA synthesis in developing rat brain: Hormonal influences. Pychoneuroendocrinology 3:93–101. [DOI] [PubMed] [Google Scholar]
- Baker JD, Azorlosa JL. 1996. The NMDA antagonist MK801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci 110:618–620. [DOI] [PubMed] [Google Scholar]
- Balazs R, Cotterrell M. 1972. Effect of hormonal state on cell number and functional maturation of the brain. Nature 236:348–350. [DOI] [PubMed] [Google Scholar]
- Barad M, Gean P-W, Lutz B. 2006. The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry 60:322–328. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. 1972. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol 81:281–290. [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux JE. 1994. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. Exp Brain Res 98:275–286. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. 2006. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol Psychiatry 60:352–360. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. 2007. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron 53:871–880. [DOI] [PubMed] [Google Scholar]
- Calandreau L, Desmedt A, Decorte L, Jaffard R. 2005. A different recruitment of the lateral and basolateral amygdala promotes contextual or elemental conditioned association in Pavlovian fear conditioning. Learn Mem 12:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. 2000. The Effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology 22:219–229. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. 1986. Neuronal architecture in the rat central nucleus of the amygdala: A cytological, hodological, and immunocytochemical study. J Comp Neurol 246:478–499. [DOI] [PubMed] [Google Scholar]
- Collins DR, Pare D. 2000. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS+ and CS. Learn Mem 7:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. 2007. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci 27:840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Rosenfeld P, Van Ekelen AM, Sutanto W, Levine S. 1988. Stress, glucorticoids and development. Prog Brain Res 73:101–120. [DOI] [PubMed] [Google Scholar]
- De Jongh R, Geyer M, Olivier B, Groenink L. 2005. The effects of sex and neonatal maternal separation on fear-potentiated and light-enhanced startle. Behav Brain Res 161:190–196. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. 1992. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci 12:854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ. 1994. Acquisition of contextual pavlovian fear conditioning is blocked by application of an nmda receptor antagonist d, l-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav Neurosci 108:210–212. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. 2005. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492:145–177. [DOI] [PubMed] [Google Scholar]
- Garcia R, Chang C-h, Maren S. 2006. Electrolytic lesions of the medial prefrontal cortex do not interfere with long-term memory of extinction of conditioned fear. Learn Mem 13:14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb R, Kolb B. 2005. Neonatal handling alters brain organization but does not influence recovery from perinatal cortical injury. Behav Neurosci 119:1375–1383. [DOI] [PubMed] [Google Scholar]
- Hays WL. 1994. Statistics. Fort Worth: Harcourt Brace College Publishers. [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. 2002. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res 950:52–63. [DOI] [PubMed] [Google Scholar]
- Iwata J, Ledoux JE, Reis DJ. 1986. Destruction of intrinsic neurons in the lateral hypothalamus disrupts the classical conditioning of autonomic but not behavioral emotional responses in the rat. Brain Res 368:161–166. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. 2002. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav 73:131–140. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. 1996. Behavioral stress modifies hippocampal plasticity through N-Methyl-DAspartate receptor activation. Proc Natl Acad Sci USA 93:4750–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. 1993. Effects of amygdala, hippocampus, and periaqueductal gray lesions on shortand long-term contextual fear. Behav Neurosci 107:1093–1098. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Song EY, Kosten TA. 2006. Stress effects in the hippocampus: Synaptic plasticity and memory. Stress 9:1–11. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Lee HJ, Kim JJ. 2006. Early life stress impairs fear conditioning in adult male and female rats. Brain Res 1087:142–150. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Lee HJ, Kim JJ. 2007. Neonatal handling alters learning in adult male and female rats in a task-specific manner. Brain Res 1154:144–153. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Misserendino MJD, Bombace JC, Lee HJ, Kim JJ. 2005. Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behav Brain Res 157:235–244. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. 2004. Long-Term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol Psychiatry 55:367–375. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. 1988. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8:2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Choi J-S, Brown TH, Kim JJ. 2001. Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. J Neurosci 21:4116–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim JJ. 1998. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci 18:8444–8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Williams DI. 1975. Long term changes in nest condition and pup grouping following handling of rat litters. Dev Psychobiol 8:91–95. [DOI] [PubMed] [Google Scholar]
- Lee MH, Williams DI. 1977. A longitudinal study of mother-young interaction in the rat: The effects of infantile stimulation, diurnal rhythms, and pup maturation. Behaviour 63:241–266. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. 2000. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci 3:199–806. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, et al. 1997. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitaryadrenal responses to stress. Science 277:1659–1662. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Lopez JM, Yang C, Schatzberg AF. 2000. Stress-level cortisol treatment impairs inhibitory control of behavior in monkeys. J Neurosci 20:7816–7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. 1995. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 1:89–98. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Togashi H, Konno K, Koseki H, Hirata R, Izumi T, Yamaguchi T, et al. 2008. Early postnatal stress alters the extinction of context-dependent conditioned fear in adult rats. Pharmacol Biochem Behav 89:247–252. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. 2003. Designing Experiments and Analyzing Data: A Model Comparison Perspective. Mahwah, New Jersey: Lawrence Erlbaum Associates. [Google Scholar]
- McIntosh J, Anisman H, Merali Z. 1999. Short-and longperiods of neonatal maternal separation differentially effect anxiety and feeding in adult rats: Gender-dependent effects. Brain Res Dev Brain Res 113:97–106. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. 1997. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390:607–611. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Tatarewicz JE, Sapolsky RM. 1985. Early postnatal handling alters glucorticoid receptor concentrations in selected brain regions. Behav Neurosci 99:765–770. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Sapolsky RM, McEwen BS. 1985. The development of the glucocorticoid receptor system in the rat limbic brain. II. An autoradiographic study. Dev Brain Res 18:165–168. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Horvath KM, Nagy GM, Bohus B, Koolhaas JM. 1999. The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. J Neuroendocrinol 11:925–933. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. 2002. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420:70–74. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. 2004. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci 118:389–394. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. 2006. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem 85:213–218. [DOI] [PubMed] [Google Scholar]
- Miserendino MJD, Sananes CB, Melia KR, Davis M. 1990. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature 345:716–718. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. 1995. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109:681–688. [DOI] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. 1997. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci 111:683–691. [DOI] [PubMed] [Google Scholar]
- O’Donnell D, Larocque S, Seckl JR, Meaney MJ. 1994. Postnatal handling alters glucocorticoid, but not mineralocorticoid messenger RNA expression in the hippocampus of adult rats. Mol Brain Res 26:242–248. [DOI] [PubMed] [Google Scholar]
- Oliveira LC, Nobre MJ, Brandao ML, Landeira-Fernandez J. 2004. Role of amygdala in conditioned and unconditioned fear generated in the periaqueductal gray. Neuroreport 15:2281–2285. [DOI] [PubMed] [Google Scholar]
- Osborne MC, Verhovshek T, Sengelaub DR. 2007. Androgen regulates trkB Immunolabeling in spinal motoneurons. J Neurosci Res 85:303–309. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1998. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press, p249. [Google Scholar]
- Price JL, Amaral DG. 1981. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci 1:1242–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Feldon J. 2001. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Dev Psychobiol 38:239–251. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschend D, Nanz-Bahr NI, Feldon J. 2003. Comparison of the effects of early handling and early deprivation on conditioned stimulus, context, and spatial learning and memory in adult rats. Behav Neurosci 117:883–893. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. 1997. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron 19:613–624. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. 2000. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 20:6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. 2001. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci 4:724–731. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS. 2005. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci 25:11757–11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. 2001. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning.[erratum appears in J Neurosci 2002 Nov 15;22(22):1A]. J Neurosci 21:6889–6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Clugnet M-C, Bordi F, LeDoux JE. 1993. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci 107:444–450. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Carmi O, McGaugh JL. 1996. Adrenocortical suppression blocks the memory-enhancing effects of amphetamine and epinephrine. Proc Natl Acad Sci USA 93:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Griffith QK, Buranday J, De Quervain DJ-F, McGaugh JL. 2003. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: Dependence on the basolateral amygdala. Proc Natl Acad Sci USA 100:1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, de Ortiz SP, Quirk GJ. 2004. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci 24:5704–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaber JS, Kapp BS, Higgins GA, Rapp PR. 1982. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci 2:1424–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NRW, Everitt BJ, Jarrard LE, Robbins TW. 1991. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience 42:335–350. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Mathew PR. 1998. NMDA receptor antagonism in the lateral/basolateral but not central nucleus of the amygdala prevents the induction of facilitated learning in response to stress. Learn Mem 5:220–230. [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Seib TB, Levine S, Thompson RF. 1989. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science 244:224–226. [DOI] [PubMed] [Google Scholar]
- Swanson LW. 1981. A direct projection from Ammon’s horn to prefrontal cortex in the rat. Brain Res 217:150–154. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kimoto T, Tanabe N, Hattori T, Yasumatsu N, Kawato S. 2002. Corticosterone acutely prolonged Nmethyl-D-aspartate receptor-mediated Ca2+ elevation in cultured rat hippocampal neurons. J Neurochem 83: 1441–1451. [DOI] [PubMed] [Google Scholar]
- Uysal N, Ozdemir D, Dayi A, Yalaz G, Baltaci AK, Bediz CS. 2005. Effects of maternal deprivation on melatonin production and cognition in adolescent male and female rats. Neuroendocrinol Lett 26:555–560. [PubMed] [Google Scholar]
- Van de Kar L, Piechowski R, Rittenhouse P, Gray T. 1991. Amygdaloid lesions: Differential effect on conditioned stress and immobilization-induced increases in corticosterone and renin secretion. Neuroendocrinology 54:89–95. [DOI] [PubMed] [Google Scholar]
- Vertes RP. 2002. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol 442:163–187. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. 1999. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav 66:293–302. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Southwood C, Sokoloff G, Steinmetz JE, Wellman CL. 2007. Neonatal maternal separation alters adult eyeblink conditioning and glucocorticoid receptor expression in the interpositus nucleus of the cerebellum. Dev Neurobiol 67:1751–1764. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. 1999. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci 19:48RC41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Holscher C, Anwyl R, Rowan MJ. 1998. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proc Natl Acad Sci USA 95:3204–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-H, Huang C-C, Hsu K-S. 2004. Behavioral stress modifies hippocampal synaptic plasticity through corticosterone-induced sustained extracellular signal-regulated kinase/mitogen-activated protein kinase activation. J Neurosci 24:11029–11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziabreva I, Schnabel R, Braun K. 2000. Parental deprivation induces N-methyl-D-aspartate-receptor upregulation in limbic brain areas of Octodon degus: Protective role of the maternal call. Neural Plast 7:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]