Abstract
Background
Olfactory and gustatory dysfunction has been reported as characteristic symptoms of coronavirus disease 2019 (COVID-19). This study evaluated olfactory and gustatory dysfunction in mild COVID-19 patients using validated assessment methods.
Methods
A prospective surveillance study was conducted for mild COVID-19 patients who were isolated at the Gyeonggi International Living and Treatment Support Center (LTSC), Korea. Olfactory function was assessed using the Korean version of the Questionnaire of Olfactory Disorders (QOD) and Cross-Cultural Smell Identification Test (CC-SIT). Gustatory function was assessed using an 11-point Likert scale and 6-n-propylthiouracil, phenylthiocarbamide, and control strips. All patients underwent nasal and oral cavity endoscopic examination.
Results
Of the 62 patients at the LTSC, 15 patients (24.2%) complained of olfactory or gustatory dysfunction on admission. Four of 10 patients who underwent functional evaluation did not have general symptoms and 2 were asymptomatic. The mean short version of QOD-negative statements and QOD-visual analogue scale scores were 13 ± 6 and 4.7 ± 3.6, respectively. The mean CC-SIT score was 8 ± 2. No patients showed anatomical abnormalities associated with olfactory dysfunction on endoscopic examination. The mean Likert scale score for function was 8 ± 2, and there were no abnormal lesions in the oral cavity of any patient.
Conclusions
The prevalence of olfactory and gustatory dysfunction was 24.2% in mild COVID-19 patients. All patients had hyposmia due to sensorineural olfactory dysfunction, which was confirmed using validated olfactory and gustatory evaluation methods and endoscopic examination. Olfactory and gustatory dysfunction may be characteristic indicators of mild COVID-19.
Keywords: COVID-19, SARS-CoV-2, Olfaction Disorders, Taste Disorders
Graphical Abstract
INTRODUCTION
Since the first report of coronavirus disease 2019 (COVID-19) in Wuhan, China in December 2019, the disease has become widespread and global threat.1 During this ongoing COVID-19 pandemic situation, several studies regarding virology, clinical characteristics, and transmission have been reported. The main clinical manifestations of COVID-19 are symptoms of upper and lower respiratory infection, including fever, cough, sputum, and fatigue, and range in severity, from asymptomatic to severe respiratory failure.2 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is different from other viruses in Coronaviridae because it can cause asymptomatic infections and show high viral loads during the early phase, which leads difficulty in controlling the disease.3,4 This led us to consider methods for the early diagnosis of asymptomatic or mild infection, which account for the majority of COVID-19 infections.
Other characteristic symptoms of COVID-19 infection are olfactory and gustatory dysfunction. Although olfactory and gustatory dysfunction were initially not recognized as symptoms of COVID-19, they were seen in a case series of confirmed patients and data from Korea have reported anosmia or ageusia in 30% of 2,000 patients.5,6 Subsequent studies have reported that olfactory and gustatory dysfunction may be characteristic symptoms of COVID-19 infection, but there are limitations in assessments by validated methods.6,7,8,9,10,11 In a study by Moein et al.,12 the University of Pennsylvania Smell Identification Test (UPSIT) was used in the assessment of smell dysfunction, and olfactory and gustatory dysfunction were identified in 90% (59/60) and 25% (15/60) of COVID-19 patients, respectively. However, the olfactory test was performed in the recovery period and the severity of the disease was heterogeneous. Therefore, this study was designed to assess olfactory and gustatory dysfunction in mild COVID-19 patients using validated olfactory and gustatory evaluation methods and endoscopic examination.
METHODS
Study design, study population, and data collection
This prospective surveillance study included mild COVID-19 patients who were isolated at Gyeonggi International Living and Treatment Support Center (LTSC) after confirmed diagnosis of COVID-19 by SARS-CoV-2 real-time reverse transcription polymerase chain reaction (RT-PCR) using respiratory samples; all patients were interviewed daily regarding changes in olfactory and gustatory symptoms and their medical records were reviewed. All patients were Korean nationals who had returned from foreign countries and quarantined on arrival at the airport in April 2020. The LTSC is a Korean community-based isolation and treatment facility for mild COVID-19 patients. All patients were assessed for symptoms including olfactory and gustatory dysfunction at the time of LTSC admission. Olfactory and gustatory evaluations and nasal endoscopic evaluation via portable endoscopic smartphone systems were performed in patients complaining of olfactory and taste disorders. The olfactory and gustatory function tests and nasal endoscopic examinations were performed by one rhinologist who was wearing personal protective equipment.
Olfactory and gustatory function evaluation
Subjective olfactory function was evaluated using the Korean version of the Questionnaire of Olfactory Disorders (QOD).13 The QOD was originally developed by Thomas Hummel and Johannes Frasnelli in 2005, and the Korean version was validated by Choi et al. in 2018.13,14 For subdomains of the QOD, we performed assessments using the QOD-visual analogue scale (QOD-VAS) and the short version of QOD-negative statements (sQOD-NS). The QOD-VAS, composed of five questions, is a measure to record the severity of symptoms using the VAS scale for each question, and the validated Korean version uses an 11-point Likert scale instead of the VAS scale. The sQOD-NS is composed of seven questions including social, eating, annoyance, and anxiety and assessed using a 4-point Likert scale (0–3). A higher score means better olfactory specific QOL.9 Additionally, we performed the Cross-Cultural Smell Identification Test (CC-SIT; Sensonics International, Haddon Heights, NJ, USA) for odor identification as an objective measurement of olfactory function.15 The CC-SIT is composed of 12 items (banana, chocolate, cinnamon, gasoline, lemon, onion, paint thinner, pineapple, rose, soap, smoke, and turpentine) based on items from the UPSIT, with the answer for each question chosen from a possible of four answers. According to the criteria, anosmia was defined as a score of 0–4, hyposmia as a score of 5–10, and normosmia as a score of 11–12.13 Subjective gustatory function was evaluated using an 11-point Likert scale (0–10), and objective gustatory function was evaluated using 6-n-propylthiouracil (PROP), phenylthiocarbamide (PTC), and control strips (Sensonics International). PROP and PTC showed a significant positive relationship with other bitter compounds used for detecting bitter compounds in screening for ageusia.16,17,18
Endoscopic examination of nasal cavity
Endoscopic nasal and oral cavity examinations were performed using a 4-mm 30° endoscope, which was connected to a Galaxy S8 smartphone (Samsung Electronics, Suwon, Korea) using the Smart Scope system (Karl Storz, Tuttlingen, Germany). During the endoscopic examination, we checked for sinonasal diseases such as chronic rhinosinusitis with or without nasal polyps, deviated nasal septum, nasal turbinate hypertrophy, and any tumorous condition. The olfactory fissure area was also specifically examined.
Ethics statement
This study protocol was approved by the Institutional Review Board of the Korea University Ansan Hospital (2020AS0122). Written informed consent was obtained from each study participant.
RESULTS
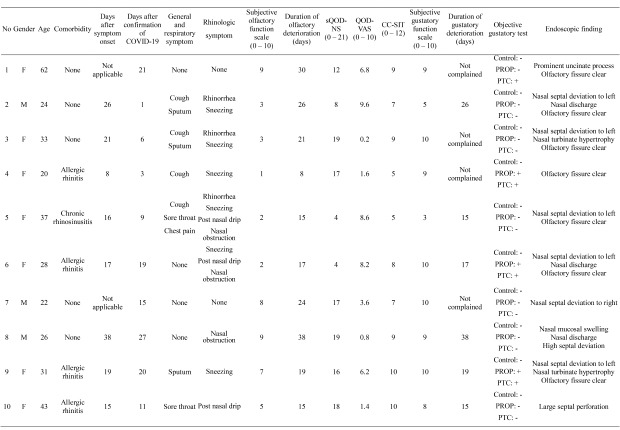
Sixty-two mild COVID-19 patients were assessed on April 28, 2020. At the time of admission, cough, sore throat, sputum, and headache were identified in 20 (32.3%), 19 (30.6%), 9 (14.5%), and 9 (14.5%) patients, respectively. Rhinorrhea and nasal congestion were identified 10 (16.1%) and 7 (11.2%) patients, respectively. Fever was identified in 3 patients, and 16 patients were asymptomatic. Fifteen patients (24.2%) complained of acute olfactory dysfunction. A total of 10 patients participated in this study, except for 5 patients whose symptoms improved after admission. The demographic and clinical characteristics of the study population are shown in Table 1. The mean patient age was 33 ± 12 years (interquartile range [IQR], 20–62 years), and 7 of 10 patients were female (70%). At initial surveillance, all the 10 patients reported no fever and 2 patients had no symptoms, except olfactory or gustatory symptoms. Six patients complained of respiratory symptoms including cough, sputum, and sore throat. Of the 10 patients, 4 patients complained of both subjective olfactory and gustatory symptoms, and 6 patients had only olfactory symptoms without gustatory dysfunction. The mean time between the diagnosis of COVID-19 and the olfactory evaluation was 12 ± 9 days (IQR, 7–19 days). All patients had no previously experienced olfactory or gustatory dysfunction before COVID-19, although 4 patients had a history of allergic rhinitis and 1 patient had chronic rhinosinusitis.
Table 1. Clinical characteristics of mild COVID-19 patients with olfactory or gustatory dysfunction.
| No. | Sex | Age | Comorbidity | Days after symptom onset | Days after confirmation of COVID-19 | General and respiratory symptom | Rhinologic symptom | Subjective olfactory function scale (0–10) | Duration of olfactory dysfunction, day | sQOD-NS (0–21) | QOD-VAS (0–10) | CC-SIT (0–12) | Subjective gustatory function scale (0–10) | Duration of gustatory dysfunction, day | Objective gustatory test | Endoscopic finding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 | None | Not applicable | 21 | • None | • None | 9 | 30 | 12 | 6.8 | 9 | 9 | Not complained | Control: − | • Prominent uncinate process |
| PROP: − | • Olfactory fissure clear | |||||||||||||||
| PTC: + | ||||||||||||||||
| 2 | M | 24 | None | 26 | 1 | • Cough | • Rhinorrhea | 3 | 26 | 8 | 9.6 | 7 | 5 | 26 | Control: − | • Nasal septal deviation to left |
| • Sputum | • Sneezing | PROP: − | • Nasal discharge | |||||||||||||
| PTC: − | • Olfactory fissure clear | |||||||||||||||
| 3 | F | 33 | None | 21 | 6 | • Cough | • Rhinorrhea | 3 | 21 | 19 | 0.2 | 9 | 10 | Not complained | Control: − | • Nasal septal deviation to left |
| • Sputum | • Sneezing | PROP: − | • Nasal turbinate hypertrophy | |||||||||||||
| PTC: − | • Olfactory fissure clear | |||||||||||||||
| 4 | F | 20 | Allergic rhinitis | 8 | 3 | • Cough | • Sneezing | 1 | 8 | 17 | 1.6 | 5 | 9 | Not complained | Control: − | • Olfactory fissure clear |
| PROP: + | ||||||||||||||||
| PTC: + | ||||||||||||||||
| 5 | F | 37 | Chronic rhinosinusitis | 16 | 9 | • Cough | • Rhinorrhea | 2 | 15 | 4 | 8.6 | 5 | 3 | 15 | Control: − | • Nasal septal deviation to left |
| • Sore throat | • Sneezing | PROP: − | • Olfactory fissure clear | |||||||||||||
| • Chest pain | • Post-nasal drip | PTC: − | ||||||||||||||
| • Nasal obstruction | ||||||||||||||||
| 6 | F | 28 | Allergic rhinitis | 17 | 19 | • None | • Sneezing | 2 | 17 | 4 | 8.2 | 8 | 10 | 17 | Control: − | • Nasal septal deviation to left |
| • Post-nasal drip | PROP: + | • Nasal discharge | ||||||||||||||
| • Nasal obstruction | PTC: + | • Olfactory fissure clear | ||||||||||||||
| 7 | M | 22 | None | Not applicable | 15 | • None | • None | 8 | 24 | 17 | 3.6 | 7 | 10 | Not complained | Control: − | • Nasal septal deviation to right |
| PROP: − | ||||||||||||||||
| PTC: − | ||||||||||||||||
| 8 | M | 26 | None | 38 | 27 | • None | • Nasal obstruction | 9 | 38 | 19 | 0.8 | 9 | 9 | 38 | Control: − | • Nasal mucosal swelling |
| PROP: − | • Nasal discharge | |||||||||||||||
| PTC: − | • High septal deviation | |||||||||||||||
| 9 | F | 31 | Allergic rhinitis | 19 | 20 | • Sputum | • Sneezing | 7 | 19 | 16 | 6.2 | 10 | 10 | 19 | Control: − | • Nasal septal deviation to left |
| PROP: + | • Nasal turbinate hypertrophy | |||||||||||||||
| PTC: + | • Olfactory fissure clear | |||||||||||||||
| 10 | F | 43 | Allergic rhinitis | 15 | 11 | • Sore throat | • Post-nasal drip | 5 | 15 | 18 | 1.4 | 10 | 8 | 15 | Control: − | • Large septal perforation |
| PROP: − | ||||||||||||||||
| PTC: − |
COVID-19 = coronavirus disease 2019, sQOD-NS = short version of QOD-negative statements, QOD-VAS = QOD-visual analogue scale, CC-SIT = Cross-Cultural Smell Identification Test, PROP = 6-n-propylthiouracil, PTC = phenylthiocarbamide.
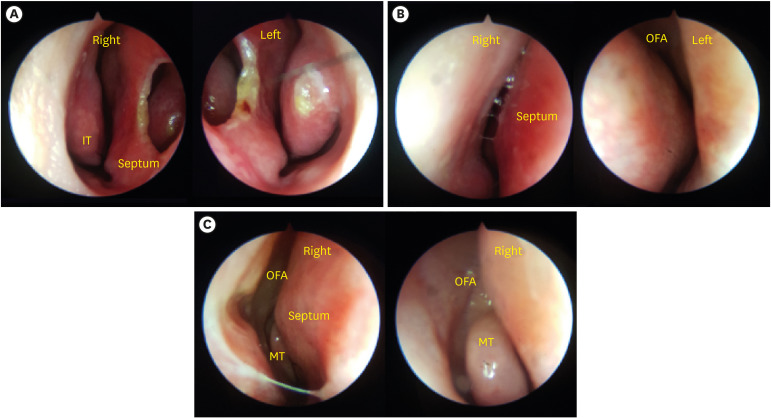
The mean sQOD-NS and QOD-VAS scores were 13 ± 6 (IQR, 9–18) and 4.7 ± 3.6 (range, 1.5–7.9), respectively. The mean CC-SIT score was 8 ± 10 (IQR, 7–9), and all patients were classified as having hyposmia according to the CC-SIT. No patients had signs of suspicious sinusitis or nasal polyps in nasal endoscopic findings. A large septal perforation was observed in 1 patient (patient number 10) and the olfactory fissure area could not be examined in 1 patient (patient number 8) because of high septal deviation. Nine patients who underwent endoscopic evaluation of the olfactory fissure area showed no anatomical abnormalities (Fig. 1).
Fig. 1. Smart portable nasal endoscopic system findings. (A) Large septal perforation prior to septal cartilage harvest for rhinoplasty. (B) Olfactory fissure is obscured by high septal deviation. (C) Right olfactory fissure area is well observed.
IT = inferior turbinate, OFA = olfactory fissure area, MT = middle turbinate.
The mean Likert scale score for gustatory function was 8 ± 2 (IQR, 8–10). In the initial questionnaire, 4 patients reported gustatory dysfunction, but an objective gustatory function test showed abnormal findings in 6 patients. No patients showed abnormal lesions of the oral cavity or tongue in the oral endoscopic examinations.
DISCUSSION
To our knowledge, this study is the first to assess both olfactory and gustatory function with validated methods, with simultaneous nasal and oral endoscopic examination in mild COVID-19 patients. We were able to evaluate whether olfactory and gustatory dysfunction were associated with anatomical abnormality using endoscopy. In our study, the prevalence of olfactory dysfunction was 24.2% in mild COVID-19 patients and all patients were classified as having hyposmia. In the recent studies by Yan et al.,10 Moein et al.,12 and Lee et al.,19 smell disturbances were reported in 16% (367/2,342), 68% (40/59), and 90% (59/90) of COVID-19 patients, respectively. In our study, the prevalence of olfactory dysfunction was markedly low, probably because the study population consisted of only mild COVID-19 patients. Further, 4 of 10 patients who complained of olfactory or gustatory dysfunction had no systemic symptoms and 2 patients were asymptomatic, which suggests olfactory or gustatory dysfunction may be possible indicators of COVID-19.
The perception of smell requires stimulation of olfactory neurons in the olfactory epithelium, which are located in the olfactory fissure. Olfactory disorders are largely divided into two types—quantitative and qualitative disorders.18 Quantitative disorders refer to decreases in the degree of smell and are subdivided according to severity into hyposmia (reduced olfaction) and anosmia (absent olfaction). Qualitative olfactory disorders refer to distortion in odor quality and are subdivided into parosmia (distortion of an odor perception) and phantosmia (perception of an odor in the absence of a stimulus).20 Currently, the olfactory disorders in COVID-19 have been reported to be quantitative disorders. Quantitative olfactory disorders have two etiologies, which show either conductive or sensorineural olfactory dysfunction. Conductive olfactory dysfunction occurs when the smell cannot reach the olfactory epithelium because of nasal mucosal edema after viral infection or underlying chronic rhinosinusitis, with or without nasal polyps. In our study, we were able to exclude conductive olfactory dysfunction by examining the olfactory fissure area using nasal endoscopy in all patients, except 1 patient who could not be tested because of high septal deviation. Thus, we concluded that all patients had the sensorineural type of olfactory dysfunction. Sensorineural olfactory dysfunction is a common form of post-infectious olfactory dysfunction (PIOD), which occurs after viral infection of the upper respiratory tract, including infection by coronaviruses.21 It may involve the olfactory neurons related to the central nervous system or non-neuronal olfactory epithelial cells.10,22 When the viral infection occurs in olfactory neurons, permanent olfactory dysfunction may occur and even if there is recovery, it may take a long time. Therefore, the location of olfactory neurons with sensorineural olfactory dysfunction can be inferred through the clinical course. Yan et al.10 have suggested that SARS-CoV-2 invades the non-neuronal olfactory epithelium because the majority of patients recover rapidly. However, they assessed olfaction with a non-objective method without long-term follow-up. Our study complemented previous studies by performing objective assessment using both olfactory and gustatory function tests. Further studies and long-term follow-up observations are needed.
The limitations of our study are as follows. First, the study population was small; hence, the results might be biased. Anosmia was not identified by the CC-SIT in this study. When we consider random responding, the expected minimum score is only 3 (multiple choice from 4 answers means that 25% of all answers should be correct).15 Currently, the most validated olfactory function test is to assess the odor threshold, discrimination, and identification and then sum the results of the 3 tests to give the threshold-discrimination-identification (TDI) score. However, this test kit is too bulky and non-disposable; hence, it is difficult to use for isolated COVID-19 patients. Thus, the CC-SIT was optimal for testing COVID-19 patients as it can be disposed and quickly performed. Second, we assessed objective gustatory function using PTC and PROP strips. Although these strips were used for screening tests for gustatory function, approximately 15%–30% of normal people are genetically non-tasters for these compounds.23,24,25 The possibility of underestimation must be considered. Furthermore, we could only detect patients did not have the sense of taste with this test; hence, we could not evaluate the mildly deteriorated patients. Third, the study population was composed of mild COVID-19 patients and has limitations in the application of the results. Further research is needed to reflect the disease severity.
In conclusion, the prevalence of olfactory dysfunction was 24.2% in mild COVID-19 patients and all patients had hyposmia due to sensorineural olfactory dysfunction, which was confirmed using validated olfactory and gustatory evaluation methods and endoscopic examination. Of the patients who complained of olfactory and taste dysfunction, 20% had no symptoms other than olfactory and taste disorders, and 40% had no systemic symptoms; this means that olfactory and gustatory dysfunction may be characteristic indicators of asymptomatic or mild COVID-19. Further studies are needed on the association of disease severity and long-term outcomes in COVID-19 patients with olfactory and gustatory dysfunction in more patients.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Seo MY, Lee SH, Choi WS.
- Data curation: Seo MY, Hwang SJ.
- Formal analysis: Choi HK, Jeon JH.
- Methodology: Choi HK, Jeon JH.
- Software: Choi HK, Jeon JH.
- Validation: Sohn JW, Park DW.
- Writing - original draft: Seo MY, Seok H.
- Writing - review & editing: Seo MY, Seok H, Lee SH, Choi WS.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020;58(3):299–301. doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 6.Russell B, Moss C, Rigg A, Hopkins C, Papa S, Van Hemelrijck M. Anosmia and ageusia are emerging as symptoms in patients with COVID-19: what does the current evidence say? Ecancermedicalscience. 2020;14:ed98. doi: 10.3332/ecancer.2020.ed98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 8.Eliezer M, Hautefort C, Hamel AL, Verillaud B, Herman P, Houdart E, et al. Sudden and complete olfactory loss of function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;146(7):674–675. doi: 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- 9.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JM, Lee SJ. Olfactory and gustatory dysfunction in a COVID-19 patient with ankylosing spondylitis treated with etanercept: case report. J Korean Med Sci. 2020;35(21):e201. doi: 10.3346/jkms.2020.35.e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10(8):944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi WR, Jeong HY, Kim JH. Reliability and validity of the Korean version of the Questionnaire of Olfactory Disorders. Int Forum Allergy Rhinol. 2018;8(12):1481–1485. doi: 10.1002/alr.22186. [DOI] [PubMed] [Google Scholar]
- 14.Frasnelli J, Hummel T. Olfactory dysfunction and daily life. Eur Arch Otorhinolaryngol. 2005;262(3):231–235. doi: 10.1007/s00405-004-0796-y. [DOI] [PubMed] [Google Scholar]
- 15.Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT) Laryngoscope. 1996;106(3 Pt 1):353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Chang WI, Chung JW, Kim YK, Chung SC, Kho HS. The relationship between phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP) taster status and taste thresholds for sucrose and quinine. Arch Oral Biol. 2006;51(5):427–432. doi: 10.1016/j.archoralbio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Leach EJ, Noble A. Comparison of bitterness of caffeine and quinine by a time–intensity procedure. Chem Senses. 1986;11(3):339–345. [Google Scholar]
- 18.Gent JF, Bartoshuk LM. Sweetness of sucrose, neohesperidin dihydrochalcone, and saccharin is related to genetic ability to taste the bitter substance 6-n-propylthiouracil. Chem Senses. 1983;7(3-4):265–272. [Google Scholar]
- 19.Lee Y, Min P, Lee S, Kim SW. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020;35(18):e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitcroft KL, Hummel T. Clinical diagnosis and current management strategies for olfactory dysfunction: a review. JAMA Otolaryngol Head Neck Surg. 2019;145(9):846. doi: 10.1001/jamaoto.2019.1728. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 23.Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15(5):497–503. doi: 10.1007/s00520-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 24.Hall MJ, Bartoshuk LM, Cain WS, Stevens JC. PTC taste blindness and the taste of caffeine. Nature. 1975;253(5491):442–443. doi: 10.1038/253442a0. [DOI] [PubMed] [Google Scholar]
- 25.Cavazzana A, Knaapila A, Roßkopf F, Han P, Hummel T. Detection thresholds for quinine, PTC, and PROP measured using taste strips. Eur Arch Otorhinolaryngol. 2019;276(3):753–759. doi: 10.1007/s00405-018-05266-8. [DOI] [PubMed] [Google Scholar]