Abstract
Background
Salt, a common environmental stress factor, inhibits plant growth and reduces yields. Melatonin is a pleiotropic molecule that regulates plant growth and can alleviate environmental stress in plants. All previous research on this topic has focused on the use of melatonin to improve the relatively low salt tolerance of glycophytes by promoting growth and enhancing antioxidant ability. It is unclear whether exogenous melatonin can increase the salt tolerance of halophytes, particularly recretohalophytes, by enhancing salt secretion from the salt glands.
Results
To examine the mechanisms of melatonin-mediated salt tolerance, we explored the effects of exogenous applications of melatonin on the secretion of salt from the salt glands of Limonium bicolor (a kind of recretohalophyte) seedlings and on the expression of associated genes. A pretreatment with 5 μM melatonin significantly improved the growth of L. bicolor seedlings under 300 mM NaCl. Furthermore, exogenous melatonin significantly increased the dry weight and endogenous melatonin content of L. bicolor. In addition, this treatment reduced the content of Na+ and Cl− in leaves, but increased the K+ content. Both the salt secretion rate of the salt glands and the expression level of genes encoding ion transporters (LbHTK1, LbSOS1, LbPMA, and LbNHX1) and vesicular transport proteins (LbVAMP721, LbVAP27, and LbVAMP12) were significantly increased by exogenous melatonin treatment. These results indicate that melatonin improves the salt tolerance of the recretohalophyte L. bicolor via the upregulation of salt secretion by the salt glands.
Conclusions
Our results showed that melatonin can upregulate the expression of genes encoding ion transporters and vesicle transport proteins to enhance salt secretion from the salt glands. Combining the results of the current study with previous research, we formulated a novel mechanism by which melatonin increases salt secretion in L. bicolor. Ions in mesophyll cells are transported to the salt glands through ion transporters located at the plasma membrane. After the ions enter the salt glands, they are transported to the collecting chamber adjacent to the secretory pore through vesicle transport and ions transporter and then are secreted from the secretory pore of salt glands, which maintain ionic homeostasis in the cells and alleviate NaCl-induced growth inhibition.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12870-020-02703-x.
Keywords: Ion homeostasis, Limonium bicolor, Melatonin, Salt gland, Salt secretion
Background
Plants are challenged by various biotic and abiotic stresses throughout their growth and development [1–3]. Salt stress is a ubiquitous abiotic environmental stress that negatively affects plants by inducing first osmotic stress and then ionic stress and oxidative stress, resulting in growth retardation, yield losses, and plant death [4–7].
Salt-tolerant plants called halophytes have evolved a variety of mechanisms that allow them to meet this challenge and limit the adverse effects of salt on their metabolism [8]. One type of halophyte, the recretohalophytes, secrete excess salt from salt glands or salt bladders located mainly on their leaves, thereby ensuring a lower Na+ content in their shoots and avoiding the excessive accumulation of ions in their cells, which protects against salt-associated damage [9, 10].
Although salt glands play a key role in the salt tolerance of plants, the mechanism of salt secretion is unclear. Three mechanisms have been proposed to explain this process: an osmotic mechanism [11], exocytosis [12–14], and transfer systems similar to fluid flow in animals [15]. Yuan et al. [9] proposed that membrane-bound translocating proteins such as plasma membrane (PM) H+-ATPase (PMA) and the Na+/H+ antiporter (SOS1, salt overly sensitive) participate in the salt secretion process. PMA produces a H+ electrochemical potential gradient that drives the Na+/H+ antiporter to expel excess Na+ from the cells, which could cause newly acquired ions to enter the salt gland [16, 17]. Ding et al. [17] showed that salt secretion from the salt glands of Limonium bicolor, a typical recretohalophyte with a 16-cell salt gland, may be an ion-active transport process, in which PMA and SOS1 play a coordinated role. The increased salt secretion function in the recretohalophyte Avicennia marina is accompanied by the upregulated expression of genes encoding PMA, SOS1, NHX1 (tonoplast Na+/H+ antiporter 1), and vacuolar H+-ATPase subunit c (VHA-c1) [18]. Tan et al. [19] used an immunoblot analysis to demonstrate that the protein levels of PMA and NHX1 significantly increased as the rate of salt secretion increased. An RNA-sequencing (RNA-seq) study revealed that the candidate genes encoding membrane-bound ion translocating proteins were associated with the salt secretion function of the salt gland in the recretohalophyte Reaumuria trigyna [20]. In the recretohalophyte Mesembryanthemum crystallinum L., the increased NHX and H+-ATPase (V-ATPase) activity of the vacuoles increased the Na+ content in the salt bladder [21]. These results indicate that ion transporters play an important role during salt gland secretion.
There is also substantial evidence supporting the role of vesicle transport in salt gland secretion [9]. In Limonium Mill., many small transport vesicles were observed in the secretory cells of the salt glands [12, 22, 23], suggesting that these vesicles may participate in salt gland secretion. Thomson and Liu [24] treated Tamarix aphylla with rubidium and observed that the resulting electron-dense region was concentrated in small vesicles. A large number of small vesicles are present in the salt glands of L. bicolor, typically fusing with the PM [22]. When L. bicolor leaves were treated with brefeldin A, a vesicle secretion inhibitor, the secretion rate of the salt glands was severely inhibited [22]. Similarly, Flowers et al. [25] reported results that supported the hypothesis that vesicle transport plays a key role in salt gland secretion, although they also indicated that transporters may be simultaneously involved. Vesicle transport consumes less energy than transport proteins, and ions can accumulate to a high concentration in the vesicles [26]. Recently, LbSYP61 (syntaxin from plants 61, a vesicle transport protein) was shown to directly regulate salt secretion levels from the salt glands of the recretohalophyte L. bicolor [26].
Many factors influence salt secretion; for example, both the salt secretion ability and the density of salt glands of Glaux maritima L. increased under salt stress [27]. The secretion ability of L. bicolor salt glands was promoted by Ca2+ and K+ under salt stress [17], whereas the stress-induced accumulation of K+ in L. bicolor salt gland cells (mainly in the nucleus and cytoplasm) increased their salt secretion ability [22]. The detailed mechanisms underlying these changes in secretion are not clear, however.
Melatonin (N-acetyl-5-methoxy-tryptamine) was first discovered in plants in 1995 [28, 29], and has since been identified in a wide variety of plant species. Over the past few years, many functions of melatonin have been revealed in different plant tissues and biological processes [30–32]. Melatonin regulates plant growth and development [33–35], and plays an important role in plant resistance to stresses, especially salt stress [31], drought stress [36], cold stress [37], oxidation stress [38, 39], and nutrient deficiencies [40]. The melatonin content increases in plants under stress, improving the plant’s ability to adapt to these challenges [41].
Melatonin-related regulatory mechanisms have been reported in some studies [42], but more research is needed on the regulation and function of melatonin in plant stress tolerance. All previous research on this topic has focused on the use of melatonin to improve the relatively low salt tolerance of glycophytes by promoting growth and enhancing antioxidant ability [7]. It is unclear whether exogenous melatonin can increase the salt tolerance of halophytes, particularly recretohalophytes, by enhancing salt secretion from the salt glands. If exogenous melatonin increases the salt-secreting ability of recretohalophytes, whether it is by regulating the ion transporters and vesicular transport proteins. With its high salt tolerance and ornamental and medicinal value, L. bicolor is a typical pioneer plant that grows on saline alkali land and has a wide planting range [9]. In this study, we examined the effects of melatonin on the salt tolerance of L. bicolor, in an effort to elucidate the molecular mechanisms involved in its salt secretion.
Results
Melatonin alleviates the NaCl-induced growth inhibition of L. bicolor seedlings
The growth of L. bicolor seedlings was significantly inhibited by a 300 mM NaCl treatment; compared with the control (0 mM NaCl), the salt-treated seedlings had significantly lower dry weights (Fig. 1). Exogenous melatonin (5 μM) significantly promoted seedling growth, with treated seedlings reaching a greater biomass than the controls, both under the 0 or 300 mM NaCl treatments. Exogenous melatonin increased the shoot dry weight of the seedlings exposed to 0 mM NaCl by 17.33%, whereas it increased the dry weight of the salt-treated seedlings by 20.9%.
Fig. 1.
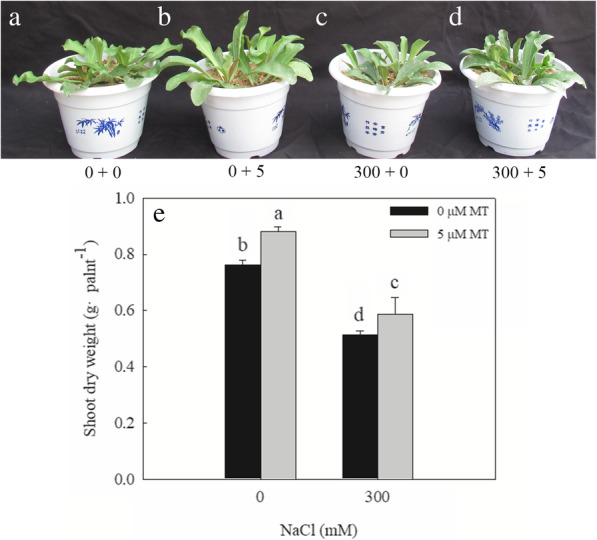
Effects of exogenous melatonin on the growth of L. bicolor seedlings under salt stress. a-d, the L. bicolor seedlings, after the six-leaf seedlings with NaCl and melatonin (MT) treatment for 15 consecutive days. a, 0 + 0, 0 mM NaCl+ 0 μM MT; b, 0 + 5, 0 mM NaCl+ 5 μM MT; c, 300 + 0, 300 mM NaCl+ 0 μM MT; D, 300 + 5, 300 mM NaCl+ 5 μM MT; e, the shoot dry weight of L. bicolor seedlings. The values are the average of five biological repeats ± standard deviation. According to Duncan’s multi-range tests, bars that there were significant differences at P < 0.05 are labeled with different letters
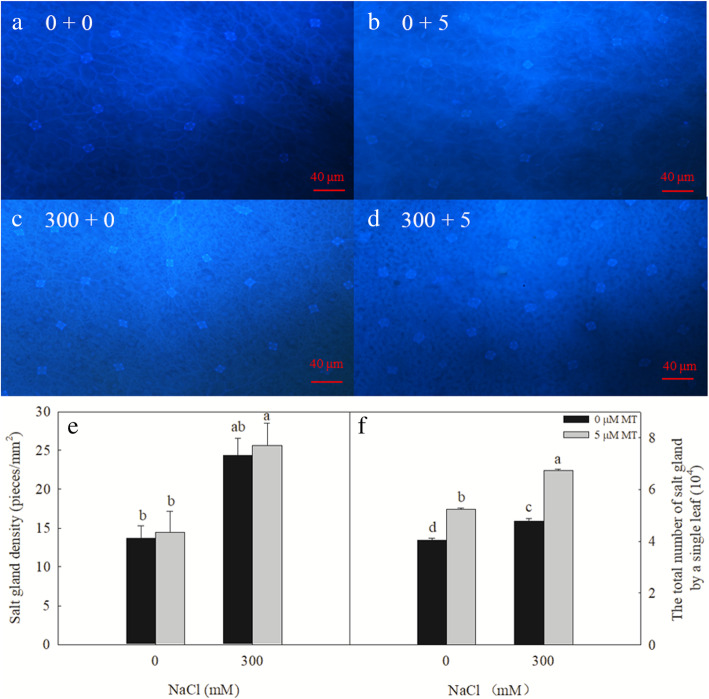
Melatonin significantly increases the number of salt glands
The 300 mM NaCl treatment significantly increased the density of salt glands produced on the L. bicolor leaves, and the total number of salt glands on a single leaf. The exogenous melatonin treatment had no significant effect on the density of the salt glands on the leaves of plants subjected to either 0 or 300 mM NaCl (Fig. 2a–e). In contrast, the melatonin treatment significantly increased the total number of salt glands produced by each leaf (25.5 and 41.2% increases in the 0- and 300-mM NaCl-treated plants, respectively; Fig. 2f).
Fig. 2.
Effects of NaCl and melatonin on the density of salt gland and total number of salt glands of a single leaf. a-d, the density of salt glands of L. bicolor leaves, a, 0 + 0, 0 mM NaCl+ 0 μM MT; b, 0 + 5, 0 mM NaCl+ 5 μM MT; c, 300 + 0, 300 mM NaCl+ 0 μM MT; d, 300 + 5, 300 mM NaCl+ 5 μM MT; e, the density of salt glands of L. bicolor leaves; f, total number of salt glands of a single leaf. Bar = 40 μm. Arrows indicate salt glands. The values are the average of five biological repeats ± standard deviation. According to Duncan’s multi-range tests, bars that there were significant differences at P < 0.05 are labeled with different letters
NaCl and exogenous melatonin increase the endogenous content of melatonin in L. bicolor leaves
The endogenous melatonin content of the L. bicolor leaves significantly increased under the 300 mM NaCl treatment (Fig. 3). The exogenous melatonin treatment significantly increased the endogenous melatonin content of the L. bicolor leaves, with increases of 97.5% in the 0 mM NaCl-treated plants and 19.1% in the 300 mM NaCl-treated plants.
Fig. 3.
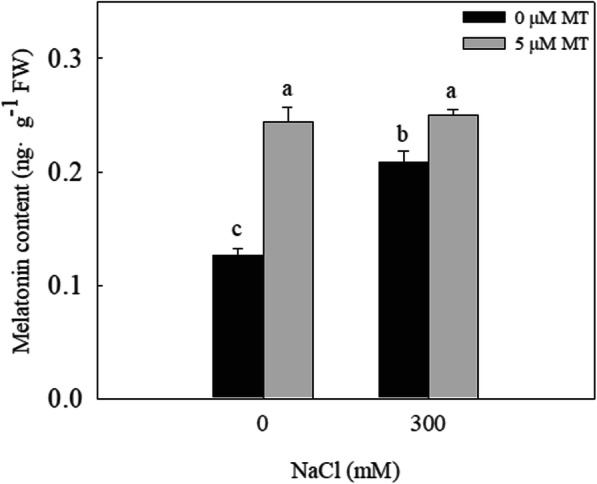
The melatonin content of L. bicolor leaves under melatonin and NaCl treatment. The values are the average of five biological repeats ± standard deviation. According to Duncan’s multi-range tests, bars that there were significant differences at P < 0.05 are labeled with different letters
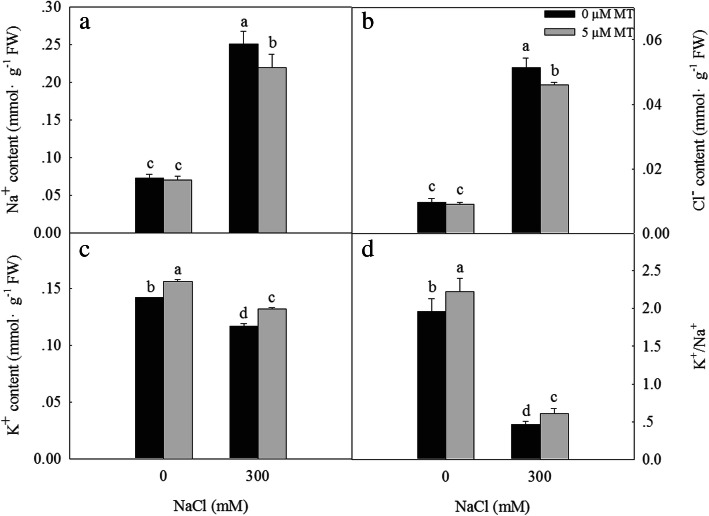
Melatonin improves ionic homeostasis under NaCl stress
Salt stress leads to an ionic imbalance in plants by increasing the content of Na+ and decreasing the content of K+, resulting in the inhibition of plant growth and development, and possibly even plant death. Treatment with 300 mM NaCl significantly increased the Na+ (Fig. 4a) and Cl− (Fig. 4b) contents of the L. bicolor leaves, while significantly decreasing the K+ (Fig. 4c) content and the K+/Na+ ratio (Fig. 4d). The exogenous melatonin treatment significantly decreased the Na+ and Cl− contents of seedlings subjected to the 300 mM NaCl treatment, while significantly increasing the K+ content and the K+/Na+ ratio. These results indicate that melatonin maintains the ion homeostasis of L. bicolor leaves under salt stress.
Fig. 4.
Effects of NaCl and exogenous melatonin on Na+ (a), Cl− (b), K+ (c) content and K+/Na+ (d) of L. bicolor leaves. The values are the average of five biological repeats ± standard deviation. According to Duncan’s multi-range tests, bars that there were significant differences at P < 0.05 are labeled with different letters
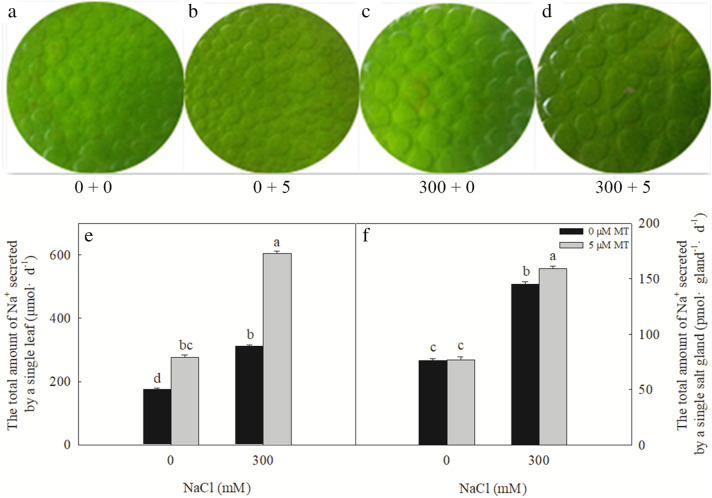
Melatonin promotes salt secretion from the salt glands
L.bicolor is a typical recretohalophyte and its growth and development in high-salt environments are closely related to its salt-secretion ability. The 300 mM NaCl treatment significantly increased the amount of salt secreted from a single leaf and a single salt gland (Fig. 5c, e, and f). Salt secretions also significantly increased following the 5 μM melatonin treatment, both in the 0- and 300-mM NaCl-treated plants. Under 0 mM NaCl, 5 μM melatonin had no significant effect on the amount of salt secreted from a single salt gland, although the melatonin treatment significantly increased the amount of salt secreted from a single salt gland in the salt-stressed plants.
Fig. 5.
Effects of NaCl and melatonin on salt secretion of salt glands of L. bicolor leaves. a-d, the ability of salt secretion of leaves with leaf disc method, a, 0 + 0, 0 mM NaCl+ 0 μM MT; b, 0 + 5, 0 mM NaCl+ 5 μM MT; c, 300 + 0, 300 mM NaCl+ 0 μM MT; d, 300 + 5, 300 mM NaCl+ 5 μM MT; e, The total amount of Na+ secreted by a single leaf; f, the total amount of Na+ secreted by a salt gland. The values are the average of five biological repeats ± standard deviation. According to Duncan’s multi-range tests, bars that there were significant differences at P < 0.05 are labeled with different letters
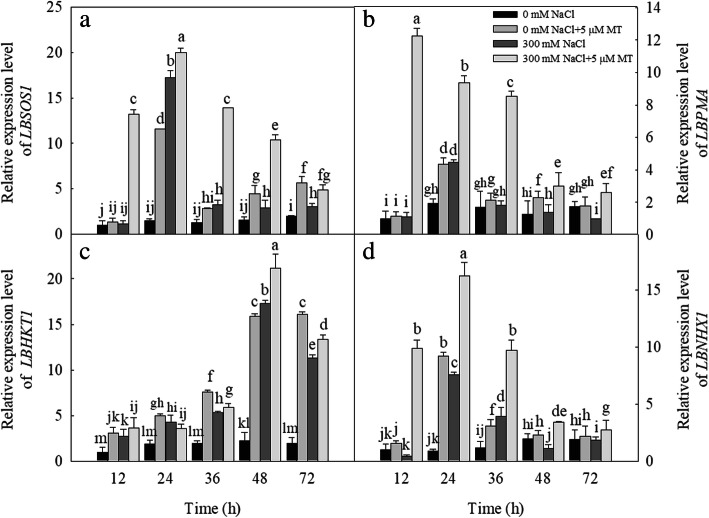
Melatonin upregulates the expression of ion transporter and vesicle transport genes
We examined the effect of melatonin treatment on the expression levels of ion transporter-related genes in salt-stressed plants. Following the 300 mM NaCl treatment, the expression levels of LbSOS1 were significantly upregulated after 24, 36, 48, and 72 h (Fig. 6a); LbPMA was upregulated after 24 h (Fig. 6b); LbHKT1 (high-affinity potassium transporter 1) was upregulated after 12, 24, 36, 48, and 72 h (Fig. 6c); and LbNHX1 was upregulated after 24 h and 36 h (Fig. 6d), relative to the control. The 5 μM melatonin treatment significantly upregulated the expression levels of LbSOS1 after 24, 36, 48, and 72 h (Fig. 6a); LbPMA after 24 h (Fig. 6b); LbHKT1 after 12, 24, 36, 48, and 72 h (Fig. 6c); and LbNHX1 after 24 h and 36 h (Fig. 6d), relative to the control. The combination of the 300 mM NaCl and 5 μM melatonin treatments significantly upregulated the expression levels of LbSOS1, LbPMA, LbHKT1, and LbNHX1 after 12, 24, 36, 48, and 72 h (Fig. 6).
Fig. 6.
Effects of NaCl and melatonin on ions transporter genes (LBSOS1, LBPMA, LBHKT1 and LBNHX1) expression in L. bicolor leaves in 6-wk-old seedlings at different time points after NaCl (300 mM) and melatonin (5 μM) treatment. (a) Transcript level of LBSOS1 (salt overly sensitive 1), (b) Transcript level of LBPMA (H+-ATPase genes), (c) Transcript level of LBHKT1 (high-affinity K+ transporter 1), (d) Transcript level of LBNHX1 (tonoplast Na+/H+ antiporter). The values are the average of three biological repeats ± standard deviation. According to Duncan’s multi-range tests, bars that there were significant differences at P < 0.05 are labeled with different letters
We also measured the expression levels of the vesicle transport-related genes in plants subjected to various salt and melatonin treatments. The 300 mM NaCl treatment significantly upregulated the expression levels of LbVAMP721 after 24, 36, 48, and 72 h; LbVAP27 after 24 h; and LbVAMP121 after 24 and 48 h. The 5 μM melatonin treatment significantly upregulated the expression levels of LbVAMP721 after 24, 36, and 48 h; LbVAP27 after 24 h; and LbVAMP121 after 24 and 36 h. The combination of the 300 mM NaCl and 5 μM melatonin treatments significantly upregulated the expression levels of LbVAMP721 after 12, 24, 36, 48, and 72 h (Fig. 7a); LbVAP27 after 12, 24, 36, and 48 h (Fig. 7b); and LbVAMP121 after 24, 36, and 48 h (Fig. 7c).
Fig. 7.
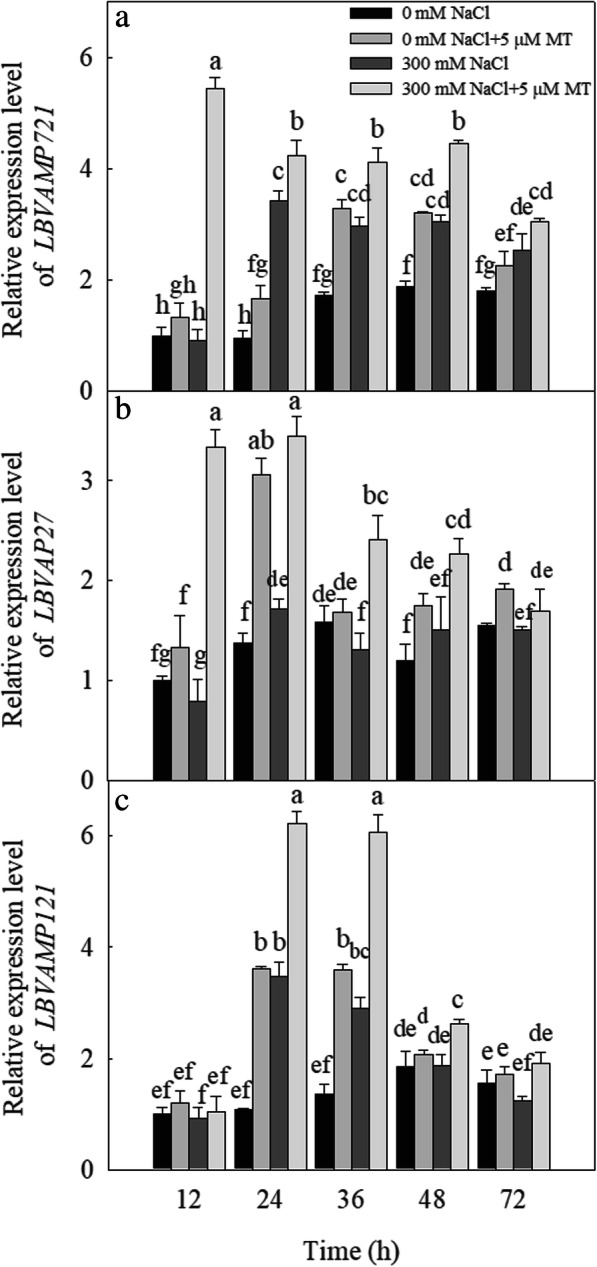
Effects of NaCl and melatonin on vesicle transport related genes LBVAMP721 (LB00297), LBVAP27 (LB11044) and LBVAMP121 (LB11277) expression in L. bicolor leaves in 6-wk-old seedlings at different time points after NaCl (300 mM) and melatonin (5 μM) treatment. (a) Transcript level of LBVAMP721 (vesicle-associated membrane protein 721), (b) Transcript level of LBVAP27 (vesicle-associated protein 27), (c) Transcript level of LBVAMP121 (vesicle-associated membrane protein 121). The values are the average of three biological repeats ± standard deviation. According to Duncan’s multi-range tests, bars that there were significant differences at P < 0.05 are labeled with different letters
Discussion
Salinity is a wide-ranging environmental stress factor that severely inhibits plant growth and development, reduces crop yield, and even causes plant death [1, 43, 44]. Many plants have developed mechanisms to secrete or tolerate the salts in their cells. The recretohalophyte L. bicolor has a strong but poorly understood salt secretion ability, and can therefore be used as a model species to study the development and function of |salt glands [3]. In this study, we revealed that exogenous melatonin significantly increased the ability of the L. bicolor salt glands to secrete salt by upregulating the expression of genes encoding ion transporters and vesicle transport proteins, which can increase the salt tolerance of this plant and enable it to grow well in heavily saline soils. This discovery provides a basis for us to further elucidate the salt secretion mechanism of the salt glands in future studies.
Salinity impairs plant growth first by causing osmotic stress, and then ion stress as the salt ions enter the plant body [45–47]. Yuan et al. [3, 23] reported that the growth of L. bicolor is inhibited under high-salt conditions (200 mM or more). In the present study, we established that exogenous melatonin application alleviated the inhibition of L. bicolor growth caused by treatment with 300 mM NaCl (Fig. 1). This is the first report of this phenomenon in a halophyte. Since even halophytes cannot tolerate large amounts of Na+ and Cl− in their cytoplasm, they either compartmentalize excess ions into vacuoles or transport ions into different tissues to maintain cytoplasmic ion homeostasis [1, 48, 49].
As a typical recretohalophyte, L. bicolor can excrete excess salts via its salt glands [3, 9], which reduces its Na+ and Cl− contents while increasing the K+/Na+ ratio observed in the leaves (Fig. 4), promoting salt tolerance. The salt secretion rate of the leaves depends on the density and function of the salt glands [10]. Here, we showed that the density of the L. bicolor salt glands increased significantly under the independent salt stress, independent melatonin, and combined salt and melatonin treatment (Fig. 2). The increased number and function of salt glands correspondingly enhanced the salt secretion ability of the leaves, which decreased their salt contents and promoted the growth of L. bicolor (Figs. 1 and 5).
The secretion rate of the salt glands is affected by many factors [8]. In the present study, exogenous melatonin significantly increased the salt secretion rate of the salt glands and the amount of salt secreted by a single leaf of L. bicolor compared with leaves under salt stress alone (Fig. 5). Previous studies have indicated that ion transporters and vesicular transport proteins are involved in salt secretion from the salt glands [12, 17]. In L. bicolor leaves, the expression levels of the ion transport-related genes, LbSOS1, LbPMA, LbHKT1, and LbNHX1, were upregulated after the application of exogenous melatonin (Fig. 6). SOS1, HKT1, and NHX1 are important ion transport proteins in plants [50]. PMA can provide the driving force for SOS1, and their expression level is positively related to the salt tolerance of plants [50]. Liu et al. [31] showed that exogenous melatonin can upregulate the expression of genes encoding important Na+-detoxification transporters under salt stress [31]. Recently, studies have indicated that the exogenous application of melatonin can improve the ion homeostasis of plants under salt stress by upregulating the expression of genes encoding NHX, SOS and other proteins with related functions [30, 51, 52]. Yuan et al. [9] proposed that LbSOS1, LbPMA, and LbHKT1 participate in the salt secretion process, which is consistent with the finding that exogenous melatonin increases the salt secretion rate of the salt glands by upregulating the expression of the ion transport genes. These results indicate that ion transport proteins participate in the salt secretion process in L. bicolor.
Ziegler and Lüttge [12] reported that vesicle transport proteins mediate the salt secretion process in the salt glands in the related species L. vulgare. Yuan et al. [9] proposed that small vesicles may be involved in transporting salt into and out of the salt glands, which is supported by the results of Lu et al. [26] for L. bicolor. We established that the expression levels of the vesicle transport-related genes LbVAMP721, LbVAP27, and LbVAMP121 were also upregulated by the melatonin treatment (Fig. 7), which suggested that the vesicle transport proteins can participate in the salt secretion process in this species; however, further research is needed to decipher the mechanism by which exogenous melatonin regulates Na+ secretion from the salt gland.
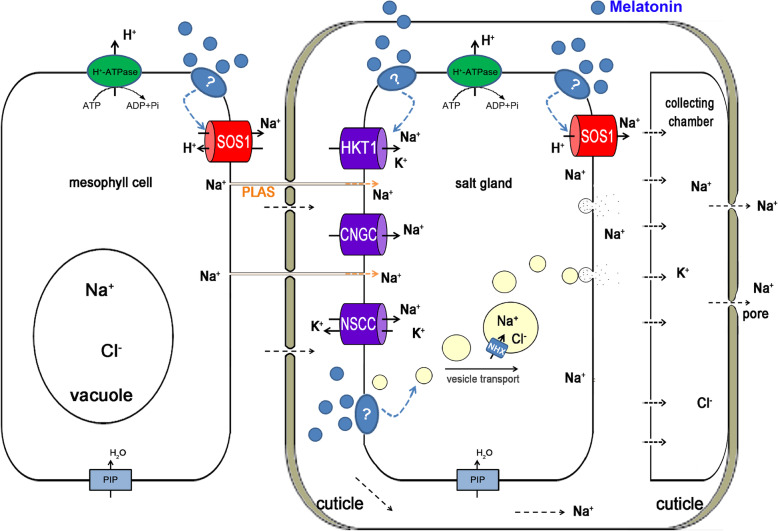
Our results showed that melatonin can upregulate the expression of genes encoding ion transporters and vesicle transport proteins to enhance salt secretion from the salt glands. Combining the results of the current study with previous research, we formulated a novel mechanism by which melatonin increases salt secretion in L. bicolor (Fig. 8). Melatonin upregulates the expression of genes encoding ion transporters and vesicle transport proteins. The ion transporters transport ions into the salt glands. Ions in the salt glands are transported to the collecting chamber adjacent to the secretory pore through vesicle transport and ions transporter and then are secreted from the secretory pore of salt glands, which maintain ionic homeostasis in the cells and alleviate NaCl-induced growth inhibition.
Fig. 8.
The possible pathway of melatonin increase salt secretion of salt gland (modified on the basis of Yuan et al. 2016). The melatonin up-regulates genes expression related to ions transporters and vesicle transport proteins. The ion transporters transport ions into the salt glands. Ions in the salt glands are transported to the collecting chamber adjacent to the secretory pore through vesicle transport and ions transporter and then are secreted from the secretory pore of salt glands. Blue round indicates melatonin; Green oval located on the plasma membrane indicates LBPMA; Red cylinder located on the plasma membrane indicates LBSOS1; Violet cylinder located on the plasma membrane indicates LBHKT1, LBCNGC (cyclic nucleotide-gated cation channel), LBNSCC (non-selective cationic channel) respectively; Blue rectangle located on the plasma membrane indicates LBPIP (plasma membrane intrinsic protein, aquaporin); Blue cylinder located on the vesicles indicates LBNHX
Methods
Plant materials and growth conditions
L. bicolor seeds were kindly provided by Professor Xu Hualing, Dongying Academy of Agricultural Sciences, Shandong Province. The seeds were sterilized in 0.5% (w/v) sodium hypochlorite solution for 15 min and then cleaned with sterile-distilled water. The seeds were sown on well-washed river sand in plastic pots (16 cm in diameter; after the leaves emerged, the plants were watered with Hoagland’s nutrient solution), which were placed in a growth chamber with 600 μmol m− 2 s− 1 light (15-h day/9-h night photoperiod), a temperature of 25 ± 3 °C/22 ± 3 °C (day/night), and a relative humidity of 60/80% (day/night).
Combined NaCl and melatonin treatment
When the seedlings reached the six-leaf stage, they were subjected to NaCl and melatonin treatments. For the NaCl treatment, the seedlings were treated with Hoagland’s nutrient solution containing NaCl, which was increased by 50 mM every 12 h to a final concentration of 300 mM to avoid salt shock. When the NaCl concentration reached 300 mM, melatonin treatment starts, which will treat for 15 days. The control seedlings were treated with Hoagland’s nutrient solution only. To research the effects of melatonin on salt tolerance in L. bicolor, the NaCl-treated (the NaCl concentration gradually increased to 300 mM) and control seedlings were irrigated with 0 or 5 μM melatonin (based on the pre-test with various concentrations of melatonin, Fig. S1), which dissolves in the above Hoagland’s nutrient solution. The L. bicolor seedlings were treated with various combinations of salt and melatonin every 12 h for 15 consecutive days. Five replicates (3 plants per replicate) were performed for each treatment. After 15 days, the leaves were collected to determine the biological indicators.
Physiological index measurements
The dry weights of seedlings after 15 days of the treatments were measured as described by Yuan et al. [10]
Melatonin quantification
The melatonin content of L. bicolor leaves was quantified as described by Sun et al. [53]. Briefly, 0.3 g L. bicolor leaves were ground into powder in liquid nitrogen, mixed well with 1.5 mL methanol, and incubated overnight at 4 °C. The solutions were centrifuged for 10 min at 10,000×g at 4 °C, after which the supernatant was transferred into new test tubes and the liquid was allowed to evaporate. The remaining residues were dissolved in 0.75 mL methanol. A fluorescence detector system (L3000; Rigol Technologies, Beijing, China) was used to determine the melatonin concentration by the area of the peaks identified during high-performance liquid chromatography.
Ion content measurement
Leaf samples (5 g) were placed into test tubes containing 10 mL ddH2O and the tubes were placed in a boiling water bath for 3 h, after which the samples were filtered through filter paper. The supernatant was made up to a volume of 25 mL with ddH2O. The Na+ and K+ contents were measured using a flame spectrophotometer (Model 2655–00 Digital Flame Analyzer; Cole-Parmer Instrument Company, Vernon Hills, Illinois, USA), and the Cl− content was measured using an ion chromatograph (ICS-1100 ion chromatograph; Thermo Fisher Scientific, Waltham, MA, USA), as described by Lin et al. [54]
Characterization of the L. bicolor salt glands
L. bicolor leaves (Fig. S2) were cleared using Carnoy’s solution (mixed solution of ethanol and acetic acid (3:1, v/v)), as described by Kuwabara and Nagata [55](2016), after which they were fixed onto microscope slides using Hoyer’s solution [56](Yuan et al., 2014). The diameters and densities of the salt glands were determined using a Nikon fluorescence microscope (ECLIPSE 80i; Nikon, Tokyo, Japan) at × 200 and × 100 magnifications with a standard DAPI filter set under UV excitation (330–380 nm). Digital images were taken using a charge-coupled device camera. Fifteen leaves were measured per treatment, with the measurements taken at the same position on each leaf. The salt glands were counted for a given leaf area to calculate the salt gland density on the abaxial surfaces of the leaves [17]. The total number of salt glands is equal to the salt gland density multiplied by the leaf area. The salt secretion levels under different NaCl and melatonin treatments were determined using the leaf disk method, as described by Lu et al. [26].
qRT-PCR analysis
The nucleotide sequences of ion homeostasis (LbHTK1, LbSOS1, LbPMA, and LbNHX1) and vesicle transport (LbVAMP721 (vesicle-associated membrane protein 721), LbVAP27 (syntaxin from plants 27), and LbVAMP121 (vesicle-associated membrane protein 721)) genes in L. bicolor were obtained according to the second-and third-generation RNA sequences [3]. A BLAST search for homologous genes was carried out in both L. bicolor and other species, and homologous sequences were downloaded. Primers were designed for cloning the conserved region sequences (800 bp). The conserved region sequences of LbHTK1, LbSOS1, LbPMA, LbNHX1, LbVAMP721, LbVAP27, and LbVAMP121 were obtained. Beacon Designer (Premier Biosoft, Palo Alto, California, USA) was used to design primers for these seven genes (Table S1). AceQ Universal SYBR Green qPCR Master Mix (Vazyme Biotech, Nanjing, China) and a real-time quantitative PCR instrument (Bio-Rad Laboratories, Hercules, California, USA) were used to perform the real-time PCR. The relative expression of each gene was calculated using the 2–△△Ct method [17, 57], with the housekeeping gene LbTUBULIN used as an internal reference.
Statistical analysis
The statistical analysis was performed using the SPSS software package (version 19.0; IBM, Armonk, New York, USA). The statistical significance was determined using an analysis of variance (ANOVA), and significant differences (P < 0.05) between the values were determined using Duncan’s multiple range test.
Supplementary information
Additional file 1: Figure S1. Pre-test with various concentrations of melatonin and found that for the species, 5 μM melatonin significantly improved the growth under control and NaCl treatment.
Additional file 2: Figure S2. Leaves for analyzing characterization of the L. bicolor salt glands.
Additional file 3: Table S1. Primers of candidate genes used for real-time qPCR analysis.
Additional file 4. All data generated during this study.
Acknowledgements
Not applicable.
Abbreviations
- MT
melatonin
- PM
plasma membrane
- PMA
plasma membrane H+-ATPase
- SOS1
salt overly sensitive
- NHX1
tonoplast Na+/H+ antiporter 1
- HKT1
high-affinity potassium transporter 1
- VAMP721
vesicle-associated membrane protein 721
- VAP27
syntaxin from plants 27
- VAMP121
vesicle-associated membrane protein 721
Authors’ contributions
MC and BW designed the research, JL, FY and YL performed the experiments, JL wrote the paper with contributions from the other authors. MZ, YL, YZ analyzed the data. BW revised the paper. All authors read and approved the final manuscript.
Funding
This work was supported by Shandong Provincial “Bohai Granary” Science and Technology Demonstration Project (2019BHLC004), Agricultural Variety Improvement Project of Shandong Province (2019LZGC009) and the major projects of science and technology in Shandong province (2017CXGC0311), and the Program for Scientific Research Innovation Team in Colleges and Universities of Shandong Province.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junpeng Li, Fang Yuan and Yanlu Liu contributed equally to this work.
Contributor Information
Baoshan Wang, Email: bswang@sdnu.edu.cn.
Min Chen, Email: chenminrundong@126.com.
References
- 1.Zhu JK. Regulation of ion homeostasis under salt stress. CurrOpin Plant Biol. 2003;6:441–445. doi: 10.1016/s1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 2.Song J, Wang BS. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann Bot. 2015;115(3):541–553. doi: 10.1093/aob/mcu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan F, Lyu MJA, Leng BY, Zhu XG, Wang BS. The transcriptome of NaCl-treated Limonium bicolor, leaves reveals the genes controlling salt secretion of salt gland. Plant Mol Biol. 2016;91:241–256. doi: 10.1007/s11103-016-0460-0. [DOI] [PubMed] [Google Scholar]
- 4.Evelin H, Kapoor R, Giri B. Arbuscular mycorrhizal fungi inalleviation of salt stress: a review. Ann Bot. 2009;104:1263–1280. doi: 10.1093/aob/mcp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song J, Shi GW, Gao B, Fan H, Wang BS. Waterlogging and salinity effects on two Suaeda salsa populations. Physiol Plant. 2011;141:343–351. doi: 10.1111/j.1399-3054.2011.01445.x. [DOI] [PubMed] [Google Scholar]
- 6.Sui N, Tian S, Wang W, Wang M, Fan H. Overexpression of glycerol-3-phosphate acyltransferase from Suaeda salsa improves salt tolerance in Arabidopsis. Front Plant Sci. 2017;8:1337. doi: 10.3389/fpls.2017.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Liu J, Zhu T, Zhao C, Li L, Chen M. The role of melatonin in salt stress responses. Int J Mol Sci. 2019;20(7):1735. doi: 10.3390/ijms20071735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levitt J. Responses of plants to environmental stresses. New York: Academic Press; 1972. [Google Scholar]
- 9.Yuan F, Leng BY, Wang BS. Progress in studying salt secretion from the salt glands in recretohalophytes: how do plants secrete salt? Front Plant Sci. 2016;7:977–988. doi: 10.3389/fpls.2016.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan F, Liang X, Li Y, Yin S, Wang B. Methyl jasmonate improves tolerance to high salt stress in the recretohalophyte Limonium bicolor. Funct Plant Biol. 2019;46:82–92. doi: 10.1071/FP18120. [DOI] [PubMed] [Google Scholar]
- 11.Arisz W, Camphuis I, Heikens H, van Tooren AJ. The secretion of the salt glands of Limonium latifolium Ktze. Acta Botanica Neerlandica. 1955;4:322–338. doi: 10.1111/j.1438-8677.1955.tb00334.x. [DOI] [Google Scholar]
- 12.Ziegler H, Lüttge U. Die Salzdrüsen von Limonium vulgare. Planta. 1967;74(1):1–17. doi: 10.1007/BF00385168. [DOI] [PubMed] [Google Scholar]
- 13.Shimony C, Fahn A. Light-and electron-microscopical studies on the structure of salt glands of Tamarix aphylla L. Bot J Linn Soc. 1968;60(383):283–288. doi: 10.1111/j.1095-8339.1968.tb00090.x. [DOI] [Google Scholar]
- 14.Feng Z, Sun Q, Deng Y, Sun S, Zhang J, Wang B. Study on pathway and characteristics of ion secretion of salt glands of Limonium bicolor. Acta Physiol Plant. 2014;36:2729–2741. doi: 10.1007/s11738-014-1644-3. [DOI] [Google Scholar]
- 15.Levering CA, Thomson WW. The ultrastructure of the salt gland of Spartina foliosa. Planta. 1971;97:183–196. doi: 10.1007/BF00389200. [DOI] [PubMed] [Google Scholar]
- 16.Shi H, Quintero FJ, Pardo JM, Zhu JK. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding F, Chen M, Sui N, Wang BS. Ca2+ significantly enhanced development and salt-secretion rate of salt glands of Limonium bicolor under NaCl treatment. S Afr J Bot. 2010;76:95–101. doi: 10.1016/j.sajb.2009.09.001. [DOI] [Google Scholar]
- 18.Gao C, Wang Y, Jiang B, et al. A novel vacuolar membrane H+-ATPase c subunit gene (ThVHAc1) from Tamarix hispida confers tolerance to several abiotic stresses in Saccharomyces cerevisiae. Mol Biol Rep. 2011;38(2):957–963. doi: 10.1007/s11033-010-0189-9. [DOI] [PubMed] [Google Scholar]
- 19.Tan WK, Lim TM, Loh CS. A simple, rapid method to isolate salt glands for three-dimensional visualization, fluorescence imaging and cytological studies. Plant Methods. 2010;6:24–35. doi: 10.1186/1746-4811-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang ZH, Zheng LL, Wang J, Gao Z, Wu SB, Qi Z, et al. Transcriptomic profiling of the salt-stress response in the wild recretohalophyte Reaumuria trigyna. BMC Genomics. 2013;14:29. doi: 10.1186/1471-2164-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barkla BJ, Zingarelli L, Blumwald E, Smith JAC. Tonoplast Na+/H+ antiporter activity and its energization by the vacuolar H+-ATPase in the halophytic plant Mesembryanthemum crystallinum L. Plant Physiol. 1995;109:549–556. doi: 10.1104/pp.109.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng ZT, Deng YQ, Zhang SC, Liang X, Yuan F, Hao JL, Zhang JC, Sun SF, Wang BS. K+ accumulation in the cytoplasm and nucleus of the salt gland cells of Limonium bicolor accompanies increased rates of salt secretion under NaCl treatment using NanoSIMS. Plant Sci. 2015;238:286–296. doi: 10.1016/j.plantsci.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Yuan F, Lyu MJ, Leng BY, Zheng GY, Feng ZT, Li PH, Zhu XG, Wang BS. Comparative transcriptome analysis of developmental stages of the Limonium bicolor leaf generates insights into salt gland differentiation. Plant Cell Environ. 2015;38:1637–1657. doi: 10.1111/pce.12514. [DOI] [PubMed] [Google Scholar]
- 24.Thomson WW, Liu LL. Ultrastructural features of the salt gland of Tamarix aphylla L. Planta. 1967;73:201–220. doi: 10.1007/BF00387033. [DOI] [PubMed] [Google Scholar]
- 25.Flowers TJ, Glenn EP, Volkov V. Could vesicular transport of Na+ and cl− be a feature of salt tolerance in halophytes? Ann Bot. 2019;123(1):1–18. doi: 10.1093/aob/mcy164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu C, Feng Z, Yuan F, Han G, Guo J, Chen M, Wang B. The SNARE protein LbSYP61 participates in salt secretion in Limonium bicolor. Environmental and Experimental Botany Available online 24 April 2020, 104076, 10.1016/j.envexpbot.2020.104076.
- 27.Rozema J, Riphagen I. Physiology and ecologic relevance of salt secretion by the salt gland of Glaux maritima L. Oecologia. 1977;29(4):349–357. doi: 10.1007/BF00345808. [DOI] [PubMed] [Google Scholar]
- 28.Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Boil Int. 1995;35:627–634. [PubMed] [Google Scholar]
- 29.Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J Pineal Re. 1995;18:28–31. doi: 10.1111/j.1600-079X.1995.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Wang P, Wei Z, Liang D, Liu C, Yin L, Jia D, Fu M, Ma F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J Pineal Res. 2012;53:298–306. doi: 10.1111/j.1600-079X.2012.00999.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu N, Gong B, Jin Z, Wang X, Wei M, Yang F, Li Y, Shi Q. Sodic alkaline stress mitigation by exogenous melatonin in tomato needs nitric oxide as a downstream signal. J Plant Physiol. 2015;186-187:68–77. doi: 10.1016/j.jplph.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Wen D, Gong B, Sun S, Liu S, Wang X, Yang F, Li Y, Shi Q. Promoting Roles of Melatonin in Adventitious Root Development of Solanum lycopersicum L by Regulating Auxin and Nitric Oxide Signaling Frontiers in Plant Science, 2016; 7, 718. [DOI] [PMC free article] [PubMed]
- 33.Zhang N, Zhang HJ, Zhao B, Sun QQ, Cao YY, Li R, Wu XX, Weeda S, Li L, Ren S, et al. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J Pineal Res. 2014;56:39–50. doi: 10.1111/jpi.12095. [DOI] [PubMed] [Google Scholar]
- 34.Wang LY, Liu JL, Wang WX, Sun Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica. 2016;54(1):19–27. doi: 10.1007/s11099-015-0140-3. [DOI] [Google Scholar]
- 35.Yan Y, Jing X, Tang H, Li X, Gong B, Shi Q. Using transcriptome to discover a novel melatonin-induced sodic alkaline stress resistant pathway in Solanum lycopersicum L. Plant Cell Physiol. 2019;60:2051–2064. doi: 10.1093/pcp/pcz126. [DOI] [PubMed] [Google Scholar]
- 36.Wang P, Yin L, Liang D, Li C, Ma F, Yue Z. Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate-glutathione cycle. J Pineal Res. 2012;53:11–20. doi: 10.1111/j.1600-079X.2011.00966.x. [DOI] [PubMed] [Google Scholar]
- 37.Bajwa VS, Shukla MR, Sherif SM, Murch SJ, Saxena PK. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J Pineal Res. 2014;56:238–245. doi: 10.1111/jpi.12115. [DOI] [PubMed] [Google Scholar]
- 38.Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpi’nski S, Mittler R. ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol. 2016;171:1606–1615. doi: 10.1104/pp.16.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong B, Yan Y, Wen D, Shi Q. Hydrogen peroxide produced by NADPH oxidase:a novel downstream signaling pathway in melatonin-induced stress toelrance in Solanum lycopersicum. Physiol Plant. 2017;160:396–409. doi: 10.1111/ppl.12581. [DOI] [PubMed] [Google Scholar]
- 40.Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, a pineal factor that lightens melanocytes. J Am Chem Soc. 1958;80:2587. doi: 10.1021/ja01543a060. [DOI] [Google Scholar]
- 41.Li, J., Zhao, C., Zhang, M., Yuan, F., Chen, M. Exogenous melatonin improves seed germination in Limonium bicolor under salt stress. Plant Signaling Behavior, 2019; e1659705. [DOI] [PMC free article] [PubMed]
- 42.Fan J, Xie Y, Zhang Z, Chen L. Melatonin: a multifunctional factor in plants. Int J Mol Sci. 2018;19:1528. doi: 10.3390/ijms19051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan F, Chen M, Yang J, Song J, Wang BS. The optimal dosage of60co gamma irradiation for obtaining salt gland mutants of exo-recretohalophyte Limonium bicolor (Bunge) O. Kuntze Pak J Bot 2015; 47:71–76.
- 44.Leng BY, Yuan F, Dong XX, Wang J, Wang BS. Distributionpattern and salt excretion rate of salt glands in two recretohalophyte species of Limonium (Plumbaginaceae) S Afr J Bot. 2018;115:74–80. doi: 10.1016/j.sajb.2018.01.002. [DOI] [Google Scholar]
- 45.Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 46.Sun ZB, Qi XY, Wang ZL, Li PH, Wu CX, Zhang H, Zhao YX. Overexpression of TsGOLS2, a galactinol synthase, in Arabidopsis thaliana enhances tolerance to high salinity and osmotic stresses. Plant Physiol Biochem. 2013;69:82–89. doi: 10.1016/j.plaphy.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Shao Q, Han N, Ding TL, Zhou F, Wang BS. SsHKT1; 1 is a potassium transporter of the C3 halophyte Suaeda salsa that is involved in salt tolerance. Funct Plant Biol. 2014;41(8):790–802. doi: 10.1071/FP13265. [DOI] [PubMed] [Google Scholar]
- 48.Han GL, Wang MJ, Yuan F, Sui N, Song J, Wang BS. The CCCH zinc finger protein gene AtZFP1 improves salt resistance in Arabidopsis thaliana. Plant Mol Biol. 2014;86:237–253. doi: 10.1007/s11103-014-0226-5. [DOI] [PubMed] [Google Scholar]
- 49.Han GL, Yuan F, Guo JR, Zhang Y, Sui N, Wang BS. AtSIZ1 improves salt tolerance by maintaining ionic homeostasis and osmotic balance in Arabidopsis. Plant Sci. 2019;285:55–67. doi: 10.1016/j.plantsci.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Gong Z, Xiong L, Shi H, Yang S, Herrera-Estrella L, Xu G, Chao D-Y, Li J, Wang P, Qin F, Li B, Ding Y, Shi Y, Wang Y, Yang Y, Guo Y, Zhu J-K. Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci. 2020;63. 10.1007/s11427-020-1683-x. [DOI] [PubMed]
- 51.Jiang C, Cui Q, Feng K, Xu D, Li C, Zheng Q. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol Plant. 2016;38:82. doi: 10.1007/s11738-016-2101-2. [DOI] [Google Scholar]
- 52.Zhao G, Zhao Y, Yu X, Kiprotich F, Han H, Guan R, Wang R, Shen W. Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int. J. Mol. Sci. 2018; 19, 1912. [DOI] [PMC free article] [PubMed]
- 53.Sun S, Wen D, Yang W, et al. Overexpression of Caffeic Acid O -Methyltransferase 1 ( COMT1 ) Increases Melatonin Level and Salt Stress Tolerance in Tomato Plant. J Plant Growth Regul, 2019; 1–15.
- 54.Lin J, Li JP, Yuan F, Yang Z, Wang BS, Chen M. Transcriptome profiling of genes involved in photosynthesis in Elaeagnus angustifolia L under salt stress Photosynthetica, 2018; 56, 998–1009.
- 55.Kuwabara A, Nagata T. Cellular basis of developmental plasticity observed in heterophyllous leaf formation of Ludwigia arcuate (Onagraceae) Planta. 2006;224:761–770. doi: 10.1007/s00425-006-0258-4. [DOI] [PubMed] [Google Scholar]
- 56.Yuan F, Chen M, Yang J, Ling BY, Wang BS. A system for the transformation and regeneration of the recretohalophyte Limonium bicolor. In Vitro Cell Dev-Pl. 2014;50:610–617. doi: 10.1007/s11627-014-9611-7. [DOI] [Google Scholar]
- 57.Gao W, Zhang Y, Feng Z, Bai Q, He J, Wang Y. Effects of melatonin on antioxidant capacity in naked oat seedlings under drought stress. Molecules. 2018;23:1580. doi: 10.3390/molecules23071580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Pre-test with various concentrations of melatonin and found that for the species, 5 μM melatonin significantly improved the growth under control and NaCl treatment.
Additional file 2: Figure S2. Leaves for analyzing characterization of the L. bicolor salt glands.
Additional file 3: Table S1. Primers of candidate genes used for real-time qPCR analysis.
Additional file 4. All data generated during this study.
Data Availability Statement
Not applicable.