Abstract
Background
Vitamin D deficiency is diagnosed by total serum 25-hydroxyvitamin D (25(OH)D) concentration and is associated with poor health and increased mortality; however, some populations have low 25(OH) D concentrations without manifestations of vitamin D deficiency. The Vitamin D Metabolite Ratio (VMR) has been suggested as a superior indicator of vitamin D status. Therefore, VMR was determined in a population with type 2 diabetes at high risk for vitamin D deficiency and correlated with diabetic complications.
Research design and methods
Four hundred sisty patients with type 2 diabetes (T2D) were recruited, all were vitamin D3 supplement naive. Plasma concentration of 25-hydroxyvitamin D3 (25(OH)D3) and its metabolites 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) and 24,25-dihydroxyvitamin D3 (24,25(OH)2D3) and its epimer, 3-epi-25-hydroxyvitamin D3 (3-epi-25(OH)D3), were measured by LC-MS/MS analysis. VMR-1 was calculated as a ratio of 24,25(OH)2D3:25(OH)D3; VMR-2 as a ratio of 1,25(OH)2D3:25(OH)D3; VMR-3 was calculated as a ratio of 3-epi-25(OH)D3: 25(OH)D3.
Results
An association means that there were significant differences between the ratios found for those with versus those without the various diabetic complications studied. VMR-1 was associated with diabetic retinopathy (p = 0.001) and peripheral artery disease (p = 0.012); VMR-2 associated with hypertension (p < 0.001), dyslipidemia (p < 0.001), diabetic retinopathy (p < 0.001), diabetic neuropathy (p < 0.001), coronary artery disease (p = 0.001) and stroke (p < 0.05). VMR-3 associated with hypertension (p < 0.05), dyslipidemia (p < 0.001) and coronary artery disease (p < 0.05).
Conclusions
In this cross sectional study, whilst not causal, VMR-2 was shown to be the superior predictor of diabetic and cardiovascular complications though not demonstrative of causality in this cross-sectional study population over VMR-1, VMR-3 and the individual vitamin D concentration measurements; VMR-2 associated with both microvascular and cardiovascular indices and therefore may have utility in predicting the development of diabetic complications.
Supplementary Information
Supplementary information accompanies this paper at 10.1186/s12902-020-00641-1.
Keywords: Vitamin D, Vitamin D metabolites, Vitamin D deficiency, Vitamin D metabolite ratio, Diabetic complications
Background
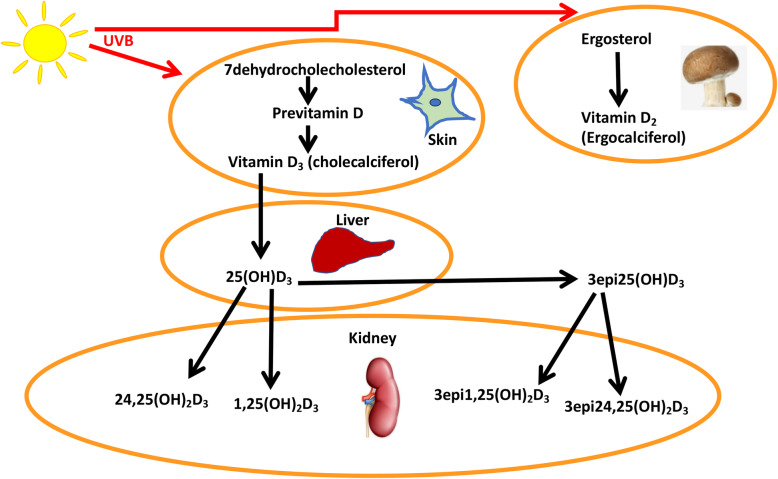
Vitamin D comprises a group of fat-soluble steroids, vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) being the two major compounds in humans. Vitamin D’s major role is facilitation of intestinal absorption of calcium, magnesium and phosphate, and it is therefore central to calcium homeostasis and bone metabolism [1]. Whilst some foods such as mushrooms and fungi contain vitamin D2 [2], most vitamin D (Vitamin D3 (25(OH)D3)) is derived from conversion of cholesterol to cholecalciferol in the skin, a process activated by UVB radiation from sunlight exposure. 25(OH)D3 is inert and must undergo hydroxylation in the kidney to its active form, 1,25 dihydroxyvitaminD3 (1,25(OH)D3 [3] or to 24,25-dihydroxyvitamin D (24,25(OH)2D3) by 24 alpha hydroxylase in the renal tubular cells (Fig. 1) [4]. While vitamin D deficiency represents a worldwide health issue [5], it is exacerbated in some parts of the world, such as the Middle East, where a notably high prevalence is consequent upon local cultural norms requiring full body coverage [6–9].
Fig. 1.
The Vitamin D3 pathway. In the skin, 7-dehydrocholesterol is converted to previtamin D3 and then to vitamin D3. This is transported to the liver, converted to 25 hydroxyvitamin D (25(OH)D3) and then transported to the kidney. In the kidney, 25(OH)D3 undergoes conversion to the active 1,25 (OH)2D3, and 24,25(OH)2D3
Vitamin D deficiency has been associated with a range of negative health outcomes, including osteoporosis and type 2 diabetes, as well as increased mortality that may be addressed by vitamin D supplementation [10]. Diagnosis is widely based upon measurement of total serum 25-hydroxyvitamin D (vitamin D2 plus vitamin D3), a value of < 20 ng/ml (< 48.4 nmol/l) being indicative of vitamin D insufficiency. However, certain ethnic groups appear to have low serum concentrations of 25(OH) D whilst maintaining healthy bone mineral density. African Americans represent one such group, with typically low concentrations of 25(OH) D [11–13] and yet with a higher bone mineral density and a lower risk of osteoporosis and fractures than their white counterparts [14–17]. 1,25-dihydroxyvitamin D concentrations (1,25(OH)2D) are related not only to kidney function but also to vitamin D status, and patients who are vitamin D deficient or insufficient have normal or even high concentrations of 1,25(OH)2D due to secondary hyperparathyroidism [3]. 24,25(OH)2D is not only related to the blood concentrations of 25-hydroxyvitamin D but are also related to the blood concentrations of 1,25(OH)2D because it induces 24 hydroxylase [3]. Whilst 1,25(OH)2D is the active metabolite of 25(OH) D, 24,25(OH)2D3 is also an active metabolite (it can be converted to 1,24,25-trihydroxyvitamin D3 through the C24 oxidation pathway [18]) as it has been shown to induce non-genomic signalling pathways, a mechanism active in many tissues, playing, for example, a physiological role in growth plate formation [3] and in activating rapid insulin release from pancreatic beta cells in response to increases in glycemia [19].
3- epimerase isomerizes the C-3 hydroxy group of 25(OH) D from the α to the β orientation leading to 3epi25(OH) D [3, 20] that may be measured inadvertently whilst measuring 25(OH) D [21]. 3epi25(OH) D is thought to be less potent physiologically when compared with 25(OH) D and 1,25(OH)2–3-epi-D; however, whilst data is sparse on the biological potency and role of the C3 epimers, we have reported that it was not associated with diabetes complications [22].
We have previously shown that type 2 diabetes (T2D) complications are associated with differing metabolites of vitamin D: diabetic retinopathy associated with lower 25(OH)D3 and 1,25(OH)2D3 concentrations; hypertension associated with lower 1,25(OH)2D3, and dyslipidemia associated with lower 25(OH)D3, 1,25(OH)2D3 and 24,25(OH)2D3 [22]. The vitamin D metabolite ratio (VMR), a ratio of 24,25(OH)2D: 25(OH) D, has been proposed at a better indicator of vitamin D status [23]. This is particularly important in ethnic groups where low 25(OH) D is prevalent, as VMR can identify individuals who are functionally deficient from those who are not. We therefore sought to determine the following three vitamin D metabolite ratios: 24,25(OH)2D3: 25(OH)D3 (termed VMR-1), 1,25(OH)2D3: 25(OH)D3 (termed VMR-2) and 3-epi-25(OH)D3: 25(OH)D3 (termed VMR-3) in a cohort of Middle Eastern type 2 diabetic subjects with normal renal function where low 25(OH)D3 is the norm.
Research design and methods
Study population
460 Middle Eastern type 2 diabetic subjects were recruited from June 2012–2013 from the Hamad outpatient clinic, Doha, Qatar as part of a study designed primarily to investigate gene expression and genomics in diabetic subjects (Table 1) [24].
Table 1.
Demographic data, Vitamin D3 levels and Vitamin D3 Metabolite Ratios (VMR) for Type 2 Diabetes (n = 460) patients
| Type 2 Diabetes n = 460 | |
|---|---|
|
Age (years) mean (SD) |
55.2 (9.9) |
|
Male Gender N (%) |
227 (49.4) |
|
BMI (kg/m2) median (IQR) |
32.4 (28.6–37.2) |
|
HbA1c (%) median (IQR) |
7.9 (6.7–9.5) |
|
Glucose (mmol/l) median (IQR) |
8.6 (6.4–12.2) |
|
1,25(OH)2D3 (ng/dl) median (IQR) |
0.02 (0.01–0.04) |
|
25(OH)D3 (ng/dl) median (IQR) |
6.5 (3.4–13.6) |
|
24,25(OH)2D3 (ng/dl) median (range) |
0.3 (0.2–0.6) |
|
3-epi-25(OH)D3 median (IQR) |
0.4 (0.2–0.8) |
| VMR-1 [24,25(OH)2D3:25(OH)D3] median (IQR) | 0.05 (0.04–0.07) |
| VMR-2 [1,25(OH)2D3:25(OH)D3] median (IQR) | 0.002 (0.001–0.004) |
| VMR-3 [3-epi-25(OH)D3:25(OH)D3 median (IQR) | 0.07 (0.05–0.10) |
1,25(OH)2D3 1,25-Dihydroxyvitamin D3; 25(OH)D3 25-hydroxyvitamin D3; 24,25(OH)2D3 24,25-dihydroxyvitamin D3; 3-epi-25(OH)D3 3-epi-25-hydroxyvitamin D3; IQR Interquartile range
Males or females aged 30 years or older were included in the study; all had normal renal function and none were taking vitamin D3 supplements. A diagnosis of T2D was based upon WHO guidelines [25] with one or more of the following criteria: fasting plasma glucose > 7 mmol/l, HbA1c > 6.5%, or a diagnostic glucose tolerance test. Exclusion criteria were a diagnosis of type 1 diabetes or secondary diabetes, such as gestational diabetes or that due to steroid treatment.
The study was approved by Weill Cornell IRB (IRB# 13–00063) and all participants provided written informed consent. The conduct of this study was in accordance with ICH GCP and the Declaration of Helsinki.
Study design
At the baseline visit, blood samples were collected following an overnight fast and weight and blood pressure were measured. Blood pressure measurement was standardised with the non-smoking patient in a seated position, resting quietly for 5 min prior to the first reading. The arm was supported with the elbow at the level of the heart. Readings were taken 3 times, and the lowest reading was selected for analysis. A wide cuff sphygmomanometer was used in obese patients. Fasting venous blood was collected into fluoride oxalate and serum gel tubes. Samples were centrifuged at 2000 g for 15 min at 4 °C, with aliquots stored at − 80 °C within 1 h of collection. Blood pressure was measured with an automated device (NPB-3900; Nellcor Puritan Bennett, Pleasanton, CA) at each study visit.
Serum vitamin D3 measurement
Measurement of vitamin D and its metabolites have previously been described in detail [22]. In brief, serum vitamin D3 concentrations were quantified using isotope-dilution liquid chromatography tandem mass spectrometry (LC-MS/MS). “25 μL of internal standards (d6-1calcitriol (1.5 ng/mL), d6-25OHD3 (50 ng/mL) and d6-epi-25(OH)D3 (20 ng/mL)) were added into each microcentrifuge tube containing 250 μL of calibration standards, Quality Control or serum samples, and kept for 30 min to reach binding equilibrium. The samples were diluted with 250 μL of pretreatment solution (isopropanol and water; 50:50 v/v) and left to stand for at least 15 min to displace binding protein.
300 μL of pre-treated samples were loaded onto ISOLUTE® supported liquid extraction (SLE+) columns (Biotage), followed by elution with 1.8 mL of n-heptane (2 × 900 μL) into a collection tube already containing 200 μL of 0.25 mg/mL PTAD solution in ethyl acetate and heptane (8:92 v/v). The eluate was evaporated to dryness using turbovap under nitrogen gas heated at 38 °C. Once dried, 50 μL of reconstituted solution consisting of methanol and deionized water, 70:30 v/v, and 0.006% methylalamine were added into all tubes. The derivatized extracts were transferred into LC insert vials and 10 μL from each was injected into the LC-MS/MS system. Data for the 25(OH)D3 and metabolite validation is shown in Supplementary Table 1.”
Study outcomes
Statistical analyses
Data trends were visually and statistically evaluated for normality. A Student’s t-test was used for normally distributed data; when those data were not normally distributed, then the Kolmogorov-Smirnov Test and non-parametric tests (Mann Whitney U) were utilised. Statistical analysis was performed using SPSS for Windows, version 24.0. All values are given as mean ± SD or as mean with 95% confidence interval (CI) unless specified.
Results
The baseline characteristics, including the demographics, for the type 2 diabetes patients are shown in Table 1.
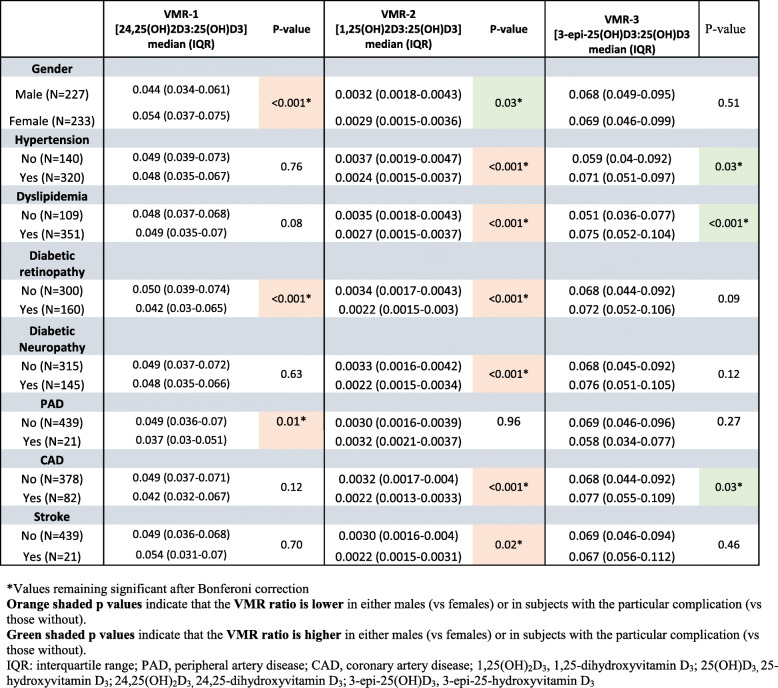
subjects. The relationship of Vitamin D3 Metabolite Ratios (VMRs) with diabetes complications in this cohort (n = 460) of subjects with Type 2 Diabetes is shown in Table 2.
Table 2.
Relationship of Vitamin D3 Metabolite Ratios (VMR) with diabetes complications in the cohort (n = 460) of subjects with Type 2 Diabetes
As we have previously reported, concentrations of 25(OH)D3, 1,25(OH)D3, 24,25(OH)2D3 and 3epi-25(OH)D3 were all lower in females (p = 0.003); however, despite the lower vitamin D concentrations measured in females, there was no difference in diabetes complication rates between males and females [22].
An association here means that there was a significant difference between the ratios found in those with versus without the diabetic complications discussed. The VMR-2 ratio showed a striking association with diabetic complications, namely hypertension (p < 0.001), dyslipidemia (p < 0.001), diabetic retinopathy (p < 0.001), diabetic neuropathy (p < 0.001), coronary artery disease (p < 0.001) and stroke (p < 0.018). By way of comparison, 25(OH)D3 associated with dyslipidaemia (p < 0.04) and diabetic retinopathy (p < 0.03), while 1,25(OH)D3 alone was associated with hypertension (p < 0.009), dyslipidaemia (p < 0.003), retinopathy (p < 0.006) and coronary artery disease (p = 0.012), as we have previously reported [22].
The VMR-1 ratio showed a relationship with diabetic retinopathy (p = 0.001) and peripheral artery disease (p = 0.012) that was not revealed using 24,25(OH)2D3 concentrations alone [22].
The VMR-3 ratio showed a relationship with hypertension (p = 0.03), dyslipidaemia (p < 0.001) and coronary artery disease (p = 0.034). For comparison, 3epi-25(OH)D3 alone associated only with diabetic neuropathy, as previously reported (p = 0.03) [22].
In view of the potential confounding influence of renal function, estimated glomerular filtration rate (eGFR) was correlated to vitamin D3 and its metabolites. The normal range for eGFR is 100–130 mL/min/1.73m2 in men and 90–120 mL/min/1.73m2 in women below the age of 40 years. eGFR decreases with age, decreasing to a mean of 99 mL/min/1.73m2 in the 40–49 years age range, 93 mL/min/1.73m2 from 50 to 59 years and 85 mL/min/1.73m2 from 60 to 69 years. All the subjects in this study had eGFR in the normal range for age and gender. The only correlation with eGFR was found with 24,25(OH)2D3 (1,25(OH)2D3: R = 0.067 p = 0.24; 25(OH)D3: R = -0.032, p = 0.51; 24,25(OH)2D3: R = 0.148, p = 0.002).
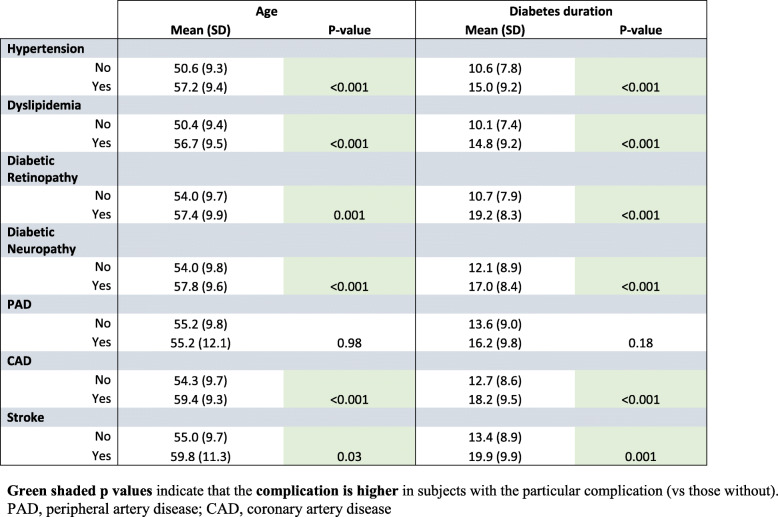
The effect of age and duration of diabetes on the VMR ratios and each of the reported complications was also considered. Age did influence VMR-2 (r2 = − 0.26, p < 0.001) but had no influence on VMR-1 (r2 = 0.004, p = 0.93) or VMR-3 (r2 = 0.07, p = 0.21). Duration of diabetes had an effect on all three VMRs (VMR-1: r2 = − 0.14, p = 0.004; VMR-2: r2 = − 0.22, p < 0.001; VMR-3: r2 = 0.14, p = 0.011).
There was a relationship of age with each of the following complications: hypertension (p < 0.001), dyslipidemia (p < 0.001), diabetic retinopathy (p = 0.001), diabetic neuropathy (p < 0.001), CAD (p < 0.001) and stroke (p = 0.03), the only complication not showing a relationship with age being PAD (p = 0.98) (Table 3). Likewise diabetes duration showed a relationship with the same complications as age: hypertension (p < 0.001), dyslipidemia (p < 0.001), diabetic retinopathy (p < 0.001), diabetic neuropathy (p < 0.001), CAD (p < 0.001) and stroke (p = 0.001); again, the only complication not showing a relationship with diabetes duration being PAD (p = 0.18) (Table 3).
Table 3.
Relationship of age and diabetes duration with diabetes complications in the cohort (n = 460) of subjects with Type 2 Diabetes. The data is normally distributed so the mean and SD have been reported
Discussion
The VMR-2 ratio showed a striking relationship between diabetic complications being lower in diabetic retinopathy and diabetic neuropathy, and the cardiovascular complications of hypertension, dyslipidemia, coronary artery disease and stroke. These findings suggest that the VMR-2 ratio is a superior predictor for development of diabetic and cardiovascular complications. We have previously reported the association of diabetic microvascular complications with 1,25(OH)2D3, 25(OH)D3 and its epimers [22]. In this current study, we hypothesized that an alternative VMR ratio (termed VMR-2) comparing 1,25(OH)2D3 to 25(OH)D3 may have even greater predictive power for diabetic and cardiovascular complications, as 1,25(OH)2D3 is the active metabolite of vitamin D3 (25(OH)D3) [3]. It should also be noted that 1,25(OH)2D3 to 25(OH)D3 appeared to be independent of the estimated glomerular function and therefore VMR-2 was unaffected. Here, it should be noted that serum calcitriol concentration is tightly regulated and would not be expected to vary when renal function is in the normal range, as is the case in this study cohort [26]. Further, all target tissues activate vitamin D, where it has paracrine and autocrine functions locally, but whether such calcitriol enters the circulation is unclear [27, 28].
The VMR-1 ratio was less discriminatory than VMR-2, associating only with diabetic retinopathy and peripheral artery disease and, in addition, the 24,25(OH)2D3 correlated to the eGFR, suggesting that this VMR-1 ratio would be affected by renal function. The VMR-3 ratio did not prove to be a usefully discriminatory measure, though it did associate with more complications than 3epi-25(OH)D3 alone.
This Middle East population is an ethnic group at high risk for vitamin D deficiency with all the associated negative outcomes such as increased risks of bone disease as well as diabetic and cardiovascular complications [5, 8, 9]. However, given the fact that the circulating concentrations of 25-hydroxyvitamin D3 (25(OH)D3) are universally low in this population [8], a measure of vitamin D status that could distinguish healthy individuals despite their having a low 25(OH)D3 versus individuals with low 25(OH)D3 at high risk for development of diabetic and cardiovascular complications, would be clinically useful.
When compared with Caucasian Americans, African Americans tend to have lower concentrations of 25(OH) D [11–13], often meeting the criteria for vitamin D insufficiency, and yet have more robust bone health [14–17]. Therefore, 25(OH) D alone is not always a discriminatory test depending on the population group. The metabolite of 25(OH) D, 24,25(OH)2D, has been proposed as an additional useful measure for several reasons [29, 30]. Firstly, concentrations of 24,25(OH)2D and 25(OH) D are closely correlated [31]. Secondly, 25(OH) D is converted to 24,25(OH)2D by CYP24A1, a 24-hydroxylase enzyme which is partially regulated by vitamin D receptor activity [32] [33]. However, the VMR-1 ratio was not found to be of greater value than VMR-2 for predicting risk of diabetic and cardiovascular complications.
Strengths of this study are the well-characterized, homogeneous Middle East population with well-recognized vitamin D deficiency, and that vitamin D3 and its metabolites were measured on state-of-the-art equipment. Limitations of this study include the fact that it was a cross sectional design and the relatively modest numbers of subjects for such a population-based study, but this limitation is mitigated by the homogeneous nature of the population studied. A prospective study would be necessary to validate the results found here. Furthermore, our findings here may not be applicable to other ethnic groups or countries, since Middle Easterners have low vitamin D status, in part, because of their primarily vegetable-based diet, near total skin coverage and tendency to stay indoors to avoid the hot summer sun [34].
Conclusion
In conclusion, in type 2 diabetes, VMR-2 was shown to be the superior predictor for development of diabetic and cardiovascular complications in this study population over VMR-1, VMR-3 and the individual vitamin D concentration measurements, associating with both microvascular and cardiovascular indices and therefore may have utility in predicting the development of diabetic complications.
Supplementary Information
Acknowledgements
None.
Abbreviations
- VMR
Vitamin D Metabolite Ratio
- 25(OH)D3
25-hydroxyvitamin D3
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- 24,25(OH)2D3
24,25-dihydroxyvitamin D3
- 3-epi-25(OH)D3
3-epi-25-hydroxyvitamin D3
- T2D
Type 2 Diabetes
- LC
liquid chromatography
- LC-MS/MS
Liquid chromatography-mass spectrometry/mass spectrometry
- HbA1c
glycosylated hemoglobin
- WHO
World Health Organization
- IRB
Internal Review Board
- ICH GCP
International Conference on Harmonization Good Clinical Practice
- SLE
supported liquid extraction
- PTAD
4-(4-(2-Azidoethoxy)phenyl)-1,2,4-triazolidine-3,5-dione
- SPSS
Statistical Package for the Social Sciences
- eGFR
estimated glomerular filtration rate
- CAD
coronary artery disease
- PAD
peripheral artery disease
- CYP24A1
Cytochrome P450 family 24 subfamily A member 1
Authors’ contributions
LHMA and AEB collated the data and wrote the manuscript. SRD performed the statistical analysis. AL performed the measurements of the vitamin D3 metabolite profiles. OMC collated the data. SLA and CAK designed the study and contributed to the discussion. All authors have seen and approved the final version of this report and give consent to its publication. Stephen L. Atkin is the guarantor of this work.
Funding
Dr. Abi Khalil’s lab is funded by the Qatar National Research Fund (QNRF), NPRP Grant 9–169–3-024. The funding source did not have a role in the design of the study, decision to publish or writing of the manuscript.
Availability of data and materials
All data underlying this study will be made available upon reasonable request to the corresponding author.
Ethics approval and consent to participate
The study was approved by Weill Cornell IRB (IRB# 13–00063) and all participants provided written informed consent. The conduct of the trial was in accordance with ICH GCP and the Declaration of Helsinki.
Consent for publication
All authors gave their consent for publication of this manuscript.
Competing interests
There are no competing interests. The authors have nothing to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stephen L. Atkin and Charbel Abi Khalil are Joint senior authors.
References
- 1.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 2.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 dietary reference intakes for calcium and vitamin D: what dietetics practitioners need to know. J Am Diet Assoc. 2011;111(4):524–527. doi: 10.1016/j.jada.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 6.el-Sonbaty MR, Abdul-Ghaffar NU. Vitamin D deficiency in veiled Kuwaiti women. Eur J Clin Nutr. 1996;50(5):315–318. [PubMed] [Google Scholar]
- 7.Mirsaeid Ghazi AA, Rais Zadeh F, Pezeshk P, Azizi F. Seasonal variation of serum 25 hydroxy D3 in residents of Tehran. J Endocrinol Investig. 2004;27(7):676–679. doi: 10.1007/BF03347502. [DOI] [PubMed] [Google Scholar]
- 8.Bassil D, Rahme M, Hoteit M, Fuleihan GH. Hypovitaminosis D in the Middle East and North Africa: prevalence, risk factors and impact on outcomes. Dermatoendocrinol. 2013;5(2):274–298. doi: 10.4161/derm.25111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakhtoura M, Rahme M, Chamoun N, El-Hajj FG. Vitamin D in the Middle East and North Africa. Bone Rep. 2018;8:135–146. doi: 10.1016/j.bonr.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. PMID: 24414552. 10.1002/14651858.CD007470.pub3. [DOI] [PMC free article] [PubMed]
- 11.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell DM, Henao MP, Finkelstein JS, Burnett-Bowie SA. Prevalence and predictors of vitamin D deficiency in healthy adults. Endocr Pract. 2012;18(6):914–923. doi: 10.4158/EP12072.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zahiri Z, Sharami SH, Milani F, Mohammadi F, Kazemnejad E, Ebrahimi H, et al. Metabolic syndrome in patients with polycystic ovary syndrome in Iran. Int J Fertil Steril. 2016;9(4):490–496. doi: 10.22074/ijfs.2015.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116(9):634–639. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Cauley JA, Danielson ME, Boudreau R, Barbour KE, Horwitz MJ, Bauer DC, et al. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women's Health Initiative (WHI) J Bone Miner Res. 2011;26(10):2378–2388. doi: 10.1002/jbmr.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293(17):2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 18.Tieu EW, Tang EK, Tuckey RC. Kinetic analysis of human CYP24A1 metabolism of vitamin D via the C24-oxidation pathway. FEBS J. 2014;281(14):3280–3296. doi: 10.1111/febs.12862. [DOI] [PubMed] [Google Scholar]
- 19.Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin N Am. 2014;43(1):205–232. doi: 10.1016/j.ecl.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamao M, Tatematsu S, Hatakeyama S, Sakaki T, Sawada N, Inouye K, et al. C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1alpha or C-24 hydroxylation. J Biol Chem. 2004;279(16):15897–15907. doi: 10.1074/jbc.M311473200. [DOI] [PubMed] [Google Scholar]
- 21.Schleicher RL, Encisco SE, Chaudhary-Webb M, Paliakov E, McCoy LF, Pfeiffer CM. Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin Chim Acta. 2011;412(17–18):1594–1599. doi: 10.1016/j.cca.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Butler AE, Dargham SR, Latif A, Mokhtar HR, Robay A, Chidiac OM, Jayyousi A, Al Suwaidi J, Crystal RG, Abi Khalil C, Atkin SL. Association of vitamin D3 and its metabolites in patients with and without type 2 diabetes and their relationship to diabetes complications. Ther Adv Chronic Dis. 2020;11:2040622320924159. PMID: 33062234; PMCID: PMC7534081.10.1177/2040622320924159. [DOI] [PMC free article] [PubMed]
- 23.Abbas S, Linseisen J, Rohrmann S, Beulens JW, Buijsse B, Amiano P, et al. Dietary vitamin D intake and risk of type 2 diabetes in the European prospective investigation into Cancer and nutrition: the EPIC-InterAct study. Eur J Clin Nutr. 2014;68(2):196–202. doi: 10.1038/ejcn.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Beirne SL, Salit J, Rodriguez-Flores JL, Staudt MR, Abi Khalil C, Fakhro KA, et al. Type 2 diabetes risk allele loci in the Qatari population. PLoS One. 2016;11(7):e0156834. doi: 10.1371/journal.pone.0156834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deckers JG, Schellevis FG, Fleming DM. WHO diagnostic criteria as a validation tool for the diagnosis of diabetes mellitus: a study in five European countries. Eur J Gen Pract. 2006;12(3):108–113. doi: 10.1080/13814780600881268. [DOI] [PubMed] [Google Scholar]
- 26.Souberbielle JC, Cavalier E, Delanaye P, Massart C, Brailly-Tabard S, Cormier C, et al. Serum calcitriol concentrations measured with a new direct automated assay in a large population of adult healthy subjects and in various clinical situations. Clin Chim Acta. 2015;451(Pt B):149–153. doi: 10.1016/j.cca.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Pike JW, Meyer MB. The unsettled science of nonrenal calcitriol production and its clinical relevance. J Clin Invest. 2020;130(9):4519–4521. doi: 10.1172/JCI141334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3–5):316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 29.Wagner D, Hanwell HE, Schnabl K, Yazdanpanah M, Kimball S, Fu L, et al. The ratio of serum 24,25-dihydroxyvitamin D (3) to 25-hydroxyvitamin D (3) is predictive of 25-hydroxyvitamin D (3) response to vitamin D (3) supplementation. J Steroid Biochem Mol Biol. 2011;126(3–5):72–77. doi: 10.1016/j.jsbmb.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Bosworth CR, Levin G, Robinson-Cohen C, Hoofnagle AN, Ruzinski J, Young B, et al. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int. 2012;82(6):693–700. doi: 10.1038/ki.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371(1):22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014;55(1):13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pike JW, Meyer MB. Regulation of mouse Cyp24a1 expression via promoter-proximal and downstream-distal enhancers highlights new concepts of 1,25-dihydroxyvitamin D (3) action. Arch Biochem Biophys. 2012;523(1):2–8. doi: 10.1016/j.abb.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowe FL, Steur M, Allen NE, Appleby PN, Travis RC, Key TJ. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: results from the EPIC-Oxford study. Public Health Nutr. 2011;14(2):340–346. doi: 10.1017/S1368980010002454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data underlying this study will be made available upon reasonable request to the corresponding author.