Abstract
Objectives
We aimed to compare the antiviral effect of hydroxychloroquine (HCQ) and lopinavir/ritonavir (LPV/r) in patients with COVID-19.
Methods
Nationwide retrospective case-control study was conducted to compare the effect of HCQ and LPV/r on viral shedding duration among patients with mild-to-moderate COVID-19 using the reimbursement data of National Health Insurance Service. After propensity score matching (PSM), multivariate analysis was conducted to determine statistically significant risk factors associated with prolonged viral shedding.
Results
Overall, 4197 patients with mild-to-moderate COVID-19 were included. Patients were categorized into three groups: LPV/r (n = 1268), HCQ (n = 801), and standard care without HCQ or LPV/r (controls, n = 2128). The median viral shedding duration was 23 (IQR 17–32), 23 (IQR 16–32), and 18 (IQR 12–25) days in the LPV/r, HCQ, and control groups, respectively. Even after PSM, the viral shedding duration was not significantly different between LPV/r and HCQ groups: 23 (IQR, 17–32) days versus 23 (IQR, 16–32) days. On multivariate analysis, old age, malignancy, steroid use, and concomitant pneumonia were statistically significant risk factors for prolonged viral shedding.
Conclusion
The viral shedding duration was similar between HCQ and LPV/r treatment groups. There was no benefit in improving viral clearance compared to the control group.
Keywords: COVID-19, SARS-CoV-2, Hydroxychloroquine, Lopinavir, Ritonavir
Introduction
Since the first emerging in December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly around the world, infecting over 38 million people globally and resulting in 1,089,047 deaths as of October 15, 2020 (WHO, 2020). To bring an end to this health crisis, vaccines are under development, but they are likely 1–2 years away. It is thus very important to minimize person-to-person transmission.
Antiviral agents are commonly used for improving clinical symptoms or ameliorating disease severity. Furthermore, they have an important clinical implication to suppress disease transmission by reducing the viral shedding duration, as shown from the effect of oseltamivir for influenza (Beigel et al., 2020, Meschi et al., 2011). Owing to the highly transmissible property of SARS-CoV-2, even asymptomatic and presymptomatic patients have transmitted the virus to their family members and colleagues (Bai et al., 2020, Gandhi et al., 2020). Moreover, as the pandemic has progressed, the number of deaths in high-risk groups has increased dramatically. Therefore, the treatment strategy for COVID-19 needs to be approached in two ways: reduction of mortality through combined antiviral therapy for severe patients and blockage of transmission through early antiviral treatment for patients with mild-to-moderate cases.
In the early pandemic periods, the most hopeful antiviral candidates were hydroxychloroquine (HCQ) and lopinavir/ritonavir (LPV/r), which had already been on the market for decades with other indications (Kim et al., 2020, Sanders et al., 2019. Both candidates were expected to interfere viral replication theoretically (Kim et al., 2020, Sanders et al., 2019), and showed good in vitro activity against SARS-CoV-2 (Huang et al., 2020, Ul Qamar et al., 2020). Under urgent needs, many clinical trials using either candidate have been conducted, but there are still insufficient data to recommend HCQ or LPV/r use. Furthermore, most studies mainly focused on patients with moderate-to-severe COVID-19, and antiviral agents were administered at more than 7 days later from symptom onset (Borba et al., 2020, Cao et al., 2020, Hung et al., 2020, Tang et al., 2020). It is necessary to comparatively evaluate the effect of viral suppression when the antiviral agent is administered in the early stage of symptom development. If effective for viral suppression, it has a very important meaning in terms of infection control and treatment (Gautret et al., 2020, Li et al., 2020).
This study aimed to compare the effect of HCQ and LPV/r on the viral shedding duration among patients with mild-to-moderate COVID-19 cases using South Korea’s National Health Insurance Service (NHIS) database.
Methods
Data source
This study used reimbursement data from the National Health Insurance Service (NHIS) of South Korea for the period from January 12, 2020 to May 15, 2020. The NHIS covers 97–98% of the population (51 million people). Data included age, sex, dates of admission and discharge, diagnoses coded according to the International Classification of Disease and Related Health Problems, 10th edition (ICD-10), and prescription of medications covered by NHIS.
Currently, the NHIS aggregates datasets for real-time reverse transcriptase polymerase chain reaction (rRT-PCR)-confirmed COVID-19 cases from information provided by the epidemiological investigation of Korea Centers for Disease Control and Prevention (KCDC) (2020). All subjects with KCD-7 codes for COVID-19 were classified into the 5 categories according to the Centers for Disease Control and Prevention (CDC) (2020) interim guidance: critical (extracorporeal membrane oxygenation, death), severe (mechanical ventilator), moderate grade 2 (high flow oxygen therapy), moderate grade 1 (oxygen therapy), and mild (remaining laboratory confirmed subjects) (Supplementary Table 1).
Study design
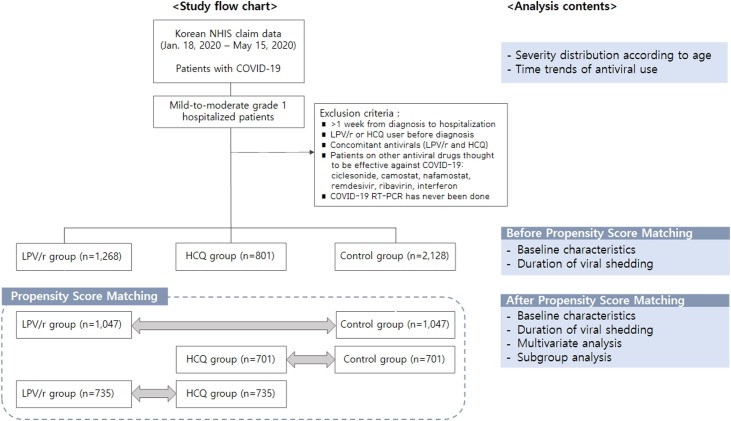
This nationwide retrospective study included patients with laboratory-confirmed COVID-19 diagnoses who were discharged during the study period from January 12, 2020 to May 15, 2020. Among these, only mild-to-moderate grade 1 patients were included in the analysis, and the effect of LPV/r or HCQ use on viral shedding duration was evaluated (Figure 1 ). We strictly included patients with mild or moderate grade 1 COVID-19, excluding severe patients for the following reasons: in severe cases, anti-viral agents might have been administered following late aggravation after initial supportive standard care and hospital stay may have been extended due to complications, although SARS-CoV-2 rRT-PCR test converted to be negative. Furthermore, the following two inclusion criteria should be met: (i) adults aged ≥19 years and (ii) hospitalization within 1 week after laboratory diagnosis for COVID-19. The criteria of ≤1 week from diagnosis to hospitalization is needed to assess the effect of early antiviral treatment. Exclusion criteria were as follows: (i) concomitant LPV/r and HCQ treatment; (ii) patients on LPV/r or HCQ medication prior to diagnosis; or (iii) those who received other antiviral agents thought to be effective against COVID-19 (ciclesonide, camostat, nafamostat, remdesivir, ribavirin, or interferon). For patients with multiple episodes of hospitalization, the first admission was only included for the analysis.
Figure 1.
Study flow chart.
All included patients were categorized according to LPV/r or HCQ exposure: LPV/r-group, HCQ-group, and control group (supportive standard care only). LPV/r or HCQ use was defined as at least one prescription being recorded in the claim data. Data on the prescription of LPV/r, HCQ, or other drugs were extracted using drug codes based on the Anatomical Therapeutic Chemical Classification in the claim data of the study periods. Comorbidities were identified using ICD-10 codes entered within 1 year prior to COVID-19 diagnosis (Supplementary Table 2). Charlson Comorbidity Index (CCI) was also calculated to assess the general health status of study subjects (Supplementary Table 3). A subgroup analysis was conducted for mild cases only, moderate grade 1 cases only, and patients with pneumonia (defined as ICD-10 codes).
We defined patient’s length of hospitalization as viral shedding duration, which was assessed using rRT-PCR. This is reasonable, because all COVID-19 patients in South Korea required undetectable RNA from two consecutive nasopharyngeal swab specimens (24 h apart) to be discharged, according to the regulation of KCDC (Choi et al., 2020b).
This study protocol was exempted for review by the Institutional Review Board of the Korea University Guro Hospital according to the exemption criteria (IRB No. 2020GR0260).
Statistical analysis
The data are presented using descriptive statistics for continuous and categorical variables. Differences between groups were analyzed with an analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables.
Considering the significant differences in baseline characteristics among study groups, propensity score matching (PSM) was taken between two groups to be compared. To compare the viral shedding duration, we created propensity scores for the LPV/r-group, HCQ-group, and control group. All sets of propensity scores were estimated via multinomial logistic regression using baseline covariates including age, sex, comorbidities, disease severity, and concomitant pneumonia. To compare the viral shedding duration, three data sets were made (LPV/r-group vs. HCQ-group; LPV/r-group vs. control group; HCQ-group vs. control group), and each of the sets were propensity score matched in 1:1 proportion. Age and sex were perfectly matched, and Greedy nearest neighbor matching was used for other covariates on the logit of the propensity score.
After PSM, multiple linear regression was used to determine statistically significant factors associated with viral shedding duration. Variables included in the models were age, sex, comorbidities, disease severity, concomitant pneumonia, concomitant use of steroid, azithromycin or oseltamivir, and elapsed days from laboratory diagnosis to hospitalization.
All tests were two tailed, and results were considered statistically significant at p-value <0.05. SAS version 9.4 (SAS Institute Inc, Cary, NC) was used for the analyses.
Results
During study periods, a total of 5720 COVID-19 patients were discharged (or death occurred) in South Korea. Most cases (>95%) were hospitalized within a day from laboratory diagnosis, and more than 98% were hospitalized within 7 days. Regarding disease severity, children and adolescents were milder in severity: 99.1% (214/216) were mild, 1.4% (2/216) were moderate grade 1, and none were moderate grade 2 or severe (Supplementary Table 4). Similarly, mild cases accounted for the large portion in adults aged 19–64 year (91.7%, 4037/4402). However, in the elderly, less than two-thirds of cases (59.5%, 656/1102) were mild, while the rest required oxygen therapy; one-third of cases on oxygen therapy required high flow oxygen supply or mechanical ventilation. Since the first emergence of COVID-19 in South Korea, the prescription trend of LPV/r and HCQ is shown in Supplementary Figure 1. Overall, LPV/r and HCQ prescription tended to decrease, and preference appeared to change significantly depending on the literature published at that time. Sequentially, LPV/r was replaced by HCQ and supportive standard care.
Baseline characteristics of patients with mild-to-moderate grade 1 COVID-19
A total of 4197 patients with mild-to-moderate grade 1 COVID-19 were included in this study. Patients were categorized into three different groups: those treated with LPV/r (LPV/r-group, n = 1268), those treated with HCQ (HCQ-group, n = 801), and those with supportive standard care without HCQ or LPV/r (control group, n = 2128) (Supplementary Table 5). There were some significant differences among the three groups in the baseline characteristics. Compared to LPV/r or HCQ-groups, the control group was significantly younger, had fewer comorbidities, and included more males. The oseltamivir combination rate was less than 0.5% in all groups.
The median time of viral RNA shedding was 23 (IQR 17–32) days in the LPV/r-group, 23 (IQR 16–32) days in the HCQ-group, and 18 (IQR 12–25) days in the control group. There was no significant difference between the LPV/r-group and the HCQ-group, but the viral shedding duration was estimated to be significantly longer in both treatment groups compared to the control group.
Propensity score matched comparison of the antiviral effect on viral shedding duration — LPV/r versus HCQ treatment
As the baseline characteristics showed significant difference across the three groups, we computed propensity scores for LPV/r use and HCQ use based on age and sex. After PSM, most of the baseline characteristics were similar, including comorbidities. However, the disease severity and proportion of accompanying pneumonia were still significantly higher in the LPV/r and HCQ-group, especially in the LPV/r-group (Table 1 ).
Table 1.
Baseline characteristics after propensity score matching between the two groups.
| LPV/r-group (n = 1047) | Control (n = 1047) | p-Value | HCQ-group (n = 701) | Control (n = 701) | p-Value | LPV/r-group (n = 735) | HCQ-group (n = 735) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 45.95 (15.64) | 45.95 (15.64) | 1 | 48.73 (16.33) | 48.73 (16.33) | 1 | 50.72 (16.78) | 50.72 (16.78) | 1 |
| Age, groups | 1 | 1 | 1 | ||||||
| 19–49 years | 565 (53.96%) | 565 (53.96%) | – | 315 (44.94%) | 315 (44.94%) | – | 301 (40.95%) | 301 (40.95%) | – |
| 50–64 years | 367 (35.05%) | 367 (35.05%) | – | 290 (41.37%) | 290 (41.37%) | – | 298 (40.54%) | 298 (40.54%) | – |
| ≥65 years | 115 (10.98%) | 115 (10.98%) | – | 96 (13.69%) | 96 (13.69%) | – | 136 (18.50%) | 136 (18.50%) | – |
| Male, no (%) | 404 (38.59%) | 404 (38.59%) | – | 246 (35.09%) | 246 (35.09%) | 1 | 246 (33.47%) | 246 (33.47%) | 1 |
| From diagnosis to admission, days | 0.09 (0.63) | 0.07 (0.57) | 0.4895 | 0.03 (0.27) | 0.02 (0.29) | 0.8497 | 0.14 (0.84) | 0.20 (0.26) | 0.0003 |
| CCI (SD) | 1.15 (1.47) | 1.07 (1.34) | 0.1925 | 1.35 (1.62) | 1.2 (1.49) | 0.0594 | 1.38 (1.63) | 1.39 (1.61) | 0.8341 |
| Comorbidities | 578 (49.15%) | 598 (50.85%) | 0.3784 | 412 (58.77%) | 406 (57.92%) | 0.7451 | 458 (62.31%) | 449 (61.09%) | 0.6292 |
| Diabetes | 159 (15.19%) | 139 (13.28%) | 0.2109 | 127 (18.12%) | 132 (18.83%) | 0.7308 | 126 (17.14%) | 139 (18.91%) | 0.3778 |
| Thyroid disease | 69 (6.59%) | 68 (6.49%) | 0.9296 | 75 (10.70%) | 76 (10.84%) | 0.9313 | 47 (6.39%) | 79 (10.75%) | 0.0029 |
| Cardiac disease | 62 (5.92%) | 75 (7.16%) | 0.2506 | 65 (9.27%) | 59 (8.42%) | 0.5725 | 61 (8.30%) | 68 (9.25%) | 0.5187 |
| Chronic respiratory disease | 305 (29.13%) | 331 (31.61%) | 0.2166 | 185 (26.39%) | 176 (25.11%) | 0.5825 | 253 (34.42%) | 199 (27.07%) | 0.0023 |
| Renal disease | 20 (1.91%) | 22 (2.10%) | 0.7552 | 16 (2.28%) | 15 (2.14%) | 0.8559 | 28 (3.81%) | 16 (2.18%) | 0.0662 |
| Chronic liver disease | 134 (12.8%) | 107 (10.22%) | 0.0645 | 109 (15.55%) | 94 (13.41%) | 0.2549 | 96 (13.06%) | 118 (16.05%) | 0.1037 |
| Chronic neurologic disease | 97 (9.26%) | 81 (7.74%) | 0.2099 | 108 (15.41%) | 106 (15.12%) | 0.8819 | 88 (11.97%) | 130 (17.69%) | 0.0021 |
| Malignancy | 45 (4.30%) | 53 (5.06%) | 0.4078 | 40 (5.71%) | 38 (5.42%) | 0.8157 | 36 (4.90%) | 39 (5.31%) | 0.7221 |
| Rheumatologic disease | 29 (2.77%) | 28 (2.67%) | 0.8932 | 22 (3.14%) | 14 (2.00%) | 0.1768 | 2 (2.99%) | 22 (2.99%) | 1 |
| Anemia | 78 (7.45%) | 95 (9.07%) | 0.1772 | 69 (9.84%) | 60 (8.56%) | 0.4056 | 73 (9.93%) | 70 (9.52%) | 0.7917 |
| Hematologic disease | 18 (1.72%) | 12 (1.15%) | 0.2699 | 4 (0.57%) | 1 (0.14%) | 0.3741 | 19 (2.59%) | 7 (0.95%) | 0.0176 |
| Mental and behavioral disorder | 184 (17.57%) | 173 (16.52%) | 0.5227 | 147 (20.97%) | 163 (23.25%) | 0.3032 | 129 (17.55%) | 167 (22.72%) | 0.0135 |
| HIV | 1 (0.10%) | 1 (0.10%) | 1 | 1 (0.14%) | 1 (0.14%) | 1 | 1 (0.07%) | 1 (0.07%) | 1 |
| Disease severity | <0.0001 | 0.0025 | <0.0001 | ||||||
| Mild | 911 (87.01%) | 1012 (96.66%) | – | 645 (92.01%) | 672 (95.86%) | – | 600 (81.63%) | 680 (92.52%) | – |
| Moderate, grade 1 | 136 (12.99%) | 35 (3.34%) | – | 56 (7.99%) | 29 (4.14%) | – | 135 (18.37%) | 55 (7.48%) | – |
| Pneumonia, no (%) | 602 (57.50%) | 129 (12.32%) | <0.0001 | 305 (43.51%) | 108 (15.41%) | <0.0001 | 501 (68.16%) | 313 (42.59%) | <0.0001 |
| Steroid use, no (%) | 33 (3.15%) | 13 (1.24%) | 0.0029 | 20 (2.85%) | 10 (1.43%) | 0.065 | 24 (3.27%) | 18 (2.45%) | 0.3476 |
| Azithromycin use, no (%) | 211 (20.15%) | 12 (1.15%) | <0.0001 | 222 (31.67%) | 10 (1.43%) | <0.0001 | 82 (11.16%) | 212 (28.84%) | <0.0001 |
| Oseltamivir use, no (%) | 5 (0.48%) | 1 (0.1%) | 0.2181 | 2 (0.29%) | 1 (0.14%) | 1 | 5 (0.68%) | 2 (0.27%) | 0.452 |
LPV/r = lopinavir/ritonavir; HCQ = hydroxychloroquine; SD = standard deviation; CCI = Charlson Comorbidity Index.
Total dosage of LPV/r was 3884/971 mg on average, which was considered to have been administered for about 5 days when calculated based on 800/200 mg/day as recommended by the guidelines (Kim et al., 2020). HCQ was used on average of 2376 mg, which was equivalent to dosage for 5–6 days, calculated based on 400 mg/day recommended by the guideline.
The median time of viral RNA shedding was not significantly different between the LPV/r and HCQ-group: 23 (IQR, 17–32) days versus 23 (IQR, 16–32) days (Table 2 ). Neither agent shortened the viral shedding duration compared to the control group.
Table 2.
Comparison of viral shedding duration based on antiviral treatment groups among patients with mild-to-moderate grade 1 COVID-19.
| LPV/r-group | HCQ-group | Control | p-Value | ||
|---|---|---|---|---|---|
| Duration of viral shedding | Before PSM, days (IQR) | 23 (17–32)a | 23 (16–32)a | 18 (12–25)b | <0.0001 |
| After PSM, days (IQR) | 22 (17–32) | 18 (12–26) | <0.0001 | ||
| 22 (16–31) | 18 (11–27) | <0.0001 | |||
| 23 (17–32) | 22 (16–32) | 0.1527 |
LPV/r = lopinavir/ritonavir; HCQ = hydroxychloroquine; PSM = propensity score matching; IQR = interquartile range.
The same superscript letters indicate non-significant differences between groups based on post-hoc analysis.
Subgroup and multivariate analyses for the viral shedding duration
On multivariate analysis using propensity score-matched data sets comparing each antiviral group versus control group, LPV/r or HCQ still showed a significantly longer viral shedding duration compared to the control group. However, the significance due to the use of antiviral agents disappeared in the subgroup analysis which includes only moderate cases or pneumonia cases (Supplementary Tables 6 and 7).
On multivariate analysis using dataset comparing LPV/r and HCQ groups, neither of the agents showed a significant difference in terms of the viral shedding duration. The factors that significantly influence the viral shedding duration were age, malignancy, steroid use, and concomitant pneumonia (Table 3 ). As the elapsed time from diagnosis to hospitalization is longer, in-hospital shedding duration was much shorter. In the subgroup analysis for patients with moderate grade 1 severity or concomitant pneumonia, cardiac disease was identified as a factor that significantly increased the viral shedding duration.
Table 3.
Multivariate analysis for the viral shedding duration among antiviral users (LPV/r or HCQ) for COVID-19.
| LPV/r-group compared to HCQ-group | Estimate | p-Value | ||
|---|---|---|---|---|
| All cases (n = 1470) | Univariate analysis | Intercept | 25.29 | <0.0001 |
| LPV/r-group | 1.01 | 0.1527 | ||
| HCQ-group | 0 | |||
| Multivariate analysis (significant factors) | LPV/r-group | 0.59 | 0.4408 | |
| Elapsed days from diagnosis to admission | −1.24 | 0.0249 | ||
| Age | 0.09 | 0.0004 | ||
| Pneumonia | 2.48 | 0.0008 | ||
| Steroid use | 11.79 | <0.0001 | ||
| Malignancy | 3.46 | 0.0295 | ||
| Mild (n = 1280) | Univariate analysis | Intercept | 25.0 | <0.0001 |
| LPV/r-group | 0.49 | 0.4914 | ||
| HCQ-group | 0 | |||
| Multivariate analysis | LPV/r-group | 0.4764 | 0.5331 | |
| Age | 0.085 | 0.0005 | ||
| Pneumonia | 2.14 | 0.004 | ||
| Steroid use | 15.56 | <0.0001 | ||
| Chronic neurologic disease | 2.86 | 0.0179 | ||
| Malignancy | 4 | 0.0173 | ||
| Moderate, grade 1 (n = 190) | Univariate analysis | Intercept | 28.9 | <0.0001 |
| LPV/r-group | 0.99 | 0.7221 | ||
| HCQ-group | 0 | |||
| Multivariate analysis | LPV/r-group | 0.42 | 0.89 | |
| Cardiac disease | 9.32 | 0.0075 | ||
| Hematologic disease | 18.78 | 0.0417 | ||
| Concomitant pneumonia (n = 814) | Univariate analysis | Intercept | 26.71 | <0.0001 |
| LPV/r-group | 1.11 | 0.2557 | ||
| HCQ-group | 0 | |||
| Multivariate analysis | LPV/r-group | 1.41 | 0.1721 | |
| Age | 0.095 | 0.0056 | ||
| Steroid use | 9.7 | 0.0002 | ||
| Cardiac disease | 1.74 | 0.0002 | ||
LPV/r = lopinavir/ritonavir; HCQ = hydroxychloroquine.
Discussion
Currently, no specific antiviral agent is available for the prevention or treatment of COVID-19, so drug repurposing has been considered as a promising approach to rapidly identify an effective therapy. HCQ and LPV/r are the candidates at the forefront of drug repurposing. This nationwide retrospective study was conducted to evaluate the antiviral effect of HCQ and LPV/r in the treatment of patients with mild COVID-19 using the NHIS reimbursement dataset. In this study, the viral shedding duration of SARS-CoV-2 was similar between HCQ and LPV/r treatment groups. When analyzing the effect of antiviral agents, the timing of antiviral therapy is an important issue to be considered. Early (within 7 days from symptom onset) initiation of antiviral therapy may be critical in reducing SARS-CoV-2 viral load, as previously noted (Fu et al., 2020, Keyaerts et al., 2004, Yan et al., 2020). Due to the limitation of our database, it was difficult to know the initiation timing of antiviral treatment in each individual patient; however, the government of South Korea had launched a series of aggressive measures to perform tight contact tracing and mass screening tests, which enabled early diagnosis within 3–5 days from symptom onset (Ryu et al., 2020). Based on the expert’s guideline, most patients were treated within 7 days after symptom development (Kim et al., 2020). As shown in this study, >95% were hospitalized within a day from laboratory diagnosis. It took more than 1 week to be hospitalized in only a small number of patients, and those were excluded in this study considering the timing issue.
HCQ or LPV/r monotherapy showed no benefit for improving viral clearance compared to the control group. The viral shedding duration seemed to be rather prolonged in the treatment groups (median viral shedding duration, 22–23 days in the antiviral treatment groups vs. 18 days in the control group). However, it would be possible that the viral shedding duration in the control group was estimated to be shorter than it really is because of the following reasons. First, community treatment centers (CTC), which were introduced in Korea as a measure to efficiently distribute limited medical resources during the declared epidemic starting in early March 2020, could make a bias in the claim database. Some patients with mild symptoms were transferred from the hospital to CTCs if they were medically stable but needed to maintain isolation (Choi et al., 2020b). Although supportive care is maintained in CTCs, some CTCs might not claim reimbursement due to neglectable medical costs and complex process, potentially contributing to the shortened length of hospitalization in the control group. Second, during the earlier period of the COVID-19 pandemic in South Korea, many mild COVID-19 patients diagnosed at the airport quarantine received supportive care without antiviral treatment at the CTC. Actually, a majority of them had symptoms for more than a week before traveling, so the viral shedding duration might have been estimated shorter. In the previous studies including mild COVID-19 patients in CTCs, the mean viral shedding duration from symptom onset was 21–24.5 days, which is longer than the results of our control group (Lee et al., 2020, Noh et al., 2020). When we compared the viral shedding duration between the HCQ or LPV/r-groups and the control group in subgroup analyses including only moderate cases or those with concomitant pneumonia, there was no significant difference, which reflected the selection bias of mild cases who were mainly included in the control groups.
One of the most effective treatment strategies would be to stop the viral replication at the beginning, thereby minimizing the peak viral load and shedding duration (Chu et al., 2004, Kim et al., 2020). It is unclear why HCQ or LPV/r did not show favorable antiviral effect in this study. One possible reason is that a higher dose is required to successfully suppress SARS-CoV-2 in patients as shown in vitro cytotoxicity test (Cao et al., 2020, Keyaerts et al., 2004, Wang et al., 2020). In particular, the in vitro antiviral activity of HCQ at concentrations commonly used in humans was reported minimal (Kang et al., 2020). Insufficient data are available regarding the optimal dose to ensure the safety and efficacy of both drugs for COVID-19. Another possible reason is the inadequate target tissue concentration of those antiviral agents. LPV/r is an anti-HIV drug, innovated to get high plasma and lymphatic tissue concentration, not lung tissue (Freeling et al., 2014). There are no pharmacokinetic data on respiratory tract concentration of LPV/r. Although pharmacokinetic data indicate HCQ exhibits extensive tissue distribution, the tissue concentration of the respiratory tract might be variable depending on intestinal resorption and hepatic first-pass metabolism (Klimke et al., 2020, Maharaj et al., 2020). In comparison, there are several encouraging reports of HCQ on reducing mortality (Arshad et al., 2020, Catteau et al., 2020, COVID-19 RISK and Treatments (CORIST) Collaboration, 2020, Mikami et al., 2020). The inverse association of HCQ with mortality was more evident in elderly, in patients who experienced more severe manifestation or especially having elevated C-reactive protein. Furthermore, the beneficial impact was observed even in the late treatment groups, suggesting that the anti-inflammatory and anti-thrombotic potential of HCQ may have had more important role rather than its antiviral properties.
On multivariate analysis, old age, malignancy, steroid use, and concomitant pneumonia were identified as risk factors for prolonged viral shedding in this study, consistent with previous studies (Fu et al., 2020, Yan et al., 2020, Zhou et al., 2020). Old age, comorbidities, and steroid use might blunt the host immune response, thereby promoting viral replication. In the subgroup analyses, chronic neurologic diseases were also associated with increased risk of prolonged viral shedding in the cases with mild COVID-19, while cardiovascular disease was identified as a risk factor in the moderate cases or cases with concomitant pneumonia. Since ACE2, the SARS-CoV-2 binding receptors, is widely expressed in the various organ including the lungs, heart, and vessels, it is possible that greater number of ACE2 receptors—along with blunted host response encountered in many comorbid conditions—might promote viral replication, resulting in prolonged viral shedding. Recent studies suggested that the negative outcomes in patients with underlying cerebrovascular disease might be due to elevated expression of ACE2 (Choi et al., 2020a). Besides prolonged viral shedding, pre-existing cardiovascular diseases were associated with worse outcomes of COVID-19 (Fu et al., 2020). Although unclear, COVID-19 might trigger acute coronary syndrome, arrhythmia, or acute exacerbation of heart failure, similar to influenza viral infection (Madjid et al., 2020). SARS-CoV-2 itself might induce new cardiac pathology or exacerbation of underlying cardiovascular diseases under the systemic and/or localized inflammatory host response, resulting in cytokine storm in some severe cases (Madjid et al., 2020). Furthermore, although HCQ is known to be less toxic than chloroquine, HCQ-related cardiotoxicity might be considered (Di Girolamo et al., 2018, Nord et al., 2004). In this study, cardiovascular disease was a significant risk factor for longer hospitalization only in the analyses including the HCQ-group. Although generally safe when used for approved indications, including autoimmune inflammatory rheumatic diseases or malaria, the safety and benefit of HCQ treatment are poorly evaluated in COVID-19, and potential safety hazards have been announced recently, especially among severe patients and/or high-dose users (Borba et al., 2020, Mehra et al., 2020).
This study has some limitations. First, this was a retrospective cohort study. Although large number of cases were included, with propensity score matched for relevant variables, we could not rule out residual confounders. Second, we did not compare the viability of SARS-CoV-2 with the duration of infectivity. This study focused on the comparison of rRT-PCR-based viral clearance between LPV/r and HCQ. Third, this study did not include cases with severe COVID-19, having a limitation in evaluating the potential anti-inflammatory impact of HCQ or LPV/r in the prevention of complications and fatality. Fourth, environmental factors were not considered in this study. Environmental factors such as temperature, humidity and food might influence transmission, severity and mortality of COVID-19 (Eslami and Jalili, 2020, Roviello and Roviello, 2020). As the severity of COVID-19 and the effect of antiviral treatment may vary by region with different environments, further studies from various countries or regions would be required. Fifth, there was significant differences in baseline characteristics between HCQ and LPV/r groups. To overcome the differences, this study used PSM and subgroup/multivariate analyses. Finally, because of the limitation of study design using claim database, data on drug concentration and related metabolic factors were not available.
In conclusion, we compared the antiviral effect of LPV/r and HCQ in patients with mild-to-moderate COVID-19 using a large sample size health insurance database. The viral shedding duration was similar between HCQ and LPV/r groups. Neither HCQ nor LPV/r monotherapy showed benefits in improving viral clearance compared to the control group. Given such a limited effectiveness of HCQ or LPV/r monotherapy, a combination strategy needs to be considered. In fact, studies have shown beneficial effects when combining ribavirin and interferon rather than LPV/r alone (34), and several combination therapies have been tried.
Funding source
This work was supported by the National Research Foundation of Korea (NRF) grant [2020M3A9I2081699] to JYS and grants from the Gachon University Gil Medical Center [2018−17 and 2019−11] to JJ.
Ethical approval
This study protocol was exempted for review by the Institutional Review Board of the Korea University Guro Hospital according to the exemption criteria (IRB No. 2020GR0260).
Conflict of interest
We declare no conflict of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.10.062.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Manosuthi W., Beeler J., Bao Y., Hoppers M., Ruxrungtham K. Effect of oral oseltamivir on virological outcomes in low-risk adults with influenza: a randomized clinical trial. Clin Infect Dis. 2020;70(11):2317–2324. doi: 10.1093/cid/ciz634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catteau L., Dauby N., Montourcy M., Bottieau E., Hautekiet J., Goetghebeur E. Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: a nationwide observational study of 8075 participants. Int J Antimicrob Agents. 2020;56(4) doi: 10.1016/j.ijantimicag.2020.106144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2020. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19) Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. [Accessed 26 June 2020] [Google Scholar]
- Choi J.Y., Lee H.K., Park J.H., Cho S.J., Kwon M., Jo C. Altered COVID-19 receptor ACE2 expression in a higher risk group for cerebrovascular disease and ischemic stroke. Biochem Biophys Res Commun. 2020;528(3):413–419. doi: 10.1016/j.bbrc.2020.05.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W.S., Kim H.S., Kim B., Nam S., Sohn J.W. Community treatment centers for isolation of asymptomatic and mildly symptomatic patients with coronavirus disease, South Korea. Emerg Infect Dis. 2020;26(10) doi: 10.3201/eid2610.201539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.H. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 RISK, Treatments (CORIST) Collaboration Use of hydroxychloroquine in hospitalized COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study. Eur J Intern Med. 2020;S0953-6205(20):30335–30336. doi: 10.1016/j.ejim.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo F., Claver E., Olivé M., Salazar-Mendiguchía J., Manito N., Cequier Á. Dilated cardiomyopathy and hydroxychloroquine-induced phospholipidosis: from curvilinear bodies to clinical suspicion. Rev Esp Cardiol. 2018;71(6):491–493. doi: 10.1016/j.rec.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Eslami H., Jalili M. The role of environmental factors to transmission of SARS-CoV-2 (COVID-19) AMB Express. 2020;10(1):1–8. doi: 10.1186/s13568-020-01028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeling J.P., Koehn J., Shu C., Sun J., Ho R.J. Long-acting three-drug combination anti-HIV nanoparticles enhance drug exposure in primate plasma and cells within lymph nodes and blood. AIDS. 2014;28(17):2625–2627. doi: 10.1097/QAD.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Han P., Zhu R., Bai T., Yi J., Zhao X. Risk factors for viral RNA shedding in COVID-19 patients. Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01190-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med. 2020;382(22):2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Tang T., Pang P., Li M., Ma R., Lu J. Treating COVID-19 with chloroquine. J Mol Cell Biol. 2020;12(4):322–325. doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C.K., Seong M.W., Choi S.J., Kim T.S., Choe P.G., Song S.H. In vitro activity of lopinavir/ritonavir and hydroxychloroquine against severe acute respiratory syndrome coronavirus 2 at concentrations achievable by usual doses. Korean J Intern Med. 2020;35(4):782–787. doi: 10.3904/kjim.2020.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323(1):264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.B., Huh K., Heo J.Y., Joo E.J., Kim Y.J., Choi W.S. Interim guidelines on antiviral therapy for COVID-19. Infect Chemother. 2020;52(2):281–304. doi: 10.3947/ic.2020.52.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimke A., Hefner G., Will B., Voss U. Hydroxychloroquine as an aerosol might markedly reduce and even prevent severe clinical symptoms after SARS-CoV-2 infection. Med Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korea Centers for Disease Control and Prevention . 2020. Infection control guidlines for healthcare professional about COVID-19. Available at: http://www.gidcc.or.kr/epvbr/%EC%BD%94%EB%A1%9C%EB%82%98%EB%B0%94%EC%9D%B4%EB%9F%AC%EC%8A%A4%EA%B0%90%EC%97%BC%EC%A6%9D-19covid-19/. [Accessed 26 June 2020] [Google Scholar]
- Lee Y.H., Hong C.M., Kim D.H., Lee T.H., Lee J. Clinical course of asymptomatic and mildly symptomatic patients with coronavirus disease admitted to community treatment centers, South Korea. Emerg Infect Dis. 2020;26(10) doi: 10.3201/eid2610.201620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xie Z., Lin W., Cai W., Wen C., Guan Y. An exploratory randomized controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI) medRxiv. 2020 https://www.medrxiv.org/content/10.1101/2020.03.19.20038984v2 p. 2020.03.19.20038984. [Google Scholar]
- Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- Maharaj A.R., Wu H., Hornik C.P., Balevic S.J., Hornik C.D., Smith P.B. Simulated assessment of pharmacokinetically guided dosing for investigational treatments of pediatric patients with coronavirus disease 2019. JAMA Pediatr. 2020:e202422. doi: 10.1001/jamapediatrics.2020.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra M.R., Ruschitzka F., Patel A.N. Retraction-hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;395(10240):1820. doi: 10.1016/S0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschi S., Selleri M., Lalle E., Bordi L., Valli M.B., Ferraro F. Duration of viral shedding in hospitalized patients infected with pandemic H1N1. BMC Infect Dis. 2011;11(1):140. doi: 10.1186/1471-2334-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami T., Miyashita H., Yamada T., Harrington M., Steinberg D., Dunn A. Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med. 2020:1–10. doi: 10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J.Y., Yoon J.G., Seong H., Choi W.S., Sohn J.W., Cheong H.J. Asymptomatic infection and atypical manifestations of COVID-19: comparison of viral shedding duration. J Infect. 2020;81(5):816–846. doi: 10.1016/j.jinf.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord J.E., Shah P.K., Rinaldi R.Z., Weisman M.H. Hydroxychloroquine cardiotoxicity in systemic lupus erythematosus: a report of 2 cases and review of the literature. Semin Arthritis Rheum. 2004:336–351. doi: 10.1016/j.semarthrit.2003.09.012. Elsevier. [DOI] [PubMed] [Google Scholar]
- Roviello V., Roviello G.N. Lower COVID-19 mortality in Italian forested areas suggests immunoprotection by mediterranean plants. Environ Chem Lett. 2020:1–12. doi: 10.1007/s10311-020-01063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S., Ali S.T., Jang C., Kim B., Cowling B.J. Effect of nonpharmaceutical interventions on transmission of severe acute respiratory syndrome coronavirus 2, South Korea, 2020. Emerg Infect Dis. 2020;26(10) doi: 10.3201/eid2610.201886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2019;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ul Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.L. Structural basis of SARS-CoV-2 3CL(pro) and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020;10(4):313–319. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Coronavirus disease (COVID-19) weekly epidemiological update, 12 October 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20201012-weekly-epi-update-9.pdf. [Accessed: 15 October 2020] [Google Scholar]
- Yan D., Liu X.-Y., Zhu Y.-N., Huang L., Dan B.-T., Zhang G.-J. Factors associated with prolonged viral shedding and impact of lopinavir/ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection. Eur Respir J. 2020;56(1):2000799. doi: 10.1183/13993003.00799-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., He X., Zhang J., Xue Y., Liang M., Yang B. Prolonged SARS-CoV-2 viral shedding in patients with COVID-19 was associated with delayed initiation of arbidol treatment: a retrospective cohort study. MedRxiv. 2020 doi: 10.1007/s11596-021-2434-y. https://www.medrxiv.org/content/10.1101/2020.06.09.20076646v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.