Abstract
AIM
To assess the efficacy and safety of parafoveal retinal massage combined with autologous whole blood cover in the treatment of refractory macular holes (MHs) and present the surgical procedure.
METHODS
Patients with giant (minimum diameter ≥800 µm), recurrent or persistent MHs who underwent PPV combined with parafoveal retinal massage and autologous whole blood cover using C3F8 as tamponade agent from February 2018 to May 2019 were enrolled in this retrospective study. After surgery, all patients were informed to maintain a prone position for at least 7d. Preoperative and postoperative best-corrected visual acuities (BCVAs) were compared and MH closure rate was measured as the main outcome.
RESULTS
A total of 13 MH patients consisted of 6 giant MHs, 4 persistent holes and 3 recurrent holes (5 men and 8 women; average age was 56.40±11.72y) were enrolled in this study. MH closure was achieved in 11 eyes by this modified surgical technique while 2 eyes failed. Revitrectomy with autologous neurosensory retinal patch transplantations was applied for those 2 patients and then both holes were closed. No intraoperative complications were observed. BCVA improved from 1.73 logMAR to 0.74 logMAR at 6mo postoperation. There was significant difference in BCVA before versus after the surgery (P<0.05). There were no adverse events occurred during the follow-up period.
CONCLUSION
With easier surgical procedure, parafoveal retinal massage combined with autologous whole blood cover is an effective addition to the surgical options for the management of refractory MHs.
Keywords: giant macular hole, persistent macular hole, recurrent macular hole, parafoveal retinal massage, autologous blood cover, vitrectomy
INTRODUCTION
With the current gold standard of pars plana vitrectomy (PPV), induction of posterior vitreous detachment, internal limiting membrane (ILM) peeling and gas tamponade, the macular holes (MHs) closure rate after primary surgery commonly exceeds 90%[1]–[2]. However, treatment options for refractory MHs (including giant, recurrent, or persistent MHs) have not yet come to a consensus because of the lack of randomized clinical trials with a sufficiently large sample size[3]–[4]. The giant MH was generally recognized as diameter larger than 800 µm and it had a lower closure rate after a standard PPV or PPV combined with peeling of the ILM[5]. The rate of persistent MH varies between 8% and 44%[6]–[7] and has been found to be positively correlated with the size of the initial MHs[8]–[9]. In addition, late reopening of MH primarily closed after vitrectomy surgery was described in 5%-10% of cases[10]. Refractory MHs still remain a surgical challenge.
Recently, surgical technique of parafoveal retinal massage and autologous blood cover was described respectively in the management of those refractory MHs[11]–[12]. Although MH closure rates were improved by above-mentioned skills, the surgical technique still need to be modified for making it easier to perform. Thus, we presented a new surgical technique which combined these two skills into one surgical procedure to treat the refractory MHs and got an acceptable closure rate. The aims of this study were evaluating the efficacy and safety of parafoveal retinal massage combined with autologous whole blood cover in the management of patients with refractory MHs and presenting the surgical technique.
SUBJECTS AND METHODS
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and was approval by the Research Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University. All patients had been fully informed of the purpose and methods of the present study and provided written informed consent from themselves or other their guardians.
Eligibility of Patients
Patients with giant (MH minimum diameter ≥800 µm), persistent (unclosed holes had underwent one or more times vitreoretinal surgeries) or recurrent (late reopening of primarily closed MHs) MHs were enrolled in this retrospective study. All the enrolled patients were recruited from the First Affiliated Hospital of Xi'an Jiaotong University from February 2018 to May 2019 and treated with this modified surgical technique. Patients with significant ocular comorbidities (such as diabetic retinopathy, retinal vascular occlusion, vitreous hemorrhage, uveitis, ocular tumors, or glaucoma), incomplete chart records, or serious systemic conditions precluding further operative intervention were excluded. Preoperative and postoperative best-corrected visual acuity (BCVA) were compared and the MH closure rates were measured as the main outcomes.
The gender, age, ocular history, preoperative and postoperative clinical manifestations, spectral-domain ocular coherence tomography (OCT, by Cirrus, Carl Zeiss, Dublin, USA) results, initial and final BCVA were collected. The diameter of the MH was calculated in microns on OCT by keeping the calipers on the nearest diametrically opposite edges of MH. MH closure was defined as a complete sealing of the MH without bare retinal pigment epithelium (RPE) by OCT imaging.
Surgical Procedure
The surgical technique mainly involved parafoveal retinal massage (tapping the hole edge slightly by vit probe or flute needle to bring its edges closer, with or without ILM peeling) and autologous blood cover on the top of MH in PPV, using C3F8 as tamponade agent (Figure 1). All patients were informed to maintain a prone position for at least 7d postoperation. All the surgeries were performed under retrobulbar anesthesia by an experienced surgeon. The detailed surgical procedure was described below. A standard three-port, 23- or 25-gauge PPV (Constellation Vitrectomy System; Alcon Laboratories, Fort Worth, TX, USA) was performed. First, a core vitrectomy was performed and the peripheral vitreous was further cut off, using a wide-angle viewing system (the Resight 700, Carl Zeiss Meditec AG, Jena, Germany) and scleral indentation. For patients with persistent or recurrent MHs who had ever underwent one or more times vitreoretinal surgeries and ILM peeling, the surgeon should conduct a complete removal of the residual posterior hyaloids, and inspected repeatedly to determine whether there were any retinal breaks or degenerative areas at the peripheral retina. For patients with giant MHs who had not underwent vitreoretinal surgery, a range close to the vascular arch of ILM peeling was performed. Then the soft flute needle was put onto the hole edge and passive suction and blow was applied slightly to make the MH generate a cuff of subretinal fluid intraoperatively for all enrolled patients. Following this step, the surgeon gently massaged the parafoveal retina toward the center with vit probe or soft flute needle to convert the hole into a smaller size and make its edges get stretched and come closer. Then a drop of autologous whole blood draw from the upper arm vein was injected into the posterior vitreous cavity with a 30-gauge needle to fully cover the MH. Thorough fluid-air exchange was performed to drain off the balanced salt solution in the vitreous cavity, and 0.4-0.7 mL C3F8 was injected into the vitreous cavity as intraocular tamponade agent which made the gas concentration around 10%. Finally, the cannulas were removed. Absorbable suture 8-0 was used to watertight the incision if necessary. Patients with visual significant cataracts were required to have concurrent cataract extraction and intraocular lens (IOL) implantation.
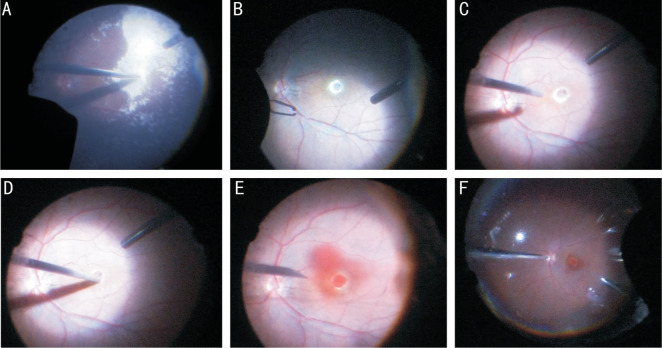
Figure 1. The patient who suffered with rhegmatogenous retinal detachment primary with macular hole (diameter=341 µm, right eye) had underwent previous vitreoretinal surgeries twice. The first surgery was vitrectomy and ILM peeling with silicone oil for tamponade, and then the hole closed. Following silicone oil extraction combined with phacoemulsification and IOL implantation was performed 6mo later. However, recurrent macular hole with diameter of 818 µm was discovered at 1wk after the second surgery.
A: Triamcinolone acetonide was used for staining and identifying whether there was residual vitreous gel; B: Little left ILM could be seen by indocyanine green staining; C: The flute needle was put onto the hole edge to make a cuff of MH with passive suction and slightly blow; D: Gently massage on the parafoveal retina toward the center with vit probe; E: Autologous whole blood was injected into vitreous cavity and the macular hole was fully covered; F: Thorough fluid-air exchange was performed to drain off the liquid of vitreous cavity.
All patients were followed up at 1d, 1, 2wk, every month for the first 3mo, and 3mo intervals postoperatively. Extra visits were scheduled as needed for anytime. Examinations included Snellen visual acuity (VA), anterior segment assessment, intraocular pressure, and clinical evaluation of the posterior segment. Examination of OCT imaging for macula was performed once the gas bubble cleared from the macular area when patient was sitting.
In order to accomplish statistical analysis, all the Snellen VA values were converted to the logarithm of the minimum angle of resolution (logMAR). VA of counting fingers was assigned 2.3, hand movements 2.6, and light perception 2.9[13]. All the continuous data were expressed as mean±standard deviation. All data were analyzed using SPSS 23.0 statistical software (SPSS Inc., Chicago, IL, USA). Paired t-tests were used in our research. P<0.05 was considered statistically significant.
RESULTS
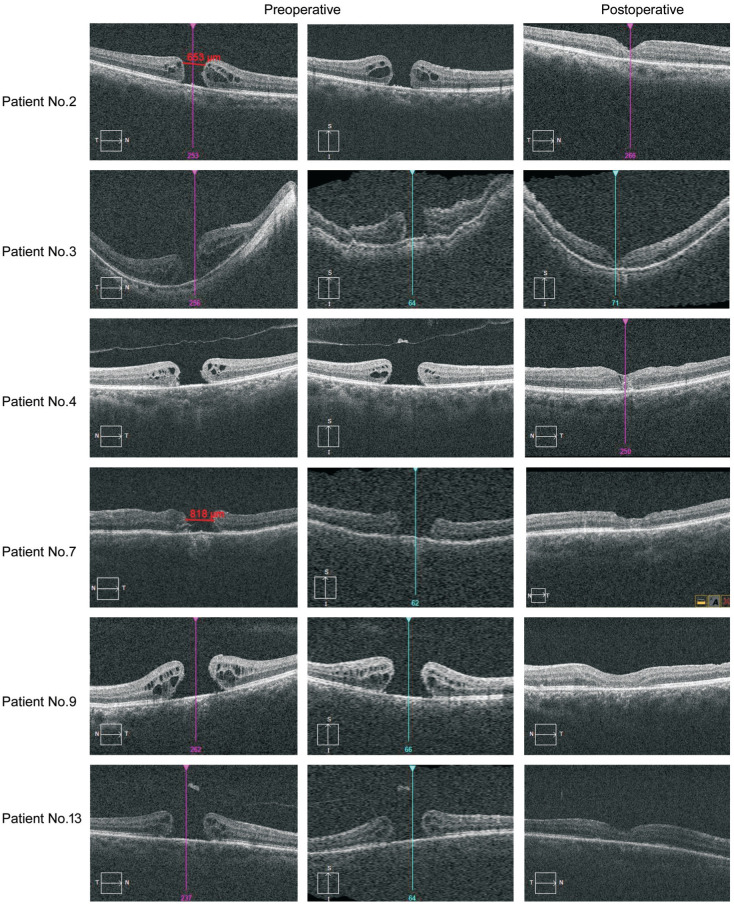
A total of 13 patients (5 men and 8 women; average age 56.4±11.72y) with MHs consisted of 6 giant MHs, 4 persistent holes and 3 recurrent holes were enrolled in this study. All of them underwent this modified surgery during the research period. All the 6 giant MH patients who were diagnosed with idiopathic MH did not have a history of vitreoretinal surgery. All the 4 persistent MH patients had previous vitreoretinal operations once with ILM peeling and gas tamponade. Two of the 3 recurrent MH patients had previous vitreoretinal surgery once with ILM peeling and gas tamponade, and the other one patient who suffered with retinal detachment primary had previously vitreoretinal surgery twice which were vitrectomy combined with silicone oil tamponade and following silicone oil extraction combined with phacoemulsification and IOL implantation (patient No.7). The ILM had been peeled at previous surgeries in all persistent and recurrent hole patients. The mean preoperative VA was 1.73±0.53 logMAR, and the mean holes size were 815.0±228.7 µm. The characteristics of all included patients are presented in Table 1. Spectral domain optical coherence tomography images (SD-OCT) in Figure 2 showed part of the typical MHs status before and after surgery.
Table 1. Baseline characteristics of included patients and their surgical results.
| Patient No. | Age (y) | Sex | Eye | Type of MH | Diameter of MH (µm) | Lens status preop. | Axial length | Preop. BCVA | MH status Postop. | Postop. BCVA at 6mo | Postop. BCVA last visit | Follow-up time (mo) |
| 1 | 67 | F | OD | G | 1317 | Phakic | 27.68 | 2.3 | Open | 2.3 | 2.3 | 16 |
| 2 | 53 | M | OS | P | 653 | Phakic | 22.89 | 1.68 | Closed | 0.59 | 0.51 | 14 |
| 3 | 47 | F | OS | R | 663 | Phakic | 28.09 | 1.49 | Closed | 0.79 | 0.73 | 13 |
| 4 | 64 | F | OD | G | 854 | Phakic | 24.31 | 1.59 | Closed | 0.68 | 0.61 | 12 |
| 5 | 52 | F | OS | P | 912 | Pseudophakic | 24.61 | 2.3 | Open | 1.35 | 1.21 | 12 |
| 6 | 59 | F | OD | G | 955 | Phakic | 22.98 | 1.89 | Closed | 0.79 | 0.75 | 11 |
| 7 | 57 | M | OD | R | 818 | Pseudophakic | 22.58 | 1.77 | Closed | 0.61 | 0.49 | 11 |
| 8 | 58 | F | OD | P | 609 | Pseudophakic | 22.65 | 1.64 | Closed | 0.71 | 0.64 | 10 |
| 9 | 56 | M | OS | G | 805 | Phakic | 22.78 | 1.74 | Closed | 0.57 | 0.51 | 9 |
| 10 | 60 | M | OS | G | 969 | Phakic | 23.07 | 1.86 | Closed | 0.72 | 0.66 | 9 |
| 11 | 49 | M | OS | P | 588 | Phakic | 29.29 | 1.15 | Closed | 1.15 | 1.15 | 8 |
| 12 | 53 | F | OD | R | 409 | Pseudophakic | 23.74 | 1.08 | Closed | 0.45 | 0.41 | 7 |
| 13 | 59 | F | OS | G | 872 | Phakic | 23.88 | 1.87 | Closed | 0.70 | 0.65 | 7 |
MH: Macular hole; BCVA: Best corrected visual acuity; F: Female; M: Male; G: Giant; R: Recurrent; P: Persistent; Preop.: Preoperative; Postop.: Postoperative.
Figure 2. Part of the MHs status before and after surgery showed by SD-OCT.
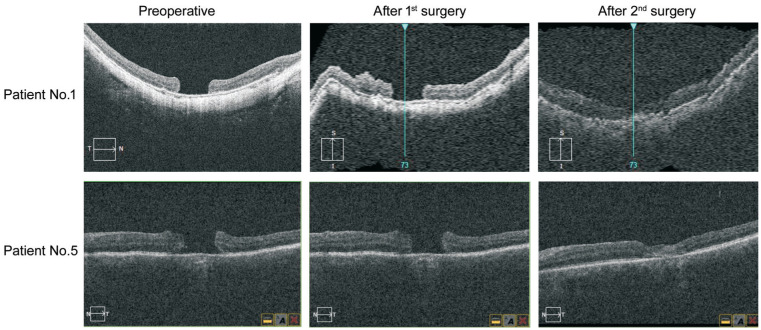
MH closure was achieved in 11 eyes by this modified surgical technique while 2 eyes failed to close (patient No.1, 5). Revitrectomy with autologous neurosensory retinal patch transplantations was applied for those 2 patients one month later after the initial operation and then both holes closed (Figure 3). Primary and final MH closure rate were 84.6% and 100% respectively. Totally 5 (38.5%) eyes with cataracts were performed concurrent cataract extraction and IOL implantation, and 4 eyes (30.8%) were pseudophakic. No intraoperative complication was observed. The mean follow-up time was 9.9±3.7mo. BCVA was improved from 1.73±0.53 logMAR preoperative to 0.74±0.19 logMAR postoperative at 6mo. The final follow-up logMAR BCVA was 0.65±0.17. There was a significant difference in BCVA before versus after the surgery at 6mo (t=19.29, P<0.001). There were no adverse events occurred during follow-up period. Table 2 shows the preoperative and postoperative parameters of all included patients.
Figure 3. SD-OCT showed status of the 2 initially failed holes before and after surgery.
Table 2. Demographics and surgical results among all patients.
| Variable | Data |
| MH size, mean±SD (µm) | 815.0±228.7 |
| Axial length, mean±SD (mm) | 24.58±2.17 |
| Preoperative logMAR BCVA (Snellen equivalent) | 1.73±0.53 (20/1176) |
| Primary MH closure, yes:no (%) | 11:2 (84.6) |
| Final MH closure (%) | 100 |
| Postoperative logMAR BCVA (Snellen equivalent, 6mo postoperation) | 0.74±0.19 (20/156) |
| Final follow-up logMAR BCVA (Snellen equivalent) | 0.65±0.17 (20/141) |
| Follow-up duration, mean±SD (mo) | 9.9±3.7 |
MH: Macular hole; BCVA: Best corrected visual acuity; logMAR: Logarithm of minimal angle of resolute.
n=13
DISCUSSION
Kumar et al[12] suggested that using the technique of parafoveal retinal massage in PPV could improve the closure rate of large MHs in 2013. In their study, hole closure was seen in 89.3% (25/28) of eyes, with significant improvement of VA from logMAR 0.86±0.2 (20/145) to logMAR 0.43±0.22 (20/54) postoperatively. The surgical skill mainly involved tapping on the hole edge to make it get stretched and come closer, so the hole would likely easy to close. Recently, various surgical techniques using ILM have been introduced to improve surgical outcomes in refractory MHs[14]–[16]. Lai et al[11] presented the surgical technique of vitrectomy combined with ILM repositioning and autologous blood cover to treat refractory MHs and obtained a better outcome than before. They suggested that autologous blood was rich in growth factors and could provide growth scaffolds to stimulate the proliferation of glial cells which would fill MHs. However, it is worth noting that the inverted ILM flap may detach spontaneously during the fluid-air exchange and it may bring difficulties to the operation[17]. Lyu et al[18] use ILM transplantation combined with autologous whole blood cover for repair of refractory MHs (with MH of the minimum diameter of >700 µm, the maximum base diameter of 1000 µm and hole formation factor is >0.6) and received 100% hole closure rate. According to previous surgical experience, ILM flap dissociation and dislocation were encountered sometimes when trying to use it cover or insert into the MH. Usually, heavy water must be used for free ILM flap transplantation, which may increase the medical cost and the complexity of the surgical procedure. Moreover, these procedures are not feasible in cases where the ILM around the MH has already been removed, such as cases of recurrent or persistent MHs after failed surgery. Persistent and recurrent MHs are often associated with poor visual prognosis, and the mechanism behind persistent or reopening of MHs remains be poorly understood[19]–[20]. Regardless of the surgical approach, the goals of techniques described above aid in stimulating or inducing gliosis, providing growth scaffolds, and striving for maximize closure rate. So, we integrated the two skills above into one modified surgical technique as parafoveal retinal massage combined with autologous whole blood cover in the management of giant, persistent or recurrent MHs in order to present an easier surgical procedure and obtain an acceptable outcome.
In our study, MH closure was achieved in 11 eyes by this modified surgical technique. The primary MH closure rate was 84.6%. Two eyes failed to close including one patient who initially suffered with giant MH with diameter of 1317 µm and another patient who suffered with persistent MH with diameter of 912 µm respectively. Revitrectomy was performed for the two failed patients. With indocyanine green staining in operation, we found there was no ILM left for transplantation. So we selected an autologous neurosensory retinal patch from peripheral fundus for repairing the MH[21]–[23] and then both of the holes closed. Finally, 11 patients had improved VA, and 2 patients had an unchanged VA (Patient No.1 & 11). As we know, modern approaches for refractory MHs were using a plug of ILM peeled from the periphery of the posterior pole[14], a neurosensory retinal free flap[22]–[23], or capsular lens fragments[24]–[25] to cover or insert into the MHs. With above-mentioned procedures, anatomical MH closure rate was achieved from 60% to 90% of all cases, and BCVA improvement account for 80% of successful cases. So, we can conclude that MH closure rate in our study (84.6%) was comparable to the previous studies, but the surgical procedure is easier to be performed. BCVA improvement rate was 84.6% in our study that was also comparable to the above-mentioned procedures. Although VA was improved significantly after the surgery, the final follow-up logMAR BCVA in our study was lower than that of part previous studies above (0.65±0.17 vs 0.43±0.22 to 1.07±0.35). The probable reason may be the low logMAR baseline VA between our patients and those of previous studies (1.73±0.53 vs 0.86±0.2 to 1.53±0.39) due to the different sample composition.
We tried to analyze the mechanism of this modified technique. The pathogenesis of MH closure primarily involves centripetal movement of retinal tissue to occupy the fovea. Parafoveal retinal massage is just an effectively physical way to make MH shrink quickly. Peyman et al[26] have demonstrated high closure rate in chronic holes using passive suction to the edges of the MH. It has also been observed that MH with cuff of subretinal fluid was more likely to close[27]–[28]. In the case of an MH with a cuff of subretinal fluid, there will not be any adhesion between the MH edges and the underlying RPE. So, we used flute needle to suck and blow the hole edge to make the MH generate a cuff of subretinal fluid intraoperatively. Autologous blood was used in our study as a growth scaffold for the repair of MH. Based on above, we could explain why the usage of parafoveal retinal massage combined with autologous blood cover could obtain a relatively satisfied outcome in the management of refractory MHs.
There were some reasons for postoperative VA improvement in our study. First, postoperative recovery of the outer retinal structure was the decisive factor affecting postoperative vision[5], while parafoveal retinal massage was just an effectively physical way to make MH come smaller and it was beneficial to the repair of MH. Of course, there are also concerns that parafoveal retinal massage could damage important retinal structures around the macula fovea. Considering the severe impact of failed MH closure on visual acute, these potential injuries could be placed on a relatively minor position. However, a randomized controlled study should be conducted to draw more reliable conclusions about the severity of macular injury caused by parafoveal retinal massage compared with MH surgery without macular massage. Second, autologous whole blood was rich in growth factors which could promote better growth and repair of MH. Third, autologous blood could provide a growth scaffold to stimulate the proliferation of glial cells which would fully fill and heal the MHs. As the reasons mentioned above, the MHs had more chance to get anatomical repair, and hole closure with VA improvement was achieved more possibly.
There were several limitations in our study. It was a retrospective study with small sample size and there was no control group. Because of the small sample of the study, we could not subgroup the patients for comparing with previous studies of giant, persistent or recurrent MHs respectively. In addition, all the enrolled patients were from a single center, and all the surgeries were performed by one single surgeon, so it may have certain selection bias.
In conclusion, we consider that parafoveal retinal massage combined with autologous whole blood cover is an easier surgical procedure and effective addition to the surgical options for the management of giant, persistent or recurrent MHs. Further prospective randomized case-control studies with a larger sample size should be undertaken.
Acknowledgments
Conflicts of Interest: Wang H, None; Ji M, None; Di R, None; Qi Y, None; Pei C, None; Gao S, None; Liu SW, None; Xie AM, None; Cheng YH, None.
REFERENCES
- 1.Oh H. Idiopathic macular hole. Dev Ophthalmol. 2014;54:150–158. doi: 10.1159/000360461. [DOI] [PubMed] [Google Scholar]
- 2.García-Layana A, García-Arumí J, Ruiz-Moreno JM, Arias-Barquet L, Cabrera-López F, Figueroa MS. A review of current management of vitreomacular traction and macular hole. J Ophthalmol. 2015;2015:809640. doi: 10.1155/2015/809640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madi HA, Masri I, Steel DH. Optimal management of idiopathic macular holes. Clin Ophthalmol Auckl N Z. 2016;10:97–116. doi: 10.2147/OPTH.S96090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grewal DS, Mahmoud TH, Fine HF. Management of challenging macular holes: current concepts and new surgical techniques. Ophthalmic Surg Lasers Imaging Retina. 2016;47(6):508–513. doi: 10.3928/23258160-20160601-01. [DOI] [PubMed] [Google Scholar]
- 5.Lee SM, Kwon HJ, Park SW, Lee JE, Byon IS. Microstructural changes in the fovea following autologous internal limiting membrane transplantation surgery for large macular holes. Acta Ophthalmol. 2018;96(3):e406–e408. doi: 10.1111/aos.13504. [DOI] [PubMed] [Google Scholar]
- 6.Ip MS, Baker BJ, Duker JS, Reichel E, Baumal CR, Gangnon R, Puliafito CA. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol Chic Ill. 2002;120(1):29–35. doi: 10.1001/archopht.120.1.29. [DOI] [PubMed] [Google Scholar]
- 7.Lim LS, Tsai A, Wong D, Wong E, Yeo I, Loh BK, Ang CL, Ong SG, Lee SY. Prognostic factor analysis of vitrectomy for retinal detachment associated with myopic macular holes. Ophthalmology. 2014;121(1):305–310. doi: 10.1016/j.ophtha.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Ullrich S, Haritoglou C, Gass C, Schaumberger M, Ulbig MW, Kampik A. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002;86(4):390–393. doi: 10.1136/bjo.86.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117(10):2018–2025. doi: 10.1016/j.ophtha.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 10.D'Souza MJ, Chaudhary V, Devenyi R, Kertes PJ, Lam WC. Re-operation of idiopathic full-thickness macular holes after initial surgery with internal limiting membrane peel. Br J Ophthalmol. 2011;95(11):1564–1567. doi: 10.1136/bjo.2010.195826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai CC, Chen YP, Wang NK, Chuang LH, Liu L, Chen KJ, Hwang YS, Wu WC, Chen TL. Vitrectomy with internal limiting membrane repositioning and autologous blood for macular hole retinal detachment in highly myopic eyes. Ophthalmology. 2015;122(9):1889–1898. doi: 10.1016/j.ophtha.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Tinwala S, Gogia, Sehra SV. Tapping of macular hole edges: the outcomes of a novel technique for large macular holes. Asia - Pac J Ophthalmol. 2013;2(5):305–309. doi: 10.1097/APO.0b013e31829a1919. [DOI] [PubMed] [Google Scholar]
- 13.Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30(2):287–290. doi: 10.1016/j.jcrs.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Morizane Y, Shiraga F, Kimura S, Hosokawa M, Shiode Y, Kawata T, Hosogi M, Shirakata Y, Okanouchi T. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am J Ophthalmol. 2014;157(4):861–869.e1. doi: 10.1016/j.ajo.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Kuriyama S, Hayashi H, Jingami Y, Kuramoto N, Akita J, Matsumoto M. Efficacy of inverted internal limiting membrane flap technique for the treatment of macular hole in high myopia. Am J Ophthalmol. 2013;156(1):125–131.e1. doi: 10.1016/j.ajo.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Shin MK, Park KH, Park SW, Byon IS, Lee JE. Perfluoro-n-octane–assisted single-layered inverted internal limiting membrane flap technique for macular hole surgery. Retina. 2014;34(9):1905–1910. doi: 10.1097/IAE.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 17.Tam ALC, Yan P, Gan NY, Lam WC. The current surgical management of large, recurrent, or persistent macular holes. Retina. 2018;38(7):1263–1275. doi: 10.1097/IAE.0000000000002020. [DOI] [PubMed] [Google Scholar]
- 18.Lyu WJ, Ji LB, Xiao Y, Fan YB, Cai XH. Treatment of refractory giant macular hole by vitrectomy with internal limiting membrane transplantation and autologous blood. Int J Ophthalmol. 2018;11(5):818–822. doi: 10.18240/ijo.2018.05.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bottoni F, Zanzottera E, Carini E, Cereda M, Cigada M, Staurenghi G. Re-accumulation of macular pigment after successful macular hole surgery. Br J Ophthalmol. 2016;100(5):693–698. doi: 10.1136/bjophthalmol-2015-307153. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Moreno JM, Arias L, Araiz J, García-Arumí J, Montero JA, Piñero DP. Spectral-domain optical coherence tomography study of macular structure as prognostic and determining factor for macular hole surgery outcome. Retina. 2013;33(6):1117–1122. doi: 10.1097/IAE.0b013e318285cc3b. [DOI] [PubMed] [Google Scholar]
- 21.Thomas AS, Mahmoud TH. Subretinal transplantation of an autologous retinal free flap for chronic retinal detachment with proliferative vitreoretinopathy with and without macular hole. Retina. 2018;38(Suppl 1):S121–S124. doi: 10.1097/IAE.0000000000002026. [DOI] [PubMed] [Google Scholar]
- 22.Grewal DS, Mahmoud TH. Autologous neurosensory retinal free flap for closure of refractory myopic macular holes. JAMA Ophthalmol. 2016;134(2):229–230. doi: 10.1001/jamaophthalmol.2015.5237. [DOI] [PubMed] [Google Scholar]
- 23.Wu AL, Chuang LH, Wang NK, Chen KJ, Liu L, Yeung L, Chen TL, Hwang YS, Wu WC, Lai CC. Refractory macular hole repaired by autologous retinal graft and blood clot. BMC Ophthalmol. 2018;18(1):213. doi: 10.1186/s12886-018-0898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng J, Chen CL, Jin HY, Zhang HT, Zhao PQ. Autologous lens capsular flap transplantation combined with autologous blood application in the management of refractory macular hole. Retina. 2018;38(11):2177–2183. doi: 10.1097/IAE.0000000000001830. [DOI] [PubMed] [Google Scholar]
- 25.Chen SN, Yang CM. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina. 2016;36(1):163–170. doi: 10.1097/IAE.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 26.Peyman GA, Cheema RA, Conway MD. Closure of chronic macular holes using passive aspiration to the edges of the macular hole. Ophthalmic Surg Lasers. 2001;32(6):486–489. [PubMed] [Google Scholar]
- 27.Rishi P, Reddy S, Rishi E. Repeat gas insufflation for successful closure of idiopathic macular hole following failed primary surgery. Indian J Ophthalmol. 2014;62(3):363–365. doi: 10.4103/0301-4738.116452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hillenkamp J, Kraus J, Framme C, Jackson TL, Roider J, Gabel VP, Sachs HG. Retreatment of full-thickness macular hole: predictive value of optical coherence tomography. Br J Ophthalmol. 2007;91(11):1445–1449. doi: 10.1136/bjo.2007.115642. [DOI] [PMC free article] [PubMed] [Google Scholar]