Abstract
The epidemiology of pulmonary vascular disease (PVD) remains unclear in Africa, where health systems do not reach the majority of the population and heath information systems are poorly developed. In this context, registries are particularly important in gathering crucial information on PVD, aiming at improving knowledge of the epidemiology and/or quality of care. While population-based registries are the main tool to identify incident cases, and be a better indicator of pulmonary vascular disease burden, hospital-based registries can give an indication of the demand for specific care services, which is useful for health policy and planning.
The only registry for pulmonary hypertension in Africa – the Pan African Pulmonary Hypertension Cohort (PAPUCO) – involved four countries, and was a pragmatic study that revealed a unique pattern of environmental risks, issues related to low access to health care, and ill-equipped health facilities for diagnosis and management of pulmonary hypertension. In addition, disease specific registries for conditions such as congenital heart disease and rheumatic heart disease uncovered high occurrence of PVD that can be managed and/or prevented with improvements in community awareness, surveillance, management and prevention.
It is suggested that existing networks of experts and researchers develop regional registries to determine the epidemiology of PVD in Africa, assess geographic, environmental and seasonal differentials, as well as inform policy and care provision in the continent.
Background
Registries use observational methods to collect uniform data on individuals with a specific disease, and are of two major types: hospital-based and population-based. They serve as clinical information systems that identify relevant sub-populations for proactive care, facilitate individual patient care planning, allow sharing of information to coordinate care, and monitor performance of practice team and care system.
With progress in information and communication technologies, registries can be collected at national, regional and global levels, allowing better characterization of disease burden and profile, as well as raising attention to similarities and differences among geographically separated regions.
In areas where the health information systems are poorly developed, registries are particularly important in gathering of crucial information on specific conditions, aiming at improving knowledge of the epidemiology and/or quality of care. However, the establishment of effective population-based registries (PBRs) in low-resource settings is challenging. Access to health care in rural remote areas is low, lack of knowledge and awareness of common conditions among patients cause them not to look promptly for health care, and – in many countries – there is lack of accurate population censuses. Thus, hospital-based registries (HBRs) – more frequently used in these under-resourced areas – have a different and complementary role to PBRs, focusing mainly on clinical information about patients as well as on their outcomes. HBRs can provide data about the mode of diagnosis, the clinical features of the condition, use of therapeutic options, patient follow-up details, and can be a source of information for PBRs.
The diagnosis of pulmonary vascular disease (PVD) is technically demanding and definitions have been evolving1, partially explaining why the global epidemiology remains unclear. In Africa, where the health systems do not reach the majority of the population, PBRs would be the main tool to identify incident cases and be a better indicator of PVD burden. Alternatively, HBRs can give an indication of the demand for specific care services, which is useful for health policy and planning2.
However, due to limited local resources, low availability of accurate information regarding the number of patients affected, lack of expertise and equipment for accurate diagnosis, and low availability of accurate data about the population, information about PVD burden on the African continent is scarce. A search on the PubMed for registries in different countries in Africa carried out based on proper keywords in English – including “hospital-based”, “clinical” and “registry” and “pulmonary hypertension” – reveals only one hospital-based registry of pulmonary hypertension (PH)3, and there are no studies on its incidence.
Historical perspective
The Heart of SOWETO study published in 2006 looked at the general profile of cardiovascular disease in an urban community in South Africa and described high occurrence of right heart failure (RHF) and PH4. This clinical registry captured data from 5328 de novo presentations of heart disease over two years (2006–2008), and detected 697 (28%) patients with RHF (50% primary diagnosis) among the 2505 cases of heart failure (47% of total cohort).
Among the many pathways to RHF the most common were:
-
i.
concurrent left-sided heart disease (213 cases, 31%) such as hypertensive heart disease, dilated cardiomyopathy, peripartum cardiomyopathy and rheumatic heart valve disease
-
ii.
chronic lung disease (179 cases, 26% including COPD and tuberculosis); and
-
iii.
pulmonary arterial hypertension (PAH) (141 cases; 20%) – with women being almost two-fold more likely to present with PAH (OR 1.72, 95% CI [1.17–2.55]; P = 0.006)4.
In Mozambique, Mocumbi et al.5 retrospectively studied 534 patients with congenital heart disease assisted at a referral unit between 2001 and 2007, collecting epidemiological, clinical, echocardiographic and surgical data from hospital files. A pattern of late presentation was revealed - median age at diagnosis was 4 years – with high occurrence of complications, including fixed PAH in 45 (8.4%) patients out of the 481 with indication for surgery. More recently in Senegal, PH was found in 50% of patients (N=50) aged at least 16 years and followed for congenital heart disease in the cardiology department in Dakar between May 2003 and March 20156.
Two registries of rheumatic heart disease show the relevance of preventable conditions as cause of PH in Africa; the diagnosis of PH was based on echocardiography.
First, the VALVAFRIC study7, a multicenter, hospital-based retrospective registry of patients with RHD hospitalized in 12 cardiology departments from seven African countries, having at least one mild RHD lesion seen on echocardiography between 2004 and 2008. Out of the 3441 patients included in the study, 1385 had severe lesions (803 women; mean age 29.3 ±15.6 years) and combined valvular lesions were observed in 13% of cases. PH was present in 28.7% patients and dilatation of the right cardiac chambers in 19.8%. The ratio of severe to any RHD valvular lesion was higher in countries with the lowest gross domestic product. Although 1200 patients required valve repair or replacement, only 27 had surgery.
The second registry – the Global Registry of Rheumatic Heart Disease8 – enrolled prospectively 3343 patients presenting between January 2010 and November 2012 to 25 centers in 12 African countries (apart from India and Yemen), who were then followed for two years to assess mortality and other adverse outcomes. Overall 3343 patients (median age 28 years; 66.2% female) were enrolled, the majority (63.9%) with moderate-to-severe multivalvular disease. PH was present in 28.8% patients at diagnosis, being the second commonest complication after congestive heart failure (33.4%)8.
The utilization of valvuloplasty and valve surgery was low overall, being higher in upper-middle compared with lower-income countries. The vital status at 24 months was known for 2960 (88.5%) patients – again two-thirds were female; although patients were young (median age at death was 28.7 years) and the case fatality rate was high (500 deaths, 16.9%), representing a mortality rate at 116.3/1000 patient-years in the first year and 65.4/1000 patient-years in the second year9. Although PH was not among the independent predictors of death it surely contributed to higher morbidity and low quality of life.
The screening of the titles and abstracts of original research into PH published on PubMed in the last 5 years (2014–2019), found 14 publications that provide data on the occurrence of PH in several heart and lung conditions10–23, which varied between 8% among HIV infected individuals and sickle cell anemia, to as high as 68.7% in patients undergoing cardiac surgery (Table 1). Most studies used transthoracic cardiac ultrasound to estimate the pulmonary arterial pressure.
Table 1. Summary of studies published between 2014 and 2019 with data on occurrence of pulmonary hypertension in Africa.
| Author | Study Design, Number of Patients, Heart Condition and Age of Participants | % with PH |
|---|---|---|
| Ifeoluwa et al. 201910 | Case-Control; 79 hypertensive heart disease and 92 healthy controls (adults) | 32.9 |
| Lamina et al. 201911 | Case-Control; 200 sickle cell disease and 200 normal controls; patients 1-12 years old | 8.0 |
| Mahomed et al. 201912 | cross-sectional study; 228 COPD - adults | 63.0 |
| Bigna et al. 201913 | Systematic review; 42,642 HIV infected adolescents and adults from 17 countries | 8.3 |
| Kushimo et al. 201914 | Observational; 219 patients with heart failure - adults | 8.8 |
| Dzudie et al. 201815 | Prospective cohort study; 2194 patients adults | 15.6 |
| Bigna et al. 201716 | Systematic review of observational studies; 11,163 people w/ cardiac complains - adults | 9.8 |
| 937 HIV positive | 10.6 | |
| 2077 heart failure | 32.9 | |
| 259 undergoing heart surgery | 68.7 | |
| 51 COPD | 62.7 | |
| Jinji et al. 201717 | Cross-sectional; 178 adults | 25.3 |
| Sokunbi et al. 201718 | Case-Control; 175 sickle cell diseases; 5-18 years old | 22.9 |
| Amadi et al. 201719 | 92 sickle cell disease –adults | 23.9 |
| Bigna et al.201620 | Systematic review and meta-analysis; 664 adult patients (3 studies) | 14.0 |
| Ejim et al. 201621 | 259 patients w/ degenerative mitral valve disease | 30.0 |
| Adem et al. 201422 | Retrospective review of records; ultrasound department evaluations in adults | 32.6 |
| Enakpene et al. 201423 | 90; patients w/sickle cell disease - adults | 12.2 |
The Pan African Pulmonary Hypertension Cohort (PAPUCO) study
The Pan African Pulmonary Hypertension Cohort (PAPUCO) research group was established in 2011 to create a prospective registry cohort study of de novo pulmonary hypertension (PH) cases in Africa.3 Recognizing that the use of medical records would jeopardize the collection of minimal data for diagnosis, and acknowledging the challenges of archiving medical records in some hospitals in the participating countries, the team decided to do a prospective registry allowing collection of detailed data from patients with definite diagnosis based on criteria agreed by all participants and subjected to quality control.
The multinational, multi-centre, registry-type cohort study was established and tailored to resource-constraint settings to describe disease presentation, disease severity and etiologies of PH, comorbidities, diagnostic and therapeutic management, and the natural course of PH in Africa3. PH was diagnosed by specialist cardiologists using echocardiography (right ventricular systolic pressure >35 mmHg, absence of pulmonary stenosis and acute right heart failure), usually accompanied by shortness of breath, fatigue, peripheral oedema and other cardiovascular symptoms, electrocardiogram and chest X-ray changes in keeping with PH as per European Society of Cardiology and European Respiratory Society (ESC/ERS) guidelines.
Complementary investigations such as computerized tomography (CT) scan, ventilation/perfusion scan or right heart catheterization were performed at the discretion of the treating physician and the site capacities. Functional tests included a 6 min walk test and the Karnofsky Performance Score. The WHO classification system for PH was applied to describe the different aetiologies of PH. All local ethics committees of the participating centres approved the protocol.
Consecutive patients from nine specialist care referral centers in Cameroon (109), Mozambique (35), Nigeria (33) and South Africa (43) were recruited over 24 months. A pragmatic approach to diagnosis was agreed, and the inclusion criteria were the following:
-
i.
newly diagnosed with PH based on standardized clinical and echocardiography criteria;
-
ii.
capacity to return for 6-month follow-up if alive.
Participating center’s eligibility was based on availability of echocardiography with experience of assessing of right heart function; experience in diagnosing PH according to World Health Organization (WHO) classification; experience in clinical management of patients with RHF; and availability of resources for 6-month follow-up3.
Two hundred–twenty patients were recruited; 209 were adults (median age 48 years), 97% of African descent and 124 (59%) women. Two-thirds presented in WHO FC III or IV; 1/3 could not walk further than 300 m on 6MWT; of those with 6-month follow-up data (N=189) 39 (21%) had died. Indoor cooking and/or heating was reported by 66 patients (32%), with predominance of women (52;44% vs 14;17% for men; p=0.0001).
Previous or concurrent pulmonary tuberculosis was reported by 47 (23%) - of which 10 patients (5%) had concurrent tuberculosis when included in the study. At the time of diagnosis 134 (64%) were subjected to active screening for HIV, revealing 47 patients with positive test (35%). Interestingly, despite 167 patients (80%) living in areas endemic for schistosomiasis only one patient was diagnosed with this condition.
An interesting aspect of multimorbidity was the finding of systemic hypertension and diabetes in 87 (42%) and 17 (8%) patients, respectively3 (Table 2). The 11 children recruited presented with dyspnoea, fatigue, cough, and palpitations; six children had concurrent PH associated congenital heart disease and three were diagnosed with left heart disease.
Table 2. Selected risk factors profile and clinical findings of 209 adults (85 female; median age 48 years) presenting with PH.
| Characteristic | Number of Patients |
|---|---|
| Risk Factors | |
| African descent | 203 (97%) |
| Primary education or less | 125 (60%) |
| Systemic Hypertension | 87 (42%) |
| Chronic Obstructive Pulmonary Disease | 24 (12%) |
| Previous or current pulmonary tuberculosis | 57 (28%) |
| HIV infection (*134 tested) | 47 (35%) |
| Chronic exposure to Biomass Fuel | 66 (32%) |
| Smokers | 26 (12%) |
| Symptoms & Signs | |
| Dyspnea | 194 (93%) |
| Cyanosis | 26 (12%) |
| Fatigue | 184 (88%) |
| Palpitations | 153 (73%) |
| WHO Functional Class III | 92 (44%) |
| WHO Functional Class IV | 46 (22%) |
| 6-minute Walking Test <300m | 71 (34%) |
| Right Heart Failure | 78 (37%) |
| Raised Jugular Venous Pressure or Peripheral Edema | 174 (83%) |
| Systolic murmur | 119 (57%) |
The PAPUCO registry showed major gaps in usage of evidence-based interventions to prevent diagnose and/or manage PH. There was high prescription rate for loop diuretics and spironolactone, in line with the high percentage of patients with congestive heart failure.
No responsiveness to CCB testing was done in most countries, due to unavailability of cardiac catheterization labs or technical expertise to perform the procedure. There was very low usage of disease modifying agents: sildenafil and high dose calcium channel blockers were essentially prescribed in Group 1 and 3; other drugs approved for primary arterial hypertension were not available. Similarly, we unveiled low usage of beta-blockers and anticoagulants in patients with heart failure and atrial fibrillation, respectively.
This registry concluded that in sub-Saharan Africa PVD derives from a high burden of and interaction between non-communicable and infectious diseases. It affects mostly the young – and in particular females – presenting in advanced stages of HF. Survival is very poor, linked to profound lack of suitable and affordable management options, especially in the context of various etiologies requiring technically challenging diagnostic procedures and open heart surgery for their management. The authors proposed the design of pragmatic management guidelines to improve outcomes, particularly considering that access to right heart catheterization is limited and PH-specific therapies remain largely unaffordable in the region; the Pediatric and Congenital Heart Disease Task Forces of the Pulmonary Vascular Research Institute partially addressed this request in their consensus statements, by providing guidelines for the minimal investigative procedures for repair of congenital heart disease with associated PH in children24 and indications for cardiac catheterization in children with pulmonary hypertensive vascular disease25.
Similar guidelines with such indications and cut-offs for surgery of multiple rheumatic valve heart disease, management of secondary pulmonary hypertension, management of tuberculosis-related PVD, etc. are lacking and may come from well conceived and implemented registries from endemic places such as the African continent.
We briefly present two case reports (see boxes) illustrating how multifactorial PVD can present in Africa, and how important is the role of infectious PVD as a cause of disability and premature death in the region.
Case Report Box 1.
Infectious pulmonary vascular disease: Sequelae of perinatal HIV infection, recurrent pulmonary tuberculosis and drop-out of antiretroviral treatment
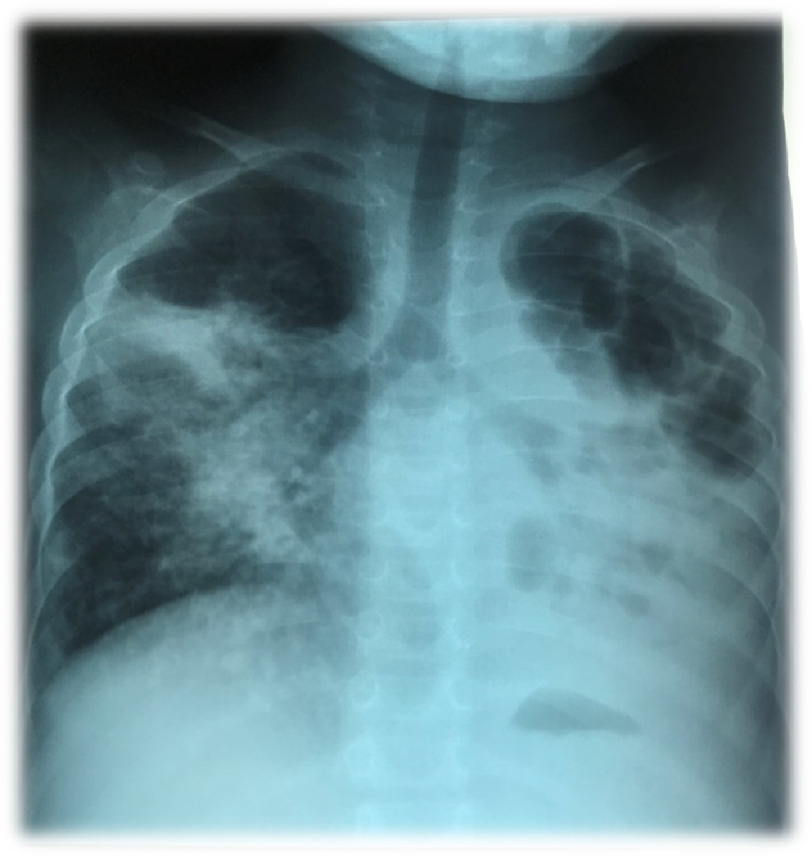
A 7-year-old Mozambican girl, daughter of HIV-positive parents, living in a suburb of a poor urban setting in a low-income country, presented to our cardiac clinic brought by her grandmother in September 2018 on NYHA functional class III-IV with a history of ten weeks of fever, productive cough and grade III dyspnea. On physical examination she presented tachypnea, regular tachycardia at 108/minute, intercostal drawing and mild peripheral hypoxia (SPO2 92%) at rest on ambient air. She also had peripheral cyanosis, clubbed fingers, lower abdominal distension, hepatomegaly (three cm below the costal border) and lower extremity edema. The chest auscultation revealed absence of palpable heart impulse, systolic murmur 3/6 over the tricuspid area, apical tubal murmur, abundant bilateral bullous snores in the lung fields and discrete basal crepitations. The HIV test performed at that time was inconclusive/undetermined, with a CD4 count of 197 cells/mm3 (corresponding to 13%). Other relevant exams revealed a white blood cell count of 13 × 103, hypochromic and mycrocitic anemia (hemoglobin 7.8 g/dl; MCV 67, CMHC 21); normal renal and liver function. No ECG was available and the chest X-ray (Figure 1) revealed severe lung parenchyma destruction, cavernous aspect large cavities in the apical regions, heterogeneous opacities and presence of centrilobular nodules, and bronchiectasis. The transthoracic cardiac ultrasound revealed marked hypertrophy and dilatation of the right ventricle with preserved systolic function, dilatation of the right atrium without any evidence of thrombi, normal pulmonary valve, dilatation of the pulmonary arteries and severely elevated pulmonary pressures – as estimated by velocity gradient across the tricuspid valve regurgitation, at 4,7 m/s; the right filling pressure was increased, as measured by the distended inferior vena cava, and the evaluation excluded any congenital heart disease with left-to-right shunt.
The child had been exposed to continuous indoor biomass fuel pollutants from birth, mainly coal and wood, had first TB treatment in 2012, previous respiratory infections and two previous admissions. She had started pulmonary tuberculosis re-treatment and second-line antiretroviral therapy in October 2017 (after being non-adherent). According to the grandmother, the family was facing social and economic issues: the child’s mother had to be home taking care of a 2-year-old HIV-infected sibling who was also sick, while being pregnant of her third child; therefore, the grandmother decided to take the little girl from her parents to help with day-to-day care. The grandmother (also HIV infected but following ART with undetectable viral loads) was motivated to take care of her granddaughter, but the parents wanted the child back.
The diagnosis of cor pulmonale associated with chronic obstructive pulmonary disease caused by recurrent pulmonary tuberculosis (and possibly other recurrent pulmonary infections) was considered. The girl was treated with diuretics (furosemide and potassium sparing amiloryde) in addition to antiretroviral therapy, antibiotics, prophylaxis for pneumocystis (jirovecii) pneumonia with cotrimoxazole, and correction of anemia with ferrous salt and folic acid. She failed to present to the following appointment, and after contacting the grandmother via cellphone we learned that she had died in January 2019.
Case Report Box 2.
Multifactorial pulmonary hypertension: Left endomyocardial fibrosis with severe pulmonary hypertension and active schistosomiasis affecting multiple organs
We have previously reported on a 13-year-old Mozambican boy presented to Maputo Central Hospital with right diastolic cardiac failure, referred from an high endemic zone of endomyocardial fibrosis (EMF)26. In summary, the boy reported a 3-month history of progressive exertional dyspnea, chest pain exacerbated by exercise, and abdominal distension. He reported no past medical history and no family history of cardiac problems. On examination he was apyretic with a heart rate of 108 bpm, regular, blood pressure of 90/60 mmHg, respiratory rate of 16 breaths per minute. Cardiac examination revealed a raised jugular venous pressure with prominent CV wave, a visible pulsating, palpable, non-displaced apex beat, and a mild holo-systolic murmur on auscultation. A three cm hepatomegaly was observed, but no pedal edema was present, as is usually the case in children with EMF.
HIV and malaria tests were negative; erythrosedimentation rate was at 55 mm/h; leucocytes at 16.9 × 103/μL with 40.6% of eosinophil’s. The chest X-ray showed increased cardio-thoracic rate due to enlargement of atria, and pulmonary congestion. On the electrocardiogram there was sinus tachycardia, signs of bi-atrial enlargement and features of important left sub-endocardial lesion. The echocardiography revealed a homogeneous mass like a thrombus occupying the apex of the left ventricle, a small right ventricle due to amputation of the apex by extensive fibrosis, large dilatation of both atria, and severe tricuspid regurgitation allowing the estimation of systolic pulmonary pressure at nearly the systemic values.
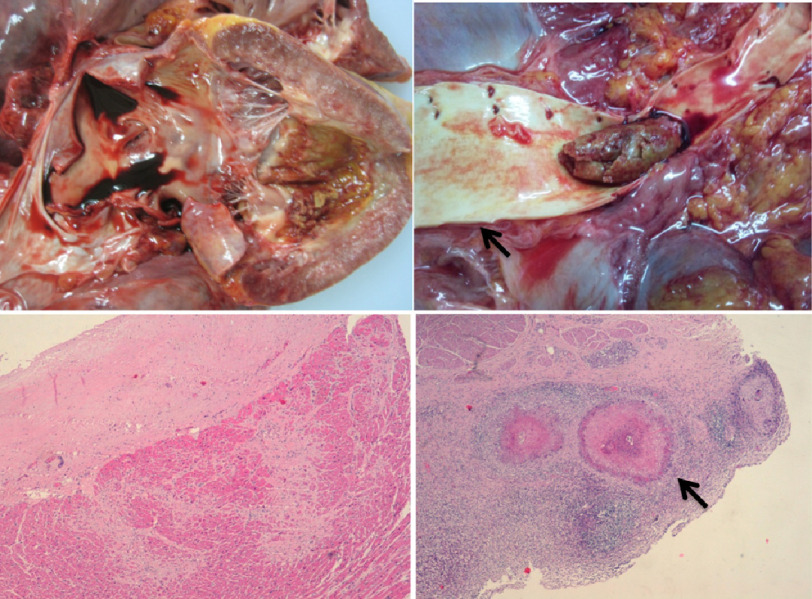
Due to his severe condition and in the absence of consensus regarding the indication for surgery immediate surgery the patient was admitted and treated with diuretics, but despite the resolution of the dyspnea he died suddenly on day 6 after admission. On autopsy there was an enlarged hearth due to bi-atrial dilatation, the RV was amputated by fibrosis with marked retraction of the apex and the endocardium and inner myocardium of the LV thickened with fibrosis. The surface of the LV apex was anfractuous, in an area corresponding to the base of a thrombus that had detached and embolized to the abdominal aorta. Histological studies with Hematoxylin-Eosin, Van Gieson, and Masson Trichromic stains in cardiac samples revealed right ventricle endocardial thickening by dense fibrosis with hyalinization, large fibrosis bands penetrating to the myocardium, neovascularization and scarce chronic inflammatory infiltrated. The same features were seen in the LV, but the neovascularization and the chronic inflammatory infiltrate was more intense; in addition, there was fibrin deposits in the thrombus detachment zone. Eosinophilic granulomas were found in the myocardium, liver and bladder. Additionally, there was chronic passive congestion of the liver and spleen; pulmonary chronic congestion; hepatic periportal fibrosis associated with the presence of eosinophilic granulomas; and active polipoyd cystitis with eosinophilic granulomas centered by Schistosoma eggs (Figure 2).
This case of concomitant active schistosomiasis and chronic endomyocardial fibrosis with major diastolic dysfunction that leads to severe pulmonary hypertension represents another example of multifactorial pulmonary vascular disease in Africa.
Discussion and lessons learned
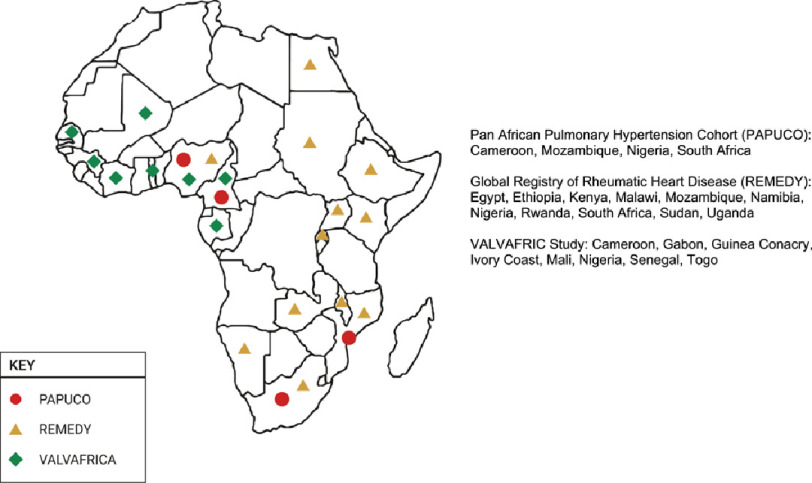
There are only three multinational registries of cardiovascular diseases dedicated exclusively to African patients from which data on the occurrence of PH could be obtained3, 7, 8. Among the lessons learned with the implementation of these registries – and in particular from the only registry dedicated to pulmonary hypertension, the PAPUCO study - is the diverse etiology of PVD in Africa. Although not covering all countries in the continent (Figure 3), these registries have uncovered some unique features of PVD in the region. Their results suggest that, at least partially, this high occurrence of interstitial and vascular pulmonary disease that results in PH is related to; chronic exposure to indoor air pollution, multiple respiratory illnesses including tuberculosis, high prevalence of schistosomiasis and HIV, as well as other poverty-related environmental and/or occupational hazards.
Figure 3. Countries participating in the multinational registries in Africa that assessed the occurrence of pulmonary vascular disease.
PAPUCO is, to date, the only all Africa disease specific registry for pulmonary hypertension, while REMEDY and VALVAFRIC were both for rheumatic heart disease, with the latter involving exclusively African countries.
Figure 1. Chest X-ray of a 7-years old girl with perinatal HIV infection, followed by recurrent pulmonary tuberculosis and persistent high viral load associated with poor adherence to therapy for both HIV and tuberculosis.
The image shows apical pleural thickening and cardiac silhoute not well defined due to heterogeneous opacities and centrilobular nodular pattern affecting the right medial and basal middle lobe, due to possible transbronchial dissemination from the left lung to the middle lobe of the right lung, suggesting a re-activation of tuberculosis. Left lung “destroyed” as a result of tuberculosis, in the upper lobe there are areas of emphysema and fibrosis, as well as consolidation of the lower lobe with cavitation (cavernous aspect) and bronchiectasis.
Figure 2. Left ventricle anfractuous surface (upper left, arrow) in an area that corresponds to the basis of a thrombus previously detached to the aorta (upper right).
Hematoxylin-Eosin 100x right ventricular endomyocardial sample revealing marked hyaline thickness of endocardium, scarce cellularity and large fibrosis bands into the myocardium (lower left). Eosinophilic granulomas centered by viable Schistossoma eggs (lower right, arrow) in a sample of the urinary bladder.
Case 1: Mozambique has a high prevalence of HIV, especially in the adolescent population aged between 15 and 19 years; the prevalence is around 2.5% in boys and 6.2% in girls. Moreover, HIV infection is a major cause of death for those aged between 15 and 24 years.26 The ambitious goal of reaching HIV treatment goals by 90–90–90 by 2020 – ie 90% of people living with HIV know they are HIV positive, 90% of people who know they are HIV positive are 90% of people on ART have viral suppression27 – was set in Mozambique, and progress towards these goals has been measured through the IMASIDA surveys28. The results of the 2015 cycle were:
-
i.
40% of women and men aged 15–49 living with HIV knew their HIV status;
-
ii.
86% of women and men aged 15–49 who knew their HIV status were on ART;
-
iii.
68% of women and men aged 15–49 who took ART had suppressed viral load28.
HIV testing for pregnant women, a key step in reducing mother-to-child transmission of HIV usually performed during antenatal visits, increased from 44% in IMASIDA 2009 to 67% in IMASIDA 201528. Only 44% of HIV-positive and pregnant/lactating women started ART, and 2% of 6–23 month olds are HIV positive, prior to systematic testing of pregnant women and prevention of maternal to child transmission in Mozambique.
The severe and fatal pulmonary vascular disease in the child we presented resulted from multiple factors namely chronic exposure to biomass fuel, perinatal HIV infection, superimposed recidicant pulmonary tuberculosis and recurrent interstitial pneumonia, all known to be risk factors for pulmonary hypertension29–32.
Low health literacy of parents/carers, weak social support, and poor adherence to therapy explain the recurrence of tuberculosis. Poor control of HIV infection and severe pulmonary complications early in life, resulting in severe right heart failure, major disability and premature mortality.
Case 2: EMF, a restrictive cardiomyopathy of unclear etiology that affects children and young adults, with high prevalence in some parts of sub-Saharan Africa33, is a major cause of disability in those affected. PH is in fact the commonest cause of death in patients with severe left EMF34, due to both left ventricular diastolic dysfunction; chronic thromboembolism is a second mechanism related to detachment of emboli from thrombus inside an aneurismal right atrium. The patient we present had also schistosomiasis, still a common cause of PVD in Africa35, despite mass and targeted administration of praziquantel to schoolchildren and adults in many countries36–38.
These cases underline the need not only for major investments in prevention of environmental and infectious dterminants of PVD in Africa, but also improvement in health care and social support for those with lower socioeconomic background.
They stress the need for an equity agenda to address the stage of complications, including universal health coverage to ensure continuous specialized care dor chronic condition such as PH. Moreover, they illustrate the challenges for the management of PVD in under-resourced settings in Africa where uncontrolled infections coexist with poverty-related non-communicable diseases.
Finally, they stress the need for prevention and early detection to prevent adverse outcomes, as well as registries to explore the potential synergistic effects of prevalent risk factors such as chronic exposure to biomass fuel and infectious agents in determining cardiopulmonary disease in general, and severe pulmonary vascular disease in particular. Such registries may help understanding the natural history and progression of PVD caused by such endemic conditions, thus allowing the identification of new therapeutic targets and better outcomes.
Perspectives
The unacceptably high burden of PVD in Africa highlights the need for the development of affordable and scalable algorithms for early diagnosis and adequate management of those detected in underserved areas. Recently, the concept of cardiopulmonary disease (CPD) as a broad spectrum of conditions concurrently affecting the heart and lungs has been suggested. This entity ranges from people at “high risk” of developing RHF and mortality due to the presence of largely asymptomatic mild-to-moderate elevation of pulmonary pressures, to those who have already developed concurrent and symptomatic lung/cardiac pathology39.
Common left heart diseases with reduced ventricular ejection fraction (dilated cardiomyopathy, peripartum cardiomyopathy, ischaemic heart disease), left ventricular diastolic dysfunction (severe uncontrolled systemic hypertension) or abnormal valve function (rheumatic heart disease) often result in pulmonary oedema and subsequent poor lung compliance. Additionally, multifactorial CPD can also occur in some regions in Africa due to high occurrence of pulmonary tuberculosis, sickle cell disease and particular conditions such as endomyocardial fibrosis. Finally, the HIV epidemic has triggered new pathways to CPD via immunosuppression and opportunistic infections, cardiac dysfunction linked to HIV-related cardiomyopathy, transplacental exposure to drugs like zidovudine and primary PH in those untreated.40 This said, CPD remains an under-appreciated contributor to poor health outcomes due to a shortage of human and material resources to screen for and diagnose this complex entity.
With this new knowledge Africa’s record of diagnosing severe PH in patients already in functional classes III and IV of the NYHA at the few specialized centres can now be challenged. Newly-identified thresholds of pulmonary pressure elevation that correspond to high-risk for premature mortality, associated with often silent and untreated PH,41 support the idea of combining early detection of at risk patients and long-term follow up to clarify the observed pattern of CPD and high incidence of RHF in Africa. Pragmatic screening protocols for identifying early stages of CPD have already been proposed for use in these contexts, aiming at allowing prevention and early recognition of PVD and/or people at risk39, 42.
The growth in use of information and communication technology in the continent has allowed the some registries to be performed using web-based databases3, 9. In parallel, pioneer studies were performed in Africa using ultrasound for community-based research on neglected cardiovascular diseases in low-income settings43, 44. Moreover, the addition of rapid testing and point-of-care devices for these community-based research efforts is currently possible in poor settings. This experience and the constant progress in technology and knowledge allow us to foresee in the near future research into the incidence, prevalence and natural history of PVD in Africa.
Existing networks of experts and researchers in the cardiovascular and pulmonary fields - such as the Pan African Society of Cardiology (PASCAR), the Non-Communicable Diseases and Injury of the Poorest Billion (NCDI Poverty), the Global Alliance for Respiratory Diseases (GARD) and the Pulmonary Vascular Research Institute (PVRI) – may link professionals geographically apart in joint efforts not only to define the profile of PVD in the region, but also to explore geographic, environmental and seasonal differentials that may inform policy and care provision within various regions of the continent. Particularly for PVD registries, PVRI may act as a promoter of high quality research using pragmatic clinical and ultrasound diagnostic algorithms, coupled with point-of-care detection of biomarkers of current and past infections, as well as of asymptomatic heart and respiratory failure.
References
- 1.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curado MP. Importance of hospital cancer registries in Africa. Ecancermedicalscience. 2019;13:948. doi: 10.3332/ecancer.2019.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thienemann F, Dzudie A, Mocumbi AO, Blauwet L, Sani MU, Karaye KM, Ogah OS, Mbanze I, Mbakwem A, Udo P, Tibazarwa K, Ibrahim AS, Burton R, Damasceno A, Stewart S, Sliwa K. Rationale and design of the Pan African Pulmonary hypertension Cohort (PAPUCO) study: implementing a contemporary registry on pulmonary hypertension in Africa. BMJ Open. 2014;4(10):e005950. doi: 10.1136/bmjopen-2014-005950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart S, Mocumbi AO, Carrington MJ, Pretorius S, Burton R, Sliwa K. A not-so-rare form of heart failure in urban black Africans: pathways to right heart failure in the Heart of Soweto Study cohort. Eur J Heart Fail. 2011;13(10):1070–7. doi: 10.1093/eurjhf/hfr108. [DOI] [PubMed] [Google Scholar]
- 5.Mocumbi AO, Lameira E, Yaksh A, Paul L, Ferreira MB, Sidi D. Challenges on the management of congenital heart disease in developing countries. Int J Cardiol. 2011;148(3):285–8. doi: 10.1016/j.ijcard.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Mbaye A, Bodian M, Ngaïdé AA, Abdourafiq H, Leye MCBO, Savodogo S, Aw F, Ndiaye M, Kouamé I, Babaka K, Dioum M, Gaye ND, Sarr SA, Ndiaye MB, Kane AD, Kane A. Congenital heart disease in adolescents and adults: Management in a general cardiology department in Senegal. Ann Cardiol Angeiol. 2017;66(4):217–22. doi: 10.1016/j.ancard.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Kingué S, Ba SA, Balde D, Diarra MB, Anzouan-Kacou JB, Anisubia B, Damorou JM, Ndobo P, Menanga A, Kane A, Kakou-Guikahué M, Kenfack M, Metogo B, Chelo D, Yangnigni E, Tantchou C, Bertr E, Monsuez JJ, Working Group on Tropical Cardiology of the Société française de cardiologie The VALVAFRIC study: A registry of rheumatic heart disease in Western and Central Africa. Arch Cardiovasc Dis. 2016;109(5):321–9. doi: 10.1016/j.acvd.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Zühlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B, Mauff K, Islam S, Joachim A, Daniels R, Francis V, Ogendo S, Gitura B, Mondo C, Okello E, Lwabi P, Al-Kebsi MM, Hugo-Hamman C, Sheta SS, Haileamlak A, Daniel W, Goshu DY, Abdissa SG, Desta AG, Shasho BA, Begna DM, ElSayed A, Ibrahim AS, Musuku J, Bode-Thomas F, Okeahialam BN, Ige O, Sutton C, Misra R, Abul Fadl A, Kennedy N, Damasceno A, Sani M, Ogah OS, Olunuga T, Elhassan HH, Mocumbi AO, Adeoye AM, Mntla P, Ojji D, Mucumbitsi J, Teo K, Yusuf S, Mayosi BM. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study) Eur Heart J. 2015;36(18):1115–22a. doi: 10.1093/eurheartj/ehu449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zühlke L, Karthikeyan G, Engel ME, Rangarajan S, Mackie P, Cupido-Katya Mauff B, Islam S, Daniels R, Francis V, Ogendo S, Gitura B, Mondo C, Okello E, Lwabi P, Al-Kebsi MM, Hugo-Hamman C, Sheta SS, Haileamlak A, Daniel W, Goshu DY, Abdissa SG, Desta AG, Shasho BA, Begna DM, ElSayed A, Ibrahim AS, Musuku J, Bode-Thomas F, Yilgwan CC, Amusa GA, Ige O, Okeahialam B, Sutton C, Misra R, Abul Fadl A, Kennedy N, Damasceno A, Sani MU, Ogah OS, Elhassan TO, Mocumbi AO, Adeoye AM, Mntla P, Ojji D, Mucumbitsi J, Teo K, Yusuf S, Mayosi BM. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: two-year follow-up of the Global Rheumatic Heart Disease Registry (the REMEDY study) Circulation. 2016;134(19):1456–1466. doi: 10.1161/CIRCULATIONAHA.116.024769. [DOI] [PubMed] [Google Scholar]
- 10.Ifeoluwa AA, Adewole AA, Abiodun AM, Akinyemi A. Right ventricular systolic function in Nigerians with heart failure secondary to hypertensive heart disease. Afr Health Sci. 2019;19(2):2130–2139. doi: 10.4314/ahs.v19i2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamina MO, Animasahun BA, Akinwumi IN, Njokanma OF. Doppler echocardiographic assessment of pulmonary artery pressure in children with sickle cell anaemia. Cardiovasc Diagn Ther. 2019;9(3):204–213. doi: 10.21037/cdt.2019.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamed MF, Ali A, Abbas A, Awad MS, Gouda M, Sediq AM. Mean platelet volume as a predictor of pulmonary hypertension in patients with stable COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:1099–108. doi: 10.2147/COPD.S176413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bigna JJ, Nansseu JR, Noubiap JJ. Pulmonary hypertension in the global population of adolescents and adults living with HIV: a systematic review and meta-analysis. Sci Rep. 2019;9(1):7837. doi: 10.1038/s41598-019-44300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushimo OA, Mbakwem AC, Ajuluchukwu JN, Amadi CE. Clinical and echocardiographic correlates of pulmonary hypertension among heart failure patients in Lagos, south-western Nigeria. Cardiovasc J Afr. 2019;30(1):9–14. doi: 10.5830/CVJA-2018-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzudie A, Dzekem BS, Tchoumi CT, Aminde LN, Mocumbi AO, Abanda M, Thienemann F, Kengne AP, Sliwa K. Pulmonary hypertension as seen in a rural area in sub-Saharan Africa: high prevalence, late clinical presentation and a high short-term mortality rate during follow up. Cardiovasc J Afr. 2018;29(4):208–12. doi: 10.5830/CVJA-2018-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bigna JJ, Noubiap JJ, Nansseu JR, Aminde LN. Prevalence and etiologies of pulmonary hypertension in Africa: a systematic review and meta-analysis. BMC Pulm Med. 2017;17(1):183. doi: 10.1186/s12890-017-0549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jingi AM, Noubiap JJ, Tankeu AT, Mfeukeu-Kuate L, Nkoke C, Kamdem P, Menanga AP, Kingue S. Prevalence and determinants of pulmonary hypertension in a group of Cameroonian patients without chronic lung disease: a cross-sectional echocardiographic study. BMC Res Notes. 2017;10(1):571. doi: 10.1186/s13104-017-2903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sokunbi OJ, Ekure EN, Temiye EO, Anyanwu R, Okoromah CAN. Pulmonary hypertension among 5 to 18 year old children with sickle cell anaemia in Nigeria. PLoS One. 2017;12(9):e0184287. doi: 10.1371/journal.pone.0184287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amadi VN, Balogun MO, Akinola NO, Adebayo RA, Akintomide AO. Pulmonary hypertension in Nigerian adults with sickle cell anemia. Vasc Health Risk Manag. 2017;13:153–60. doi: 10.2147/VHRM.S92799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bigna JJ, Nansseu JR, Um LN, Noumegni SR, Simé PS, Aminde LN, Koulla-Shiro S, Noubiap JJ. Prevalence and incidence of pulmonary hypertension among HIV-infected people in Africa: a systematic review and meta-analysis. BMJ Open. 2016;6(8):e011921. doi: 10.1136/bmjopen-2016-011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ejim EC, Oguanobi NI. The burden of pulmonary hypertension in patients with degenerative mitral valve disease in Enugu South-East Nigeria: An echocardiographic based study. Ann Med Health Sci Res. 2016;6(3):172–5. doi: 10.4103/2141-9248.183945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adem A, Abebe S, Hailu A, Feleke B, Berhe M, Atsibeha M, Davilla VG. Heart diseases in North Ethiopia pattern of echocardiographic abnormalities among adult cardiac patients - an experience from ayder hospital of mekelle university. Ethiop Med J. 2014;52(4):173–83. [PubMed] [Google Scholar]
- 23.Enakpene EO, Adebiyi AA, Ogah OS, Olaniyi JA, Aje A, Adeoye MA, Falase AO. Non-invasive estimation of pulmonary artery pressures in patients with sickle cell anaemia in Ibadan, Nigeria: an echocardiographic study. Acta Cardiol. 2014;69(5):505–11. doi: 10.1080/ac.69.5.3044877. [DOI] [PubMed] [Google Scholar]
- 24.Lopes AA, Barst RJ, Haworth SG, Rabinovitch M, Al Dabbagh M, DelCerro MJ, Ivy D, Kashour T, Kumar K, Harikrishnan S, D’Alto M, Thomaz AM, Zorzanelli L, Aiello VD, Mocumbi AO, Santana MV, Galal AN, Banjar H, Tamimi O, Heath A, Flores PC, Diaz G, Sandoval J, Kothari S, Moledina S, Gonçalves RC, Barreto AC, Binotto MA, Maia M, Habshan FAl, Adatia I. Repair of congenital heart disease with associated pulmonary hypertension in children: what are the minimal investigative procedures? Consensus statement from the Congenital Heart Disease and Pediatric Task Forces, Pulmonary Vascular Research Institute (PVRI) Pulm Circ. 2014;4(2):330–41. doi: 10.1086/675995. . Erratum in: Pulm Circ. 4(3) (2014) 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DelCerro MJ, Moledina S, Haworth SG, Ivy D, AlDabbagh M, Banjar H, Diaz G, Heath-Freudenthal A, Galal AN, Humpl T, Kulkarni S, Lopes A, Mocumbi AO, Puri GD, Rossouw B, Harikrishnan S, Saxena A, Udo P, Caicedo L, Tamimi O, Adatia I. Cardiac catheterization in children with pulmonary hypertensive vascular disease: consensus statement from the Pulmonary Vascular Research Institute, Pediatric and Congenital Heart Disease Task Forces. Pulm Circ. 2016;6(1):118–25. doi: 10.1086/685102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mocumbi AO, Goncalves C, Damasceno A, C. Carrilho. Active schistosomiasis, severe hypereosinophilia and rapid progression of chronic endomyocardial fibrosis. Cardiovasc J Afr. 2016;27(5):e4–e6. doi: 10.5830/CVJA-2016-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. [17 April 2020]. Instituto Nacional de Estatística. Mortalidade em Moçambique: Inquérito nacional sobre causas de mortalidade 2007–08 http://www.ine.gov.mz/operacoes-estatisticas/inqueritos/inquerito-sobre-causas-de-mortalidade/inquerito-nacional-sobre-causas-de-mortalidade-2007-8.pdf/at_download/file .
- 28. [17 April 2020]. https://www.unaids.org/en/resources/909090 .
- 29. [17 April 2020]. IMASIDA Inquérito de Indicadores de Imunizacçaão, Malária e HIV/SIDA em Mocçambique Relatorio de indicadores. https://mz.usembassy.gov/wp-content/uploads/sites/182/2017/06/IMASIDA-2016_Relatorio-de-Indicadores-Basicos-for-Web.pdf .
- 30.Liu S, Zhou Y, Wang X, etal Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South Chin. Thorax. 2007;62:889–897. doi: 10.1136/thx.2006.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almodovar S, Hsue PY, Morelli J, Huang L, Flores SC, Lung HIV Study Pathogenesis of HIV-associated pulmonary hypertension: potential role of HIV-1 Nef. Proc Am Thorac Soc. 2011;8(3):308–12. doi: 10.1513/pats.201006-046WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todd NW, Lavania S, Park MH, etal Variable prevalence of pulmonary hypertension in patients with advanced interstitial pneumonia. J Heart Lung Transplant. 2010;29(2):188–94. doi: 10.1016/j.healun.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mocumbi AO. Recent trends in the epidemiology of endomyocardial fibrosis in Africa. Paediatr Int Child Health. 2012;32(2):63–4. doi: 10.1179/204690512X13345408275810. [DOI] [PubMed] [Google Scholar]
- 34.Mocumbi AO, Carrilho C, Burke MM, Wright G, Yacoub MH. Emergency surgical treatment of advanced endomyocardial fibrosis in Mozambique. Nat Clin Pract Cardiovasc Med. 2009;6(3):210–4. doi: 10.1038/ncpcardio1449. [DOI] [PubMed] [Google Scholar]
- 35.Papamatheakis DG, Mocumbi AO, Kim NH, Mandel J. Schistosomiasis-associated pulmonary hypertension. Pulm Circ. 2014;4(4):596–11. doi: 10.1086/678507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cribb DM, Clarke NE, Doi SAR, Vaz Nery S. Differential impact of mass and targeted praziquantel delivery on schistosomiasis control in school-aged children: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2019;13(10):e0007808. doi: 10.1371/journal.pntd.0007808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palha De Sousa CA, Brigham T, Chasekwa B, etal Dosing of praziquantel by height in sub-Saharan African adults. Am J Trop Med Hyg. 2014;90(4):634–7. doi: 10.4269/ajtmh.13-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabuyaya M, Chimbari MJ, Mukaratirwa S. Efficacy of praziquantel treatment regimens in pre-school and school aged children infected with schistosomiasis in sub-Saharan Africa: a systematic review. Infect Dis Poverty. 2018;7:73. doi: 10.1186/s40249-018-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart S, Al-Delaimy W, Sliwa K, Yacoub M, Mocumbi A. Clinical algorithm to screen for cardiopulmonary disease in low-income settings. Nat Rev Cardiol. 2019;16(11):639–41. doi: 10.1038/s41569-019-0268-0. [DOI] [PubMed] [Google Scholar]
- 40.Githinji LN, etal Cardiopulmonary dysfunction in perinatally HIV-infected South African adolescents on antiretroviral therapy: baseline findings from the Cape Town Adolescent Antiretroviral Cohort. J Int AIDS Soc. 2019;22:e25340. doi: 10.1002/jia2.25340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strange G, Stewart S, Celermajer DS, Prior D, Scalia GM, Marwick TH, Gabbay E, Ilton M, Joseph M, Codde J, Playford D, NEDA Contributing Sites Threshold of pulmonary hypertension associated with increased mortality. J Am Coll Cardiol. 2019;73(21):2660–72. doi: 10.1016/j.jacc.2019.03.482. [DOI] [PubMed] [Google Scholar]
- 42.Dzudie A, Kengne AP, Lamont K, Dzekem BS, Aminde LN, Abanda MH, Thienemann F, Sliwa K. A diagnostic algorithm for pulmonary hypertension due to left heart disease in resource-limited settings: can busy clinicians adopt a simple, practical approach? Cardiovasc J Afr. 2019;30(1):61–7. doi: 10.5830/CVJA-2018-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, Paquet C, Jacob S, Sidi D, Jouven X. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357(5):470–6. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 44.Mocumbi AO, Ferreira MB, Sidi D, Yacoub MH. A population study of endomyocardial fibrosis in a rural area of Mozambique. N Engl J Med. 2008;359(1):43–9. doi: 10.1056/NEJMoa0708629. [DOI] [PubMed] [Google Scholar]