Abstract
The parasitic mite Sarcoptes scabiei is an economically highly significant parasite of the skin of humans and animals worldwide. In humans, this mite causes a neglected tropical disease (NTD), called scabies. This disease results in major morbidity, disability, stigma and poverty globally and is often associated with secondary bacterial infections. Currently, anti-scabies treatments are not sufficiently effective, resistance to them is emerging and no vaccine is available. Here, we report the first high-quality genome and transcriptomic data for S. scabiei. The genome is 56.6 Mb in size, has a a repeat content of 10.6% and codes for 9,174 proteins. We explored key molecules involved in development, reproduction, host-parasite interactions, immunity and disease. The enhanced ‘omic data sets for S. scabiei represent comprehensive and critical resources for genetic, functional genomic, metabolomic, phylogenetic, ecological and/or epidemiological investigations, and will underpin the design and development of new treatments, vaccines and/or diagnostic tests.
Author summary
Scabies is a highly significant parasitic disease caused by the mite S. scabiei. This NTD has a major adverse impact in disadvantaged communities around the world, particularly when associated with secondary bacterial infections and clinical complications. Here we report the first high-quality genome and transcriptomic data for S. scabiei and explore molecular aspects of S. scabiei/scabies. This genome (56.6 Mb, encoding ~ 9,200 proteins) provides a solid foundation for fundamental investigations of the molecular biology of the mite, host-parasite interactions and disease processes as well as for translational research to develop new treatments, vaccines and diagnostic tests.
Introduction
Sarcoptes scabiei is a parasitic mite of the skin that causes scabies, one of the commonest dermatological diseases worldwide that results in major morbidity, disability, stigma and poverty [1, 2]. Of the 15 most burdensome dermatologic conditions, assessed in disability-adjusted life years (DALYs), scabies ranks higher than keratinocyte carcinoma and melanoma [3]. The prevalence of scabies can be very high (35%) in disadvantaged communities, including those in remote tropical regions in northern Australia [2, 4]. Scabies is often associated with secondary, opportunistic bacterial infections, a major concern in children in hyperendemic situations [2, 5]. Here, scabies poses a high risk of potentially life-threatening Staphylococcus aureus bacteraemia and severe post-streptococcal sequelae [6, 7], including rheumatic fever, heart disease and/or glomerulonephritis, representing a substantial mortality burden [8]. In spite of this knowledge, current epidemiological data underrepresent the actual scabies burden [9] due to an absence of accurate diagnostic tools and serious gaps in disease surveillance. In 2017, WHO’s recommendation to include scabies in the highest NTD category came with an urgent call for research and drug development [10].
There is no vaccine, and only a small number of treatments are used to combat this highly contagious disease. Topical permethrin and systemic/topical ivermectin are ‘broad-spectrum’ compounds of choice [11]. However, permethrin is not recommended for use in infants, and ivermectin is contra-indicated in patients with severely impaired liver or kidney function and the safety of its use in pregnant women and in children of < 15 kg body weight is only beginning to be investigated [12, 13]. Some other agents, such as sulphur, crotamiton, malathion and benzyl benzoate are presently available for topical application in children, but their clinical efficacies and tolerability have not been adequately assessed. Moreover, currently available drugs kill motile stages (larvae, nymphs and adults) of S. scabiei by interfering with the mite’s muscle function and/or nervous system [14–17]. These drugs often fail because the eggs of the mite are not susceptible to treatment, and drugs have short half-lives in the skin. Thus, eggs can hatch and perpetuate infection. Resistances to drugs are emerging in S. scabiei [18], which emphasises the urgency of finding novel scabicides to improve the treatment and management of scabies at the individual-patient, household and community levels. The discovery of new scabicides has been challenging, predominantly because of difficulties in producing adequate amounts of the mite for experimentation and drug screening/testing, and also due to a limited understanding of the mite’s biology and how it interacts with its host at the molecular level.
Given these abovementioned challenges, there is an urgent need to search for new drug targets encoded as proteins in the S. scabiei genome. Although three draft genomes have been assembled and/or annotated for S. scabiei from different host animals including human, dog and pig [19, 20], all of them are fragmented, limiting their utility for critical fundamental and applied investigations. Here, we report the first high-quality draft genome for S. scabiei, complemented by its transcriptome, to underpin fundamental and applied investigations of this parasitic mite at the molecular level. This genome is expected to provide a substantially enhanced resource to the research community for genetic, functional genomic, evolutionary, biological, ecological and epidemiological investigations, and a basis for the discovery of new drug and vaccine targets against scabies.
Results and discussion
Genome assembly
We sequenced the genome of S. scabiei var. suis from Australia at 114-fold long read and 443-fold short read coverage (S1 Table), producing a final draft assembly of 56.6 Mb (scaffold N50: 2.97 Mb; Table 1) with a mean GC-content of 33.3%. The present assembly was represented by a total of 66 contiguous sequences, compared with 4,268, 3,138 and 18,860 contigs for previous assemblies for S. scabiei var. suis, var. hominis and var. canis, respectively [19, 20]. As S. scabiei var. suis cells appear to contain 17–18 chromosomes [21], this assembly of 21 contigs (Table 1; L90 = 21 for S. scabiei var. suis) indicates that we have achieved a near chromosomal-level assembly. The estimated repeat content for this genome is 10.6%, equating to 6.0 Mb of DNA. The assembly contained 3.1% (1.8 Mb) interspersed and 7.9% (4.4 Mb) simple and low complexity repeats (S1 Table), the latter of which is in accord with findings for the house dust mite, Dermatophagoides pteronyssinus (9.2%; ~ 4.8 Mb) [22]. DNA transposons are more abundant (0.89%; 506 kb) in identified retrotransposon sequences (S1 Table) than long terminal repeats (LTRs) (0.38%; 215 kb), long interspersed elements (LINEs) (0.11%; 61 kb) and short interspersed elements (SINEs) (0.04%; 22kb). We also identified 915 kb (1.7%) of unclassified repeat elements (S1 Table).
Table 1. Features of Sarcoptes scabiei draft genome.
| Description | Sarcoptes scabiei var. suis | Dermatophagoides pteronyssinus | Tetranychus urticae | Psoroptes ovis | Sarcoptes scabiei var. canis |
|---|---|---|---|---|---|
| NCBI accession identifier | WVUK01000000 | GCF_001901225.1 | GCF_000239435.1 | GCA_002943765.1 | GCA_000828355.1 |
| Genome size (bp) | 56,576,587 | 70,778,228 | 90,828,597 | 63,414,655 | 56,262,437 |
| Number of scaffolds | 66 | 1,373 | 641 | 134 | 18,860 |
| N50 (bp); L50 | 2,965,819; 5 | 450,436; 33 | 2,993,488; 10 | 2,279,290; 8 | 11,557; 972 |
| N90 (bp); L90 | 703,488; 21 | 51,383; 206 | 732,742; 34 | 560,979; 29 | 1,270; 7,002 |
| Genome GC content (%) | 33.3 | 30.9 | 32.3 | 28.3 | 33.3 |
| Repetitive sequences (%) | 10.6 | ||||
| Exonic proportion; incl. introns (%) | 28.0; 44.4 | 26.0; 45.0 | 19.3; 47.5 | 23.5; 28.5 | 21.3; 27.1 |
| Number of putative protein-coding genes | 9,174 | 11,159 | 11,428 | 12,037 | 10,460 |
| Mean; median gene size (bp) | 2,735; 1,601 | 2,852; 1,576 | 3,836; 1,656 | 1,501; 1,107 | 1,459; 1,025 |
| Mean; median CDS length (bp) | 1,729; 1,305 | 1,646; 1,251 | 1,547; 1,209 | 1,236; 915 | 1,146; 830 |
| Mean exon number per protein-coding gene | 4.0 | 3.6 | 3.9 | 3.3 | 3.1 |
| Mean; median exon length (bp) | 431; 241 | 458; 253 | 396; 196 | 373; 186 | 372; 207 |
| Mean; median intron length (bp) | 334; 71 | 464; 71 | 788; 98 | 120; 70 | 147; 71 |
| Coding GC content (%) | 37.2 | 33.1 | 37.7 | 33.5 | 37.6 |
| Number or transfer RNAs | 294 | ||||
| BUSCO completeness: complete; partial (%) | 90.8; 92.6 | 92.3; 93.7 | 91.5; 92.8 | 84.5; 87.4 | 80.8; 87.5 |
Gene set
Given the fragmentation in published draft genome assemblies of S. scabiei variants [19, 20], we elected to predict genes and annotate them independently. We used transcriptomic data for egg, and adult stages of S. scabiei var. suis and protein sequences in UniProtKB/SwissProt (14 May 2019) [23] to support gene predictions. In total, we annotated 9,174 protein-encoding genes consisting of ~ 4.0 exons per gene (Table 1; S2 Table). In the predicted gene set, we inferred 967 (90.8%) of 1,066 complete core essential genes using the program Benchmarking Universal Single-Copy Orthologs (BUSCO) [24] for arthropods, which suggested that the genome is near complete. These findings accord with the numbers of BUSCO orthologs for D. pteronyssinus (984; 92.3%) [25] and Tetranychus urticae (975; 91.5%) [26] (Table 1). The statistics for the gene models of S. scabiei were similar to those of the well-assembled and annotated genome for D. pteronyssinus [25]: mean/median lengths of gene regions (2,735/1,601 bp), coding sequences (1,729/1,305 bp), exons (431/241 bp) and introns (334/71 bp)–excluding untranslated regions (UTRs)–were comparable with those of D. pteronyssinus (i.e. 2,852/1,576 bp, 1,646/1,251 bp, 458/253 bp and 464/71 bp, respectively), but distinct from those of T. urticae in which genes were larger (3,836/1,656 pb) due to longer intron sizes (788/98 bp) and coding sequences (1,547/1,209 bp), but exons (396/196 bp) were shorter (Table 1; Fig 1). Among these three mite species, S. scabiei shared more orthologous genes (OrthoMCL; BLASTp E-value of ≤ 10−8) with the genome of D. pteronyssinus (n = 7,203; 75.3%) than with that of T. urticae (n = 4,797; 52.0%) (Fig 2). Conspicuous are 822 protein-encoding genes (9.6%) that are unique to S. scabiei (Fig 2) for the acarines compared; 47 of these genes encode excretory/secretory (ES) proteins.
Fig 1. Characteristics of coding sequences, exons and introns.
Density diagrams–showing the distribution of data–were used to compare coding sequences, exons and introns for the gene models of the mite species Sarcoptes scabiei var. suis (black), Dermatophagoides pteronyssinus (blue), Psoroptes ovis (red), Tetranychus urticae (green) and Sarcoptes scabiei var. canis (yellow). The NCBI accession identifiers for the genomes of the taxa included here are: WVUK01000000, GCF_001901225.1, GCA_002943765.1, GCF_000239435.1 and GCA_000828355.1, respectively.
Fig 2. Comparison of orthologous proteins among selected mite species.
VENN diagram showing numbers of homologous groups of proteins among Sarcoptes scabiei var. suis, Sarcoptes scabiei var. canis, Psoroptes ovis, Dermatophagoides pteronyssinus and Tetranychus urticae. Protein-coding genes of S. scabiei var. suis are indicated in parentheses. NCBI accession identifiers for the genomes of the taxa included here are: WVUK01000000, GCA_000828355.1, GCA_002943765.1, GCF_001901225.1 and GCF_000239435.1, respectively.
Genetic relationships
We studied the molecular phylogenetic relationships of select free-living and parasitic mite species for which comparative genomic sequence data sets were available. Using data for protein-encoding single-copy orthologous genes (SCOs; n = 2,314), we showed that S. scabiei var. suis is genetically similar to S. scabiei var. canis, phylogenetically related to the dust mite (Dermatophagoides pteronyssinus) and the scab mite (Psoroptes ovis), and is distant from the spider mite (Tetranychus urticae) and the predatory mite (Metaseiulus occidentalis) (Fig 3). These relationships are in accord with the numbers of shared orthologous genes, with S. scabiei var. suis sharing most (n = 7,685) with S. scabiei var. canis and least (n = 5,016) with T. urticae (Fig 2). Density diagrams for coding sequence-, exon- and intron- lengths of S. scabiei var. suis were compared with those of S. scabiei var. canis, D. pteronyssinus and T. urticae. The distributions for S. scabiei were most similar to those for D. pteronyssinus; the distributions reflected long introns in T. urticae and short coding regions in S. scabiei var. canis compared with the other mite species studied (Fig 1). Previous results from a phylogenetic analysis of 350 astigmatid mite taxa using concatenated sequence data for five house-keeping genes (8942 nt) [27] suggested that a single common ancestor of the pyroglyphid (dust) mites evolved from a permanent, parasitic life style to become secondarily free-living.
Fig 3. Genetic relationships of selected species of mites.
The phylogenetic tree was constructed using data for shared single-copy orthologous protein sequences (n = 2,314) representing Sarcoptes scabiei var. suis, Sarcoptes scabiei var. canis, Dermatophagoides pteronyssinus (dust mite), Psoroptes ovis (sheep mite), Tetranychus urticae (spider mite) and Metaseiulus occidentalis (predatory mite). All nodes had absolute support values (posterior probability = 1 and bootstrap support = 100%) for both the Bayesian and maximum likelihood inference methods.
Intervention targets
The excessive and uncontrolled use of a small number of drug classes for the treatment of scabies has led to drug resistances to some of these compounds [28]. Unfortunately, only a small number of scabicides, permethrin and ivermectin in particular, have been available for treatment [14, 29–31]. However, these drugs do not kill eggs and have short half-lives in skin. As a foundation to explore novel intervention targets for S. scabiei, we identified and manually curated some key groups of proteins inferred to be encoded in this mite, including peptidases, peptidase inhibitors, kinases, G-protein coupled receptors (GPCRs) and ion channels.
Peptidases (n = 217) represented five key classes (aspartic, cysteine, metallo-, serine and threonine), with the metallo- (n = 68; 31.3%) and serine peptidases (n = 74; 34.1%) predominating (S3 Table). Notable were excreted peptidases, such as cathepsins (C01A; n = 3), serine peptidases (S09; n = 2), threonine peptidases (T01A; n = 7) and aminopeptidases (M17; n = 2), which are likely to be involved in cutaneous establishment, protein degradation, immune evasion and/or activation of inflammation, based on knowledge of the biology of S. scabiei [18]. Identified protease inhibitors (n = 30) included immunosuppressive factors, such as cytotoxic T-lymphocyte antigen-2 alpha (I29; n = 7), alpha-2-macroglobulin (I39; n = 3), subtilisin (I08; n = 7) and aprotinin (I02; n = 2), as well as genes homologous to those encoding serpins (I04; n = 2; SAR_2327s and SAR_4743s), which are known to inhibit activation pathways of the human complement system [32] (S4 Table).
Kinases (n = 251) represented mainly the groups CAMK (n = 53), CMGC (n = 26), tyrosine (TK; n = 21), AGC (n = 19), STE (n = 17), TKL (n = 16) and atypical (n = 8) kinases (S5 Table), which have significant potential as drug targets in parasites due to their role in pivotal cellular processes [33, 34]. GPCRs (n = 106) representing the rhodopsin classes A (n = 73), B1 (n = 9), class B2 (n = 7), class C (n = 8), class F (n = 4) (S6 Table) are intensively studied drug targets [35], and are known to bind molecules critically involved in key biological processes including signalling proteins (e.g., chemokines), neuropeptides (e.g., bombesin, galanin, neuromedin U, neuropeptide Y, neurotensin and tachykinin), lipids (e.g., lysophosphatidylinositol and cannabinoid), hormones (e.g., adrenaline, calcitonin, cholecystokinin, corticotropin-releasing, glucagon, oxytocin, gonadotropins, somatostatin, thyrotropin-releasing and vasopressin), amino acids (gamma-aminobutyric acid and metabotropic glutamate) and/or compounds such as acetylcholine, dopamine, histamine and 5-hydroxytryptamine. Since 2012, > 69 drugs that target GPCRs have been approved by the U.S. Food and Drug Administration (FDA) [36]. Ion channel proteins (n = 126), including voltage-gated ion channels (VGICs; n = 27) and ligand-gated ion channels (LGICs; n = 48), were also identified (S7 Table). Such channels are known targets for endo- and ecto-cidal compounds, including permethrin which targets voltage-gated sodium channels (VGSC) [15, 37], and macrocyclic lactones (e.g., ivermectin and moxidectin) which target glutamate-gated chloride channels (GluCls) [16, 30, 31]. We expect some of these peptidases, peptidase inhibitors, kinases, GPCRs and ion channels to be intervention target candidates that warrant detailed evaluation in S. scabiei in the future.
The host-pathogen interplay and immunogens/allergens
Excretory/secretory proteins are central to the host-mite relationship [28, 38]. A proteomic analysis of faecal matter from S. scabiei var. suis revealed totals of 236 excretory proteins (representing the ‘excretome’) (S8 Table) and 373 secretory proteins (‘secretome’) (S9 Table), with 14 proteins being common to both protein sets. The excretome includes 20 proteases, including 7 threonine-, 4 metallo-, 4 cysteine-, 4 serine- and 1 aspartic peptidases (S3 Table; S8 Table), and 5 peptidase inhibitors (including 2 immunosuppressive factors representing cytotoxic T-lymphocyte antigen-2 alpha), 2 subtilisin inhibitors and 1 trypsin inhibitor (aprotinin) (S4 Table; S8 Table). Many of these peptidases and inhibitors are likely involved in the degradation/digestion of skin, tissue barriers and nutrients, and also proposed to play critical roles in the growth, development, moulting and survival of S. scabiei on the host animal and immunomodulation by this mite [28, 38].
We inferred 85 putative allergens (S10 Table) to be encoded in the genome of S. scabiei var. suis, many of which are homologs of known allergens in D. farinae (22 of 48; 45.8%; S11 Table) and D. pteronyssinus (20 of 37; 54.0%; S12 Table) [25, 39]. The inferred excretome contained 28 of these homologs, whereas the secretome contained four. Interestingly, the inferred allergens are amongst the most highly-transcribed genes in the genome, and 22 of them appear to be unique to S. scabiei (S10 Table).
Apolipoprotein, glutathione S-transferases, cysteine- and serine proteases and serine protease inhibitors have been hypothesised as vaccine candidates against scabies [40]. Here, we identified apolipoproteins Ssag1 and Ssag2 [41], the first of which (SAR_333s) is inferred to be an excreted allergen, but the second (SAR_1661s) is not (S10 Table). We inferred a previously-discovered glutathione S-transferase [42] to be an allergen (SAR_5548); of 11 other glutathione S-transferases identified here, 8 are likely allergens, 3 of which are predicted to be excreted (S8 Table; S10 Table). We also identified a serine protease (cf. accession no. AY333071), an inactive cysteine protease (AY525155) and an active cysteine protease (AY525149) [43, 44], all of which are inferred to be allergens (SAR_9234s, SAR_6923s and SAR_5356s, respectively) (S3 Table). We also identified two serine protein inhibitors (serpins; accession nos. JF317220.1 and JF317222.1) [32], one of which is inferred to be an allergen (SAR_4743s; S4 Table) and the other (SAR_1449s) not.
Functional genomics and double-stranded RNA interference (RNAi) machinery
Prioritised target candidates (S10 Table) could first be tested for essentiality in S. scabiei using RNAi, which might support the development of a scabicide. Moreover, functional analysis of the ~ 22% of S. scabiei protein-encoding genes proposed to be parasite-specific, some of which might be involved in host-parasite interactions, could be facilitated by gene knockdown experiments. The recent establishment of an RNAi assay for S. scabiei [45] should underpin integrative functional genomic, transcriptomic and proteomic analyses [46] of distinct stages of S. scabiei in the future. To provide a foundation for such studies, we explored RNAi pathways in this mite.
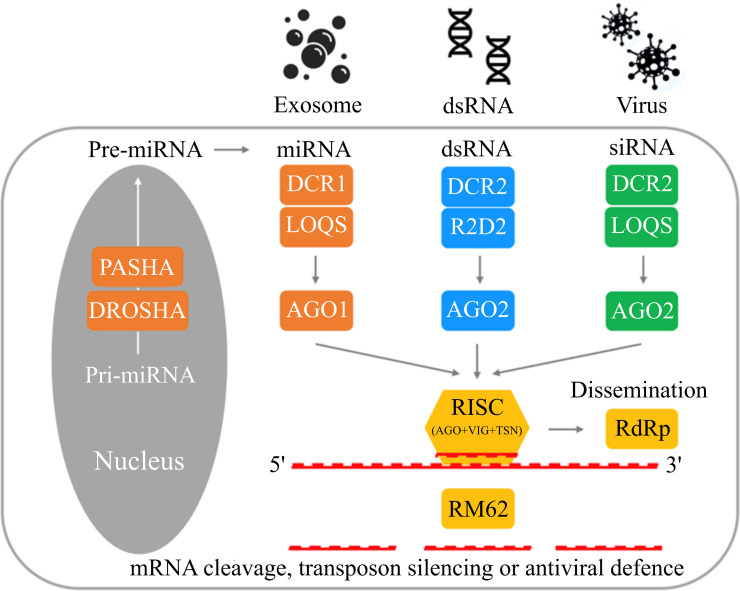
Typically, the RNAi machinery of eukaryotic organisms comprises the canonical microRNA (miRNA), small-interfering RNA (siRNA) and/or piwi-interacting RNA (piRNA) pathways [47, 48]. These RNAi pathways regulate a range of biological processes at post-transcriptional level via essential cofactors, the Dicer- and Argonaute-family proteins [49, 50]. Although RNAi pathways have been defined in the model arthropod Drosophila melanogaster [51], very little is known about them in S. scabiei. Here, we identified gene homologues (n = 29) encoding core components of RNAi pathways in S. scabiei (S13 Table). The results revealed relatively conserved miRNA, dsRNA, viRNA and/or piRNA pathways (Fig 4). Although components [i.e., systemic RNAi defective gene (sid), synthetic secondary siRNA-deficient argonaut mutant (sago) and RNAi spreading defective gene (rsd)] that are known to function in dsRNA/siRNA uptake and secondary siRNA dissemination in nematodes [52] were not detected in S. scabiei, the presence of the RNA-dependent RNA polymerase coding gene (rdrp) suggested an endogenous synthetic machinery for secondary siRNAs, which might link to a novel spreading mechanism. In addition, although homologous piRNA-binding proteins aubergine (AUB) and PIWI were not detected (Fig 4), the genes ago-1, -2 and/or -3 encoding similar protein domains to those of AUB and PIWI may play complementary roles in a piRNA-like pathway in S. scabiei. The lack of a canonical piRNA pathway in S. scabiei is consistent with findings for dust mites [53].
Fig 4. Proposed RNA interference machinery of Sarcoptes scabiei.
Proteins PASHA and DROSHA are involved in the endogenous synthesis of miRNA. Endogenous or exogenous miRNA, dsRNA and viral siRNA are recognised and diced by endoribonucleases DCR1 or DCR2, mediated by proteins LOQS or R2D2, and transferred to argonaut protein (AGO1 or AGO2), forming the RNA-induced silencing complex (RISC). The RISC facilitates targeting specific transcripts, leading to mRNA cleavage and antiviral defence via ATP-dependent RNA helicase (RM62). The silencing effect can be disseminated to other cells via a key component RNA-dependent RNA polymerase (RdRp); miRNA, dsRNA and virus-derived siRNA pathways are indicated in orange, blue and green, respectively. Silencing and dissemination modules are indicated in yellow.
Concluding remarks
The present genomic and molecular exploration of S. scabiei provides improved insights into the molecular landscape of one of the most important mite pathogens of animals worldwide. This study has inferred molecules involved in host-parasite interactions and immune responses/allergy. The improved genome assembly and associated data sets for S. scabiei should accelerate post-genomic explorations of molecules involved in mite reproduction and development, metabolism, parasite-host interactions, disease pathogenesis, and the genetics and mechanisms of drug resistance.
Inferring the RNAi machinery in S. scabiei could assist functional genomic work on selected stages (e.g., eggs) of the parasite. Given that gene-specific knockdown by double-stranded RNA interference (RNAi) has been demonstrated [45], we believe that genome-assisted drug target or drug discovery could provide a complementary approach to the screening of whole mites for new scabicides, similar to approaches proposed for parasitic helminths [54]. The aim is to identify genes or molecules whose inactivation by one or more drugs would selectively kill S. scabiei but not harm the host animal. Combined with the bioinformatic prediction and prioritisation of essential genes from functional information (e.g., lethality) available for other metazoan organisms, particularly D. melanogaster, using machine learning approaches [55], RNAi-based screening of S. scabiei stages provides a powerful functional genomics tool to validate prioritised targets. Focusing on groups of molecules, such as the complex array of peptidases, GPCRs, kinases and ion channels, and understanding their involvement in the host-mite interplay would likely assist in the design of new drugs or a vaccine against scabies. Moreover, future studies should focus on defining a spectrum of key molecules involved in pathways associated with the development of the nervous system in different life-stages of the mite, and on evaluating their potential as drug targets. The availability of a gene knockdown system [45], a drug screening platform [56, 57] and an in vivo pig-scabies model [58] provide a particularly useful context to assess prioritised intervention targets and then to evaluate drug candidates both in vitro and in vivo. Although the present study focused on S. scabiei, the results and methods employed here should be readily applicable to other ectoparasites of major animal and human health importance. We believe that the substantially improved genome of S. scabiei should accelerate both fundamental and applied investigations of scabies, enabling the development of new interventions for this important neglected tropical disease.
Materials and methods
Ethics approval
Animal ethics approval was granted by the QIMR Berghofer Medical Research Institute (permit nos. P630 and P2159) and the Ethics Committee of the Queensland Animal Science Precinct (permit SA 2015/03/504).
Production and procurement of S. scabiei
Sarcoptes scabiei was produced on pigs (3 months of age), isolated and stored using a well-established protocol [21]. Mites (n = 1000; approximately equal proportion of larvae, nymphs and adults) were isolated from skin crusts from S. scabiei-infected pigs, washed extensively, and directly snap frozen and stored at -70°C. In addition, faecal samples (n = 5) were collected from five different batches of mites (same number and stages) isolated from skin crusts taken from pigs on different days; from these faecal samples, crude protein extracts were prepared, freeze-dried and resuspended in 200 μl 8M urea in 100 mM triethylammonium bicarbonate (pH 8.5) with protease inhibitor cocktail set I (Merck, Denmark) [59].
Genomic DNA library construction and sequencing
High molecular weight genomic DNA was isolated from six samples each containing 1,000 motile adults, nymphs, larvae and eggs, collected on different days, using the Gentra Puregene Tissue Kit (Qiagen) according to manufacturer’s instructions. Total DNA amount was determined using a Qubit fluorometer dsDNA HS Kit (Invitrogen), according to the manufacturer’s instructions. Genomic DNA integrity was verified by agarose gel electrophoresis and using a Bioanalyzer 2100 (Agilent). Long-read sequencing of libraries constructed using the 20 kb Template Preparation employing BluePippin Size-Selection System was conducted using an established Pacific Biosciences (PacBio) protocol [60]. Short-read paired-end (PE) libraries (100 bp-inserts) were constructed, checked for size distribution and quality using Bioanalyzer 2100 and sequenced with Illumina HiSeq 2500 using an established method [20]. Jumping libraries (with 3-, 5-, and 7-kb inserts; see S1 Table) were constructed and sequenced using an established method [61]. Library preparation and long-read sequencing was conducted at the Centre for Clinical Genomics at the Translational Research Institute, Diamantina Institute in Wooloongabba, Queensland, Australia. Library preparation and long-read sequencing was conducted using a 20Kb PacBio RSII, Bluepipin size-selected SMRT bell library preparation and sequencing on 10 SMRT cells. The average number of reads per SMRT cell was 51,128 bp; the mean read length was 12,663 bp, and the N50 read length was 18,857 bp.
RNA isolation and RNA-seq
Total RNA was isolated separately from eggs (n = 16,000) and mixed larvae, nymphs and adults (n = 16,000) of S. scabiei var. suis employing the ToTally RNA Kit (Ambion). RNA yields were estimated spectrophotometrically (NanoDrop 1000), and the integrity of RNA was verified using a BioAnalyzer 2100 (Agilent). Following mRNA isolation using the MicroPolyAPurist kit (Ambion), RNA-seq was carried out as described previously [20]. Sequence data were assessed for quality and adaptors removed.
Liquid chromatography/tandem mass spectrometry (LC-MS/MS) analysis
The proteome of faecal matter (“excretome”) from S. scabiei eggs, nymphs and adults was investigated using an established in-solution digestion protocol [62]. In brief, the five samples (i.e. biological replicates; 50 μg of protein each) were reduced, alkylated and double-digested with Lys-C/trypsin mix (Promega, USA) at 37°C for 16 h. The tryptic samples were then acidified with 1.0% (v/v) formic acid and purified using Oasis HLB cartridges (Waters, USA). Using an established technique [63], tryptic peptides were analysed using a Q Exactive Plus Orbitrap mass spectrometer (Thermo Fisher, USA). Protein- and peptide- level fractionation and LC-MS/MS analysis of whole mite preparations was undertaken at the Institute of Bioinformatcs ain Bangalore, India, and egg preparations underwent on-tip strong-cation exchange chromatography-based fractionation and were analyzed on Orbitrap Fusion Lumos mass spectrometer interfaced with Easy nLC 1200 UPLC system (Thermo Scientific, Bremen, Germany) at Johns Hopkins University.
Excretory/Secretory proteins and allergens
Excretory/secretory proteins were inferred from LC-MS/MS (faecal matter) data against the proteome inferred from the genome of S. scabiei. First, raw LC-MS/MS data were processed with the program MaxQuant using the Andromeda search engine [64]. Fixed modifications of carbamidomethylation of cysteine (+57 Da) and variable modifications of methionine oxidation (+16 Da) were used. Results were compiled at targeted false discovery rate (FDR) of < 0.01 on both the peptide spectrum match (PSM) and the protein level. Proteins identified with ≥ 2 peptides were accepted. Secreted proteins were predicted using the programs SignalP 4.0 [65] and MultiLoc2 [66]. To classify a secreted protein, a predicted signal peptide and predicted extracellular location were required. Allergens were identified using BLASTp v2.2.30+ searches (E-value ≤ 10−8) against the NCBI protein nr database, the allergens identified for S. scabiei var. canis [19], and known allergens of Dermatophagoides farinae and D. pteronyssinus [67]; gene models of identified allergens were manually curated using available transcriptomic data.
Genomic assembly
An established pipeline [68] was used to create an assembly from PacBio sequence read data. In brief, these data were assembled using the program Canu v1.6 [69], polished using both PacBio raw reads and Illumina PE reads employing the programs SmrtLink v5.0.1 [70] and Pilon v1.22 [71], and sequences representing redundant haplotypes were removed using the program HaploMerger2 (build_20160512) [72]. The assembly was then scaffolded using Illumina mate-pair reads (3-, 5- and 7-kb inserts), and gaps were closed with Illumina PE reads in two iterations employing the programs SSPACE v3.0 [73] and GapCloser v1.12 [74].
Gene prediction
The S. scabiei protein-coding gene set was inferred utilizing available evidence data, including the transcriptomic data for egg and mixed-sex, motile stages, and protein sequence data were deposited in the UniProtKB/SwissProt database (May 14, 2019) [23]. First, known interspersed repeats in Repbase v.17.02 [75] and simple repeats were masked using the program RepeatMasker [76]. Transcriptomic evidence data were collected from both cDNA [77, 78] and RNAseq experiments; cDNA sequences were assembled using the program CAP3 (version 10/15/07) [79] and RNAseq data using the program Trinity v2.4.0 [80]. CAP3-assembled transcripts were concatenated with de novo and genome-guided transcript assemblies acquired using the Trinity pipeline. Transcripts with unknown nucleotide positions (“Ns”) were removed, and cd-hit-est [81] was used to reduce transcript redundancy by 1%. Open reading frames (ORFs) were inferred from the remaining 99% of transcripts employing the program TransDecoder [80], and cd-hit-est was used to reduce redundancy by 1%. This final set of ORFs (≥ 500 bp in length) was used as transcriptomic evidence data for gene predictions and mapped to the genome using BLAT [82]. The validity of splice sites was verified, and ORF-sequences were then used to train the de novo-gene prediction program AUGUSTUS [83] that produces a Hidden Markov Model (HMM) for gene prediction. The non-redundant ORFs and the proteome of T. urticae were also given to MAKER3 [84] to provide evidence for predicted genes. The resultant HMM, the ORFs and the proteome were subjected to analysis using MAKER3 to provide a consensus set of genes for S. scabiei. Genes inferred to encode peptides of ≥ 30 amino acids in length were preserved. Next, the PASA pipeline [85] employed non-redundant ORFs to improve predicted gene models in three iterations. The gene set was compared against original MAKER3 gene models, and those that did not overlap with the PASA-improved gene models were added to the gene set. Isoforms were removed from this gene set by preserving the longest isoform to represent each gene. For NCBI submission, UTR-regions were removed, and the gene set was verified using the programs GAG v2.0.1 [86] and tbl2asn [87].
Functional annotation
First, following the prediction of the protein-coding gene set for S. scabiei, each inferred amino acid sequence was assessed for conserved protein domains using InterPro (release 75.0) [88] employing default settings. Then, amino acid sequences were subjected to BLASTp (E-value ≤ 10−8) against the following protein databases: Swiss-Prot within UniProtKB [23]; Kyoto Encyclopedia of Genes and Genomes (KEGG) [89, 90]; and NCBI protein nr [91]. Genes encoding proteases, protease inhibitors, G-protein-coupled receptors (GPCR), kinases and ion channels were manually curated.
Curation of gene annotations for key protein groups
Gene models were curated employing protein domain architecture information from the InterPro database (release 75.0) and from transcriptomic data. Kinase gene models were curated using an established approach [92]–i.e. kinases were first inferred and classified into groups, families and subfamilies using Kinannote [93], and the PANTHER [94] and InterPro databases were then employed for unclassified kinases. GPCR gene models were identified and manually curated using an established approach [95] and assigned to class, family and/or subfamily based on information from GPCRdb (March 2019 release) [96]. Peptidase gene models were inferred by searching MEROPS peptidase and peptidase inhibitor databases (release 12.1) (BLASTp; E-value ≤ 10−8) [97] and manually curated. Ion channel gene models were manually curated and classified based on information from the PANTHER (release 14.1), Pfam (release 32.0) [98] and InterPro (release 76.0) databases.
Prediction of repeat regions
Genomic repeats specific to S. scabiei were inferred using the program RepeatModeler [99] that merges repeat predictions from the programs RECON [100] and RepeatScout [101]. Custom repeats and known repeats in Repbase v.17.02 [75] were then masked in the S. scabiei genome assembly using the program RepeatMasker [76].
Inferred protein sequence homology
Homologs among S. scabiei, T. urticae and D. pteronyssinus were inferred by comparison among all proteins using the program OrthoMCL v2.0.4 (BLASTp; E-value ≤ 10−8). The counts for shared homologous genes among these species were displayed in a Venn diagram.
Phylogenetic analysis
Single-copy orthologous (SCO) genes were inferred from homologous genes shared by S. scabiei var. suis, S. scabiei var. canis [19], D. pteronyssinus [25], Metaseiulus occidentalis [102], Psoroptes ovis [103] and Tetranychus urticae [26], and conceptually translated into amino acid sequences. The 1,859 clusters of SCO sequences representing all six species were individually aligned using the program AQUA [104], employing the programs MUSCLE v3.8.31 [105] and MAFFT v.7.271 [106] for the alignment and RASCAL v1.34 [107] for the refinement of alignments. Each gene cluster of SCO sequences with an alignment score of ≥ 0.8 obtained from the program NorMD [108] were merged using the program PartitionFinder v2.1.1 [109] to assign each merged partition to a replacement matrix. Partitions that did not contain all 20 amino acids, or represented mitochondrial or viral sequences, were removed. Remaining partitions were then subjected to separate phylogenetic analyses using the Bayesian inference (BI) and maximum likelihood (ML) tree-building methods. BI analysis was conducted using the program MrBayes v3.2.6 [110] from four independent Markov chains, run for 1,000,000 metropolis-coupled MCMC iterations, for which trees were sampled every 1000 iterations. The resultant tree was inferred by, first, discarding 250,000 sampled trees (25%) as burn-in, and using the remaining sampled trees to infer tree topology, branch lengths and to calculate Bayesian posterior probabilities (BPP). ML analysis was conducted using the program RAxML v8.2.6 [111] and the same replacement matrices were used as for BI analysis. The phylogram was prepared using FigTree v.1.31 (http://tree.bio.ed.ac.uk/software/figtree).
Density diagrams of gene features
Density diagrams were created using standard commands in the R language [112]. Gene-, exon- and intron- lengths were inferred from the gene models of D. pteronyssinus [25], S. scabiei var. canis [19], S. scabiei var. suis and T. urticae [26].
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors thank Milou Dekkers, Scott Cullen and Sheree Boisen at at the Queensland Animal Science Precinct, University of Queensland, Gatton Campus, Australia, for maintaining S. scabiei on pigs. The authors thank Dr Lawrie Wheeler for PacBio sequencing.
Data Availability
This Whole Genome Shotgun project has been deposited in DDBJ/ENA/GenBank under the accession WVUK00000000. The project data are available in the GenBank database through BioProject PRJNA598457. Raw genomic and RNA-seq reads have been submitted to the NCBI SRA (SRR10821142-SRR10821157). Mass spectrometry proteomic data are available in ProteomeXchange Consortium via the PRIDE [113] repository (PXD016925).
Funding Statement
Support from the Australian Research Council (ARC) and the National Health and Medical Research Council (NHMRC) of Australia is gratefully acknowledged (K.F, R.B.G., N.D.Y. and P.K.K). K.F. held an ARC Future Fellowship followed by an NHMRC Senior Research Fellowship and. P.K.K. held an NHMRC Early Career Research Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Investig Dermatol. 2014;134(6):1527–34. 10.1038/jid.2013.446 [DOI] [PubMed] [Google Scholar]
- 2.Romani L, Steer AC, Whitfeld MJ, Kaldor JM. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15(8):960–7. 10.1016/S1473-3099(15)00132-2 [DOI] [PubMed] [Google Scholar]
- 3.Karimkhani C, Dellavalle RP, Coffeng LE, Flohr C, Hay RJ, Langan SM, et al. Global skin disease morbidity and mortality: an update from the global burden of disease study 2013. JAMA Dermatol. 2017;153(5):406–12. 10.1001/jamadermatol.2016.5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tasani M, Tong SY, Andrews RM, Holt DC, Currie BJ, Carapetis JR, et al. The importance of scabies coinfection in the treatment considerations for impetigo. Pediatr Infect Dis J. 2016;35(4):374–8. 10.1097/INF.0000000000001013 [DOI] [PubMed] [Google Scholar]
- 5.Clucas DB, Carville KS, Connors C, Currie BJ, Carapetis JR, Andrews RM. Disease burden and health-care clinic attendances for young children in remote Aboriginal communities of northern Australia. SciELO Public Health; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gear R, Carter J, Carapetis J, Baird R, Davis J. Changes in the clinical and epidemiological features of group A streptococcal bacteraemia in Australia's Northern Territory. TM & IH. 2015;20(1):40–7. [DOI] [PubMed] [Google Scholar]
- 7.Boyd R, Patel M, Currie B, Holt D, Harris T, Krause V. High burden of invasive group A streptococcal disease in the Northern Territory of Australia. Epidemiol Infect. 2016;144(5):1018–27. 10.1017/S0950268815002010 [DOI] [PubMed] [Google Scholar]
- 8.Lynar S, Currie BJ, Baird R. Scabies and mortality. Lancet Infect Dis. 2017;17(12):1234. [DOI] [PubMed] [Google Scholar]
- 9.Fuller LC. Epidemiology of scabies. Curr Opin Infect Dis. 2013;26(2):123–6. 10.1097/QCO.0b013e32835eb851 [DOI] [PubMed] [Google Scholar]
- 10.WHO. Report of the Tenth Meeting of the WHO Strategic and Technical Advisory Group for Neglected Tropical Diseases. Geneva: World Health Organization; 2017. [Google Scholar]
- 11.Rosumeck S, Nast A, Dressler C. Ivermectin and permethrin for treating scabies. Cochrane Database Syst Rev. 2018;4:CD012994 10.1002/14651858.CD012994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy M, Martin L, Bursztejn AC, Chiaverini C, Miquel J, Mahé E, et al. Ivermectin safety in infants and children under 15 kg treated for scabies: a multicentric observational study. Br J Dermatol. 2019. 10.1111/bjd.18369 [DOI] [PubMed] [Google Scholar]
- 13.Morris-Jones R. Oral ivermectin for infants and children under 15 kg appears to be a safe and effective treatment for scabies. Br J Dermatol. 2019. 10.1111/bjd.18788 [DOI] [PubMed] [Google Scholar]
- 14.Mounsey KE, McCarthy JS. Treatment and control of scabies. Curr Opin Infect Dis. 2013;26(2):133–9. 10.1097/QCO.0b013e32835e1d57 [DOI] [PubMed] [Google Scholar]
- 15.Zlotkin E. The insect voltage-gated sodium channel as target of insecticides. Annu Rev Entomol. 1999;44(1):429–55. [DOI] [PubMed] [Google Scholar]
- 16.Geary TG. Ivermectin 20 years on: maturation of a wonder drug. Trends Parasitol. 2005;21(11):530–2. 10.1016/j.pt.2005.08.014 [DOI] [PubMed] [Google Scholar]
- 17.Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. New Eng J Med. 2010;362(8):717–25. 10.1056/NEJMct0910329 [DOI] [PubMed] [Google Scholar]
- 18.Fischer K, Holt D, Currie B, Kemp D. Scabies: important clinical consequences explained by new molecular studies. Adv Parasitol. 2012;79:339–73. 10.1016/B978-0-12-398457-9.00005-6 [DOI] [PubMed] [Google Scholar]
- 19.Rider SD, Morgan MS, Arlian LG. Draft genome of the scabies mite. Parasit Vectors. 2015;8(1):585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mofiz E, Holt DC, Seemann T, Currie BJ, Fischer K, Papenfuss AT. Genomic resources and draft assemblies of the human and porcine varieties of scabies mites, Sarcoptes scabiei var. hominis and var. suis. Gigascience. 2016;5(1):23 10.1186/s13742-016-0129-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mounsey KE, Willis C, Burgess ST, Holt DC, McCarthy J, Fischer K. Quantitative PCR-based genome size estimation of the astigmatid mites Sarcoptes scabiei, Psoroptes ovis and Dermatophagoides pteronyssinus. Parasit Vectors. 2012;5(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randall TA, Mullikin JC, Mueller GA. The draft genome assembly of Dermatophagoides pteronyssinus supports identification of novel allergen isoforms in Dermatophagoides species. Int Arch Allergy Immunol. 2018;175(3):136–46. 10.1159/000481989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magrane M, Consortium U. UniProt knowledgebase: a hub of integrated protein data. Database: the journal of biological databases and curation. 2011. 10.1093/database/bar009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simao FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–2. 10.1093/bioinformatics/btv351 [DOI] [PubMed] [Google Scholar]
- 25.Waldron R, McGowan J, Gordon N, McCarthy C, Mitchell EB, Doyle S, et al. Draft genome sequence of Dermatophagoides pteronyssinus, the European house dust mite. Genome Announc. 2017;5(32):e00789–17. 10.1128/genomeA.00789-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grbić M, Van Leeuwen T, Clark RM, Rombauts S, Rouzé P, Grbić V, et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature. 2011;479(7374):487–92. 10.1038/nature10640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klimov PB, OConnor B. Is permanent parasitism reversible?—Critical evidence from early evolution of house dust mites. Syst Biol. 2013;62(3):411–23. 10.1093/sysbio/syt008 [DOI] [PubMed] [Google Scholar]
- 28.Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10(1):297 10.1186/s13071-017-2234-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007;3:CD000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chabala JC, Mrozik H, Tolman RL, Eskola P, Lusi A, Peterson LH, et al. Ivermectin, a new broad-spectrum antiparasitic agent. J Med Chem. 1980;23(10):1134–6. 10.1021/jm00184a014 [DOI] [PubMed] [Google Scholar]
- 31.Prichard R, Ménez C, Lespine A. Moxidectin and the avermectins: consanguinity but not identity. Int J Parasitol Drugs Drug Resist. 2012;2:134–53. 10.1016/j.ijpddr.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mika A, Reynolds SL, Mohlin FC, Willis C, Swe PM, Pickering DA, et al. Novel scabies mite serpins inhibit the three pathways of the human complement system. PLoS One. 2012;7(7):e40489 10.1371/journal.pone.0040489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramamoorthi R, Graef KM, Dent J. Repurposing pharma assets: an accelerated mechanism for strengthening the schistosomiasis drug development pipeline. Future Med Chem. 2015;7(6):727–35. 10.4155/fmc.15.26 [DOI] [PubMed] [Google Scholar]
- 34.Stroehlein AJ, Young ND, Gasser RB. Advances in kinome research of parasitic worms-implications for fundamental research and applied biotechnological outcomes. Biotechnol Adv. 2018;36(4):915–34. 10.1016/j.biotechadv.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 35.Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, et al. A comprehensive map of molecular drug targets. Nat Rev Drug Discov. 2017;16(1):19 10.1038/nrd.2016.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16(12):829 10.1038/nrd.2017.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasay C, Arlian L, Morgan M, Vyszenski-Moher D, Rose A, Holt D, et al. High-resolution melt analysis for the detection of a mutation associated with permethrin resistance in a population of scabies mites. Med Vet Entomol. 2008;22(1):82–8. 10.1111/j.1365-2915.2008.00716.x [DOI] [PubMed] [Google Scholar]
- 38.Morgan MS, Arlian LG, Rider SD Jr, Grunwald Jr WC, Cool DR. A proteomic analysis of Sarcoptes scabiei (Acari: Sarcoptidae). J Med Entomol. 2016;53(3):553–61. 10.1093/jme/tjv247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan T-F, Ji K-M, Yim AK-Y, Liu X-Y, Zhou J-W, Li R-Q, et al. The draft genome, transcriptome, and microbiome of Dermatophagoides farinae reveal a broad spectrum of dust mite allergens. J Allergy Clin Immunol. 2015;135(2):539–48. 10.1016/j.jaci.2014.09.031 [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Walton S, Mounsey K. Vaccine against scabies: necessity and possibility. Parasitology. 2014;141(6):725–32. 10.1017/S0031182013002047 [DOI] [PubMed] [Google Scholar]
- 41.Harumal P, Morgan M, Walton SF, Holt DC, Rode J, Arlian LG, et al. Identification of a homologue of a house dust mite allergen in a cDNA library from Sarcoptes scabiei var. hominis and evaluation of its vaccine potential in a rabbit/S. scabiei var. canis model. Am J Trop Med Hyg. 2003;68(1):54–60. [PubMed] [Google Scholar]
- 42.Pettersson EU, Ljunggren EL, Morrison DA, Mattsson JG. Functional analysis and localisation of a delta-class glutathione S-transferase from Sarcoptes scabiei. Int J Parasitol. 2005;35(1):39–48. 10.1016/j.ijpara.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 43.Holt DC, Fischer K, Pizzutto SJ, Currie BJ, Walton SF, Kemp DJ. A multigene family of inactivated cysteine proteases in Sarcoptes scabiei. J Investig Dermatol. 2004;123(1):240–1. 10.1111/j.0022-202X.2004.22716.x [DOI] [PubMed] [Google Scholar]
- 44.Beckham SA, Boyd SE, Reynolds S, Willis C, Johnstone M, Mika A, et al. Characterization of a serine protease homologous to house dust mite group 3 allergens from the scabies mite Sarcoptes scabiei. J Biol Chem. 2009;284(49):34413–22. 10.1074/jbc.M109.061911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernando DD, Marr EJ, Zakrzewski M, Reynolds SL, Burgess ST, Fischer K. Gene silencing by RNA interference in Sarcoptes scabiei: a molecular tool to identify novel therapeutic targets. Parasit Vectors. 2017;10(1):289 10.1186/s13071-017-2226-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma G, Wang T, Korhonen PK, Young ND, Nie S, Ang C-S, et al. Dafachronic acid promotes larval development in Haemonchus contortus by modulating dauer signalling and lipid metabolism. PLoS Pathog. 2019;15(7):e1007960 10.1371/journal.ppat.1007960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–55. 10.1016/j.cell.2009.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10(2):94 10.1038/nrg2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tijsterman M, Plasterk RH. Dicers at RISC: the mechanism of RNAi. Cell. 2004;117(1):1–3. 10.1016/s0092-8674(04)00293-4 [DOI] [PubMed] [Google Scholar]
- 50.Meister G. Argonaute proteins: functional insights and emerging roles. Nat Rev Genet. 2013;14(7):447–59. 10.1038/nrg3462 [DOI] [PubMed] [Google Scholar]
- 51.Czech B, Malone CD, Zhou R, Stark A, Schlingeheyde C, Dus M, et al. An endogenous small interfering RNA pathway in Drosophila. Nature. 2008;453(7196):798–802. 10.1038/nature07007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwarz EM, Korhonen PK, Campbell BE, Young ND, Jex AR, Jabbar A, et al. The genome and developmental transcriptome of the strongylid nematode Haemonchus contortus. Genome Biol. 2013;14(8):R89 10.1186/gb-2013-14-8-r89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mondal MMH. Divergent RNAi Biology in Mites and Development of Pest Control Strategies. 2018. Available from: https://aquila.usm.edu/dissertations/1539. [Google Scholar]
- 54.Shanmugam D, Ralph SA, Carmona SJ, Crowther GJ, Roos DS, Agüero F. Integrating and mining helminth genomes to discover and prioritize novel therapeutic targets In: Caffrey CR, editor. Parasitic Helminths: Targets, Screens, Drugs and Vaccines. 3 Published Online: Wiley-VCH Verlag GmbH & Co. KGaA; 2012. p. 43–59. [Google Scholar]
- 55.Campos TL, Korhonen PK, Gasser RB, Young ND. An evaluation of machine learning approaches for the prediction of essential genes in eukaryotes using protein sequence-derived features. Comput Struct Biotechnol J. 2019;17:785–96. 10.1016/j.csbj.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Denecke S, Nowell CJ, Fournier-Level A, Perry T, Batterham P. The wiggle index: An open source bioassay to assess sub-lethal insecticide response in Drosophila melanogaster. PLoS One. 2015;10(12):e0145051 10.1371/journal.pone.0145051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Preston S, Jabbar A, Nowell C, Joachim A, Ruttkowski B, Baell J, et al. Low cost whole-organism screening of compounds for anthelmintic activity. Int J Parasitol. 2015;45:333–43. 10.1016/j.ijpara.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 58.Mounsey K, Ho M-F, Kelly A, Willis C, Pasay C, Kemp DJ, et al. A tractable experimental model for study of human and animal scabies. PLoS neglected tropical diseases. 2010;4(7):e756 10.1371/journal.pntd.0000756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang T, Ma G, Ang C-S, Korhonen PK, Xu R, Nie S, et al. Somatic proteome of Haemonchus contortus. Int J Parasitol. 2019;49(3–4):311–20. 10.1016/j.ijpara.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 60.Quail MA, Smith M, Coupland P, Otto TD, Harris SR, Connor TR, et al. A tale of three next generation sequencing platforms: comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genomics. 2012;13(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li R, Fan W, Tian G, Zhu H, He L, Cai J, et al. The sequence and de novo assembly of the giant panda genome. Nature. 2010;463(7279):311–7. 10.1038/nature08696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ang C-S, Binos S, Knight MI, Moate PJ, Cocks BG, McDonagh MB. Global survey of the bovine salivary proteome: integrating multidimensional prefractionation, targeted, and glycocapture strategies. J Proteome Res. 2011;10(11):5059–69. 10.1021/pr200516d [DOI] [PubMed] [Google Scholar]
- 63.Wang T, Ma G, Ang C-S, Korhonen PK, Koehler AV, Young ND, et al. High throughput LC-MS/MS-based proteomic analysis of excretory-secretory products from short-term in vitro culture of Haemonchus contortus. J Proteomics. 2019;204:103375 10.1016/j.jprot.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 64.Tyanova S, Temu T, Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc. 2016;11(12):2301 10.1038/nprot.2016.136 [DOI] [PubMed] [Google Scholar]
- 65.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–6. 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 66.Blum T, Briesemeister S, Kohlbacher O. MultiLoc2: integrating phylogeny and Gene Ontology terms improves subcellular protein localization prediction. BMC Bioinform. 2009;10:274 10.1186/1471-2105-10-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pomés A, Davies JM, Gadermaier G, Hilger C, Holzhauser T, Lidholm J, et al. WHO/IUIS Allergen Nomenclature: Providing a common language. Mol Immunol. 2018;100:3–13. 10.1016/j.molimm.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korhonen PK, Hall RS, Young ND, Gasser RB. Common Workflow Language (CWL)-based software pipeline for de novo genome assembly from long-and short-read data. Gigascience. 2019;8(4):giz014 10.1093/gigascience/giz014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koren S, Walenz B, Berlin K, Miller J, Phillippy A. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27(5):722–36. 10.1101/gr.215087.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chin C-S, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10(6):563–9. 10.1038/nmeth.2474 [DOI] [PubMed] [Google Scholar]
- 71.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9(11):e112963 10.1371/journal.pone.0112963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang S, Chen Z, Huang G, Yu T, Yang P, Li J, et al. HaploMerger: reconstructing allelic relationships for polymorphic diploid genome assemblies. Genome research. 2012;22(8):1581–8. 10.1101/gr.133652.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27(4):578–9. 10.1093/bioinformatics/btq683 [DOI] [PubMed] [Google Scholar]
- 74.Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1(1):18 10.1186/2047-217X-1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005;110(1–4):462–7. 10.1159/000084979 [DOI] [PubMed] [Google Scholar]
- 76.Smit AFA, Hubley R, Green P. RepeatMasker. http://www.repeatmasker.org: Institute of Systems Biology; 1996–2010. [Google Scholar]
- 77.Fischer K, Holt DC, Harumal P, Currie BJ, Walton SF, Kemp DJ. Generation and characterization of cDNA clones from Sarcoptes scabiei var. hominis for an expressed sequence tag library: identification of homologues of house dust mite allergens. Am J Trop Med Hyg. 2003;68(1):61–4. [PubMed] [Google Scholar]
- 78.Fischer K, Holt D, Wilson P, Davis J, Hewitt V, Johnson M, et al. Normalization of a cDNA library cloned in λZAP by a long PCR and cDNA reassociation procedure. BioTechniques. 2003;34(2):250–4. 10.2144/03342bm03 [DOI] [PubMed] [Google Scholar]
- 79.Huang X, Madan A. CAP3: A DNA sequence assembly program. Genome Res. 1999;9(9):868–77. 10.1101/gr.9.9.868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 2013;8(8):1494–512. 10.1038/nprot.2013.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22(13):1658–9. 10.1093/bioinformatics/btl158 [DOI] [PubMed] [Google Scholar]
- 82.Kent WJ. BLAT—The BLAST-like alignment tool. Genome Res. 2002;12(4):656–64. 10.1101/gr.229202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stanke M, Keller O, Gunduz I, Hayes A, Waack S, Morgenstern B. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 2006;34(Web Server issue):W435-9. 10.1093/nar/gkl200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holt C, Yandell M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinform. 2011;12:491 10.1186/1471-2105-12-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haas BJ, Delcher AL, Mount SM, Wortman JR, Smith RK Jr, Hannick LI, et al. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucl Acids Res. 2003;31(19):5654–66. 10.1093/nar/gkg770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hall B, DeRego T, Geib S. GAG: the Genome Annotation Generator. 1.0 ed. http://genomeannotation.github.io/GAG2014. [DOI] [PMC free article] [PubMed]
- 87.Benson DA, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucl Acids Res. 2015;43(Database issue):D30–5. 10.1093/nar/gku1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitchell AL, Attwood TK, Babbitt PC, Blum M, Bork P, Bridge A, et al. InterPro in 2019: improving coverage, classification and access to protein sequence annotations. Nucl Acids Res. 2018;47(D1):D351–D60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular datasets. Nucleic Acids Res. 2012;40:D109–D14. 10.1093/nar/gkr988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2013;41(Database issue):D8–20. 10.1093/nar/gks1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stroehlein AJ, Young ND, Gasser RB. Improved strategy for the curation and classification of kinases, with broad applicability to other eukaryotic protein groups. Sci Rep. 2018;8(1):6808 10.1038/s41598-018-25020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goldberg JM, Griggs AD, Smith JL, Haas BJ, Wortman JR, Zeng Q. Kinannote, a computer program to identify and classify members of the eukaryotic protein kinase superfamily. Bioinformatics. 2013;29(19):2387–94. 10.1093/bioinformatics/btt419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8(8):1551 10.1038/nprot.2013.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Campos TD, Young ND, Korhonen PK, Hall RS, Mangiola S, Lonie A, et al. Identification of G protein-coupled receptors in Schistosoma haematobium and S. mansoni by comparative genomics. Parasit Vectors. 2014;7:242 10.1186/1756-3305-7-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Munk C, Isberg V, Mordalski S, Harpsøe K, Rataj K, Hauser A, et al. GPCRdb: the G protein-coupled receptor database–an introduction. Br J Pharmacol. 2016;173(14):2195–207. 10.1111/bph.13509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rawlings ND, Barrett AJ, Thomas PD, Huang X, Bateman A, Finn RD. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucl Acids Res. 2017;46(D1):D624–D32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44(D1):D279–D85. 10.1093/nar/gkv1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smit AFA, Robert H, Kas A, Siegel A, Gish W, Price A, et al. RepeatModeler. 1.0.5 ed. http://www.repeatmasker.org: Institute of Systems Biology; 2011. [Google Scholar]
- 100.Bao Z, Eddy SR. Automated de novo identification of repeat sequence families in sequenced genomes. Genome Res. 2002;12(8):1269–76. 10.1101/gr.88502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Price AL, Jones NC, Pevzner PA. De novo identification of repeat families in large genomes. Bioinformatics. 2005;21 Suppl 1:i351–8. 10.1093/bioinformatics/bti1018 [DOI] [PubMed] [Google Scholar]
- 102.Hoy MA, Waterhouse RM, Wu K, Estep AS, Ioannidis P, Palmer WJ, et al. Genome sequencing of the phytoseiid predatory mite Metaseiulus occidentalis reveals completely atomized Hox genes and superdynamic intron evolution. Genome Biol Evol. 2016;8(6):1762–75. 10.1093/gbe/evw048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burgess ST, Bartley K, Marr EJ, Wright HW, Weaver RJ, Prickett JC, et al. Draft genome assembly of the sheep scab mite, Psoroptes ovis. Genome Announc. 2018;6(16):e00265–18. 10.1128/genomeA.00265-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muller J, Creevey CJ, Thompson JD, Arendt D, Bork P. AQUA: automated quality improvement for multiple sequence alignments. Bioinformatics. 2009;26(2):263–5. 10.1093/bioinformatics/btp651 [DOI] [PubMed] [Google Scholar]
- 105.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res. 2004;32(5):1792–7. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thompson JD, Thierry J-C, Poch O. RASCAL: rapid scanning and correction of multiple sequence alignments. Bioinformatics. 2003;19(9):1155–61. 10.1093/bioinformatics/btg133 [DOI] [PubMed] [Google Scholar]
- 108.Thompson JD, Plewniak F, Ripp R, Thierry J-C, Poch O. Towards a reliable objective function for multiple sequence alignments1. J Mol Biol. 2001;314(4):937–51. 10.1006/jmbi.2001.5187 [DOI] [PubMed] [Google Scholar]
- 109.Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol. 2016;34(3):772–3. [DOI] [PubMed] [Google Scholar]
- 110.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–4. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- 111.Stamatakis A, Ludwig T, Meier H. RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics. 2005;21(4):456–63. 10.1093/bioinformatics/bti191 [DOI] [PubMed] [Google Scholar]
- 112.Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: the R Foundation for Statistical Computing; ISBN: 3-900051-07-0. Available online at http://www.R-project.org/. 2.15 ed: Vienna, Austria; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
This Whole Genome Shotgun project has been deposited in DDBJ/ENA/GenBank under the accession WVUK00000000. The project data are available in the GenBank database through BioProject PRJNA598457. Raw genomic and RNA-seq reads have been submitted to the NCBI SRA (SRR10821142-SRR10821157). Mass spectrometry proteomic data are available in ProteomeXchange Consortium via the PRIDE [113] repository (PXD016925).