Background.
Rates of kidney transplantation vary substantially across dialysis facilities in the United States. Whether distance between the dialysis facility and transplant center associates with variations in transplantation rates has not been examined.
Methods.
We performed a retrospective study of adults treated with dialysis between 2005 and 2015, according to the US Renal Data System. We examined the association between distance from dialysis facility to transplant center and time to kidney transplantation (primary outcome) and waitlist registration (secondary outcome) using Fine-Gray models. We also performed sensitivity analyses using the distance from each patient’s dialysis facility to the nearest transplant center as the predictor so that patients who were never registered on the waitlist (and therefore would not have a transplant center) could be included.
Results.
In total, 178 885 waitlisted patients were included for our primary analysis. As distance between dialysis facility and transplant center increased, lower hazard of transplantation (subhazard ratio [HR], 0.92; 95% confidence interval [CI], 0.91-0.94, if distance was 10 to <50 miles; sub-HR, 0.90; 95% CI, 0.88-0.92, if distance ≥50 miles compared with <10 miles) was noted. We also found a weak association between longer distance and hazard of waitlist registration (sub-HR, 0.96; 95% CI, 0.94-0.97, if distance was ≥50 miles versus <10 miles). Findings were similar in sensitivity analyses using distance between dialysis facility and the nearest transplant center (N = 1 149 721).
Conclusions.
Patients receiving dialysis in facilities located further away from transplant centers have lower hazard of kidney transplantation. Developing strategies to address barriers to transplantation in patients receiving dialysis at facilities located far away from a transplant center may help improve disparities in transplantation rates.
INTRODUCTION
Kidney transplantation is the optimal treatment for patients with end-stage kidney disease (ESKD) given its associated benefits on quality of life and survival,1–3 but barriers to transplantation can occur at the level of the patient, provider and dialysis facility, broader healthcare system, or healthcare policy.4 Identifying and overcoming barriers to transplantation can help ensure equity in access to transplantation.
The pathway to receiving a kidney transplant is complex and often long, starting from awareness of advanced chronic kidney disease, referral to and evaluation at a transplant center, waitlist registration (if deemed eligible), and, finally, surgery when a suitable organ becomes available. However, rates of referral, waitlist registration, and transplantation vary substantially across dialysis facilities in the United States,5–8 potentially reflecting both individual- and system-level barriers during this complicated process. Dialysis facility factors that have been associated with higher transplantation rates include nonprofit status, higher number of staff per facility, and higher proportion of patients managed with peritoneal dialysis.5,8 To our knowledge, the distance between the dialysis facility and transplant center (DFTC distance) has not been evaluated as a potential factor that may be associated with access to waitlist registration or kidney transplantation. If patients receiving dialysis care in facilities further from the transplant center are shown to have lower access to transplantation, then targeted dialysis facility- and system-level interventions could help optimize disparities in access to kidney transplantation.9–11
The objective of this study was to assess whether DFTC distance is associated with receipt of kidney transplantation (primary outcome) and, secondarily, waitlist registration, using data from the United States Renal Data System (USRDS). We hypothesized that as DFTC distance increases, access to kidney transplantation and waitlist registration would decrease.
MATERIALS AND METHODS
Study Population and Data Source
We performed a retrospective cohort study using data from the USRDS, the national ESKD registry that contains data on all patients treated with dialysis or kidney transplantation in the United States. We included all adults (>18 y) who started dialysis between January 2005 and December 2015 and who had both a dialysis facility and transplant center zip code according to the USRDS. Recipients of a prior kidney transplant and preemptive kidney transplant recipients (who would not have a dialysis facility) were excluded from study.
Patient demographics, dialysis type, and zip code at time of dialysis initiation were determined from the PATIENTS file in the USRDS. We used patient zip code to determine neighborhood median income according to the American Community Survey,12 as well as whether patients were living in metropolitan, micropolitan, or rural/small town areas, according to the US Department of Agriculture Economic Research Service.13,14 Insurance coverage and comorbidities were derived at the time of dialysis initiation as reported on the Centers for Medicare and Medicaid Services (CMS) 2728 form (MEDEVID file). Dialysis facility zip code was obtained from the FACILITY file in the USRDS and used to determine dialysis facility location and the United Network for Organ Sharing region in which the facility was located.15 Other dialysis facility characteristics (profit status and facility size) were based on the CMS End-stage Renal Disease Annual Facility Survey characteristics derived from the FACILITY file.
The University of California, San Francisco, Institutional Review Board considers this work not human subjects research.
Primary Predictor: Distance Between Dialysis Facility and Transplant Center (DFTC Distance)
We estimated the distance from each patient’s dialysis facility to their transplant center. The location of the dialysis facility was defined based on the zip code of the first outpatient dialysis facility where the patient received treatment, and the transplant center location was defined based on the zip code of the center where the patient was first registered on the waitlist. We calculated the geodetic distance between dialysis facility and transplant center zip codes (using the “geodist” function in Stata). We used US Census Zip Code Tabulation Areas and commercial databases to determine the coordinates of each zip code.16,17 We then categorized DFTC distance as <10, 10 to <50, and ≥50 miles (approximate tertiles of distance in our data) and used this as the primary predictor in our analyses.
The majority of dialysis patients were not registered on the kidney transplant waitlist during follow-up and therefore did not have a transplant center zip code available (which could be due to failure of providers to refer for evaluation for transplant candidacy, patient decision not to pursue transplantation, or ineligibility for kidney transplantation). To understand the relationship between DFTC distance and access to transplantation in the total population of dialysis patients and thereby enhance the generalizability of our findings, we conducted sensitivity analyses using the distance from each patient’s dialysis facility to the nearest adult transplant center (termed nearest DFTC distance henceforth) as the predictor. The approach to estimating the distance to the nearest transplant center has been used in prior studies to allow for the inclusion of dialysis patients who were never registered on the kidney transplant waitlist in distance-related analyses.18,19 We calculated the geodetic distance between the zip codes of each patient’s dialysis facility and the nearest adult transplant center, which we considered the nearest DFTC distance. We defined adult transplant centers as those that performed at least 1 kidney transplant in patients >18 y during the study period. We categorized nearest DFTC distance in the same manner as for DFTC distance. To evaluate whether these theoretical distances to the nearest transplant center are a reasonable approximation, we assessed the Spearman correlation between the DFTC distance and nearest DFTC distance among those who were registered on the transplant waitlist and for whom we had an actual zip code for the transplant center where they were waitlisted in the USRDS.
Primary Outcome: Time to Kidney Transplantation
The primary outcome was time to kidney transplantation (defined as time from dialysis initiation to first kidney transplantation, either from a living or deceased donor). We started time at the date of outpatient dialysis initiation as we were primarily interested in the length of time on dialysis before first kidney transplantation. Additionally, the vast majority of dialysis patients are never registered on the transplant waitlist and, therefore, could not be included in our sensitivity analysis if we started time at waitlist registration. We ascertained dialysis initiation, transplantation, and death dates using the USRDS PATIENTS file and treated death as a competing risk. We also evaluated time to living and deceased donor transplantation as separate outcomes of interest.
Secondary Outcome: Time to Waitlist Registration
Our secondary outcome was time to waitlist registration (defined as time from dialysis initiation to first waitlist registration for kidney transplantation). Patients registered on the waitlist before dialysis initiation were excluded from this analysis, as we were interested in the role of the dialysis facility in access to the transplant waitlist, and patients registered on the waitlist before dialysis would have been referred by their provider during the nondialysis requiring chronic kidney disease phase of illness. Dates of waitlist registration were derived from the USRDS PATIENTS file.
Statistical Analysis
Primary Analyses
We used Pearson’s chi-squared, Kruskal-Wallis, and ANOVA tests to assess for differences in patient characteristics across DFTC distance categories. We assessed the correlation between DFTC distance and nearest DFTC distance using a Spearman rank correlation coefficient.
We used Fine and Gray models to assess the subhazard ratio (HR) for time to kidney transplantation using the categorized DFTC distance as the primary predictor while accounting for the competing risk of death.20 We censored follow-up at time at death, transplantation, or administratively as of December 31, 2015.
We conducted both unadjusted and adjusted analyses. In model 1, we adjusted for patient-level factors including age, sex, race/ethnicity, dialysis treatment modality (peritoneal or hemodialysis), insurance status, distance from patient residence to transplant center, neighborhood median income by zip code, rural/urban category (metropolitan, micropolitan, rural/ small town), and comorbidities (diabetes, congestive heart failure, coronary artery disease, cerebrovascular disease, peripheral artery disease, drug or alcohol dependence, smoking status, and inability to mobilize or transfer). In model 2, we additionally adjusted for dialysis facility-level factors including profit status (nonprofit or for-profit), size of dialysis facility (number of patients receiving ESKD care at the facility in the year of the patient’s dialysis initiation), and United Network for Organ Sharing region in which the facility is located.
We then completed our sensitivity analysis using categorized nearest DFTC distance as the predictor in the larger cohort that included those who did and did not have a transplant center zip code in the USRDS. We used the same adjusted models with the exception that we adjusted for the distance from the patient residence to the nearest rather than actual transplant center. Due to the prolonged model run times in this large cohort, we divided the cohort into 50 random subsets, which we analyzed separately and then averaged the effect size.
We tested for interaction between DFTC distance and region of the United States, and rural/urban category of patient residence for our primary outcome (time to transplantation).
To understand whether known racial/ethnic disparities in access to transplantation are accounted for by variations in DFTC distance, we examined the subhazard of transplantation by race/ethnicity as well as DFTC.
Next, we evaluated access to living donor transplantation and deceased donor transplantation in separate Fine and Gray models. When evaluating access to living donor transplantation, we accounted for deceased donor transplantation and death as competing events, and when evaluating access to deceased donor transplantation, we accounted for living donor transplantation and death as competing events.
Secondary Analyses
We repeated Fine and Gray models to assess the sub-HR for time to waitlist registration using DFTC distance as the predictor in unadjusted and adjusted models, as described above. We then repeated these models using nearest DFTC distance in a sensitivity analysis. We initially excluded patients who were registered on the waitlist before dialysis initiation from these analyses, as noted previously. However, in an additional sensitivity analysis, we repeated all models including patients who were registered on the waitlist before dialysis initiation (for whom we assigned a time to waitlist registration of 1 d).
We used Stata 15 software (StataCorp LLC, College Station, TX) for all analyses.
RESULTS
Study Participants
In total, 178 885 adults started dialysis during the study period and had both a valid dialysis facility and transplant center zip code. Derivation of this cohort is shown in Figure 1. During median follow-up of 3.1 y, 77 116 patients received a transplant and 30 562 died. The mean age at the time of dialysis initiation was 52 y, and 62% were male (Table 1). The median DFTC distance was 20.5 miles (interquartile range [IQR], 7.2-68.3). There were small but statistically significant differences in age, sex, and comorbidities across DFTC distance categories. Patients in the longest DFTC distance category (≥50 miles) were more likely to be non-Hispanic white, less likely to live in metropolitan areas, more likely to live in the South, and had lower median neighborhood incomes. Median income was lower for those residing in the South (median, $45 439; IQR, 36 193-58 983) and Midwest (median, $47 607; IQR, 39 263-59 913) compared with the Northeast (median, $53 610; IQR, 39 771-71 947) and West (median, $55 883; IQR, 44 509-71 832).
FIGURE 1.
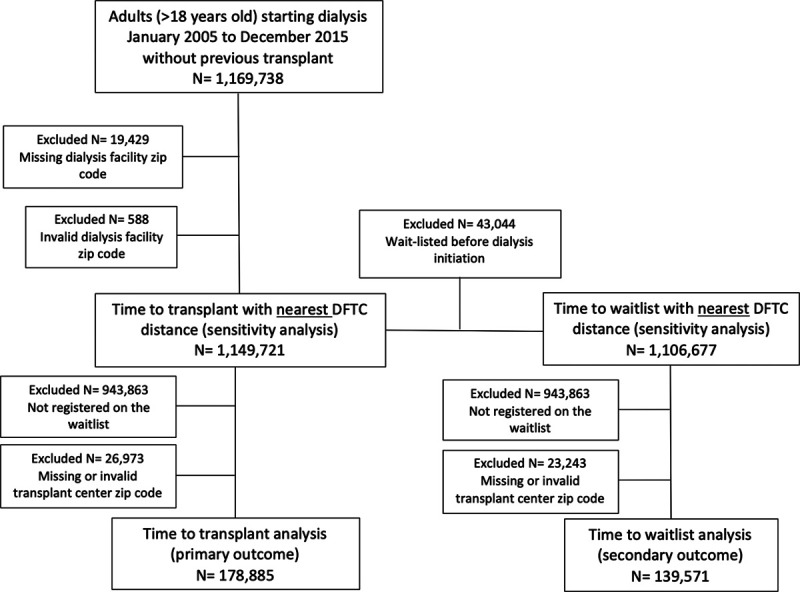
Cohort derivation. DFTC, dialysis facility to transplant center.
Table 1.
Characteristics of patients included for study in DFTC distance analyses
| % (unless otherwise specified) | DFTC distance cohort | DFTC distance category (miles) | ||
|---|---|---|---|---|
| <10 | 10 to <50 | ≥50 | ||
| No | 178 885 | 57 795 | 64 899 | 56 191 |
| Age at dialysis initiation (y), mean (SD)a | 51.6 (12.9) | 51.3 (13.0) | 52.0 (12.8) | 51.5 (13.0) |
| Sex, malea | 62.3 | 61.3 | 62.8 | 62.8 |
| Race/ethnicitya | ||||
| Asian | 6.9 | 7.7 | 8.1 | 4.7 |
| Black | 30.2 | 38.9 | 26.8 | 25.2 |
| Non-Hispanic White | 44.6 | 34.7 | 46.7 | 52.3 |
| Hispanic White | 17.0 | 17.8 | 17.5 | 15.4 |
| Other | 1.3 | 0.9 | 0.8 | 2.3 |
| Dialysis typea | ||||
| Hemodialysis | 83.2 | 82.3 | 84.6 | 82.5 |
| Peritoneal dialysis | 16.8 | 17.6 | 15.4 | 17.5 |
| Comorbidities | ||||
| Inability to ambulate or transfer | 0.9 | 0.9 | 0.8 | 0.9 |
| Diabetesa | 48.7 | 47.9 | 49.0 | 49.2 |
| CHFa | 14.2 | 14.2 | 13.6 | 14.9 |
| CADa | 9.8 | 9.5 | 9.3 | 10.6 |
| CVAa | 4.1 | 4.0 | 4.0 | 4.3 |
| PADa | 5.6 | 5.0 | 5.3 | 6.5 |
| Drug dependencea | 0.6 | 0.8 | 0.5 | 0.6 |
| Alcohol dependencea | 1.0 | 0.9 | 0.9 | 1.1 |
| Smokera | 4.4 | 4.2 | 3.8 | 5.3 |
| Dialysis facility to transplant centera | ||||
| Distance (miles), median (IQR) | 20.5 (7.2-68.3) | 4.2 (1.6-7.0) | 20.9 (14.2-32.2) | 107.5 (73.2-185.9) |
| Patient residence to transplant centera | ||||
| Distance (miles), median (IQR) | 24.6 (9.7-72.1) | 7.1 (4.0-11.9) | 22.6 (14.5-34.7) | 105.1 (71.1-179.8) |
| Rural/ urban categorya | ||||
| Metropolitan | 85.9 | 96.4 | 91.7 | 68.4 |
| Micropolitan | 7.9 | 1.9 | 5.3 | 17.3 |
| Rural/small town | 6.1 | 1.7 | 3.0 | 14.3 |
| Regiona | ||||
| West | 24.4 | 18.6 | 27.4 | 26.9 |
| Midwest | 19.5 | 20.3 | 18.5 | 19.8 |
| South | 36.8 | 32.6 | 33.3 | 45.0 |
| Northeast | 19.4 | 28.4 | 20.9 | 8.3 |
| Insurance typea | ||||
| None | 10.1 | 11.2 | 8.7 | 10.7 |
| Medicare | 28.7 | 26.7 | 28.8 | 30.8 |
| Medicaid | 13.6 | 16.2 | 12.3 | 12.4 |
| Private | 46.6 | 45.2 | 49.9 | 44.2 |
| VA | 0.9 | 0.7 | 0.3 | 1.9 |
| Incomea | ||||
| Median income ($1000), median (IQR) | 49.7 (38.9–65.2) | 48.4 (36.8–64.2) | 56.5 (44.5–73.1) | 44.6 (36.7–55.1) |
| Dialysis facility factors | ||||
| For-profita | 80.2 | 73.4 | 84.6 | 81.8 |
| Facility size (number of patients), median (IQR)a | 92 (60–134) | 104 (68–152) | 90.0 (60–128) | 83 (53–121) |
aP < 0.05 for difference across DFTC categories using Pearson’s chi-squared, Kruskal-Wallis, or ANOVA tests.
CAD, coronary artery disease; CHF, congestive heart failure; CVA, cerebrovascular disease; DFTC, dialysis facility to transplant center; IQR, interquartile range; PAD, peripheral artery disease; SD, standard deviation; VA, Veterans Affairs.
In total, 1 149 721 patients had a valid dialysis facility zip code according to the USRDS and were included in our sensitivity analyses using nearest DFTC as the predictor (Figure 1, Table S1A, SDC, http://links.lww.com/TXD/A278). This cohort was older, more likely to receive hemodialysis as the initial treatment modality, and had more comorbidities compared with the smaller cohort of waitlisted patients.
When we compared the correlation between the DFTC distance and nearest DFTC distance in the subset of patients who were registered on the kidney transplant waitlist, we found that the correlation was robust (Spearman r = 0.78; 95% confidence interval [CI], 0.78-0.79).
Access to Kidney Transplantation
As the DFTC distance increased, access to kidney transplantation decreased. In our fully adjusted model, we observed lower sub-HRs for kidney transplantation if the DFTC was 10 to <50 miles (sub-HR, 0.92; 95% CI, 0.91-0.94) or ≥50 miles (sub-HR, 0.90; 95% CI, 0.88-0.92) compared with our reference group (DFTC of <10 miles; Table 2).
Table 2.
Subhazard ratio (95% CI) for kidney transplantation, living donor transplantation, deceased donor transplantation, and waitlist registration by dialysis facility to transplant center distance, accounting for competing risks
| Dialysis facility to transplant center distance (miles) | |||
|---|---|---|---|
| <10 (reference) | 10 to <50 | ≥50 | |
| Transplanta | |||
| Unadjusted (N = 178,885) | 1 | 0.99 (0.98-1.01) | 0.95 (0.94-0.97) |
| Model 1 (N = 174,536) | 1 | 0.89 (0.88-0.91) | 0.87 (0.85-0.89) |
| Model 2 (N = 172 006) | 1 | 0.92 (0.91-0.94) | 0.90 (0.88-0.92) |
| Living donor transplantb | |||
| Model 2 (N = 172 006) | 1 | 0.97 (0.94-1.00) | 0.98 (0.95-1.02) |
| Deceased donor transplantc | |||
| Model 2 (N = 172 006) | 1 | 0.93 (0.91-0.95) | 0.88 (0.86-0.91) |
| Waitlist registrationa | |||
| Unadjustedd (N = 139 571) | 1 | 1.02 (1.01-1.04) | 0.95 (0.94-0.96) |
| Model 1d (N = 135 907) | 1 | 0.95 (0.94-0.97) | 0.92 (0.91-0.94) |
| Model 2d (N = 133 852) | 1 | 0.98 (0.96-0.99) | 0.96 (0.94-0.97) |
Model 1: adjusted for patient factors (age, sex, race/ethnicity, dialysis treatment modality [peritoneal or hemodialysis], insurance status, distance from patient residence to transplant center, neighborhood median income by zip code, rural/urban category [metropolitan, micropolitan, rural/ small town], and comorbidities [diabetes, congestive heart failure, coronary artery disease, cerebrovascular disease, peripheral artery disease, drug or alcohol dependence, smoking status, and inability to mobilize or transfer]).
Model 2: additionally adjusted for dialysis facility factors (profit status [nonprofit or for-profit], size of dialysis facility [number of patients receiving ESKD care at the facility in the year of the patient’s dialysis initiation], and UNOS region in which the facility is located).
aFine and Gray model with competing event of death.
bFine and Gray model with competing event of deceased donor transplant or death.
cFine and Gray model with competing event of living donor transplant or death.
dNo competing events.
CI, confidence interval; ESKD, end-stage kidney disease; UNOS, United Network for Organ Sharing.
In sensitivity analysis of the nearest DFTC distance, we observed similar associations between nearest DFTC distance and access to transplantation in our adjusted models (Table S2, SDC, http://links.lww.com/TXD/A278).
We detected a statistically significant interaction between DFTC distance and both region of the United States and rural/urban patient residence (Table 3). With respect to region of the United States, when DFTC distance exceeded 50-miles patients residing in the West and South had substantially lower access to transplantation (Table 3). This was not observed in the Midwest and Northeast. Furthermore, when DFTC distance exceeded 50-miles patients living in metropolitan or micropolitan areas had reduced access to transplantation, but patients living in rural areas did not (Table 3).
Table 3.
Results of subgroup analysis showing subhazard ratio (95% CI) for access to transplantation by DFTC distance category
| Access to transplant | Dialysis facility to transplant center distance (miles) | ||
|---|---|---|---|
| <10 miles (reference) | 10 to <50 miles | ≥50 miles | |
| Regiona | |||
| West | 1 | 0.81 (0.78-0.84) | 0.82 (0.78-0.86) |
| Midwest | 1 | 0.96 (0.92-0.99) | 1.00 (0.96-1.05) |
| South | 1 | 0.96 (0.93-0.99) | 0.89 (0.86-0.92) |
| Northeast | 1 | 0.98 (0.94-1.02) | 0.97 (0.92-1.03) |
| Rural/urban categorya | |||
| Metropolitan | 1 | 0.93 (0.91-0.95) | 0.90 (0.88-0.92) |
| Micropolitan | 1 | 0.69 (0.62-0.77) | 0.74 (0.67-0.81) |
| Rural | 1 | 1.04 (0.92-1.17) | 0.97 (0.88-1.08) |
Fine and Gray models with competing risk of death.
Model 2: adjusted for patient factors (age, sex, race/ethnicity, dialysis treatment modality [peritoneal or hemodialysis], insurance status, distance from patient residence to transplant center, neighborhood median income by zip code, rural/urban category [metropolitan, micropolitan, rural/ small town], and comorbidities [diabetes, congestive heart failure, coronary artery disease, cerebrovascular disease, peripheral artery disease, drug or alcohol dependence, smoking status, and inability to mobilize or transfer]) and dialysis facility factors (profit status [nonprofit or for-profit], size of dialysis facility [number of patients receiving ESKD care at the facility in the year of the patient’s dialysis initiation], and UNOS region in which the facility is located).
aP < 0.05 for interaction with DFTC distance.
CI, confidence interval; DFTC, dialysis facility to transplant center; ESKD, end-stage kidney disease; UNOS, United Network for Organ Sharing.
The association between race/ethnicity and transplantation remained statistically significant even after accounting for DFTC distance, suggesting that DFTC distance does not explain differences in transplantation rates in different racial/ethnic groups (Table S3, SDC, http://links.lww.com/TXD/A278).
Access to Living or Deceased Donor Kidney Transplant
There was an association between DFTC distance and receipt of transplanted kidneys from deceased donors but not living donors in our adjusted models (Table 2). Comparing DFTC distance ≥50 miles to <10 miles, the sub-HR was 0.88 (95% CI, 0.86-0.91) for receipt of deceased donor transplantation versus 0.98 (95% CI, 0.95-1.02) for receipt of living donor transplantation. We again observed similar results when the entire dialysis cohort was included using nearest DFTC distance as the predictor (Table S2, SDC, http://links.lww.com/TXD/A278).
Access to Waitlist Registration
After excluding patients registered on the waitlist before dialysis initiation, 139 571 and 1 106 677 patients were included in our secondary analyses of the association between DFTC distance or nearest DFTC distance and waitlist registration, respectively (Figure 1, Table S1B, SDC, http://links.lww.com/TXD/A278).
Longer DFTC distance was associated with lower access to waitlist registration, but the effect size was small. In adjusted analyses, the sub-HR for waitlist registration was 0.98 (95% CI, 0.96-0.99) when the DFTC distance was 10 to <50 miles and 0.96 (95% CI, 0.94-0.97) when the DFTC distance was ≥50 miles compared with our reference group with DFTC distance <10 miles (Table 2).
However, in the nearest DFTC sensitivity analysis, when DFTC distance increased, we did not observe lower access to waitlist registration (Table S2, SDC, http://links.lww.com/TXD/A278). In further sensitivity analyses that included patients who were registered on the waitlist before dialysis initiation, the results were overall unchanged (Table S4, SDC, http://links.lww.com/TXD/A278).
DISCUSSION
In this study, we found that longer distance between dialysis facility and transplant center was associated with lower access to deceased donor kidney transplantation, and, to a lesser extent, lower access to waitlist registration among patients treated with dialysis in the United States. To our knowledge, this association has not been described previously and was evident even when we accounted for the distance that the patient lives from the transplant center. Our results highlight the potential presence of system-level barriers to kidney transplantation among patients receiving dialysis in facilities located further away from transplant centers. Although we hypothesized that access to both kidney transplantation and waitlist registration would be lower with increasing DFTC, this observation was most prominent for deceased donor but not living donor transplantation.
Previous studies have demonstrated that patients residing further from the transplant center or in rural areas do not have lower likelihood of receiving a referral for kidney transplant evaluation or reduced access to waitlist registration.18,19,21 Prior studies have also examined whether there is an association between distance from patient residence to the transplant center and access to deceased or living donor transplantation.18,21 One study reported that longer distance between patient residence and the transplant center was associated with lower access to deceased donor transplantation but better access to living donor transplantation.21 Our study is novel in its focus on the distance from the dialysis facility to the transplant center, rather than distance from patient residence to the transplant center, which has been the focus of the previous studies in this field.18,19,21 Our study adds new information in our finding that increased DFTC distance is associated with barriers to deceased donor transplantation but that living donor transplantation is not as susceptible to these distance-related barriers, and furthermore that these associations remain important even after accounting for distance between patient residence and the transplant center. This may reflect the important role that the dialysis facility plays along the pathway to transplantation. Indeed, the majority of patients in the United States receive pretransplantation care at dialysis facilities, given that only 2.8% of patients needing renal replacement therapy receive preemptive kidney transplantation.22
There are a number of possible contributors to our observations. Referring providers at dialysis facilities located in close proximity to the transplant center may maintain closer communication channels which may be facilitated by shared electronic health record systems, or even direct affiliation with the nearest transplant center. This may result in more effective relay of information regarding the diagnostic tests required for waitlist registration or transplantation and changes in the patient’s health status over time. Lack of communication between dialysis and transplant teams has been identified as a barrier to access to transplantation.23 While the dialysis team is mandated to communicate with the transplant center at least annually as a condition for coverage by CMS,24 it is possible that communication between dialysis providers and the transplant center is less frequent or less effective when the dialysis facility is located further away from the transplant center.
Aside from communication with the transplant center, dialysis providers play a critical role in ensuring access to transplantation given the close patient-healthcare provider relationships that are fostered by thrice weekly encounters. Dialysis providers therefore have the opportunity to stress the importance of prompt completion of workup, encourage the search for potential living donors, and counsel on behavior changes, such as smoking cessation and weight loss, that may improve candidacy for transplantation.25–27 Unfortunately, the vast majority of nephrologists report spending less time discussing transplantation with patients than they would ideally like.28 It is possible that this barrier is exaggerated among providers practicing further away from transplant centers, as these providers may have a greater burden of patients and consequently less time to discuss transplantation or oversee pretransplant workup.
The reasons why DFTC distance was not associated with lower access to living donor kidney transplantation remain unclear, although it is possible that patients who undergo living donor transplantation are more proactive in seeking transplantation options or completing the required evaluations. It is also possible that patients seeking living donor transplantation may be from higher socioeconomic status and have better understanding of the benefits of transplantation.21 These individuals may be less prone to barriers in education and referral at the level of the dialysis facility.
Recently, multicomponent interventions targeting dialysis facilities with the aim of increasing referral rates for kidney transplantation have been developed and tested. The Reducing Disparities In Access to KidNey Transplantation Community Study (RaDIANT) was a randomized trial that tested 1 such intervention in dialysis units in Georgia and demonstrated increased referral rates in the dialysis facilities randomized to the intervention.9,11 The Allocation System Changes for Equity in KidNey Transplantation study is a study testing a similar multicomponent intervention in dialysis facilities nationally.10 The Allocation System Changes for Equity in KidNey Transplantation study has been completed with dialysis facilities treating over 42 000 patients enrolled, and the results are eagerly anticipated.29 However, it is yet unclear if these strategies will increase transplantation rates or if they will mitigate disparities in access to transplantation observed in our study.
Interestingly, there was effect modification by region of the United States in the association between DFTC distance and subhazard of transplantation. When DFTC distance exceeded 50 miles, patients living in the West and South had the lowest access to transplantation, whereas access was not lower in the Midwest or Northeast. This does not seem to be associated with regional differences in the density of transplant centers per ESKD patient.5 Our findings are concerning given that the largest proportion of patients receiving dialysis treatment in a dialysis facility located over 50 miles from their transplant center were in the South, which is known to have the lowest transplant rates in the United States.4–6
The strengths of this study include the large size of the cohort and the novelty of our focus, but a few limitations should be noted. We do not have data on the date of referral from dialysis facilities to the transplant center for transplant evaluation. However, given that the DFTC distance seemed less strongly associated with access to waitlist registration, we believe that the differential access we observed was less likely to be at the level of referral for transplantation and more likely due to differences in care after waitlist registration. We do not have data on whether more providers per dialysis facility were affiliated with transplant centers when the DFTC distance was shorter. For our nearest DFTC analyses, we acknowledge that patients may not always go to the nearest transplant center for transplant care for a variety of reasons, although we did observe similar results when we used the actual (rather than the nearest) transplant center. We also note that our study period encompassed data before the implementation of the new Kidney Allocation System (KAS) in December 2014. We are unable to fully assess the association between DFTC distance and deceased donor transplantation following this KAS change due to the short follow-up duration for outcomes and likelihood that those who were registered on the waitlist after 2014 and received a transplant before the end of our follow-up would not be representative of most waitlisted individuals in the United States, since the median waiting time for kidney transplantation is 4.0 y.22 Studies to assess changes in all types of geographic disparity following the changes in KAS will be required as we accumulate more data. Finally, given that our study is observational, residual confounding may be present.
In conclusion, we observed that patients receiving dialysis therapy in facilities located further away from transplant centers had lower access to deceased donor transplantation, and to a lesser extent, lower access to waitlist registration. These distance-related barriers to transplantation appear to be most important during the frequently prolonged period between waitlist registration and transplantation when continued communication between providers as well as continued encouragement of patient engagement with the transplant center and of behaviors that promote transplantation could have the greatest impact. The association between DFTC distance and transplantation also varied by region of the United States. Further studies are needed to understand these regional variations in our observations. While we are unable to pinpoint the exact reasons for our findings, we believe there may be important geography-related barriers that contribute to disparities in access to transplantation that warrant further investigation.
Footnotes
Published online 17 September, 2020.
E.K. and K.L.J. were funded by National Institutes of Health R01 DK 115629. E.K. was funded by the National Institutes of Health K23 HL 131023. K.L.J. was funded by National Institutes of Health K24 DK 085153. This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR001872. The data reported here have been supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
The authors declare no conflicts of interest.
A.M.W., K.L.J., and E.K. designed the study. A.M.W, C.E.M, B.G., and E.K. developed the analysis plan. A.M.W., B.G., and E.K. were involved in analysis of the data. A.M.W. wrote the first draft of the article. A.M.W., K.L.J., D.A., C.U.N., G.R.R., S.S., and E.K. revised the article. Each author contributed important intellectual content during article drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
REFERENCES
- 1.Schnuelle P, Lorenz D, Trede M, et al. Impact of renal cadaveric transplantation on survival in end-stage renal failure: evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. J Am Soc Nephrol. 1998; 9:2135–2141 [DOI] [PubMed] [Google Scholar]
- 2.Port FK, Wolfe RA, Mauger EA, et al. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 1993; 270:1339–1343 [PubMed] [Google Scholar]
- 3.Orandi BJ, Luo X, Massie AB, et al. Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med. 2016; 374:940–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patzer RE, Pastan SO. Kidney transplant access in the Southeast: view from the bottom Am J Transplant. 2014; 14:1499–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patzer RE, Plantinga L, Krisher J, et al. Dialysis facility and network factors associated with low kidney transplantation rates among United States dialysis facilities. Am J Transplant. 2014; 14:1562–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patzer RE, Plantinga LC, Paul S, et al. Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. JAMA. 2015; 314:582–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg PP, Frick KD, Diener-West M, et al. Effect of the ownership of dialysis facilities on patients’ survival and referral for transplantation. N Engl J Med. 1999; 341:1653–1660 [DOI] [PubMed] [Google Scholar]
- 8.Gander JC, Zhang X, Ross K, et al. Association between dialysis facility ownership and access to kidney transplantation. JAMA. 2019; 322:957–973 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Patzer RE, Paul S, Plantinga L, et al. ; Southeastern Kidney Transplant Coalition; Southeastern Kidney Transplant Coalition. A randomized trial to reduce disparities in referral for transplant evaluation. J Am Soc Nephrol. 2017; 28:935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patzer RE, Smith K, Basu M, et al. The ASCENT (Allocation System Changes for Equity in Kidney Transplantation) Study: a randomized effectiveness-implementation study to improve kidney transplant waitlisting and reduce racial disparity. Kidney Int Rep. 2017; 2:433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patzer RE, Gander J, Sauls L, et al. ; Southeastern Kidney Transplant Coalition; Southeastern Kidney Transplant Coalition. The RaDIANT community study protocol: community-based participatory research for reducing disparities in access to kidney transplantation. BMC Nephrol. 2014; 15:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.University of Michigan Population Studies Center.. Zip code characteristics: Mean and median household income 2006-2010. Available at https://www.psc.isr.umich.edu/dis/census/Features/tract2zip/index.html. Accessed December 21, 2014
- 13.Documentation: 2010 Rural-Urban Commuting Area (RUCA) Codes.. United States Department of Agriculture Economic Research Service website. Available at https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/. Accessed June 13, 2019
- 14.Center for Rural Health, School of Medicine and Health Sciences, University of North Dakota website.; Temporary ZIP RUCA 3.10 File. Available at https://ruralhealth.und.edu/ruca. Accessed June 7, 2019. [Google Scholar]
- 15.Organ Procurement and Transplantation Network website; Regional Data. Available at https://optn.transplant.hrsa.gov/data/view-data-reports/regional-data/. Accessed October 9, 2019. [Google Scholar]
- 16.United States Census Bureau. Gazetteer Files: 2015. Available at https://www.census.gov/geographies/reference-files/time-series/geo/gazetteer-files.2015.html. Accessed July 8, 2019
- 17.CivicSpace Labs website; US Zip Code Latitude and Longitude. Available at https://public.opendatasoft.com/explore/dataset/us-zip-code-latitude-and-longitude/export/. Accessed July 8, 2019. [Google Scholar]
- 18.Tonelli M, Klarenbach S, Rose C, et al. Access to kidney transplantation among remote- and rural-dwelling patients with kidney failure in the United States. JAMA. 2009; 301:1681–1690 [DOI] [PubMed] [Google Scholar]
- 19.McPherson LJ, Barry V, Yackley J, et al. ; Southeastern Kidney Transplant Coalition; Southeastern Kidney Transplant Coalition. Distance to kidney transplant center and access to early steps in the kidney transplantation process in the Southeastern United States. Clin J Am Soc Nephrol. 2020; 15:539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A propotional hazards model for the subdistribution of a competing risk J Am Stat Assoc. 1999; 94:496–509 [Google Scholar]
- 21.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol. 2010; 5:2276–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United States Renal Data System 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. 2018, Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases [Google Scholar]
- 23.Sandal S, Charlebois K, Fiore JF, Jr, et al. Health professional-identified barriers to living donor kidney transplantation: a qualitative study. Can J Kidney Health Dis. 2019; 6:2054358119828389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Department of Health and Human Services. Medicare and Medicaid Programs; conditions for coverage for end-stage renal disease facilities; final rule Federal Register. 2008; 73:20369–20484 [PubMed] [Google Scholar]
- 25.Segev DL, Simpkins CE, Thompson RE, et al. Obesity impacts access to kidney transplantation. J Am Soc Nephrol. 2008; 19:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansen KL. Obesity and body composition for transplant wait-list candidacy–challenging or maintaining the BMI limits? J Ren Nutr. 2013; 23:207–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasiske BL, Klinger D. Cigarette smoking in renal transplant recipients. J Am Soc Nephrol. 2000; 11:753–759 [DOI] [PubMed] [Google Scholar]
- 28.Balhara KS, Kucirka LM, Jaar BG, et al. Disparities in provision of transplant education by profit status of the dialysis center. Am J Transplant. 2012; 12:3104–3110 [DOI] [PubMed] [Google Scholar]
- 29.U.S. National Library of Medicine, National Institutes of Health. ClinicalTrials.gov; Allocation System Changes for Equity in kidNey Transplantation (ASCENT) Available at https://clinicaltrials.gov/ct2/show/NCT02879812. Accessed February 6, 2020. [Google Scholar]


