Abstract
Background
Advanced light-chain (AL) amyloidosis is associated with poor prognosis, with a 5-year survival rate of <25%. Prognostication is based on the revised Mayo (rMayo) staging according to serum cardiac biomarkers.
Objectives
This study sought to determine whether global longitudinal strain (GLS) can provide incremental prognostic value in patients with advanced disease.
Methods
Baseline (pre-treatment) clinical, 2-dimensional echocardiogram with GLS and laboratory data were collected prospectively in 94 patients with newly diagnosed AL amyloidosis with rMayo stage III or IV disease. Overall survival (OS) was defined as time from baseline echocardiography to death.
Results
Of 94 patients, 60% (n = 56) had rMayo stage III and 40% (n = 38) had stage IV disease. Ninety of the 94 patients underwent plasma cell-directed therapy. The median left ventricular ejection fraction (LVEF) was 60%, and the median GLS was 13.2%. Of 94 patients, 64 died during follow-up. The median OS was 11.2 months, with an estimated 5-year OS of 21%. In univariable analysis, brain natriuretic peptides, GLS, LVEF, E/e′ ratio, and rMayo stage were significantly associated with OS. In Cox regression, GLS provided incremental value over brain natriuretic peptide, troponin, and LVEF for predicting OS. Patients with GLS < –14.2% had a corresponding median OS and 5-year OS rate of 33.2 months and 39%, respectively, versus 7.7 months and 6% for those with GLS ≥ –14.2%. This difference was maintained despite further stratification by rMayo stage.
Conclusions
Baseline GLS is an independent predictor of OS beyond the circulating biomarkers and can identify groups with different survival outcomes beyond the Mayo Staging.
Key Words: amyloidosis, cardiomyopathy, echocardiography, global longitudinal strain, prognosis
Abbreviations and Acronyms: AL, light chain; BNP, B-type natriuretic peptide; CI, confidence interval; dFLC, difference between involved and uninvolved free light chains; GLS, global longitudinal strain; HCT, hematopoietic cell transplantation; HR, hazard ratio; LV, left ventricular; LVEF, left ventricular ejection fraction; OS, overall survival; rMayo, revised Mayo; Tn, troponin; TnI, troponin I
Central Illustration
Light-chain (AL) amyloidosis is a rare, potentially fatal plasma cell dyscrasia characterized by tissue deposition of amyloid fibrils derived from monoclonal ALs leading to progressive organ failure (1). Current treatment primarily targets the pathologic plasma cells to terminate monoclonal free light-chain production, with outcomes largely dependent on hematologic and organ response to treatment (2,3). Cardiac involvement occurs in 50% of cases and is the major determinant of survival (4, 5, 6). Advanced cardiac disease often precludes patients from aggressive treatment such as autologous hematopoietic cell transplantation (HCT) and clinical trials because of increased treatment-related risks and poor overall outcome (2,5,7,8).
Serum cardiac biomarkers such as troponin (Tn) and N-terminal pro (NT-pro) and B-type natriuretic peptides (BNP) reflect the extent of cardiac involvement and play a central role in assessing prognosis in AL amyloidosis (9, 10, 11, 12, 13, 14). In combination with hematologic disease burden, as measured by the absolute difference between involved and uninvolved free light chains (dFLC), these biomarkers are incorporated in the prognostically validated revised Mayo (rMayo) staging system, which is currently used as the standard for risk stratification and prediction of survival of patients with newly diagnosed AL amyloidosis. rMayo stage III or IV is associated with very poor prognosis, because only an estimated 25% of patients are alive 5 years after diagnosis with treatment (13). However, a previous study has shown heterogeneity in outcomes among patients with AL amyloidosis within each Mayo stage (15). Hence, a more refined assessment of prognosis, particularly among high-risk patients, would be highly useful in guiding optimal risk-adapted treatment strategies and more clearly identifying a population with an unmet medical need.
Left ventricular (LV) global longitudinal strain (GLS) is a robust prognostic marker that provides incremental value beyond standard clinical and echocardiographic parameters among patients with AL amyloidosis (15, 16, 17). However, whether GLS can provide independent prognostic information among the highest-risk patients based on the current staging system remains unknown. Thus, we examined GLS in patients with newly diagnosed rMayo stage III and IV AL amyloidosis to determine its role in risk stratification beyond conventional clinical, serological, and echocardiographic indices.
Patients and Methods
Study design and patient selection
Ninety-four consecutive patients with newly diagnosed biopsy-proven AL amyloidosis evaluated at Memorial Sloan Kettering Cancer Center between May 2007 and January 2018 were included in this study. All patients were classified as having rMayo stage III or IV disease at the time of diagnosis. Disease staging was based on the rMayo score, which uses circulating cardiac biomarkers and dFLC. Patients were classified as having rMayo stage III or IV disease if 2 or 3 of the following criteria were met: BNP ≥400 pg/ml, troponin I (TnI) ≥0.1 ng/ml, and dFLC ≥18 mg/dl (10,13). Baseline clinical, echocardiographic, and laboratory test results were collected before or within 1 month of the start of light-chain treatment. Clinical and treatment data were extracted from a prospectively maintained database of an ongoing institutional review board–approved protocol that collects clinical characteristics and outcomes of patients with systemic AL amyloidosis.
Echocardiography
The conventional 2-dimensional and Doppler echocardiography protocols have been previously described (15). Briefly, the studies were performed by using commercially available standard ultrasound scanners (Vivid E9, General Electric Medical Systems [Chicago, Illinois] and iE33, Philips Medical Systems [Andover, Massachusetts]), according to the standardized American Society Echocardiography protocol (18). LV ejection fraction (LVEF) was calculated using the modified Simpson method. The mitral inflow velocity pattern was recorded from the apical 4-chamber view with the pulsed-wave Doppler sample volume positioned at the tips of the leaflets during diastole. Peak early filling (E-wave) and late diastolic filling (A-wave) velocities were measured, and their ratio (mitral E/A) was derived. Doppler tissue imaging of the mitral annulus was performed with measurement of the early (e′) diastolic velocity at the lateral annulus.
Myocardial strain measurement
The methods of image acquisition and post-processing of strain measurements have been previously described (15). Briefly, GLS measurements were performed offline using vendor independent 2D Cardiac Performance Analysis software (Tom Tec Imaging Systems, Unterschleissheim, Germany). The endocardial border was traced in end diastole in the 3 standard apical views, which allowed the software to track myocardial movement throughout the cardiac cycle (19). After careful inspection, manual correction was performed if the myocardial tracking was suboptimal. Each view was divided into 6 segments, for a total of 18 segments representing the entire LV. Longitudinal strain curves were generated for each segment. GLS was calculated as the average value of the peak negative systolic strain values for all the segments within the 3 standard apical views. Relative apical sparing was calculated as the ratio of the (average apical segments)/(average basal + mid segments). GLS was measured by 2 experienced operators (K.L. and J.Y.). All echocardiographic measurements were made with the operator blinded to the clinical and outcome data.
Repeatability and reproducibility
Interobserver agreement (reproducibility) was assessed by comparing the original GLS calculations (made by K.L. and J.Y.) with those calculated by a blinded second observer (J.L.) in 20 randomly selected patients. Intraobserver variability was calculated by repeated measurements in 20 patients by the primary reviewer 3 weeks after the initial measurement. Intraclass coefficient was calculated as a measure of both interobserver and intraobserver agreement.
Statistical analysis
Overall survival (OS) was defined as time from baseline echocardiogram to death. Date of death was obtained through hospital records or Social Security Death Index (SSDI). Those who were lost to follow-up (no clinical encounter ≥1 year before the end of chart review on May 15, 2018) with no documented death date were censored as being alive on the last day of available Social Security Death Index records (March 19, 2014) or last clinical encounter, whichever was later. Continuous variables are presented as median (interquartile range) and categorical variables are presented as number (percentage). The correlation between GLS and selected continuous variables was estimated using the Spearman rank correlation coefficient. Associations between baseline variables and OS were calculated using a univariable proportional hazards Cox regression model. Continuous variables that did not follow a normal distribution (TnI, BNP, and dFLC) were log-transformed for the purposes of Cox regression interpretation. Cox regression model results are presented with hazard ratio (HR) (95% confidence interval [CI]). Tests of the proportional hazards assumption were conducted for all Cox regression models by using Schoenfeld residuals. The incremental value of GLS over other cardiac and echocardiographic markers was assessed with chi-square tests of overall difference between log likelihoods of models. Time-dependent receiver operating characteristic curve analysis was used to identify the optimal cutoff for the GLS level that best predicted prognosis. Survival analysis was performed using the Kaplan-Meier method and log-rank test. Statistical significance was defined as a 2-sided p value of <0.05. Statistical analyses were performed with R Core Team (2019), version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline patient characteristics
The baseline clinical and echocardiographic characteristics are summarized in Tables 1 and 2. Of 94 patients, 60% (56) had rMayo stage III and 40% (38) had stage IV disease. Ninety (96%) of the 94 patients underwent plasma cell-directed therapy. Among the 4 patients who did not receive treatment, 2 died of complications of amyloidosis before initiation of treatment, and 2 were lost to follow-up. Of the 90 patients who received treatment, 23 (26%) underwent hematopoietic cell transplantation (HCT)—8 underwent up-front HCT, and 15 received chemotherapy before HCT (14 received bortezomib-based regimens). Among the 67 patients who did not have HCT, first-line treatment included melphalan plus dexamethasone (n = 20); bortezomib plus dexamethasone (n = 12); melphalan, bortezomib, and dexamethasone (n = 4); cyclophosphamide, bortezomib, and dexamethasone (n = 25); immunomodulatory drugs (n = 5); and rituximab (n = 1). The median baseline LVEF was 60%, with 16 (17%) patients having an LVEF of <50%, and the median baseline GLS was –13.2%. Among the 94 patients, 88 (94%) had adequate echocardiographic views for GLS measurement. GLS was significantly correlated with BNP (ρ = 0.22; p = 0.018) and LVEF (ρ = –0.55; p < 0.001) but not with TnI (ρ = –0.06; p = 0.800).
Table 1.
Baseline Clinical and Serological Characteristics (N = 94)
| Male | 61 (65) |
| Age, yrs | 64 (54–70) |
| Body mass index, kg/m2 | 26.4 (23.4–30.1) |
| Systolic blood pressure, mm Hg | 112 (10–126) |
| Diastolic blood pressure, mm Hg | 70 (64–77) |
| Heart rate, beats/min | 83 (75–92) |
| Troponin I, ng/ml | 0.17 (0.08–0.26) |
| ≥0.1 | 69 (73) |
| BNP, pg/ml | 659 (424–1315) |
| ≥400 | 74 (79) |
| FLC difference, mg/dl | 38 (23–77) |
| ≥18 | 74 (79) |
| Serum creatinine, mg/dl | 1.30 (1.00–1.98) |
| Revised Mayo stage | |
| III | 56 (60) |
| IV | 38 (40) |
| Hypertension | 47 (50) |
| Hyperlipidemia | 38 (40) |
| Chronic kidney disease | 40 (43) |
| Atrial fibrillation | 16 (17) |
| Heart failure | 40 (43) |
| Coronary artery disease | 12 (13) |
| Cancer | 3 (3.2) |
| Cerebrovascular disease | 7 (7.4) |
Values are n (%) or median (interquartile range).
BNP = B-type natriuretic peptide; FLC = free light chain.
Table 2.
Baseline Echocardiographic Characteristics
| n | Median (Interquartile Range) | |
|---|---|---|
| GLS, % | 88 | –13.2 (–9.8 to –16.5) |
| LVEF, % | 94 | 60 (55 to 66) |
| IVS thickness, cm | 94 | 1.40 (1.20 to 1.60) |
| Mitral E-wave, cm/s | 93 | 91 (74 to 105) |
| Mitral A-wave, cm/s | 86 | 57 (33 to 82) |
| E/A ratio | 86 | 1.50 (0.98 to 2.90) |
| Lateral e′, cm/s | 85 | 5.70 (4.50 to 6.90) |
| Septal e′, cm/s | 83 | 4.60 (3.85 to 5.80) |
| Average E/e′ ratio | 88 | 17 (13 to 22) |
| RALS | 79 | 0.50 (0.41 to 0.59) |
| Deceleration time, s | 78 | 0.17 (0.14 to 0.21) |
| LA volume index, ml/m2 | 78 | 37 (31 to 44) |
| TAPSE, cm | 51 | 1.7 (1.3 to 2.0) |
| RV TDI, cm/s | 30 | 1.23 (1.02 to 1.44) |
| Stroke volume, ml | 78 | 52 (42 to 73) |
| Stroke volume index, ml/m2 | 78 | 29 (22 to 36) |
| Cardiac output, l/min | 78 | 4.30 (3.26 to 5.42) |
| Cardiac index, l/min/m2 | 78 | 2.30 (1.90 to 2.80) |
GLS = global longitudinal strain; IVS = interventricular septum; LA = left atrium; LVEF = left ventricular ejection fraction; RALS = relative apical longitudinal sparing; RV TDI = right ventricular tissue Doppler imaging; TAPSE = tricuspid annular plane systolic excursion.
Survival analysis
Of 94 patients, 64 died during follow-up. The median OS was 11.2 months (95% CI: 7.4 to 27.2 months), with an estimated 5-year OS of 21% (95% CI: 13% to 34%). The median OS and 5-year OS rate were substantially better among patients with rMayo stage III versus stage IV disease: 15 months (95% CI: 9.7 to 43.0 months) and 27% (95% CI: 16% to 44%) versus 6.9 months (95% CI: 3.7 to 30.9 months) and 7% (95% CI: 1% to 44%), respectively. In univariable analysis (Table 3), BNP, GLS, LVEF, E/e′ ratio, and rMayo stage were significantly associated with OS. The univariable analysis demonstrates that for each percentage point of GLS improvement, the HR for overall mortality decreased by 12% (HR: 0.88; 95% CI: 0.82 to 0.95). In Cox regression models that included GLS with each of the other significantly associated markers (BNP, LVEF, E/e′ ratio, and rMayo stage), GLS demonstrated incremental predictive value over each of those markers. (Table 4). Conversely, when each of the markers was assessed for incremental predictive value over GLS alone, none of them added independent incremental predictive value. When added to the multivariable Cox regression model of variables used clinically to predict OS such as Tn, BNP, and LVEF, GLS also demonstrated incremental value for predicting OS (p = 0.037) (Table 5).
Table 3.
Univariable Analysis for Predictors of Overall Survival
| Hazard Ratio | 95% CI | p Value | |
|---|---|---|---|
| Age | 1.01 | (0.99–1.03) | 0.379 |
| Male (vs. female) | 0.86 | (0.52–1.41) | 0.544 |
| Hypertension | 0.83 | (0.51–1.37) | 0.473 |
| Hyperlipidemia | 0.78 | (0.47–1.31) | 0.350 |
| Chronic kidney disease | 1.02 | (0.63–1.68) | 0.926 |
| Atrial fibrillation | 1.75 | (0.94–3.26) | 0.076 |
| Heart failure | 0.91 | (0.55–1.49) | 0.696 |
| Coronary artery disease | 0.92 | (0.44–1.94) | 0.833 |
| Cerebrovascular disease | 0.60 | (0.22–1.65) | 0.319 |
| Body mass index | 1.01 | (0.95–1.06) | 0.863 |
| Systolic blood pressure | 1.00 | (0.98–1.01) | 0.505 |
| Diastolic blood pressure | 0.99 | (0.97, 1.01) | 0.374 |
| BNP∗ | 1.30 | (1.01–1.68) | 0.041 |
| ≥400 (vs. <400 pg/ml) | 1.71 | (0.89–3.29) | 0.105 |
| Troponin I∗ | 1.09 | (0.89–1.33) | 0.411 |
| ≥0.1 (vs. <0.1 ng/ml) | 1.06 | (0.61–1.86) | 0.826 |
| FLC difference∗ | 1.02 | (0.84–1.23) | 0.869 |
| ≥18 mg/dl (vs. <18 mg/dl) | 1.35 | (0.71–2.60) | 0.361 |
| Serum creatinine | 1.00 | (0.84–1.19) | 0.995 |
| GLS | 0.88 | (0.82–0.95) | 0.001 |
| LVEF | 0.97 | (0.94–0.99) | 0.008 |
| IVS thickness | 1.81 | (0.87–3.77) | 0.111 |
| E/A ratio | 1.11 | (0.96–1.27) | 0.148 |
| Average E/e′ ratio | 1.04 | (1.00–1.07) | 0.044 |
| RALS | 0.98 | (0.73–1.32) | 0.962 |
| Deceleration time | 0.01 | (0.00–1.51) | 0.070 |
| LA volume index | 1.00 | (0.98–1.02) | 0.808 |
| TAPSE | 0.49 | (0.12–1.93) | 0.306 |
| RV TDI | 0.92 | (0.74–1.13) | 0.415 |
| Stroke volume | 0.98 | (0.97–1.00) | 0.052 |
| rMayo stage | 0.028 | ||
| III | 1.00 | — | |
| IV | 1.77 | (1.07–2.94) |
Table 4.
Incremental Value of GLS Over Other Statistically Significant Clinical and Echocardiographic Markers in Cox Regression Models
| HR | 95% CI | Incremental p Value | |
|---|---|---|---|
| Model 1 | |||
| Log BNP | 1.21 | 0.90–1.61 | 0.200 |
| GLS | 0.89 | 0.83–0.96 | 0.002 |
| Model 2 | |||
| LVEF | 0.99 | 0.96–1.02 | 0.388 |
| GLS | 0.90 | 0.83–0.98 | 0.016 |
| Model 3 | |||
| Average E/e′ ratio | 1.02 | 0.99–1.06 | 0.220 |
| GLS | 0.90 | 0.84–0.98 | 0.009 |
| Model 4 | |||
| rMayo stage IV | 1.51 | 0.88–2.60 | 0.136 |
| GLS | 0.89 | 0.83–0.96 | 0.002 |
Table 5.
Incremental Value of GLS Over Clinically Important Markers in Cox Regression Model
| HR | 95% CI | p Value | |
|---|---|---|---|
| Baseline log BNP | 1.23 | 0.92–1.65 | 0.169 |
| Baseline log troponin | 1.03 | 0.83–1.29 | 0.766 |
| Baseline LVEF | 0.99 | 0.95–1.02 | 0.374 |
| GLS | 0.91 | 0.84–0.99 | 0.037 |
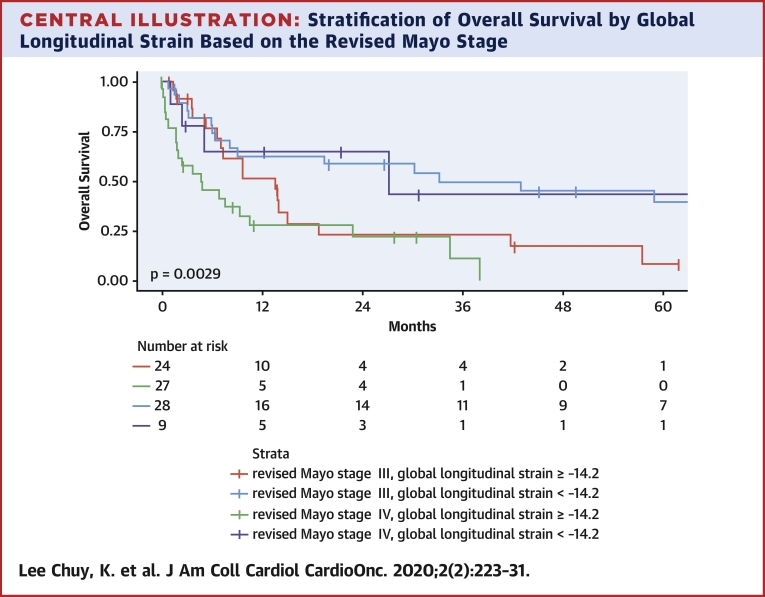
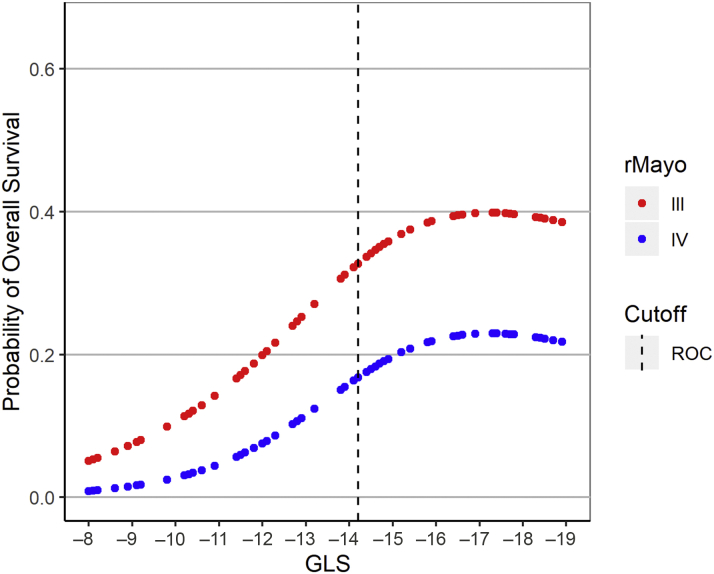
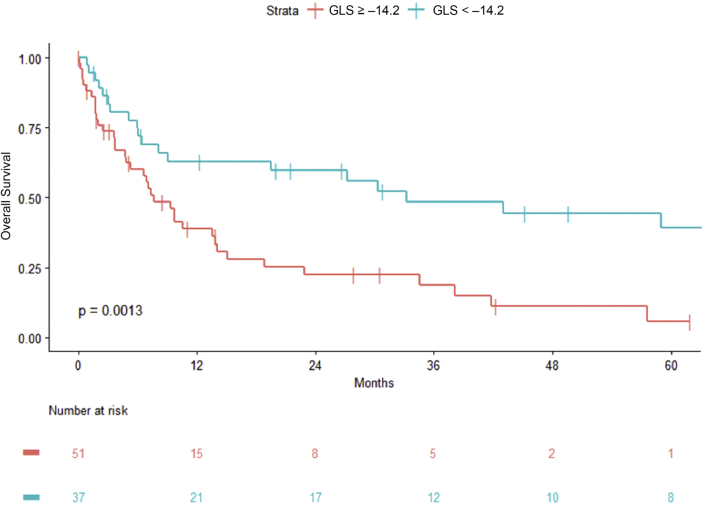
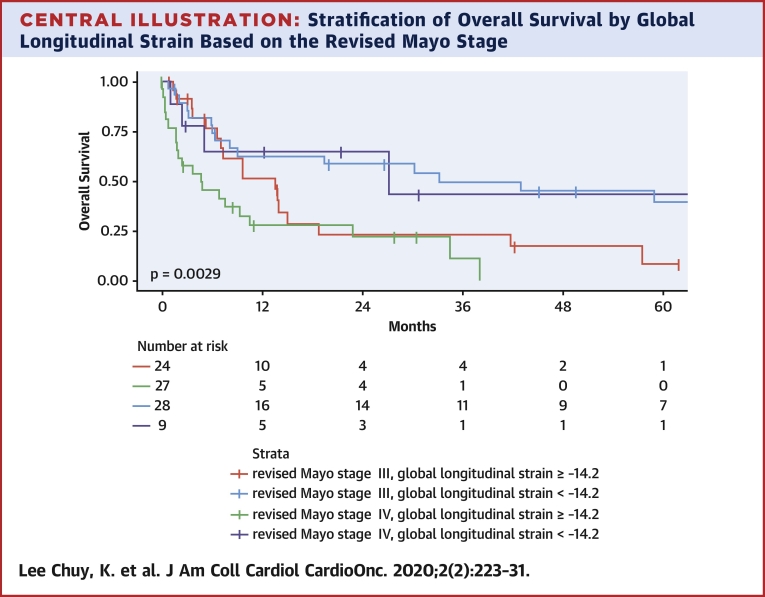
In addition, GLS provided powerful discriminatory segregation curves for estimating survival, with –14.2% identified as the value that best discriminated survivors from nonsurvivors, with an area under the curve of 0.78 (Figure 1). Patients with GLS of <–14.2% had a corresponding median OS and 5-year OS rate of 33.2 months and 39%, respectively, versus 7.7 months and 6% for those with GLS of ≥–14.2% (Table 6, Figure 2). This difference was maintained despite further stratification by rMayo stage (Central Illustration). Patients with rMayo stage IV and GLS ≥–14.2% had the worst prognosis, with a median OS of 4.7 months (95% CI: 1.8 to 22.9 months).
Figure 1.
The Relationship of GLS With 5-Year OS for Patients with rMayo Stage III and IV Disease
GLS provided powerful discriminatory segregation curves for estimating survival, with –14.2% identified as the value that best discriminated survivors from nonsurvivors (area under the curve: 0.78). GLS = global longitudinal strain; OS = overall survival; rMayo = revised Mayo stage; ROC = receiver operating characteristic.
Table 6.
OS by GLS Cutoff (AUC: 0.78) and rMayo Stage
| n (%) | OS Estimate |
Multivariable Cox Regression |
||
|---|---|---|---|---|
| Median OS, months (95% CI) | HR (95% CI) | p Value | ||
| All patients | 94 | 11.2 (7.4–27.2) | ||
| GLS, % | 0.007 | |||
| ≥–14.2 | 51 (58) | 7.7 (5.3–14.1) | ||
| <–14.2 | 37 (42) | 33.2 (9.0 to not reached) | 0.45 (0.25–0.80) | |
| rMayo stage | 0.140 | |||
| III | 56 (60) | 15 (9.7–43) | — | |
| IV | 38 (40) | 6.9 (3.7–30.9) | 1.51 (0.87–2.61) | |
Figure 2.
OS Based on the GLS Cutoff of 14.2%
Patients with GLS of <–14.2% had a corresponding median OS and 5-year OS rate of 33.2 months and 39%, respectively, versus 7.7 months and 6% for those with GLS of ≥–14.2%. Abbreviations as in Figure 1.
Central Illustration.
Stratification of Overall Survival by Global Longitudinal Strain Based on the Revised Mayo Stage
The difference in overall survival based on global longitudinal strain stratification was maintained despite further stratification by revised Mayo stage.
To date, 10 patients survived beyond 5 years (range 5.15 to 13 years). All had GLS of ≤–14.2% (range –14.2% to –21.5%) except for 1 patient missing GLS. Nine patients had rMayo stage III disease, and 1 had rMayo stage IV disease.
Intraobserver and interobserver variability
Substantial agreement for intraobserver and interobserver GLS measurement had previously been reported in a cohort of patients with AL amyloidosis of mixed stage (15). Here, we confirm the high repeatability and reproducibility for GLS measurement in this advanced-stage cohort: the intraclass correlation coefficient for interobserver agreement was 0.96 (95% CI: 0.90 to 0.98), and the intraclass correlation coefficient for intraobserver agreement was 0.96 (95% CI: 0.90 to 0.98).
Discussion
To our knowledge, this is the first study to evaluate the prognostic value of GLS when added to the current biomarker-based staging system among patients with advanced AL amyloidosis. The main findings of this study are as follows:
-
•
GLS is a robust and independent predictor of OS among patients with rMayo stage III and IV disease, above and beyond well-established prognostic parameters such as Tn, BNP, and LVEF.
-
•
OS among patients with either rMayo stage III or stage IV disease was further stratified by incorporating GLS, demonstrating heterogenous outcome within each stage.
-
•
GLS of <–14.2% was associated with improved OS in a linear fashion during the 5-year follow-up post-treatment.
The effect on OS plateaued at GLS of <–14.2% (Figure 1). A GLS cut point of –14.2% best discriminated survivors from nonsurvivors, identifying a 4-fold difference in OS among patients with rMayo stage III and IV disease.
This study demonstrates that GLS significantly contributes to risk stratification and prediction of outcome among patients with AL amyloidosis. It predicted outcomes beyond the rMayo staging system and identified a 4-fold difference in the 5-year OS among this group of high-risk patients with advanced disease. The inclusion of patients with rMayo stage III or IV disease, an uniform and well-defined cohort, distinguishes these data from those of other studies. GLS has been shown to predict outcome among patients with AL amyloidosis with and without evidence of cardiac involvement (16). Our findings confirm that GLS is an independent predictor of survival, providing incremental value beyond established clinical, echocardiographic, and circulating cardiac biomarker risk parameters. Furthermore, the results of this study suggest that GLS may be the strongest predictor of survival compared to other validated risk factors such as troponin, BNP, dFLC, and LVEF among patients with advanced disease.
GLS, an index of longitudinal LV function, is considered a better measure of myocardial contractile function than ejection fraction, particularly in hearts with small cavity size and thick walls, phenotypic of amyloid cardiomyopathy. LVEF can remain normal despite significant reduction in longitudinal and circumferential shortening, given its dependence on LV geometry (20), as observed in the current study, with many patients having preserved or near-normal LVEF despite advanced cardiac involvement. Furthermore, longitudinal contraction is a marker of subendocardial function because the majority of the longitudinally oriented fibers are in the subendocardium, which is most sensitive and vulnerable to myocardial disease. As such, GLS has shown to be superior to LVEF and wall motion score for the prediction of all-cause mortality among patients undergoing echocardiogram for suspected or known LV impairment in the general population (21), as well as in specific cardiovascular conditions.
Despite emerging data on the prominent role of GLS in predicting survival, cardiac Tn and BNP, biochemical markers of cardiac injury and LV dysfunction, remain the indices utilized in the current staging system for prognostic classification. Our findings indicate that pre-treatment GLS may be the strongest parameter predicting survival over the serological cardiac and hematologic biomarkers. Incorporation of GLS, a marker of cardiac contractile function, can significantly improve risk stratification in high-risk patients with advanced disease.
Clinical implications
The findings of this study have important clinical implications. We have shown that the addition of GLS to the current rMayo staging system clearly allows better classification of patients in terms of outcome. This study focused on patients with Mayo stage III and IV disease, given their poor prognosis with unmet medical needs. Although the early stages of disease were not included, GLS is likely to have a similar impact on survival among patients with stage I and stage II disease (15,16). Better discrimination of patients will allow the development and evaluation of treatment strategies targeted toward specific patient groups based on risk and reduce population differences when comparing outcomes of available therapy. Moreover, improved risk classification may help refine the optimal selection of patients for risk-adapted therapeutic approaches, particularly among patients with advanced disease, who are often deemed ineligible for aggressive treatment given their risk with treatment and expected poor prognosis based on the current staging system. However, whether GLS is useful for guiding the selection of therapeutic approaches warrants further investigation.
The median OS of 7.7 months for patients with GLS ≥–14.2% despite contemporary therapy is a sobering finding. Advances in the diagnosis and treatment of AL amyloidosis have led to improved short- and long-term survival over the past 15 to 20 years (2,22), but this is limited to patients with the early stages of disease because the mortality trends for patients with advanced cardiac disease at diagnosis have remained relatively unchanged (22, 23, 24). Current therapies target clonal plasma cells to stop production of the monoclonal light chains to prevent further organ deposition and damage. However, improvement in cardiac structure and function may be evident only 4 to 5 years after successful treatment (25) or may not occur at all and even deteriorate in some cases, despite successful plasma cell–directed therapy with complete hematologic remission. The persistent poor outcome of patients with advanced disease underscores the need to better understand the mechanisms and pathophysiology of the myocardial toxicity that results from circulating immunoglobulin and amyloid deposits and to develop treatments that can facilitate organ recovery and improve outcome.
Study limitations
It is a single center study with a relatively small number of patients. However, despite the small sample size, the number of events (patient deaths) was high enough to enable adequate power to determine the independent prognostic value of key clinical, laboratory, and imaging markers. Given the lack of GLS standardization and the variation in strain analysis platform, the cutoff value identified in the present study may not be applicable when using a different system and must be considered in the context of the study population. Larger studies are required to validate the current observations and better define the prognostic GLS cutoff value. These data were generated based on the rMayo staging system, which may not be identical to the standard definition, because BNP was used instead of NT-pro BNP although both forms of the biomarker have been prognostically validated. Although baseline or pre-treatment GLS was shown to be a strong prognostic marker, the study was not able to address whether improvement in GLS after treatment predicts better survival.
Conclusions
We have shown the value of incorporating GLS into the currently used rMayo staging system among patients with advanced AL amyloidosis. Baseline GLS is a strong independent predictor of OS beyond the circulating biomarkers, which can further stratify risk beyond the Mayo staging and allow better discrimination of groups with different survival outcomes. GLS should be considered as part of staging and incorporated into the standard prognostic classification. Further work is needed to assess whether GLS is useful in the selection of optimal risk-adapted therapy in this especially high-risk cohort.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Baseline GLS is a strong independent marker and provides incremental prognostic value beyond the current staging system among patients with advanced AL amyloidosis. Refinement of prognostic classification with GLS may assist clinical decision making for the selection of optimal risk-adapted therapy in this high-risk cohort.
TRANSLATIONAL OUTLOOK: Further studies should determine if risk-adapted treatment based on GLS prognostication can affect clinical outcomes.
Footnotes
Dr. Landau has received research funding from Amgen, Spectrum Pharmaceuticals, and Takeda Pharmaceuticals; has provided consultancy for Karyopharm; and has served on the advisory board for Janssen, Celgene, Caelum Pharmaceuticals, Takeda Pharmaceuticals, and Spectrum Pharmaceuticals. Dr. Hassoun has received research funding and served on the advisory board for Takeda and Celgene; and has provided consultancy for Novartis. Dr. Yu has provided consultancy for Eidos Therapeutics, Genentech, and Glenmark. Dr. Liu has served on the advisory board for Pfizer; and provided consultancy for Bay Labs. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Falk R.H., Comenzo R.L., Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A., Buadi F., Kumar S.K. Treatment of immunoglobulin light chain amyloidosis: Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement. Mayo Clin Proc. 2015;90:1054–1081. doi: 10.1016/j.mayocp.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Palladini G., Dispenzieri A., Gertz M.A. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30:4541–4549. doi: 10.1200/JCO.2011.37.7614. [DOI] [PubMed] [Google Scholar]
- 4.Kyle R.A., Gertz M.A., Greipp P.R. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997;336:1202–1207. doi: 10.1056/NEJM199704243361702. [DOI] [PubMed] [Google Scholar]
- 5.Palladini G., Merlini G. What is new in diagnosis and management of light chain amyloidosis? Blood. 2016;128:159–168. doi: 10.1182/blood-2016-01-629790. [DOI] [PubMed] [Google Scholar]
- 6.Falk R.H., Alexander K.M., Liao R., Dorbala S. A.L. (light-chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol. 2016;68:1323–1341. doi: 10.1016/j.jacc.2016.06.053. [DOI] [PubMed] [Google Scholar]
- 7.Gertz M.A., Lacy M.Q., Dispenzieri A. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone Marrow Transplant. 2013;48:557–561. doi: 10.1038/bmt.2012.170. [DOI] [PubMed] [Google Scholar]
- 8.Dispenzieri A., Dingli D., Kumar S.K. Discordance between serum cardiac biomarker and immunoglobulin-free light-chain response in patients with immunoglobulin light-chain amyloidosis treated with immune modulatory drugs. Am J Hematol. 2010;85:757–759. doi: 10.1002/ajh.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palladini G., Campana C., Klersy C. Serum N-terminal pro-brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation. 2003;107:2440–2445. doi: 10.1161/01.CIR.0000068314.02595.B2. [DOI] [PubMed] [Google Scholar]
- 10.Dispenzieri A., Kyle R.A., Gertz M.A. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet. 2003;361(9371):1787–1789. doi: 10.1016/S0140-6736(03)13396-X. [DOI] [PubMed] [Google Scholar]
- 11.Dispenzieri A., Gertz M.A., Kyle R.A. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22(18):3751–3757. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Dispenzieri A., Gertz M.A., Kyle R.A. Prognostication of survival using cardiac troponins and N-terminal pro-brain natriuretic peptide in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2004;104:1881–1887. doi: 10.1182/blood-2004-01-0390. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S., Dispenzieri A., Lacy M.Q. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S.K., Gertz M.A., Dispenzieri A. Validation of Mayo Clinic staging system for light chain amyloidosis with high-sensitivity troponin. J Clin Oncol. 2018;37:171–173. doi: 10.1200/JCO.18.01398. [DOI] [PubMed] [Google Scholar]
- 15.Pun S.C., Landau H.J., Riedel E.R. Prognostic and added value of two-dimensional global longitudinal strain for prediction of survival in patients with light chain amyloidosis undergoing autologous hematopoietic cell transplantation. J Am Soc Echocardiogr. 2018;31:64–70. doi: 10.1016/j.echo.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barros-Gomes S., Williams B., Nhola L.F. Prognosis of light chain amyloidosis with preserved LVEF: added value of 2D speckle-tracking echocardiography to the current prognostic staging system. J Am Coll Cardiol Img. 2017;10:398–407. doi: 10.1016/j.jcmg.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Buss S.J., Emami M., Mereles D. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical and biochemical markers. J Am Coll Cardiol. 2012;60:1067–1076. doi: 10.1016/j.jacc.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 18.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Mor-Avi V., Lang R.M., Badano L.P. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Stokke T.M., Hasselberg N.E., Smedsrud M.K. Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. J Am Coll Cardiol. 2017;70:942–954. doi: 10.1016/j.jacc.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 21.Stanton T., Leano R., Marwick T.H. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2(5):356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 22.Muchtar E., Gertz M.A., Kumar S.K. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129:2111–2119. doi: 10.1182/blood-2016-11-751628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palladini G., Sachchithanantham S., Milani P. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126:612–615. doi: 10.1182/blood-2015-01-620302. [DOI] [PubMed] [Google Scholar]
- 24.Sperry B.W., Ikram A., Hachamovitch R. Efficacy of chemotherapy for light-chain amyloidosis in patients presenting with symptomatic heart failure. J Am Coll Cardiol. 2016;67:2941–2948. doi: 10.1016/j.jacc.2016.03.593. [DOI] [PubMed] [Google Scholar]
- 25.Madan S., Kumar S.K., Dispenzieri A. High-dose melphalan and peripheral blood stem cell transplantation for light-chain amyloidosis with cardiac involvement. Blood. 2012;119:1117–1122. doi: 10.1182/blood-2011-07-370031. [DOI] [PMC free article] [PubMed] [Google Scholar]