Abstract
Context:
Many inflammatory cytokines are also elevated in degenerated or herniated intervertebral discs. Among biomarkers, interleukin-6 (IL-6) plays an essential role in the inflammatory process of disc herniation. Some studies have suggested that an increase in serum IL-6 levels occurs in sustained radicular pain.
Aims:
The aim of this study was to determine the relationship between changes in IL-6 serum level and pain and disability index in patients with radicular pain in acute herniated lumbar disc before and after lumbar disc surgery.
Settings and Design:
This is a descriptive-analytic prospective study to examine the association between IL-6 serum levels on pain and disability before and after the surgery in patients admitted with acute herniated lumbar intervertebral discs from 2015 to 2018 in Imam Khomeini Hospital, Sari, Mazandaran, Iran.
Subjects and Methods:
The blood level of IL-6, the severity of pain based on visual analog score, and disability based on the Oswestry disability index were measured before and 3 months after surgery.
Statistical Analysis Used:
All data were analyzed using SPSS version 24.
Results:
Thirty-two patients were enrolled in the study. Seventeen patients were male. The mean age was 39.53 ± 8.89 years. IL-6 concentration, 4.36 and 1.16 pg/ml were determined as cutoff before and after the surgery.
Conclusions:
The acceptable sensitivity and specificity of IL were obtained in this study. Our findings revealed that IL-6 could be used as a biomarker for predicting postoperative pain relief and disability improvement.
Keywords: Herniated disc, interleukin-6, neurosurgery
Introduction
The compression of neural elements made by herniated disc can produce numerous complications for the patients, but the pain is the most common manifestation.[1] Back pain causes a serious load on the health-care structure.[2] Low back pain (LBP) is the second source of referred to clinics in the USA with an estimated 50–100 billion dollars in health-care charges.[3,4] Finally, LBP is the main cause of disability in the world.[5]
Radicular pain relief seems to be influenced by several physical, psychosocial, surgical, and clinical factors.[4] The role of biological agents in the improvement of lumbar disc herniation is not fully understood.[4] Mental stress, fear of movement, lumbar pain, long-term symptoms, smoking, clinical factors such as muscle weakness, and nonsurgical treatment do not improve the lumbar disc radicular pain.[3] In contrast, magnetic resonance imaging (MRI) findings are helpful in predicting postoperative prognosis.[6]
Many inflammatory cytokines are also elevated in degenerated or herniated the intervertebral discs,[5,6] including interleukin (IL)-1, IL-6, IL-17, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). Inflammatory cytokines also advocate the enlistment of immune cells to the disc in its processes.[7] Infiltrated and activated immune cells result in refinement of an inflammatory response and the release of neurotrophic factors that contribute to nerve sensitization of spinal nerves.[8]
Several in vitro and animal studies of pain have confirmed the influence of pro-inflammatory mediators on peripheral and central nociception.[9] In human studies, focal and diffuse induced cytokines have been concerned as intermediaries of pain.[6] Conversely, although the impact of TNF-α to prolonged pain has been revealed frequently, documents on the roles of IL-1b and IL-6 in human radicular pain are inadequate.[10]
Clinically, the IL-6 concentration has been used as a marker of surgical magnitude after major surgery, but the kinetics of the IL-6 concentration differs based on the surgical procedure.[6] There have been few studies evaluating the kinetics of IL-6 after spinal surgery.[11] Among biomarkers, IL-6 plays an essential role in the inflammatory process of disc herniation. Several studies have recently reported the relationship between IL-6 and radicular pain.[3,5,11] Some studies have suggested that an increase in serum IL-6 levels occurs in sustained radicular pain.[12] A cross-sectional study has shown that increased levels of IL-6 in patients with chronic lumbar pain cause disc herniation.[3] IL-6 concentrations have also been shown to increase in the cerebrospinal fluid and serum in the 1st days after the disc herniation.[13]
Therefore, the aim of this study was to determine the relationship between changes in IL-6 serum level and pain and disability index in patients with radicular pain in acute herniated lumbar disc before and after lumbar disc surgery so by results of this study this biomarker can be used as a predictor of patient recovery.
Subjects and Methods
Study design
This is a descriptive-analytic prospective study to examine the association between IL-6 serum levels on pain and disability before and after surgery in patients admitted with acute herniated lumbar intervertebral discs from 2015 to 2018 in Imam Khomeini hospital, Sari, Mazandaran, Iran. The blood level of IL-6, the severity of pain based on visual analog score (VAS), and disability based on the Oswestry disability index (ODI) were measured before and 3 months after surgery.
Ethical consideration
The study was submitted to the Department of Research and Technology, Mazandaran University of Medical Sciences, and approved by the ethics committee on biomedical research, code IR.MAZUMS.REC.94.1509. Patients were informed about the study objectives and procedures. The confidentiality of patients' information was guaranteed. Study participants were provided written informed consent. All the experimental procedures involving human samples were conducted with strict adherence to the guidelines of the Declaration of Helsinki.
Patients
Patients who selected informed consent and all information as confidential. Entering the study had no interaction with the patient's treatment process and was safe. Patients aged 18–60 years, who had acute radicular and LBP for <3 months, the herniated lumbar disc had been confirmed by MRI, were eventually candidates for surgery, entered the study. Patients with spinal cord stenosis, history of spinal surgery, generalized musculoskeletal pain, pregnant, congenital syndromes, scoliosis, vertebral fracture, and pain due to systemic diseases (e.g., inflammatory spondyloarthropathies, spinal cord infection, and tumor) were excluded.
Laboratory procedures
Venous blood samples were taken before and 3 months after surgery. Samples were collected in heparinized tubes and centrifuged at 3000 rpm for 10 min. They were then stored at −20°C until assayed. Serum IL-6 was measured by enzyme immunoassay. All procedures were conducted in a blind fashion.
Questionnaires
ODI (also known as the Oswestry LBP disability questionnaire) is an essential tool measuring the patient's permanent functional disability. The test is considered the “gold standard” of low back functional outcome tools includes ten items. The total score of the questionnaire is expressed as a percentage. In our study, <20% were considered as minimal, 21%–40% moderate, and over 41% severe.
VAS is a measurement instrument that tries to measure a characteristic or attitude that is believed to range across a continuum of values and cannot easily be directly measured. A person rates their pain on a scale of 0–10 or 0–5. Zero means “no pain,” and 5 or 10 means “the worst possible pain. In this study, this scale was used for the evaluation of pain before and after surgery.
Statistical analysis
Normality distribution of data was evaluated using the Kolmogorov–Smirnov test. All quantitative data except age have not normal distribution (P < 0.05). Therefore, on-parametric tests were used during the study to analysis them. Furthermore, in this study, the relationship between quantitative variables based on the Chi-square test was investigated. Mann–Whitney test and one-way ANOVA were used to examine the relationship between quantitative variables. The roc curve test was used to determine the cutoff point; all analyses were performed with IBM® SPSS® Statistics V24.0 (IBM, Chicago, Illinois, USA).
Results
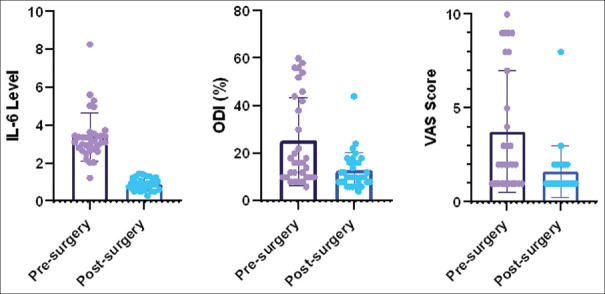
Thirty-two patients were enrolled in the study based on inclusion and exclusion criteria. The frequency (percentage) of males and females was 17 (53.1) and 15 (46.9), respectively. The mean age was 39.53 ± 8.89 years. The frequency of disc herniation at levels L3-L4, L4-L5, and L5-S1 were 2 (6.3%), 12 (37.5%), and 18 (56.3%), respectively. Serum levels mean of IL-6 were evaluated before and after surgery 3.45 ± 1.22 pg/ml and 0.91 ± 0.29 pg/ml, respectively. The mean and standard deviation of the VAS score before and after the surgery were 3.24 ± 3.24 and 1.37 ± 1.40; and ODI score were 25.00 ± 18.60 and 12.62 ± 7.71, respectively. There was a significant difference between serum IL-6 levels, VAS, and ODI before and after surgery (P < 0.05) [Figure 1].
Figure 1.
The mean of serum IL-6 levels, VAS score, and ODI percentage before and after surgery
Correlation between serum IL-6 level, VAS, and ODI score before and after surgery was evaluated. The results are shown presurgery IL-6 had Inverse and statistically significant correlation with difference (post-pre) in VAS (correlation coefficient (CC) = −0.443 and P = 0.011) and difference in ODI (post–pre) (CC = −0.460 and P = 0.008). That is, presurgery IL-6 levels decreased with decreasing pain and improvement inability to perform activities and had a positive significant relationship with presurgery VAS (CC = 0.532 and P = 0.002). This means that higher presurgery VAS score was associated with higher presurgery IL-6 levels [Table 1].
Table 1.
Correlation between serum interleukin-6 level before and after surgery with visual analogue score and percentage of the Oswestry disability index and changes after and before surgery
| IL-6 | Post-pre difference | VAS | ODI | |||
|---|---|---|---|---|---|---|
| VAS | ODI | Presurgery | Postsurgery | Presurgery | Postsurgery | |
| Presurgery | ||||||
| CC | −0.443 | −0.460 | 0.523 | 0.299 | 0.340 | −0.039 |
| P | 0.011 | 0.008 | 0.002 | 0.097 | 0.057 | 0.833 |
| Postsurgery | ||||||
| CC | −0.079 | 0.054 | 0.078 | 0.161 | 0.001 | 0.042 |
| P | 0.669 | 0.770 | 0.670 | 0.377 | 0.995 | 0.820 |
Analyzed with Spearman’s test, Significant level is <0.05. IL-6 – Interleukin-6; VAS – Visual analog score; ODI – Oswestry disability index; CC – Correlation coefficient
Serum IL-6 levels before and after surgery were assessed according to the interpretation status of the ODI. There was a significant difference in presurgery IL-6 variable with the interpretation of the ODI questionnaire before surgery so that presurgery IL-6 levels were higher in patients with severe disability (4.73 pg/ml) than in minimal (3.03 pg/ml) and moderate (2.46 pg/ml) (P = 0.0001). However, there was not a significant difference in postsurgery IL-6 with the interpretation of the ODI after surgery (P = 0.304).
Patients were divided into two groups based on the interpretation of ODI after surgery as severe and (both minimal and moderate). The cutoff point of serum level of IL-6 for postsurgery severe disability was measured. Based on the results of presurgery IL-6 concentration, 4.36 pg/ml with a sensitivity of 66% and specificity of 90% was determined as severe disability after surgery. According to the ROC diagram, the area below the diagram is 0.598. Furthermore, about the postsurgery IL-6 concentration level variable, the concentration of 1.16 pg/ml with 66.7% sensitivity and 80% specificity was determined as the cutoff for severe disability after surgery. According to the ROC diagram, the area below the diagram is 0.667. Moreover, these cutoff points were significant, given the area below the graph [Figure 2].
Figure 2.
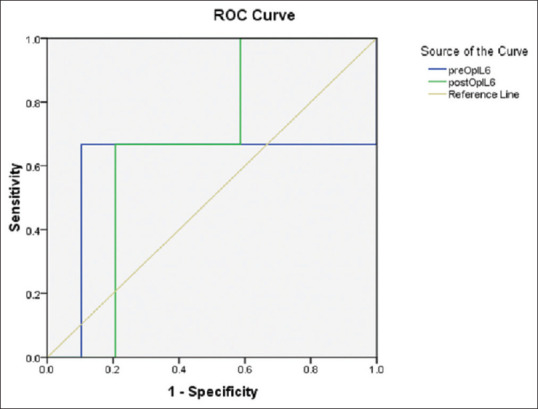
Receiver operating characteristic curve for measuring the cut-off point of serum level of interleukin-6 for postsurgery in severe disability
Discussion
There was a significant difference between serum IL-6 levels, VAS, and ODI before and after surgery. The results showed that improvement in patients' symptoms based on VAS and ODI was correlated with serum levels of IL-6. Serum levels of IL were also lower in patients with low presurgery VAS and ODI scores. Furthermore, patients with low severity of pain and disability in postsurgery had low IL levels. These relationships have been reported in previous studies.[5,6]
Previous studies have shown that an increase in the level of inflammatory cytokines (IFN-γ, IL-1β, IL-2, IL-6, and TNF-α) is associated with an increase in pain score in various types of neurological and rheumatologic disorders.[14] For example, Binshtok et al.[15] have shown that, in addition to producing inflammation and sensitizing sensory receptors for pain stimuli, inflammatory cytokines such as IL-1 rapidly and directly activate sensory receptors for pain and substantially increase pain sensitivity. Interestingly, increased IL-1β release may also increase the level of other inflammatory cytokines such as IL-6.
One of our remarkable results was about 66% sensitivity and high specificity of IL-6 before and after surgery that it can be a predictor for prognosis of injury and improvement of the patient. In this regard, the results of Schistad et al.[6] showed that high levels of IL-6 had a negative effect on recovery rate in patients with a long-term herniated disc, and the modic changes and disc degeneration had no effect on patients' recovery over a year.
In another study by Ohtori et al.,[4] the effect of epidural injection of monoclonal antibody receptor monoclonal antibody, on radicular pain in patients with spinal stenosis were studied. Sixty patients with radicular pain were enrolled. Thirty patients received lidocaine and monoclonal antibody receptor, and 30 patients received lidocaine and dexamethasone. The results of this study showed that this drug reduces the pain of radicular legs, numbness, and lumbar pain. Therefore, IL6 seems to induce pain in people with spinal canal stenosis. There have been inconsistent findings among the studies, but most of them have emphasized the association between IL-6 and discopathy-related pain and functional disability; however, further studies with larger sample sizes are needed.
Conclusions
The acceptable sensitivity and specificity of IL were obtained in this study. Our findings revealed that IL-6 could be used as a biomarker for predicting postoperative pain relief and disability improvement.
Financial support and sponsorship
Mazandaran University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank the vice president of research at the Mazandaran University of Medical Science, who contribute us to conduct this study by approving and financing this. Gratitude is expressed to all participants who have helped in the completion of this research.
References
- 1.Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine (Phila Pa 1976) 1996;21:218–24. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 2.Weber KT, Alipui DO, Sison CP, Bloom O, Quraishi S, Overby MC, et al. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Ther. 2016;18:3. doi: 10.1186/s13075-015-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demura S, Takahashi K, Kawahara N, Watanabe Y, Tomita K. Serum interleukin-6 response after spinal surgery: Estimation of surgical magnitude. J Orthop Sci. 2006;11:241–7. doi: 10.1007/s00776-006-1002-4. [DOI] [PubMed] [Google Scholar]
- 4.Ohtori S, Miyagi M, Eguchi Y, Inoue G, Orita S, Ochiai N, et al. Efficacy of epidural administration of anti-interleukin-6 receptor antibody onto spinal nerve for treatment of sciatica. Eur Spine J. 2012;21:2079–84. doi: 10.1007/s00586-012-2183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade P, Hoogland G, Garcia MA, Steinbusch HW, Daemen MA, Visser-Vandewalle V. Elevated IL-1β and IL-6 levels in lumbar herniated discs in patients with sciatic pain. Eur Spine J. 2013;22:714–20. doi: 10.1007/s00586-012-2502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schistad EI, Espeland A, Pedersen LM, Sandvik L, Gjerstad J, Røe C. Association between baseline IL-6 and 1-year recovery in lumbar radicular pain. Eur J Pain. 2014;18:1394–401. doi: 10.1002/j.1532-2149.2014.502.x. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto H, Saura R, Doita M, Kurosaka M, Mizuno K. The role of cyclooxygenase-2 in lumbar disc herniation. Spine (Phila Pa 1976) 2002;27:2477–83. doi: 10.1097/00007632-200211150-00011. [DOI] [PubMed] [Google Scholar]
- 8.Doita M, Kanatani T, Ozaki T, Matsui N, Kurosaka M, Yoshiya S. Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine (Phila Pa 1976) 2001;26:1522–7. doi: 10.1097/00007632-200107150-00004. [DOI] [PubMed] [Google Scholar]
- 9.Geiss A, Varadi E, Steinbach K, Bauer HW, Anton F. Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent discectomy. Neurosci Lett. 1997;237:65–8. doi: 10.1016/s0304-3940(97)00810-0. [DOI] [PubMed] [Google Scholar]
- 10.Dang L, Chen Z, Liu X, Guo Z, Qi Q, Li W, et al. Lumbar Disk Herniation in Children and Adolescents: The Significance of Configurations of the Lumbar Spine. Neurosurgery. 2015;77:954–9. doi: 10.1227/NEU.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi A, Kikuchi S, Konno S. Correlation between inflammatory cytokines released from the lumbar facet joint tissue and symptoms in degenerative lumbar spinal disorders. J Orthop Sci. 2007;12:154–60. doi: 10.1007/s00776-006-1105-y. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–94. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brisby H, Olmarker K, Larsson K, Nutu M, Rydevik B. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J. 2002;11:62–6. doi: 10.1007/s005860100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krämer HH, Eberle T, Uçeyler N, Wagner I, Klonschinsky T, Müller LP, et al. TNF-α in CRPS and 'normal' trauma–significant differences between tissue and serum. Pain. 2011;152:285–90. doi: 10.1016/j.pain.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–73. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]