Abstract
Background
Acute myocardial injury and heart failure characterized by elevated cardiac troponin and decreased heart pump function are significant clinical features and prognostic factors of coronavirus disease-19 (COVID-19). Triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio is an indicator of insulin resistance. This study aimed to explore the association of the TG/HDL-C ratio with cardiovascular risk and prognosis in COVID-19.
Methods
Ninety-eight laboratory-confirmed patients with COVID-19 admitted in a tertiary teaching hospital in Wuhan, China, were enrolled in this retrospective study. Regression models were used to investigate the association between TG/HDL-C ratio with myocardial injury, heart failure, severity, and mortality in COVID-19.
Results
Among the 98 patients, the mean age was 63.9±1.4 years, and male sex (58, 59%) was predominant. Forty-six patients (47%) were admitted to the intensive care unit (ICU), 32 (33%) and 46 (47%) patients suffered from myocardial injury and heart failure, respectively, and 36 (37%) patients died. The TG/HDL-C ratio was increased in patients with myocardial injury, heart failure, severe illness, and fatal outcome (P<0.05 for each). Baseline TG/HDL-C ratio significantly correlated with log transformed levels of plasma high-sensitivity cardiac troponin I (r=0.251, P=0.018), N-terminal brain natriuretic propeptide (r=0.274, P=0.008), glycated hemoglobin (r=0.239, P=0.038), and interleukin-6 (r=0.218, P=0.042). Multivariate logistic regression analysis showed that an increased TG/HDL-C ratio was independently associated with the risk of myocardial injury [odds ratio (OR)=2.73; P=0.013], heart failure (OR=2.64; P=0.019), disease severity (OR=3.01; P=0.032), and fatal outcome (OR=2.97; P=0.014).
Conclusion
Increased TG/HDL-C ratio was independently associated with myocardial injury, heart failure, disease severity, and mortality in patients with COVID-19, and it may be a useful marker for early identification of patients with high risk and poor outcome.
Keywords: TG/HDL-C, insulin resistance, myocardial injury, heart failure, mortality, COVID-19
Introduction
In December 2019, a novel coronavirus pneumonia caused by a newly identified coronavirus emerged in Wuhan, China;1 subsequently, it was named coronavirus disease-2019 (COVID-19) by the World Health Organization. At present, the disease has become a global pandemic and has posed a great threat to the world. As of August 19, 2020, 22 million confirmed cases worldwide have been reported, with 786,000 (3.5%) deaths. Although the mortality rate of COVID-19 is lower than those of severe acute respiratory syndrome (SARS) (10%) and Middle Eastern respiratory syndrome (MERS) (36%),2,3 the total number of deaths due to COVID-19 is much greater than that due to SARS and MERS, and is still growing fast. Therefore, early identification of the severity of COVID-19 and lowering of its mortality rate is an urgent need in public health.
Some striking common characteristics have been found among patients who have died, including advanced age and comorbidities, especially cardiovascular diseases and diabetes.4–7 Chen et al7 summarized the clinical characteristics of 113 deceased patients who died in the early days of the outbreak in Wuhan, and found that hypertension and other cardiovascular comorbidities were more frequent among the deceased (48% and 14%) than in patients who recovered (24% and 4%). Chen et al summarized the characteristics of 1590 confirmed COVID-19 cases throughout China and found that age ≥75 years (HR=7.86), age between 65–74 years (HR=3.43), and coronary heart disease (HR=4.28) were independent risk factors associated with mortality.4 Similarly, diabetes has also been found to be positively correlated with mortality in COVID-19 patients.5,6
The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio has been widely used as a reliable marker of insulin resistance8 and has a positive correlation with the incidence of type 2 diabetes and metabolic syndrome.9 Meanwhile, TG/HDL ratio has also been demonstrated to be correlated with cardiovascular diseases, including hypertension,10 coronary heart disease (CHD),11 new-onset heart failure,12 and myocardial infarction.13
As mentioned above, previous studies revealed that cardiovascular diseases and diabetes were positively correlated with mortality in COVID-19; however, no study to date has indicated the relationship between TG/HDL-C ratio and the rate of progression and mortality of the disease. In this context, it is of great importance to investigate whether TG/HDL ratio could be used as a predictive indicator of outcome in COVID-19, for early identification of severe cases and reduction of mortality.
In this study, we retrospectively analyzed the clinical data of 98 confirmed inpatients admitted to Sino-French Branch of Tongji Hospital (Wuhan, China), a designated hospital for severe or critically ill patients with COVID-19. The purpose of this study was to investigate the predictive value of TG/HDL ratio in the severity and mortality of COVID-19.
Patients and Methods
Study Design and Participants
A consecutive case series of 113 adult inpatients with laboratory-confirmed COVID-19 were admitted to two inpatient wards of the Sino-French Branch of Tongji Hospital, Wuhan, China. Ninety-eight patients, who had completed medical records and follow-up data, were enrolled in this retrospective study. Follow-up began on February 6, 2020 until February 28, 2020 when all the patients enrolled in this study had definite outcomes (discharge or death).
All the patients were categorized on the basis of whether he/she needed intensive care unit (ICU) treatment (defined as severe cases in this study, n=52) or not (non-severe cases, n=46). The criteria for ICU treatment are listed in the definitions section of the manuscript, and all ICU treatment needs were met within appropriate times. The therapeutic schedule was formulated according to the patient’s condition and not influenced by the study. Finally, all the clinical data were retrospectively evaluated and analyzed.
To clarify, all patients enrolled in this study were severe or critically ill patients according to the Guidelines for diagnosis and management of COVID-19 (6th edition, in Chinese) released by the National Health Commission of China.14 However, we regrouped the patients into severe cases (needing ICU treatment) and non-severe cases (no ICU treatment needed) owing to the study requirement.
All participants provided oral informed consent due to the urgency of the communicable disease outbreak. The study and the oral informed consent process was approved by the Ethics Commission of Tongji Medical College, Huazhong University of Science and Technology (No.S130), and complied with the Declaration of Helsinki.
Definitions
According to the Guidelines for diagnosis and management of COVID-19 issued by the National Health Commission of China,14 all 98 patients were confirmed with COVID-19 referring to the following diagnostic criteria: 1) epidemiologic history, 2) fever or other respiratory symptoms and typical computed tomography (CT) appearance of viral pneumonia, and 3) positive real-time reverse transcription polymerase chain reaction (RT-PCR) detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA.
In this study, patients needing ICU treatment were defined as severe cases. The criteria for ICU treatment were as follows (any one of the following): respiratory failure requiring invasive mechanical ventilation; cardiovascular shock; newly diagnosed acute failure of extrapulmonary organs requiring organ support therapy; other conditions for which expert specialists recommended ICU treatment according to the patient’s situation.
Cardiovascular disease was defined as having a history of cardiovascular system diseases that may cause myocardial damage and/or affect the functioning of the heart, such as coronary atherosclerotic heart disease, myocardial infarction, cardiomyopathy, arrhythmia, and valvular heart disease. Chronic respiratory disease was defined as having a history of respiratory system diseases that may cause pulmonary ventilation and gas exchange function disorders, such as chronic obstructive pulmonary disease (COPD), lung cancer, interstitial lung disease, pulmonary tuberculosis, and bronchial asthma. Chronic kidney disease was defined as having a history of kidney diseases that may lead to abnormalities of kidney structure or function, such as chronic glomerulonephritis, uremia, polycystic kidney, interstitial nephritis, diabetic nephropathy, and hypertensive nephropathy. Chronic liver disease was defined as having a history of kidney diseases that may cause injury of liver function, such as chronic viral hepatitis, autoimmune hepatitis, liver cirrhosis, liver cancer, and schistosomiasis liver disease.
Acute myocardial injury was defined as serum levels of hypersensitive cardiac troponin I (hs-cTnI) being higher than 34 pg/mL, or the presence of new abnormalities in electrocardiography or segmental ventricular wall motion anomaly were shown.15 Heart failure was defined as acute dyspnea combined with a significant increase in NT-proBNP with the cut-off points of 450 pg/mL (age<50), 900 pg/mL (age=50–75), and 1800 pg/mL (age>75), respectively, according to Heart Failure Association of the European Society of Cardiology practical guidance.16
Data Collection
We collected epidemiological and demographic data, and data regarding comorbidities (hypertension, cardiovascular disease, diabetes, chronic kidney disease, chronic respiratory disease, chronic liver disease, and malignancy), clinical symptoms and signs (fever, cough, fatigue, myalgia, headache, edema, chest tightness, dyspnea, hemoptysis, diarrhea, nausea or vomiting, anorexia, and palpitation), vital signs on admission (systolic pressure, heart rate, respiratory rate, and percutaneous oxygen saturation), laboratory measurements [glycated hemoglobin (HbA1C), fasting blood glucose (FBG), complete blood count, urine routine test, liver function, renal function, electrolytes, lactate dehydrogenase (LDH), lipid profile, coagulation function test, N-terminal brain natriuretic propeptide (NT-proBNP), hs-cTnI, hypersensitive C-reactive protein (hs-CRP), procalcitonin (PCT), serum ferritin, interleukin-6 (IL-6), and erythrocyte sedimentation rate (ESR)], electrocardiography, and echocardiography. Blood samples except fasting blood glucose and lipids were collected once the patients were admitted, and plasma samples for fasting blood glucose tests and serum samples for lipids tests were collected under fasting state on the next day. The frequency of laboratory examinations was determined by the severity of illness. The clinical outcome was obtained from the electronic medical records using standard data collection forms; the collected data were checked independently by two researchers.
Statistical Analysis
Data are presented as mean±standard error (SE) or median (interquartile range, IQR) for continuous variables and number (percentage) for categorical variables. Comparisons between the groups of non-ICU/non-severe vs ICU/severe patients and non-survivors vs survivors were analyzed using Student’s t-test, Mann–Whitney U-test, chi-squared test, and Fisher’s exact test. Chi-squared test, one-way analysis of variance, and Jonckheere-Terpstra test were used to compare the baseline characteristics of patients classified by tertiles of the TG/HDL-C ratio. Pearson's correlation analysis was used to explore the relationship of the TG/HDL-C ratio with selected covariates (levels of hs-cTnI, NT-proBNP, HbA1C, IL-6, and TG/HDL-C were log transformed). Binary logistic regression analysis was used to evaluate the independent association of TG/HDL-C ratio with myocardial injury, heart failure, severity, and fatal outcome in patients with COVID-19 after adjustment for several confounders. The adjusted confounders were selected according to previous literature and the results of the univariate analysis in this study. Receiver operating characteristic curve (ROC) analysis was constructed to assess the performance of TG/HDL-C ratio for prediction of fatal outcome based on the value of area under the ROC curve (AUC). SPSS version 20.0 software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Statistical significance was defined by a two-sided P-value<0.05.
Results
Demographics and Baseline Characteristics of Patients with COVID-19
Of the 98 patients with confirmed COVID-19 included in this study cohort, 46 (47%) were severe patients (admitted to ICU) and 52 (53%) were non-severe patients admitted to one of the isolation wards (non-ICU). And finally, 62 (63%) patients were discharged and 36 (37%) died in total.
The demographic and baseline characteristics of the patients recorded at admission are presented in Table 1. The patients had a mean age of 63.9±1.4 years, and 58 (59%) were men. Comorbidities were present in 74 (76%) patients. Hypertension (43%) was the most common comorbidity, followed by diabetes (37%) and cardiovascular disease (17%). The most common manifestations at disease onset were fever (81%), dry cough (77%), fatigue (35%), chest tightness (37%), and dyspnea (58%). Less common symptoms were myalgia, headache, nausea or vomiting, and anorexia. Severe patients had a higher respiratory rate than non-severe patients at hospital admission (P=0.020). The non-survivors were older and also more likely to manifest with a higher respiratory rate (P=0.017) and a lower percutaneous oxygen saturation (P=0.011) at hospital admission than the survivors.
Table 1.
Demographics and Baseline Characteristics of Patients with COVID-19
| Characteristics | Severity | Mortality | |||||
|---|---|---|---|---|---|---|---|
| Total (n=98) |
Non-ICU/Severe (n=46) |
ICU/Severe (n=52) |
P | Survivor (n=62) |
Non-Survivor (n=36) |
P | |
| Age, years | 63.9±1.4 | 61.1±2.2 | 66.3±1.8 | 0.074 | 60.0±1.9 | 70.5±1.7 | 0.000 |
| Male | 58 (59%) | 24 (52%) | 34 (65%) | 0.184 | 35 (57%) | 23 (64%) | 0.470 |
| Comorbidity | |||||||
| Hypertension | 42 (43%) | 19 (41%) | 23 (44%) | 0.770 | 28 (45%) | 14 (39%) | 0.545 |
| Diabetes | 36 (37%) | 14 (30%) | 22 (42%) | 0.224 | 20 (32%) | 16 (44%) | 0.228 |
| Cardiovascular disease | 17 (17%) | 7 (15%) | 10 (19%) | 0.601 | 8 (13%) | 9 (25%) | 0.127 |
| Chronic respiratory disease | 11 (11%) | 5 (11%) | 6 (12%) | 0.917 | 4 (7%) | 7(19%) | 0.093 |
| Chronic kidney disease | 10 (10%) | 8 (17%) | 2 (4%) | 0.042 | 8 (13%) | 2 (6%) | 0.317 |
| Chronic liver disease | 3 (3%) | 0 (0%) | 3 (6%) | 0.245 | 1 (2%) | 2 (6%) | 0.552 |
| Signs and symptoms | |||||||
| Fever | 79 (81%) | 36 (78%) | 43 (83%) | 0.580 | 52 (84%) | 27 (75%) | 0.284 |
| Cough | 75 (77%) | 36 (78%) | 39 (75%) | 0.704 | 48 (77%) | 27 (75%) | 0.785 |
| Fatigue | 34 (35%) | 26 (57%) | 8 (15%) | 0.000 | 26 (42%) | 8 (22%) | 0.048 |
| Myalgia | 24 (25%) | 19 (41%) | 5 (10%) | 0.000 | 20 (32%) | 4 (11%) | 0.019 |
| Headache | 11 (11%) | 10 (22%) | 1 (2%) | 0.003 | 9 (15%) | 2 (6%) | 0.319 |
| Chest tightness | 36 (37%) | 21 (46%) | 15 (29%) | 0.085 | 28 (45%) | 8 (22%) | 0.023 |
| Dyspnea | 57 (58%) | 26 (57%) | 31 (60%) | 0.757 | 33 (53%) | 24 (67%) | 0.193 |
| Diarrhea | 27 (28%) | 15 (33%) | 12 (23%) | 0.292 | 16 (26%) | 11 (31%) | 0.612 |
| Nausea or vomiting | 11 (11%) | 10 (22%) | 1 (2%) | 0.003 | 9 (15%) | 2 (6%) | 0.176 |
| Anorexia | 15 (15%) | 11 (24%) | 4 (8%) | 0.046 | 10 (16%) | 5 (14%) | 0.767 |
| Vital signs on admission | |||||||
| Systolic pressure, mm Hg | 132±2.3 | 131±3.6 | 133±2.9 | 0.724 | 134±2.7 | 129±4.2 | 0.247 |
| Heart rate, bpm | 95±2.0 | 92±3.0 | 98±2.6 | 0.189 | 93±2.1 | 99±3.9 | 0.158 |
| Respiratory rate, bpm | 24±0.6 | 22±0.6 | 25±1.0 | 0.020 | 23±0.7 | 26±1.2 | 0.017 |
| Percutaneous oxygen saturation, % | 96 (90–98) | 96 (93–98) | 95 (87–98) | 0.318 | 96 (93–98) | 91 (78–97) | 0.011 |
Notes: Data are presented as mean±SE or median (interquartile range) for continuous variables and n (%) for categorical variables. P-values comparing non-ICU/severe and ICU/severe or non-survivors and survivors are from Student’s t-test, Mann–Whitney U-test, χ2 test, or Fisher’s exact test.
Abbreviations: COVID-19, coronavirus Disease 2019; bpm, beats per minute.
Baseline Laboratory Parameters of Patients with COVID-19
The severe patients and non-survivors showed a significantly decreased level of lymphocyte count (P=0.000) and increased levels of white blood cell count (WBC), neutrophil count (NEU), aspartate aminotransferase (AST), LDH, as well as multiple indicators of inflammation, such as hs-CRP, serum ferritin, PCT, and IL-6 compared with the survivors (P<0.05 for each) (Table 2). In terms of cardiometabolic indicators, non-survivors had higher levels of hs-cTnI, NT-proBNP, FBG, HbA1C, and total cholesterol (TC), and a lower level of high-density lipoprotein cholesterol (HDL-C) (P<0.05 for each) compared with non-severe patients and survivors, respectively. It is noteworthy that the TG/HDL-C ratio, calculated by TG and HDL-C values, was markedly increased in the severe patients vs non-severe patients (P=0.007) and in the non-survivors vs in the survivors (P=0.001).
Table 2.
Baseline Laboratory Findings of Patients with COVID-19
| Characteristics | Normal Range | Severity | Mortality | ||||
|---|---|---|---|---|---|---|---|
| Non-ICU/Severe (n=46) | ICU/Severe (n=52) |
P | Survivor (n=62) |
Non-Survivor (n=36) |
P | ||
| Hematologic and biochemical | |||||||
| White blood cell count, ×109/L | 3.5–9.5 | 5.4 (4.7–7.0) | 10.5 (6.3–13.4) | 0.000 | 5.8 (4.7–7.4) | 11.7 (7.3–13.9) | 0.000 |
| Neutrophil count, ×109/L | 1.8–6.3 | 3.8 (2.8–5.0) | 9.7 (5.3–12.3) | 0.000 | 3.9 (2.8–5.4) | 10.5 (6.2–12.6) | 0.000 |
| Lymphocyte count, ×109/L | 1.1–3.2 | 1.2 (0.7–1.5) | 0.6 (0.5– 0.8) | 0.000 | 1.0 (0.7–1.4) | 0.6 (0.4–0.7) | 0.000 |
| Alanine aminotransferase, U/L | ≤41 | 23 (14–40) | 32 (18–50) | 0.052 | 26 (15–41) | 30 (17–63) | 0.225 |
| Aspartate aminotransferase, U/L | ≤40 | 24 (19–36) | 39 (25–64) | 0.000 | 26 (20–41) | 44 (25–75) | 0.002 |
| Creatinine, μmol/L | 59–104 | 70 (60–94) | 79 (59–130) | 0.623 | 69 (56–89) | 89 (66–158) | 0.014 |
| Lactate dehydrogenase, U/L | 135–225 | 269 (222–362) | 481 (329–758) | 0.000 | 285 (227–379) | 501 (373–770) | 0.000 |
| Inflammatory indicators | |||||||
| High-sensitivity C-reactive protein, mg/L | <1 | 12 (3– 66) | 85 (36–177) | 0.000 | 27 (4–85) | 101 (29–192) | 0.000 |
| Serum ferritin, μg/L | 30–400 | 542 (239–1093) | 1150 (723–2722) | 0.005 | 683 (289–1139) | 1362 (768–2722) | 0.000 |
| Procalcitonin, ng/mL | 0.02–0.05 | 0.12 (0.05–0.70) | 0.24 (0.11–1.11) | 0.030 | 0.13 (0.06–0.51) | 0.29 (0.12–2.17) | 0.005 |
| Interleukin 6, pg/mL | <7 | 14 (4–41) | 57 (22–137) | 0.000 | 19 (4–47) | 80 (39–181) | 0.000 |
| Cardiometabolic indicators | |||||||
| Hypersensitive cardiac troponin I, pg/mL | ≤34 | 6 (3–12) | 27 (9–482) | 0.000 | 6 (3–18) | 111 (20–1162) | 0.000 |
| N-terminal pro-brain natriuretic peptide, pg/mL | <285 | 179 (68–798) | 762 (190–2513) | 0.002 | 184 (75–671) | 1573 (542–7487) | 0.000 |
| Fasting blood glucose, mmol/L | 4.1–6.0 | 5.5 (4.9–6.4) | 7.9 (6.1–11.3) | 0.000 | 5.7 (5.0–7.6) | 7.9 (6.3–12.4) | 0.000 |
| Glycated hemoglobin, % | 4.0–6.0 | 6.1 (5.7–6.5) | 6.4 (6.0–7.1) | 0.040 | 6.0 (5.7–6.5) | 6.5 (6.1–7.4) | 0.009 |
| Total cholesterol, mmol/L | <5.2 | 3.8 (3.4–4.3) | 3.3 (2.7–3.9) | 0.002 | 3.8 (3.3–4.3) | 3.0 (2.6–3.9) | 0.001 |
| Triglycerides, mmol/L | <1.7 | 1.3 (1.0–1.8) | 1.5 (1.1–2.2) | 0.326 | 1.3 (1.0–1.8) | 1.6 (1.2–2.2) | 0.038 |
| High-density lipoprotein cholesterol, mmol/L | 1.0–1.6 | 1.0 (0.8–1.1) | 0.8 (0.5–1.0) | 0.001 | 0.9 (0.8–1.1) | 0.7 (0.5–1.0) | 0.002 |
| TG/HDL-C | – | 1.4 (1.0–2.2) | 2.0 (1.3–3.8) | 0.007 | 1.5 (1.0–2.2) | 2.3 (1.5–4.5) | 0.001 |
Notes: Data are presented as median (interquartile range) for continuous variables. P-values comparing non-ICU/severe and ICU/severe patients or non-survivors and survivors are from Mann–Whitney U-test.
Abbreviations: COVID-19, coronavirus Disease 2019; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol.
Complications and Treatments of Patients with COVID-19
In addition, compared with non-severe patients and survivors, the severe patients and non-survivors were more likely to develop acute cardiac injury, heart failure, acute respiratory distress syndrome, acute kidney injury, acute liver injury, disseminated intravascular coagulation, and secondary infection (P<0.05 for each, Table 3) during the period of follow-up. Thus, patients were more likely to use antibiotics, corticosteroids, non-invasive mechanical ventilation, and invasive mechanical ventilation, but less likely to use antiviral treatment in the severe patients vs non-severe patients and in the non-survivors vs in the survivors (P<0.05 for each).
Table 3.
Complications and Treatments of Patients with COVID-19
| Severity | Mortality | ||||||
|---|---|---|---|---|---|---|---|
| Total (n=98) |
Non-ICU/Severe (n=46) |
ICU/Severe (n=52) |
P | Survivor (n=62) |
Non-Survivor (n=36) |
P | |
| Complications | |||||||
| Acute cardiac injury | 32 (33%) | 3 (7%) | 29 (56%) | 0.000 | 4 (7%) | 28 (78%) | 0.000 |
| Heart failure | 46 (47%) | 10 (22%) | 36 (69%) | 0.000 | 11 (18%) | 35 (97%) | 0.000 |
| Acute cardiac injury and Heart failure | 29 (30%) | 2 (4%) | 27 (52%) | 0.000 | 2 (3%) | 27 (75%) | 0.000 |
| Acute respiratory distress syndrome | 45 (46%) | 3 (7%) | 42 (81%) | 0.000 | 11 (18%) | 34 (94%) | 0.000 |
| Acute kidney injury | 29 (30%) | 5 (11%) | 24 (46%) | 0.000 | 5 (8%) | 24 (67%) | 0.000 |
| Acute liver injury | 41 (42%) | 11 (24%) | 30 (58%) | 0.001 | 19 (31%) | 22 (61%) | 0.003 |
| Disseminated intravascular coagulation | 20 (20%) | 1 (2%) | 19 (37%) | 0.000 | 3 (5%) | 17 (47%) | 0.000 |
| Secondary infection | 58 (59%) | 16 (35%) | 42 (81%) | 0.000 | 24 (39%) | 34 (94%) | 0.000 |
| Treatments | |||||||
| Antibiotics | 68 (69%) | 16 (35%) | 52 (100%) | 0.000 | 32 (52%) | 36 (100%) | 0.000 |
| Antiviral treatment | 75 (77%) | 45 (98%) | 30 (58%) | 0.000 | 58 (94%) | 17 (47%) | 0.000 |
| Corticosteroids | 43 (44%) | 3 (7%) | 40 (77%) | 0.000 | 15 (24%) | 28 (78%) | 0.000 |
| Intravenous immunoglobin | 30 (31%) | 3 (7%) | 27 (52%) | 0.000 | 15 (24%) | 15 (42%) | 0.070 |
| Non-invasive mechanical ventilation | 35 (36%) | 1 (2%) | 34 (65%) | 0.000 | 8 (13%) | 27 (75%) | 0.000 |
| Invasive mechanical ventilation | 32 (33%) | 0 (0%) | 32 (62%) | 0.000 | 5 (8%) | 27 (75%) | 0.000 |
| Renal replacement therapy | 8 (8%) | 3 (7%) | 5 (10%) | 0.719 | 5 (8%) | 3 (8%) | 1.000 |
Notes: Data are presented as n (%) for categorical variables. P-values comparing non-ICU/severe and ICU/severe or non-survivors and survivors are from χ2 test or Fisher's exact test.
Abbreviations: COVID-19, coronavirus Disease 2019; ICU, intensive care unit.
The TG/HDL-C Ratio Correlated with Myocardial Injury, Heart Failure, Severity, and Mortality in Patients with COVID-19
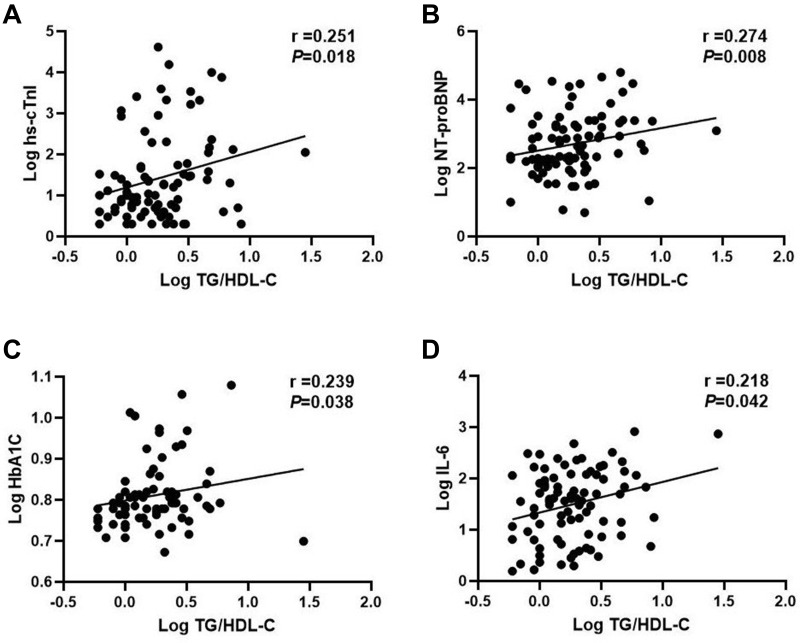
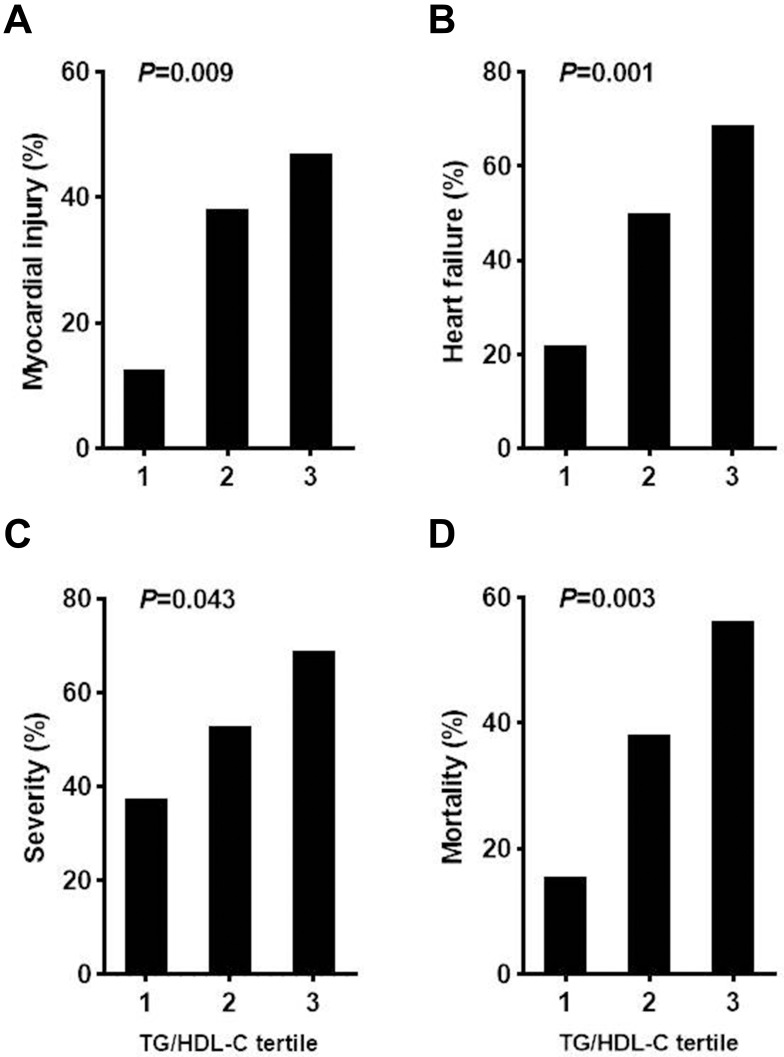
It is noteworthy that the TG/HDL-C ratio, calculated by TG and HDL-C values, was higher in patients with myocardial injury and heart failure than in patients without myocardial injury [2.1, IQR (1.5, 4.6) vs 1.5, IQR (1.0, 2.4), P=0.002] and heart failure [2.1, IQR (1.5, 4.5) vs 1.3, IQR (1.0, 2.1), P=0.000], respectively. In addition, the TG/HDL-C ratio was markedly increased in the severe patients vs non-severe patients [2.0, IQR (1.3, 3.8) vs 1.4, IQR (1.0, 2.2), P=0.007] and in the non-survivors vs in the survivors [2.3, IQR (1.5, 4.5) vs 1.5, IQR (1.0, 2.2), P=0.001] (Table 2). We further found that the baseline TG/HDL-C ratio in all patients with COVID-19 significantly correlated with log transformed levels of plasma hs-cTnI (r=0.251, P=0.018), NT-proBNP (r=0.274, P=0.008), HbA1C (r=0.239, P=0.038), and IL-6 (r=0.218, P=0.042) (Figure 1). After stratifying the TG/HDL-C ratio into tertiles [T1 (<1.3), T2 (1.3–2.3), and T3 (≥2.3); Table 4], compared with patients in tertile 1 (T1) of the TG/HDL-C ratio, those in the higher tertile (T3) had higher levels of WBC, NEU, serum ferritin, hs-cTnI, NT-proBNP, and FBG (P<0.05 for each). Coincidentally, patients with increasing TG/HDL-C ratio had higher incidence of myocardial injury, heart failure, severe cases, and fatal outcome (P<0.05 for each, Figure 2).
Figure 1.
Correlation of the TG/HDL-C ratio with cardiometabolic and inflammatory indicators ((A) hs-CnTI; (B). NT-proBNP; (C) HbA1C; (D) IL-6.) Variables were log transformed.
Abbreviations: hs-CnTI, hypersensitive cardiac troponin I; NT-proBNP, N-terminal pro-brain natriuretic peptide; HbA1C, glycated hemoglobin; IL-6, interleukin-6.
Table 4.
Baseline Characteristics of Patients with COVID-19 Based on Tertiles of TG/HDL-C Ratio
| Characteristics | TG/HDL-C Ratio | P | ||
|---|---|---|---|---|
| T1 (<1.3) (n=32) | T2 (1.3–2.3) (n=33) | T3 (≥2.3) (n=33) | ||
| Age, years | 63.7±2.6 | 64.6±2.4 | 63.4±2.6 | 0.847 |
| Male, n (%) | 18 (31%) | 22 (38%) | 18 (31%) | 0.556 |
| White blood cell count, ×109/L | 5.1 (4.5–6.8) | 7.3 (4.8–11.6) | 8.4 (6.6–13.7) | 0.000 |
| Neutrophil count, ×109/L | 3.8 (2.6–5.6) | 5.3 (3.1–10.6) | 6.8 (4.4–12.5) | 0.001 |
| Lymphocyte count, ×109/L | 0.8 (0.7–1.2) | 0.7 (0.5–1.2) | 0.7 (0.5–1.2) | 0.524 |
| Alanine aminotransferase, U/L | 26 (13–49) | 29 (17–53) | 28 (17–41) | 0.593 |
| Aspartate aminotransferase, U/L | 27 (22–45) | 33 (22–50) | 38 (19–58) | 0.467 |
| Creatinine, μmol/L | 69 (55–87) | 70 (60–96) | 81 (66–207) | 0.081 |
| Lactate dehydrogenase, U/L | 306 (246–398) | 353 (276–522) | 456 (240–709) | 0.045 |
| High-sensitivity C-reactive protein, mg/L | 40 (2–90) | 63 (11–118) | 44 (10–180) | 0.110 |
| Serum ferritin, μg/L | 650 (285–1046) | 874 (470–1381) | 1093 (517–2719) | 0.016 |
| Procalcitonin, ng/mL | 0.16 (0.05–0.46) | 0.13 (0.09–0.72) | 0.22 (0.10–2.06) | 0.060 |
| Interleukin 6, pg/mL | 33 (6–79) | 38 (12–77) | 53 (11–137) | 0.112 |
| Hypersensitive cardiac troponin I, pg/mL | 9 (4–18) | 11 (4–195) | 30 (5–140)– | 0.048 |
| N-terminal pro-brain natriuretic peptide, pg/mL | 176 (83–630) | 486 (166–1772) | 864 (215–2476) | 0.008 |
| Fasting blood glucose, mmol/L | 5.9 (5.0–6.6) | 6.4 (5.3–10.0) | 7.4 (5.5–9.7) | 0.058 |
| Glycated hemoglobin, % | 6.1 (5.6–6.4) | 6.4 (5.9–7.3) | 6.3 (6.0–7.3) | 0.096 |
| Total cholesterol, mmol/L | 3.7 (3.0–4.2) | 3.6 (3.0–4.1) | 3.4 (2.8–4.2) | 0.643 |
| Triglycerides, mmol/L | 1.0 (0.9–1.2) | 1.4 (1.3–1.7) | 2.2 (1.8–2.8) | 0.000 |
| High-density lipoprotein cholesterol, mmol/L | 1.1 (1.0–1.1) | 0.9 (0.7–1.0) | 0.7 (0.4–0.8) | 0.000 |
Notes: Data are presented as mean±SE or median (interquartile range) for continuous variables and n (%) for categorical variables. P-values indicate differences among the tertiles of TG/HDL-C ratio.
Abbreviations: COVID-19, coronavirus Disease 2019; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; T, tertile.
Figure 2.
Frequency of COVID-19 patients with myocardial injury (A), heart failure (B), severe illness (C), and fatal outcome (D) according to tertiles of the TG/HDL-C ratio.
Independent Association Between the TG/HDL-C Ratio and Myocardial Injury, Heart Failure, Severity, and Mortality in Patients with COVID-19
Next, the independent association between the TG/HDL-C ratio and myocardial injury, heart failure, severity of disease, and mortality was determined by logistic regression analysis. As shown in Table 5, after adjusting for age, gender, lymphocyte count, HbA1C, hs-CRP, NT-proBNP, and Cr, increased TG/HDL-C ratio remained independently associated with myocardial injury, heart failure, severity, and mortality in patients with COVID-19. As seen in Table 5, in model 3, the ORs and 95% CIs for tertile 3 vs tertile 1 were: OR=20.72 (95% CI=1.57–272.99; P=0.021), OR=13.38 (95% CI=1.97–91.07; P=0.008), OR=20.71 (95% CI=1.69–279.63; P=0.018), and OR=14.81 (95% CI=1.82–120.36; P=0.012) for myocardial injury, heart failure, severity, and mortality, respectively. Additionally, when the TG/HDL-C ratio was examined as a continuous variable with each single-unit increase in the TG/HDL-C ratio, the adjusted ORs of myocardial injury, heart failure, severity, and mortality were 4.52 (95% CI=1.27–16.18; P=0.020), 2.64 (95% CI=1.17–5.94; P=0.019), 3.01 (95% CI=1.08–8.38; P=0.035), and 2.94 (95% CI=1.22–7.12; P=0.017), respectively. The independent association was also significant in the patients without diabetes (Supplemental Table 1).
Table 5.
Association of TG/HDL-C Ratio with Myocardial Injury, Heart Failure, Severity, and Mortality in All Patients with COVID-19
| TG/HDL-C | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Myocardial injury | ||||||
| T1 | 1.00 | 0.016 | 1.00 | 0.009 | 1.00 | 0.069 |
| T2 | 4.33 (1.24–15.21) | 0.022 | 4.56 (1.20–17.35) | 0.026 | 4.83 (0.57–40.75) | 0.147 |
| T3 | 6.18 (1.76–21.71) | 0.005 | 8.71 (2.18–34.74) | 0.002 | 20.72 (1.57–272.99) | 0.021 |
| Per unit increase | 1.40 (1.07–1.84) | 0.014 | 1.61 (1.17–2.23) | 0.004 | 4.52 (1.27–16.18) | 0.020 |
| Heart failure | ||||||
| T1 | 1.00 | 0.001 | 1.00 | 0.001 | 1.00 | 0.026* |
| T2 | 3.57 (1.22–10.46) | 0.020 | 3.78 (1.20–11.89) | 0.023 | 5.01 (0.91–27.54) | 0.064* |
| T3 | 7.86 (2.56–24.15) | 0.000 | 11.47 (3.26–40.40) | 0.000 | 13.38 (1.97–91.07) | 0.008* |
| Per unit increase | 1.85 (1.27–2.71) | 0.002 | 2.04 (1.36–3.06) | 0.001 | 2.64 (1.17–5.94) | 0.019* |
| Severity | ||||||
| T1 | 1.00 | 0.048 | 1.00 | 0.036 | 1.00 | 0.050 |
| T2 | 1.88 (0.70–5.01) | 0.210 | 1.77 (0.64–4.86) | 0.271 | 1.32 (0.27–6.42) | 0.732 |
| T3 | 3.67 (1.30–10.32) | 0.014 | 4.13 (1.40–12.18) | 0.010 | 20.71 (1.69–279.63) | 0.018 |
| Per unit increase | 1.51 (1.09–2.01) | 0.014 | 1.57 (1.13–2.19) | 0.008 | 3.01 (1.08–8.38) | 0.035 |
| Mortality | ||||||
| T1 | 1.00 | 0.006 | 1.00 | 0.002 | 1.00 | 0.036 |
| T2 | 3.43 (1.03–10.86) | 0.045 | 3.54 (1.01–12.55) | 0.050 | 2.12 (0.35–12.87) | 0.412 |
| T3 | 6.94 (2.13–22.65) | 0.001 | 10.87 (2.84–41.56) | 0.000 | 14.81 (1.82–120.36) | 0.012 |
| Per unit increase | 1.45 (1.10–1.93) | 0.009 | 1.65 (1.20–2.28) | 0.002 | 2.94 (1.22–7.12) | 0.017 |
Notes: Model 1: unadjusted; Model 2: adjusted for age and gender; Model 3: adjusted for age, gender, lymphocyte count, HbA1C, hs-CRP, NT-proBNP, and creatinine. *Odds ratio for heart failure was adjusted for age, gender, lymphocyte count, HbA1C, hs-CRP, and creatinine.
Abbreviations: TG/HDL-C, triglyceride/high-density lipoprotein cholesterol; COVID-19, coronavirus disease 2019; OR, odds ratio; CI, confidence interval; T, tertile.
Finally, the ROC analysis was performed to evaluate the discriminatory efficiency of the TG/HDL-C ratio for identification of risk of fatal outcome in patients with COVID-19. For prediction of fatal outcome in all the patients, the AUC value of the TG/HDL-C ratio was 0.70 (95% CI=0.60–0.79, P=0.000). When the Youden index reached the maximum, the optimal cut-off was >2.5 with a corresponding sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 47.2%, 83.9%, 63.0%, and 73.2%, respectively. In the subgroups of patients with and without diabetes, the AUC value for fatal outcome prediction was 0.725 (95% CI=0.56–0.89, P=0.008) with sensitivity, specificity, PPV, and NPV of 93.8%, 40.0%, 55.6%, and 88.9%, and 0.698 (95% CI=0.55–0.84, P=0.009) with sensitivity, specificity, PPV, and NPV of 50.0%, 85.7%, 62.5%, and 78.3%, respectively.
Discussion
In our study, we explored the associations between TG/HDL-C ratio with myocardial injury, heart failure, disease severity (expressed by ICU treatment needs), and fatal outcome in a consecutive case series of 98 patients with COVID-19. We demonstrated for the first time that patients with increasing TG/HDL-C ratio showed increased prevalence of myocardial injury, heart failure, ICU treatment needs, and fatal outcome. Baseline TG/HDL-C ratio significantly positively correlated with plasma high-sensitivity cardiac troponin I, N-terminal brain natriuretic propeptide, glycated hemoglobin, and interleukin-6 (P<0.05 for each). After adjusting for confounding factors, TG/HDL-C ratio was independently associated with an increased risk of myocardial injury, heart failure, disease severity, and mortality in patients with COVID-19.
The TG/HDL-C ratio is considered an explicit reflection of insulin resistance, which has been validated as an important risk factor for cardiovascular disease (CVD).17 The mechanisms by which insulin resistance leads to CVD are complex. First, it is generally acknowledged that as a result of insulin resistance, the islet cells attempt to lower blood sugar by secreting increasing amounts of insulin, resulting in hyperinsulinemia.18 Then, hyperinsulinemia and insulin resistance can lead to cardiovascular disease through the following mechanisms: 1) stimulates sympathetic nerve activity and leads to elevated blood pressure;19 2) increases release of cytokines, generation of plasminogen activator inhibitor 1, leucocytes adhesion, and so on, and consequently arousing inflammatory damage to vascular endothelial cells and myocardial cells and promoting atherosclerosis.20–23 In addition, elevated serum triglycerides or decreased HDL cholesterol have been demonstrated to directly contribute to endothelial damage and atherosclerosis.24,25 Through the above mechanism, increasing the TG/HDL-C ratio contributes to cardiovascular diseases including hypertension, cardiac remodeling, atherosclerosis, coronary heart disease (CHD), ischemic cardiomyopathy, and heart failure.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infects cells by binding directly to angiotensin-converting enzyme 2 (ACE2),26 which is highly expressed in alveolar epithelial cells and in the heart. It is interesting that ACE2 expression is increased in the ischemic myocardium,27 meanwhile, the SARS-CoV-2 spike protein has approximately 10- to 20-fold higher affinity than severe acute respiratory syndrome (SARS) coronavirus to ACE2.28 These two findings may reveal why heart injury is more common and more severe in patients with COVID-19 than in those with SARS, and may be one of the reasons why patients with cardiovascular disease are more likely to develop severe disease in COVID-19. In this study, we found that the prevalence of myocardial injury and heart failure, and mortality rate increased with the increase of TG/HDL-C ratio. Simultaneously, heart injury in ICU patients and non-survivors was significantly more common than in non-ICU patients and survivors. Additional mechanisms such as the direct enhancement of viral invasion capacity into cardiomyocytes by elevated triglycerides and/or reduced HDL-C need to be further explored.
Elevated TG/HDL-C ratio is positively associated with insufficient glycemic control leading to diabetes.8,9,29 Chen et al retrospectively analyzed the health check data of 114,787 adults from a Chinese study cohort, which includes 6 years of medical check data, and found that TG/HDL-C is positively associated with diabetes risk (HR=1.159, 95% CI=1.104–1.215)29. Several studies have proved that diabetes and insufficient glycemic control were positively correlated with disease severity and mortality in COVID-19.5,6,30 HbA1C as a measure of glycemic control reflects long-term glucose concentrations over the preceding months prior to admission,30 whereas fasting blood glucose after admission reflects short-term blood glucose control during hospitalization. It has been proved that fasting blood glucose was positively correlated with the incidence of multi-organ injury and poor prognosis of COVID-19, which might be explained by the following mechanisms: 1) Patients with severe disease are generally in a more severe state of stress which may elevate the fasting blood glucose;31 2) SARS-CoV-2 could directly damage the islet cells, and the damage may be more severe in severe and critically ill patients.32 In this study, both the HbA1C level (stands for glycemic control before admission) and fasting blood glucose level (expressing glycemic control during period of hospitalization) in ICU patients and non-survivors were significantly higher than those of non-ICU patients and survivors, which might be an explanation for increased frequency of ICU care needs and mortality in patients with elevated TG/HDL-C ratio.
In the pathophysiological course of infection with SARS-CoV-2, markedly increased levels of cytokines may be released from inflammatory cells and lead to cytokine storm, which can result in multiple organ dysfunction and are considered to be one of the leading causes of disease progression and death in COVID-19 patients.33 Among the different kinds of cytokines, elevation of interleukin-6 (IL-6) is considered to have predictive value in disease progression.34 Meanwhile, IL-6 has been listed as a possible therapeutic target in COVID-19, and tocilizumab or Janus kinase inhibitors for lowering IL-6 have been used as off-label treatment in patients with severe COVID-19.35 IL-6 is increased in conditions of chronic inflammation including insulin resistance, diabetes, and cardiovascular disease.19,35 Additionally, IL-6 was found to be more elevated in patients with COVID-19 with diabetes and cardiovascular disease than in those without these comorbidities.35,36 In our study, we found that IL-6 increased gradually with the increase of TG/HDL-C ratio, meanwhile, the IL-6 levels in ICU patients and non-survivors were significantly higher than in non-ICU patients and survivors. Based on the above findings, we can speculate that more severe inflammation might be an important reason for increased severity and mortality in patients with an elevated TG/HDL-C ratio.
A few limitations of this study should be acknowledged. First, this is a retrospective analysis using a single-center dataset and the sample size was relatively small. Hence, further validation is needed with a larger sample size, multi-centered study and, ideally, involving patients of multiple ethnic origins. Second, since Tongji Hospital was a designated hospital for severe cases, all the patients enrolled in this study were severe or critically ill patients defined according to the Guidelines for diagnosis and management of COVID-19 (6th edition, in Chinese) issued by the National Health Commission of China; thus, the conclusions of this study can only be generalized to patients with severe COVID-19. Third, data on the effect of metabolic control on outcome was unavailable because attention was mostly focused on treating pneumonia and metabolic disorders were overlooked, owing to the rapid emergence of the pandemic. Fourth, this study indicated that an increased TG/HDL-C ratio was positively associated with myocardial injury and heart failure, which was mainly manifested as elevated hs-cTnI and NT-proBNP levels in patients with COVID-19, however, the previous levels of hs-cTnI and NT-proBNP before SARS-CoV-2 infection were unknown in this population.
Conclusions
Our study revealed that the TG/HDL-C ratio, an indicator of insulin resistance, was independently associated with myocardial injury, heart failure, disease severity (needing ICU treatment), and fatal outcome in patients with COVID-19. Our findings suggested that evaluation of the TG/HDL-C ratio is necessary for the clinical management in adult patients with COVID-19. Close monitoring and intensive treatment may be beneficial for the patients with an elevated TG/HDL-C ratio to improve the risk of myocardial injury, heart failure, and poor outcome. However, this inference needs to be confirmed by further prospective studies.
Acknowledgments
We thank the National Natural Science Foundation of China (81700727 to L.L. and 81700207 to B.Z.) and China Diabetes Young Scientific Talent Research Project of China International Medical Foundation (2018-N-01 to L.L.). We also gratefully acknowledge all the healthcare workers on the front line and all the patients involved in the study.
Abbreviations
ACE2, angiotensin-converting enzyme 2; AST, aspartate aminotransferase; AUC, area under the ROC curve; CHD, coronary heart disease; CI, confidence interval; COVID-19, coronavirus disease; Cr, creatinine; CT, computed tomography; ESR, erythrocyte sedimentation rate; FBG, fasting blood glucose; HbA1C, glycated hemoglobin, HDL-C high-density lipoprotein cholesterol; hs-CRP, hypersensitive C-reactive protein; hs-cTnI, hypersensitive cardiac troponin I; ICAM-1, intercellular adhesion molecule 1; ICU, intensive care unit; IL-6, interleukin-6; IQR, interquartile range; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; MERS, Middle Eastern respiratory syndrome; NEU, neutrophil count; NT-proBNP, N-terminal brain natriuretic propeptide; OR, odds ratio; PCT, procalcitonin; ROC, Receiver operating characteristic curve; ROS, reactive oxygen species; RT-PCR, reverse transcription polymerase chain reaction; SARS, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SE, standard error; TC, total cholesterol; TG, triglyceride; TG/HDL-C ratio, triglyceride to high-density lipoprotein cholesterol ratio; VCAM-1, vascular cell adhesion protein 1; WBC, white blood cell count.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lew TW, Kwek TK, Tai D, et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290(3):374–380. doi: 10.1001/jama.290.3.374 [DOI] [PubMed] [Google Scholar]
- 3.Hui DS, Azhar EI, Kim YJ, et al. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18(8):e217–227. doi: 10.1016/S1473-3099(18)30127-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with Coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. doi: 10.1016/j.chest.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marotta T, Russo BF, Ferrara LA. Triglyceride-to-HDL-cholesterol ratio and metabolic syndrome as contributors to cardiovascular risk in overweight patients. Obesity (Silver Spring). 2010;18(8):1608–1613. doi: 10.1038/oby.2009.446 [DOI] [PubMed] [Google Scholar]
- 9.Hajian-Tilaki K, Heidari B, Bakhtiari A. Triglyceride to high-density lipoprotein cholesterol and low-density lipoprotein cholestrol to high-density lipoprotein cholesterol ratios are predictors of cardiovascular risk in Iranian adults: evidence from a population-based cross-sectional study. Caspian J Intern Med. 2020;11(1):53–61. doi: 10.22088/cjim.11.1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-Inigo L, Navarro-Gonzalez D, Pastrana-Delgado J, et al. Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J Hypertens. 2016;34(7):1257–1265. doi: 10.1097/HJH.0000000000000941 [DOI] [PubMed] [Google Scholar]
- 11.Farrell SW, Finley CE, Barlow CE, et al. Moderate to high levels of cardiorespiratory fitness attenuate the effects of triglyceride to high-density lipoprotein cholesterol ratio on coronary heart disease mortality in men. Mayo Clin Proc. 2017;92(12):1763–1771. doi: 10.1016/j.mayocp.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 12.Yunke Z, Guoping L, Zhenyue C. Triglyceride-to-HDL cholesterol ratio. Predictive value for CHD severity and new-onset heart failure. Herz. 2014;39(1):105–110. doi: 10.1007/s00059-013-3788-0 [DOI] [PubMed] [Google Scholar]
- 13.Wan GX, Xia WB, Ji LH, et al. Triglyceride to high density lipoprotein cholesterol ratio may serve as a useful predictor of major adverse coronary event in female revascularized ST-elevation myocardial infarction. Clin Chim Acta. 2018;485:166–172. doi: 10.1016/j.cca.2018.06.049 [DOI] [PubMed] [Google Scholar]
- 14.New coronavirus pneumonia prevention and control program (6th ed.) (in Chinese); 2020. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/fil463es/b218cfeb1bc54639af227f922bf6b817.pdf.
- 15.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21(6):715–731. doi: 10.1002/ejhf.1494 [DOI] [PubMed] [Google Scholar]
- 17.Salazar MR, Carbajal HA, Espeche WG, et al. Insulin resistance: the linchpin between prediabetes and cardiovascular disease. Diab Vasc Dis Res. 2016;13(2):157–163. doi: 10.1177/1479164115610057 [DOI] [PubMed] [Google Scholar]
- 18.Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32(8):1754–1759. doi: 10.1161/ATVBAHA.111.241885 [DOI] [PubMed] [Google Scholar]
- 19.Huggett RJ, Scott EM, Gilbey SG, et al. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circualtion. 2003;108(25):3097–3101. doi: 10.1161/01.CIR.0000103123.66264.FE [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Lee S, Zhang H, et al. Interaction of IL-6 and TNF-alpha contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS One. 2017;12(11):e187189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487 [DOI] [PubMed] [Google Scholar]
- 22.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. doi: 10.1038/nrendo.2014.29 [DOI] [PubMed] [Google Scholar]
- 23.Shrader CD, Bailey KM, Konat GW, et al. Insulin enhances proliferation and viability of human umbilical vein endothelial cells. Arch Dermatol Res. 2009;301(2):159–166. doi: 10.1007/s00403-008-0921-7 [DOI] [PubMed] [Google Scholar]
- 24.Olivecrona G, Olivecrona T. Triglyceride lipases and atherosclerosis. Curr Opin Lipidol. 2010;21(5):409–415. doi: 10.1097/MOL.0b013e32833ded83 [DOI] [PubMed] [Google Scholar]
- 25.Zhou M, Learned RM, Rossi SJ, et al. Therapeutic FGF19 promotes HDL biogenesis and transhepatic cholesterol efflux to prevent atherosclerosis. J Lipid Res. 2019;60(3):550–565. doi: 10.1194/jlr.M089961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulot JS. COVID-19 in patients with cardiovascular diseases. Arch Cardiocasc Dis. 2020;113(4):225–226. doi: 10.1016/j.acvd.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Hu H, Chen M, et al. Association of Triglyceride to high-density lipoprotein cholesterol ratio and incident of diabetes mellitus: a secondary retrospective analysis based on a Chinese cohort study. Lipids Health Dis. 2020;19(1):33. doi: 10.1186/s12944-020-01213-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rollins KE, Varadhan KK, Dhatariya K, Lobo DN. Systematic review of the impact of HbA1c on outcomes following surgery in patients with diabetes mellitus. Clin Nutr. 2016;35(2):308–316. doi: 10.1016/j.clnu.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Li H, Zhang J, et al. The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycemia with coronavirus disease 2019: a single-centre, retrospective, observational study in Wuhan. Diabetes Obes Metab. 2020;22(8):1443–1454. doi: 10.1111/dom.14086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Han Y, Nilsson-Payant BE, et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(1):125–136. doi: 10.1016/j.stem.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Fei D, Li X, et al. IL-6 may be a good biomarker for earlier detection of COVID-19 progression. Intensive Care Med. 2020;46(7):1475–1476. doi: 10.1007/s00134-020-06065-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maddaloni E, Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020;e33213321. doi: 10.1002/dmrr.3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, Dong Y, Wang H, et al. Cardiovascular disease potentially contributes to the progression and poor prognosis of COVID-19. Nutr Metab Cardiovasc Dis. 2020;30(7):1061–1067. doi: 10.1016/j.numecd.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]