Abstract
BACKGROUND:
Ureaplasma parvum infection is a prevalent cause of intrauterine infection associated with preterm birth, preterm premature rupture of membranes, fetal inflammatory response syndrome, and adverse postnatal sequelae. Elucidation of diagnostic and treatment strategies for infection-associated preterm labor may improve perinatal and long-term outcomes for these cases.
OBJECTIVE:
This study assessed the effect of intraamniotic Ureaplasma infection on fetal hemodynamic and cardiac function and the effect of maternal antibiotic treatment on these outcomes.
STUDY DESIGN:
Chronically catheterized pregnant rhesus monkeys were assigned to control (n=6), intraamniotic inoculation with Ureaplasma parvum (107 colony-forming units/mL, n=15), and intraamniotic infection plus azithromycin treatment (12.5 mg/kg twice a day intravenously, n=8) groups. At approximately 135 days’ gestation (term=165 days), pulsed and color Doppler ultrasonography was used to obtain measurements of fetal hemodynamics (pulsatility index of umbilical artery, ductus venosus, descending aorta, ductus arteriosus, aortic isthmus, right pulmonary artery, middle cerebral artery and cerebroplacental ratio, and left and right ventricular cardiac outputs) and cardiac function (ratio of peak early vs late transmitral flow velocity [marker of ventricular function], Tei index [myocardial performance index]). These indices were stratified by amniotic fluid proinflammatory mediator levels and cardiac histology.
RESULTS:
Umbilical and fetal pulmonary artery vascular impedances were significantly increased in animals from the intraamniotic inoculation with Ureaplasma parvum group (P<.05). Azithromycin treatment restored values to control levels. Amniotic fluid prostaglandin F2 alpha levels were significantly higher in animals with abnormal umbilical artery pulsatility index (>1.1) than in those with normal blood flow (P<.05; Spearman ρ=0.6, P<.05). In the intraamniotic inoculation with Ureaplasma parvum group, left ventricular cardiac output was significantly decreased (P<.001), and more animals had abnormal right-to-left ventricular cardiac output ratios (defined as >1.6, P<.05). Amniotic fluid interleukin-6 concentrations were elevated in cases of abnormal right-to-left ventricular cardiac output ratios compared with those in normal cases (P<.05).
CONCLUSION:
Fetal hemodynamic alterations were associated with intraamniotic Ureaplasma infection and ameliorated after maternal antibiotic treatment. Doppler ultrasonographic measurements merit continuing investigation as a diagnostic method to identify fetal cardiovascular and hemodynamic compromise associated with intrauterine infection or inflammation and in the evaluation of therapeutic interventions or clinical management of preterm labor.
Keywords: azithromycin, chorioamnionitis, Doppler ultrasound, preterm birth
Intrauterine infection is a major cause of early preterm birth, involved in more than 70% of births at less than 30 weeks’ gestation.1,2 Intrauterine infection occurs when microbes invade the amniotic cavity, characterized by a robust inflammatory response, chorioamnionitis, fetal inflammatory response syndrome (FIRS), and preterm labor.1,3,4 FIRS is a risk factor for adverse outcomes in preterm infants and has been linked to hypoxic-ischemic brain damage and neonatal cardiorespiratory failure.5–7 Increased amniotic fluid (AF) proinflammatory mediators double the risk of severe neonatal morbidity, including cortical white matter damage.8 Ureaplasma parvum infection is a prevalent cause of early preterm delivery that causes intrauterine and fetal inflammation, manifested by increased proinflammatory mediators in AF, increased fetal cord blood interleukin (IL)-6, and fetal lung injury.9,10
Antibiotics and anti-inflammatory agents are therapies for intrauterine infection that can reduce fetal inflammation and delay preterm delivery.11–13 In the current nonhuman primate (NHP) model of intrauterine U parvum infection, we found that maternal azithromycin treatment effectively clears intraamniotic Ureaplasma infection in an average of 4 days, inhibits preterm labor, and reduces the severity of histologic chorioamnionitis and fetal lung injury.14 Furthermore, recent studies in women have reported that maternal antibiotic treatment can resolve intrauterine infection and delay premature labor without short-term neonatal sequelae.15,16 However, the risk of long-term adverse consequences continues to be a clinical concern when using antibiotics to treat preterm labor because of the potential for residual inflammation.17–19 Therefore, methods to evaluate fetal well-being during and after treatment could inform the use of antibiotics for intrauterine infection and preterm labor.
Multiple studies have found that fetal hemodynamic and cardiovascular dysfunction occurs in the setting of intrauterine infection, inflammation, and FIRS,20–25 which could be useful for identifying fetal inflammation by ultrasonography.26–28 For example, fetal ventricular filling characteristics are considered an indirect measure of cardiac diastolic function, whereas the Tei index takes into account both diastolic and systolic functional properties and is used as an indicator of global cardiac function.29,30 Abnormalities in fetal hemodynamic indices that indicate blood flow impedance in fetal vessels (eg, umbilical artery [UA] pulsatility index [PI] or middle cerebral artery [MCA] PI) can be indicative of poor outcomes31 but have not been used or well defined for intrauterine infection.
Our objective was to use Doppler ultrasonography, in an NHP model, to assess the effect of intraamniotic Ureaplasma infection and maternal antibiotic therapy on fetal hemodynamic and cardiac function. We hypothesized that fetal hemodynamic compromise in the setting of intrauterine infection will improve with antibiotic treatment and that Doppler ultrasound may be a useful aid in the evaluation of therapeutic interventions for preterm labor.
Materials and Methods
Ethics statement
Animal studies were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University West Campus and performed in strict accordance with the Animal Welfare Act and Regulations and the recommendations in the Guide for the Care and Use of Laboratory Animals published by the National Research Council. All operations were performed in keeping with best veterinary practices, and all efforts were made to minimize pain and discomfort.
Animal model
Animals were allocated to the study by assignment from the Oregon National Primate Research Center (ONPRC) breeding colony and divided into control (control, n=6), intraamniotic infection with U parvum (IAI, n=15), and intraamniotic infection plus antibiotic treatment (AZI, n=8) groups. Doppler ultrasound assessments of fetoplacental blood flow and fetal cardiac function were performed.
Using a long-established NHP model,32 time-mated pregnant rhesus monkeys (Macaca mulatta) were adapted to a vest and mobile catheter protection system before intrauterine surgery was performed at 110±8 days’ gestation to implant catheters into the amniotic cavity and maternal femoral artery and vein.14,33 A standard postoperative regimen of intravenous antibiotics (cefazolin sodium) and tocolytic medications (terbutaline sulfate, atosiban) was administered.4,14 Fetuses were delivered by cesarean delivery based on the signs of imminent preterm labor (uterine activity, cervical dilatation) or gestational age (GA) for neonatal survival studies.
Ureaplasma parvum for intraamniotic inoculation
At 123±6 days’ gestation (term gestation=165 days), animals in the IAI and AZI groups were inoculated, through intraamniotic catheters, with a low-passaged clinical isolate of U parvum serovar 1 (1 mL of 1.4×105–7 colony-forming units (CFU)/mL in 2-sucrose-phosphate media supplied by the Mycoplasma Laboratory, University of Alabama at Birmingham, Birmingham, AL). Animals in the control group received sterile media. After 15±7 days of inoculation, animals from the AZI group received maternal azithromycin treatment (intravenous infusion of 12.5 mg/kg, every 12 hours for 10 days). For the IAI plus AZI group, ultrasound studies were performed in animals after 2–12 days of azithromycin therapy (average length of treatment when ultrasonography was performed was 4 days).
Ultrasound imaging
All scans were performed at ONPRC (by J.P.R. and A.E.F.) using a standardized protocol and blinded to treatment group. Image-directed pulsed and color Doppler ultrasonography (GE Voluson 730 Expert, Kretztechnik, Zipf, Austria) was used as published34 to obtain fetoplacental hemodynamic and cardiac measurements. Pregnant animals were sedated with intramuscular administration of 10 mg/kg ketamine and placed in the dorsal recumbency. All animals received the same sedation protocol, and vital signs remained stable throughout each procedure.
Measurements of fetal hemodynamics
Blood flow velocity waveforms of the UA, MCA, right pulmonary artery (RPA), aortic isthmus, ductus arteriosus, descending aorta, inferior vena cava, left hepatic vein, and ductus venosus were obtained. The PI ([peak systolic velocity-end-diastolic velocity]/time-averaged maximum velocity) was determined in each vessel. Cerebroplacental ratio (CPR=MCA-PI/UA-PI) was also calculated.
UA-PI was considered abnormal if it exceeded 1.1, based on data from human studies35 taking the 90th percentile of the UA-PI in the third trimester of human pregnancy because there is no standard available in NHPs. Using this threshold, all control animals were in the reference range.
Measurements of fetal cardiac function
Echocardiography was performed to assess fetal cardiac function.34 Peak E and A wave velocities were recorded for atrioventricular valves, and the tricuspid and mitral valve E/A ratios calculated. To measure the left ventricular Tei index, mitral and aortic valve blood flow velocity waveforms were simultaneously obtained. The Tei index was calculated as follows: isovolumic contraction time-+isovolumic relaxation time/ejection time.36 To measure ventricular shortening fractions (SFs), the end-diastolic dimension (EDD) and end-systolic dimension (ESD) of the left and right ventricles were measured by M-mode technique.37 The SFs of the left and right ventricles were derived using the following equation: SF=([EDD-ESD]/EDD)×100.37 Cardiac output (CO) was calculated by measuring the diameter (d) of the aortic and pulmonary valves twice on frozen real-time images taken during systole using the leading-edge-to-edge method. Mean values were used in analysis. Aortic and pulmonary valve velocity time integrals (VTIs) and heart rates (HRs) were calculated. Left and right COs were derived from the equation: CO (mL/min)=VTI (cm)×π×d (cm)2/4×HR (beats/min).38 We considered the right-to-left CO ratio (RCO:LCO) greater than 1.6 as abnormal based on data from human studies.39 Fetal biometrics, including biparietal diameter, abdominal circumference, and femur length, were also assessed.
Quantification of amniotic fluid and fetal blood proinflammatory cytokines and prostaglandins
Serial AF samples were collected, and the concentrations of proinflammatory mediators tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and prostaglandins (PGE2 and PGF2α) were measured by enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions.14,33 For control and IAI groups, the peak values were selected from time points between 130 and 150 days GA, and for the AZI group, the peak values from 48 hours after treatment to 2 days before delivery were chosen. Fetal cord blood IL-6 concentrations were measured for n=4 per group. ELISA intra- and interassay coefficients of variance were <5% and <15%, respectively.
Statistical analyses
SPSS version 19.0 (SPSS Institute, Chicago, IL) and Prism 6 for Mac OSX (GraphPad Software Inc, San Diego, CA) were used for statistical analyses. Discrete data were analyzed using chi-square or Fisher exact tests and continuous variables using a one-way analysis of variance (ANOVA) or Student’s t-test for normally distributed data (as determined by D’Agostino-Pearson omnibus normality test) and Kruskal-Wallis or Mann-Whitney U tests for nonparametric data as appropriate. For analysis of proinflammatory mediators measured by ELISA, the lowest detectable value was used for all samples that had values below this level. Post hoc Bonferroni or Fisher least significant difference was performed to identify differences by group in cases where one-way ANOVA was P<.05, and Dunn multiple comparison test was used for nonparametric data. Quartile regression was used to estimate in 25%, 50%, and 75% quartile of concentrations of fetal and AF proinflammatory mediators for each group. Comparison between groups at each quartile was based on rank tests. Normal, Wilcoxon, and sign functions were implemented and suitable for iid error models, and tau score function was implemented and appropriate for non-iid error models. SAS 9.4 (SAS Institute Inc, Cary, NC) was used for analysis. Correlations between ultrasound measurements and proinflammatory mediator levels were calculated using Spearman rank correlation. P<.05 was considered statistically significant.
Results
There were no differences in GA, fetal sex, biparietal diameter, abdominal circumference, femur length, or fetal HR among the 3 groups (Table 1).
TABLE 1.
Fetal biometry, heart rate, and sex distribution
| GA (d) | 133±3 | 134±4 | 136±5 | .433 |
| BPD (mm) | 46.0±1.4 | 44.2±2.1 | 44.3±1.9 | .156 |
| AC (mm) | 134.9±7.1 | 129.6±11.6 | 133.2±21.0 | .752 |
| FL (mm) | 36.2±1.8 | 35.5±2.5 | 37.2±2.1 | .332 |
| Fetal heart rate (bpm) | 181 ±29 | 181 ±13 | 179±7 | .960 |
| Fetal sex (male) | 42.9% | 53.3% | 37.5% | .749 |
P<.05 was considered significant. Data are expressed as mean±SD or %.
AC, abdominal circumference; BPD, biparietal diameter; bpm, beats per minute; FL, femur length; GA, gestational age; IAI, intraamniotic infection; IAI+AZI, azithromycin treatment group.
Increased umbilical and pulmonary artery impedance in animals exposed to infection
UA-PI was significantly elevated in the IAI group compared with that in the control group (P<.01) (Table 2). Significantly more IAI animals also had UA impedance in the abnormal range (UA-PI>1.1) than did control animals. Pulmonary artery impedance (RPA-PI) was significantly elevated in the IAI group compared with both that in both control and IAI plus AZI animals (P=.034) (Table 2). Descending aorta PI was significantly elevated in the AZI animals compared with that in the control group (P<.05). There were no significant differences in the MCA, the ductus arteriosus, and aortic isthmus PI values or in the CPR among the 3 groups (Table 2).
TABLE 2.
Uteroplacental and fetal hemodynamic parameters
| UA-PI | 0.95±0.09 | 1.26±1.84* | 1.10±0.15 | .008 |
| Abnormal UA-PI (>1.1) | 0.0% | 78.6%* | 50.0% | .017 |
| MCA-PI | 1.48±0.32 | 1.45±0.14 | 1.47±0.12 | .970 |
| CPR | 1.30±0.19 | 1.18±0.19 | 1.24±0.19 | .471 |
| RPA-PI | 6.28±3.07 | 10.05±3.52*,# | 6.71 ±2.39 | .034 |
| Ductus arteriosus PI | 2.30±0.33 | 2.40±0.39 | 2.55±0.39 | .551 |
| Aortic isthmus PI | 1.96±0.33 | 3.06±1.32 | 3.55±1.53 | .110 |
| Descending aorta PI | 1.43±0.18 | 1.89±0.37 | 2.08±0.52^ | .017 |
P<.05 was considered significant by one-way analysis of variance. Symbols indicate significant differences between groups by post hoc analysis:
Control vs IAI;
Control vs IAI+AZI;
IAI vs IAI+AZI. Data are expressed as mean±SD or %.
CPR, cerebroplacental ratio; IAI, intraamniotic infection; IAI+AZI, azithromycin treatment group; MCA, middle cerebral artery; PI, pulsatility index; RPA, right pulmonary artery; UA, umbilical artery; UtA, uterine artery.
Decreased left ventricular CO in fetuses exposed to infection
Left ventricular CO (LCO) was significantly decreased in the IAI group compared with that in the control and AZI groups (P<.001) (Table 3). When the ratio of bilateral CO (RCO:LCO) was calculated, there were significantly more animals with abnormal left-to-right CO ratios (RCO:LCO>1.6) in the IAI vs control and AZI groups (P<.05). The mitral valve E:A ratio was decreased in the IAI group (P<.05) (Table 3). There were no significant differences in other measurements of cardiac function among the 3 groups.
TABLE 3.
Fetal cardiac functional parameters and cardiac outputs
| Tricuspid E:A ratio | 0.74±0.09 | 0.80±0.11 | 0.74±0.16 | .577 |
| Mitral valve E:A ratio | 0.73±0.08 | 0.68±0.09# | 0.81 ±0.10 | .045 |
| Left ventricular TEI | 0.47±0.04 | 0.45±0.08 | 0.40±0.07 | .235 |
| Right SF (%) | 29.22±4.22 | 38.05±10.51 | 33.34±5.76 | .077 |
| Left SF (%) | 29.95±3.18 | 34.98±9.79 | 32.89±10.67 | .079 |
| Right SF/left SF | 1.77±1.15 | 1.16±0.44 | 1.11 ±0.44 | .987 |
| RCO (mL/min) | 92.61 ±27.60 | 100.04±15.82 | 109.86±26.76 | .989 |
| LCO (mL/min) | 93.48±18.03 | 61.93±12.36*,# | 72.98±8.29 | <.001 |
| CCO (mL/min) | 189.14±62.67 | 162.52±24.73 | 169.92±31.89 | .369 |
| RCO:LCO | 1.33±0.21 | 1.60±0.33 | 1.38±0.21 | .092 |
| Abnormal RCO:LCO (>1.6) | 0.0% | 53.3%*,# | 27.6% | .022 |
P<.05 was considered significant by one-way analysis of variance. Symbols indicate significant differences between groups by post hoc analysis:
Control vs IAI;
IAI vs IAI+AZI. Data are expressed as mean±SD or %.
AZI, azithromycin treatment group; CCO, combined cardiac output; E:A ratio, ratio of peak velocity of early diastolic transmitral flow to the peak velocity of late diastolic transmitral flow; IAI, intraamniotic infection; LCO, left cardiac output; RCO, right cardiac output; SF, shortening fraction; TEI, isovolumic contraction time plus isovolumic relaxation time divided by ejection time.
Amniotic fluid and fetal blood proinflammatory mediators are elevated in animals with placental hemodynamic and fetal cardiac changes
The peak concentrations in AF of all proinflammatory mediators (Table 4) were significantly increased with infection (P<.05) at the 25%, 50%, and 75% quartiles. Concentrations of TNF-α, IL-1β, IL-6, PGE2, and PGF2α were also significantly elevated in the IAI plus AZI group, compared with those in the control group, at the 50% and 75% quartiles; however, these values were not significantly increased at the 25th percentile.
TABLE 4.
Estimation by quartile regression of amniotic fluid proinflammatory mediators following intraamniotic infection and azithromycin treatment
| Estimate | Lower | Upper | Estimate | Lower | Upper | Estimate | Lower | Upper | IAI vs Con | IAI+AZI vs Con | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNF-α | 25% quartile | 0.47 | −∞ | 0.47 | 0.47 | 0.47 | 44.92 | 20.50 | −∞ | 116.33 | .0155 | .0821 |
| 50% quartile | 0.47 | 0.47 | 0.47 | 54.20 | 18.25 | 266.45 | 84.70 | 24.79 | 211.68 | <.0001 | .0006 | |
| 75% quartile | 0.47 | 0.47 | ∞ | 323.60 | 74.21 | 1681.11 | 202.00 | 116.17 | ∞ | <.0001 | <.0001 | |
| IL-1β | 25% quartile | 0.17 | −∞ | 0.17 | 0.17 | 0.17 | 6.21 | 0.40 | −∞ | 20.06 | .0155 | .0821 |
| 50% quartile | 0.17 | 0.17 | 0.17 | 11.60 | 1.26 | 119.23 | 17.30 | 0.51 | 152.35 | <.0001 | .0006 | |
| 75% quartile | 0.17 | 0.17 | ∞ | 145.60 | 27.40 | 468.69 | 24.00 | 20.04 | ∞ | <.0001 | <.0001 | |
| IL-6 | 25% quartile | 1.38 | −∞ | 1.38 | 1.38 | 1.38 | 13638.74 | 1.38 | −∞ | 1382.55 | .0216 | .0989 |
| 50% quartile | 1.38 | 1.38 | 1.38 | 14281.60 | 57.88 | 40579.90 | 1598.20 | 0.95 | 76125.50 | <.0001 | .0010 | |
| 75% quartile | 1.38 | 1.38 | ∞ | 47130.60 | 25674.59 | 60766.71 | 45858.60 | 1483.25 | ∞ | <.0001 | <.0001 | |
| PGE2 | 25% quartile | 15.00 | −∞ | 15.00 | 15.00 | 15.00 | 187.41 | −0.14 | −∞ | −0.08 | .0278 | .2736 |
| 50% quartile | 15.00 | 15.00 | 88.68 | 196.70 | 15.91 | 1006.35 | −1.19 | −∞ | ∞ | <.0001 | .0363 | |
| 75% quartile | 15.00 | 15.00 | ∞ | 1134.60 | 368.62 | 3258.51 | −0.14 | −∞ | ∞ | <.0001 | .0001 | |
| PGF2α | 25% quartile | 10.00 | −∞ | 10.00 | 10.00 | 10.00 | 90.52 | 0.10 | −∞ | 216.79 | .0216 | .2636 |
| 50% quartile | 10.00 | 10.00 | 10.00 | 176.00 | −∞ | 567.37 | 0.10 | −∞ | ∞ | <.0001 | .0183 | |
| 75% quartile | 10.00 | 10.00 | ∞ | 596.80 | 270.47 | 912.72 | 173.80 | 3.36 | In | <.0001 | <.0001 |
P<.05 was considered significant.
AZI, azithromycin treatment group; IAI, intraamniotic infection; IL, interleukin; ND, not detectable; PG, prostaglandin; TNF, tumor necrosis factor.
Based on rank tests. Normal, Wilcoxon, and sign functions are implemented and suitable for iid error models, and tau score function is implemented and appropriate for non-iid error models.
We also examined intraamniotic inflammation in reference to UA impedance values and CO. In animals with abnormal UA impedance (defined as UAPI>1.1) (Figure 1, A), PGF2α concentrations were significantly higher than in animals with normal UA-PI values (P<.01) (Figure 1, B). The correlation coefficient (Spearman, ρ) between UA-PI and PGF2α was 0.7 (P<.05). AF PGE2 was also significantly higher with abnormal UA-PI values (P<.05). In animals with abnormal RCO:LCO ratios (shown in Figure 2, A), AF IL-6 concentrations were significantly higher than in those with normal output ratios (P<.05) (Figure 2, B).
FIGURE 1. Amniotic fluid proinflammatory cytokines for animals with normal and abnormal umbilical artery pulsatility index.
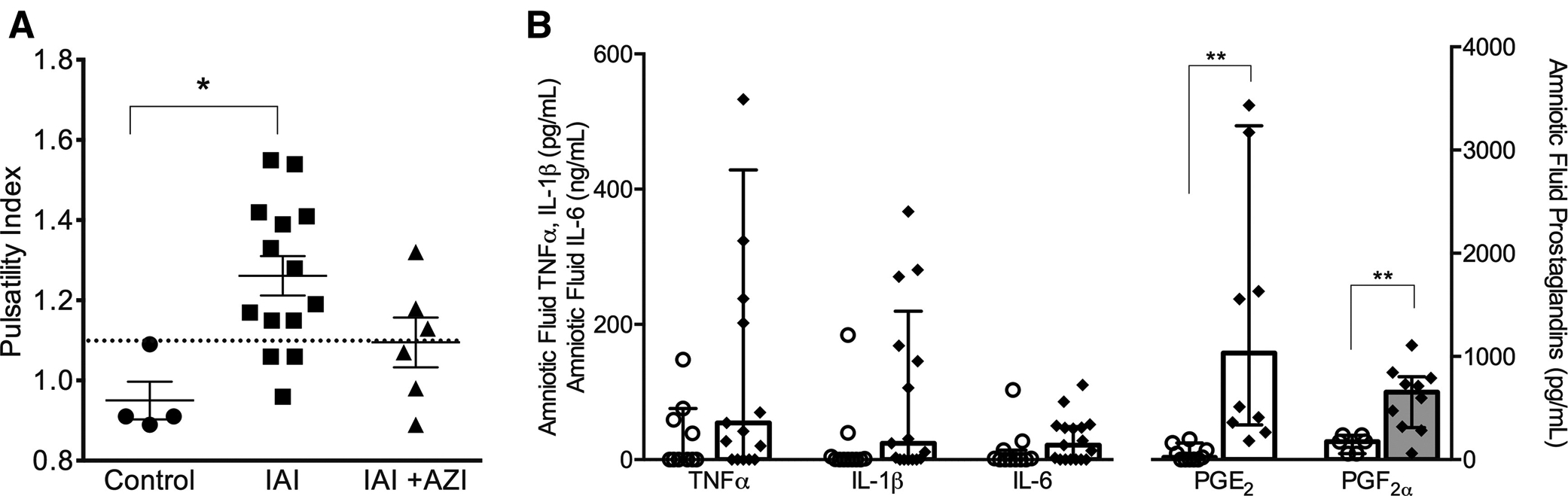
A, Umbilical artery pulsatility index (PI) is shown for animals in control, with intraamniotic infection, and with intraamniotic infection and maternal azithromycin treatment. The threshold for abnormal values was PI >1.1 (indicated by dotted horizontal line). Values are expressed as mean±SEM. * indicates significant difference between controls and IAI animals (P<.05). B, The concentrations of amniotic fluid proinflammatory mediators are shown for animals with normal (open circles) vs abnormal (diamonds) umbilical artery PI values. Values are expressed as median (interquartile range) with statistical difference in mediator concentration indicated by *P<.05 or ** P<.01.
IAI, intraamniotic infection; IAI+AZI, intraamniotic infection and maternal azithromycin treatment; IL, interleukin; PG, prostaglandin; TNF, tumor necrosis factor.
FIGURE 2. Concentrations of amniotic fluid proinflammatory cytokines for animals with normal and abnormal cardiac output ratios.
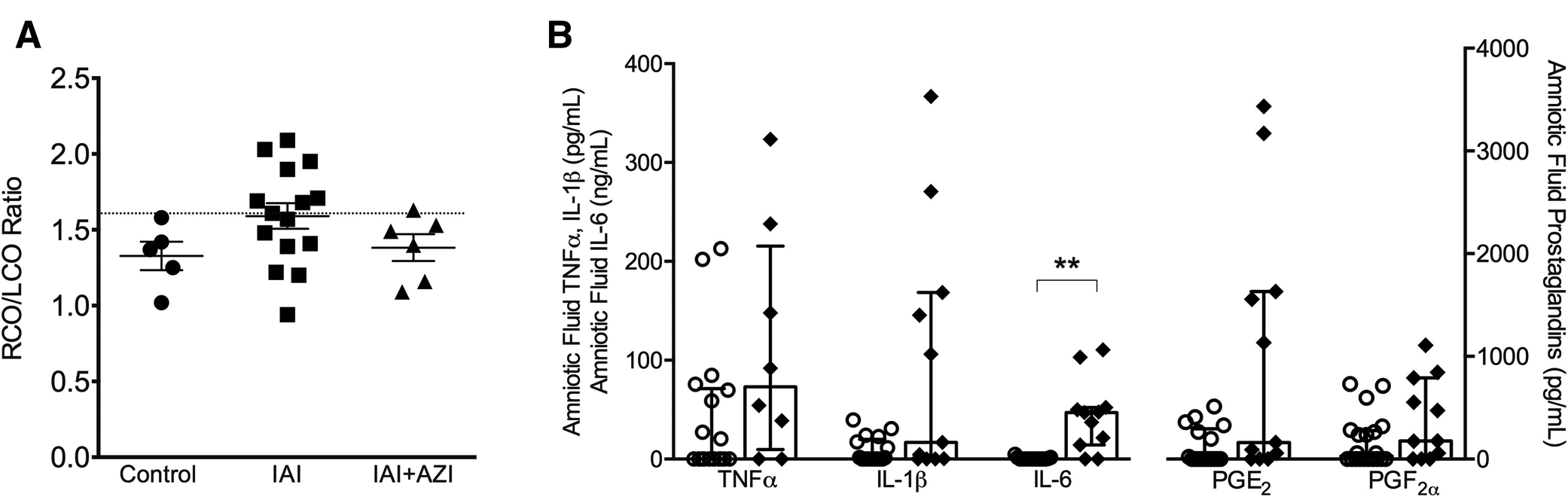
A, The ratio of right to left ventricular cardiac output is shown for animals in control, with intraamniotic infection, and with intraamniotic infection and maternal azithromycin treatment (IAI+AZI). The threshold for abnormal values was pulsatility index of >1.6 (indicated by dotted horizontal line). Values are expressed as mean±SEM. B, The concentrations of amniotic fluid proinflammatory mediators are shown for animals with normal (open circles) vs abnormal (diamonds) RCO:LCO ratios. Values are expressed as median (interquartile range) with statistical difference in mediator concentration indicated by **P<.01.
IAI, intraamniotic infection; IAI+AZI, intraamniotic infection and maternal azithromycin treatment; IL, interleukin; PG, prostaglandin; RCO:LCO, ratio of right to left ventricular cardiac output; TNF, tumor necrosis factor.
Discussion
Principal findings
Our Doppler ultrasound study in an NHP model of intraamniotic U parvum infection and preterm labor identified fetal and placental hemodynamic alterations that were associated with intrauterine infection and AF proinflammatory mediators. Principal findings of the study were that intraamniotic Ureaplasma infection was associated with (1) increased vascular impedance in the umbilical and fetal pulmonary arteries, (2) a decrease in fetal LCO, and (3) an association between intrauterine inflammation (AF proinflammatory mediators) and these fetal hemodynamic changes. Finally, we demonstrated that maternal azithromycin treatment partially corrected fetal CO and vascular impedance in the placental and pulmonary circulations. Taken together, these data indicate the efficacy of maternal antibiotic treatment to mitigate the fetal hemodynamic consequences of intrauterine infection, as well as the potential clinical utility of Doppler ultrasonography in detecting fetal hemodynamic compromise associated with intrauterine infection.
Results in the context of what is known
Fetal hemodynamic function has not previously been assessed with reference to the treatment of intrauterine infection in animal models or in women. However, clinical and animal studies have previously linked inflammation and chorioamnionitis with fetal hemodynamic alterations and cardiac dysfunction. Furthermore, reports of measurement of fetal hemodynamic outcomes in clinical studies have mostly been limited to pregnancies associated with preterm premature rupture of membranes (pPROM)40,41 rather than confirmed cases of intrauterine infection. The main findings in this study were hemodynamic alterations associated with intrauterine infection (ie, increased UA impedance). CO was altered, although it is suggested that this is due to reductions in venous return in the fetus because functional indices of cardiac function, such as the Tei index, were not altered (Figure 3).
FIGURE 3. Conceptual model of fetal hemodynamic responses to intraamniotic infection.
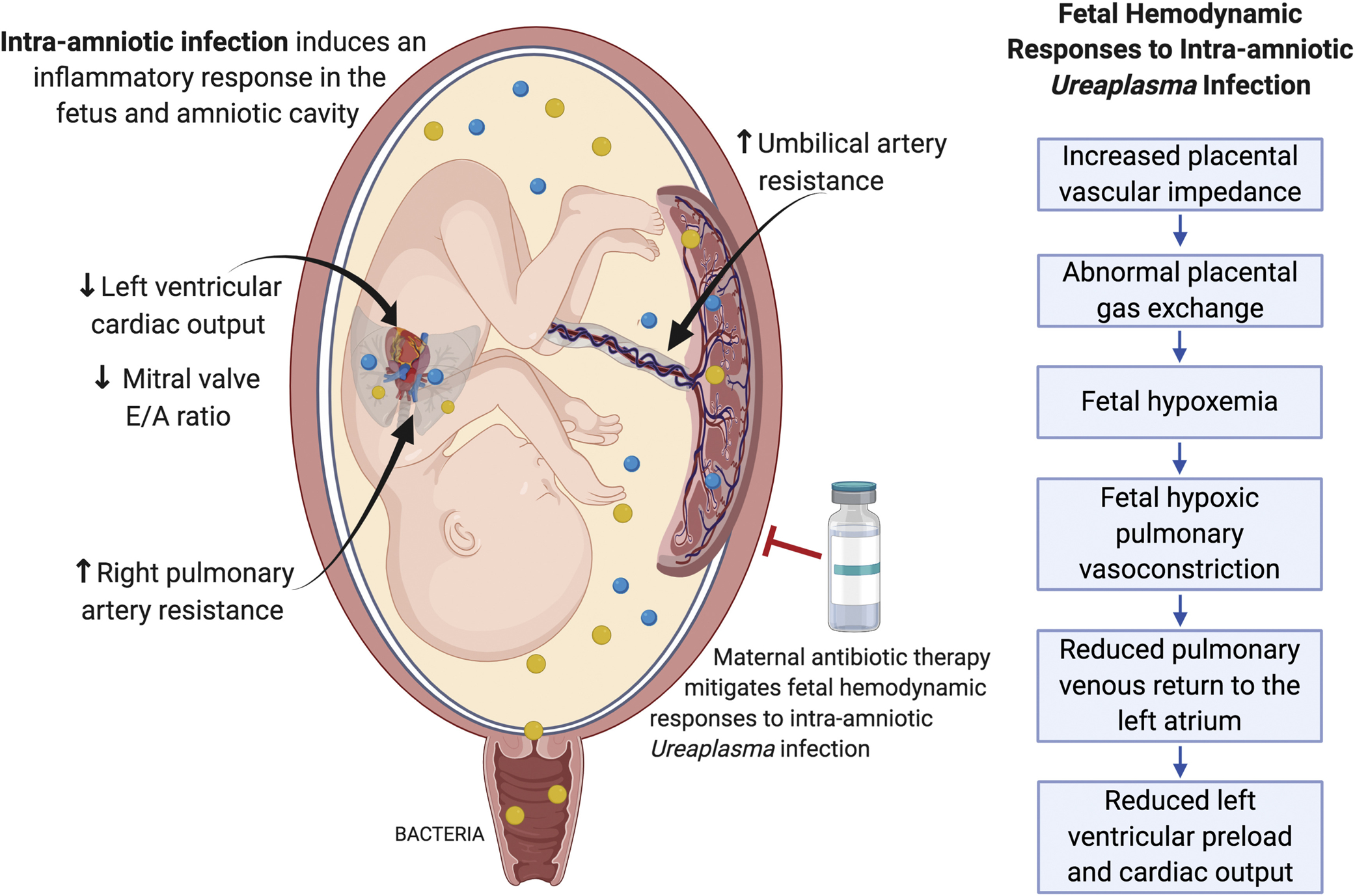
Bacteria ascend from the lower reproductive tract and invade the amniotic cavity, resulting in an immune response and production of proinflammatory mediators that can be detected in the amniotic fluid. Microbial invasion of the amniotic cavity causes a fetal inflammatory response and fetal hemodynamic changes that likely contributes to an increased risk of poor outcomes for preterm infants exposed to intrauterine infection and inflammation. Maternal antibiotic therapy mitigated the fetal hemodynamic changes observed in our nonhuman primate model of intraamniotic Ureaplasma infection, suggesting that appropriate antimicrobial treatments may be able to rescue fetal injury in addition to delaying infection-associated preterm labor.
Fetal hemodynamic responses to intrauterine infection
Given the observations in this study, we hypothesized that uterine and placental inflammation, which occurs in the presence of intrauterine Ureaplasma infection, results in perturbation of placental circulation, as evidenced by increased UA vascular impedance, which we proposed leads to abnormal gas exchange and subsequent fetal hemodynamic responses and reduced CO (Figure 3). Previous studies have linked increased umbilical vascular impedance with increased circulating maternal and fetal cytokines, placental vascular congestion, and inflammation in the endothelium of the fetoplacental microcirculation in cases of placental insufficiency.42–45 Decreased placental blood flow has also been reported in fetal sheep exposed to lipopolysaccharide as an inflammatory stimuli.46,47 Clinically, increased UA resistance is a well-established indicator of placental compromise and of poor perinatal outcome, which is routinely monitored in high-risk pregnancies by Doppler ultrasound. Therefore, the increase in UA impedance identified in this study suggests impacts on placental function in the setting of intrauterine Ureaplasma infection with potential consequences on fetal well-being.
Fetal hypoxemia is a potential consequence of placental compromise and an important mediator of fetal hemodynamic responses. We suggest that placental compromise leading to fetal hypoxemia results in the increase in pulmonary artery impedance observed with infection (ie, elevated RPA-PI), attributed to the processes of hypoxic pulmonary vasoconstriction.34 Fetal lung injury caused by Ureaplasma infection may also exacerbate these effects.48,49 Pulmonary vasoconstriction reduces the available blood volume for venous return to the left atrium, thus decreasing left ventricular preload. A reduction in left ventricular preload could then result in reduced LCO with intraamniotic Ureaplasma infection observed in this study. The foramen ovale, which shunts oxygenated blood from the right to the left atrium bypassing the fetal pulmonary circulation, functions close to its maximum capacity50,51; hence, it cannot increase left atrial flow to compensate for the drop in pulmonary venous return. The finding of decreased mitral valve E:A ratio in the infected group (ie, abnormal left ventricular filling) further supports the concept of reduced left ventricular preload contributing to decreased left CO in this model. Other potential indicators of fetal hypoxemia, such as reduced fetal cerebral blood flow impedance (MCA-PI) or reduction in CPR,52,53 were not observed in this study. However, it has previously been shown in human preterm infants that neither chorioamnionitis nor elevated circulating cytokines are associated with changes in Doppler MCA blood flow velocities.21
Alterations in fetal cardiac function were not identified in animals exposed to intraamniotic infection, despite previous studies in women and animal models reporting that cardiac performance could be impaired with intrauterine infection and inflammation, including signs of fetal congestive heart failure.20,54,55 In this study, the left ventricular Tei index, which detects changes in myocardial performance (eg, cardiac contractility and relaxation parameters), was similar between the groups, suggesting that reduction in LCO was not due to direct cardiac dysfunction. The absence of histologic evidence on myocardial injury supports this hypothesis. In addition, measures of right ventricular cardiac function remained normal. Therefore, we propose that decreases in mitral valve E:A ratio and LCO with infection observed in this study are due to fetal hemodynamic changes rather than abnormal myocardial performance. Altered fetal blood flow distribution and reduced CO may have significant consequences on fetal well-being and by reducing blood flow to peripheral organs may contribute to adverse postnatal outcomes and underdevelopment of organ systems that are particularly vulnerable to injury associated with Ureaplasma infection, intrauterine inflammation, and prematurity (eg, necrotizing enterocolitis, bronchopulmonary dysplasia).56,57
Proinflammatory mediators associated with fetal hemodynamic responses
In this study, we have demonstrated that abnormal distribution of CO and increased UA vascular impedance were correlated with increased AF levels of IL-6 and PGF2α, respectively. Azithromycin treatment of intraamniotic infection reduces AF concentrations of proinflammatory cytokines and fetal cord blood IL-6, which is associated with a delay in preterm labor and diminished signs of chorioamnionitis14 and improvement in fetal hemodynamic function observed in this study. Intrauterine, placental, and fetal inflammation are associated with fetal hemodynamic function in this model.
Antibiotic treatment of infection-associated preterm labor
Our results extend previous studies on fetal cardiovascular function in cases of intrauterine infection and inflammation to address the question of whether antimicrobial treatment of intrauterine infection—to delay preterm labor—can also improve the negative consequences of fetal inflammation. Randomized controlled trials of antibiotic treatment for preterm labor in women have produced mixed results and identified potential adverse outcomes for the fetus.19,58,59 More success has been reported in prolonging gestation without negative consequences on the fetus in cases of pPROM.60 Infection is a significant cause of preterm labor, present in up to half of preterm births earlier than 28 weeks of gestation. However, trials and epidemiologic studies are not always able to select patients with confirmed intrauterine infection, which potentially limits the ability of large clinical studies to identify effective treatments. Azithromycin has demonstrated anti-inflammatory actions61 and has been found to cross the placenta and accumulate in AF after maternal intravenous administration,62 which may directly treat inflammation in the placenta and fetal lung and therefore improve the negative fetal hemodynamic consequences of infection. Thus, the observation in this study that maternal azithromycin treatment mitigates changes in fetal hemodynamics in a translational model of known infection may have important clinical relevance to the use of antibiotic treatment for preterm labor.
Clinical implications
The findings of this study support the use of maternal antibiotic therapy for intrauterine infection to improve fetal outcomes and suggest that using Doppler ultrasound to monitor fetal well-being, as occurs commonly for pregnancies at high risk for fetal growth restriction, may aid the clinical management of women in preterm labor. However, Doppler ultrasound parameters have not been well defined for the diagnosis of intrauterine infection and inflammation. A recent study of ultrasound assessment for pPROM management found no differences in MCA-PI and CPR and only a slight increase in the UA-PI values in women with ruptured membranes with suspected chorioamnionitis.40 Although we found no changes in MCA or CPR indices in this study, with a known infection in a clinically relevant NHP model, we did identify significantly increased UA impedance, with most animals in the infection group in a defined abnormal range for this index. Recent clinical studies evaluating the use of antibiotics for the treatment of preterm labor highlighted the importance of direct identification of intrauterine infection by amniocentesis for evaluating the efficacy of antibiotic treatment to target infection-associated preterm labor.15,16,63 The choice of antimicrobial agent is also an important consideration because of microbial sensitivity and development of potential resistance. Studies in women have suggested that newer generation macrolide antibiotics, such as clarithromycin, may have more efficacy with better neonatal outcomes.64
Research implications
Cardiovascular decline in the fetus after intrauterine infection may contribute to injury in multiple organ systems by exacerbating the effects of inflammation on these tissues. Further study on fetal brain, lung, gut, and other organs along with placental vascular changes may provide information about the sensitivity of the fetus to alterations in blood flow during infection and after antibiotic treatment. In vivo imaging of placental blood oxygen concentrations—techniques used in an NHP model of Zika virus infection—has potential for evaluating functional placental gas exchange in cases of intrauterine Ureaplasma infection.65,66 In vivo measurement of fetal oxygenation during infection and postnatal pulmonary function may also provide evidence of mechanisms involved in fetal hemodynamic responses to infection that we identified in this study. Furthermore, Mitchell et al67 recently reported elevated levels of IL-6 and IL-8 in the myocardium and changes in the expression of cardiac genes in fetal monkeys exposed to intrauterine infection despite a lack of histopathologic inflammation, suggesting that fetal cardiac inflammation may be present without overt evidence of injury. Evaluation of postnatal cardiovascular function will be important in determining whether programming of postnatal cardiovascular disease occurs in this model, despite absence of histologic cardiac injury, and whether hemodynamic improvements observed in the fetal period with antibiotic treatment persist after birth.
Strengths and limitations
The strength of this study lies in our utilization of clinically relevant Doppler techniques in a translational animal model to examine direct fetal consequences of antibiotic treatment for intrauterine infection. NHPs share many similarities with human pregnancy (placentation, endocrinology, labor mechanisms). These advantages, our ability to perform serial AF sampling, the direct application of Doppler measurements used in human pregnancies, and the known timing of intrauterine infection overcome some of the limitations of epidemiologic studies (eg, amniocentesis to confirm infection, retrospective data analysis). Furthermore, our NHP model recapitulates clinical features of infection-associated preterm labor with a robust amniotic and fetal inflammatory response, increased uterine contractility, cervical effacement, chorioamnionitis, and fetal lung injury.4,14 However, because of the clinical nature of this model, a limitation we faced was in coordinating the timing of treatment (based on clinical signs of preterm labor) with ultrasound time points. For this reason, sample size was not uniform between the infection and treatment groups. In addition, the mode of infection of direct AF inoculation used in this study potentially bypasses the early stages of ascending reproductive tract infection. This is a limitation of this experimental model compared with natural infection in women; however, this is balanced by the known timing of infection, which is difficult to ascertain in observational clinical studies in women with intrauterine infection. Ongoing studies by the authors also include choriodecidual Ureaplasma inoculation to replicate an earlier stage of ascending intrauterine infection in this NHP model. We also acknowledge that the direct mechanisms involved in inducing fetal hemodynamic compromise have not been addressed in this study and in particular the examination of placental vascular alterations—a potential mediator of the fetal hemodynamic responses to infection we observed—may be important to our understanding and implementation of therapeutic strategies.
Conclusion
Our study advances the current understanding of mechanisms of FIRS as it evaluates changes in fetal hemodynamic and cardiac function in NHPs exposed to intrauterine Ureaplasma infection and with subsequent antibiotic therapy. This study supports the use of maternal antibiotic therapy for the treatment of intrauterine infection and suggests that Doppler ultrasound may be a useful tool for identifying inflammation-mediated fetal cardiovascular dysfunction. Studies in women with known infection confirmed by amniocentesis have shown varied efficacy of treatment with broad-spectrum antibiotics,15,16 indicating that the development of prognostic techniques to identify whether individual cases are responding to therapies could be beneficial when defining clinical management plans for intrauterine infection and in improving neonatal and long-term outcomes associated with preterm birth and pPROM.
AJOG at a Glance.
Why was this study conducted?
The study utilized Doppler ultrasonography in a nonhuman primate model of preterm labor to determine whether intrauterine infection and inflammation were associated with altered fetal hemodynamic and cardiovascular function and whether these effects would be ameliorated in utero by maternal antibiotics.
Key findings
Intrauterine Ureaplasma infection alters the fetal hemodynamic profile, with potential compromise of cardiovascular function, which was mitigated by maternal antibiotics.
What does this add to what is known?
Our study provides additional new evidence that Doppler ultrasonography is a useful method to evaluate fetal cardiovascular status in the context of intrauterine infection and preterm labor and, in this setting, to assess the efficacy and safety of therapeutic interventions.
Acknowledgments
The authors thank Byung Park for his assistance with statistical analyses.
This work was performed at the Oregon National Primate Research Center, Beaverton, OR. Research reported in this publication was supported by the Office of the Director of the National Institutes of Health under award number P51OD011092 to the Oregon National Primate Research Center and from the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award numbers R01 HD006159, R01 HD069610, and K99 HD090229.
Footnotes
The authors report no conflict of interest.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
Data from this manuscript have been presented at the Society for Maternal-Fetal Medicine 39th Annual Meeting on Pregnancy, Las Vegas, NV, February 11–16, 2019, and at the 39th Annual Meeting of the American Society for Reproductive Immunology, Grand Rapids, MI, June 12–15, 2019.
Contributor Information
Meredith A. Kelleher, Division of Reproductive and Development Sciences, Oregon National Primate Research Center, Beaverton, OR.
Ji Yeon Lee, Integrated Research Center for Fetal Medicine, Department of Gynecology and Obstetrics, Johns Hopkins University School of Medicine, Baltimore, MD.
Victoria H. J. Roberts, Division of Reproductive and Development Sciences, Oregon National Primate Research Center, Beaverton, OR.
Christopher M. Novak, Integrated Research Center for Fetal Medicine, Department of Gynecology and Obstetrics, Johns Hopkins University School of Medicine, Baltimore, MD.
Ahmet A. Baschat, Johns Hopkins Center for Fetal Therapy, Department of Gynecology and Obstetrics, Johns Hopkins University, School of Medicine, Baltimore, MD.
Terry K. Morgan, Department of Obstetrics and Gynecology, Oregon Health and Science University, Portland, OR.
Miles J. Novy, Division of Reproductive and Development Sciences, Oregon National Primate Research Center, Beaverton, OR.
Juha P. Räsänen, Department of Obstetrics and Gynecology, Oregon Health and Science University, Portland, OR; University of Helsinki, Helsinki, Finland.
Antonio E. Frias, Division of Reproductive and Development Sciences, Oregon National Primate Research Center, Beaverton, OR; Department of Obstetrics and Gynecology, Oregon Health and Science University, Portland, OR.
Irina Burd, Integrated Research Center for Fetal Medicine, Department of Gynecology and Obstetrics, Johns Hopkins University School of Medicine, Baltimore, MD; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD.
References
- 1.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342:1500–7. [DOI] [PubMed] [Google Scholar]
- 2.Gonçalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 2002;8: 3–13. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–29. [DOI] [PubMed] [Google Scholar]
- 4.Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 2009;16:56–70. [DOI] [PubMed] [Google Scholar]
- 5.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202. [DOI] [PubMed] [Google Scholar]
- 6.Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000;183:1124–9. [DOI] [PubMed] [Google Scholar]
- 7.Cytokines Hallman M., pulmonary surfactant and consequences of intrauterine infection. Biol Neonate 1999;76(Suppl 1):2–9. [DOI] [PubMed] [Google Scholar]
- 8.Dammann O, Leviton A. Role of the fetus in perinatal infection and neonatal brain damage. Curr Opin Pediatr 2000;12:99–104. [DOI] [PubMed] [Google Scholar]
- 9.Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 1998;179: 1254–60. [DOI] [PubMed] [Google Scholar]
- 10.Waites KB, Schelonka RL, Xiao L, Grigsby PL, Novy MJ. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Semin Fetal Neonatal Med 2009;14:190–9. [DOI] [PubMed] [Google Scholar]
- 11.Yoneda S, Shiozaki A, Yoneda N, et al. Antibiotic therapy increases the risk of preterm birth in preterm labor without intra-amniotic microbes, but may prolong the gestation period in preterm labor with microbes, evaluated by rapid and high-sensitive PCR system. Am J Reprod Immunol 2016;75:440–50. [DOI] [PubMed] [Google Scholar]
- 12.Oliver RS, Lamont RF. Infection and antibiotics in the aetiology, prediction and prevention of preterm birth. J Obstet Gynaecol 2013;33: 768–75. [DOI] [PubMed] [Google Scholar]
- 13.Sato TA, Keelan JA, Blumenstein M, Mitchell MD. Efficacy and specificity of nonsteroidal anti-inflammatory drugs for the inhibition of cytokine-stimulated prostaglandin E(2) secretion by amnion-derived WISH cells. Pros-taglandins Leukot Essent Fatty Acids 2002;66: 525–7. [DOI] [PubMed] [Google Scholar]
- 14.Grigsby PL, Novy MJ, Sadowsky DW, et al. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol 2012;207:475.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon BH, Romero R, Park JY, et al. Antibiotic administration can eradicate intraamniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol 2019;221:142.e1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh KJ, Romero R, Park JY, et al. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intraamniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol 2019;221:140.e1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bookstaver PB, Bland CM, Griffin B, Stover KR, Eiland LS, McLaughlin M. A review of antibiotic use in pregnancy. Pharmacotherapy 2015;35:1052–62. [DOI] [PubMed] [Google Scholar]
- 18.Marlow N, Pike K, Bower E, et al. Characteristics of children with cerebral palsy in the ORACLE children study. Dev Med Child Neurol 2012;54:640–6. [DOI] [PubMed] [Google Scholar]
- 19.Kenyon S, Pike K, Jones DR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet 2008;372:1319–27. [DOI] [PubMed] [Google Scholar]
- 20.Romero R, Espinoza J, Gonçalves LF, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2004;16:146–57. [DOI] [PubMed] [Google Scholar]
- 21.Yanowitz TD, Jordan JA, Gilmour CH, et al. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res 2002;51:310–6. [DOI] [PubMed] [Google Scholar]
- 22.Mäkikallio K, Rounioja S, Vuolteenaho O, Paakkari J, Hallman M, Räsänen J. Fetal cardiac natriuretic peptide expression and cardiovascular hemodynamics in endotoxin-induced acute cardiac dysfunction in mouse. Pediatr Res 2006;59:180–4. [DOI] [PubMed] [Google Scholar]
- 23.Abdulkadir AA, Kimimasa T, Bell MJ, Macpherson TA, Keller BB, Yanowitz TD. Placental inflammation and fetal hemodynamics in a rat model of chorioamnionitis. Pediatr Res 2010;68:513–8. [DOI] [PubMed] [Google Scholar]
- 24.Galinsky R, Hooper SB, Polglase GR, Moss TJ. Intrauterine inflammation alters fetal cardiopulmonary and cerebral haemodynamics in sheep. J Physiol 2013;591:5061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polglase GR, Hooper SB, Gill AW, et al. Intrauterine inflammation causes pulmonary hypertension and cardiovascular sequelae in preterm lambs. J Appl Physiol (1985) 2010;108: 1757–65. [DOI] [PubMed] [Google Scholar]
- 26.Mastrolia SA, Erez O, Loverro G, et al. Ultrasonographic approach to diagnosis of fetal inflammatory response syndrome: a tool for at-risk fetuses? Am J Obstet Gynecol 2016;215: 9–20. [DOI] [PubMed] [Google Scholar]
- 27.Crispi F, Valenzuela-Alcaraz B, Cruz-Lemini M, Gratacós E. Ultrasound assessment of fetal cardiac function. Australas J Ultrasound Med 2013;16:158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letti Müller AL, Barrios Pde M, Kliemann LM, Valério EG, Gasnier R, Magalhães JA. Tei index to assess fetal cardiac performance in fetuses at risk for fetal inflammatory response syndrome. Ultrasound Obstet Gynecol 2010;36:26–31. [DOI] [PubMed] [Google Scholar]
- 29.Tei C, Ling LH, Hodge DO, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function—a study in normals and dilated cardiomyopathy. J Cardiol 1995;26: 357–66. [PubMed] [Google Scholar]
- 30.Friedman D, Buyon J, Kim M, Glickstein JS. Fetal cardiac function assessed by Doppler myocardial performance index (Tei Index). Ultrasound Obstet Gynecol 2003;21:33–6. [DOI] [PubMed] [Google Scholar]
- 31.Eloundou SN, Lee J, Wu D, et al. Placental malperfusion in response to intrauterine inflammation and its connection to fetal sequelae. PLoS One 2019;14:e0214951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ducsay CA, Cook MJ, Novy MJ. Simplified vest and tether system for maintenance of chronically catheterized pregnant rhesus monkeys. Lab Anim Sci 1988;38:343–4. [PubMed] [Google Scholar]
- 33.Kelleher MA, Liu Z, Wang X, et al. Beyond the uterine environment: a nonhuman primate model to investigate maternal-fetal and neonatal outcomes following chronic intrauterine infection. Pediatr Res 2017;82:244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arraut AM, Frias AE, Hobbs TR, McEvoy C, Spindel ER, Rasanen J. Fetal pulmonary arterial vascular impedance reflects changes in fetal oxygenation at near-term gestation in a nonhuman primate model. Reprod Sci 2013;20: 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acharya G, Wilsgaard T, Berntsen GK, Maltau JM, Kiserud T. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am J Obstet Gynecol 2005;192:937–44. [DOI] [PubMed] [Google Scholar]
- 36.Pellett AA, Tolar WG, Merwin DG, Kerut EK. The Tei index: methodology and disease state values. Echocardiography 2004;21:669–72. [DOI] [PubMed] [Google Scholar]
- 37.Godfrey ME, Messing B, Valsky DV, Cohen SM, Yagel S. Fetal cardiac function: M-mode and 4D spatiotemporal image correlation. Fetal Diagn Ther 2012;32:17–21. [DOI] [PubMed] [Google Scholar]
- 38.Lee JY, Kim YL, Jeong JE, Ahn JW. Prediction of pregnancy complication occurrence using fetal cardiac output assessments made by ultrasonography at 20 to 24 weeks of gestation. Obstet Gynecol Sci 2017;60:336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng M, Schaal M, Chen Y, et al. Real-time 3-dimensional echocardiographic assessment of ventricular volume, mass, and function in human fetuses. PLoS One 2013;8:e58494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aviram A, Quaglietta P, Warshafsky C, et al. The utility of ultrasound assessment in the management of preterm prelabor rupture of the membranes. Ultrasound Obstet Gynecol 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 41.Santolaya J, Sampson M, Nobles G, Font G, Ramakrishnan V, Warsof SL. Doppler evaluation of the fetoplacental circulation in the latent phase of preterm premature rupture of membranes. J Ultrasound Med 1991;10: 327–30. [DOI] [PubMed] [Google Scholar]
- 42.Dubiel M, Seremak-Mrozikiewicz A, Breborowicz GH, Drews K, Pietryga M, Gudmundsson S. Fetal and maternal Doppler velocimetry and cytokines in high-risk pregnancy. J Perinat Med 2005;33:17–21. [DOI] [PubMed] [Google Scholar]
- 43.Trudinger B, Wang J, Athayde N, Beutler L, Wang X. Association of umbilical placental vascular disease with fetal acute inflammatory cytokine responses. J Soc Gynecol Investig 2002;9:152–7. [PubMed] [Google Scholar]
- 44.Wang X, Athayde N, Trudinger B. A proinflammatory cytokine response is present in the fetal placental vasculature in placental insufficiency. Am J Obstet Gynecol 2003;189: 1445–51. [DOI] [PubMed] [Google Scholar]
- 45.Rounioja S, Räsänen J, Ojaniemi M, Glumoff V, Autio-Harmainen H, Hallman M. Mechanism of acute fetal cardiovascular depression after maternal inflammatory challenge in mouse. Am J Pathol 2005;166: 1585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duncan JR, Cock ML, Rees S, Harding R. The effects of repeated endotoxin exposure on placental structure in sheep. Placenta 2003;24: 786–9. [DOI] [PubMed] [Google Scholar]
- 47.Dalitz P, Harding R, Rees SM, Cock ML. Prolonged reductions in placental blood flow and cerebral oxygen delivery in preterm fetal sheep exposed to endotoxin: possible factors in white matter injury after acute infection. J Soc Gynecol Investig 2003;10:283–90. [DOI] [PubMed] [Google Scholar]
- 48.Kafetzis DA, Skevaki CL, Skouteri V, et al. Maternal genital colonization with Ureaplasma urealyticum promotes preterm delivery: association of the respiratory colonization of premature infants with chronic lung disease and increased mortality. Clin Infect Dis 2004;39:1113–22. [DOI] [PubMed] [Google Scholar]
- 49.Viscardi RM, Hasday JD. Role of Ureaplasma species in neonatal chronic lung disease: epidemiologic and experimental evidence. Pediatr Res 2009;65:84R–90R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashima JN, Rogers V, Langley SM, et al. Fetal ventricular interactions and wall mechanics during ductus arteriosus occlusion in a sheep model. Ultrasound Med Biol 2015;41:1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lantto J, Erkinaro T, Haapsamo M, et al. Foramen ovale blood flow and cardiac function after main pulmonary artery occlusion in fetal sheep. Exp Physiol 2019;104:189–98. [DOI] [PubMed] [Google Scholar]
- 52.Meyberg R, Tossounidis I, Ertan AK, Friedrich M, Schmidt W. The clinical significance of antenatal pathological Doppler findings in the fetal middle cerebral artery in cases with peripheral reduced diastolic Doppler flow but no absence of end-diastolic flow in the umbilical artery or fetal aorta. Clin Exp Obstet Gynecol 2001;28:17–9. [PubMed] [Google Scholar]
- 53.Kalafat E, Khalil A. Clinical significance of cerebroplacental ratio. Curr Opin Obstet Gynecol 2018;30:344–54. [DOI] [PubMed] [Google Scholar]
- 54.Rounioja S, Räsänen J, Glumoff V, Ojaniemi M, Mäkikallio K, Hallman M. Intra-amniotic lipopolysaccharide leads to fetal cardiac dysfunction. A mouse model for fetal inflammatory response. Cardiovasc Res 2003;60: 156–64. [DOI] [PubMed] [Google Scholar]
- 55.Tare M, Bensley JG, Moss TJ, et al. Exposure to intrauterine inflammation leads to impaired function and altered structure in the preterm heart of fetal sheep. Clin Sci (Lond) 2014;127:559–69. [DOI] [PubMed] [Google Scholar]
- 56.Okogbule-Wonodi AC, Gross GW, Sun CC, et al. Necrotizing enterocolitis is associated with Ureaplasma colonization in preterm infants. Pediatr Res 2011;69:442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viscardi RM, Kallapur SG. Role of Ureaplasma respiratory tract colonization in bronchopulmonary dysplasia pathogenesis: current concepts and update. Clin Perinatol 2015;42: 719–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romero R, Sibai B, Caritis S, et al. Antibiotic treatment of preterm labor with intact membranes: a multicenter, randomized, double-blinded, placebo-controlled trial. Am J Obstet Gynecol 1993;169:764–74. [DOI] [PubMed] [Google Scholar]
- 59.Kenyon SL, Taylor DJ, Tarnow-Mordi W; ORACLE Collaborative Group. Broad-spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. ORACLE Collaborative Group. Lancet 2001;357:989–94. [DOI] [PubMed] [Google Scholar]
- 60.Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev 2013;12: CD001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 2014;143:225–45. [DOI] [PubMed] [Google Scholar]
- 62.Acosta EP, Grigsby PL, Larson KB, et al. Transplacental transfer of azithromycin and its use for eradicating intra-amniotic Ureaplasma infection in a primate model. J Infect Dis 2014;209:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gravett MG. Successful treatment of intraamniotic infection/inflammation: a paradigm shift. Am J Obstet Gynecol 2019;221:83–5. [DOI] [PubMed] [Google Scholar]
- 64.Chang KH, Kim HJ, Yu HJ, et al. Comparison of antibiotic regimens in preterm premature rupture of membranes: neonatal morbidity and 2-year follow-up of neurologic outcome. J Matern Fetal Neonatal Med 2017;30:2212–8. [DOI] [PubMed] [Google Scholar]
- 65.Schabel MC, Roberts VHJ, Lo JO, et al. Functional imaging of the nonhuman primate placenta with endogenous blood oxygen level-dependent contrast. Magn Reson Med 2016;76:1551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirsch AJ, Roberts VHJ, Grigsby PL, et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat Commun 2018;9:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitchell T, MacDonald JW, Srinouanpranchanh S, et al. Evidence of cardiac involvement in the fetal inflammatory response syndrome: disruption of gene networks programming cardiac development in nonhuman primates. Am J Obstet Gynecol 2018;218:438. e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


