Abstract
Cell cycle is a cellular process that is subject to stringent control. In contrast to the wealth of knowledge of proteins controlling the cell cycle, very little is known about the molecular role of lncRNAs (long noncoding RNAs) in cell-cycle progression. By performing genome-wide transcriptome analyses in cell-cycle-synchronized cells, we observed cell-cycle phase-specific induction of >2000 lncRNAs. Further, we demonstrate that an S-phase-upregulated lncRNA, SUNO1, facilitates cell-cycle progression by promoting YAP1-mediated gene expression. SUNO1 facilitates the cell-cycle-specific transcription of WTIP, a positive regulator of YAP1, by promoting the co-activator, DDX5-mediated stabilization of RNA polymerase II on chromatin. Finally, elevated SUNO1 levels are associated with poor cancer prognosis and tumorigenicity, implying its pro-survival role. Thus, we demonstrate the role of a S-phase up-regulated lncRNA in cell-cycle progression via modulating the expression of genes controlling cell proliferation.
Research organism: None
Introduction
Cell-cycle progression is a vital cellular process, subject to stringent control, as aberrant cell-cycle progression usually results in genome instability, contributing to cancer progression (Robertson et al., 1990; Cho et al., 2001; Dyson, 1998; Frolov and Dyson, 2004; Sánchez and Dynlacht, 1996). The eukaryotic cell cycle is controlled by a regulatory network, which proceeds through tightly regulated transitions to make sure that specific events occur in an orderly fashion. The activity of genes that control cell proliferation is strictly regulated through the cell-cycle-dependent oscillation of their expression (Robertson et al., 1990; Cho et al., 2001; Dyson, 1998; Frolov and Dyson, 2004; Sánchez and Dynlacht, 1996). Such dynamic changes in gene expression during cell cycle are essential for efficient cell-cycle progression (Robertson et al., 1990; Cho et al., 2001; Dyson, 1998; Frolov and Dyson, 2004; Sánchez and Dynlacht, 1996). For example, studies have established the role of transcription factors (TFs) such as the E2F and TEAD family of proteins in regulating the transcription of genes controlling cell cycle and cell proliferation (Frolov and Dyson, 2004; Chen et al., 2009; Harbour and Dean, 2000; Meng et al., 2016). Extensive studies on the identification of protein-coding genes exhibiting periodic expression patterns during cell cycle have led to improved understanding of the basic cell-cycle process and its regulatory mechanism, exemplified by studies on cyclins (Pines and Hunter, 1989). Understanding the mode of cell cycle-regulated gene expression is also central to the study of many diseases, most prominently cancer. Thus, characterization of the genome-wide changes in the transcriptional program during the cell cycle is a critical step toward a deeper mechanistic understanding of the cell proliferation process and its role in cancer.
One of the most unexpected discoveries in the genomics era of biology is the extensive transcription of RNA from non-protein-coding regions of the genome (www.gencodegenes.org). Tens of thousands of long noncoding RNAs (lncRNAs), defined as transcripts larger than 200 nt with no or low protein-coding potential, have been identified in mammalian cells. Pioneering studies on a small proportion of lncRNAs revealed that lncRNAs are an integral part of the cellular control network that co-exists along with proteins (Goff and Rinn, 2015; Yao et al., 2019; Kopp and Mendell, 2018; Sun et al., 2018a; Quinn and Chang, 2016; Rinn and Chang, 2012) and play important roles in cancer (Gutschner et al., 2013). Mechanistically, the RNA sequence and structure offer lncRNAs two inherent functional properties: (1) sequence-mediated interaction with genomic DNA or other RNA, and (2) secondary/tertiary structure-mediated interaction with RNA-binding proteins. With these properties, lncRNAs modulate the recruitment of TFs, cofactors or chromatin modifiers to specific genomic locus, to regulate gene expression transcriptionally or epigenetically; or to regulate the binding of RNA processing factors or microRNAs to pre-mRNAs or mRNAs, thereby influencing gene expression at the post-transcriptional level (Batista and Chang, 2013). Functionally, lncRNAs control several biological functions, including but not limited to processes such as dosage compensation, genomic imprinting, cell metabolism, differentiation and stem cell pluripotency (Goff and Rinn, 2015; Kopp and Mendell, 2018; Sun et al., 2018a; Quinn and Chang, 2016; Rinn and Chang, 2012).
In contrast to the wealth of knowledge of proteins involved in the regulation of the cell cycle, and associated with oncogenic mutations, very little is known about the molecular role of cell-cycle phase-regulated lncRNAs. Recent studies have indicated that several lncRNAs regulate vital biological processes such as cell cycle, cell proliferation and DNA-damage response, via either directly regulating DNA replication or indirectly controlling the expression of critical cell-cycle regulatory genes (Schmitt and Chang, 2016; Li et al., 2016; Kitagawa et al., 2013). Examples include Y RNA, which is involved in the activation of replication initiation (Kowalski and Krude, 2015), MALAT1 that promotes the expression and activity of TFs such as E2F and B-Myb (Tripathi et al., 2013; Ji et al., 2003), and the recently reported CONCR, a lncRNA whose expression is periodic during cell cycle, controls sister chromatid cohesion by regulating the activity of DDX11 helicase (Marchese et al., 2016). In addition, LncRNAs such as p15-AS, lincRNA-p21, RoR, PANDA, DINO and NORAD are known to regulate cell-cycle progression through modulating the tumor-suppressor and growth-arrest pathways during senescence and in response to DNA damage (Petermann et al., 2010; Zhang et al., 2013; Schmitt et al., 2016; Lee et al., 2016). Also, elegant studies have demonstrated that a subset of lncRNAs transcribed from or near the promoters of cell-cycle-regulated protein-coding genes were shown to have coordinated transcription with their respective protein-coding genes, in response to diverse perturbations, including oncogenic stimuli, stem cell differentiation or DNA damage, suggesting their potential biological functions (Schmitt et al., 2016; Hung et al., 2011; Goyal et al., 2017). Finally, by performing CRISPR/Cas9- or CRISPRi-mediated of depletion of >1000 s of lncRNAs in multiple cancer cell lines, a recent study had reported that ~ 100 lncRNAs regulate cell growth and cell viability in a cell type-specific manner, though the molecular function of these lncRNAs is yet to be determined (Liu et al., 2017a). Despite these studies, our understanding on the mechanistic role of lncRNAs during cell-cycle progression remains extremely limited. A comprehensive characterization of the expression of lncRNAs during cell cycle would generate a rich resource for further characterizing lncRNA-mediated regulatory networks, contributing to cell-cycle progression. In addition, such a dataset would provide insights into how lncRNAs are exploited by tumorigenic mutations that drive malignancy.
Here, we systematically profiled the expression of both protein-coding and lncRNA genes during cell cycle by performing deep RNA-seq of cell-cycle-synchronized (G1, G1/S, S, G2 and M-phases) cancer cells, and identified >2000 lncRNAs that displayed periodic expression, peaking during specific phases of the cell cycle. Mechanistic studies on a S-phase-upregulated novel lncRNA that we named as SUNO1 (S-phase-Upregulated NOn-coding-1) revealed its vital role in modulating the Hippo/Yap1 signaling pathway, thereby promoting cell-cycle progression.
Results
Transcriptome analyses of cell-cycle-synchronized cells reveal cell-cycle-regulated expression of protein-coding and noncoding genes
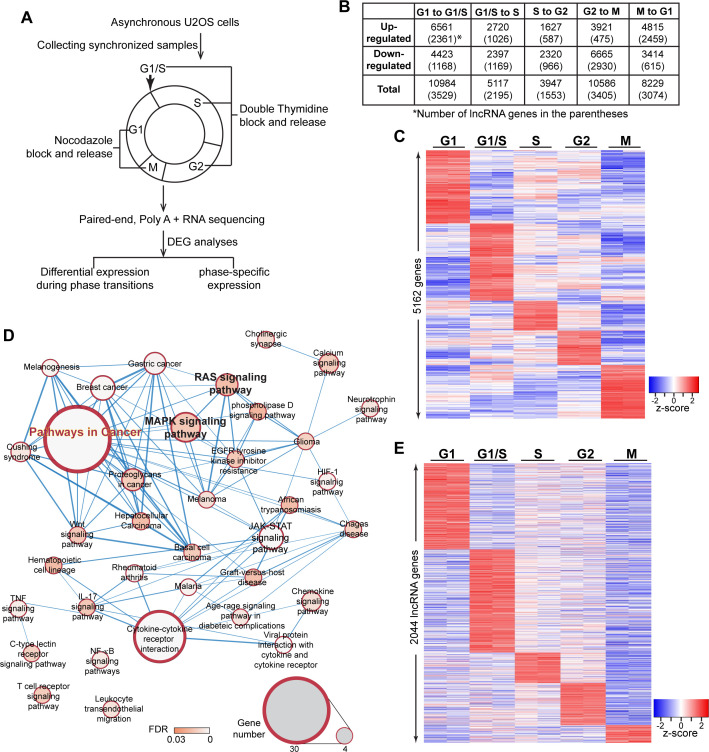
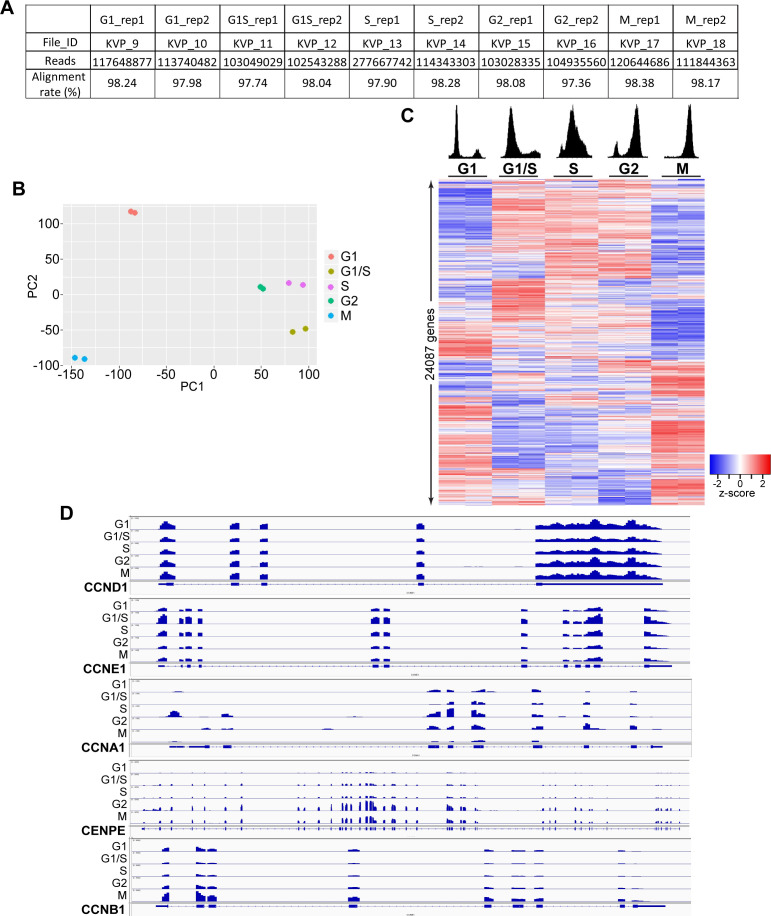
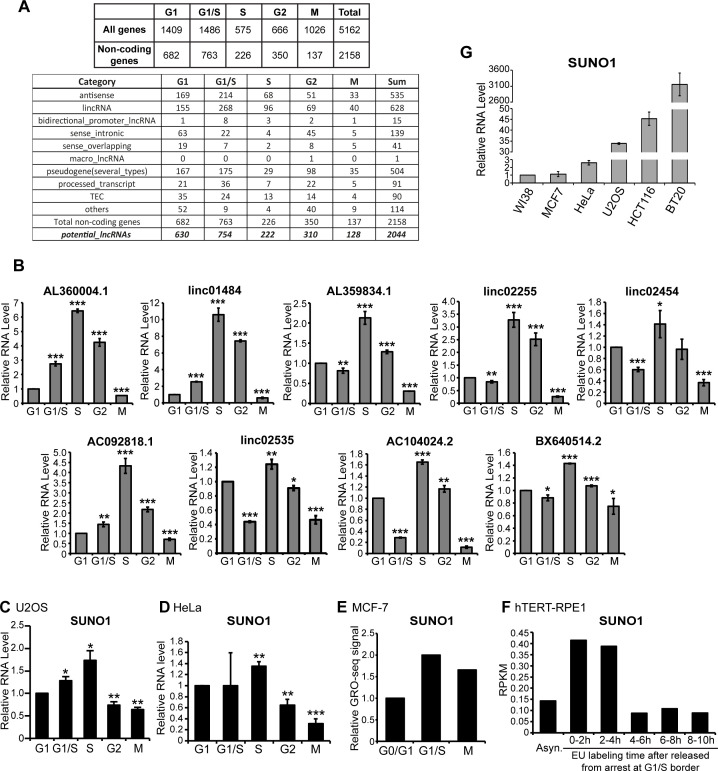
To determine non-random cyclical changes in gene expression during cell-cycle progression, we performed paired-end deep RNA-sequencing (>100 million paired-end reads/sample) of the osteosarcoma cells U2OS that were synchronized into discrete cell-cycle stages: G1, G1/S, S, G2 and M (please see Materials and methods for synchronization details). (Figure 1A and Figure 1—figure supplement 1A). Principal component analysis confirmed that the data set from biological replicates was highly consistent in our RNA-seq data sets (Figure 1—figure supplement 1B). U2OS cells showed quantifiable expression (CPM ≥ 0.075 in at least two samples) of ~24,087 genes, including 15,780 coding and 8307 non-coding genes, including 7836 potential lncRNAs (Figure 1—figure supplement 1C; Supplementary file 1). Transcriptome profiling revealed dynamic expression of genes during cell-cycle progression (Figure 1—figure supplement 1C). In order to assess the biological processes/pathways that are activated/repressed during cell-cycle transition, we performed differential expression analyses between two adjacent cell-cycle stages (for example, G1 to G1/S or G1/S to S) (Figure 1B; Supplementary files 2 and 3). In this case, we defined differentially expressed genes (DEGs) as genes that displayed |fold change| ≥ 1.5 and FDR < 0.05, in statistical analysis. We observed differential expression of several thousands of genes during cell-cycle stage transition (10984 DEGs between G1 to G1/S; 5117 DEGs between G1/S to S; 3947 DEGs between S to G2; 10586 DEGs between G2 to M; and 8229 DEGs between M to G1), including the established cell-cycle regulators such as cyclins (Figure 1B and Figure 1—figure supplement 1D; Supplementary file 3). Interestingly, we observed that ~ 35–40% of the genes that showed differential expression during a particular cell-cycle stage transition consisted of lncRNAs (3529 in G1 to G1/S; 2195 in G1/S to S; 1553 in S to G2; 3405 in G2 to M and 3074 in M to G1 transition) (Figure 1B; Supplementary file 3), implying potential roles played by thousands of lncRNAs during cell-cycle progression.
Figure 1. Transcriptome landscape of U2OS cells during cell-cycle progression.
(A) Schematic of sample preparation and analyses pipeline of RNA-seq. U2OS cells are synchronized to different phases of cell cycle (G1, G1/S, S, G2, M) in biological replicates, then subject to paired-end, polyA+, and high depth RNA-seq. Differential expression analyses are performed using gene count data to identify differentially expressed genes comparing every two adjacent phases. Phase-specific genes are further defined as detailly described in Materials and method. (B) Table representing the number of differentially expressed genes (DEGs) between every two adjacent cell-cycle phases. The number in the parenthesis refers to long non-coding DEGs. Detailed DEG information is available in Supplementary file 3. (C) Heatmap of all phase-specific genes. Full list of all 5162 phase-specific genes are listed in Supplementary file 5. (D) Top events from Kegg pathway analysis of S-phase-specific genes. Full results are listed in Supplementary file 4. (E) Heatmap showing cell-cycle phase-specific expression of lncRNAs in U2OS cells.
Figure 1—figure supplement 1. Cell-cycle-specific expression of genes in U2OS cells.
Figure 1—figure supplement 2. Pathways and biological processes of genes that showed differential expression during cell cycle.
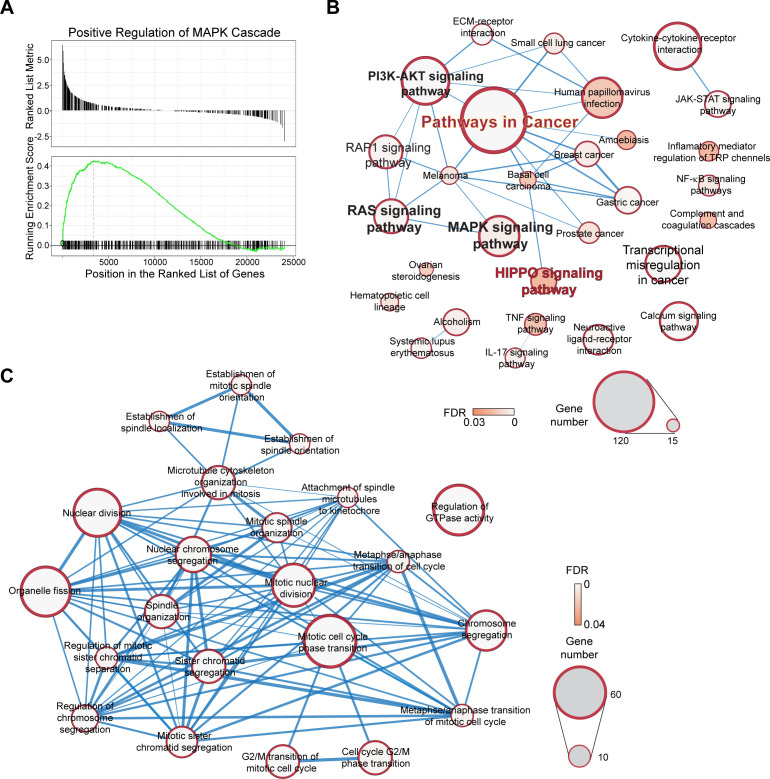
Next, we performed bioinformatic analyses to gain insights into the biological pathways that were associated with the DEGs during cell-cycle stage transition. GSEA analyses revealed that pro-proliferative and oncogenic pathways, such as positive regulators of MAPK cascade were activated during G1/S to S-phase transition (Figure 1—figure supplement 2A; Supplementary file 4). Pathway analyses indicated that DEGs during G1/S to S transition were enriched for biological processes that promote cancer progression, including the MAPK, RAS and Hippo signaling pathways (Figure 1—figure supplement 2B; Supplementary file 4), implying an intimate link between differential expression of genes during G1/S to S transition and cancer.
In order to determine if a particular gene participates in a cellular function during a specific cell-cycle phase, we further identified the cell-cycle phase-specific expressed genes from the DEGs described above, by utilizing the following criteria: The genes showing (1) the highest expression in one particular cell-cycle stage compared to rest of the cell-cycle stages, and (2) significantly (FDR < 0.05) higher expression (|Fold change| ≥ 1.5) in a particular cell-cycle phase compared to adjacent cell-cycle phases. By this approach, we identified 5162 genes (1409 genes in G1, 1486 genes in G1/S, 575 genes in S, 666 genes in G2, and 1026 genes during M phase) that display phase-specific expression (Figure 1C; also see Figure 2—figure supplement 1A and Supplementary file 5). Pathway and Gene ontology analyses revealed important functions attributed to the phase-specifically expressed genes. For instance, S-phase-specific genes participated in several pro-proliferation and cancer promoting pathways, (Figure 1D, Supplementary file 4). Similarly, M-phase-expressed genes are detected to be relevant to mitotic cell-related biological processes (Figure 1—figure supplement 2C, Supplementary file 4).
Transcriptome analyses revealed phase-specific expression of lncRNAs during cell cycle
At present, little is known about the role of lncRNAs that show enhanced expression during a particular cell-cycle phase. We demonstrated a microRNA-independent role for the G1 phase-enriched MIR100 host gene lncRNA in G1/S transition by modulating HuR-mediated mRNA stability and/or translation (Sun et al., 2018b). Recently, we reported that MIR222HG lncRNA promoted the cell cycle re-entry post quiescence by modulating ILF3/2 activity, further supporting the role of lncRNAs in cell-cycle progression (Sun et al., 2020). Our RNA-seq analyses revealed that ~ 42% (2158 out of 5162 genes) of genes that show elevated expression during a particular cell-cycle phase categorized into non-coding genes, in which the majority of them (2044 out of 2158) belonged to one of the several classes of lncRNA genes (630 in G1; 754 in G1/S; 222 in S; 310 in G2, and 128 during M phase) (Figure 1E and Figure 2—figure supplement 1A; Supplementary file 5).
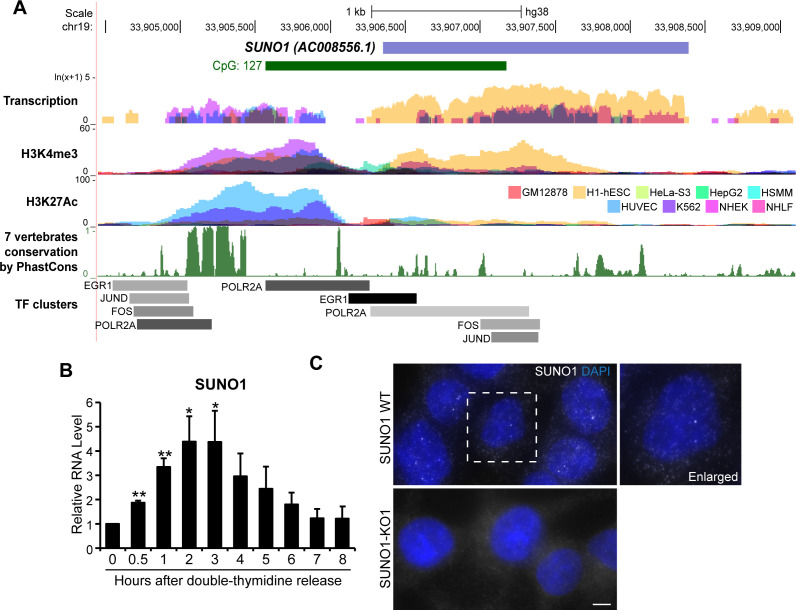
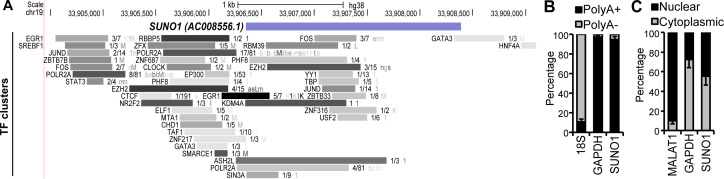
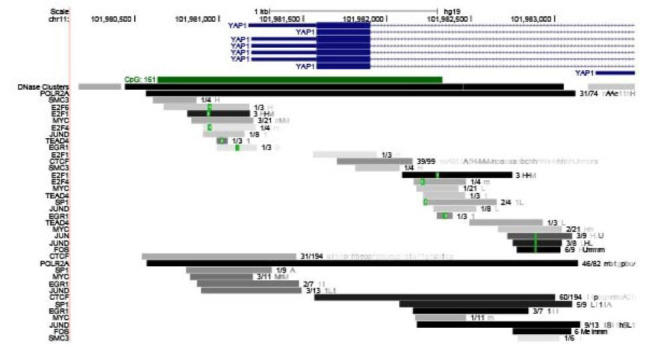
In order to test the hypothesis that similar to protein-coding genes, cell-cycle phase-specific-expressed lncRNAs perform vital roles during cell proliferation, we focused on characterizing the function of an S-phase-enriched lncRNA. By performing RT-qPCR we validated the RNA-seq data, showing S-phase-specific elevated expression of several candidate lncRNAs (Figure 2—figure supplement 1B). Further, we performed mechanistic studies to determine the role of one of the novel S-phase-upregulated lncRNAs, SUNO1 (S-phase-Upregulated NOn-coding-1) (AC008556.1; ENSG00000277013; LNCipedia gene ID: lnc-KCTD15-2) (Figure 2A) in cell-cycle progression. RNA-seq analyses revealed elevated levels of SUNO1 in U2OS cells that were synchronized into S-phase (Supplementary files 3 and 5). RT-qPCR analyses of cell-cycle-synchronized cells also indicated that SUNO1 showed elevated levels during G1/S and early stages of S-phase (Figure 2B and Figure 2—figure supplement 1C). The SUNO1 gene is a long intergenic lncRNA (lincRNA), located on human Chromosome 19. Analyses, including RT-qPCR, GRO-seq and EU-pulse labeling followed by nascent RNA-seq in various human cell lines (HeLa, MCF7 [Liu et al., 2017b] and hTERT-RPE1 [Yildirim et al., 2020]) revealed G1/S- and/or S-phase induced expression of SUNO1, implying that cell-cycle phase-specific expression of SUNO1 is not unique to a particular cell line (Figure 2—figure supplement 1D–F). RNA-seq data from nine ENCODE human cell lines (Figure 2A) as well as RT-qPCR in other human cell lines revealed cell line-specific expression of SUNO1 (Figure 2—figure supplement 1G). For example, the tumorigenic human breast cancer cell line BT-20 showed the highest expression of SUNO1, whereas HCT116 and U2OS showed moderate levels of SUNO1 compared to other cell lines, such as the WI-38 human diploid fibroblasts (Figure 2—figure supplement 1G). We utilized HCT116 (pseudo-diploid) and U2OS (aneuploid) cell lines for most of the downstream functional studies. A genome-wide histone-tail modification map indicated significant H3K4me3 and H3K27ac marks on the promoter of SUNO1 in multiple cell lines (Figure 2A). SUNO1’s 5’end, including the promoter is located within a long stretch of a CpG island (>1 kb), a scenario normally observed for house-keeping genes (Figure 2A). The SUNO1 sequence seems to be conserved only in primate lineage, except for sequence elements within the 5’end of the gene, including the promoter, which was reasonably conserved among vertebrates (Figure 2A). Further, the SUNO1 promoter showed significant enrichment of RNA pol II (POL2RA) and TFs, such as FOS, JUND and EGR1, which are known to induce the expression of genes promoting cell-cycle progression (Figure 2A and Figure 2—figure supplement 2A).
Figure 2. SUNO1 is an S-phase-induced lncRNA.
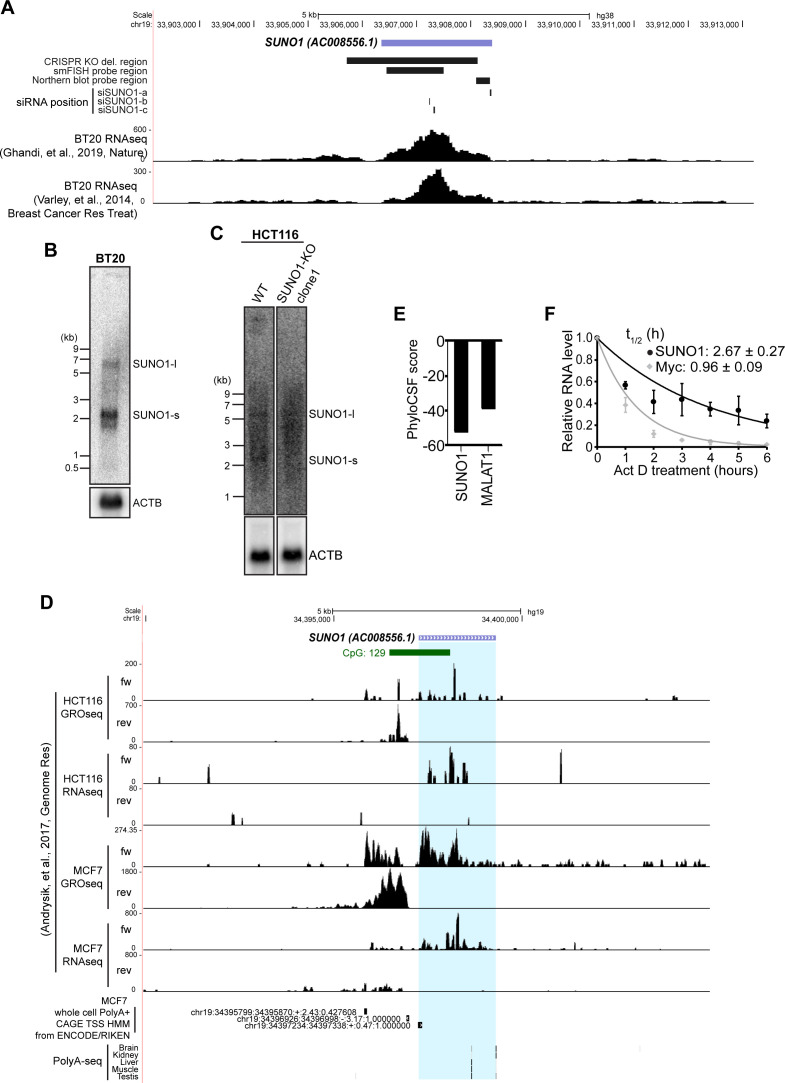
(A) UCSC genomic browser view of SUNO1 genomic locus, showing position of CpG island, transcription in 9 ENCODE cell lines, H3K4me3 ChIP-seq data set, H327Ac ChIP-seq data set, vertebrate conservation, and clusters of Pol II and cell-cycle-regulating transcription factors (TFs) from ENCODE data sets. (B) RT-qPCR to detect relative levels of SUNO1 in U2OS cells post double-thymidine block for indicated time points (hours). Data are presented as Mean ± SD, n = 2. Unpaired two-tail t-tests are performed. *p<0.05, **p<0.01, ***p<0.001. (C) Single-molecule RNA-FISH (smRNA-FISH) to detect SUNO1 RNA in wild-type and SUNO1 knock-out HCT116 cells. SUNO1 KO1 cells used as a negative control for SUNO1 RNA smRNA-FISH. DNA is counterstained with DAPI. Scale bar: 5 μm.
Figure 2—figure supplement 1. SUNO1 is upregulated during S-phase.
Figure 2—figure supplement 2. Basic characterization of SUNO1.
Figure 2—figure supplement 3. Basic characterization of SUNO1.
Cellular fractionation followed by RT-qPCR analyses revealed that SUNO1 is a poly A+ RNA that is present in both the nucleus and cytoplasm (Figure 2—figure supplement 2B–C). Single-molecule (sm)-RNA-FISH revealed that SUNO1 was preferentially enriched as 2–3 well-separated puncta in the nucleus (Figure 2C). The nuclear puncta signal, detected by the SUNO1 smRNA-FISH probe set was absent in SUNO1 knock-out (KO) cells, confirming the specificity of SUNO1 localization (Figure 2C; Figure 2—figure supplement 3A for sm-FISH probe position and also the deleted region in the CRISPR KO cells). Northern blot with a SUNO1-unique probe in BT-20 and HCT116 cells hybridized to discrete bands of >2 kb and >5 kb in length (Figure 2—figure supplement 3B–C). These bands were absent in SUNO1 HCT116 KO cells, implying that SUNO1 primarily codes for two isoforms. Publicly available RNA-seq data from multiple cell lines (BT-20 [Ghandi et al., 2019; Varley et al., 2014], HCT116 and MCF7 [Andrysik et al., 2017]) revealed >2 kb transcript to be the predominant isoform of SUNO1, with the higher molecular weight isoform present in lower levels, further confirming our Northern blot data (Figure 2—figure supplement 3A & D). Furthermore, GRO-seq (Andrysik et al., 2017), CAGE as well as poly A+ seq data sets confirmed defined transcription start site (located within the CpG island) and the 3’end of SUNO1 (Figure 2—figure supplement 3D). Estimation of protein-coding potential using PhyloCSF revealed that similar to the well-characterized MALAT1 lncRNA, SUNO1 did not show any protein-coding potential (Figure 2—figure supplement 3E). Finally, RNA stability assays revealed SUNO1 to be a relatively stable poly A+ RNA with a half-life of >2.6 hr (Figure 2—figure supplement 3F). Altogether, our results indicate that SUNO1 is a G1/S to S-phase-induced low copy but relatively stable poly A+ lncRNA and is preferentially enriched in the nucleus as 2–3 puncta.
Depletion of SUNO1 results in defective cell-cycle progression and hypersensitivity to DNA damage
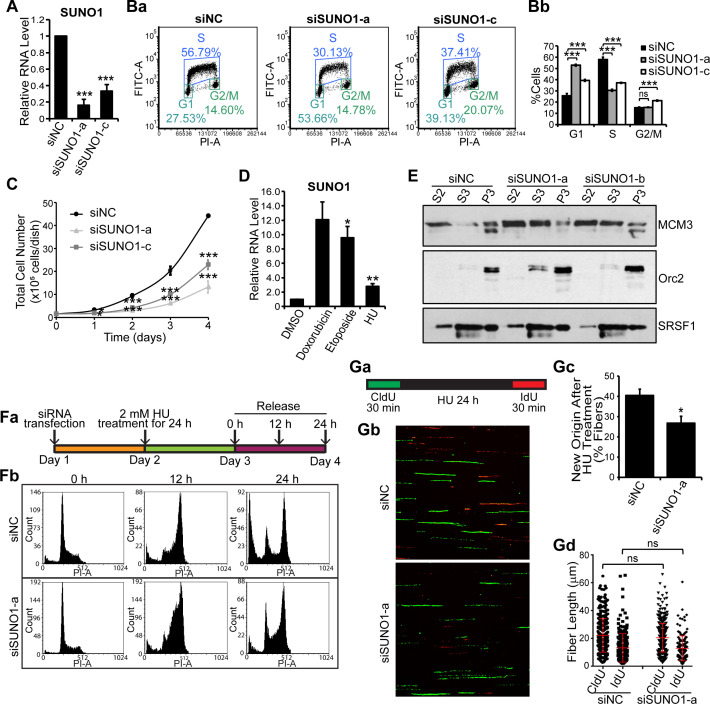
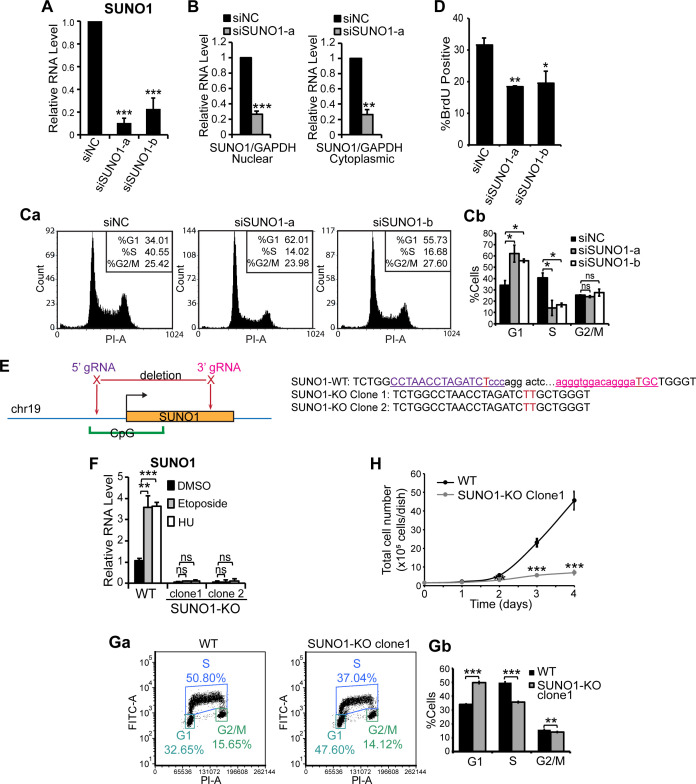
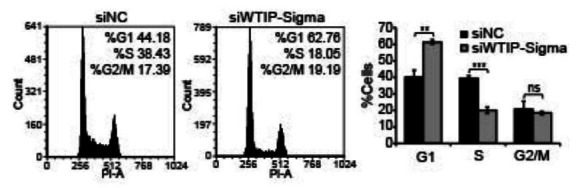
We next determined whether SUNO1 was required for normal cell-cycle progression. We successfully depleted SUNO1 using multiple independent siRNAs targeting different regions of SUNO1 (siSUNO1) in U2OS or HCT116 cells (Figure 3A, Figure 3—figure supplement 1A and also see Figure 2—figure supplement 3A for siRNA positions). SUNO1-specific siRNA-treated cells showed significant downregulation of both the nuclear and the cytoplasmic pool of SUNO1 (Figure 3—figure supplement 1B). Propidium Iodide (PI)- as well as BrdU-PI-flow cytometry analyses revealed that SUNO1-depleted U2OS and HCT116 cells showed reduced number of cells in S-phase and a concomitant increase in G1 population, suggesting a defect in efficient progression into S-phase (Figure 3a-b and Figure 3—figure supplement 1C a-b). Furthermore, reduced number of cells in S-phase upon SUNO1 depletion was confirmed by BrdU incorporation followed by immunostaining in control and SUNO1-depleted cells (Figure 3—figure supplement 1D). SUNO1-depleted cells also showed reduced cell proliferation compared to control cells, indicating that defects in cell-cycle progression upon SUNO1 depletion contribute to defects in cell proliferation (Figure 3C). Finally, independent clones of SUNO1 KO cells (both in HCT116 and U2OS) generated via CRISPR/Cas9-mediated genome-editing also displayed G1 or G1/S arrest and reduced cell proliferation (Figure 3—figure supplement 1E–H) similar to SUNO1 knockdown cells, further supporting the involvement of SUNO1 in S-phase entry.
Figure 3. SUNO1 depletion results in cell-cycle arrest and defects in S-phase entry.
(A) RT-qPCR to quantify SUNO1 levels in control (siNC) and SUNO1-specific siRNA (a and c)-treated HCT116 cells. Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. *p<0.05, **p<0.01, ***p<0.001. (B) BrdU-PI-flow cytometry analyses of control (siNC) and SUNO1-specific siRNA (a and c)-treated HCT116 cells. Dot graphs from one of the replicates are shown (Ba). Population of G1, S and G2/M cells are quantified (Bb). Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. ns, not significant; *p<0.05; **p<0.01; ***p<0.001. (C) Growth curve assay of control (siNC) and SUNO1-specific siRNA (a and c)-treated HCT116 cells. Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. *p<0.05, **p<0.01, ***p<0.001. (D) RT-qPCR to quantify SUNO1 levels in U2OS cells that are incubated with DMSO (control) and drugs (Doxorubicin [0.5 μM for 24 hr], Etoposide [20 μM for 24 hr] and Hydroxyurea [HU; 2 mM for 24 hr]), all of which induce double-strand DNA breaks. Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. *p<0.05, **p<0.01, ***p<0.001. (E) Cellular fractionation to determine the chromatin loading of MCM3 and ORC2 in control (siNC) and SUNO1-depleted U2OS cells. S2 = cytoplasmic fraction; S3 = soluble nuclear fraction; P3 = insoluble chromatin fraction. SRSF1 is used as control. Refer to Figure 3—source data 1. (Fa) Flow chart showing the experimental plan. (Fb) PI-flow cytometry analyses to assess cell-cycle progression in U2OS cells transfected with siNC or siSUNO1-a, followed by 24 hr of 2 mM HU treatment, and released in fresh medium for 0, 12 and 24 hr. (G) Data from DNA fiber experiments in control and SUNO1-depleted U2OS cells. (Ga) DNA fiber experimental plan. DNA fiber experiments of U2OS cells treated with siNC or siSUNO1-a. U2OS cells are transfected with siNC or siSUNO1-a, pulse-labeled with CldU (green) for 30 min, followed by 24 hr of 2 mM HU treatment, and then released for 30 min in presence of IdU (red). DNA fiber spreads are prepared in biological triplicates. Representative images from one of the replicates are shown (Gb). The percentage of new origins (Gc) and the tract length of CldU and IdU fibers (Gd) are determined by counting 200 fibers per replicate. Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. ns, not significant; *p<0.05; **p<0.01; ***p<0.001. Refer to Figure 3—source data 2.
Figure 3—figure supplement 1. Depletion of SUNO1 results in cell-cycle arrest and DNA damage.
Figure 3—figure supplement 2. SUNO-1depleted cells show DNA damage.
Figure 3—figure supplement 3. SUNO1-depleted cells are sensitive to drug-induced DNA damage.
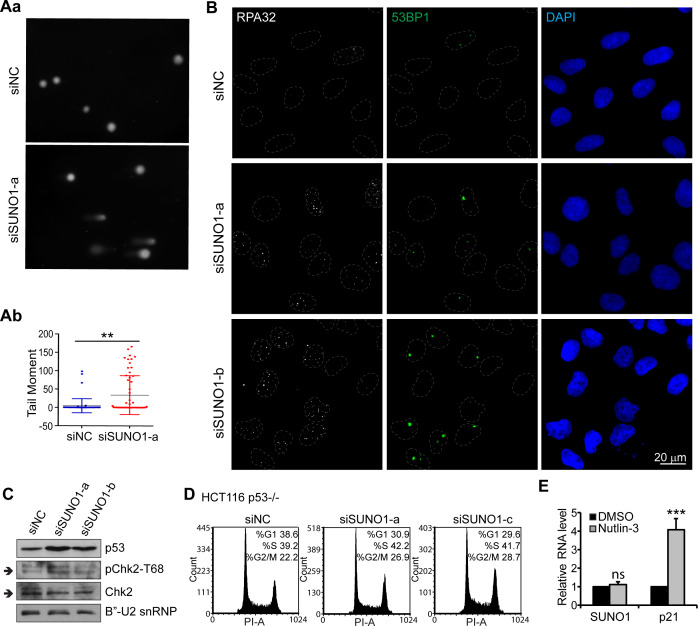
S-phase of the cell cycle is an intrinsically challenging phase for cells, given that any defect during the initial stages of DNA replication could give rise to DNA damage that could induce G1 or G1/S arrest (Macheret and Halazonetis, 2015). In order to determine if the accumulation at G1 or G1/S observed upon SUNO1 depletion is a result of enhanced DNA damage, we determined whether SUNO1-depleted cells were more prone to DNA damage. We found that SUNO1-depleted asynchronous cells showed significant increase in the levels of DNA damage as observed by DNA comet assays (Figure 3—figure supplement 2Aa-b). SUNO1-depleted cells also showed increased number of RPA32- (+ve cells, control = 7.2%; siSUNO1-a = 65.8%; siSUNO1-b 36.5%; n ≥ 75) and 53BP1- (+ve cells, control = 26.6%; siSUNO1-a = 53.5%; siSUNO1-b 64.5%; n ≥ 70) decorated nuclear foci, indicative of DNA damage (Figure 3—figure supplement 2B). Finally, SUNO1-depleted cells also showed increased levels of p53 as well as phospho-Chk2, consistent with increased DNA damage (Figure 3—figure supplement 2C). To specify whether the induction of p53 upon SUNO1 depletion contributes to G1 or G1/S arrest, we determined the extent of G1 or G1/S arrest in SUNO1-depleted p53+/+ or p53-/- HCT116 cells. PI-flow cytometry analyses revealed that unlike p53 wild-type cells, SUNO1-depleted p53 -/- HCT116 cells failed to arrest in G1 (Figure 3—figure supplement 2D) but showed increase in G2/M population. This result indicates that the G1 or G1/S arrest observed in SUNO1-depleted cells requires functional p53, implying SUNO1-depleted cells elicit intra-G1 or G1/S checkpoint.
The increased DNA damage observed in SUNO1-depleted cells prompted us to investigate whether SUNO1 levels were sensitive to DNA damage. Cells treated with drugs that induced double-strand breaks such as doxorubicin (DNA intercalator and topoisomerase II inhibitor), or Etoposide (topoisomerase II inhibitor), or hydroxyurea (HU; 2 mM for 24 hr for inducing replication fork collapse) (Petermann et al., 2010) showed pronounced induction of SUNO1 (Figure 3D and Figure 3—figure supplement 1F). Several lncRNAs participate in the p53-mediated stress response, and their induction upon DNA damage is dependent on the integrity of the p53 pathway (Huarte et al., 2010; Hung et al., 2011). However, we observed a significant induction of SUNO1 in both wild-type (WT; p53 +/+) and p53 -/- HCT116 cells upon DNA damage (data not shown). In addition, treatment of cells with Nutlin-3, a stabilizer of p53 did not induce SUNO1, further indicating that SUNO1 activation was not mediated by p53 (Figure 3—figure supplement 2E).
We demonstrated that SUNO1 was upregulated during S-phase and upon DNA damage, and loss of SUNO1 led to cell-cycle arrest with increased DNA damage, resulting in cell-cycle checkpoint activation. One likely scenario is that SUNO1 is required for entry into S-phase and perhaps for S-phase progression as well. Without SUNO1, the cells have difficulty entering S-phase and hence arrest at the G1/S boundary.
To gain molecular insights into why G1 accumulation is observed in SUNO1-depleted cells, we performed chromatin fractionation of pre-replicative complex proteins in control cells and in ones lacking SUNO1. It is known that defects in the pre-RC complex levels or their chromatin loading could compromise the origin assembly and/or firing. We observed reduced chromatin loading of the Mini Chromosome Maintenance 3 (MCM3), core component of the MCM helicase complex but not (Origin recognition complex 2) ORC 2, member of the ORC complex in SUNO1-depleted cells (Figure 3E), supporting the model that aberrant G1 or G1/S arrest observed upon SUNO1 depletion could be partially due to defects in pre-RC assembly. This is consistent with our results that fewer origins are licensed in the absence of SUNO1 leading to an accumulation in G1 phase.
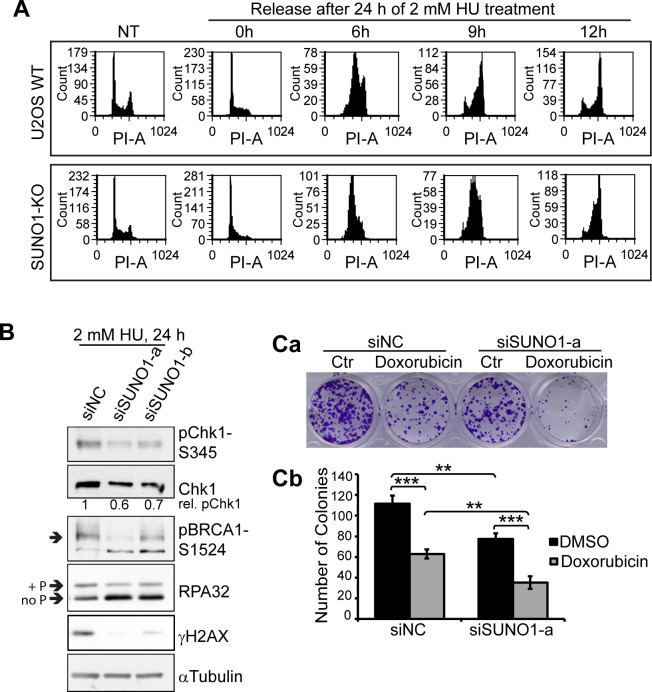
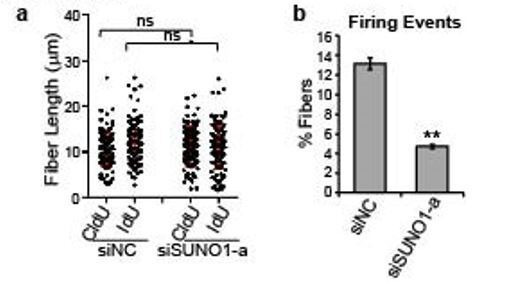
To address why there was increased DNA damage in the absence of SUNO1, we addressed if SUNO1 is involved in sensing and/or repairing DNA damage or the phenotype is a consequence of fewer licensed origins. To test this, we treated control and SUNO1-depleted or SUNO1-KO cells with HU for 24 hr, a condition that elicits strong replication stress by causing replication fork collapse (Petermann et al., 2010), and analyzed the recovery of cells post-HU-release (Figure 3Fa and Figure 3—figure supplement 3A). By PI-flow cytometry, we observed that control cells, post-HU-release, resumed DNA replication, with majority of them reaching G2/M phase by 12 hr post- HU-release (Figure 3Fb and Figure 3—figure supplement 3A). However, SUNO1-depleted and knockout cells showed slow S-phase progression, as observed by the accumulation of a significant fraction of cells in S-phase 12 hr (hr) post-HU-release (Figure 3Fb and Figure 3—figure supplement 3A). Reduced S-phase progression upon SUNO1 deletion could be due to the inability to repair DNA damage or due to defective fork progression. To test this, we performed a DNA fiber combing assay (Figure 3G). Control and SUNO1-depleted cells were first incubated with the thymidine analog 5-chloro-2'-deoxyuridine (CldU) for 30 min to label the replicating DNA strands. CldU was then washed off, and cells were treated with HU for 24 hr to induce replication fork collapse and double strand breaks. Then, cells were released into fresh medium containing another thymidine analog, 5-iodo-2'-deoxyuridine (IdU) for 30 min (Figure 3Ga). By this, the newly synthesized DNA strands will be labeled with IdU. The DNA Fiber assay revealed that SUNO1-depleted cells showed reduced number of tracks that incorporated only IdU (red) post-HU treatment, implying a reduced number of dormant replication origins firing (Figure 3Gb-c). At the same time, both control and SUNO1-depleted cells showed comparable length in the CldU-labeled fibers (green), indicating a normal rate of replication fork progression (Figure 3Gb and d), suggesting that SUNO1 is not required for S-phase progression, once the replication is initiated. Based on the results from the DNA fiber assay, we conclude that the defects in the S-phase progression observed in SUNO1-depleted cells post-HU-release are due to inefficient firing of dormant replication origins.
Interestingly, SUNO1-depleted cells failed to elicit some of the key DNA damage-induced checkpoint responses. For example, compared to control cells, SUNO1-depleted cells post-HU treatment (2 mM for 24 hr) showed reduced Chk1 phosphorylation at Ser345, BRCA1 phosphorylation at Ser1524, RPA32 phosphorylation and γH2AX induction, indicative of defective ATR-mediated check-point activation (Figure 3—figure supplement 3B). Furthermore, cell viability (MTT assay) and long-term cell survival assays (clonogenic assay) with and without DNA damage (Doxorubicin) revealed that SUNO1 acted as a pro-survival gene. SUNO1-depleted cells showed reduced cell growth/survival under both normal and after DNA-damage conditions. (Figure 3—figure supplement 3BCa-b; data not shown), These data revealed that SUNO1-depleted cells are more sensitive to drug-induced DNA damage, implying that SUNO1 is involved in DNA-damage response (DDR), and its loss causes defects in the cells’ ability to recover from DNA damage. Our results demonstrate that SUNO1 is required for entry into S-phase, and its depletion renders cells to become more sensitive to DNA damage, resulting in the inability to reinitiate DNA replication upon replicative stress.
SUNO1 regulates cell proliferation by promoting the expression of YAP1-target genes
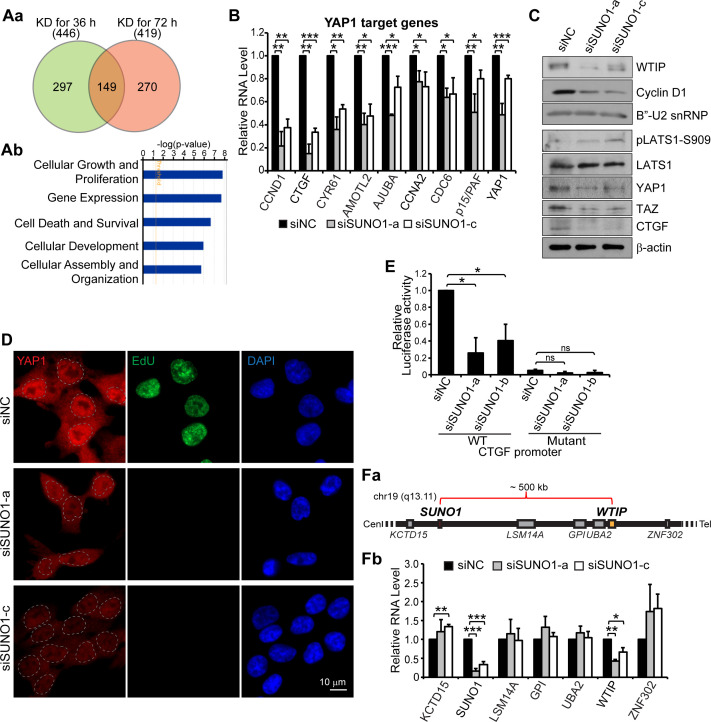
In order to understand the underlying molecular mechanism by which nuclear-enriched SUNO1 regulates cell proliferation, we analyzed the gene expression changes at the steady state levels in control and SUNO1-depleted cells. We isolated RNA from control and SUNO1-depleted HCT116 cells from early and late time points (36 and 72 hr after first round of siRNA treatment) and performed transcriptome-wide microarray analyses. Gene expression changes observed at the earlier time point would help to identify the primary targets of SUNO1. Cells collected 36 hr post siRNA treatment showed efficient depletion of SUNO1 but did not show any observable cell-cycle defect phenotype, assessed by PI-flow cytometry analyses (data not shown). On the other hand, cells treated with SUNO1-specific siRNA for 72 hr showed pronounced cell-cycle arrest (Figure 3B). To identify primary targets of SUNO1, we looked for common target genes whose expression was altered in both early (36 hr) and late time (72 hr) points (Supplementary file 6). We observed 149 common genes that displayed reduced expression after 36 and 72 hr post SUNO1 depletion (Figure 4Aa and Supplementary file 6). Further, Gene ontology (GO) analyses of genes that were downregulated even during early time point post SUNO1 depletion (when there was no cell-cycle defect) revealed that they regulate cellular pathways, including Cellular Growth and Proliferation and Cell Death and Survival pathways (Figure 4Ab).
Figure 4. SUNO1 promotes cell proliferation by regulating the expression of WTIP, a positive regulator of YAP1.
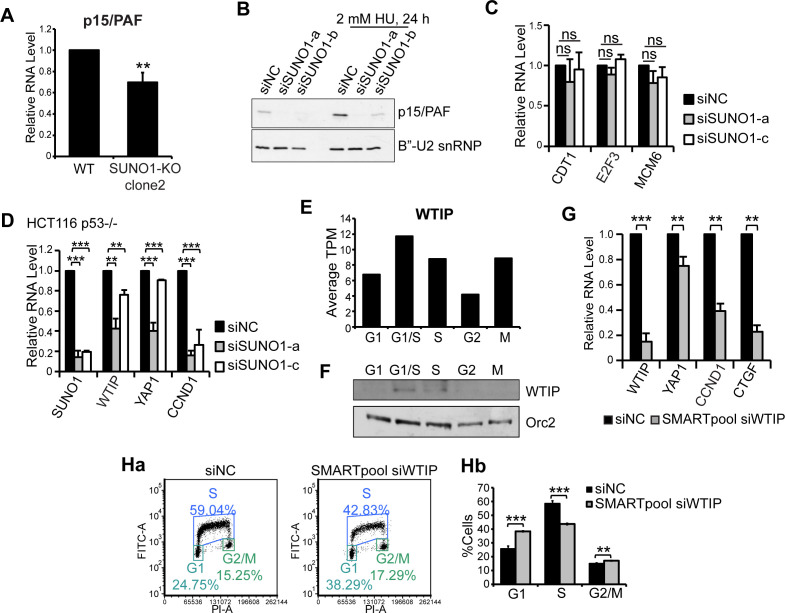
(Aa) Venn diagram showing significantly downregulated genes in SUNO1-depleted wild-type (WT) HCT116 cells (36 and 76 hr post siRNA treatment). 149 common genes showed significant downregulation at both early (36 hr) and late (72 hr) time points post SUNO1 depletion. (Ab) Gene ontology (GO) analysis of all of the genes downregulated after 36 hr post SUNO1 knockdown. (B) RT-qPCR to show the levels of several YAP1 target gene mRNAs in control and SUNO1-depleted WT HCT116 cells. Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. *p<0.05, **p<0.01, ***p<0.001. (C) Western blot to detect WTIP, Cyclin D1, YAP1, LATS1, pLATS1, TAZ, and CTGF in control and SUNO1-depleted WT HCT116 cell. B’-U2 snRNP and β-actin are used as loading control. Refer to Figure 4—source data 1. (D) Immunofluorescence staining to assess the cellular localization of YAP1 coupled with EdU incorporation assay. Cells in S-phase were labeled by EdU. Scale bar: 10 μm. (E) CTGF promoter luciferase assay. WT CTGF promoter (WT) or TEAD-binding sites mutated CTGF promoter (mutant) were cloned upstream of the luciferase reporter gene. WT or mutant reporters are transfected into control and SUNO1-depleted (siSUNO1-a or siSUNO1-b) U2OS cells, and the relative luciferase activity is quantified. Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. ns, not significant; *p<0.05; **p<0.01; ***p<0.001. (Fa) Diagram showing relative genomic position of SUNO1 and other genes near SUNO1 locus. (Fb) RT-qPCR to show the relative mRNA levels from SUNO1 and other genes that are located in the genomic proximity of SUNO1 gene locus in control and SUNO1-depleted WT HCT116 cells. Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. *p<0.05, **p<0.01, ***p<0.001.
Figure 4—figure supplement 1. SUNO1-depleted cells show defects in cell proliferation by downregulating the levels of YAP1 target genes.
Figure 4—figure supplement 2. Stable overexpression of WTIP partially rescues the cell-cycle phenotype caused by SUNO1 depletion.
Since SUNO1 appears to promote cell proliferation, we analyzed whether genes that are part of a particular cell growth controlling pathway were overrepresented in the list of 149 genes, the expression of which was altered upon SUNO1 depletion. We observed that several known YAP1 (Yes-associated protein 1) target genes showed reduced expression in SUNO1-depleted cells (Figure 4B). YAP1 is a transcription co-activator that positively regulates TEAD- or FOS-mediated transcription of genes, thereby promoting cell proliferation (Ehmer and Sage, 2016). For example, CCND1, CTGF, CYR61and AMOTL2 are the known targets of YAP1 (Harvey et al., 2013; Zhao et al., 2008), and we found that these genes were significantly downregulated in SUNO1-depleted cells (Figure 4B). In support of the gene expression data, SUNO1-depleted cells also showed reduced protein levels of YAP1 targets, including Cyclin D1, CTGF (Figure 4C). In addition, another potential YAP1 target, p15/PAF, a PCNA-associated factor that plays crucial roles in S-phase progression and DNA-damage repair (Xie et al., 2014; Chang et al., 2013; De Biasio et al., 2015; Povlsen et al., 2012; Jung et al., 2013), also showed reduced expression in SUNO1-depleted control and DNA-damaged cells (Figure 4B and Figure 4—figure supplement 1A–B). We consistently observed reduced mRNA and protein levels of YAP1 in SUNO1-depleted cells (Figure 4B–C). A recent study indicated that YAP1 positively autoregulates its own expression (Vázquez-Marín et al., 2019). In support of this observation, YAP1 promoter contains several TEAD4 binding sites (data not shown), implying that YAP1 expression could be regulated by TEAD/YAP1 axes. Finally, SUNO1-depleted cells also showed reduced levels of TAZ, the YAP1 paralog, which also promotes cell proliferation by co-activating the TEAD-mediated transcription (Figure 4C).
During cell cycle, active YAP1 protein is imported into the nucleus, where it positively regulates the TEAD- and FOS-mediated transcription of genes controlling cell proliferation (Meng et al., 2016; Kim et al., 2019). We therefore tested whether SUNO1-depleted cells alter the nuclear and cytoplasmic levels of YAP1 by immunostaining. Control cells showed nuclear as well as cytoplasmic distribution of YAP1 (Figure 4D). However, we observed decrease in the levels of YAP1, including the nuclear pool upon SUNO1 depletion, implying that SUNO1-depleted cells reduced active pool of YAP1 (Figure 4D). It is established that phosphorylated LATS1 (at serine-909) kinase by phosphorylating YAP1, inhibits its nuclear import, ultimately resulting in the YAP1 degradation (Meng et al., 2016). We therefore quantified the pLATS1 levels in control and SUNO1-depleted cells. We observed increased levels of pLATS1 in SUNO1-depleted cells (Figure 4C). This result suggests that increased pLATS1 could also contribute to the reduced levels of YAP1 in SUNO1-depleted cells.
Next, to test whether the reduced expression of YAP1 target genes observed in SUNO1-depleted cells is due to G1 arrest, we examined the expression of several cell-cycle genes whose expression is controlled by other cell proliferation-promoting and cell-cycle-regulated TFs, such as E2Fs in control and SUNO1-depleted cells. We observed no significant changes in the levels of E2F target mRNA (CDT1, E2F3 and MCM6) in SUNO1-depleted cells (Figure 4—figure supplement 1C). In addition, we also observed downregulation of YAP1 and its target mRNA like CCND1 even in SUNO1-depleted HCT116 p53-/- cells (Figure 4—figure supplement 1D), where in the absence of p53, SUNO1 depletion did not induce G1 arrest (Figure 3—figure supplement 2D), further supporting that the downregulation of YAP1 targets in SUNO1-depleted cells is not a consequence of G1 arrest.
Finally, to test the status of YAP1/TEAD-mediated transcription activity in presence or absence of SUNO1, we employed a reporter system where the CTGF promoter was cloned upstream of a luciferase reporter. In addition to the reporter with the wild-type CTGF promoter, a mutant reporter with TEAD-binding sites mutated in the CTGF promoter was used as negative control (Zhao et al., 2008). We transfected wild-type and mutant reporters into control and SUNO1-depleted U2OS cells and quantified the reporter activities by luciferase assay. The knock-down of SUNO1 resulted in the significant decrease of luciferase activity driven by the CTGF wild-type promoter (Figure 4E). Notably, mutation of the TEAD sites itself caused a strong decrease of transactivation, which was not significantly further decreased by SUNO1 knock-down (Figure 4E). Altogether, our data support the model that SUNO1 promotes TEAD-mediated transcription via modulating YAP1 activity.
SUNO1 promotes YAP1-mediated transcription of cell-cycle genes by regulating WTIP expression
SUNO1 lncRNA is a low abundant transcript (based on RNA-seq analyses and smRNA-FISH) and is preferentially enriched as 2–3 nuclear puncta. We hypothesized that like several other low abundant lncRNAs, SUNO1 could function in cis, via regulating the expression of protein-coding genes located at its genomic proximity (Wang and Chang, 2011). To test this, we analyzed the microarray data from control and SUNO1-depleted cells and determined potential changes in the expression of genes that were located near SUNO1 genomic locus (~1 Mb window) (Figure 4Fa). Out of the six protein-coding genes that are located near SUNO1 locus, we observed consistent reduced expression of only the WTIP (Wilms tumor 1-interacting protein), a gene located ~500 kb downstream of the SUNO1 locus, in SUNO1-depleted cells (Figure 4Fb). Immunoblot analyses confirmed reduced WTIP protein in SUNO1-depleted cells (Figure 4C). Reduced levels of WTIP were also observed in SUNO1-depleted HCT116 p53-/- cells, indicating that the change in WTIP levels was not a consequence of cell-cycle arrest at G1 (Figure 4—figure supplement 1D).
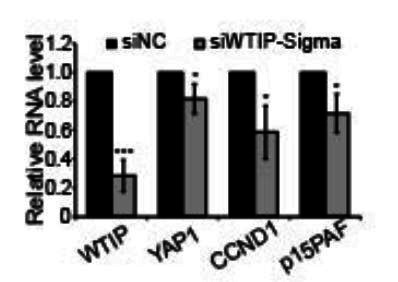
WTIP is a member of the mammalian Ajuba LIM family proteins, along with Ajuba and LIMD1 (Das Thakur et al., 2010). In Drosophila, Ajuba promotes cell proliferation by positively regulating YAP1 activity (Das Thakur et al., 2010). Ajuba LIM family proteins are adaptor proteins, which communicate cell adhesive events with nuclear responses to antagonize the LATS1-medited inhibitory phosphorylation of YAP1, thereby negatively regulating the Hippo signaling pathway (Harvey et al., 2013; Das Thakur et al., 2010). Ajuba LIM family proteins stabilizes YAP1 by negatively regulating the interaction between pLATS1 and YAP1 (Harvey et al., 2013). Given this crucial role of WTIP in regulating YAP1/Hippo signaling, we hypothesized that WTIP could be an important cis target of SUNO1, mediating SUNO1’s positive impact on cell proliferation. In support of this, we observed that WTIP expression was also regulated during cell cycle, with highest levels of WTIP mRNA and protein observed during G1/S and S-phases, a time window that coincided with the elevated levels of SUNO1 (Figure 4—figure supplement 1E–F). Furthermore, depletion of WTIP resulted in downregulation of YAP1, and YAP1 target mRNAs, such as CCND1 and CTGF (Figure 4—figure supplement 1G). Finally, both WTIP- and SUNO1-depleted cells showed similar cell-cycle phenotypes (G1 or G1/S arrest and reduced S-phase) (Figure 4—figure supplement 1Ha-b), implying potential epistatic regulation.
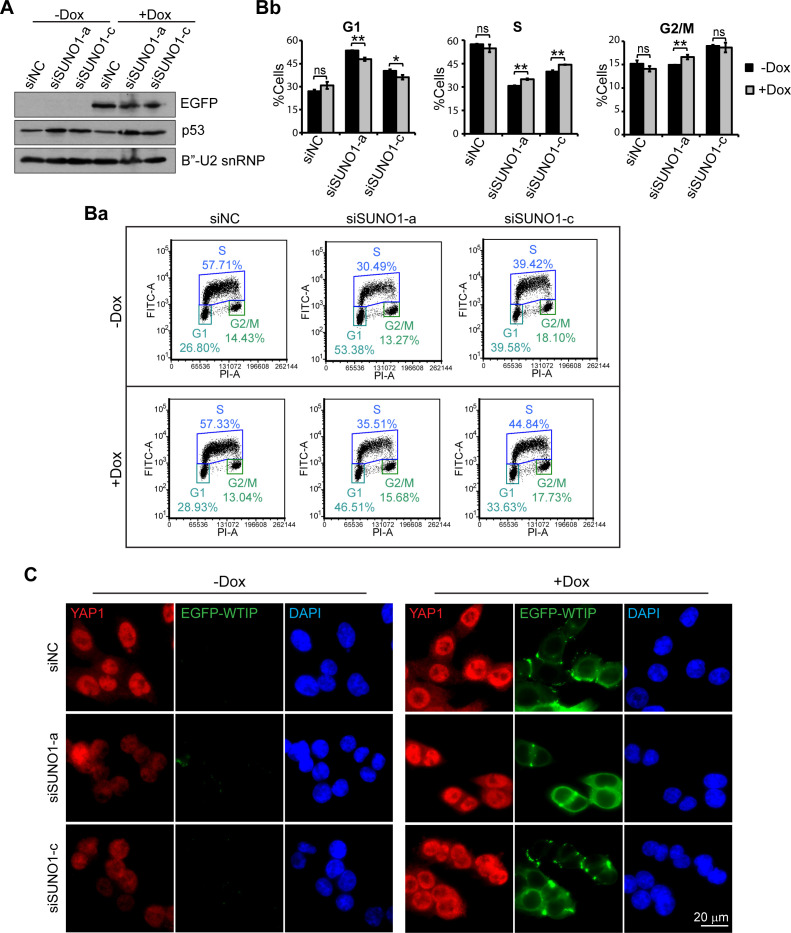
Finally, we have attempted to rescue the defects in cell cycle as well as cellular levels of YAP1 observed in SUNO1-depleted cells by stably overexpressing WTIP. To achieve this, we stably expressed a doxycycline (Dox)-inducible version of EGFP-WTIP cDNA (Ibar et al., 2018) in HCT116 cells. Upon treating the cells with Dox, we achieved stable overexpression of WTIP in control and SUNO1-depleted cells (Figure 4—figure supplement 2A). BrdU-PI-flow cytometry analyses revealed that overexpression of WTIP in cells depleted of SUNO1 partially rescued the cell-cycle defects. We observed a significant reduction in the G1 population (with a concomitant increase in S population) in SUNO1-depleted cells overexpressing WTIP (Figure 4—figure supplement 2Ba-b). However, the SUNO1-depleted cells, overexpressing WTIP continued to show p53 induction, and the p53 levels were comparable to SUNO1-depleted cells with no WTIP overexpression (Figure 4—figure supplement 2A). The absence of a complete rescue of cell-cycle defects in WTIP-overexpressed cells could be attributed to p53-mediated checkpoint activation. These results suggest that the DNA-damage phenotype observed in SUNO1-depleted cells may not be entirely due to defects in the WTIP/YAP1 pathway.
Next, we tested whether overexpression of WTIP in SUNO1-depleted cells rescues the cellular pool of YAP1. Immunofluorescence imaging revealed that SUNO1-depleted cells overexpressing EGFP-WTIP showed significant increase in the cellular pool of YAP1 compared to SUNO1-alone depleted cells (Figure 4—figure supplement 2C). These results indicate that SUNO1 modulates YAP1 levels by regulating the expression of WTIP.
SUNO1 promotes WTIP transcription via regulating DDX5-RNA polymerase II interaction on the chromatin
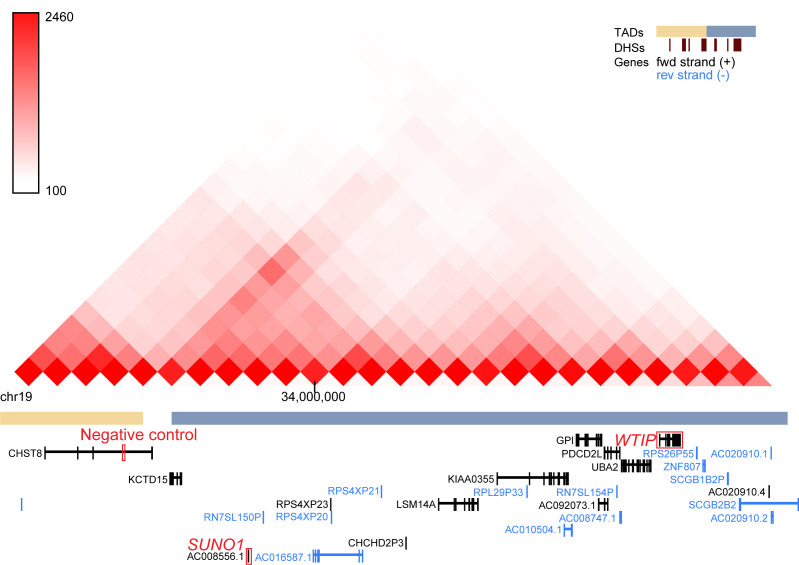
We proposed that physical association between SUNO1 and WTIP genes would facilitate the recruitment of the low-copy SUNO1 lncRNA to the WTIP gene locus for its regulatory function. Chromosome confirmation capture (3C) analyses revealed potential physical interaction between SUNO1 and WTIP gene locus in a SUNO1 lncRNA-independent manner (Figure 5A). The 3C data was further supported by the publicly available Hi-C data set in HCT116 showing that both SUNO1 and WTIP genes are located within a single TAD (Rao et al., 2017; Figure 5—figure supplement 1). On the other hand, the negative control gene locus, located next to the SUNO1 locus, but were part of a different TAD did not interact with the SUNO1 (Figure 5A and Figure 5—figure supplement 1).
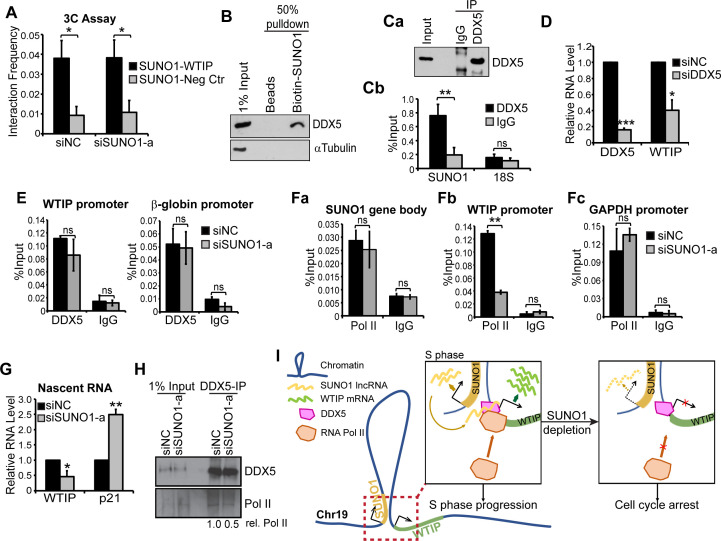
Figure 5. SUNO1 promotes WTIP transcription by stabilizing the interaction between DDX5 and RNA polymerase II on chromatin.
(A) 3C analyses to quantify the physical interaction frequency between SUNO1 and WTIP genes in presence or absence of SUNO1 RNA in WT HCT116 cells. Data are presented as Mean ± SD, n = 2. Unpaired one-tail t-tests are performed. *p<0.05, **p<0.01, ***p<0.001. (B) Western blot analysis to detect DDX5 and αTubulin in biotinylated RNA pulldown of SUNO1 in WT HCT116 cells. αTubulin serves as a negative control. (C) DDX5-RIP in WT HCT116 cells followed by (Ca) western blot to detect DDX5 and (Cb) RT-qPCR to quantify the levels of SUNO1 and 18S rRNA. 18S rRNA serves as a negative control for the binding of DDX5 to non-specific RNAs. Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. ns, not significant; *p<0.05; **p<0.01; ***p<0.001. (D) RT-qPCR to quantify relative levels of DDX5 and WTIP mRNAs in control and DDX5-depleted WT HCT116 cells. Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. *p<0.05, **p<0.01, ***p<0.001. (E) DDX5 ChIP-qPCR to quantify DDX5 association at the WTIP and β-globin promoter in control and SUNO1-depleted cells. IgG ChIP-qPCR on the same target genes serves as negative control. Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. ns, not significant. (F) RNA pol II ChIP-qPCR to quantify RNA pol II association at the SUNO1 gene body (Fa), WTIP promoter (Fb), and GAPDH promoter (Fc) in control and SUNO1-depleted cells. IgG ChIP-qPCR on the same target genes serves as negative control. Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. ns, not significant; *p<0.05; **p<0.01; ***p<0.001. (G) Click-iT nascent RNA capture assays followed by RT-qPCR to quantify relative pre-mRNA levels of WTIP and p21 in control versus SUNO1-depleted WT HCT116 cells. Note: increased levels of p21 nascent RNA (a direct target of p53) in SUNO1-depleted cells confirm DNA-damage-induced p53-mediated check-point activation upon SUNO1 depletion. Data are presented as Mean ± SD, n = 3. Unpaired two-tail t-tests are performed. *p<0.05, **p<0.01, ***p<0.001. (H) DDX5-IP on chromatin followed by DDX5 and RNA pol II immunoblot assays to detect the relative levels of RNA pol II that are associated with DDX5 on chromatin in control and SUNO1-depleted WT HCT116 cells. (I) Model depicting the mode of action of SUNO1 in regulating the transcription of WTIP. During S-phase, enhanced levels of SUNO1 lncRNA promotes WTIP transcription by stabilizing the chromatin interactions between DDX5 and RNA pol II on promoters of genes such as WTIP. In the absence of SUNO1, WTIP transcription is compromised due to defects in the loading of RNA pol II.
Figure 5—figure supplement 1. SUNO1 and WTIP locate in a single TAD. Hi-C data covering SUNO1, WTIP, and the negative control genomic loci in HCT116 cells (Rao et al., 2017; GSE104334) is visualized by 3D Genome Browser (http://promoter.bx.psu.edu/hi-c/view.php).
Figure 5—figure supplement 2. SUNO1 interacts with DDX5.
LncRNAs regulate the expression of genes by facilitating the recruitment or stabilization of TFs, co-factors, chromatin regulators or RNA-binding proteins to chromatin or RNA (Sun et al., 2018a; Chen and Carmichael, 2010). In order to determine the molecular mechanism utilized by SUNO1 to promote WTIP transcription, we searched for SUNO1-interacting proteins that could regulate WTIP transcription. For this, in vitro transcribed biotinylated- SUNO1 RNA (2.1 Kb isoform) was incubated with cell lysate, then SUNO1-interacting proteins were pulled down by streptavidin affinity purification followed by mass spectrometry analysis. Biotin-labeled YFP RNA was used as negative control. We identified several proteins that were enriched in the SUNO1 RNA pull down (Supplementary file 7). We focused on the interaction between SUNO1 and one of its interactors, DDX5 (also known as p68), because of its known function as a transcription co-activator of cell-cycle genes (Fuller-Pace, 2013; Figure 5—figure supplement 2A–B). The interaction between DDX5 protein and SUNO1 lncRNA was confirmed by western blot analysis (Figure 5B) as well as RNA-Immunoprecipitation (RIP) using antibody against DDX5 followed by RT-qPCR to detect SUNO1 (Figure 5Ca-b). DDX5 is a DEAD box RNA helicase, and also acts as a transcriptional co-factor to modulate the activity of several cell proliferation-promoting TFs (Fuller-Pace, 2013). For example, DDX5 has been reported to promote E2F1-, p53-, Androgen receptor- and β-catenin-mediated transcription of genes controlling cell-cycle progression and DDR (Nicol et al., 2013; Clark et al., 2013; Wagner et al., 2012; Bates et al., 2005; Mazurek et al., 2012). In addition, studies have reported the involvement of ncRNAs in regulating the co-activator activity of DDX5 (Caretti et al., 2006). Based on this, we hypothesized that SUNO1 may facilitate the DDX5-mediated transcription of WTIP during the cell cycle. Cells depleted of DDX5 showed reduced levels of WTIP mRNA, indicating that DDX5 positively regulates WTIP expression (Figure 5D). DDX5 ChIP-qPCR in control cells revealed the association of DDX5 to the WTIP and β-globin (positive control) promoters (Figure 5E). However, DDX5 continued to associate with both WTIP and β-globin promoters even in SUNO1-depleted cells, implying that SUNO1 did not recruit/stabilize DDX5 to WTIP regulatory elements (Figure 5E). Recent studies have reported that DDX5 promotes the transcription of cell-cycle genes by recruiting or stabilizing RNA polymerase II (RNA pol II) (Clark et al., 2013; Mazurek et al., 2012; Rossow and Janknecht, 2003). We therefore quantified the RNA pol II association to WTIP promoter in the presence or absence of SUNO1. Initially, we determined the association of RNA pol II in the SUNO1 gene body of cells treated with control siRNA as well as siRNA targeting the 3’end of SUNO1. ChIP-qPCR assay revealed that RNA pol II showed comparable levels of binding to the SUNO1 gene body in control and SUNO1 siRNA-treated cells (Figure 5Fa). These results imply that siRNA targeting the 3’end of the SUNO1 gene (Figure 2—figure supplement 3A for siSUNO1a position) only degraded SUNO1 lncRNA and did not affect the transcription from the SUNO1 locus. In support of this, a recent study demonstrated that antisense oligonucleotides targeting the 3’end of the gene normally degrade only the transcript without impacting the transcription from the locus (Lee and Mendell, 2020). On the other hand, we observed significantly reduced association of RNA pol II to WTIP promoter in SUNO1-depleted cells (Figure 5Fb), compared to the control GAPDH promoter, which showed comparable levels of RNA pol II in control and SUNO1-depleted cells (Figure 5Fc). Nascent RNA capture followed by RT-qPCR revealed that SUNO1-depleted cells showed a significant reduction in the levels of nascent WTIP pre-mRNA (Figure 5G), further supporting the earlier result that SUNO1 depletion reduced RNA pol II activity at WTIP locus. Increased levels of p21 pre-mRNA observed in SUNO1-depleted cells, due to p53-mediated G1 checkpoint activation, was used as a positive control. We then examined whether SUNO1 influenced the DDX5-mediated recruitment/stabilization of RNA pol II to gene promoters. Towards this, we tested the DDX5-RNA pol II interaction on chromatin in control versus SUNO1-depleted cells by DDX5-chromatin-IP in formaldehyde-crosslinked cell lysate followed by immunoblot assays. Control cells showed specific interaction between DDX5 and RNA pol II on chromatin (Figure 5H). However, SUNO1-depleted cells significantly compromised the interaction between DDX5 and RNA pol II on chromatin (Figure 5H). Based on this, we conclude that SUNO1 lncRNA influences DDX5-mediated recruitment/stabilization of RNA pol II on the promoter in cis, thereby enhancing WTIP transcription (Figure 5I).
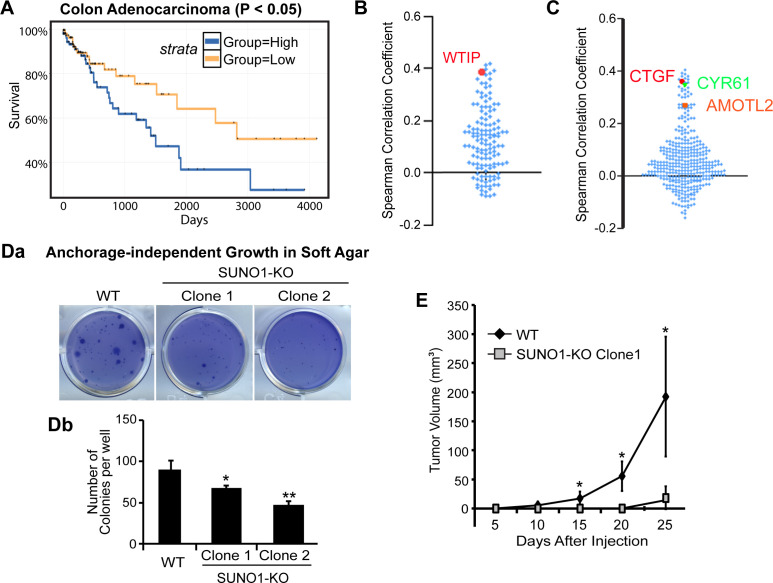
SUNO1 promotes tumorigenicity in colon cancer cells
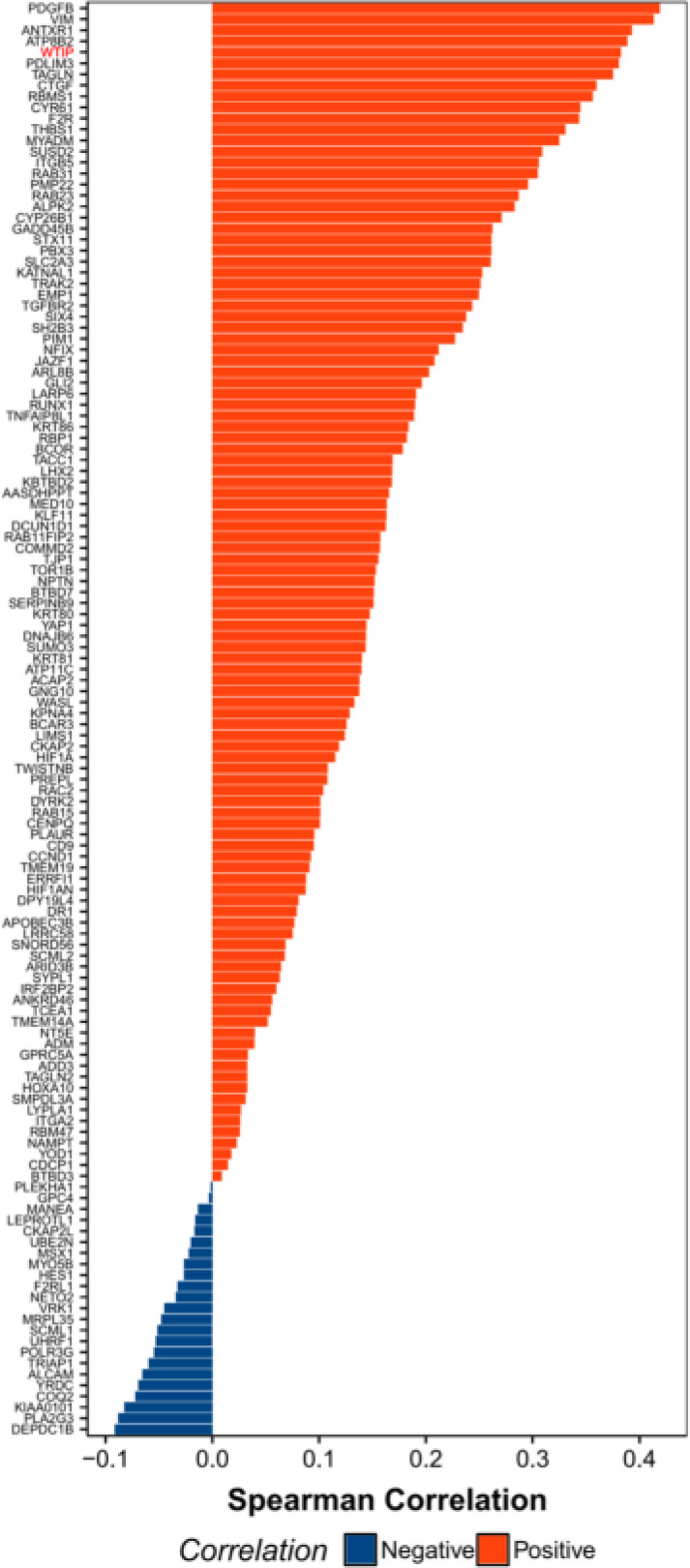
Our results indicate a pro-proliferative function of SUNO1. Since we demonstrated that SUNO1 facilitates the well-established oncogene YAP1-mediated transcription of genes promoting cell proliferation in colon carcinoma cells (HCT116), we wondered whether SUNO1 contributes to tumor progression. Patient survival analyses using the colon adenocarcinoma samples from the TCGA data set revealed that patients with higher SUNO1 levels displayed significantly shorter survival compared to patients with lower SUNO1 expression, indicating that a high SUNO1 level is associated with poor prognosis in colon adenocarcinoma (Figure 6A). Next, we tested whether the ~149 genes that showed reduced expression in SUNO1-depleted cells also exhibited synchronous change in expression patterns in the TCGA colon cancer patient cohort. Interestingly, a major fraction of these genes (71%), including WTIP, showed positive correlation in expression with SUNO1 across colon cancer patients, implying that SUNO1 potentially regulates the expression of these genes even in cancer tissue samples (Figure 6B and Figure 6—figure supplement 1). Finally, we also observed a positive correlation between the levels of SUNO1 and a significant number of YAP1-target mRNAs in the same patients, supporting our data that SUNO1 regulates YAP1-mediated transcriptional program (Figure 6C; Zhao et al., 2008; Kapoor et al., 2014; Shao et al., 2014; Zanconato et al., 2015).
Figure 6. SUNO1 contributes to tumorigenicity under in vitro and in vivo conditions.
(A) Kaplan-Meier analyses to depict the survival rate in TCGA colon adenocarcinoma patients with high and low levels of SUNO1. Expression levels are separated into high and low levels across cancer samples based on median. (B) Spearman correlation of the expression levels of the 149 genes that are downregulated in SUNO1-depleted cells (Figure 4A; Supplementary file 6) with SUNO1 in colon adenocarcinoma patient tumor samples. Each dot represents one of the downregulated genes upon SUNO1 knockdown, and its Spearman correlation coefficient with SUNO1 is plotted. All of the included positively correlated genes with SUNO1 exhibited a p-value<0.01 at a 5% FDR. WTIP is highlighted in red. (C) Spearman correlation of the expression levels of YAP1/TAZ/TEAD target genes with SUNO1 in colon adenocarcinoma patient tumor samples. Each dot represents one of the YAP1/TAZ/TEAD direct target genes, and its Spearman correlation coefficient with SUNO1 is plotted. CTGF, CYR61 and AMOTL2 is highlighted. (Da–b) Long-term anchorage-independent colony formation assay in soft agar of wild-type and SUNO1-CRISPR KO HCT116 (Clone one and Clone 2) cells. (E) Tumor formation of wild-type control and SUNO1-CRISPR KO HCT116 (clone 1) cells in mouse xenograft experiments. Data are presented as Mean ± SD, n = 5. Paired two-tail t-tests are performed. *p<0.05, **p<0.01, ***p<0.001.
Figure 6—figure supplement 1. Distribution of spearman correlation values for various genes with respect to SUNO1 across the colon adenocarcinoma cancer samples from the TCGA project.
Further, to test the involvement of SUNO1 in tumor progression, we performed anchorage-independent growth assays in wild-type and SUNO1-KO HCT116 cells. In contrast to wild-type HCT116 cells, SUNO1-KO cells significantly lost their ability to form colonies in soft agar, revealing the requirement of SUNO1 for the tumorigenicity of HCT116 cells under in vitro conditions (Figure 6D). We next performed tumor xenograft assay to examine the effect of SUNO1 deletion on primary tumor growth in vivo. SUNO1-KO HCT116 and control HCT116 cells were injected subcutaneously into the flanks of immune compromised mice, and the tumor sizes were monitored for 25–30 days post-injection. The tumor growth in SUNO1-KO cells was significantly compromised compared to control HCT116 cells (Figure 6E). These data collectively support the model that SUNO1 participates in tumorigenesis.
Discussion
In this study, we performed a comprehensive analysis to understand human lncRNA expression during the cell cycle. We identified >2000 lncRNAs with periodic expression patterns peaking at a specific cell-cycle phase. To demonstrate that the cell-cycle phase-specific expressed lncRNAs regulate vital cellular processes, we characterized the function of SUNO1, an S-phase-enriched lncRNA in cell proliferation. We observed that SUNO1 regulated the expression of WTIP, a member of AJUBA family of proteins that repress Hippo signaling pathway. Furthermore, we have provided evidence indicating that SUNO1 promoted transcription by facilitating the co-activator, DDX5-mediated recruitment/stabilization of RNA pol II on chromatin.
DDX5 is an established RNA helicase involved in multiple processes of RNA metabolism, including pre-mRNA splicing, rRNA and miRNA processing (Fuller-Pace, 2013). In addition, it is becoming increasingly clear that DDX5 also acts as transcription co-activator or co-repressor in a context-dependent manner via interacting with specific TFs or RNA pol II (Fuller-Pace, 2013). For example, in response to DNA damage, it interacts with and co-activates p53 to mediate cell-cycle arrest (Nicol et al., 2013). However, during normal cell-cycle progression, DDX5 stimulates the recruitment/stabilization of RNA pol II to the promoters of E2F1-regulated DNA replication factor genes, thereby promoting cell proliferation (Mazurek et al., 2012). Several other studies have also demonstrated the involvement of DDX5 in regulating RNA pol II activity, though the exact mechanism is yet to be established (Clark et al., 2013; Rossow and Janknecht, 2003). Interestingly, DDX5 is known to interact with ncRNAs (Caretti et al., 2006; Das et al., 2018). DDX5 facilitates the transcriptional activity of MyoD by forming a complex with the ncRNA SRA in muscle cells (Caretti et al., 2006). In the present study, we demonstrated that early S-phase- upregulated SUNO1, by forming a complex with DDX5, promotes the association between DDX5 and RNA pol II on chromatin, thereby promoting transcription of genes such as WTIP. Reduced WTIP mRNA level in DDX5-depleted cells further support the role of DDX5 as a regulator of WTIP transcription. Future studies will address how SUNO1 influences the DDX5-mediated recruitment of RNA pol II specifically at the WTIP or other gene promoters. It is possible that the SUNO1-DDX5 RNP complex at WTIP promoter may either confer specificity in recruiting RNA pol II to WTIP promoter, and/or stimulate the transcriptional co-activator activity of DDX5. Earlier studies, demonstrating the role of the ncRNA, SRA in promoting DDX5 activity support such a model (Caretti et al., 2006). In addition, a recent study showed that the CONCR lncRNA interacts with another helicase, DDX11, and regulates its enzymatic activity (Marchese et al., 2016). We therefore speculate that the mode of action of SUNO1 may represent a wider spread mechanism in which lncRNAs interact with DEAD box family DNA/RNA helicases to modulate their location and activity.
The Hippo pathway controls organ size and tissue homeostasis in diverse species through regulating cell proliferation, apoptosis and stemness, whereas its deregulation contributes to tumor progression in a broad range of human carcinomas. Despite the fact that Hippo pathway activity is frequently deregulated in different human cancers, somatic or germline mutations in Hippo pathway genes are uncommon (Harvey et al., 2013; Yu et al., 2015). Here, by identifying the lncRNA SUNO1 as a cis activator of WTIP, that positively regulates YAP1, we hypothesize that SUNO1 acts as an oncogene via inhibiting the Hippo pathway. Our hypothesis is supported by the observation that elevated expression of SUNO1 correlates with poor prognosis in colon adenocarcinoma, and further tumor assays revealed that SUNO1 is required for the tumorigenicity of colon cell lines.
We observed that SUNO1 was induced upon DNA damage. Furthermore, SUNO1-depleted cells showed slow S-phase progression post release from HU-mediated DNA damage, due to defects in replication origin re-activation. Also, SUNO1-depleted cells showed enhanced sensitivity to DNA damage. At present, the role of SUNO1 in DDR is unclear. Interestingly, SUNO1-depleted cells failed to activate ATR-mediated DNA-damage checkpoint during HU treatment, as observed by reduced phosphorylation of several of ATR and CHK1 substrates. This could be due to the fact that SUNO1-depleted cells fail to enter S-phase as the ATR-mediated check point is active during S and G2 phase of the cell cycle (Buisson et al., 2015; Zeman and Cimprich, 2014). We propose that SUNO1 contributes to DDR by modulating the expression of genes that regulate DDR. In support of this, SUNO1-depleted cells showed reduced expression of several genes (GADD45B, CEBPA, UHRF1, P51/PAF) that contribute to DDR (data not shown) (Supplementary file 6), though the mode of action is yet to be determined. Alternatively, the above-described phenotypes observed in SUNO1-depleted cells could be a consequence of aberrant replication stress. Dormant replication origins are activated following replication stress to ensure completion of DNA replication at stalled forks (Zeman and Cimprich, 2014). However, SUNO1-depleted cells showed reduced number of new origins firing post-HU treatment, and this could result in delayed S-phase progression. Future studies will test whether SUNO1 actually plays a role in DDR or the aberrant DNA-damage phenotype observed in SUNO1-depleted cells is a consequence of error in origin licensing.
LncRNAs regulate cell proliferation and survival, by regulating the expression of cell-cycle-regulated protein-coding genes, such as cyclins or CDKs or CDK inhibitors (Kitagawa et al., 2013). Also, lncRNAs that are transcribed from the promoters of cell-cycle regulators have coordinated transcription of their respective protein-coding gene partners (Hung et al., 2011). A recent study using nascent DNA strand sequencing, identified >1000 s of lncRNAs to be induced during S-phase, and a significant number of these RNAs showed differential expression in pan-cancer samples (Ali et al., 2018). Further, loss-of-function studies revealed that several of the lncRNAs contribute to cancer progression, underpinning the important roles played by cell-cycle-regulated ncRNAs in cancer (Ali et al., 2018). Similarly, an independent study from the RIKEN group reported that depletion of a significant number of lncRNAs resulted in cell-cycle defects, further supporting the involvement of lncRNAs in cell-cycle progression (Ramilowski et al., 2020). These individual examples, though strengthened the argument about the importance of lncRNAs in cell cycle, failed to provide a genome-wide understanding of the crucial roles played by thousands of uncharacterized lncRNAs in cell proliferation. We have identified several hundreds of lncRNAs that displayed cell-cycle phase-specific expression. As a proof of principle, we have demonstrated the vital role for SUNO1 in promoting YAP1-mediated expression of genes controlling cell proliferation. It is evident that similar to proteins, lncRNAs could constitute organized programs of biological activities that are required for efficient cell proliferation. Our study would be the first step in the continuum of research that is expected to lead to the functional characterization of a large number of cell-cycle-regulated lncRNAs.
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Anti-BrdU (Mose monoclonal) | Sigma-Aldrich | B9434 | IF (1:800) |
| Antibody | Anti-MCM3 (Rabbit polyclonal) | Stillman B. lab, CSHL | clone 738 | WB (1:1000) |
| Antibody | Anti-Orc2 (Rabbit polyclonal) | Stillman B. lab, CSHL | clone 205–6 | WB (1:500) |
| Antibody | Anti-SRSF1 (Mouse monoclonal) | Krainer A. lab, CSHL | clone 96 | WB (1:1000) |
| Antibody | Anti-p53 (Mouse monoclonal) | Santa Cruz | sc-126 | WB (1:500) |
| Antibody | Anti-B’-U2 snRNP (Mouse polyclonal) | Spector lab, CSHL | clone 4G3 | WB (1:250) |
| Antibody | Anti-Chk1 (Rabbit polyclonal) | Cell Signaling | #2345 | WB (1:500) |
| Antibody | Anti-pChk1-S345 (Rabbit polyclonal) | Cell Signaling | #2348 | WB (1:500) |
| Antibody | Anti-Chk2 (Rabbit polyclonal) | Cell Signaling | #2662 | WB (1:500) |
| Antibody | Anti-pChk2-T68 (Rabbit polyclonal) | Cell Signaling | #2661 | WB (1:500) |
| Antibody | Anti-pBRCA1-S1524 (Rabbit polyclonal) | Cell Signaling | #9009 | WB (1:400) |
| Antibody | Anti-RPA32 (Rat polyclonal) | Cell Signaling | #2208 | WB (1:700), IF (1:500) |
| Antibody | Anti-γH2AX (Rabbit monoclonal) | Cell Signaling | #9718 | WB (1:700) |
| Antibody | Anti-αTubulin (Mouse monoclonal) | Sigma-Aldrich | T5168 | WB (1:5000) |
| Antibody | Anti-WTIP (Mouse polyclonal) | Sigma-Aldrich | SAB1411722 | WB (1:200) |
| Antibody | Anti-Cyclin D1 (Rabbit polyclonal) | Cell Signaling | #2922 | WB (1:500) |
| Antibody | Anti-LATS1 (Mouse monoclonal) | Santa Cruz | sc-398560 | WB (1:100) |
| Antibody | Anti-pLATS1-S909 (Rabbit polyclonal) | Cell Signaling | #9157 | WB (1:1000) |
| Antibody | Anti-YAP1 (Mouse monoclonal) | Santa Cruz | sc-376830 | WB (1:100), IF (1:50) |
| Antibody | Anti-TAZ (Mouse monoclonal) | Santa Cruz | sc-518036 | WB (1:100) |
| Antibody | Anti-CTGF (Mouse monoclonal) | Santa Cruz | sc-365970 | WB (1:100) |
| Antibody | Anti-β-Actin (Mouse monoclonal) | Santa Cruz | sc-47778 | WB (1:300) |
| Antibody | Anti-p15/PAF (Rabbit polyclonal) | Santa Cruz | sc-9996 | WB (1:200) |
| Antibody | Anti-GFP (Mouse monoclonal) | Santa Cruz | sc-67280 | WB (1:100) |
| Antibody | Anti-DDX5 (Mouse monoclonal) | Millipore | clone204, #05–580 | WB (1:200) |
| Antibody | Anti-Pol II (Mouse monoclonal) | Millipore | clone CTD4H8, #05–623 | WB (1:1000), ChIP (5 μg/experiment) |
| Antibody | Anti-53BP1 (Rabbit polyclonal) | Cell Signaling | #4937 | IF (1:300) |
| Antibody | Anti-DDX5 (Rabbit polyclonal) | BETHYL | A300-523A | ChIP (5 μg/experiment) |
| Antibody | Anti-BrdU (CldU) (Rat monoclonal) | Bio-Rad | OBT0030G, Clone BU1/75 (ICR1) | DNA fiber assay (1:200) |
| Antibody | Anti-BrdU (IdU) (Mouse monoclonal) | BD | #347580, clone B44 | DNA fiber assay (1:200) |
| Transfected construct | pT3.5 Caggs-FLAG-hCas9 | This paper | Construct to express Cas9 for making KO cell lines | |
| Transfected construct | pCR4-TOPO-U6-gRNA | This paper | Backbone of the construct to express gRNAs for making KO cell lines | |
| Transfected construct | pcDNA-PB7 | This paper | Construct to express PiggyBac transposase for making KO cell lines | |
| Transfected construct | pPBSB-CG-Luc-GFP-Puro | This paper | Construct to express the puromycin resistent gene for making KO cell lines | |
| Transfected construct (human) | pTRIPZ-EGFP:WTIP | Addgene Ibar et al., 2018 | #66953 | Lentiviral vector for Tet-inducible EGFP:WTIP fusion protein expression |
| Commercial assay or kit | FITC BrdU Flow Kit (RUO) | BD Pharmingen | #559619 | |
| Commercial assay or kit | ChIP-IT High Sensitivity kit | Active Motif | #53040 | |
| Commercial assay or kit | CometAssay Kit | Trevigen | 4250–050 K | |
| Commercial assay or kit | Dual-Luciferase Reporter Assay System | Promega | E1910 | |
| Commercial assay or kit | Click-iT Nascent RNA Capture Kit | Invitrogen | C10365 | |
| Commercial assay or kit | FiberPrep (DNA Extraction Kit) | Genomic vision | EXTR-001 | |
| Cell line (H. sapiens) | HCT116 | ATCC | CCL-247 | |
| Cell line (H. sapiens) | BT20 | ATCC | HTB-19 | |
| Cell line (H. sapiens) | U2OS | ATCC | HTB-96 | |
| Cell line (H. sapiens) | HeLa | ATCC | CCL-2 | |
| Cell line (H. sapiens) | HCT116 p53 -/- | Vogelstein B. lab, Johns Hopkins Uni. | ||
| Chemical compound, drug | Thymidine | Sigma-Aldrich | T9250 | |
| Chemical compound, drug | Nocodazole | Sigma-Aldrich | M1404 | |
| Chemical compound, drug | Doxorubicin hydrochloride | Sigma-Aldrich | D1515 | |
| Chemical compound, drug | Etoposide | Sigma-Aldrich | E1383 | |
| Chemical compound, drug | Hydroxyurea | Sigma-Aldrich | H8627 | |
| Chemical compound, drug | Nutlin-3 | Sigma-Aldrich | N6287 | |
| Chemical compound, drug | Actinomycin D | Sigma-Aldrich | A9415 | |
| Chemical compound, drug | Doxycyline Hyclate | Sigma-Aldrich | D9891 | |
| Chemical compound, drug | BrdU | Sigma-Aldrich | B9285 | |
| Chemical compound, drug | EdU | Invitrogen | A10044 | |
| Chemical compound, drug | CldU | Sigma-Aldrich | C6891 | |
| Chemical compound, drug | IdU | MP Biomedicals | SKU02100357.2 | |
| Chemical compound, drug | Alexa Fluor 488 Azide | Invitrogen | A10266 | |
| Sequence-based reagent | SUNO1-5'gRNA | This paper | gRNA for SCRISPR KO | CCTAACCTAGATCTCCC |
| Sequence-based reagent | SUNO1-3'gRNA | This paper | gRNA for SCRISPR KO | AGGGTGGACAGGGATGC |
| Sequence-based reagent | SUNO1-F | This paper | qPCR primers | CACCAACAGACGTGAGTTCGA |
| Sequence-based reagent | SUNO1-R | This paper | qPCR primers | AGAACACTGCGAGGCTCACA |
| Sequence-based reagent | siNC | This paper | control siRNA | targeted sequence: UUCUCCGAACGUGUCACGU |
| Sequence-based reagent | siSUNO1-a | This paper | SUNO1-specific siRNA | targeted sequence: GCACGUGGUAAUACAUAAU |
| Sequence-based reagent | siSUNO1-b | This paper | SUNO1-specific siRNA | targeted sequence: GAGGAAUGCUGAUCUAGAA |
| Sequence-based reagent | siSUNO1-c | This paper | SUNO1-specific siRNA | targeted sequence: GGCGUGAUUUAGAUGGAAA |
| Transfected construct (Human) | siRNA to WTIP (SMARTpool) | Dharmacon | L-023639-02-0005 |
Cell lines
U2OS and HeLa cells were grown in DMEM medium. HCT116 WT and p53-/- cells were grown in McCoy’s 5A medium. BT-20 cells were grown in EMEM medium. All media were supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin. Cells were maintained in a 5% CO2 incubator at 37°C. Cell lines are obtained from commercial vendors such as ATCC. We confirm that the identity of all cell lines used in our study has been authenticated by STR profiling. All cell lines were checked for mycoplasma.
Generation of SUNO1 CRISPR KO cell lines
SUNO1 CRISPR KO HCT116 and U2OS clones were made by transiently transfecting pT3.5 Caggs-FLAG-hCas9, gRNA expressing plasmids (in pCR4-TOPO-U6-gRNA), PiggyBac Transposase expressing plasmid (pcDNA-PB7) and pPBSB-CG-Luc-GFP-Puro. Selection was carried out with 2 μg/ml of puromycin followed by single clone selection. The KO clones were confirmed by PCR followed by DNA sequencing.
Generation of stable cell lines
pTRIPZ-EGFP:WTIP was a gift from Kenneth Irvine (Ibar et al., 2018; Addgene plasmid #108231). HCT116 cells were incubated with the lentiviral particles for 2 days. Cells were then selected in medium containing 1 μg/ml puromycin for 3 days. EGFP-WTIP was induced by adding 0.05 μg/ml of Doxycycline (DOX) 24 hr prior to siRNA transfection.
Cell synchronization
U2OS cells were synchronized to different cell-cycle stages as previously described (Tripathi et al., 2013). Briefly, cells were synchronized to mitosis by treatment with 50 ng/ml nocodazole for 12 hr. To collect cells in G1 phase, mitotic cells were shaken off and released in fresh medium for 3.5 hr. G1/S-boundary, S-phase and G2-phase samples were collected by double-thymidine block and release. G1/S samples were collected after the second block. Cells were then released in fresh medium for 4 hr to be collected as S-phase samples and 8 hr to be collected as G2-phase samples.
RNA extraction and quantitative real-time PCR (RT-qPCR)
RNA was extracted using Trizol reagent (Invitrogen) as per manufacturer’s instructions. Samples for RNA-seq were further cleaned up by RNeasy Mini Kit (QIAGEN). RNA was reverse transcribed into cDNA by Multiscribe Reverse Transcriptase and Random Hexamers (Applied Biosystems). One-step RT-PCR was performed as previously described (Sun et al., 2018b; Caretti et al., 2006).
Bioinformatics and statistical analyses of RNA-seq data
The RNA-seq libraries were prepared with Illumina's 'TruSeq Stranded mRNAseq Sample Prep kit' (Illumina). Paired-end, polyA+ RNA-sequencing was performed on Illumina platform (Novaseq 6000, SP flowcell) at the Roy J. Carver Biotechnology Center at UIUC. The RNA-seq are deposited in GEO with accession number GSE143275. High quality of RNA-seq reads was confirmed by FASTQC. RNA-seq reads were aligned to human reference genome GRCh38 assembly using HISAT2 (Kim et al., 2015) with alignment rate ~98% for all samples. Transcript assembly and expression assessment was performed by Stringtie (Pertea et al., 2015) to get the TPM (Transcripts Per Million) values for each gene. For direct visualization of RNA-seq signals, BigWig files were generated using deepTools with bamCoverage function, with RPKM normalization (Ramírez et al., 2014). Biological duplicates were merged via bigWigMerge (ucsc-bigwigmerge tools). Final bigwig files were visualized using both UCSC genome or Integrated Genome viewer (IGV).
Categorization of gene type was extracted from GRCh38 assembly GTF file downloaded from Ensemble (v94, from https://useast.ensembl.org/info/data/ftp/index.html). We summarized all types of pseudogenes into ‘pseudogene’ category. And ‘others’ refer to all the rest classes in our summary tables. The categories that were included in ‘lncRNAs’ in this study are described in Supplementary file 1: biotype_of_24087_genes.
For statistical analyses, raw gene counts were first analyzed by HTSEQ-Count (Anders et al., 2015), then analyzed using edgeR (Robinson et al., 2010). Qualifiable expression was defined by CPM >= 0.075 in at least two samples out of total 10 samples. Normalization of library size was performed. For visualization of transcriptome, heatmaps were plotted using coolmap function from limma package (Ritchie et al., 2015), with row centering and scaling. Hierarchical clustering of genes (rows) was performed with complete-linkage method. Differential expression analyses were performed using exactTest between every two adjacent cell-cycle phases. Differentially expressed genes (DEGs) were defined by |fold change| >= 1.5 fold and FDR < 0.05. Phase-specific genes were further filtered from DEG lists by these criteria: (1) Genes show highest expression in that cell-cycle stage; (2) Significantly (FDR < 0.05) upregulated for >= 1.5 fold when compared to the two adjacent cell-cycle stages.
Gene ontology analyses (biological processes, Kegg pathway analyses) and GSEA (gene set enrichment analysis) were performed using clusterprofiler of Bioconductor (Yu et al., 2012). Specifically, gene ontology for biological process was performed using enrichGO function, Kegg pathway analyses was performed using enrichKEGG. All enrichment analyses include using background gene list containing all 24087 genes which showed qualifiable expression in the RNA-seq. Gene ontology networks results were visualized using Cytoscape. GSEA analysis was performed using gseGO function and gene lists were ranked using logFC values.
siRNA treatment
SUNO1 siRNAs (listed in File 8) (Sigma) were transfected to cells, at a final concentration of 20 nM for twice (48 hr) with a gap of 24 hr, using Lipofectamine RNAiMax reagent (Invitrogen). Then cells were further cultured for another day before harvest. WTIP SMARTpool siRNAs were transfected to cells at a final concentration of 25 nM for twice. For the short-term SUNO1 depletion, performed for the microarray analysis in Figure 4A, only one transfection of siSUNO1 was applied, then cells were harvested 36 hr post transfection.
Nuclear/cytoplasmic fractionation and chromatin fractionation
For nuclear and cytoplasmic fractionation, U2OS cells were lysed in lysis buffer (10 mM Tris-HCl (pH 7.4), 100 mM NaCl, 2.5 mM MgCl2 and 40 μg/ml digitonin) by incubation on ice for 10 min. Nuclei were collected by centrifugation at 2,000 g at 4°C and lysed in Trizol reagent (Invitrogen). The supernatant was collected as the cytoplasmic fraction and mixed with Trizol LS reagent (Invitrogen) for RNA extraction.
For chromatin fractionation, U2OS cells were resuspended with solution A (10 mM HEPES pH7.9, 10 mM KCl, 1.5 mM MgCl2, 0.34M sucrose, 1 mM DTT, 10% glycerol and 0.1% Triton X-100) and incubated on ice for 5 min. The cytoplasmic fraction (S2) was then separated from the nuclei by centrifuging at 4°C at 1,400 g for 4 min. Isolated nuclei were then washed with solution A without Triton X-100. The nuclei pellet was resuspended with solution B (3 mM EDTA, 0.2 mM EGTA, and 1 mM DTT) and incubated on ice for 30 min. The nuclear soluble fraction (S3) was then separated by centrifuging at 4°C at 1700xg for 4 min. The isolated chromatin fraction was then washed with buffer B. The chromatin pellet (P3) was resuspended in solution A and sonicated for 1 min.
Single-molecule fluorescence RNA in-situ hybridization (smFISH)
The SUNO1 smFISH probe set was designed using Stellaris Probe Designer (accession number AK124080.1), consisted of 32 20-mer DNA oligonucleotides. Oligonucleotides with a 3’amino group (LGC Biosearch Technologies) were pooled and coupled with Cy3 Mono NHS Ester (GE Healthcare).
HCT116 WT and SUNO1 KO cells were seeded on coverslips coated with poly-L-lysine two days before experiments. At harvest, cells were fixed with freshly prepared fixative (3:1 Methanol-Glacial Acetic Acid) for 10 min at room temperature and washed with washing buffer (10% formamide, 2XSSC) for 5 min. Probe was added to hybridization buffer (10% dextran sulfate, 10% formamide in 2X SSC) at a final concentration of 125 nM. Hybridization was carried out as described in Orjalo and Johansson, 2016 (Orjalo and Johansson, 2016) in a humidified chamber in the dark for 2 hr at 37°C. After hybridization, the coverslips were washed twice with wash buffer, 30 min for each wash, in the dark at 37°C. DNA was counterstained by DAPI during the second wash. The coverslips were then washed with 4XSSC for 5 min at room temperature and mounted in VectaShield Antifade Mounting Medium (Vector Laboratories). Images were taken using Zeiss Axiovert 200M microscope equipped with Cascade 512b high sensitivity camera.
Northern blotting
Poly A+ RNA was fractionated from total RNA by NucleoTrap mRNA Mini Kit (Macherey-Nagel). 5 μg of Poly A+ RNA from HCT116 WT or KO cells were separated on 1% agarose gel prepared with NorthernMax Denaturing Gel Buffer (Ambion) and run in NorthernMax Running Buffer (Ambion). RNAs were then transferred to Amersham Hybond-N+ blot (GE Healthcare) by capillary transfer in 10 x SSC and crosslinked to the blot by UV (254 nm, 120mJ/cm2).
The DNA probes were labeled with [α−32P] dCTP by Prime-It II Random Primer Labeling Kit (Stratagene) as per manufacturer’s instructions. Hybridization was carried out using ULTRAhyb Hybridization Buffer (Ambion) containing 1 × 106 cpm/ml of denatured radiolabeled probes overnight at 42°C. Blots were then washed with 2 x SSC, 0.1% SDS and 0.1 x SSC, 0.1% SDS sequentially at 42°C, and developed using phosphor-imager.
Flow cytometry
For PI flow, cells were fixed by 90% chilled ethanol overnight. Fixed cells were washed and resuspended in PBS containing 1% NGS and then incubated with 10 μg/ml of RNase A and 120 μg/ml of propidium iodine (PI) for 30 min in the dark at 37°C. For BrdU-PI flow, cells were pulsed with 50 μM BrdU for 30 min before collection. Cells were trypsinized, washed once in PBS, resuspended in 0.5 ml 0.9% NaCl and then added 0.5 ml chilled ethanol for fixation. After fixing overnight, cells were treated with 2N HCl/Triton X-100 solution for 25 min at room temperature to denature DNA. Cells were then washed once with 0.1M Na2B4O7, resuspended in 1% BSA/PBS and stained with FITC-conjugated BrdU antibody (BD) for 1 hr. Cells were again washed and resuspended in PBS with 120 μg/ml propidium iodide (PI) and 10 μg/ml RNase A for 45 min in the dark at 37°C. Samples were analyzed on BD FACS Canto II analyzer. Data were processed using De Novo FCS Express five software.
BrdU incorporation assay
For BrdU labeling, cells were incubated with 10 μM of BrdU for 20 min. Cells were then fixed with 2% PFA for 15 min at room temperature and permeabilized by 0.5% Triton X-100 for 10 min on ice. DNA was denatured by 4N HCl for 30 min at room temperature. Immunofluorescence staining of BrdU was performed using anti-BrdU antibody (Sigma) and anti-mouse Texas Red antibody. Images were taken using Axioimager.Z1 microscope (Zeiss) equipped with Hamamatsu ORCA-flash camera. Cells in S-phase (BrdU positive) were counted.
Cell proliferation assay
HCT116 cells were incubated with control or SUNO1-specific siRNAs for 48 hr. After this, cells were reseeded into 6 cm plates at a density of 1.5 × 105 cells/plate. Cell numbers were then counted every 24 hr until day 5.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) for DDX5 was performed using ChIP-IT High Sensitivity kit (Active Motif) according to manufacturer’s protocol. 50 μg of cross-linked and sheared chromatin, and 5 µg of antibody were used for precipitation. Similarly, 5 µg of IgG was used to pull 50 µg of cross-linked and sheared chromatin as a control. Pol II ChIP was performed as reported earlier (Khan et al., 2015). Briefly, cells were fixed using freshly prepared 1% Formaldehyde solution for ten minutes at room temperature followed by quenching with 0.125 M Glycine solution. Cell were lysed, sonicated and precipitated using antibodies. 50 µg of cross-linked and sheared chromatin, and 5 µg of RNA Pol II antibody were used to pull the chromatin. 5 µg IgG was also used as a non-specificity control. qPCR was performed with purified DNA and results were analyzed as percent input.
Immunoblotting
Cells were collected by scraping and lysed in lysis buffer containing protease inhibitors and phosphatase inhibitors for 10 min on ice. Loading dye was added to the lysate and samples were then heated at 95°C for 5 min before loading onto a polyacrylamide gel. Western Blotting was performed as described previously (Sun et al., 2018b). Antibodies are listed in Supplementary file 8.
Alkaline comet assay
Comet assay was performed using CometAssay Kit (Trevigen) following the manufacturer’s instructions. Briefly, cells were collected by trypsinization, embedded in low-melting agarose and placed on CometSlides. After agarose solidifying, the slides were immersed in lysis solution for 30 min, incubated in alkaline unwinding solution then subjected to electrophoresis for 30 min. After washing in water and 70% ethanol for 5 min each, the slides were allowed to dry, and DNA was stained using SYBR safe.
DNA fiber assay
Cells were labeled with 25 μM CldU for 30 min and then treated by 2 mM hydroxyurea for 24 hr followed by 30 min of labeling with 250 μM IdU. DNA fibers were prepared on vinyl-silane coated coverslips using the FiberComb molecular combing system (Genomic Vision) as per the manufacture’s protocol. To visualize the CldU and IdU tracks, DNA fibers on coverslips were denatured in denaturation solution (0.5M NaOH, 1M NaCl) for 8 min at room temperature. Coverslips were then washed with PBS and dehydrated in 70%, 90%, and 100% ethanol for 5 min each. Coverslips were blocked with 1% BSA in PBST, followed by incubating in antibodies against CldU (anti-BrdU, 1:200, Bio-Rad, OBT0030G) and IdU (anti-BrdU, 1:200, BD, 347580). After washing in BSA/PBST, the coverslips were incubated in FITC-conjugated goat anti-rat IgG and TexasRed-conjugated goat anti-mouse IgG. Images were taken using Axioimager.Z1 microscope (Zeiss) equipped with Hamamatsu ORCA-flash camera.
Immunofluorescence staining
For YAP1 immunofluorescence staining coupled with EdU incorporation assay, cells were pulse-labeled by 10 μM EdU for 30 min and then fixed by 2% PFA for 15 min at room temperature. Cells were then permeabilized by 0.5% Triton X-100 for 10 min on ice. After washing with PBS, click reaction was performed with freshly prepared click cocktail (2 mM copper sulfate, 10 μM AF488-Azide, and 100 mM sodium ascorbate in PBS) for 1 hr at room temperature. Cells were then preceded to blocking step and YAP1 was stained by anti-YAP1 antibody (Santa Cruz) and Goat anti-Mouse AF568 antibody.
For immunostaining of DNA-damage markers, cells were pre-extracted by 0.5% Triton X-100 in cytoskeletal (CSK) buffer for 3 min on ice and then fixed by 2% PFA for 15 min at room temperature. Total RPA32 was stained by anti-RPA32 antibody (Cell signaling) and Goat anti-Rat TexasRed antibody. 53BP1 was stained by anti-53BP1 antibody (Cell signaling) and Goat anti-Rabbit Dylight 488 antibody. Images were taken using Axioimager.Z1 microscope (Zeiss) equipped with Zeiss AxioCam 506 Mono camera.
Anchorage-dependent plastic colony formation assay
Cells were incubated with control or SUNO1-specific siRNAs for 48 hr. After that, cells were treated with DMSO (control) or Doxorubicin (300 nM) for 16 hr. After 16 hr, cells were washed with media to remove the drugs and were grown in fresh medium. Cells were reseeded in a 6-well plate at a density of 1000 cells per well. After 2 to 3 weeks, colonies were fixed with ice-cold 100% methanol for 5 min, stained with crystal violet and colonies were counted and analysis using ImageJ.
Microarray analyses
Total RNA from control and SUNO1-depleted HCT116 cells were isolated using the RNeasy Plus Mini kit (Qiagen). 250 ng of total RNA was used for microarray analysis. Samples were labeled using the IlluminaTotalPrep RNA amplification kit (Ambion) according the instruction by the manufacture. 750 ng of cRNA was used for hybridization on microarrays using the HumanHT-12 v4 Expression BeadChip kit (Illumina) manufacturer’s instructions and data was analyzed using the R/Bioconductor package (Bioconductor, Seattle, WA, USA). The microarray data are deposited in GEO with accession number GSE157393.
Luciferase reporter assay
Wild-type CTGF promoter (WT) or TEAD-binding sites mutated CTGF promoter (mutant) luciferase reporters (kind gift from Dr. Kun-Liang Guan, UCSD) are transfected into control and SUNO1-depleted (siSUNO1-a or siSUNO1-b) U2OS cells, and 24 hr later, the relative luciferase activity is quantified using Dual-Luciferase Reporter Assay System (Promega, E1910) following the manufacturer’s instructions.
Chromosome conformation capture (3C) assay
The 3C assay was performed as described (Dekker, 2006), with minor modifications. Briefly, one million HCT116 were cross-linked with formaldehyde (final concentration 1%) for 15 min at room temperature, and resuspended in lysis buffer (10 mM Tris, pH 8.0, 10 mM NaCl, and 0.2% NP40) and incubated on ice for 90 min. One million of the prepared nuclei were digested with EcoRI (New England Biolabs) overnight at 37°C, followed by ligation with T4 DNA ligase (New England Biolabs) at 16°C for 4 hr. The ligated DNA was incubated with Proteinase K at 65°C for >8 hr or overnight to reverse the cross-links. Following incubation, the DNA was treated with RNase A. The treated DNA was extracted with phenol:chloroform and precipitated with sodium acetate (10% vol) and ethanol (2.5–3-fold volume). The DNA concentration of the recovered 3C library was determined using Qubit dsDNA HS assay kit (Invitrogen). Quantitative real-time PCR was performed to confirm the specific ligation between two DNA fragments - between SUNO1 region and WTIP region, and between SUNO1 region and Control genomic region - in the sample libraries (SUNO1 KD and SUNO1 control) and BAC control libraries. Interaction frequencies were calculated by dividing the amount of PCR product obtained from the 3C sample library by the amount of PCR product obtained from the control library DNA generated from the corresponding BAC: Interaction frequency = 2^(dCt sample – dCt control). The primers designed for 3C assay are: SUNO1 region, 5’-TAGAACATGTTTCTTTGTCCAATAGGTGCTGAAAGGCCCG-3’; WTIP region, 5’- GGAGAGACGGGGTTTCACCATGTTGGCCAGGC-3’; and control region, 5’- ACCCCAGGCTCTCAGCAGCCGTGACCTCACAGCACCAT-3’.
RNA-affinity pulldown
RNA-affinity pulldown was performed as previously described (Sun et al., 2018b). Briefly, Biotin-labeled RNA probes were in vitro transcribed as per manufacturers’ instructions (Biotin RNA labeling Mix, Roche; T7 polymerase, Promega) and purified by G-50 column (GE Healthcare). 2 μg purified biotinylated RNA was used for each pulldown.
Cells were resuspended in lysis buffer (10 mM Tris-HCl (pH 7.4), 100 mM NaCl, 2.5 mM MgCl2, 40 μg/ml digitonin) and lysed on ice for 20 min with frequent mixing. Nuclei were then pelleted, resuspended in RIP buffer (150 mM KCl, 25 mM Tris pH 7.4, 0.5 mM DTT, 0.5% NP40), and sonicated three times for 5 s each. Debris were removed by centrifugation at 14,000 rpm for 10 min at 4°C. The nuclear lysate was then precleared by incubating with 40 μl of streptavidin beads (Dynabeads M-280 streptavidin, Invitrogen) at 4°C for 2 hr with rotation. The precleared lysate was incubated with the 2 μg biotinylated for 2 ~ 3 hr and then incubated with blocked beads at 4°C overnight. Beads were then washed with high salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl (pH 8.0), 500 mM NaCl), low salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl (pH 8.0), 150 mM NaCl) and TE buffer. RNase Inhibitors, protease inhibitors, and phosphatase inhibitors were included in all the buffers used in the previous steps. Beads were then resuspended in SDS loading buffer and heated at 95°C for 5 min. Protein samples were then analyzed by mass spectrometry or western blotting.
DDX5 RNA-immunoprecipitation
HCT116 wild-type cells from two 10 cm plate were fixed with 1% formaldehyde in PBS, at room temperature for 10 min, and then Glycine was added at final concentration of 100 mM, and further incubated for 5 min. Following one wash with PBS, cells were then resuspended with 400 μl Lysis Buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl pH8.1, supplemented with protease inhibitor cocktail and RNAase Inhibitor), and incubated at 4°C for 30 min with rotation. The lysate was then sonicated with Bioruptor Diagenode (setting ‘High’, 15 mins, three times). Centrifuge the sonicated lysate at 10, 000 rpm for 5 min to remove the debris. Then transfer the supernatant, make up volume to 1 ml with IP buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.1, 167 mM NaCl), add to 50 μl of pre-washed Gamma Bind G Sepharose beads, incubated at 4°C for 1 hr with rotation, to pre-clear the lysate. After pre-clearing, centrifuge at 10,000 rpm for 5 min, transfer the supernatant. Keep 100 μl as input, 450 μl for IgG, and 450 μl for IP with mouse monoclonal anti-DDX5 antibody (Millipore, Cat#: 05–580). Incubate at 4°C overnight with rotation. On the next day, add pre-washed Sepharose beads, incubate at 4°C for 2 hr with rotation. Then wash the beads once with IP buffer, once with High Salt Buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl pH 8.1, 500 mM NaCl), once with TE buffer, 5 min rotation at 4°C for each wash. Elute in 165 μl Elution Buffer (1% SDS, 0.1M NaHCO3, RNAase Inhibitor), incubate at 37°C for 15 min, repeat once, combine the elute. To 330 μl eluate, add 14 μl 5M NaCl. To the Input sample, add 282 μl Elution buffer and 14 μl 5M NaCl. Incubate at 65°C with vortex for 2 hr. Add 1032 μl Trizol LS (Invitrogen) to IP/IgG/Input sample. Proceed with RNA isolation following manufacturer’s instructions.
Nascent RNA capture assay
Nascent RNAs were labeled and captured using Click-iT Nascent RNA capture kit (Life Technologies) as per the manufacturer’s instructions. Expression level of nascent RNAs were quantified by qRT-PCR.
Tumor xenograft assay
Immunocompromised mice (neu-/-) were obtained from Jackson laboratory (females) and used for the xenograft experiment. A cohort of 5 mice were used for this study. The mice were injected with one million control HCT116 cells on the right flank and equal number of SUNO1 KO HCT116 cells were injected into the left flank of the same cohort of mice. Tumors were measured with a digital caliper (length (mm) X breadth (mm) X height (mm)) every five days. The graph denotes the mean of five tumor volume for each cell line.
Data analyses and statistics
Relative RNA levels were normalized to GAPDH or 18S RNA. Results are represented as mean ± SD of three independent experiments. Two-tailed Student’s t-tests were performed. *p<0.05, **p<0.01, ***p<0.001.
PCR primers, qPCR Primer, siRNA, and gRNA sequences
See Supplementary file 8 for the details.
Antibodies
See Supplementary file 8 for the details.
Acknowledgements
We thank members of Prasanth’s laboratory for their valuable comments. We thank Drs. Sayee Anak (UIUC) (YAP antibody), Erik Bolton (UIUC) (Cyclin D1 antibody), Kun-Liang Guan (UCSD) (CTGF-promoter reporter constructs), Dr. Kenneth Irvine (Rutgers University (pTRIPZ-EGFP:WTIP)) for providing reagents, and Dr. Alvaro G Hernandez (UIUC Genomic facility) for RNA-sequencing. We also thank Dr. Jian Ma, Dr. Yang Zhang and Omid Gholamalamdari for technical discussion relating to bioinformatic analyses. We thank Jon Zetterval for his assistance on the Xenograft experiments. This work was supported by National Institute of Health [R01GM088252, R01GM132458 and R21AG065748 to KVP, GM125196 to SGP and GM123314 to SCJ], Cancer center at Illinois seed grant and Prairie Dragon Paddlers to KVP, National Science Foundation [EAGER grant to KVP {1723008} and career award {1243372} and 1818286 to SGP]. AL was supported by the Intramural Research Program of the National Cancer Institute (NCI), Center for Cancer Research (CCR). Research in the SD lab is supported by Deutsche Forschungsgemeinschaft (Di 1421/7–1) and Deutsche Krebshilfe.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Kannanganattu V Prasanth, Email: kumarp@illinois.edu.
Roger J Davis, University of Massachusetts Medical School, United States.
Kevin Struhl, Harvard Medical School, United States.
Funding Information
This paper was supported by the following grants:
National Institute of General Medical Sciences GM088252 to Kannanganattu V Prasanth.
National Institute of General Medical Sciences GM125196 to Supriya G Prasanth.
National Institute of General Medical Sciences GM123314 to Sarath C Janga.
National Science Foundation 1723008 to Kannanganattu V Prasanth.
National Science Foundation 1243372 to Supriya G Prasanth.
National Institute on Aging AG065748 to Kannanganattu V Prasanth.
American Cancer Society RSG-11-174-01-RMC to Kannanganattu V Prasanth.
National Institute of General Medical Sciences R01GM132458 to Kannanganattu V Prasanth.
National Science Foundation 1818286 to Supriya G Prasanth.
Additional information
Competing interests
Reviewing editor, eLife.
No competing interests declared.
Arturo V Orjalo is affiliated with LGC Biosearch Technologies and Genentech Inc, the author has no financial interests to declare.
Hans Johansson is affiliated with LGC Biosearch Technologies. The author has no financial interests to declare.
Author contributions
Conceptualization, Data curation, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing - original draft, Writing - review and editing.
Conceptualization, Data curation, Formal analysis, Supervision, Funding acquisition, Validation, Investigation, Visualization, Methodology, Writing - original draft, Project administration, Writing - review and editing.
Resources, Data curation, Software, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing - review and editing.
Resources, Data curation, Software, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing - review and editing.
Data curation, Formal analysis.
Data curation, Software, Formal analysis, Investigation, Visualization, Methodology, Writing - review and editing.
Formal analysis, Investigation, Methodology.
Data curation, Software, Formal analysis, Validation, Investigation, Methodology, Writing - review and editing.
Data curation, Formal analysis, Validation, Investigation, Methodology, Writing - review and editing.
Data curation, Formal analysis, Validation, Investigation, Methodology, Writing - review and editing.
Data curation, Formal analysis, Validation, Investigation, Methodology, Writing - review and editing.
Validation, Investigation, Methodology, Writing - review and editing.
Formal analysis, Investigation, Methodology, Writing - review and editing.
Formal analysis, Investigation, Methodology, Writing - review and editing.
Formal analysis, Validation, Investigation, Methodology, Writing - review and editing.
Validation, Investigation, Methodology, Writing - review and editing.
Resources, Investigation, Methodology, Writing - review and editing.
Resources, Methodology.
Resources, Supervision, Investigation, Methodology.
Data curation, Formal analysis.
Conceptualization, Resources, Supervision, Investigation, Methodology.
Conceptualization, Supervision, Investigation, Methodology.
Supervision, Investigation, Methodology.
Resources, Data curation, Software, Supervision, Investigation, Methodology.
Conceptualization, Formal analysis, Supervision, Investigation, Methodology, Writing - review and editing.
Conceptualization, Resources, Data curation, Software, Supervision, Investigation, Methodology, Writing - review and editing.
Conceptualization, Formal analysis, Supervision, Funding acquisition, Investigation, Methodology, Project administration, Writing - review and editing.
Additional files
First sheet ‘gene_count_all’ contains raw counts from HTSeq-count analysis. Second sheet ‘TPM_all’ contains TPM (Transcripts Per Million) as the expression level of each gene in all samples. TPM is calculated using Stringtie. Third sheet ‘list_of_24087_genes’ includes the genes that have quantifiable expression (CPM >= 0.075 in at least two samples). Last sheet ‘biotype_of_24087_genes’ includes the detailed categorization information of these genes. The biotype information is based on Ensemble (https://useast.ensembl.org/info/genome/genebuild/biotypes.html).
Five sheets represent the full results (of 24087 genes) of differential expression tests (exactTest from edgeR) between G1 vs. G1S, G1S vs. S, S vs. G2, G2 vs. M, M vs. G1, respectively.
File three is a subset of File two and it includes only DEGs information. The gene categories are also provided as individual sheets. Statistics summarizing the categorization of each comparison (between two phases) is listed in Figure 1B.
Six sheets represent the detailed, full output from GSEA/GO/Kegg pathway analyses in this study. They correspond to data presented in Figure 1—figure supplement 2A, Figure 1—figure supplement 2B, Figure 1D, Figure 1—figure supplement 2C, respectively.
First sheet ‘all_phase_specific_with_TPM’ includes all 5162 phase-specific genes and their TPM values. Second sheet ‘2044_phase_specific_lncRNAs’ shows the list of 2044 lncRNAs only. The lncRNA categorization criteria are explained in detail in Supplementary file 1, last sheet, ‘biotype_of_24087_genes’. Statistics summarizing the categorization is listed in Figure 2—figure supplement 1A.
Data availability
Sequencing data have been deposited in GEO under accession code GSE143275. Microarray data has been deposited in GEO under the accession number GSE157393.
The following datasets were generated:
Hao Q, Prasanth KV, Sun Q. 2020. poly A+ RNA sequencing of cell cycle-synchronized RNA from U2OS cells. NCBI Gene Expression Omnibus. GSE143275
Hao Q, Prasanth KV, Sun Q. 2020. Microarray analyses to determine deregulated genes in SUNO1 KD cells compared to control cells. NCBI Gene Expression Omnibus. GSE157393
References
- Ali MM, Akhade VS, Kosalai ST, Subhash S, Statello L, Meryet-Figuiere M, Abrahamsson J, Mondal T, Kanduri C. PAN-cancer analysis of S-phase enriched lncRNAs identifies oncogenic drivers and biomarkers. Nature Communications. 2018;9:883. doi: 10.1038/s41467-018-03265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrysik Z, Galbraith MD, Guarnieri AL, Zaccara S, Sullivan KD, Pandey A, MacBeth M, Inga A, Espinosa JM. Identification of a core TP53 transcriptional program with highly distributed tumor suppressive activity. Genome Research. 2017;27:1645–1657. doi: 10.1101/gr.220533.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates GJ, Nicol SM, Wilson BJ, Jacobs AM, Bourdon JC, Wardrop J, Gregory DJ, Lane DP, Perkins ND, Fuller-Pace FV. The DEAD box protein p68: a novel transcriptional coactivator of the p53 tumour suppressor. The EMBO Journal. 2005;24:543–553. doi: 10.1038/sj.emboj.7600550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson R, Boisvert JL, Benes CH, Zou L. Distinct but concerted roles of ATR, DNA-PK, and Chk1 in countering replication stress during S Phase. Molecular Cell. 2015;59:1011–1024. doi: 10.1016/j.molcel.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretti G, Schiltz RL, Dilworth FJ, Di Padova M, Zhao P, Ogryzko V, Fuller-Pace FV, Hoffman EP, Tapscott SJ, Sartorelli V. The RNA helicases p68/p72 and the noncoding RNA SRA are coregulators of MyoD and skeletal muscle differentiation. Developmental Cell. 2006;11:547–560. doi: 10.1016/j.devcel.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Chang CN, Feng MJ, Chen YL, Yuan RH, Jeng YM. p15(PAF) is an rb/E2F-regulated S-phase protein essential for DNA synthesis and cell cycle progression. PLOS ONE. 2013;8:e61196. doi: 10.1371/journal.pone.0061196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in Cancer: an exit from cell cycle control. Nature Reviews Cancer. 2009;9:785–797. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Carmichael GG. Decoding the function of nuclear long non-coding RNAs. Current Opinion in Cell Biology. 2010;22:357–364. doi: 10.1016/j.ceb.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RJ, Huang M, Campbell MJ, Dong H, Steinmetz L, Sapinoso L, Hampton G, Elledge SJ, Davis RW, Lockhart DJ. Transcriptional regulation and function during the human cell cycle. Nature Genetics. 2001;27:48–54. doi: 10.1038/83751. [DOI] [PubMed] [Google Scholar]
- Clark EL, Hadjimichael C, Temperley R, Barnard A, Fuller-Pace FV, Robson CN. p68/DdX5 supports β-catenin & RNAP II during androgen receptor mediated transcription in prostate Cancer. PLOS ONE. 2013;8:e54150. doi: 10.1371/journal.pone.0054150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Renganathan A, Dighe SN, Bhaduri U, Shettar A, Mukherjee G, Kondaiah P, Satyanarayana Rao MR. DDX5/p68 associated lncRNA LOC284454 is differentially expressed in human cancers and modulates gene expression. RNA Biology. 2018;15:214–230. doi: 10.1080/15476286.2017.1397261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Thakur M, Feng Y, Jagannathan R, Seppa MJ, Skeath JB, Longmore GD. Ajuba LIM proteins are negative regulators of the hippo signaling pathway. Current Biology. 2010;20:657–662. doi: 10.1016/j.cub.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasio A, de Opakua AI, Mortuza GB, Molina R, Cordeiro TN, Castillo F, Villate M, Merino N, Delgado S, Gil-Cartón D, Luque I, Diercks T, Bernadó P, Montoya G, Blanco FJ. Structure of p15(PAF)-PCNA complex and implications for clamp sliding during DNA replication and repair. Nature Communications. 2015;6:6439. doi: 10.1038/ncomms7439. [DOI] [PubMed] [Google Scholar]
- Dekker J. The three 'C' s of chromosome conformation capture: controls, controls, controls. Nature Methods. 2006;3:17–21. doi: 10.1038/nmeth823. [DOI] [PubMed] [Google Scholar]
- Dyson N. The regulation of E2F by pRB-family proteins. Genes & Development. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- Ehmer U, Sage J. Control of proliferation and Cancer growth by the hippo signaling pathway. Molecular Cancer Research. 2016;14:127–140. doi: 10.1158/1541-7786.MCR-15-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov MV, Dyson NJ. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. Journal of Cell Science. 2004;117:2173–2181. doi: 10.1242/jcs.01227. [DOI] [PubMed] [Google Scholar]
- Fuller-Pace FV. The DEAD box proteins DDX5 (p68) and DDX17 (p72): Multi-tasking transcriptional regulators. Biochimica Et Biophysica Acta (BBA) - Gene Regulatory Mechanisms. 2013;1829:756–763. doi: 10.1016/j.bbagrm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Ghandi M, Huang FW, Jané-Valbuena J, Kryukov GV, Lo CC, McDonald ER, Barretina J, Gelfand ET, Bielski CM, Li H, Hu K, Andreev-Drakhlin AY, Kim J, Hess JM, Haas BJ, Aguet F, Weir BA, Rothberg MV, Paolella BR, Lawrence MS, Akbani R, Lu Y, Tiv HL, Gokhale PC, de Weck A, Mansour AA, Oh C, Shih J, Hadi K, Rosen Y, Bistline J, Venkatesan K, Reddy A, Sonkin D, Liu M, Lehar J, Korn JM, Porter DA, Jones MD, Golji J, Caponigro G, Taylor JE, Dunning CM, Creech AL, Warren AC, McFarland JM, Zamanighomi M, Kauffmann A, Stransky N, Imielinski M, Maruvka YE, Cherniack AD, Tsherniak A, Vazquez F, Jaffe JD, Lane AA, Weinstock DM, Johannessen CM, Morrissey MP, Stegmeier F, Schlegel R, Hahn WC, Getz G, Mills GB, Boehm JS, Golub TR, Garraway LA, Sellers WR. Next-generation characterization of the Cancer cell line encyclopedia. Nature. 2019;569:503–508. doi: 10.1038/s41586-019-1186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff LA, Rinn JL. Linking RNA biology to lncRNAs. Genome Research. 2015;25:1456–1465. doi: 10.1101/gr.191122.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Fiškin E, Gutschner T, Polycarpou-Schwarz M, Groß M, Neugebauer J, Gandhi M, Caudron-Herger M, Benes V, Diederichs S. A cautionary tale of sense-antisense gene pairs: independent regulation despite inverse correlation of expression. Nucleic Acids Research. 2017;45:12496–12508. doi: 10.1093/nar/gkx952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschner T, Hämmerle M, Diederichs S. MALAT1 -- a paradigm for long noncoding RNA function in Cancer. Journal of Molecular Medicine. 2013;91:791–801. doi: 10.1007/s00109-013-1028-y. [DOI] [PubMed] [Google Scholar]
- Harbour JW, Dean DC. The rb/E2F pathway: expanding roles and emerging paradigms. Genes & Development. 2000;14:2393–2409. doi: 10.1101/gad.813200. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The hippo pathway and human Cancer. Nature Reviews Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, Attardi LD, Regev A, Lander ES, Jacks T, Rinn JL. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, Wang Y, Kong B, Langerød A, Børresen-Dale AL, Kim SK, van de Vijver M, Sukumar S, Whitfield ML, Kellis M, Xiong Y, Wong DJ, Chang HY. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nature Genetics. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibar C, Kirichenko E, Keepers B, Enners E, Fleisch K, Irvine KD. Tension-dependent regulation of mammalian hippo signaling through LIMD1. Journal of Cell Science. 2018;131:jcs214700. doi: 10.1242/jcs.214700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Müller-Tidow C. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- Jung HY, Jun S, Lee M, Kim HC, Wang X, Ji H, McCrea PD, Park JI. PAF and EZH2 induce wnt/β-catenin signaling hyperactivation. Molecular Cell. 2013;52:193–205. doi: 10.1016/j.molcel.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, Chang Q, Chu GC, Al-Khalil R, Jiang S, Xia H, Fletcher-Sananikone E, Lim C, Horwitz GI, Viale A, Pettazzoni P, Sanchez N, Wang H, Protopopov A, Zhang J, Heffernan T, Johnson RL, Chin L, Wang YA, Draetta G, DePinho RA. Yap1 activation enables bypass of oncogenic kras addiction in pancreatic Cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Giri S, Wang Y, Chakraborty A, Ghosh AK, Anantharaman A, Aggarwal V, Sathyan KM, Ha T, Prasanth KV, Prasanth SG. BEND3 represses rDNA transcription by stabilizing a NoRC component via USP21 deubiquitinase. PNAS. 2015;112:8338–8343. doi: 10.1073/pnas.1424705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nature Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Cho YS, Wang X, Park O, Ma X, Kim H, Gan W, Jho EH, Cha B, Jeung YJ, Zhang L, Gao B, Wei W, Jiang J, Chung KS, Yang Y. Hippo signaling is intrinsically regulated during cell cycle progression by APC/CCdh1. PNAS. 2019;116:9423–9432. doi: 10.1073/pnas.1821370116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, Kitagawa K, Kotake Y, Niida H, Ohhata T. Cell cycle regulation by long non-coding RNAs. Cellular and Molecular Life Sciences. 2013;70:4785–4794. doi: 10.1007/s00018-013-1423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski MP, Krude T. Functional roles of non-coding Y RNAs. The International Journal of Biochemistry & Cell Biology. 2015;66:20–29. doi: 10.1016/j.biocel.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kopp F, Chang TC, Sataluri A, Chen B, Sivakumar S, Yu H, Xie Y, Mendell JT. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164:69–80. doi: 10.1016/j.cell.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Mendell JT. Antisense-Mediated transcript knockdown triggers premature transcription termination. Molecular Cell. 2020;77:1044–1054. doi: 10.1016/j.molcel.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tian H, Yang J, Gong Z. Long noncoding RNAs regulate cell growth, proliferation, and apoptosis. DNA and Cell Biology. 2016;35:459–470. doi: 10.1089/dna.2015.3187. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Horlbeck MA, Cho SW, Birk HS, Malatesta M, He D, Attenello FJ, Villalta JE, Cho MY, Chen Y, Mandegar MA, Olvera MP, Gilbert LA, Conklin BR, Chang HY, Weissman JS, Lim DA. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science. 2017a;355:eaah7111. doi: 10.1126/science.aah7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen S, Wang S, Soares F, Fischer M, Meng F, Du Z, Lin C, Meyer C, DeCaprio JA, Brown M, Liu XS, He HH. Transcriptional landscape of the human cell cycle. PNAS. 2017b;114:3473–3478. doi: 10.1073/pnas.1617636114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheret M, Halazonetis TD. DNA replication stress as a hallmark of Cancer. Annual Review of Pathology: Mechanisms of Disease. 2015;10:425–448. doi: 10.1146/annurev-pathol-012414-040424. [DOI] [PubMed] [Google Scholar]
- Marchese FP, Grossi E, Marín-Béjar O, Bharti SK, Raimondi I, González J, Martínez-Herrera DJ, Athie A, Amadoz A, Brosh RM, Huarte M. A long noncoding RNA regulates sister chromatid cohesion. Molecular Cell. 2016;63:397–407. doi: 10.1016/j.molcel.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek A, Luo W, Krasnitz A, Hicks J, Powers RS, Stillman B. DDX5 regulates DNA replication and is required for cell proliferation in a subset of breast Cancer cells. Cancer Discovery. 2012;2:812–825. doi: 10.1158/2159-8290.CD-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Moroishi T, Guan K-L. Mechanisms of hippo pathway regulation. Genes & Development. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol SM, Bray SE, Derek Black H, Lorimore SA, Wright EG, Lane DP, Meek DW, Coates PJ, Fuller-Pace FV. The RNA helicase p68 (DDX5) is selectively required for the induction of p53-dependent p21 expression and cell-cycle arrest after DNA damage. Oncogene. 2013;32:3461–3469. doi: 10.1038/onc.2012.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjalo AV, Johansson HE. Stellaris RNA fluorescence in situ hybridization for the simultaneous detection of immature and mature long noncoding RNAs in adherent cells. Methods in Molecular Biology. 2016;1402:119–134. doi: 10.1007/978-1-4939-3378-5_10. [DOI] [PubMed] [Google Scholar]
- Pertea M, Pertea GM, Antonescu CM, Chang T-C, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nature Biotechnology. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann E, Orta ML, Issaeva N, Schultz N, Helleday T. Hydroxyurea-Stalled replication forks become progressively inactivated and require two different RAD51-Mediated pathways for restart and repair. Molecular Cell. 2010;37:492–502. doi: 10.1016/j.molcel.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J, Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989;58:833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Povlsen LK, Beli P, Wagner SA, Poulsen SL, Sylvestersen KB, Poulsen JW, Nielsen ML, Bekker-Jensen S, Mailand N, Choudhary C. Systems-wide analysis of ubiquitylation dynamics reveals a key role for PAF15 ubiquitylation in DNA-damage bypass. Nature Cell Biology. 2012;14:1089–1098. doi: 10.1038/ncb2579. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nature Reviews Genetics. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- Ramilowski JA, Yip CW, Agrawal S, Chang J-C, Ciani Y, Kulakovskiy IV, Mendez M, Ooi JLC, Ouyang JF, Parkinson N, Petri A, Roos L, Severin J, Yasuzawa K, Abugessaisa I, Akalin A, Antonov IV, Arner E, Bonetti A, Bono H, Borsari B, Brombacher F, Cameron CJF, Cannistraci CV, Cardenas R, Cardon M, Chang H, Dostie J, Ducoli L, Favorov A, Fort A, Garrido D, Gil N, Gimenez J, Guler R, Handoko L, Harshbarger J, Hasegawa A, Hasegawa Y, Hashimoto K, Hayatsu N, Heutink P, Hirose T, Imada EL, Itoh M, Kaczkowski B, Kanhere A, Kawabata E, Kawaji H, Kawashima T, Kelly ST, Kojima M, Kondo N, Koseki H, Kouno T, Kratz A, Kurowska-Stolarska M, Kwon ATJ, Leek J, Lennartsson A, Lizio M, López-Redondo F, Luginbühl J, Maeda S, Makeev VJ, Marchionni L, Medvedeva YA, Minoda A, Müller F, Muñoz-Aguirre M, Murata M, Nishiyori H, Nitta KR, Noguchi S, Noro Y, Nurtdinov R, Okazaki Y, Orlando V, Paquette D, Parr CJC, Rackham OJL, Rizzu P, Sánchez Martinez DF, Sandelin A, Sanjana P, Semple CAM, Shibayama Y, Sivaraman DM, Suzuki T, Szumowski SC, Tagami M, Taylor MS, Terao C, Thodberg M, Thongjuea S, Tripathi V, Ulitsky I, Verardo R, Vorontsov IE, Yamamoto C, Young RS, Baillie JK, Forrest ARR, Guigó R, Hoffman MM, Hon CC, Kasukawa T, Kauppinen S, Kere J, Lenhard B, Schneider C, Suzuki H, Yagi K, de Hoon MJL, Shin JW, Carninci P. Functional annotation of human long noncoding RNAs via molecular phenotyping. Genome Research. 2020;30:1060–1072. doi: 10.1101/gr.254219.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Dündar F, Diehl S, Grüning BA, Manke T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Research. 2014;42:W187–W191. doi: 10.1093/nar/gku365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SSP, Huang S-C, Glenn St Hilaire B, Engreitz JM, Perez EM, Kieffer-Kwon K-R, Sanborn AL, Johnstone SE, Bascom GD, Bochkov ID, Huang X, Shamim MS, Shin J, Turner D, Ye Z, Omer AD, Robinson JT, Schlick T, Bernstein BE, Casellas R, Lander ES, Aiden EL. Cohesin loss eliminates all loop domains. Cell. 2017;171:24–320. doi: 10.1016/j.cell.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annual Review of Biochemistry. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H, Wheeler J, Morley AR. In vivo bromodeoxyuridine incorporation in normal mouse kidney: immunohistochemical detection and measurement of labelling indices. The Histochemical Journal. 1990;22:209–214. doi: 10.1007/BF02386007. [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow KL, Janknecht R. Synergism between p68 RNA helicase and the transcriptional coactivators CBP and p300. Oncogene. 2003;22:151–156. doi: 10.1038/sj.onc.1206067. [DOI] [PubMed] [Google Scholar]
- Sánchez I, Dynlacht BD. Transcriptional control of the cell cycle. Current Opinion in Cell Biology. 1996;8:318–324. doi: 10.1016/S0955-0674(96)80004-4. [DOI] [PubMed] [Google Scholar]
- Schmitt AM, Garcia JT, Hung T, Flynn RA, Shen Y, Qu K, Payumo AY, Peres-da-Silva A, Broz DK, Baum R, Guo S, Chen JK, Attardi LD, Chang HY. An inducible long noncoding RNA amplifies DNA damage signaling. Nature Genetics. 2016;48:1370–1376. doi: 10.1038/ng.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt AM, Chang HY. Long Noncoding RNAs in Cancer Pathways. Cancer Cell. 2016;29:452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, Zwang Y, Roberts TM, Root DE, Jacks T, Hahn WC. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Hao Q, Prasanth KV. Nuclear long noncoding RNAs: key regulators of gene expression. Trends in Genetics. 2018a;34:142–157. doi: 10.1016/j.tig.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Tripathi V, Yoon J-H, Singh DK, Hao Q, Min K-W, Davila S, Zealy RW, Li XL, Polycarpou-Schwarz M, Lehrmann E, Zhang Y, Becker KG, Freier SM, Zhu Y, Diederichs S, Prasanth SG, Lal A, Gorospe M, Prasanth KV. MIR100 host gene-encoded lncRNAs regulate cell cycle by modulating the interaction between HuR and its target mRNAs. Nucleic Acids Research. 2018b;46:10405–10416. doi: 10.1093/nar/gky696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Hao Q, Lin Y-C, Song YJ, Bangru S, Arif W, Tripathi V, Zhang Y, Cho J-H, Freier SM, Jenkins L, Ma J, Yoon J-H, Kalsotra A, Lal A, Prasanth SG, Prasanth KV. Antagonism between splicing and microprocessor complex dictates the Serum-induced processing of Lnc-MIRHG for efficient cell cycle Re-entry. RNA. 2020;1:rna.075309.120. doi: 10.1261/rna.075309.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi V, Shen Z, Chakraborty A, Giri S, Freier SM, Wu X, Zhang Y, Gorospe M, Prasanth SG, Lal A, Prasanth KV. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLOS Genetics. 2013;9:e1003368. doi: 10.1371/journal.pgen.1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley KE, Gertz J, Roberts BS, Davis NS, Bowling KM, Kirby MK, Nesmith AS, Oliver PG, Grizzle WE, Forero A, Buchsbaum DJ, LoBuglio AF, Myers RM. Recurrent read-through fusion transcripts in breast Cancer. Breast Cancer Research and Treatment. 2014;146:287–297. doi: 10.1007/s10549-014-3019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Marín J, Gutiérrez-Triana JA, Almuedo-Castillo M, Buono L, Gómez-Skarmeta JL, Mateo JL, Wittbrodt J, Martínez-Morales JR. yap1b , a divergent Yap/Taz family member, cooperates with yap1 in survival and morphogenesis via common transcriptional targets. Development. 2019;146:dev173286. doi: 10.1242/dev.173286. [DOI] [PubMed] [Google Scholar]
- Wagner M, Rid R, Maier CJ, Maier RH, Laimer M, Hintner H, Bauer JW, Onder K. DDX5 is a multifunctional co-activator of steroid hormone receptors. Molecular and Cellular Endocrinology. 2012;361:80–91. doi: 10.1016/j.mce.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Wang KC, Chang HY. Molecular Mechanisms of Long Noncoding RNAs. Molecular Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Yao M, Dong Q. Proliferating cell unclear antigen-associated factor (PAF15): A novel oncogene. The International Journal of Biochemistry & Cell Biology. 2014;50:127–131. doi: 10.1016/j.biocel.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Yao R-W, Wang Y, Chen L-L. Cellular functions of long noncoding RNAs. Nature Cell Biology. 2019;21:542–551. doi: 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]
- Yildirim O, Izgu EC, Damle M, Chalei V, Ji F, Sadreyev RI, Szostak JW, Kingston RE. S-phase enriched Non-coding RNAs regulate gene expression and cell cycle progression. Cell Reports. 2020;31:107629. doi: 10.1016/j.celrep.2020.107629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R Package for Comparing Biological Themes Among Gene Clusters. OMICS: A Journal of Integrative Biology. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F-X, Zhao B, Guan K-L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, Piccolo S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nature Cell Biology. 2015;17:1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman MK, Cimprich KA. Causes and consequences of replication stress. Nature Cell Biology. 2014;16:2–9. doi: 10.1038/ncb2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Zhou N, Huang J, Liu Q, Fukuda K, Ma D, Lu Z, Bai C, Watabe K, Mo Y-Y. The human long non-coding RNA-RoR is a p53 repressor in response to DNA damage. Cell Research. 2013;23:340–350. doi: 10.1038/cr.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang C-Y, Chinnaiyan AM, Lai Z-C, Guan K-L. TEAD mediates YAP-dependent gene induction and growth control. Genes & Development. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]