Abstract
Purpose
The 6-min walk test (6MWT) is a useful tool to assess the physiologic function in patients with chronic obstructive pulmonary disease (COPD). The recent study showed that patients with COPD with oxygen desaturation during the 6MWT had an increased risk of exacerbation and death compared with those without oxygen desaturation. This study aimed to explore the potential risk factors for exercise-induced desaturation (EID) in patients with COPD.
Patients and Methods
Adult patients with COPD were enrolled from the Chang Gung Research Database between January 2013 and January 2017. Age, sex, body mass index, underlying diseases, medications, and results of the pulmonary function tests and 6MWT were retrospectively collected and analyzed.
Results
Among 1768 patients with COPD, 932 (52.7%) had oxygen desaturation, and the other 836 (47.3%) had no desaturation during the 6MWT. The patients with EID had a shorter 6-min walk distance than those without desaturation (352.08±120.29 vs 426.56±112.56, p<0.0001). In the multivariate logistic regression analysis, older age, female sex, lower forced expiratory volume in 1 s, and comorbidity with atrial fibrillation (AF) were associated with oxygen desaturation during the 6MWT. Patients with EID had higher exacerbation frequency than those without desaturation in the 1-year follow-up period (0.59±1.50 vs 0.34±1.26, p<0.0001). Patients with COPD with AF also had a higher rate of exacerbation requiring emergency department visit or hospitalization in the 1-year follow-up.
Conclusion
This study demonstrates that older age, low FEV1, and female sex are risk factors for EID. Desaturation during 6MWT is related to frequent acute exacerbation of COPD in the 1-year follow-up.
Keywords: 6-minute walk test, A-fib, exacerbation, hypoxia
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by chronic airflow limitation and several comorbidities, such as cardiovascular disease, lung cancer, osteoporosis, and diabetes.1–3 Acute exacerbation of COPD could become more frequent as the COPD severity increased. Exacerbation of COPD was also an independent indicator of poor prognosis.4,5 Exercise-induced desaturation and hypoxia are largely associated with reduced quality of life, increased rate of acute exacerbation requiring hospitalization, diminished exercise tolerance, and decreased survival rate in the patients with COPD.6–9
The 6-min walk test (6MWT) is a useful tool to assess the physiologic function in patients with COPD, interstitial lung disease, bronchiectasis, and heart disease.10–13 The previous study showed that the 6-min walk distance (6MWD) was related to the mortality rate.9,14,15 Waatevik et al reported that patients with COPD and oxygen desaturation during the 6MWT had a higher risk of death and exacerbation than those without oxygen desaturation.16 However, the causes of exercise-induced desaturation (EID) in patients with COPD may be influenced by multiple potential factors, such as pulmonary functions, cardiovascular conditions, and medications, thus leading to the impairment of exercise tolerance.17 This study aimed to explore the potential risk factors for exercise-induced desaturation (EID) in patients with COPD.
Patients and Methods
Data Collection
This is a retrospective cohort study using the Chang Gung Research Database (CGRD). The CGRD is a de-identified database derived from multi-institutional standardized electric medical records of Chang Gung Memorial Hospital in Taiwan (http://www.chang-gung.com/en/index.aspx). The CGRD includes 6.1% of outpatients and 10.2% of hospitalized patients in Taiwan.18,19 This study was approved by the Institutional Review Board of Chang Gung Medical Foundation (IRB No. 201900152B0C501). All personally identifiable information was encrypted, and patient consent was waived for this study.
Patients
Adult patients were recruited from the CGRD between January 2013 and January 2017. Inpatient and outpatients with the diagnosis of COPD identified by the disease coding of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) 491, 492, and 496, or Tenth Revision (ICD-10) (J44). The patients with a ratio of the FEV1/forced vital capacity (FVC) more than 70%, those younger than 18 years, and those who did not receive pulmonary function tests and 6MWT were excluded. The baseline comorbidities of the subjects were retrieved from the outpatient and inpatient medical records in CGRD.
Medication Exposure
Medication exposure was assessed as of the index date for the first pulmonary function test of the COPD cases. The long-term use of cardiopulmonary drugs was assumed whenever there was any order for a reimbursement code of a prescription length of 28 days or longer. Cardiopulmonary drugs include inhaled drugs and oral medications, as listed in the following tables.
Covariates and Outcome
We divided the patients into two groups with or without oxygen desaturation during the 6MWT. Oxygen desaturation was defined as ≥4% reduction between pre- and posttest arterial oxygen saturation (Δ SpO2 ≥ 4%) and posttest SpO2 <90% measured by pulse oximetry.20,21 Covariates for chronic comorbidities and risk factors were collected from the previous one year before the indexed day of the pulmonary function test. Age, sex, body mass index (BMI), underlying diseases, results of pulmonary function tests and 6MWT, and record of emergency department visit or hospitalization were collected. The primary outcome measures were the acute exacerbation of COPD during the 1-year follow-up period. Exacerbations were defined as a worsening of respiratory symptoms requiring emergency department visit or hospitalization with disease codes of acute exacerbation of COPD (ICD-9: 49322, 49321/ICD-10: J441, J440).
Statistical Analyses
Under a retrospective case-control sampling scheme, the odds ratios estimate the risk of desaturation during the 6MWT. The odds ratios of desaturation (plus 95% confidence intervals (CI)) were estimated using conditional logistic regression analysis adjusted for all covariates. Statistical analysis was performed using SAS software, version 9.3 (SAS Institute Inc.). For comparing baseline differences between patients with and without desaturation during the 6MWT, t-tests were used. P values < 0.05 were considered statistically significant. Desaturation risk (odds ratios with 95% CI) was calculated with the univariate and multivariable logistic regression analyses.
The variables used in the logistic regression model were age, sex,22 FEV1%,22 BMI,23 common comorbidities,23 and medication. Variables included in multivariate analysis were those that were significant at p<0.05 in univariate analysis by stepwise method. Stepwise regression is a combination of the forward and backward selection techniques. The method is discussed in standard textbooks,24,25 and is available in all major statistical software programs for several types of commonly used epidemiological models, such as linear, logistic, Cox, and Poisson regression. The risk of later exacerbations (incidence rate ratios with 95% CI for yearly exacerbations) was analyzed using the univariate and multivariate negative binomial regression models. Multivariate adjustment included baseline sex, age, height, weight, BMI, pulmonary function, and 6MWD. The Kaplan–Meier (K–M) methods based on the logistic regression after adjustment for sex, age, and BMI were used for the analysis of the association of desaturation in the 6MWT and acute exacerbation of COPD in the 1-year follow-up.
Results
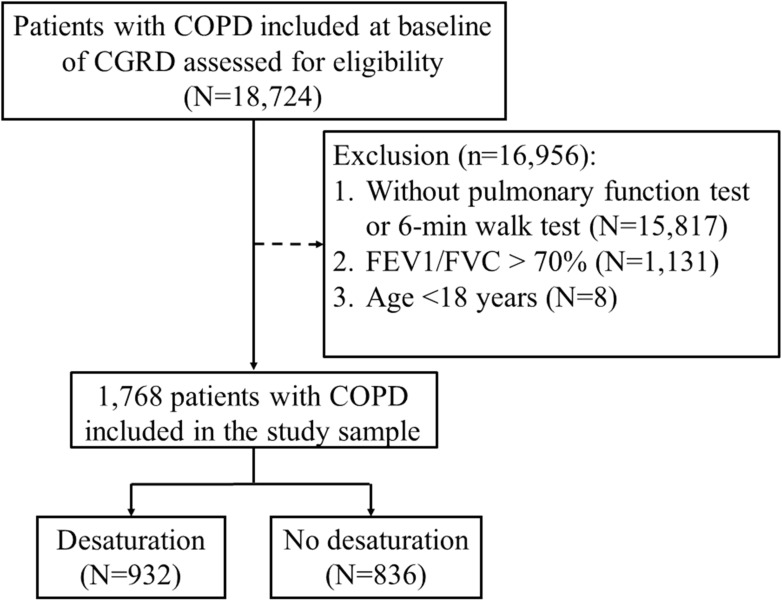
The study design is depicted in the CONSORT flow diagram in Figure 1. The characteristics of the 1768 participating patients with COPD are shown in Table 1. Among the 1768 patients with COPD, 932 (52.7%) were desaturators (desaturation during the 6MWT), and 836 (47.3%) were non-desaturators (no desaturation during the 6MWT). There was no significant difference in sex between the groups. The mean age of the non-desaturators (68.61±10.98 years) was significantly younger than that of the desaturators (70.57±10.91 years, p value: 0.0002). As shown in Table 1, the proportions of some cardiovascular diseases such as hypertension, AF, and heart failure were higher in the desaturators (19.1%, 4.0%, and 5.6%, respectively) than in the non-desaturators (13.6%, 2.0%, and 2.4%; p value: 0.0020, 0.0182, and 0.0007, respectively). On the other hand, there was no significant difference in the comorbidities such as diabetes, angina pectoris, myocardial infarction, ischemic heart disease, stroke, or peripheral arterial diseases. We also analyzed the common pulmonary medications prescribed in COPD. The use of short-acting β-agonists (SABA), both inhaled and oral SABA, in the desaturators (13.3%) was more than that in the non-desaturators (5.5%). The proportions of the use of short- and long-acting muscarinic antagonists, inhaled corticosteroids and long-acting β-agonists (LABA), xanthine, and oral steroid are more in the desaturators than in the non-desaturators. The proportion of the use of calcium channel blockers (CCB) was more in the desaturators (11.4%) than in the non-desaturators (10.7%). No significant difference was noted between dihydropyridine (DHP) CCB and non-DHP CCB in further analysis. Also, there was no significant difference in the use of other typical cardiac medications such as β-blockers (with or without β1-selectivity), amiodarone, or digoxin between the two groups.
Figure 1.
Flowchart of the study design and patients with chronic obstructive pulmonary disease (COPD) who performed the 6-min walk test (6MWT).
Table 1.
Characteristics of Patients with Chronic Obstructive Pulmonary Disease with or without Desaturation During the 6MWT (N=1768)
| Total (N=1768) | No Desaturation (N=836) |
Desaturation (N=932) |
P value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 1478 | 705 (84.3) | 773 (82.94) | 0.4306 |
| Female | 290 | 131 (15.67) | 159 (17.06) | |
| Age (years) | 69.64±10.99 | 68.61±10.98 | 70.57±10.91 | 0.0002 |
| BMI (BW/BH(m)2) | 23.75±4.34 | 24.17±4.12 | 23.38±4.50 | 0.0001 |
| Comorbidities | ||||
| Hypertension | 292 | 114 (13.64) | 178 (19.10) | 0.0020 |
| Diabetes | 134 | 58 (6.94) | 76 (8.15) | 0.3345 |
| Angina pectoris | 70 | 36 (4.31) | 34 (3.65) | 0.4786 |
| Myocardial infarction | 18 | 11 (1.32) | 7 (0.75) | 0.2376 |
| Ischemic heart disease | 69 | 28 (3.35) | 41 (4.40) | 0.2551 |
| Atrial fibrillation | 54 | 17 (2.03) | 37 (3.97) | 0.0182 |
| Heart failure | 72 | 20 (2.39) | 52 (5.58) | 0.0007 |
| Stroke | 29 | 11 (1.32) | 18 (1.93) | 0.3090 |
| Peripheral arterial disease | 6 | 1 (0.12) | 5 (0.54) | 0.2221 |
| Medication use | ||||
| SABA (total) | 170 | 46 (5.50) | 124 (13.30) | <0.0001 |
| SABA (oral) | 69 | 22 (2.63) | 47 (5.04) | 0.0090 |
| SABA (inhaled) | 160 | 44 (5.26) | 116 (12.45) | <0.0001 |
| SAMA | 128 | 35 (4.19) | 93 (9.98) | <0.0001 |
| SABA+SAMA | 85 | 23 (2.75) | 62 (6.65) | 0.0001 |
| Ultra-LABA | 124 | 57 (6.82) | 67 (7.19) | 0.7606 |
| LAMA | 332 | 117 (14.00) | 215 (23.07) | <0.0001 |
| LABA+LAMA | 79 | 40 (4.78) | 39 (4.18) | 0.5420 |
| ICS+LABA | 335 | 122 (14.59) | 213 (22.85) | <0.0001 |
| Xanthine | 226 | 89 (10.65) | 137 (14.70) | 0.0108 |
| Oral steroid | 210 | 73 (8.73) | 137 (14.70) | 0.0001 |
| Amiodarone | 9 | 3 (0.36) | 6 (0.64) | 0.5125 |
| β-blocker | 91 | 40 (4.78) | 51 (5.47) | 0.5137 |
| β1-selectivity | 68 | 29 (3.47) | 39 (4.18) | 0.4347 |
| Non-β1-selectivity | 26 | 12 (1.44) | 14 (1.50) | 0.9073 |
| CCB | 195 | 89 (10.65) | 106 (11.37) | 0.0001 |
| DHP | 148 | 73 (8.73) | 75 (8.05) | 0.6037 |
| Non-DHP | 42 | 16 (1.91) | 26 (2.79) | 0.2273 |
Note: Data are presented as N, N (%), or mean±SD.
Abbreviations: CCB, calcium channel blockers; DHP, dihydropyridine; SABA, short-acting β-agonists; SAMA, short-acting muscarinic antagonists; LABA, long-acting β-agonists; LAMA, long-acting muscarinic antagonists; ICS, inhaled corticosteroids.
Comparing the results of the pulmonary function test between the desaturators and non-desaturators during the 6MWT (Table 2), the desaturators were characterized by a significantly lower FEV1 (2.06±1.76 L) and FVC (2.07±3.14 L) than the non-desaturators (3.04±1.80 and 2.50±1.22 L). The baseline and post-6MWT SpO2 of the desaturators (93.3% and 82.6%) were also lower than those of the non-desaturators (95.8% and 92.6%, p<0.0001). The pre- and post-6MWT heart rates of the desaturators (88.2 and 116.1 bpm) were more rapid than those of the non-desaturators (83.3 and 107.6 bpm). The mean 6MWD of the desaturators (352.1 m) was shorter than that of the non-desaturators (426.6 m, p<0.0001). In addition, the walking distance of 76.1% of the non-desaturators could reach >350 m, while only 56.1% of the desaturators could walk >350 m (p<0.0001).
Table 2.
The Outcomes of 1768 Patients with Chronic Obstructive Pulmonary Disease Who Underwent the 6-Min Walk Tests
| No Desaturation (N=836) |
Desaturation (N=932) |
P value | |
|---|---|---|---|
| FVC% predicted | 64.68±18.16 | 61.53±17.38 | 0.0003 |
| FEV1% predicted | 59.29±18.20 | 45.83±18.83 | <0.0001 |
| FEV1/FVC (%) | 51.25±21.04 | 49.46±17.04 | 0.1125 |
| FEV1 (L) | 3.04±1.80 | 2.06±1.76 | <0.0001 |
| FVC (L) | 2.50±1.22 | 2.07±3.14 | 0.0012 |
| Pre-6MWT SpO2 (%) | 95.76±1.69 | 93.30±3.22 | <0.0001 |
| Post-6MWT SpO2 (%) | 92.63±1.85 | 82.61±6.08 | <0.0001 |
| Pre-6MWT heart rate (bpm) | 83.27±14.87 | 88.23±15.44 | <0.0001 |
| Post-6MWT heart rate (bpm) | 107.62±17.93 | 116.09±27.58 | <0.0001 |
| 6-min walk distance (m) | 426.56±112.56 | 352.08±120.29 | <0.0001 |
| Walk distance > 350 m | 636 (76.08%) | 523 (56.12%) | <0.0001 |
Note: Data are presented as N, N (%) or mean±SD.
Abbreviations: 6MWT, 6-min walk test; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SpO2, oxygen saturation measured by pulse oximetry.
The binary logistic regression of the characteristics and medications of patients with COPD with or without desaturation during the 6MWT is shown in Table 3. Full model of multivariate regression analysis of variables associated with oxygen desaturation during the 6MWT in patients with COPD showed significance in female sex (p<0.0001), older age (p=0.0013), and FEV1 (p<0.0001) (Table 4). After stepwise selection, the multivariate logistic regression showed female sex (p<0.0001), older age (p=0.0005), lower FEV1 (p<0.0001), and atrial fibrillation (p=0.0245) are the factors associated with EID (Table 4). The desaturators were more female predominant and older than the non-desaturators. The FEV1% of the desaturators was lower than that of the non-desaturators. The proportion of AF in the desaturators was higher than in the non-desaturators.
Table 3.
Univariate Regression Analysis of Variables Associated with Oxygen Desaturation During the 6-Min Walk Test in Patients with Chronic Obstructive Pulmonary Disease
| Subjects | Univariate | ||
|---|---|---|---|
| OR | 95% CI | P value | |
| Sex (male vs female) | 0.90 | 0.70–1.16 | 0.4318 |
| Age (years) | 1.02 | 1.01–1.03 | 0.0002 |
| BMI | 0.96 | 0.94–0.98 | 0.0002 |
| FEV1% predicted | 0.96 | 0.96–0.97 | <0.0001 |
| Hypertension | 1.50 | 1.16–1.93 | 0.0021 |
| Diabetes | 1.19 | 0.84–1.70 | 0.3350 |
| Angina pectoris | 0.84 | 0.52–1.36 | 0.4782 |
| Myocardial infarction | 0.57 | 0.22–1.47 | 0.2437 |
| Ischemic heart disease | 1.33 | 0.81–2.17 | 0.2565 |
| Atrial fibrillation | 1.99 | 1.11–3.56 | 0.0204 |
| Heart failure | 2.41 | 1.43–4.07 | 0.0010 |
| Stroke | 1.48 | 0.69–3.15 | 0.3119 |
| Peripheral arterial disease | 4.50 | 0.53–38.62 | 0.1699 |
| SABA (total) | 2.64 | 1.85–3.75 | <0.0001 |
| SABA (oral) | 1.97 | 1.17–3.29 | 0.0102 |
| SABA (inhaled) | 2.56 | 1.78–3.67 | <0.0001 |
| SAMA | 2.54 | 1.70–3.79 | <0.0001 |
| SABA+SAMA | 2.52 | 1.55–4.10 | 0.0002 |
| Ultra-LABA | 1.06 | 0.73–1.53 | 0.7610 |
| LAMA | 1.84 | 1.44–2.36 | <0.0001 |
| LABA+LAMA | 0.87 | 0.55–1.36 | 0.5415 |
| ICS+LABA | 1.73 | 1.36–2.22 | <0.0001 |
| Xanthine | 1.45 | 1.09–1.92 | 0.0111 |
| Oral steroid | 1.80 | 1.33–2.43 | 0.0001 |
| β-blocker | 1.15 | 0.75–1.76 | 0.5160 |
| β1-selectivity | 1.22 | 0.75–1.98 | 0.4353 |
| Non-β1-selectivity | 1.05 | 0.48–2.28 | 0.9075 |
| CCB | 0.99 | 0.73–1.35 | 0.9613 |
| DHP | 0.92 | 0.65–1.28 | 0.6032 |
| Non-DHP | 1.47 | 0.78–2.76 | 0.2300 |
Note: Data are presented as N, N (%), or mean±SD.
Abbreviations: CCB, calcium channel blockers; DHP, dihydropyridine; SABA, short-acting β-agonists; SAMA, short-acting muscarinic antagonists; LABA, long-acting β-agonists; LAMA, long-acting muscarinic antagonists; ICS, inhaled corticosteroids.
Table 4.
Stepwise Multivariate Regression Analysis of Variables Associated with Oxygen Desaturation During the 6-Min Walk Test in Patients with Chronic Obstructive Pulmonary Disease
| Subjects | Multivariate (Enter) | Multivariate (Stepwise Selection) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Sex (male vs female) | 0.43 | 0.28–0.64 | <0.0001 | 0.43 | 0.28–0.64 | <0.0001 |
| Age (years) | 1.02 | 1.01–1.03 | 0.0013 | 1.02 | 1.01–1.04 | 0.0005 |
| BMI | 0.98 | 0.95–1.01 | 0.1862 | |||
| FEV1% predicted | 0.96 | 0.96–0.97 | <0.0001 | 0.96 | 0.95–0.97 | <0.0001 |
| Hypertension | 1.25 | 0.88–1.78 | 0.2096 | |||
| Atrial fibrillation | 2.18 | 0.84–5.66 | 0.1102 | 2.86 | 1.14–7.15 | 0.0245 |
| Heart failure | 1.68 | 0.78–3.62 | 0.1864 | |||
The exacerbation events were analyzed by the episodes of emergency department visit or hospitalization with the disease codes of acute exacerbation of COPD. As shown in Table 5, the desaturators have higher rates of emergency department visit or hospitalization with the diagnosis of acute exacerbation of COPD (59%) than the non-desaturators (34%, p<0.0001).
Table 5.
The Outcome of Patients with Chronic Obstructive Pulmonary Disease with or without Desaturation During the 6-Min Walk Tests, one-year follow-up
| Outcome: Exacerbation Events | No Desaturation | Desaturation | P value |
|---|---|---|---|
| Emergency department visit or hospitalization (times) | 0.34±1.26 | 0.59±1.50 | <0.0001 |
| Hospitalization (times) | 0.19±0.78 | 0.34±0.91 | <0.0001 |
| Emergency department visit (times) | 0.15±0.64 | 0.24±0.74 | <0.0001 |
Note: Data are presented as N, N (%), or mean±SD.
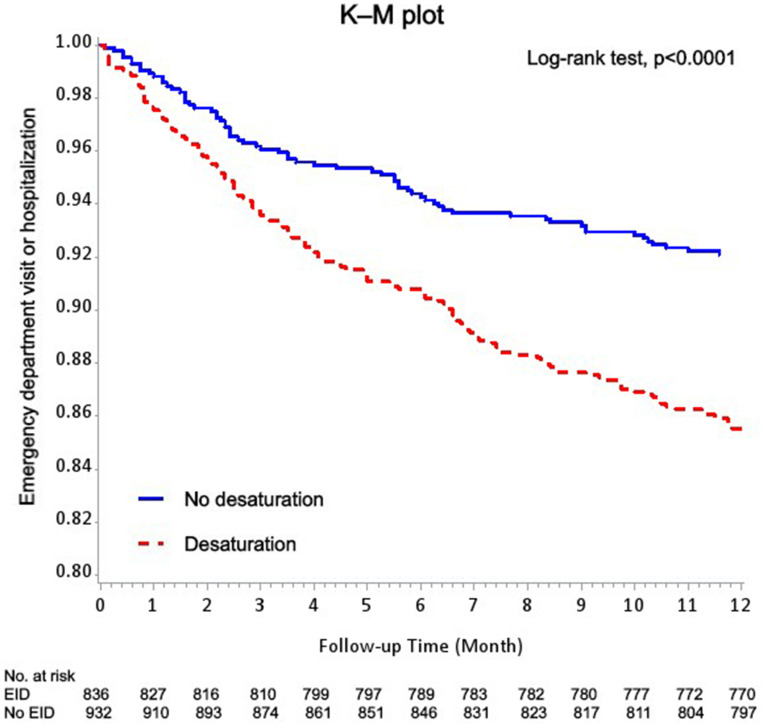
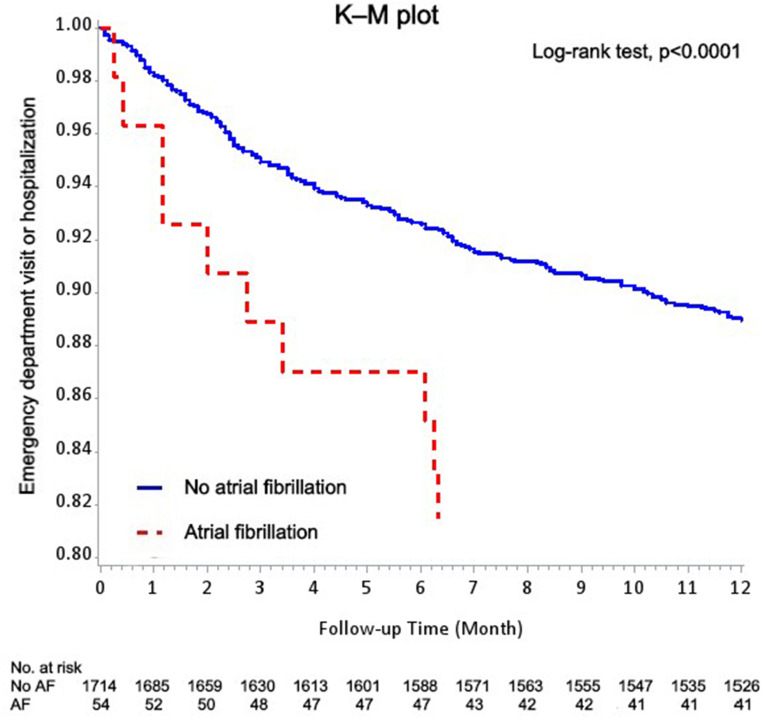
The K–M method was used to analyze the relationship between desaturation and acute exacerbation of COPD. Figure 2 shows a higher rate of emergency department visit or hospitalization in the desaturators than in the non-desaturators in the 1-year follow-up. There is also a higher rate of emergency department visit or hospitalization in patients with COPD with AF in the 1-year follow-up (Figure 3).
Figure 2.
Kaplan–Meier methods for the effect of desaturation during the 6-min walk test on the patients with emergency department visit or hospitalization based on the logistic regression after adjustment for sex, age, and BMI.
Figure 3.
Kaplan–Meier methods for the patients with COPD with or without atrial fibrillation with emergency department visit or hospitalization based on the logistic regression after adjustment for sex, age, and BMI.
Discussion
To our current knowledge, this is the first study to determine whether AF is an independent risk factor for EID in patients with COPD. There are three major findings in this study. First, 52.7% of all patients with COPD exhibited exertion desaturation during the 6MWT. Second, in the multivariate analysis with adjustment for age, sex, and FEV1, AF is the independent risk factor for desaturation during the 6MWT in patients with COPD. Moreover, patients with COPD with EID and AF had a higher rate of acute exacerbation requiring emergency department visit or hospitalization during the 1-year follow-up.
In clinical trials, the prevalence of hypoxia with oxygen use was approximately 2% in patients with COPD.26 The incidence of resting hypoxia was relatively low. Among those patients without resting hypoxia, EID could be identified during exercise testing. The results from the ECLIPSE study showed that 21% of patients had EID.27 In this study, 53% of patients had desaturation during the 6MWT. The significance of the association between female sex and desaturation showed in the multivariate analysis may be unconcluded because of the extremely low prevalence of COPD in the female population. There was no significant difference between the desaturation and non-desaturation groups using oral β-blockers and CCB although β-blockers have been reported to be associated with a reduction in COPD exacerbations.28 Cardioselective β-blockers have been reported to be safe in patients with COPD.29 Sustained use was not associated with a significant change in baseline FEV1 or worsening of respiratory symptoms or increase in the use of inhaled β-agonists.30 Our results showed compatible reports with these studies.
Several chronic comorbidities were associated with having frequent exacerbations and increased exacerbation risk in COPD. Among them, the coexistence of heart failure or atrial fibrillation (AF) with COPD in patients increased cardiovascular, non-cardiovascular, and all-cause mortality.31,32 The relationship between COPD and AF has been found in several epidemiologic studies. In the Copenhagen City Heart Study, reduced pulmonary function is a predictor for AF in patients with COPD.33 The Multi-Ethnic Study of Atherosclerosis Lung Study also found that lower FEV1 was associated with a higher risk of AF.34 The current pathological mechanisms are not fully understood. The potential mechanisms are hypoxia, hypercapnia, pulmonary hypertension, inflammation with oxidative stress, atrial remodeling, and respiratory drugs.35,36 Some researchers found that obstructive sleep apnea, with intermittent hypoxia at night, is a risk factor for AF.37 Except intermittent hypoxia, the potential mechanisms of obstructive sleep apnea and AF are also inflammation, oxidative stress, sympathetic activation, and atrial structure abnormalities.38
Although the stepwise selection revealed AF was associated with EID in patients with COPD, the association was not shown in the full model. The possible explanation is the patients with atrial fibrillation were few in the total subjects. Only 54 of 1768 patients (3%) with COPD had atrial fibrillation in the study. Older age and low FEV1 remain the predictors of EID in patients with COPD. The previous study showed that ventilation/perfusion mismatch resulting from progressive airflow limitation and emphysematous destruction of the pulmonary capillary bed is the key driver of hypoxia and the hypoxia may be exacerbated by sleep and exercise.39,40
The further analysis of the UPLIFT trial reported that the risk of AF or atrial flutter was higher after an exacerbation of COPD.41 In a prospective cohort study, the risk of AF hospitalization showed a linear correspondence with reduced pulmonary function during the 5-year follow-up.33 The rate of incident AF was also found inversely associated with forced expiratory volume in 1 seconds (FEV1) in different race, sex, and smoking status categories.42
The K–M method presents the significantly higher rate of acute exacerbation of COPD in the desaturation group than in the non-desaturation group of patients with COPD. This result supported the findings of the previous study of Waatevik et al.16 The K–M method also revealed a significantly higher rate of acute exacerbation of COPD in patients with the COPD with AF than in those without AF. This result correlated with the high acute exacerbation rate of COPD in the desaturation group. Several researches showed the association between AF and COPD.32,35,43,44 A large retrospective case-control cohort study showed that patients with COPD had 4.41- and 1.98-fold higher risks of AF and hospitalization, respectively, because of AF over an average follow-up of nearly 3 years.45 The incidence of new-onset AF in patients with COPD was also found higher than in those without COPD.43 Our reports showed compatible results of poor outcome of patients with COPD with AF.32,44 Several explanations of the association between COPD and AF included the chronic inflammation status of the coronary endothelial cell in patients with COPD and use of β-agonists contributing to the progression of AF. Other factors that induce AF in the COPD population included older age, hypoxia, smoking, and cardiovascular disease.43 In our study, older age, poorer saturation pre- and post-6MWT, and higher rates of the use of β-agonists were also found in the desaturation group of patients with COPD. It was hypothesized that desaturation during the 6MWT and AF are highly associated with acute exacerbation of COPD in patients with COPD.
This study has some limitations. First, this is a retrospective study. Some patients may be lost to follow-up in the following year. The emergency department visit or hospitalization with the diagnosis of acute exacerbation of COPD to hospitals other than Chang Gung Memorial Hospital will be underestimated in our study. Second, the data were collected from a single medical center. The disease severity may be more severe than all COPD populations. Third, the follow-up is only one year. The impact of EID on exacerbation in long term follow-up is not clear in the study. Finally, this study showed an association rather than the causal relationship between EID and AF in COPD. Further longitudinal prospective study is needed.
Conclusion
This study demonstrates that older age, low FEV1, and female sex are risk factors for EID. Desaturation during 6MWT is related to frequent acute exacerbation of COPD in the 1-year follow-up. The clinical physicians should be aware of EID in patients with COPD and educate them to avoid desaturation during exercise.
Acknowledgments
The authors thank and acknowledge the support of the Maintenance Project of the Center for Big Data Analytics and Statistics (Grant CLRPG3D0046) at Chang Gung Memorial Hospital for statistical assistance and consultation and data analysis.
Funding Statement
This project was supported by research grants from Chang Gung Memorial Hospital, Taiwan (CMRPG3K0061). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013;1(1):73–83. doi: 10.1016/S2213-2600(12)70060-7 [DOI] [PubMed] [Google Scholar]
- 2.Feary JR, Rodrigues LC, Smith CJ, et al. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–962. doi: 10.1136/thx.2009.128082 [DOI] [PubMed] [Google Scholar]
- 3.Mannino DM, Doherty DE, Buist AS. Global initiative on obstructive lung disease (GOLD) classification of lung disease and mortality: findings from the atherosclerosis risk in communities (ARIC) study. Respir Med. 2006;100(1):115–122. doi: 10.1016/j.rmed.2005.03.035 [DOI] [PubMed] [Google Scholar]
- 4.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 5.Soler-Cataluna J, Martínez-García MÁ, Sánchez PR, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim V, Benditt JO, Wise RA, et al. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):513–518. doi: 10.1513/pats.200708-124ET [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nonoyama ML, Brooks D, Guyatt GH, et al. Effect of oxygen on health quality of life in patients with chronic obstructive pulmonary disease with transient exertional hypoxemia. Am J Respir Crit Care Med. 2007;176(4):343–349. doi: 10.1164/rccm.200702-308OC [DOI] [PubMed] [Google Scholar]
- 8.Panos RJ, Eschenbacher W. Exertional desaturation in patients with chronic obstructive pulmonary disease. COPD. 2009;6(6):478–487. doi: 10.3109/15412550903341497 [DOI] [PubMed] [Google Scholar]
- 9.Takigawa N, Tada A, Soda R, et al. Distance and oxygen desaturation in 6-min walk test predict prognosis in COPD patients. Respir Med. 2007;101(3):561–567. doi: 10.1016/j.rmed.2006.06.017 [DOI] [PubMed] [Google Scholar]
- 10.Beatty AL, Schiller NB, Whooley MA. Six-minute walk test as a prognostic tool in stable coronary heart disease: data from the heart and soul study. Arch Intern Med. 2012;172(14):1096–1102. doi: 10.1001/archinternmed.2012.2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guazzi M, Dickstein K, Vicenzi M, Arena R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circ Heart Fail. 2009;2(6):549–555. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh M-H, Fang Y-F, Chung F-T, et al. Distance-saturation product of the 6-minute walk test predicts mortality of patients with non-cystic fibrosis bronchiectasis. J Thorac Dis. 2017;9(9):3168. doi: 10.21037/jtd.2017.08.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lettieri CJ, Nathan SD, Browning RF, et al. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med. 2006;100(10):1734–1741. doi: 10.1016/j.rmed.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension: comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161(2):487–492. doi: 10.1164/ajrccm.161.2.9906015 [DOI] [PubMed] [Google Scholar]
- 15.Camillo CA, Langer D, Osadnik C, et al. Survival after pulmonary rehabilitation in patients with COPD: impact of functional exercise capacity and its changes. Int J Chron Obstruct Pulmon Dis. 2016;11:2671. doi: 10.2147/COPD.S113450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waatevik M, Johannessen A, Gomez Real F, et al. Oxygen desaturation in 6-min walk test is a risk factor for adverse outcomes in COPD. Eur Respir J. 2016;48(1):82–91. doi: 10.1183/13993003.00975-2015 [DOI] [PubMed] [Google Scholar]
- 17.Du H, Wonggom P, Tongpeth J, et al. Six-minute walk test for assessing physical functional capacity in chronic heart failure. Curr Heart Fail Rep. 2017;14(3):158–166. doi: 10.1007/s11897-017-0330-3 [DOI] [PubMed] [Google Scholar]
- 18.Shao SC, Chan -Y-Y, Kao Yang Y-H, et al. The Chang Gung research database—a multi-institutional electronic medical records database for real‐world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. 2019;28(5):593–600. doi: 10.1002/pds.4713 [DOI] [PubMed] [Google Scholar]
- 19.Tsai M-S, Lin M-H, Lee C-P, et al. Chang Gung research database: a multi-institutional database consisting of original medical records. Biomed J. 2017;40(5):263–269. doi: 10.1016/j.bj.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wedzicha J. Domiciliary oxygen therapy services: clinical guidelines and advice for prescribers. Summary of a report of the Royal College of Physicians. J R Coll Physicians Lond. 1999;33(5):445–447. [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta R, Ruppel GL, Espiritu JRD. Exercise-induced oxygen desaturation during the 6-minute walk test. Med Sci. 2020;8(1):8. doi: 10.3390/medsci8010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrianopoulos V, Franssen FME, Peeters JPI, et al. Exercise-induced oxygen desaturation in COPD patients without resting hypoxemia. Respir Physiol Neurobiol. 2014;190:40–46. doi: 10.1016/j.resp.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 23.Perez T, Deslée G, Burgel PR, et al. Predictors in routine practice of 6-min walking distance and oxygen desaturation in patients with COPD: impact of comorbidities. Int J Chron Obstruct Pulmon Dis. 2019;14:1399. doi: 10.2147/COPD.S188412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic Research: Principles and Quantitative Methods. John Wiley & Sons; 1982. [Google Scholar]
- 25.Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 26.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800 [DOI] [PubMed] [Google Scholar]
- 27.Andrianopoulos V, Celli BR, Franssen FME, et al. Determinants of exercise-induced oxygen desaturation including pulmonary emphysema in COPD: results from the ECLIPSE study. Respir Med. 2016;119:87–95. doi: 10.1016/j.rmed.2016.08.023 [DOI] [PubMed] [Google Scholar]
- 28.Bhatt SP, Wells JM, Kinney GL, et al. β-Blockers are associated with a reduction in COPD exacerbations. Thorax. 2016;71(1):8–14. doi: 10.1136/thoraxjnl-2015-207251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta‐blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dransfield MT, McAllister DA, Anderson JA, et al. β-blocker therapy and clinical outcomes in patients with moderate chronic obstructive pulmonary disease and heightened cardiovascular risk. An observational substudy of SUMMIT. Ann Am Thorac Soc. 2018;15(5):608–614. doi: 10.1513/AnnalsATS.201708-626OC [DOI] [PubMed] [Google Scholar]
- 31.Yoshihisa A, Takiguchi M, Shimizu T, et al. Cardiovascular function and prognosis of patients with heart failure coexistent with chronic obstructive pulmonary disease. J Cardiol. 2014;64(4):256–264. doi: 10.1016/j.jjcc.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 32.Durheim MT, Cyr DD, Lopes RD, et al. Chronic obstructive pulmonary disease in patients with atrial fibrillation: insights from the ARISTOTLE trial. Int J Cardiol. 2016;202:589–594. doi: 10.1016/j.ijcard.2015.09.062 [DOI] [PubMed] [Google Scholar]
- 33.Buch P, Friberg J, Scharling H, et al. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J. 2003;21(6):1012–1016. doi: 10.1183/09031936.03.00051502 [DOI] [PubMed] [Google Scholar]
- 34.Chahal H, Heckbert SR, Barr RG, et al. Ability of reduced lung function to predict development of atrial fibrillation in persons aged 45 to 84 years (from the Multi-Ethnic Study of Atherosclerosis-Lung Study). Am J Cardiol. 2015;115(12):1700–1704. doi: 10.1016/j.amjcard.2015.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goudis CA. Chronic obstructive pulmonary disease and atrial fibrillation: an unknown relationship. J Cardiol. 2017;69(5):699–705. doi: 10.1016/j.jjcc.2016.12.013 [DOI] [PubMed] [Google Scholar]
- 36.Matarese A, Sardu C, Shu J, et al. Why is chronic obstructive pulmonary disease linked to atrial fibrillation? A systematic overview of the underlying mechanisms. Int J Cardiol. 2019;276:149–151. doi: 10.1016/j.ijcard.2018.10.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49(5):565–571. doi: 10.1016/j.jacc.2006.08.060 [DOI] [PubMed] [Google Scholar]
- 38.Khalyfa A, Gozal D. Connexins and atrial fibrillation in obstructive sleep apnea. Curr Sleep Med Rep. 2018;4(4):300–311. doi: 10.1007/s40675-018-0130-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbera J, Roca J, Ferrer A, et al. Mechanisms of worsening gas exchange during acute exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 1997;10(6):1285–1291. doi: 10.1183/09031936.97.10061285 [DOI] [PubMed] [Google Scholar]
- 41.Halpin DM, Decramer M, Celli B, et al. Risk of nonlower respiratory serious adverse events following COPD exacerbations in the 4-year UPLIFT® trial. Lung. 2011;189(4):261–268. doi: 10.1007/s00408-011-9301-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Agarwal SK, Alonso A, et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2014;129(9):971–980. doi: 10.1161/CIRCULATIONAHA.113.004050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao K-M, Chen C-Y. Incidence and risk factors of atrial fibrillation in Asian COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2523. doi: 10.2147/COPD.S143691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terzano C, Romani S, Conti V, Paone G, Oriolo F, Vitarelli A. Atrial fibrillation in the acute, hypercapnic exacerbations of COPD. Eur Rev Med Pharmacol Sci. 2014;18(19):2908–2917. [PubMed] [Google Scholar]
- 45.Sidney S, Sorel M, Quesenberry CP, et al. COPD and incident cardiovascular disease hospitalizations and mortality: kaiser permanente medical care program. Chest. 2005;128(4):2068–2075. doi: 10.1378/chest.128.4.2068 [DOI] [PubMed] [Google Scholar]