Abstract
Background
Currently, novel coronavirus disease (Covid-19) outbreak creates global panic across the continents, as people from almost all countries and territories have been affected by this highly contagious viral disease. The scenario is deteriorating due to lack of proper & specific target-oriented pharmacologically safe prophylactic agents or drugs, and or any effective vaccine. drug development is urgently required to back in the normalcy in the community and to combat this pandemic.
Purpose
Thus, we have proposed two novel drug targets, Furin and TMPRSS2, as Covid-19 treatment strategy. We have highlighted this target-oriented novel drug delivery strategy, based on their pathophysiological implication on SARS-CoV-2 infection, as evident from earlier SARS-CoV-1, MERS, and influenza virus infection via host cell entry, priming, fusion, and endocytosis.
Study design & Methods
An earlier study suggested that Furin and TMPRSS2 knockout mice had reduced level of viral load and a lower degree of organ damage such as the lung. The present study thus highlights the promise of some selected novel and potential anti-viral Phytopharmaceutical that bind to Furin and TMPRSS2 as target.
Result
Few of them had shown promising anti-viral response in both preclinical and clinical study with acceptable therapeutic safety-index.
Conclusion
Hence, this strategy may limit life-threatening Covid-19 infection and its mortality rate through nano-suspension based intra-nasal or oral nebulizer spray, to treat mild to moderate SARS-COV-2 infection when Furin and TMPRSS2 receptor may initiate to express and activate for processing the virus to cause cellular infection by replication within the host cell and blocking of host-viral interaction.
Keywords: Virus-host cell interaction blocking, Host cell entry inhibitor, Nasal or oral inhaler, Covid-19, TMPRSS2, FURIN
Introduction
The global data on severity and mortality of the coronavirus disease 2019 (Covid-19) pandemic shows that the people of all countries and territories have been struggling due to lack of a specific vaccine or effective prophylactic and or drug. A commercial vaccine (live attenuated, heat-killed, RNA or subunit) for the susceptible population usually takes time. While, to date, most of the anti-viral protease inhibitors (Ritonavir, Lopinavir, Remdesivir, Hydroxychloroquine, etc.) used in Covid-19 is on trial and error basis. Most cases, patients are failed to recover due to nonspecific drug binding, and adverse reaction because of patient's co-morbid conditions, including organ malfunctioning (Cameron and Castro, 2001; Zumla et al., 2016; Lenkens et al., 2020; Carter and Do, 2020, Carter et al., 2020; Zhao et al., 2018). So, drug target identification, followed by specific drug delivery for rapid healing and protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are urgent issues.
The SARS-CoV-2, the causative agent of COVID-19, is an enveloped, non-segmented positive-sense single-stranded (ss) RNA virus that contains four structural proteins: Spike (S), Envelope (E), Membrane (M), and Nucleocapsid or N (De-Wit et al., 2016; Drosten et al., 2003). It is reported that the Spike glycoprotein of CoV-2 harbors a Furin cleavage site, at the boundary between its two subunits, S1/S2, which is activated by the host cell enzyme Furin proprotein convertase to make the virus more susceptible to its primary receptor, angiotensin-converting enzyme-2 or ACE2 (Walls et al., 2020). Furin also helps the virion to bind with ACE2 efficiently, via the receptor-binding domain (RBD) S1 of S-protein, to transmit the virus as stable form and invade the host cell rapidly for further pathogenicity (Coutard et al., 2020; Walls et al., 2020). The RBD of S-protein after cleavage mediate processing, followed by activation, and is responsible for binding with ACE2; while the interaction of S-protein with host ACE2 receptor helps the virus to invade the host cell (Li et al., 2005). The binding affinity of S-protein of SARS-CoV-2 with ACE2 is about ten times higher than other Corona viruses including SARS-CoV-1(Lau and Peiris, 2005; Ge et al., 2013), indicated that CoV-2 infection depends on the expression of host cell ACE2 receptor. Furthermore, Transmembrane protease serine 2 (TMPRSS2) fuse and activate the S-protein-ACE2 complex into the host cell endosome, via proteolytic digestion of the S2 domain of S-protein's through membrane fusion (Hoffmann et al., 2020). This cascade helps to promote the pathogenicity and life-cycle, for which ACE2 and TMPRSS2 can be served as a viable target for nCoV-2 therapy. It has been found that Furin and TMPRSS2 could play a significant role for efficient attachment of cleaved S-protein with host cell ACE2 receptor via activation, priming, and fusion, followed by endosomal internalization and then to establish pathogenicity through replication, growth, and cell to cell spread of the infecting virus to different organ's. The proteolytic activity of such transmembrane serine protease, prior to cleavage, activation and fusion have been exploited by the S-protein of the virus towards stronger infection (Hoffmann et al., 2020). Our current understanding will focus on the promise of suitable ligand on those drug targets, via intra-nasal or oral nano-suspension spray-based drug delivery, to tackle this pandemic panic.
The platform of hypertension, ACE2 receptor and Covid-19
Earlier studies indicated that the pre-treated hypertensive patients, particularly with Angiotensin receptor-1 (AR1) blocker (Losartan or Telmisartan), may recover and help to protect from Covid-19 infection with reduced recovery time and mortality (Rothlin et al., 2020; Magrone et al., 2020; Tomasoni et al., 2020), because those AR1 blockers could attenuate the angiotensin-2 mediated vasoconstriction, inflammation, cell damage, lung injury and lung fibrosis. Moreover, these drugs can counterbalance the ACE/Ang-II/AT1R axis upregulation, mediated by the renin-angiotensin-aldosterone system (RAAS), a signalling disorder in the hypertensive patient. Furthermore, those agents may upregulate the ACE2/Ang1-7/MasR homeostatic axis for better recovery from hypertension, which is being suppressed during Covid-19 infection as the viral S-protein bind with ACE2 for entry to the host cell. It has been reported that ATR1 receptor antagonist could reduce the virus-driven lung injury, edema, inflammation and acute lung pneumonia (Sanchis-Gomar et al., 2020; Mousavizadeh and Ghasemi, 2020). Moreover, anti-inflammatory and anti-fibrotic agent Losartan (Aziz et al., 2018 Arjmand et al., 2020, Aziz et al., 2018; Dionísio et al., 2020), an angiotensin receptor blocker, has been reported as an anti-inflammatory and anti-fibrotic agent (Arjmand et al., 2019Dionísio et al., 2020 could ameliorating the post-infective cytokine storm and organ damage. Thus, it can be hypothesized that the ARBs (Angiotensin-II receptor blockers) treatment for Covid-19 patient may lead to early recovery.
ACE2 is known to be present in the cell membrane surface of human esophagus, lungs, heart, intestine, kidney, prostrate, and brain (Sanchis-Gomar et al., 2020). Functionally it facilitate SARS-CoV-2 invasion to host cells, and thus, ACE2 antagonist could be a promising treatment option in Covid-19 patients (Kuba et al., 2006). It will be interesting to explore those receptor blockers that could be the novel drug target for effective and safe therapeutic option to fight SARS-CoV-2 infection (Hoffmann et al., 2020; Shin and Seong, 2017; Zhang et al., 2020; Gurwitz, 2020). We thought that this approach can successfully prevent the host-virus attachment and pharmacodynamic interactions to stop further infection. This cascade may also prevent the entry of this deadly virus into the host cell via endocytosis, and its subsequent replication, thereby stop further division, assembly and life-cycle. This therapeutic strategy could be the game-changer as a prophylactic treatment, and to attenuate the mortality as well as to cope-up with SARS-CoV-2 infection, as reporetd earlier (Ho et al., 2007).
The ACE2 receptor is located in most of the major organs of human body, including esophagus and lung. The COVID-19 virus enters the human body through the nostrils and mouth to the target goblet and ciliated cells. Thus, there is an ample scope for the virion to attach with the main gateway ACE2 receptor in esophageal cells. If the immune system unable to fight back, the virus goes down the windpipe and infect lungs cells and enter the pneumocytes of the alveoli to release viral-RNA in cell cytoplasm for replication. The higher binding affinity of the Covid-19 virus to the ACE2 active site, compared to SARS-CoV-1, may result in greater endocytosis and translocation of the virus-enzyme complex into the endosomes (Wang et al. 2008; Millet and Whittaker, 2018 ; Li et al., 2020). It will be a novel approach to handle Covid-19, if any stable Phyto-molecule can be identified from the age-old traditional herbs and spices (Table 1 ), like Emodin (Ho et al., 2007), that could block the interaction between S-protein of Coronavirus and ACE2 receptor of the human host.
Table 1.
Phytopharmaceuticals derived from the traditional herbs that targets entry receptor of the host cells to block the interaction and attachment of some pathogenic viruses, to be useful in COVID-19.
| Name of Phytochemical | Chemical nature | Biological source | Target receptor of Host-cell | Mode of action | Target virus or cells |
|---|---|---|---|---|---|
| Emodin (Ho et al., 2007) | 6-methyl-1,3,8-trihydroxyanthraquinone | Rheum palmatum L, Reynoutria japonica | ACE2 receptor | Block the interaction and binding of CoV S-protein with ACE2 receptor . | SARS-CoV-1 |
| Luteolin (Peng et al., 2017; Ojha et al., 2015) | Flavonoid | Reseda luteola, Salvia tomentosa, Aiphane saculeata, Pedilanthus tithymaloides | Furin | Prevent replication of DENV and HSV. | Furin proprotein convertase of Dengue virus |
| Nicotianamine (Takahashi et al., 2015) | Metal-chelating molecule like nicotainamine | Nicotiana tabacum L. and Glycine max (Soyabean) | ACE2 | Block SARS CoV spike protein mediated cell fusion. | Corona Virus 1 |
| Andrographalide (Basak et al., 1999) | Diterpene | Andrographalis paniculata | Furin proprotein convertage | Blocking of HIV replication | HIV |
| 5,2′-dihydroxy-3′,4′-dimethoxy flavone-7-O-β-D-glucopyrano side (De et al., 2015) | Flavonoid glucoside | Phrynium placentarium | Furin | Furin inhibition mediated blocking of virus attachment in host cell | HIV, Influenza, and Dengue virus infection |
| Polyphenolics (Zhu et al., 2013) | - | Various tea, fruits, grapes and traditional medicinal plants | Host cell Furin | Furin like proprotein convertase inhibitor | Inhibition of prostate cancer. *can be projected for COVID prevention |
| Bromhexine (Maggio and Corsini, 2020) |
2,4-dibromoaniline |
Justicia adhatoda | TMPRSS2 | TMPRSS2 inhibition in Prostate cancer & Influenza virus | Inhibition of prostate cancer & influenza. *can be projected for prevention of COVID |
| Baicalein, Chrysin, Oro-xylin-A and its glycosides (Lalou et al., 2013) | Flavonoids and their glycosides | Oroxylum indicum | Furin Proprotein Convertase | Furin inhibitor (Proprotein Convertase) |
Inhibit tumor cell proliferation |
| Celastrol (Shao et al., 2013) | Pentacyclic triterpene of quinone methides. | Tripterygium wilfordii, Celastrus regelii | Prostate cancer | TMPRSS2 inhibition followed by blocking of Androgen receptor | Inhibition of prostate cancer. *can be projected for COVID prevention |
| Plant Lectin (Keyaerts et al., 2007) | Glycoprotein | Allium sativum, Lycoris radiata | Host cell ACE2 | ACE2 binding of SARS-CoV-1 to inhibit virus entry into host cells. | SARS-CoV-1 |
| Ursolic acid (Chiang et al., 2005; Bag, 2012) | Pentacyclic triterpenoid | Ocimum sanctum, Malotus peltatus | CD4 receptor | Inhibit Protease, gp120-CD4 binding & entry; HSV replication; immunomodulatory against virus | HIV, Coxa- kivirus B1, Enterovirus 71 (EV71) |
| Acetyl-11-keto-boswellic acid (Von et al., 2016; Goswami et al., 2018) | Pentacyclic terpenoid | Boswellia serrata | Specific host cell receptor for virus entry, | Able to block CHIKV entry to inhibit Chikungunya,Vesicular stomatitis virus (VSV), and HSV infections | CHIKV, VSV may block interaction and attachment of SARS-COV-2 with host cell membrane. |
| Cepharanthine (Keyaerts et al., 2007) | Bisbenzyliso-quinoline | Stephania cephalantha or other species | Specific host cell receptor in connection to virus entry, not yet recruited | Protease inhibitor, Antiinflammatory, antiviral, immunomodulatory, anti-cancer. | Cancer. Might be used for novel Corona virus |
| Sylimarin (Dai et al., 2013; Song et al., 2011) | Flavonoids | Milk thistle (Silybum marianum) | Specific host cell receptor in connection to virus entry, not yet recruited. | Inhibit Viral RNA synthesis and Influenza virus replication |
Anti-Influenza and Anti-CHKV (Lani et al, 2015); and anti-Mayaro Virus activity (Camini et al, 2018) |
| Sylibinin (Farooqi et al. 2010) | Flavonoids | Milk thistle (Silybum marianum) | InhibitTMPRSS2 expression in prostate cancer | TMPRSS2 expression inhibition following the androgen receptor blocking | Prostate cancer *May be targeted for TMPRSS2 activation blocking during corona virus entry and infection. |
| Epigallocatechin 3-gallate (Kim et al., 2013) | Polyphenolic catechin | Camelia sinensis (Green tea) | Specific host cell receptor of virus entry, not yet recruited; Block Influenza virus fusion with host cell membrane and replication |
Inhibit the TMPRSS2 expression in prostate cancer, and block influenza virus attachment in host cell | May be targeted for TMPRSS2 activation during novel Corona virus entry and infection |
| Quercetin, hypericin, rutin, isoquercetin (Ling et al., 2020) | Flavonoids | Houttuynia cordata | 3Cl Mpro and RNA dependent RNA polymerase inhibitor of SARS CoV-1 | Inhibit influenza virus entry and replication, and anti-SARS-COV-1 (Lau et al, 2008) | Influenza & SARS CoV *May be potential for SARS-COV-2 infection blocking |
| Glycyrrhizic Acid & its Derivatives | Triterpenoid saponin | Glycyrrhiza glabra | Host cell surface receptor not identified | Inhibit DENV proliferation (Baltina et al., 2019) and anti-SARS-CoV (Hoever et al., 2005) activity; and prevent infection by blocking viral replication | Dengue and SARS CoV *Might be focused to inhibit SARS-COV-2 |
| Caffeic acid & Caffeic acid phenethyl ester(Weng et al., 2019) | Hydroxy cinnamic acid. | Eucalyptus globulus, Dipsacus asperoides | Host cell surface receptor not identified | Block viral entry in host cell to prevent infection |
Human CoV NL63 *Might be focused to inhibit SARS COV-2 |
| Resveratrol and its semi synthetic derivatives (Li et al., 2006) | Stilbenoid, a type of natural phenol, and a phytoalexin | Include the skin of Grapes, Blueberries, Raspberries, Mulberries, and Peanuts | Host cell surface receptor not identified | Inhibition of SARS Coronavirus Replication | SARS Coronavirus *Might be focused and applied to inhibit SARS-COV-2 |
| Paeoniflorin (Gan et al., 2014) | Terpene glycoside | Paeonia lactiflora, Salvinia molesta | Host cell surface receptor not identified | Anti-inflammatory by suppressing inflammatory cytokines,triggered by the ROS generated by viral particle . | *Might be focused and potentially used to inhibit SARS-COV-2 |
| Tetrahydrocannabinol, Cannabidiol (Reiss, 2010) | Phytocannabinoid | Cannabis indica | Host cell surface receptor not identified | Block HSV entry and replication | HSV *Might be focused and applied potentially to inhibit SARS- COV-2 |
| Berberine (Botwina et al., 2020; Hung et al., 2019) | Benzyl-isoquinoline alkaloid | Berberis vulgaris | Inhibit viral replication and block entry of virus in host cell | Blocking of hepatitis C virus entry in host cell and hampers Influenza virus replication | *Hepatitis C virus Might be focused and applied to inhibit SARS-COV-2 |
| Taxifolin (Min et al., 2002) | Dihydroquercetin | Milk Thistle, Juglans mandshurica | Anti-human Immunodeficiency Virus-Type 1 Activity | Blocking HIV infection and killing of virus by diminishing the viral invasion | HIV *Might be focused to inhibit COV-2 |
| Sinigrin and Hesperidin (Lin et al., 2005) | Glucosinolate & Flavonoid | Sinigra alba, Brussels sprouts, Citrus fruit | Anti- SARS Corona virus | 3CLMpro inhibitor | SARS Corona virus *Might be focused and applied to inhibitCOV-2 |
| Allitridin (Kiesel and Stan, 2017) | Diallyl trisulfide organosulfur compound | Allium sativum | Chemopreventive activity against breast cancer | Furin convertase inhibitor | Breast cancer *Might be focused and potentially used to inhibit SARS-CoV-2 |
NA, Not available
However, the approach of blocking ACE2 receptor-mediated virus entry may be contraindicated for hypertensive patients, who are suffering from malfunctioning of kidney and associated RAAS disorder mediated hypertension. Angiotensin-2 receptor blocker (AT1, Losartan, telmisartan) reduces the blood pressure in hypertensive patients with kidney disorders, via competitive inhibition of angiotensin-2 signalling molecule that binds with the AT1 receptor. If the angiotensin 2 binds with AT1 receptor in the absence of ARBs, it could lead to vasoconstriction, followed by hypertension and cardiac hypertrophy as a consequence of the release of aldosterone hormone, leading to the severe lung injury (SLI) and acute lung respiratory disorders (ALRD). These could be a fatal manifestation for Covid-19 patients’ having co-morbidity of hypertension. On the other hand, excess angiotensin 2 converts into angiotensin- (1-7) by the ACE2 receptor, located in the lung, kidney, and esophagus cell surface. This angiotensin-(1-7) elicits hypotensive effect via vasodilation of artery and blood vessels by releasing Nitric Oxide.
Meanwhile, if SARS-CoV-2 invades hypertensive hosts target cell via ACE2 receptor; then ACE2-induced homeostatic mechanism and normal physiological activity would be suppressed, with disruption of the production of vasodilator, angiotensin-(1-7) molecule. Therefore, the entry of CoV-2 in hypertensive hosts may fail to protect them from lung-fibrosis and cardiac hypertension. Thus, host-virus interaction via ACE2 receptor attachment, followed by virion-induced free radical generation and inflammasome, may generate pro-inflammatory cytokine storm affecting other organs. Patients susceptible to cardiac arrest and organ failure could lead to a fatal outcome, even death, due to this type of abnormal cell signalling.
Moreover, ACE2 has a significant role in protecting major organ failure due to hypertension and angiotensin-2 mediated lung disorders (Kuba et al., 2006). Studies in mice showed that the interaction of CoV spike protein with ACE2 receptor persuades a decline in ACE2 level in cells through internalization and degradation of the protein that may contribute to lung damage (Imai et al., 2008; Jia, 2006; Zhang and Zheng-Li, 2013). Thus, ARBs therapy could reduce the chances of pneumonia, and lung damage along with the control of hypertension, pulmonary oxygen saturation, and cardiac risk in the Covid-19 patient (Saavedra, 2020). AT1 receptor antagonist treatment bind the Angiotensin 2 with the AT2 receptor to prevent lung injury or damage following the hypotensive effect. A recent study illustrated that Covid-19 patients pre-treated with ARBs have significantly reduced mortality and does not require hospitalization or intensive care (Rothlin et al., 2020; Magrone et al., 2020; Tomasoni et al., 2020). The primary function of ACE2 is to counterbalance the ACE function to reduce blood pressure and inflammation. Binding of Coronavirus S-protein with ACE2 receptor and its translocation may lead to complete loss of its hypotensive, anti-inflammatory and antioxidant property. So, S-protein binding with ACE2 receptor may augment the serious side effects, such as lung injury, fibrosis, hypertension and acute inflammation due to upregulated production of Angiotensin II in co-morbid cases. Hence, it can be postulated that ACE2 receptor blocker mediated drug therapy may not be fruitful as a prophylactic or therapeutic agent for the hypertensive patient suffering from renal dysfunction to fight Covid-19. As the down-regulation of the ACE2 receptor may trigger serious side effects like lung and other organ damage, owing to angiotensin-2 mediated hypertension and pneumonia, as observed during COVID-19 infection. Nevertheless, for healthy normal people ACE2 receptor blocker could successfully counter the cellular entry of SARS-CoV-2.
Host cell receptors and their role on Covid-19 virus entry process
It is interesting to note that after the binding of nCoV spike protein with host cells ACE2 receptor, the adjacent TMPRSS-2 receptor helps to allow the virion to enter into the intracellular endosome, by priming of S-2 domain of S protein (Heurich et al., 2014). Therefore, inhibition of TMPRSS2 by appropriate and specific phytochemicals, derived from traditional ethnomedicines including Ayurveda (Vellingiri et al., 2020) and Unani (Nikhat and Fazil, 2020) could be the potential therapeutic intervention for the management of Covid-19. Expression of this cell surface receptor helps to augment the chance of infection by facilitating the endocytosis of SARS-CoV-2 and ACE2 complex into the host cell endosome (Matsuyama et al., 2014). Moreover, the hypertensive patient may be more benefitted with the TMPRSS2 inhibitor to fight against SARS-CoV-2 infection, and its pathogenesis (Shen et al., 2017), influenza and other coronavirus infections, due to more or less similar pathophysiological symptoms. So far, many TMPRSS2 inhibitors of plant origins are reported, and some of the most promising candidates are depicted in Table 1 as possible prevention or remedy against this horrible respiratory virus. Additionally, some of those compounds (spices derived phyto-compounds) having identified mechanism of action against SARS and RNA virus has been presented in Table 2 . Prophylactic therapy with the dual blocker of ACE2 and TMPRSS2 receptor to healthy people, without cardiac and kidney disorders, may provide excellent result by blocking the virus attachment to the host cell and translocation of the virion into the intracellular endosome. Due to strong and efficient binding ability of S-protein with ACE2 receptor, particularly in lung cells, leads to lung damage with hyperactive cytokine storm mediated acute inflammation to another organ (Kuba et al., 2020). Thus, the combinatorial therapy of anti-inflammatory agent along with TMPRSS2 receptor antagonist may hold good promise for both therapeutic and prophylactic treatment to cope up with this deadly virus. Those molecules as a combination may be delivered in situ by nano-aerosol suspension inhaler via oral or intra-nasal route rapidly in target-oriented manner at the TMPRSS2 receptor-binding site of esophagus and lung thereby block the endosome internalization of the virus-ACE2 complex. This kind of therapy can be improved by the addition of competitive ACE2 receptor binding antagonist of viral S-glycoprotein.
Table 2.
Phytopharmaceuticals, derived from the traditional spices, that target host cell entry receptorto block the interaction and attachment of some pathogenic viruses, to be useful in COVID-19.
| Compounds from Spice | Source Plant | Antiviral activity | Disease | References |
|---|---|---|---|---|
| Coriandrin | Coriandrum sativum L | Inhibit viral growth and replication | HIV | Towers, 1989 |
| Carnosolic acid and carnosol | Rosmarinus officinalis L. | Inhibits HIV protease | HIV | Paris et al., 1994 |
| Eugenin/Eugenol | Syzygium aromaticum (L) Merr. & Perry | Inhibits DNA polymerase activity | HSV-1 & HSV-2 Hepatitis C | Takeshi & Tanaka, 1981; Minami et al., 2003; Nolkemper et al., 2006; Reichling et al., 2009; Gilling et al., 2011; Reichling et al., 2009 |
| Ajoene, Allicin, Allylmethyltio-sulfinate, Methyl allyltio-sulfinate | Allium sativa L. | Inhibit virus growth (Nonspecific) |
HSV, Parainfluenza 3, Vaccinia, Vesicular Stomatitis, Rhino-virus type 2 | Weber et al., 1992 |
| Coumarins | Cinnamomum spp. | Inhibit virusg Growth (Nonspecific) |
Influenza, HIV, Enterovirus 71, Coxsackie virus A16, Dengue and Chikungunya virus |
Wang et al., 2012; Riveiro et al., 2010; Li et al., 2017; Hassan et al. 2016 |
| Gingerols | Zingiber officinale Rosc. | Inhibit virus growth (Not specific) |
Human Respiratory syncytial virus (RSV) |
Chang et al., 2013; Rathinavel et al., 2020 |
| Curcumin | Curcuma longa L. | Interfere viral replication; suppress cellular signalling pathways | Different viruses | Mathew and Hsu, 2018; Mounce et al., 2017 |
However, virtual simulation analysis revealed that this highly contagious coronavirus may also invades the human body using three more cell surface receptors, including (i) Glucose Regulated Protein 78 or GRP78 (Ibrahim et al., 2020), (ii) CD26 (cluster of differentiation 26) or dipeptidyl peptidase 4 (DPP4), a key immunomodulatory player for cell hijacking and virulence (Lu et al., 2013; Vankadari and Wilce, 2020), and (iii) Basigin (BSG) or extracellular matrix metalloproteinase inducer (EMMPRIN) or CD147 (Wang et al., 2020, Wang et al., 2020). The binding of the SARS-CoV-2 spike protein with those three-cell surface receptor(s) has been illustrated, based on computational in-silico analysis. Hence, this type of attachment needs to be validated by physical laboratory experimentation with the causative virus, SARS CoV-2, responsible for Covid-19.
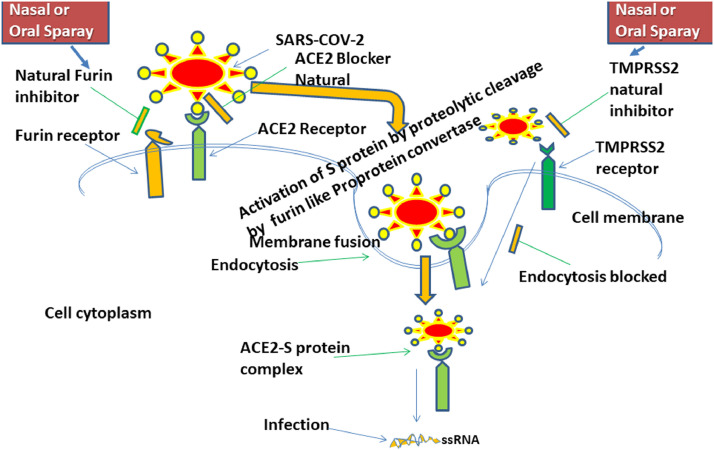
Finally, if the CD26, CD147, GRP78 receptor binding of SARS-CoV-2 is excluded due to deficiency of proper experimental validation, we may focus predominantly on the three cell surface receptors such as Furin, ACE2 and TMPRSS2 for potential therapeutic target (Shen et al., 2017) to seal the intracellular entry of the virus, as depicted in Fig. 1 . However, if we discard the ACE2 receptor blocking approach due to unavoidable limitation related to kidney disorder and diabetes, then dual inhibition of TMPRSS2 and Furin protein using safe phytochemicals like Bromhexine, an inhibitor of TMPRSS2 (Maggio and Corsini, 2020), could be the lucrative treatment strategy for the management and prevention of Covid-19. Similarly, researchers may short out several phytochemicals against host cell Furin protease for effective and safe anti-COVID-19 drug development, like Phyto-flavonoid luteolin, an inhibitor of Furin (Peng et al., 2017). Luteolin had potent activity against wild-type and clinical isolates of HSV-2 (EC50 22.4 and 27.5 µg/ml and EC99 at 40.2 and 49.6 µg/ml (Ojha et al., 2015), and replication of dengue virus via Furin proprotein convertase inhibition (Peng et al., 2017). Additionally, luteolin possess anti-seditious (Aziz et al., 2018) and immunomodulatory activity (Hosseinzade et al., 2019). However, the designed and predicted Phyto-compounds needs to have interacted with the host cell surface receptor with optimal inhibition, as well as constant and stable binding at pharmacological relevant concentration.
Fig. 1.
Infection pathway of cellular infectivity of SARS-COV-2 via Host cell protease surface receptor and subsequent drug targeting through phytopharmaceuticals mediated nasal or oral nebuliser for blocking of host-virus interaction during early endocytosis during therapeutic intervention.
These strategies could be achieved by in-silico homology modelling, molecular docking, molecular dynamics simulation, followed by the validation through in-vitro model of inhibition studies on the interaction between host cell receptor and Spike protein of novel coronavirus. Thus, our study suggests an investigation of the localization of host cell receptor and spike protein binding by ELISA, FACS, receptor binding fluorescent assay, and immuno-electron microscopy. The anti-inflammatory and immunomodulatory activity of an anti-viral agent seems to be additionally beneficial to protect the damage of organs like lungs, heart, liver and kidney from virus-driven Th1/Th2 mediated inflammatory cytokine storm during the Covid-19 (Stebbing et al., 2020; Mehta et al., 2020). Therefore, the identification of any antivirally active Phyto-compounds having anti-inflammatory and immunomodulatory activity will be of immensely helpful to control the super-spreading Covid-19. Rationally selected host cell receptor blocker may be explored as a promising treatment option on Covid-19 patient, if the compounds additionally exhibit anti-thrombotic effect alone or in combination (Galimberti et al., 2020). Recent reports suggest that the Covid-19 patient with acute infection may die due to blood clotting mediated pulmonary lung coagulopathy (Connors and Levy, 2020; Menezes‐Rodrigues et al., 2020).
Moreover, this validated receptor blockers could be delivered specifically via oral or nasal spray nebulizer or vapour nano-spray formulation-based drug delivery to the corresponding host cell receptor, located at oral mucosa, nasal membrane and lung cell surface receptor membrane. This tactic could be appropriate; because of the micronized nano-aerosol suspended drug particle would bind very rapidly to the Furin and TMPRSS2 receptor(s) of the goblet and ciliated cells in upper respiratory tract in a small concentration, via targeted novel drug delivery system. Furthermore, drug formulated in e-cigarette (Breitbarth et al., 2018) could deliver the active constituent quickly into the lung cell surface receptor for competitive binding to block the virus attachment(entry) into the host cell surface. The strategy would provide promising therapeutic intervention towards the emerging insight of prophylactic treatment as supported by the earlier report on the treatment of influenza patient with intranasal or oral delivery of anti-viral drug successfully (Peng et al., 2000; Wong et al., 2010; Dickens et al., 2007). The novel approach of such drug delivery may improve the pharmacokinetic behaviour of the anti-viral prophylactic or therapeutic agent(s) by enhancing absorption and cell permeability. Drug particle formulated as a harmonious combination of cocktail receptor inhibitors at optimal and pharmacologically relevant dose could block the host cell Furin, and TMPRSS2 receptors located in the target organs like esophagus, lungs, as well as in colon, liver, heart, kidneys, intestine and pancreas to prevent the entry of the SARS-CoV-2. Additionally, it may diminish the toxicity of the main active ingredient, as well (Remacle et al., 2010).
Most of the current anti-viral agents are nucleic acid analogues that directly act on the virion via conventional systemic tablet, while injection have poor efficacy, prone to drug-resistance (due to frequent mutation of viral RNA and spike protein) and have undesirable side effects. Thus, when it is used against Covid-19, then those directly acting drugs may enhance the adverse drug reaction due to host cell permeability issue (Lenkens et al., 2020; Zhao et al., 2020; Saha et al., 2020; Becerra-Flores and Cardozo, 2020). Hence, some selected lead phytopharmaceuticals can primarily be focused on anti-COVID-19 drug discovery and development, as mentioned in Table 1 and Table 2 based on their anti-viral activity reported against influenza, HIV, and other RNA viruses through host cell surface receptors ACE2, Furin and TMPRSS2 blocking action. Moreover, few of them had shown promising degree of antiviral activity in both in-vitro and in-vivo pre-clinical animal model as well as conferred clinical efficacy against different viral infection through clinical trial, as depicted in Table 3 & Table 4 . Based on the consolidated reports on the degree of anti-viral efficacy and safety profile of the known phytocompounds as potential agents against SARS, MERS, Influenza, respiratory syncytial virus (RSV), Herpes simplex virus (HSV), and HIV infection. A few potential Phyto-lead compounds could be explored against SARS-COV-2 infection to manage COVID-19 patients, subjected to their preclinical and clinical investigation. The concised anti-viral response study depicted in Table 3 and Table 4 suggest that lycorine, flavopiridol, berberine, EGCG, cannabidiol, celastrol,baicalein, punicalagin, mangiferin, hesperitin, silymarin,and curcumin could be proceeded further to highlight them as potential prophylactic or therapeutic agent against COVID-19. Because those compounds exerted strong anti-viral response in low IC50 (Nano or micromolar range) with high selectivity index by inhibiting the viral growth and replication without showing any toxic effect to host cells. Moreover, the high therapeutic index based effective anti-viral and anti-inflammatory activity in animal study were quite encouraging without showing any apparent adverse reaction at therapeutic dose. Few of these agents, having nutritional value, had been recruited in clinical trial phase 1, 2, or 3 for management of COVID-19 induced complications or as prophylactic agent against viral infection including SARS-COV-2 through immune modulation.
Table 3.
Practical use, in-vitro and in-vivo degree of antiviral activity, therapeutic & selectivity index, toxicity and clinical study profile of few selected marker phytocompounds derived from herbs.
| Compounds (PubChem ID) & structure | IC50/EC50 (in vitro degree of antiviral activity) [CC50: at which dose of the test compound kills 50% normal host cell SI=CC50/EC50 | In-vivo degree of antiviral activity in animal model | Toxicity | Clinical Trial/Stage of development | References |
|---|---|---|---|---|---|
Emodin (3220)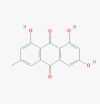
|
200 μM against SARS-CoV-1 by inhibiting interaction of Spike protein and Vero E6 cell | - | - | - | Ho et al., 2007 |
| EC50 against Human Influenza A is 4.25μg/ml, inhibit viral replication at CC50 of 182.95 μg/ml against MDCK cells. | - | Emodin is apparently non-toxic at low therapeutic dose in Preclinical Rat model due to extensive glucuronidation, however high doses may cause renal or hepatic toxicity. | Observational study conducted on human breast cancer without any confirmed result. |
Dai et al., 2017; Dong et al., 2016; https://clinicaltrials.gov/ct2/show/record/NCT01287468 |
|
| - | Emodin significantly increase the survival rate and promote recovery of HSV-infected mice; equivalent to acyclovir in vivo at 6.7 and 11.3 g/kg/day for 7 days. | - | - | Xiong et al., 2011 | |
|
Luteolin (5280445) 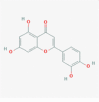
|
EC50 is 10.6 μM against wild-type SARS-CoV with selectivity index of 14.62 | 100 mg/kg for 3 days of post DENV-2 (clinical isolates) infection in mice; reduce viremia 2-fold, compared to untreated control. | Quite safe in preclinical animal study with LD50 of 456 mg/kg. Used safely with high SI without any substantial toxicity (Yi et al., 2004) | Luteolin enrich Pomegranate juice have been shown good efficacy to ameliorate the C-reactive protein in inflammatory disorders at randomized controlled trial without any serious adverse effect luteolin enrich Açaí Palm Berry extract in Phase 2 for Intervention in COVID-19 Patients. |
Yi et al., 2004; Peng et al., 2017; Sahebkar et al., 2016 https://clinicaltrials.gov/ct2/show/NCT04404218 |
|
Nicotionamine (9882882) 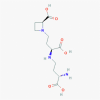
|
IC50 was found to be 84 nM for Nicotionamine to inhibit recombinant ACE2 activity of host cell. | - | Quite safe and non-toxic to normal mammalian cells at therapeutic dose without any apparent adverse effect. | A Double-Blind-Randomized, Placebo-controlled Adaptive Design Phase 2 Trial in Mild Cognitive Impairment due to Alzheimer's disease are conducted successfully. | Takahashi et al., 2015; https://clinicaltrials.gov/ct2/show/NCT03061474 |
|
Andrographolide 5318517 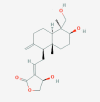
|
EC50 was 22 µM against Dengue virus. EC50 against H9N2, H5N1, H1N1 influenza viral strain was 94.3, 121.7, 110.0 μM with CC50 3085 µM in MDCK cells. |
Out of 10, nine infected mice were survived and recovered from H1N1 influenza virus infection 500 mg/kg. | Does not produce AMES toxicity, Maximum tolerated dose for human is 0.128 log mg/kg/day. Does not inhibit hERG-I and hERG-II. (LD50 2.162 mol/kg). Chronic oral rat toxicity (LOAEL) was found to be 1 log mg/kg body weight/day. Does not produce hepatotoxicity or cause skin sensitivity. LD50 >4000 mg /kg in mice without any apparent toxicity. |
In China it is used clinically to treat pain, fever and inflammation in viral infection. Phase 1 study in HIV patients and healthy volunteer showed no toxicity on normal population. Phase 4 clinical trial was conducted for Acute Exacerbation of Chronic Bronchitis and Acute Tonsillitis. |
Sukanth et al., 2020; Panraksa et al., 2017; Chen et al., 2009; Chen et al., 2010; Calabrese et al., 2000; Enmozhi et al., 2020 https://clinicaltrials.gov/ct2/show/NCT03134443 https://clinicaltrials.gov/ct2/show/NCT03132610 |
|
Polyphenolic compounds: Ellagic acid (5281855) 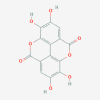 Myricetin (5281672) 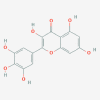 (+)-Catechin (9064) 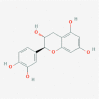
|
Dose-dependent inhibitory activity against MERS/SARS-CoV proteases with IC50 of 30.2 to 233.3 μM. | Ellagic acid, and Myricetin like polyphenols have potentials at 1.0 mg/kg as anti-influenza agents in vivo. | No pronounced toxicity was shown in animal model at 1.0 mg/kg | Catechin rich polyphenolic extract of Green tea recruited in Randomized human clinical trial for prevention of influenza infection through gargling and dietary consumption in young and adults successfully completed. |
Park et al., 2017; Park et al., 2013 ; https://clinicaltrials.gov/ct2/show/NCT01225770 https://clinicaltrials.gov/ct2/show/NCT01008020 |
|
Bromhexine (2442) 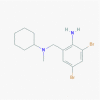
|
IC50 0.75μM against TMPRSS2 receptor expression |
Induce expectorant activity significantly at 12 mg/kg for 3 days treatment in RAT model | Induce liver toxicity when given chloroquine/HCQ with liver damage in COVID-19. Exploratory results of the study support our proposal that bromhexine hydrochloride may have a good effect on the treatment of COVID-19. |
A clinical trial (registration number NCT04273763), carried out by WEPON Pharmaceutical Co. Ltd., is the first human-based preliminary exploratory randomized-controlled clinical study on treating COVID-19 with bromhexine hydrochloride tablets (BHT). | Roberto et al., 2020. Markus et al., 2020; Depfenhart et al., 2020 https://clinicaltrials.gov/ct2/show/NCT04273763 |
|
Baicalein (5281605) 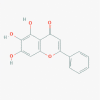
|
EC50 of Baicalein was 2.94 μM in SARS-CoV-2 clinical isolates on Vero E6 cells with CC50>200 μM | It significantly decrease viral load and mortality in HSV infection at 200 mg/kg; Als0 attenuates acute lung injury triggered by influenza A infection at 100 mg/kg in mouse model. | Single oral dose of 100–2800 mg of Baicalein were safe and well tolerated by healthy subjects (clinical trials registration number CTR20132944) | Baicalein has been registered for safety and efficacy evaluation in randomized, double-blind, placebo-controlled trial for influenza infection in Phase 2 Clinical trial. |
Su et al., 2020; Zhi et al.,2020; Luo et al., 2020 https://clinicaltrials.gov/ct2/show/NCT03830684 |
Celastrol (122724)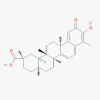
|
IC50 against nSARS-CoV 3CLpro is 10.3 μM. Celastrol inhibit replication of DENV-2 viral strain with IC50 of 0.12 ± 0.11 μM. |
It significantly attenuates the auto-immune disorder related inflammation in Trex1-deficient Wild type C57BL/6 mice at 1.0 mg/kg Celastrol given for 3 days without apparent toxicity Another study showed that Celastrol at 0.1 mg/kg defended 80% of the mice against life-threatening DENV-2 infection. | Higher doses of Celastrol, beyond therapeutic dose, shows infertility, QT prolongation, and hepatotoxicity | No clinical trial reported so far. |
Ryu et al, 2010b; Liu et al., 2020; Hou et al., 2020; Yu et al., 2017 |
|
Plant Lectins 2GTY (PDB ID) 
|
Griffithsin, a potential Lectin inhibited infectivity of MERS-CoV infection by more than 90% in cell Culture assay. |
It demonstrates remarkable activity with satisfactory survival rate against SARS-CoV-infected mice at 5 mg/kg intra nasal route followed by two daily doses at 2.5 mg/kg. H84T, a banana lectin confers more than 80% protection against broad spectrum influenza A viral infection at 0.1 mg/kg |
Griffithsin, glycoprotein-based lectin shows high therapeutic index without any considerable side effect. Banana lectin does not show any toxicity at normal mammalian cells at anti-viral doses |
A Randomized, Double-Blind Phase- 1 Safety and Pharmaco-kinetic Study of Q-Griffithsin Enema administered to prevent HIV-1 entry on Sero- negative adults was reported |
Millet et al., 2016, O'Keefe et al., 2010; Covés-Datson et al., 2020; https://clinicaltrials.gov/ct2/show/NCT04032717 |
|
Ursolic acid (64945) 
|
IC50 against Influenza virus type 1, and HIV was > 200 μg/ml and 0.3 µM respectively. Further, Ursolic acid inhibit HSV-1and HSV-2 at EC50 of 5.5 and 5.8 µg/ml at SI of 20 and 18.97 (Bag et al., 2012). |
N.D. | Parasite infected mice treated with Ursolic acid did not exhibit any alterations in biochemical parameters, support that this triterpene is non-toxic for animals. Considering the low or absent of toxicity of triterpenes for mice, as well as high Trypanocidal activity. | N.D. |
Kashyap et al., 2016 Bag et al., 2012 |
|
acetyl-11-keto-boswellic acid (11168203) 
|
IC50 against clinical isolates of Herpes simplex virus-1 was 12.1 μg/ml | N.D. | It is nontoxic at experimental therapeutic dose | It has been recruited for complementary therapy as along with Glycyrrhiza glabra extract for prevention of Covid-19. |
Goswami et al., 2018 https://clinicaltrials.gov/ct2/show/NCT04487964 |
|
Cepharanthine (10206) 
|
IC50 was 9.5 μg/ml against some Corona virus strains. | It reduces tumor growth and volume by inhibiting STAT3 expression at 20-mg/kg/day for 20 days in xenograft mice model of SaOS2 osteo-sarcoma. | It reduces the chemotherapy induced renal toxicity. While Endotoxin induced lethal toxicity can be bypassed by this compound. It is quite safe at its anti-tumor in-vivo doses. | N.D. |
Zhang et al., 2005; Bailly, 2019 |
Sylimarin (5213)
|
Silymarin showed anti-viral activity against influenza virus A/PR/8/34 with 98% protection at 100 μg/ml with IC50 of 11.12 μg/ml. |
Silybin, active constituent of Silymarin, revealed significant reduction of HCV load within 16 days of treatment at 20 mg/kg by reducing baseline mean value of viral load in Phase 3 clinical trials without any untoward effect. | It is completely safe and did not show any apparent toxicity at its anti-viral dose. | A randomized placebo-controlled trial in Phase 3 is carrying out to assess the clinical outcome in COVID-19 Pneumonia by silymarin due to its ability to inhibit p38 MAPK pathway and its antiviral, anti-inflammatory and anti-oxidant potential. |
Liu et al., 2019; Rendina et al., 2014; Song and Choi, 2011 https://clinicaltrials.gov/ct2/show/NCT04394208 |
|
Epigallocatechin 3-gallate (EGCG) (65064) 
|
Attenuate the binding activity of SARS-CoV Nucleocapsid protein with QDs-conjugated RNA oligonucleotide on immobilized protein chip at concentrations ≥0.005µg/ml, IC50: 0.05µg/ml for activity against SARS. | Reduced the core hepatitis B viral antigen expression and replication at 25 mg/kg/day for 5 days i.p. treatment. | Oral acute LD50 was 2170 mg/kg in Rat. High therapeutic dose (100 mg/kg/day) did not produce any adverse side effects, without changing body weight, genotoxicity, behavior or mortality of treated animals. |
Safety, toxicity, dosing, and antiviral effects of EGCG at 200 mg in capsule form (orally twice daily at three different doses) in HIV-1-infected Patient was carried out successfully through Randomized interventional Clinical Trial. |
Roh, 2012; Wang et al, 2020; Ferreira et al, 2012; Isbrucker et al, 2006; https://clinicaltrials.gov/ct2/show/NCT01433289 |
|
Quercetin (5280343)  Rutin (5280805) 
|
IC50 against SARS-CoV-1 3CLPro and PLpro was 52.7±4.1 μM and 8.6±3.2 μM, respectively. IC50 of Rutin was >25 μM against Feline Coronavirus replication. |
Quercetin significantly reduced viral load of Rhinovirus infection (95.84%) at 0.2 mg/mouse for 4-day treatment without showing any toxicity. | Quercetin has no adverse effect at therapeutic dose and did not show any carcinogenicity or genotoxicity | Quercetin applied in Q-Trial in HCV (Q) patients, result not yet published. |
Park et al., 2017; McDonagh et al., 2014; Ganesan et al., 2012; Harwood et al., 2007 https://clinicaltrials.gov/ct2/show/NCT01438320 |
|
Glycyrrhizic acid (14982) 
|
At EC50 of 365 µM it inhibit SARS-CoV replication in Vero cells with Selectivity Index of > 65 (CC50 >24000 µM) |
Showed remarkable protection by 70% survival against 20 LD50 of influenza A2 at 10 mg/kg per day before infection and 1- and 4-days post infection. |
GL has no apparent adverse reaction or toxicity at lower doses; and have no genotoxicity. | Dietary Supplement: Licorice extract containing Glycyrrhizic acid has been used for complementary intervention of COVID-19 in interventional Clinical Trial. | Hoever et. al, 2005; Utsunomiya et. al,1997; Ming et al., 2013 https://clinicaltrials.gov/ct2/show/NCT04487964 |
RESVERATROL (RE) (445154)
|
RE inhibit nucleocapsid protein translation of MERS-CoV without any toxicity on Vero E6 cells at IC50 of 250 μM. |
RE significantly reduces levels of Rota virus antigens in the colon, jejunum, and ileum, compared to untreated mice at 4-10-day post infection with increasing rate of survival and decreasing diarrhea scores. It may lead to weight gain at 20 mg/kg orally. | Orally administered RE at 200 mg/kg/day in Rats and 600 mg/kg/day in Dogs for 90 days, did not show any side effect. But high dose of RE acts as pro-oxidant, thyroid disruptor and a goitrogen. In a phase II clinical trial on patients with refractory multiple myeloma, a daily dose of 5.0 g of RE showed nausea, diarrhea, fatigue, and renal toxicity. |
SARS-CoV-2 Viral Load and COVID-19 Disease Severity be Reduced by Resveratrol-assisted Zinc therapy at Phase 2 trial, proposed but not yet recruited. Dietary supplement of RE at 500 mg tablet for 30 days on Double-blind Cross-over Randomized Controlled Trial reduce the systemic inflammatory state & oxidants-antioxidants imbalance of tobacco users in Phase 3 study. |
Lin et al., 2017; Huang et al., 2020; Shaito et al., 2020; https://clinicaltrials.gov/ct2/show/NCT04542993; https://clinicaltrials.gov/ct2/show/NCT01492114 |
Cannabidiol (644019)
|
EC50 against Hepatitis C virus was 3.163±0.133 μM with selectivity index of 4.954 in normal mammalian cell. | TMEV-infected mice treated for 7 consecutive days with CBD (5 mg/kg) reduced the brain leucocyte infiltration and other inflammatory cytokine and chemokine expression; thereby increase the survival rate of the virus infected mice. Both in HIV and in post-Ebola syndrome Cannabidiol has been recommended as therapeutic agent to control immune activation at doses of 10–20 mg/kg/day and 1.7–10 mg/kg/day. |
CBD shows developmental toxicity, embryo-fetal mortality, central nervous system inhibition and neurotoxicity, hepatocellular injuries, sperm reduction, organ weight alterations, male reproductive system alterations, and hypotension, at doses higher than recommended for human. Intravenous LD50 of CBD on Rhesus monkey is 212 mg/kg |
syndrome, cannabidiol has been proposed as a therapeutic agent to control immune activation at doses of 10–20 mg kg−1 day−1 and 1.7–10 mg kg−1 day−1 (100 mg day−1titrating up to 600 mg day−1), respectively Cannabis is used as medicine for Prevention and Treatment of COVID-19, has been recruited for Non-randomized clinical trial in Phase 2 with 200000 participants. |
Lowe et. Al., 2017; Costiniuk et. al, 2020; Esposito et al., 2020; Mecha et al., 2013; Huestis et al.,2019 https://clinicaltrials.gov/ct2/show/NCT03944447 |
|
Tetrahydrocannabinol (THC) (2978) 
|
- | THC reduced the sign of ARDS in SEB induced severe acute respiratory syndrome mice at 20 and 10 mg /kg i.p. dose and suppressed the inflammatory cytokine storm. | Oral LD50 of THC in mice is 482 mg/kg and in Rat oral LD50 is 666 mg/kg. THC is not genotoxic, carcinogenic or causes remarkable immunosuppression during acute inflammation and not shown any apparent side effect or acute adverse reaction at therapeutic dose in mice. |
Double-Blind, Randomized, study with oral Dronabinol Versus Placebo was conducted in the treatment or prevention of Highly Active Antiretroviral Therapy (HAART)-related nausea and vomiting in phase 2. |
Mohammed et al., 2020; Chilakapati and Farris, 2014; https://clinicaltrials.gov/ct2/show/NCT00642499 |
Berberine (2353)
|
IC50 against mouse hepatitis virus was 2.0±0.5 μM with S.I. of 34.9. IC50 against influenza A H1N1 strain was: 0.44 μM |
It drastically reduced the viral load of H1N1 influenza infection in mice lungs at 7 days post infection treatment at 20 mg/kg. | Non-toxic and safe at therapeutic dose. | Effect of Berberine on Intestinal function and Inflammatory mediators in severe COVID-19 Patients was carried out, based on prospective randomized controlled clinical trial in Phase 4. |
Mani et al., 2020; Cecil et al., 2011; Yan et al., 2018; https://clinicaltrials.gov/ct2/show/NCT04479202 |
Sinigrin (23682211)
|
IC50 against cell-free (3CLpro.) and cell-based cleavage activity of SARS-CoV was 121 and 217 μM with CC50 >10,000 μM. | N.D. | Fetotoxicity, Non-carcinogenic and non-mutagenic at safe doses. Oral LD50 is 644 mg/kg in Rat. |
N.D. on viral disease | Lin et al., 2005; https://www.chemsrc.com/en/cas/3952-98-5_673009.html |
Hesperetin (72281)
|
IC50 against cell-free (3CLpro.) and cell-based cleavage activity of SARS-CoV was 60 and 8.3 μM with CC50 -2718 μM. | Every two days at 5 mg/kg dose given to mice with cancer for 30 days, showed significant reduction of tumor without toxicity. | Does not induce any apparent toxicity within the therapeutic dose and no damage to human cell lines GES-1. | An Open-Label study to evaluate the effect of Elimune (2 capsules BID for 28 days; Hesperetin containing Biomarkers in Patients with Plaque Psoriasis. |
Lin et al., 2005; He et al., 2020 https://clinicaltrials.gov/ct2/show/NCT02251678 |
|
Allitridin (16315) 
|
Inhibition of HCMV replication via suppression of viral IE gene transcription with IC50 of 20 μg/ml and selectivity index of 16.7 on human HEL cell. | Reduced MCMV DNA load and hepatic leision in Murine cytomegalovirus model at 0.14 and 0.42 mM/kg/day for 18 and 14 days. | Intravenous use of Allitridin is safe and relatively free of negative side-effects. LD50 in mice following intravenous injection was 110.9 mg/kg |
N.D. |
Zhen et al. 2006; Liu et al. 2004; Shen et al., 1996; Li et al., 2011 |
Alvocidib (Flavopiridol) [5287969]
|
IC50 against Influenza A H7N9, pdm H1N1 and H3N2 Replication: 0.24, 0.59 and 0.70μM respectively, with selectivity index of >425, 170, and 142. EC50 against HIV replication: 8–15 nM. |
Flavopiridol (2.5 mg/kg every 12 h) for 20 days suppressed HIV-1 transcription in mouse kidney in HIV transgenic mouse model. | Flavopiridol treatment to both animals and humans at doses within the therapeutic range in vivo displays no apparent toxicity. | In cancer this molecule has been tested successfully in phase 3 for various type of lymphoma, leukemia; but in HIV infection it is not yet recruited. |
Perwitasari et al., 2015; Nelson et al., 2003; Sadaie et al., 2004 |
Ginsenoside Rg3(9918693)
|
IC50 of 20(S)-Rg3 against MHV-68 viral replication: 10.82 ±1.56 μM | At 1.0 mg/mouse 55% improvement against infection of hemagglutinating virus of Japan (HVJ) at 11 days post-infection. | The no-observed-adverse-effect of Rg3 for dogs were considered to be 7.20 mg/kg/day in 26 days intramuscular injection after recovery from tumerosis. Body weight, food intake, ophthalmoscopy, electrocardio-gram, urinalysis, hematology, serum biochemistry, gross and histopathology findings were normal. | A Randomized, Double-blind, Placebo-controlled, Parallel-group Clinical study was done to evaluate the efficacy and safety of Ginsenoside, using Rg3 Capsule in Prevention of Postoperative recurrence of Hepatocellular Carcinoma. |
Kang et al., 2018; Liu et al., 2011 https://clinicaltrials.gov/ct2/show/NCT01717066 |
Lycorine (72378)
|
EC50 15.7 nM and CC50 value 14980.0 nM against SARS-CoV EC50 0.8 μM and CC50 of 25.1 μM against DENV. |
Protect the ZIKV infected AG6 mice mortality rate by 83%, 66 and 33% following the reduction of viral load and its replication at 10, 5.0 and 1.0 mg/kg body wt respectively. |
A potential candidate for cancer therapy and other viral infection with low toxicity. It also posses anti-oxidant, anti-toxic, and hepato-protective effect with safe therapeutic window. LD50 via i.p. in mice was 112.2±0.024 mg/kg. LD50: 344 mg/kg via gastric lavage injection in mice |
N.D. |
Li et al., 2005, Li et al., 2005; Cao et al., 2013, Cao et al., 2013 Wang et al., 2014; Chen et al., 2020; |
Vitexin (5280441)
|
IC50 against Parainfluenza type 3 virus was: 20.8μg/ml with CC50 333.3 μg/ml IC50 against Rota virus was: 129 μM |
Vitexin at 2 mg/kg i.v. successfully exert 55% protection against the middle cerebral apoptosis and ischemic stroke via suppressing proinflammatory cytokines (TNF-α and IL-6) and stimulating anti-inflammatory cytokine (IL-10) in Rat model. | It completely devoid of any adverse reaction and toxicity within the therapeutic window in animal model due to its cell protecting and anti-oxidant activity. | Not yet recruited in viral diseases or associated disorders |
Gandhi et al., 2016; Li et al., 2002; Jiang et al., 2018 |
|
Chlorogenic acid (1794427) 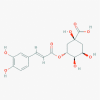
|
IC50 against SARS-CoV-2 3CLpro was 39.48 ± 5.51 µM EC50 against oseltamivir sensitive and resistant strain of influenza virus: 39.42 & 58.34 μM respectively with CC50 against MDCK cells 364.30 μM |
Intravenous injection of 100 mg/kg/day exerted promising antiviral effect in mice, conferring 60% and 50% protection from death against H1N1 and H3N2 infection, reduce virus titers and effectively attenuate inflammation in the lungs, inhibiting neuraminidase. | LD50 in Rat (i.p.) at 3000 mg/kg. It did not produce any unwanted toxicity up to 4000 mg/kg in Rat. Neither Teratogenic, nor CNS toxic. |
Dietary supplement of Açaí Berry extract containing Chlorogenic acid (3 capsules of 520 mg for 30 days) was given in Phase 2 for alleviating inflammation of Covid-19 patients. |
Su et al., 2020; Ding et al., 2017; Chaube et al., 1976; https://clinicaltrials.gov/ct2/show/NCT04404218 |
Mangiferin (5281647)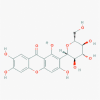
|
IC50 against HIV was: 3.59 μg/ml and CC50 in MT-2 cells: 125 μg/ml EC50 against HIV-1KM018 isolate and HIV-1A17 resistant strain: 35.40 & 22.75 μM respectively. IC50 against neuraminidase activity for influenza virus infection in host cell 0.82 μM |
Both IgG1 and IgG2b levels were significantly augmented for Th1 humoral immunity modulation by the oral treatment of Mangiferin at 100 mg/kg for 28 days. | Safe and does not display any toxic effect in experimental mice at therapeutic dose. | Not yet recruited in viral diseases or associated disorders. |
Guha et al., 1996; Wang et al., 2011; Li et al., 2007; García et al., 2003 |
Punicalagin (44584733)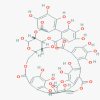
|
IC50 against EV71 infection of RD cells was 15 μg/ml and CC50 300 μg/ml. IC50 against DENV-2, RSV and MV was: 7.86±0.40; 0.54±0.04; and 25.49±2.94 (μM) along with CC50 of: 151.44 ± 9.31; 264.83 ± 23.72; 283.76 ± 11.54 μM, Respectively. |
A dosage of 5 mg/kg protected the EV71-infected mice from mortality by 38% without any clinical symptoms after 2 weeks. |
It did not show any toxicity and adverse drug reaction in therapeutic dose (180 mg/kg/day for 90 days) both in clinical and preclinical animal experiment. | In Phase 3 study Pomegranate products were tested for 16-week administering 2 oz. package daily for prevention of Common Cold, Flu or Influenza-Like Symptoms: A Double- Blind, Placebo-Controlled Randomized Clinical Trial, showed impressive results |
Yang et al., 2012; Lin et al., 2013; Patel et al., 2008; https://clinicaltrials.gov/ct2/show/NCT00617318 |
|
Wogonin (5281703) 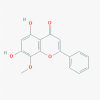
|
Nearly 73% inhibition of influenza A virus replication at 10 μg/ml with CC50 using sheep erythrocyte. Docking study suggested strong binding with Mpro catalytic side of SARS-COV-2. |
Dosage of 40 mg/kg significantly reduced liver fibrosis in mice, induced by CCL4. Effect was equivalent to standard. | High therapeutic index and other data suggest that the compound is quite safe and nontoxic in high therapeutic doses and protect the all organ and tissue damage at therapeutic dose | Not yet recruited in viral diseases or associated disorders |
Zhi et al., 2019; Huang et al.,2020; Du et al., 2019 |
Neferine (159654)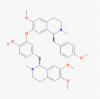
|
The EC50 against HIV replication < 0.8 µg/ml with S.I. >8.6. | The increase of MPO activity and fibrosis in lung tissue mediated by Bleomycin were significantly attenuated by n Neferine (20 mg/kg, b.i.d) on 7th and 14th days by reducing inflammatory cytokines and NF-kβ expression. |
Safe, nontoxic to liver and kidney at therapeutic dose | Not yet recruited in viral diseases or associated disorders |
Kashiwada et al., 2005; Zhao et al., 2010 |
Carnosine (439224)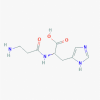
|
IC50 against Dengue and Zika viral protein: 52.3 & 59.5 μM | Inhibited MPO activity, decreased the mortality rate, suppressed TNF-α and IL-1β release, decreased H9N2 viral titer, and in the lungs of infected mice at 10 mg/kg oral dose for 7 days. | Showed minimal cytotoxicity in Huh7 cultured cells. Exogenous carnosine administration up to 2000 mg/kg was well‐tolerated in vivo. |
Not recruited in viral infection patient randomly. | Rothan et al., 2019; Xu et al., 2015 |
Harmine (5280953)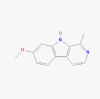
|
EC50 against dual-specific tyrosine phospho-rylation-regulated kinases (DYRKs) of viral replication at early-late stage of HCMV gene expression and HSV at 0.71 μM. Inhibit HSV-1 by blocking IE transcription (Bag et al., 2014). |
Attenuated AG129 mice against EV71 infection by single dose of (12.5 μg/ml) daily i.p. for 4 days. Recover HSV-1 and HSV-2 infected mice (Bag et al., 2014; Bag et al., 2013). |
Very safe on normal mammalian cells at the therapeutic and experimental dose without causing hepatotoxicity, cardiotoxicity and neurotoxicity. | Not yet recruited in viral diseases or associated disorders |
Hutterer et al., 2017; Bag et al., 2013; Bag et al., 2014; Chen et al., 2018 |
Table 4.
Practical use, in-vitro and in-vivo degree of antiviral activity, therapeutic or selectivity index, toxicity and clinical study profile of few selected marker phytocompounds derived from spices.
| Compounds | IC50/EC50 (in-vitro degree of anti-viral activity) [CC50: at which dose of the test compound kills 50% normal host cell S.I. =CC50/EC50 | In-vivo degree of anti-viral efficacy | Toxicity | Clinical Trial Stage of development | References |
|---|---|---|---|---|---|
|
Carnosic acid (65126) 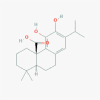
|
IC50 against RSV (Respiratory Syncytial virus) was 19.58 and 20.19 μM in A549 and HEp-2 cells by inhibiting viral proteins F, NS2, SH) respectively. IC50 against HIV-1 protease was 0.24 μM in cell free assay. |
- | Carnosic acid did not show any significant cytotoxicity at concentrations used for antiviral assay. | N.D. in anti-viral | Shin et al., 2013; Paris et al., 1994 |
|
Eugenol (3314) 
|
EC50 was 25.6 and 16.2 μg/ml for HSV-1 and HSV-2 respectively. IC50 against EBOLA virus was: 1.3 ± 0.5 with CC50 > 50 μM against HeLa cell. EC50 against Influenza A virus was 0.6392 μg/ml; while CC50 against MDCK cells was 75.28 μg/ml |
At 160 mg/kg b.w. Eugenol attenuated the lung injury in mice through i.p. route via inhibition of inflammatory cytokine. | Eugenol was virucidal and showed no cytotoxicity on normal cell at therapeutic dose. LDLo dose for i.p. route on RAT is 800 mg/kg |
Eugenol containing AV2 formulation of topical spray reduced the HPV load in uterine cervix and associated lesions to stop the cervical cancer progression significantly on the cervix through one time (2 puffs) topical application of 100µl AV2 |
Benencia and Courrèges, 2000; Lane et al., 2019; Magalhães et al., 1985 Revue Medicale de la Suisse Romande, 16 (449), 1896. |
|
Curcumin (969516) 
|
The inhibitory effect of Curcumin (EC50) SARS‐CoV replication was 10 μM with CC50 of > 250 μM. Inhibited SARS-CoV 3CLpro with IC50 value of 23.5 μM. |
At 50 mg/kg/day), beginning at 5 days prior to Reovirus 1/L infection protects CBA/J mice from the development of ALI/ARDS and suppresses subsequent fibrosis. | It is completely safe, non-toxic at high therapeutic dose without any genotoxicity, carcinogenicity due to potential anti-oxidant effect. In mice LD50 i.p. and oral route was: 1500 mg/kg and 2000 mg/kg respectively. |
Despite robust preclinical data, there is criticism concerning clinical studies performed with curcumin particularly on translation of in vitro concentrations and in vivo dosages to reproducible and achievable concentrations in humans. |
Wen et al., 2007; Avasarala et al., 2013 Heinrich et al., 2020; Tyagi et al., 2016; Bansal and Chhibber, 2010 |
Eucalyptol (2758)
|
- | Significantly increased the survival rate, reducing the mortality, attenuating the clinical symptoms in the lung pneumonia in Balb/c mice at 60 and 120 mg/kg via oral gavage at 6 days post infection of Influenza virus Anti-influenza solution containing eucalyptol was reported as preventive for further spreading of influenza infection through nose and throat via nebulizer or inhaler spray. |
Very safe at therapeutic dose without showing any sign of genotoxicity, carcinogenicity, lung toxicity. Oral LD50 in Rat: 2480 mg/kg |
A double-blind, placebo-controlled trial suggested that Eucalyptol can act as mucolytic as well as in upper and lower airway diseases |
Li et al., 2016; Juergens et al., 2003 . https://patents.google.com/patent/US7596836B2/en. |
|
Cinnamaldehyde (637511) 
|
IC50 against influenza PR8 virus in MDCK cells: 15.87μM without showing any cytotoxicity to MDCK cells at 200 μM. | Mice infected with the lung-adapted PR-8 virus, inhalation of CA at (50 mg/cage/day) and nasal inoculation (250 μg/mouse/day) significantly reduced the viral growth & infection and increased survival rates on 8 days to 100% and 70%, respectively. | Mouse LD50 in oral route was: 2225 mg/kg Inhalation of CA did not induce any adverse reactions, but showed a beneficial effect on influenza virus-induced pneumonia in mice. |
Liquid containing Cinnamaldehyde from Vapor Shark DNA 250™ e-cigarette will be recruiting by Randomized Interventional (Clinical Trial) for checking Innate Lung Host Defense (Cinimic). |
Hayashi et al., 2007; https://clinicaltrials.gov/ct2/show/NCT03700892 |
Conclusions
The nasal or oral suspension or vapour spray inhaler containing combination of phytopharmaceuticals derived from herbs, spices and traditional medicaments would deliver the active ingredient(s) specifically in the host cell receptor's (Furin, TMPRSS2) active site to block the attachment of viral S-protein via hindering the S-protein cleavage activation, and membrane fusion. This strategy may offer a steady and putative anti-viral response, permit to use lower doses of the Phyto-drug, and minimize the side effects, as reported earlier with zinc nasal spray in upper respiratory tract illness (Belongia et al., 2001). It may help to mediate successful anti-viral therapy against SARS-CoV-2 in upper respiratory tract via preventive or therapeutic treatment option (Hayden et al., 2003). Herbs containing polyherbal Phyto-components (Table 1,2,3 &4 ) & phyto-extract ( Table 5 ) may act on different stages of viral infection cycle for prevention and healing, as follows:
-
1)
May stimulate the innate immunity for killing the virus via Tc or NK cell mediated immune-modulation by immunogenic phytonutrients and amino acids.
-
2)
Could interfere the interaction of the virus with the host cell entry receptors to prevent their cellular endocytosis, replication and infection.
-
3)
Can protect the vital and target organs by executing the anti-inflammatory activity against cytokine storm induced organ damage, as anti-inflammatory activity of the natural herbs or Phytocompounds may confer the vasodilation of bronchial tube (Ignarro, 2020; Scavone et al., 2020).
Table 5.
Clinical study profile of few selected phyto-extract
| Artemisinin, Curcumin, Boswellia, and Vitamin C (ArtemiC) | Anti-oxidant, anti-inflammatory, anti-aggregate and anti-microbial property of the formulation promote these for preventive therapy against COVID-19 | Phase 2 | Kumari et al., 2020 |
| Nigella Sativa and Honey | Immunomodulator, and antiviral activiites trigger it for potential treatment against Covid-19 patient | Phase 3 | Kumari et al., 2020 |
| Natural honey | Antibacterial, antifungal, antiviral and antimycobacterial activities encourage these to use against Covid-19 patient for probable therapeutics. | Phase 3 | Kumari et al., 2020 |
| Gum Arabic powder | Immunomodulator and anti-inflammatory agent for potential use for Covid-19 treatment. | Phase 2 | Kumari et al., 2020 |
| Açaí Palm Berry extract | For Potent inhibition of Nod-like receptor family, pyrin-containing 3 (NLRP3) inflammasome owing to attenuate the cCovid-19 symptoms. | Phase 2 | Kumari et al., 2020 |
| Traditional Chinese medicines (various herbal products are included in this category) | For Immunomodulatory and anti-inflammatory mechanisms, decrease the level of cytokines such as TNF-α, IL-1β, IL-6, IL-8, and IL-10, to inhibit lung inflammation or acute lung injury | Phase 3 | Kumari et al., 2020 |
Further, the intra-nasal nanosuspension based aerosol spray inhaler (nebulizer) may help to reach the active ingredient to the lungs and esophagus rapidly; so, the formulated combination of anti-inflammatory, as well as host cell entry blocker, could protect the patient from virus-driven lung injury, fibrosis, and infection by local action. In this context, few anti-inflammatories as well as anti-viral Phyto-compounds derived from herbs and spices such as carnosic acid, Eugenol, Gingerol, Eucalyptol, Cinnamaldehyde, green tea and white tea essential oils (Table 3 & 4) may be formulated as oral or nasal nebulizer for effective drug delivery of the active medicament into the lungs, or upper respiratory tract due to their protecting ability against SARS viral infection mediated Acute respiratory distress syndrome (ARDS), Acute lung inflammation (ALI), as evident from earlier patented formulation against influenza virus infection and associated respiratory inflammation and distress (US7596836B2, patent).
Some promising leads such as Tetrahydrocannabinol, Cannabidiol, Andrographolides have been recruited in the clinical trial for the intervention of cancer and inflammation for the practical use. Bromhexine has already been marketed as commercially FDA approved medicine for practical use towards the treatment of respiratory disorders as mucolytic agent.
Moreover, few plant-based antiviral compounds (such as Ursolic acid, Vitexin,Wogonin, Baicalein,Curcumin, Carnosic acid, Gingerol, Lycorine, Ginsenoside Rg3, Hesperetin, Tetrahydrocannabinol, Cannabidiol, Resveratrol, Glycyrrhizic Acid, Epigallocatechin 3-gallate, Luteolin) also possess promising anti-oxidant, anti-inflammatory, anti-coagulant activity at various pharmacological doses. Therefore, those compounds could be the potential therapeutic option to attenuate the cytokine storm in the acute symptomatic Covid-19 patient due to their significant anti-inflammatory activity by suppressing the release of inflammatory cytokines during immunopathological response of host against viral infection. Anti-coagulant property of such anti-viral phyto-molecules could be used as probable management of the pulmonary coagulopathy and blood clotting in the blood vessels to decrease the mortality rate and assist for early recovery. Anti-oxidant and strong anti-inflammatory activity of those compounds could be potential for the practical use to manage vasculitis like manifestation of the recovered patients as per the current clinical reports.
Furthermore, electronic metered chip device could optimize the pharmacological dose of the delivered agent to the target cell and maintain the stability of the phytopharmaceuticals by nanosuspension coating of natural polymer. Thus, it could maintain the safety, accuracy and efficacy of the formulated dose. This method would be promising and less likely to produce side-effect and drug resistance, as suggested by the WHO for multi-drug therapy in tuberculosis or leprosy. Hence, it could be hypothesized that intra-oral or intra-nasal inhaler-based aerosol spray (Ignarro, 2020) could deliver the drug accurately to viral entry point as cell surface receptor blocker, which could be the potential, safe and rapid prophylactic as well as a therapeutic option against Covid-19 pandemic.
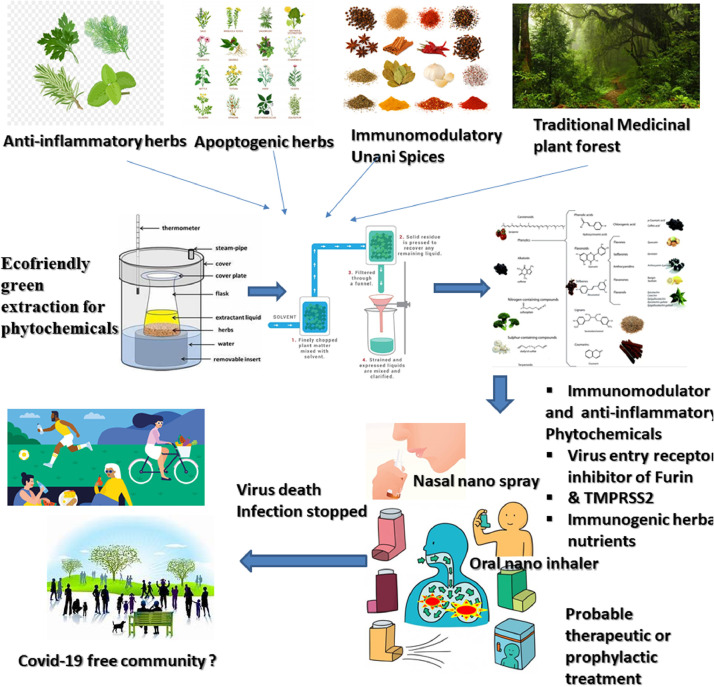
Furthermore, the selection of the drug candidate to be delivered via nasal or oral spray is very important and thus the selected pharmacological agents should possess the immunomodulatory property and block the virus entry to the host cell receptor via dual blocking of Furin protease and TMPRSS2 receptor site. For this, adaptogenic herbs from the traditional system of medicine like Ayurvedic and Unani may be useful as rational formulation. Nano-spray suspension coated with a stable polymer and suspending agents along-with receptor-ligand could stabilize the entrapped Phyto-pharmacological agents like curcumin-nano-formulation (Preis et al., 2019; Pandey, 2011; Taki et al., 2016) for longer duration avoiding pharmacokinetic failure. The application of nano-spray might be useful also to check the infection further, when mild to moderate symptoms would arise by augmenting the cell surface receptor activation (Furin and TMPRSS2). This projected drug delivery via nasal or oral nano-spray comprising of stable Phyto-immunomodulators and entry receptor blocker can be scaled up to achieve the expected recovery and stop the infection cycle of Covid-19 (Fig. 2 .) pandemic and its panic, subjected to preclinical and clinical validation.
Fig. 2.
Overview of identification and extraction of phytopharmaceuticals from Traditional medicinal plants, Adaptogenic herbs, Immunomodulatory spices, Anti-inflammatory herbs for developing probable therapeutic and prophylactic agents via nasal or oral inhaler based nano-spray phytoformulation development for eradicating covid-19.
https://www.drugs.com/medical-answers/losartan-shown-receptor-block-coronavirus-3534166/.
https://www.caymanchem.com/msdss/11707m.pdf
https://clinicaltrials.gov/ct2/show/NCT04404218
https://clinicaltrials.gov/ct2/show/NCT03061474
https://clinicaltrials.gov/ct2/show/NCT03134443
https://clinicaltrials.gov/ct2/show/NCT03132610
https://clinicaltrials.gov/ct2/show/NCT01438320
https://clinicaltrials.gov/ct2/show/NCT04487964
https://clinicaltrials.gov/ct2/show/NCT04479202
https://clinicaltrials.gov/ct2/show/NCT02251678
https://clinicaltrials.gov/ct2/show/NCT01433289
https://clinicaltrials.gov/ct2/show/NCT01717066
https://clinicaltrials.gov/ct2/show/NCT00617318
https://clinicaltrials.gov/ct2/show/NCT02346227
https://patents.google.com/patent/US7596836B2/en.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or no conflict of interest
Acknowledgements
The authors greatly thank the Universal Scientific Education and Research Network (USERN), Stockholm, Sweden for their constant guidance and preparing the manuscript.
References
- Arjmand M.H., Zahedi-Avval F., Barneh F., Mousavi S.H., Asgharzadeh F., Hashemzehi M., Soleimani A., Avan A., Fakhraie M., Nasiri S.N., Mehraban S., Ferns G.A., Ryzhikov M., Jafari M., Khazaei M., Hassanian S.M. Intraperitoneal Administration of Telmisartan Prevents Postsurgical Adhesion Band Formation. J Surg Res. 2020;248:171–181. doi: 10.1016/j.jss.2019.10.029. [DOI] [PubMed] [Google Scholar]
- Aziz N., Kim M.Y., Cho J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342–358. doi: 10.1016/j.jep.2018.05.019. [DOI] [PubMed] [Google Scholar]
- Bag P, Bag P, Chattopadhyay D, Mukherjee H, Ojha D, Mandal N, Chawla Sarkar M, Chatterjee T, Das G, Chakraborti S Anti-herpes virus activities of bioactive fraction and isolated pure constituent of Mallotus peltatus: an Ethnomedicine from Andaman Islands. Virology Journal. 2012;9(98):98–105. doi: 10.1186/1743-422X-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C. Cepharanthine: An update of its mode of action, pharmacological properties and medical applications. Phytomedicine. 2019;62 doi: 10.1016/j.phymed.2019.152956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltina L.A., Tasi Y.T., Huang S.H., Lai H.C., Baltina L.A., Petrova S.F., Lin C.W. Glycyrrhizic acid derivatives as Dengue virus inhibitors. Bioorg. Med. Chem. Lett. 2019;29 doi: 10.1016/j.bmcl.2019.126645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal S., Chhibber S. Curcumin alone and in combination with augmentin protects against pulmonary inflammation and acute lung injury generated during Klebsiella pneumoniae B5055-induced lung infection in BALB/c mice. J. Med. Microbiol. 2010;59:429–437. doi: 10.1099/jmm.0.016873-0. [DOI] [PubMed] [Google Scholar]
- Basak A., Cooper S., Roberge A.G., Banik U.K., Chrétien M., Seidah N.G. Inhibition of proprotein convertases-1,-7 and furin by diterpines of Andrographis paniculata and their succinoyl esters. Biochem. J. 1999;338:107–113. [PMC free article] [PubMed] [Google Scholar]
- Becerra-Flores M., Cardozo T. SARS-CoV-2 viral spike G614 mutation exhibits higher case fatality rate. Int J Clin Pract. 2020;10:1111. doi: 10.1111/ijcp.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belongia E.A., Berg R., Liu K. A randomized trial of zinc nasal spray for the treatment of upper respiratory illness in adults. Am J Med. 2001;111:103‐108. doi: 10.1016/s0002-9343(01)00765-3. [DOI] [PubMed] [Google Scholar]
- Benencia F., Courrèges M.C. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother Res. 2000;14:495–500. doi: 10.1002/1099-1573(200011)14:7<495::aid-ptr650>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Botwina P., Owczarek K., Rajfur Z., Ochman M., Urlik M., Nowakowska M., Pyrc K. Berberine Hampers Influenza A Replication through Inhibition of MAPK/ERK Pathway. Viruses. 2020;12:344. doi: 10.3390/v12030344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbarth A.K., Morgan J., Jones A.L. E-cigarettes—an unintended illicit drug delivery system. Drug Alcohol Depend. 2018;192:98–111. doi: 10.1016/j.drugalcdep.2018.07.031. [DOI] [PubMed] [Google Scholar]
- Calabrese C., Berman S.H., Babish J.G., Ma X., Shinto L., Dorr M., Wells K, Wenner C.A., Standish L.J. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother Res. 2000;14:333–338. doi: 10.1002/1099-1573(200008)14:5<333::aid-ptr584>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Cameron C.E., Castro C. The mechanism of action of ribavirin: lethal mutagenesis of RNA virus genomes mediated by the viral RNA-dependent RNA polymerase. Curr Opin Infect Dis. 2001;14:757–764. doi: 10.1097/00001432-200112000-00015. [DOI] [PubMed] [Google Scholar]
- Camini F.C., da Silva T.F., da Silva Caetano C.C., Almeida L.T., Ferraz A.C., Vitoreti V.M.A., de Brito Magalhães C.L. Antiviral activity of silymarin against Mayaro virus and protective effect in virus-induced oxidative stress. Antiviral Res. 2018;158:8–12. doi: 10.1016/j.antiviral.2018.07.023. [DOI] [PubMed] [Google Scholar]
- Cao Z., Yu D., Fu S., Zhang G., Pan Y., Bao M., Tu J., Shang B., Guo P., Yang P. Lycorine hydrochloride selectively inhibits human ovarian cancer cell proliferation and tumor neovascularization with very low toxicity. Toxicol Lett. 2013;218:174–185. doi: 10.1016/j.toxlet.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Cao Z., Yang P., Zhou Q. Multiple biological functions and pharmacological effects of lycorine. Sci China Chem. 2013;56:1382–1391. doi: 10.1007/s11426-013-4967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B, J.T. Collins, F. Barlow-Pay, F. Rickard, E. Bruce, A. Verduri, T.J. Quinn, E. Mitchell, A. Price, A. Vilches-Moraga, M.J. Stechman, R. Short, A. Einarsson, P. Braude, S. Moug, P.K. Myint, J. Hewitt, L. Pearce, K. McCarthy, and COPE Study Collaborators. Nosocomial COVID-19 infection: examining the risk of mortality. The COPE-Nosocomial Study (COVID in Older PEople). J Hosp Infect. 2020;106(2):376–384. doi: 10.1016/j.jhin.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K.L., Do D.V. Hydroxychloroquine-induced Retinal Toxicity. J Rheumatol. 2020;47:632. doi: 10.3899/jrheum.190538. [DOI] [PubMed] [Google Scholar]
- Cecil C.E., Davis J.M., Cech N.B., Laster S.M. Inhibition of H1N1 influenza A virus growth and induction of inflammatory mediators by the isoquinoline alkaloid berberine and extracts of goldenseal (Hydrastis canadensis) Int Immunopharmacol. 2011;11:1706–1714. doi: 10.1016/j.intimp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Chaube S., Swinyard C.A. Teratological and toxicological studies of alkaloidal and phenolic compounds from Solanum tuberosum L. Toxicol Appl Pharmacol. 1976;36:227–237. doi: 10.1016/0041-008x(76)90002-8. [DOI] [PubMed] [Google Scholar]
- Chang J.S., Wang K.C., Yeh C.F., Shieh D.E., Chiang L.C. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J Ethnopharmacol. 2013;145:146–151. doi: 10.1016/j.jep.2012.10.043. [DOI] [PubMed] [Google Scholar]
- Chen D., Tian X., Zou X., Xu S., Wang H., Zheng N, Wu Z. Harmine, a small molecule derived from natural sources, inhibits enterovirus 71 replication by targeting NF-κB pathway. Int Immunopharmacol. 2018;60:111–120. doi: 10.1016/j.intimp.2018.04.050. [DOI] [PubMed] [Google Scholar]
- Chen D.Y, Shien J.H, Tiley L, Chiou S.S., Wang S.Y., Chang T.J., Lee Y.J., Chan K.W., Hsu W.L. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010;119:1346–1351. [Google Scholar]
- Chen J.X., Xue H.J., Ye W.C., Fang B.H., Liu Y.H., Yuan S.H., Yu P., Wang Y.Q. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol Pharm Bull. 2009;32:1385–1391. doi: 10.1248/bpb.32.1385. [DOI] [PubMed] [Google Scholar]
- Chen H., Lao Z., Xu J., Li Z., Long H., Li D., Lin L., Liu X., Yu L., Liu W., Li G., Wu J. Antiviral activity of lycorine against Zika virus in vivo and in vitro. Virol. 2020;546:88–97. doi: 10.1016/j.virol.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilakapati J., Farris F.F. Third Edition. Encyclopedia of Toxicology; 2014. Cannabinoids; pp. 649–654. [Google Scholar]
- Connors, J.M., Levy, J.H., 2020. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020006000. [DOI] [PMC free article] [PubMed]
- Costiniuk C.T., Jenabian M.A. Acute inflammation and pathogenesis of SARS-CoV-2 infection: Cannabidiol as a potential anti-inflammatory treatment? Cytokine Growth Factor Rev. 2020;53:63–65. doi: 10.1016/j.cytogfr.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covés-Datson E.M., King S.R., Legendre M., Guptac A., Chand S.M., Gitlinc E., Kulkarnif V.V., Garcíaf J.P., Smeeg D.F., Lipkad E., Evansf S.E., Tarbetg E.B., Onob A., Markovit D.M. A molecularly engineered antiviral banana lectin inhibits fusion and is efficacious against influenza virus infection in vivo. Proc Natl Acad Sci U S A. 2020;117:2122–2132. doi: 10.1073/pnas.1915152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J.P., Wu L.Q., Li R., Zhao X.F., Wan Q.Y., Chen X.X., Li K.S. Identification of 23-(s)-2-amino-3-phenylpropanoyl-silybin as an antiviral agent for influenza a virus infection in vitro and in vivo. Antimicrob Agents Chemother. 2013;57:4433–4443. doi: 10.1128/AAC.00759-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J.P., Wang Q.W., Su Y., Gu L.M., Zhao Y., Chen X.X., Chen C., Li W.Z., Wang G.F., Li K.S. Emodin inhibition of influenza A virus replication and influenza viral pneumonia via the Nrf2, TLR4, p38/JNK and NF-kappaB pathways. Molecules. 2017;22:1754. doi: 10.3390/molecules22101754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523‐534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De U.C., Bhowmik J., Chowdhury S., Basak A., Dinda B. Isolation and Characterization of a New Flavonoid Glucoside from Aerial Parts of Phrynium placentarium. Chem Nat Compd. 2015;51:444–447. [Google Scholar]
- Depfenhart M., Villiers D.D., Lemperle G., Meyer M., Somma S.D. Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy? Intern Emerg Med. 2020;26:1–12. doi: 10.1007/s11739-020-02383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens, C., Asgharian, B., Kimbell, J., Price, O., Brace, G., 2007. Inventors; Consort Medical PLC, assignee, Nasal drug delivery. United States patent application US 10/553,330. Jun 7.
- Ding Y., Cao Z., Cao L., Ding G., Wang Z., Xiao W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci Rep. 2017;7:45723. doi: 10.1038/srep45723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionísio T.J., Souza G.P., Colombini-Ishikiriama B.L., Garbieri T.F., Parisi V.A., Oliveira G.M., Cano I.P., Rodini C.O., Oliveira S.H.P., Greene A.S., Santos C.F. AT1 receptor antagonism promotes bone loss attenuation in experimental periodontitis, block inflammatory mediators, upregulate antioxidant enzymes and bone formation markers. J Periodontol. 2020;91:533–544. doi: 10.1002/JPER.19-0064. [DOI] [PubMed] [Google Scholar]
- Dong X., Fu J., Yin X., Cao S., Li X., Lin L., Huyiligeqi, Ni J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother Res. 2016;30:1207–1218. doi: 10.1002/ptr.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X.S., Li H.D., Yang X.J., Li J.J., Xu J.J., Chen Y., Xu Q.Q., Yang L., He C.S., Huang C., Meng X.M., Li J. Wogonin attenuates liver fibrosis via regulating hepatic stellate cell activation and apoptosis. Int Immunopharmacol. 2019;75 doi: 10.1016/j.intimp.2019.05.056. [DOI] [PubMed] [Google Scholar]
- Enmozhi S.K., Raja K., Sebastine I., Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J Biomol Struct Dyn. 2020;2020:1–7. doi: 10.1080/07391102.2020.1760136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira N., Saraiva M.J., Almeida M.R. Epigallocatechin-3-gallate as a potential therapeutic drug for TTR-related amyloidosis: "in vivo" evidence from FAP mice models. PLoS One. 2012;7:29933. doi: 10.1371/journal.pone.0029933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti S., Baldini C., Baratè C., Ricci F., Balducci S., Grassi S., Baglietto L. The CoV-2 outbreak: how hematologists could help to fight Covid-19. Pharmacol Res. 2020;157 doi: 10.1016/j.phrs.2020.104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y., Cui X., Ma T., Liu Y., Li A., Huang M. Paeoniflorin upregulates β-defensin-2 expression in human bronchial epithelial cell through the p38 MAPK, ERK, and NF-κB signaling pathways. Inflammation. 2014;37:1468–1475. doi: 10.1007/s10753-014-9872-7. [DOI] [PubMed] [Google Scholar]
- Gandhi G.R., Barreto P.G., Lima B.D., Siqueira Quintans J.D.S., Antunes de Souza Araújo A., Narain N., Quintans Junior L.J., Gurgel R.Q. Medicinal plants and natural molecules with in-vitro and in-vivo activity against rotavirus: A systematic review. Phytomedicine. 2016;23:1830–1842. doi: 10.1016/j.phymed.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Ganesan S., Faris A.N., Comstock A.T, Wang W., Nanua S., Hershenson M.B., Sajjan U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antiviral Res. 2012;94:258–271. doi: 10.1016/j.antiviral.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García D., Leiro J., Delgado R., Sanmartín M.L., Ubeira F.M. Mangifera indica L. extract (Vimang) and mangiferin modulate mouse humoral immune responses. Phytother Res. 2003;17:1182–1187. doi: 10.1002/ptr.1338. [DOI] [PubMed] [Google Scholar]
- Ge X.Y., Li X.L., Yang A.A., Zhu C.G., Jonathan H., Epstein J.K., Mazet B.H., Peng Cheng, Zhang Yu-Ji, Z. W., Luo C.M., Wang Ning, Gary Crameri B.T., Wang Y.Z., Fa L., Y. S., Daszak P., Shi Z-Li. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535‐538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami D., Mahapatra A.D., Banerjee S., Kar A., Ojha D., Mukherjee P.K., Chattopadhyay D. Boswellia serrata oleo-gum-resin and β-boswellic acid inhibits HSV-1 infection in vitro through modulation of NF-кB and p38 MAP kinase signaling. Phytomedicine. 2018;51:94–103. doi: 10.1016/j.phymed.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020:1–4. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha S., Ghosal S., Chattopadhyay U. Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occurring glucosyl xanthone. Chemotherapy. 1996;42:443–451. doi: 10.1159/000239478. [DOI] [PubMed] [Google Scholar]
- Harwood M., Danielewska-Nikiel B., Borzelleca J.F., Flamm G.W., Williams G.M., Lines T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Hayden F.G., Turner R.B., Gwaltney J.M., Chi-Burris K., Gersten M., Hsyu P., Zalman L.S. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob Agents Chemother. 2003;47:3907–3916. doi: 10.1128/AAC.47.12.3907-3916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurich A., Hofmann-Winkle H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293‐1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P., Ma J., Liu Y., Deng H., Dong W. Hesperetin Promotes Cisplatin− Induced Apoptosis of Gastric Cancer In Vitro and In Vivo by Upregulating PTEN Expression. Front. Pharmacol. 2020;11:1326. doi: 10.3389/fphar.2020.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Tolstikov G.A., Cinatl J. Antiviral Activity of Glycyrrhizic Acid Derivatives against SARS− Coronavirus. J. Med. Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler Georg., Wu N.H., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271‐280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74:92‐101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzade A., Sadeghi O., Biregani A.N., Soukhtehzari S., Brandt G.S., Esmaillzadeh A. Immunomodulatory effects of flavonoids: possible induction of T CD4+ regulatory cells through suppression of mTOR pathway signaling activity. Front Immunol. 2019;10:51. doi: 10.3389/fimmu.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W., Liu B., Xu H. Celastrol: Progresses in structure-modifications, structure-activity relationships, pharmacology and toxicology. Eur J Med Chem. 2020;189 doi: 10.1016/j.ejmech.2020.112081. [DOI] [PubMed] [Google Scholar]
- Huang H., Liao D., Zhou G., Zhu Z., Cui Y., Pu R. Antiviral activities of resveratrol against rotavirus in vitro and in vivo. Phytomedicine. 2020;77 doi: 10.1016/j.phymed.2020.153230. [DOI] [PubMed] [Google Scholar]
- Huang YF, Bai C, He F, Xie Y, Zhou H. Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID-19) Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis M.A., Solimini R., Pichini S., Pacifici R., Carlier J., Busardò F.P. Cannabidiol Adverse Effects and Toxicity. Curr Neuropharmacol. 2019;17:974–989. doi: 10.2174/1570159X17666190603171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T.C., Jassey A., Liu C.H., Lin C.J., Lin C.C., Wong S.H., Lin L.T. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine. 2019;53:62–69. doi: 10.1016/j.phymed.2018.09.025. [DOI] [PubMed] [Google Scholar]
- Hutterer C., Milbradt J., Hamilton S., Zaja M., Leban J., Henry C., Vitt D., Steingruber M., Sonntag E, Zeitträger I., Bahsi H., Stamminger T., Rawlinson W., Strobl S., Marschall M. Inhibitors of dual-specificity tyrosine phosphorylation-regulated kinases (DYRK) exert a strong anti-herpes viral activity. Antiviral Res. 2017;143:113–121. doi: 10.1016/j.antiviral.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Imanishi N., Kashiwayama Y., Kawano A., Terasawa K., Shimada Y., Ochiai H. Inhibitory effect of cinnamaldehyde, derived from Cinnamomi cortex, on the growth of influenza A/PR/8 virus in vitro and in vivo. Antiviral Res. 2007;74:1–8. doi: 10.1016/j.antiviral.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Heinrich M., Appendino G., Efferth T., Fürst R., Izzo A.A., Kayser O., Pezzuto J.M., Viljoen A. Best practice in research - Overcoming common challenges in phytopharmacological research. J Ethnopharmacol. 2020;246 doi: 10.1016/j.jep.2019.112230. [DOI] [PubMed] [Google Scholar]
- Ibrahim I.M., Abdelmalek D.H., Elshahat M.E., Elfiky A.A. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect. 2020;80:554‐562. doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L.J. Inhaled Nitric Oxide and COVID‐19. Br. J. Pharmacol. 2020 [Google Scholar]
- Imai Y., Kuba K., Penninger J.M. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp Physiol. 2008;93:543‐548. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbrucker R.A., Bausch J., Edwards J.A., Wolz E. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 1: genotoxicity. Food Chem Toxicol. 2006;44:626–635. doi: 10.1016/j.fct.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Jiang J., Dai J., Cui H. Vitexin reverses the autophagy dysfunction to attenuate MCAO-induced cerebral ischemic stroke via mTOR/Ulk1 pathway. Biomed Pharmacother. 2018;99:583–590. doi: 10.1016/j.biopha.2018.01.067. [DOI] [PubMed] [Google Scholar]
- Juergens U.R., Dethlefsen U., Steinkamp G., Gillissen A., Repges R., Vetter H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir Med. 2003;97:250–256. doi: 10.1053/rmed.2003.1432. [DOI] [PubMed] [Google Scholar]
- Kang S., Song M.J., Min H. Antiviral activity of ginsenoside Rg3 isomers against gamma herpesvirus through inhibition of p38-and JNK-associated pathways. J. Funct. Foods. 2018;40:219–228. [Google Scholar]
- Kashiwada Y., Aoshima A., Ikeshiro Y., Chen Y.P., Furukawa H., Itoigawa M., Fujioka T., Mihashi K., Cosentino L.M., Morris-Natschke S.L., Lee K.H. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure-activity correlations with related alkaloids. Bioorg Med Chem. 2005;17:443–448. doi: 10.1016/j.bmc.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Kashyap D., Tuli H.S., Sharma A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016;146:201–213. doi: 10.1016/j.lfs.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Pannecouque C., Van Damme E., Peumans W., Egberink H., Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75:179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesel V.A., Stan S.D. Diallyl trisulfide, a chemopreventive agent from Allium vegetables, inhibits alpha-secretases in breast cancer cells. Biochem. Biophys. Res. Commun. 2017;484:833–838. doi: 10.1016/j.bbrc.2017.01.184. [DOI] [PubMed] [Google Scholar]
- Kim M., Kim S.Y., Lee H.W., Shin J.S., Kim P., Jung Y.S., Lee C.K. Inhibition of influenza virus internalization by (−)-epigallocatechin-3-gallate. Antiviral Res. 2013;100:460–472. doi: 10.1016/j.antiviral.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Jiang C., Penninger J.M. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J Mol Med. 2006;84:814‐820. doi: 10.1007/s00109-006-0094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari P., Rawat K., Saha L. Pipeline Pharmacological Therapies in Clinical Trial for COVID-19 Pandemic: a Recent Update. Curr Pharmacol Rep. 2020;6:1–13. doi: 10.1007/s40495-020-00226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalou C., Basak A., Mishra P., Mohanta B.C., Banik R., Dinda B., Khatib A.M. Inhibition of tumor cells proliferation and migration by the flavonoid Furin inhibitor isolated from Oroxylum indicum. Curr. Med. Chem. 2013;20:583–591. doi: 10.2174/0929867311320040010. [DOI] [PubMed] [Google Scholar]
- Lane T., Anantpadma M., Freundlich J.S, Davey R.A., Madrid P.B., Ekins S. The natural product eugenol is an inhibitor of the ebola virus in vitro. Pharm res. 2019;36:104. doi: 10.1007/s11095-019-2629-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lani R., Hassandarvish P., Chiam C.W., Moghaddam E., Chu J.J.H., Rausalu K., Zandi K. Antiviral activity of silymarin against chikungunya virus. Sci. Rep. 2015;5:11421. doi: 10.1038/srep11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y.L., Peiris J.S. Pathogenesis of severe acute respiratory syndrome. Curr Opin Immunol. 2005;17:404‐410. doi: 10.1016/j.coi.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K.M., Lee K.M., Koon C.M., Cheung C.S.F., Lau C.P., Ho H.M., Tsui S.K.W. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J Ethnopharmacol. 2008;118:79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenkens M., de Wit H., Danser A.H., Esselink A.C., Horikx A., Ten Oever J., Veerdonk van de F., Kramers C. Medication and comedication in COVID-19 patients. Ned Tijdschr Geneeskd. 2020;164:D4995. [PubMed] [Google Scholar]
- Lin L.T., Chen T.Y., Lin S.C., Chung C.Y., Lin T.C., Wang G.H., Anderson R., Lin C.C., Richardson C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013;13:187. doi: 10.1186/1471-2180-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.L., Ma S.C., Yang Y.T., Ye S.M., But P.P. Antiviral activities of flavonoids and organic acid from Trollius chinensis Bunge. J Ethnopharmacol. 2002;79:365–368. doi: 10.1016/s0378-8741(01)00410-x. [DOI] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864‐1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li X., Ohtsuki T., Shindo S., Sato M., Koyano T., Preeprame S., Kowithayakorn T., Ishibashi M. Mangiferin identified in a screening study guided by neuraminidase inhibitory activity. Planta med. 2007;73:1195–1196. doi: 10.1055/s-2007-981582. [DOI] [PubMed] [Google Scholar]
- Li Y., Lai Y., Wang Y., Liu N., Zhang F., Xu P. 1, 8-Cineol Protect Against Influenza-Virus-Induced Pneumonia in Mice. Inflammation. 2016;39:1582–1593. doi: 10.1007/s10753-016-0394-3. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhou W., Yang L., You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. 2020;157 doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.Q., Li Z.L., Zhao W.J., Wen R.X., Meng Q.W., Zeng Y. Synthesis of stilbene derivatives with inhibition of SARS coronavirus replication. Eur. J. Med. Chem. 2006;41:1084–1089. doi: 10.1016/j.ejmech.2006.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.Y., Chen C., Zhang H.Q., Guo H.Q., Wang H., Wang L., Zhang X., Hua S., Yu J., Xiao P.G., Li R.S., Tan X.U. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67:18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L.J., Lu Y., Zhang Y.Y., Zhu H.Y., Tu P., Li H., Chen D.F. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine. 2020;67 doi: 10.1016/j.phymed.2019.153150. [DOI] [PubMed] [Google Scholar]
- Lin C.W., Tsai F.J., Tsai C.H., Lai C.C., Wan L., Ho T.Y., Chao P.D.L. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. 2005;68:36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.C., Ho C.T., Chuo W.H., Li S., Wang T.T., Lin C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17:144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yue Y., Zhou Y., Fan Y., Fan C., Huang Y., Wu F., Liu Y. An oil-free microemulsion for intravenous delivery of diallyl trisulfide: formulation and evaluation. Int J Pharm. 2011;407:158–166. doi: 10.1016/j.ijpharm.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Liu Z.F., Fang F., Dong Y.S., Li G., Zhen H. Experimental study on the prevention and treatment of murine cytomegalovirus hepatitis by using allitridin. Antiviral Res. 2004;61:125–128. doi: 10.1016/s0166-3542(03)00087-1. [DOI] [PubMed] [Google Scholar]
- Liu Y., Xiao N., Du H., Kou M., Lin L., Huang M., Zhang S., Xu S., Li D., Chen Q. Celastrol ameliorates autoimmune disorders in Trex1-deficient mice. Biochem Pharmacol. 2020;178 doi: 10.1016/j.bcp.2020.114090. [DOI] [PubMed] [Google Scholar]
- Liu C.H., Jassey A., Hsu H.Y., Lin L.T. Antiviral Activities of Silymarin and Derivatives. Molecules. 2019;24:1552. doi: 10.3390/molecules24081552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe H.I., Toyang N.J., McLaughlin W. Potential of Cannabidiol for the Treatment of Viral Hepatitis. Pharmacognosy Res. 2017;9:116–118. doi: 10.4103/0974-8490.199780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y., Zhang Y., Zhang W., Yuan Y., Bao J., Zhang B., Shi Y., Yan J., Gao G.F. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227‐231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Kuang X., Zhou Q., Yan C., Li W., Gong H., Kurihara H., Li W., Li Y., He R. Inhibitory effects of baicalein against herpes simplex virus type 1. Acta Pharm. Sin. B. 2020;25 doi: 10.1016/j.apsb.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio R., Corsini G.U. Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection. Pharmacol. Res. Commun. 2020;157 doi: 10.1016/j.phrs.2020.104837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães C.B., Riva D.R., DePaula L.J., Brando-Lima A., Koatz† V.G., Leal-Cardoso J.H., Zin W.A., Faffe D.S. In vivo anti-inflammatory action of eugenol on lipopolysaccharide-induced lung injury. J Appl Physiol. 1985;108:845–851. doi: 10.1152/japplphysiol.00560.2009. [DOI] [PubMed] [Google Scholar]
- Magrone T., Magrone M., Jirillo E. Endocr Metab Immune Disord Drug Targets. 2020;10:2174. doi: 10.2174/1871530320666201007152628. [DOI] [PubMed] [Google Scholar]
- Magrone, T., Magrone, M., Jirillo, E., 2020. Focus on Receptors for Coronaviruses with Special Reference to Angiotensin-converting Enzyme 2 as a Potential Drug Target - A Perspective Endocr Metab Immune Disord Drug Targets 10, 2174. [DOI] [PubMed]
- Mani J.S., Johnsona J.B., Steela J.C., Broszczak D.A., Neilsen P.M., Walsh K.B., Naiker M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020;284 doi: 10.1016/j.virusres.2020.197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A., FK Alghetaa H., Miranda K., Wilson K., Singh N., Cai G., Putluri N., Nagarkatti P., Nagarkatti M. Δ9-Tetrahydrocannabinol Prevents Mortality from Acute Respiratory Distress Syndrome through the Induction of Apoptosis in Immune Cells, Leading to Cytokine Storm Suppression. Int J Mol Sci. 2020;21:6244. doi: 10.3390/ijms21176244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F., Sakata M., Tahara M., Kutsuna S., Ohmagari N., Kuroda M., Suzuki T., McDonagh P., Sheehy P.A., Norris J.M. Identification and characterization of small molecule inhibitors of feline coronavirus replication. Vet. Microbiol. 2014;174:438–447. doi: 10.1016/j.vetmic.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha M., Feliú A., Iñigo P.M., Mestre L., Carrillo-Salinas F.J., Guaza C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis. 2013;59:141–150. doi: 10.1016/j.nbd.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395:033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes‐Rodrigues F.S., Padrão Tavares J.G., Pires de Oliveira M., Guzella de Carvalho R., Ruggero Errante P., Omar Taha M., Caricati‐Neto A. Anticoagulant and antiarrhythmic effects of heparin in the treatment of COVID‐19 patients. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virol. 2018;517:3‐8. doi: 10.1016/j.virol.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Seron K., Labitt R.N., Danneels A., Palmer K.E., Whittaker G.R., Dubuisson J., Belouzard S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antiviral Res. 2016;133:1–8. doi: 10.1016/j.antiviral.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming L.J., Yin A.C. Therapeutic effects of glycyrrhizic acid. Nat Prod Commun. 2013;8:415–418. [PubMed] [Google Scholar]
- Nelson P.J., D'Agati V.D., Gries J.M., Suarez J.R., Gelman I.H. Amelioration of nephropathy in mice expressing HIV-1 genes by the cyclin-dependent kinase inhibitor flavopiridol. J Antimicrob Chemother. 2003;51:921–929. doi: 10.1093/jac/dkg175. [DOI] [PubMed] [Google Scholar]
- Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J Microbiol Immunol Infect. 2020;03:022. doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikhat S., Fazil M. Overview of Covid-19; its prevention and management in the light of Unani medicine. Sci Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha D., Das R., Sobia P., Dwivedi V., Ghosh S., Samanta A., Chattopadhyay D. Pedilanthus tithymaloides inhibits HSV infection by modulating NF-κB signaling. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe B.R., Giomarelli B., Barnard D.L., Shenoy S.R., Chan P.K.S., McMahon J.B., Palmer K.E., Barnett B.W., Meyerholz D.K., Wohlford-Lenane C.L., McCray P.B. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panraksa P., Ramphan S., Khongwichit S., Smith D.R. Activity of andrographolide against dengue virus. Antiviral Res. 2017;139:69–78. doi: 10.1016/j.antiviral.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Pandey G. Active principles and median lethal dose of curcuma longa linn. Int. Res. J. Pharm. 2011;5:239–241. [Google Scholar]
- Park S, Kim JI, Lee I, Lee S., Hwang M.W., Bae J.Y., Heo J., Kim D., Han S.Z., Park M.S. Aronia melanocarpa and its components demonstrate antiviral activity against influenza viruses. Biochem Biophys Res Commun. 2013;440:14–19. doi: 10.1016/j.bbrc.2013.08.090. [DOI] [PubMed] [Google Scholar]
- Park J.Y., Yuk H.J., Ryu H.W., Lim S.H., Kim K.S., Park K.H., Young B.R., Lee W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J Enzyme Inhib Med Chem. 2017;32:504–515. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris A., Strukelj B., Renko M., Turk V., Pukl M., Umek A., Korant B.D. Inhibitory effect of carnosic acid on HIV-1 protease in cell-free assays [corrected] [published correction appears. J Nat Prod. 1994;56:1426–1430. doi: 10.1021/np50098a031. [DOI] [PubMed] [Google Scholar]
- Patel C., Dadhaniya P., Hingorani L., Soni M.G. Safety assessment of pomegranate fruit extract: acute and subchronic toxicity studies. Food Chem Toxicol. 2008;46:2728–2735. doi: 10.1016/j.fct.2008.04.035. [DOI] [PubMed] [Google Scholar]
- Peng A.W., Milleri S., Stein D.S. Direct measurement of the anti-influenza agent zanamivir in the respiratory tract following inhalation. Antimicrob Agents Chemother. 2000;44:1974–1976. doi: 10.1128/aac.44.7.1974-1976.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Watanabe S., Chan K.W.K., He Q., Zhao Y., Zhang Z., Li G. Luteolin restricts dengue virus replication through inhibition of the proprotein convertase furin. Antivir Res. 2017;143:176–185. doi: 10.1016/j.antiviral.2017.03.026. [DOI] [PubMed] [Google Scholar]
- Perwitasari O., Yan X., O'Donnell J., Johnson S., Tripp R.A. Repurposing Kinase Inhibitors as Antiviral Agents to Control Influenza A Virus Replication. Assay Drug Dev Technol. 2015;13:638–649. doi: 10.1089/adt.2015.0003.drrr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis E., Baghdan E., Agel M.R., Anders T., Pourasghar M., Schneider M., Bakowsky U. Spray dried curcumin loaded nanoparticles for antimicrobial photodynamic therapy. Eur J Pharm Biopharm. 2019;142:531–539. doi: 10.1016/j.ejpb.2019.07.023. [DOI] [PubMed] [Google Scholar]
- Rathinavel T., Palanisamy M., Srinivasan P., Subramanian A., Thangaswamy S. Phytochemical 6-Gingerol – A promising Drug of choice for COVID-19. Int. J. Adv. Sci. Eng. 2020;4:1482–1489. [Google Scholar]
- Remacle A.G., Gawlik K., Golubkov V.S., Cadwell G.W., Liddington R.C., Cieplak P., Neugebauer W.A. Selective and potent Furin inhibitors protect cells from anthrax without significant toxicity. Int J Biochem Cell Biol. 2010;42:987–995. doi: 10.1016/j.biocel.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendina M., D'Amato M., Castellaneta A., Castellaneta N.M., Brambilla N., Giacovelli G., Rovati L., Rizzi S.F., Zappimbulso M., Bringiotti R.S., Di Leo A. Antiviral activity and safety profile of silibinin in HCV patients with advanced fibrosis after liver transplantation: A randomized clinical trial. Transpl. Int. 2014;27:696–704. doi: 10.1111/tri.12324. [DOI] [PubMed] [Google Scholar]
- Reiss C.S. Cannabinoids and viral infections. Pharmaceuticals. 2010;3(6):1873–1886. doi: 10.3390/ph3061873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Abdulrahman A.Y., Khazali A.S., Nor Rashid N., Chong T.T., Yusof R. Carnosine exhibits significant antiviral activity against Dengue and Zika virus. J Pept Sci. 2019;25:3196. doi: 10.1002/psc.3196. [DOI] [PubMed] [Google Scholar]
- Rothlin R.P., Vetulli H.M., Duarte M., Pelorosso F.G. Telmisartan as tentative angiotensin receptor blocker therapeutic for COVID-19. Drug Dev Res. 2020;10:1002. doi: 10.1002/ddr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh C. A facile inhibitor screening of SARS coronavirus N protein using nanoparticle-based RNA oligonucleotide. Int J Nanomed. 2012;7:2173–2179. doi: 10.2147/IJN.S31379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y.B., Park S.J., Kim Y.M., Lee J.Y., Seo W.D., Chang J.S., Park K.H., Rho M.C., Lee W.S. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes. from Tripterygium regelii. Bioorg. Med. Chem. Lett. 2010;20:1873–1876. doi: 10.1016/j.bmcl.2010.01.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J.M. COVID-19, Angiotensin Receptor Blockers, and the Brain. Cell Mol Neurobiol. 2020;40:667–674. doi: 10.1007/s10571-020-00861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, P., Banerjee, A. K., Tripathi, P. P., Srivastava, A.K., Ray, U., 2020. A virus that has gone viral: amino acid mutation in S protein of Indian isolate of Coronavirus COVID-19 might impact receptor binding, and thus, infectivity. Biosci. Rep 40, BSR20201312. [DOI] [PMC free article] [PubMed]
- Sadaie M.R., Mayner R., Doniger J. A novel approach to develop anti-HIV drugs: adapting non-nucleoside anticancer chemotherapeutics. Antiviral Res. 2004;61:1–18. doi: 10.1016/j.antiviral.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Sahebkar A., Gurban C., Serban A., Andrica F., Serban M.C. Effects of supplementation with pomegranate juice on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 2016;23:1095–1102. doi: 10.1016/j.phymed.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Sanchis-Gomar, F., Lavie, C.J., Perez-Quilis, C., Henry, B.M., Lippi, G., 2020. Angiotensin-Converting Enzyme 2 and Antihypertensives (Angiotensin Receptor Blockers and Angiotensin-Converting Enzyme Inhibitors) in Coronavirus Disease 2019 Mayo Clin Proc. 4. [DOI] [PMC free article] [PubMed]
- Scavone C., Brusco S., Bertini M., Sportiello L., Rafaniello C., Zoccoli A., Capuano A. Current pharmacological treatments for COVID‐19: what's next? Br. J. Pharmacol. 2020;10:1111. doi: 10.1111/bph.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaito A., Posadino A.M., Younes N., Hasan H., Halabi S., Alhababi D., Al-Mohannadi A., Abdel-Rahman W.M., Eid A.H., Nasrallah G.K., Pintus G. Potential Adverse Effects of Resveratrol: A Literature Review. Int J Mol Sci. 2020;21:2084. doi: 10.3390/ijms21062084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L., Zhou Z., Cai Y., Castro P., Dakhov O., Shi P., Wang J. Celastrol suppresses tumor cell growth through targeting an AR-ERG-NF-κB pathway in TMPRSS2/ERG fusion gene expressing prostate cancer. PloS one. 2013;8:58391. doi: 10.1371/journal.pone.0058391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Davis L., Wallace J.M., Cai Y., Lawson L.D. Enhanced diallyl trisulfide has in vitro synergy with amphotericin B against Cryptococcus neoformans. Planta Med. 1996;62:415–418. doi: 10.1055/s-2006-957929. [DOI] [PubMed] [Google Scholar]
- Shen L.W., Mao H.J., Wu Y.L., Tanaka Y., Zhang W. TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017;142:1‐10. doi: 10.1016/j.biochi.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin W.J., Seong B.L. Type II transmembrane serine proteases as potential target for anti-influenza drug discovery. Expert Opin Drug Discov. 2017;12:1139‐1152. doi: 10.1080/17460441.2017.1372417. [DOI] [PubMed] [Google Scholar]
- Shin H.B., Choi M.S., Ryu B., Lee N.R., Kim H.I., Choi H.E., Chang J., Lee K.T., Jang D.S., Inn K.S. Antiviral activity of carnosic acid against respiratory syncytial virus. Virol. J. 2013;10:303. doi: 10.1186/1743-422X-10-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.H., Choi H.J. Silymarin efficacy against influenza A virus replication. Phytomedicine. 2011;18:832–835. doi: 10.1016/j.phymed.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H.X., Yao S., Zhao W.F., Li M.J., Liu J., Shang W.J., Xie H., Ke C.Q., Hu H.C., Gao M.N., Yu K.Q., Liu H., Shen J.S., Tang W, Zhang L.K., Xiao G.F., Ni L., Wang D.W., Zuo J.P., Jiang H.L., Bai F., Wu Y., Ye Y., Xu Y.C. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol Sin. 2020;41:1167–1177. doi: 10.1038/s41401-020-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, H., Yao, S., Zhao, W., Liu, M.J., Shang, W., Xie, H., Ke, C., Gao, M., Yu, K., Liu, H., Shen, J., Tang, W., Zhang, L., Zuo, J., Jiang, H., Bai, F., Wu, Y., Ye Y., Xu, Y., 2020. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. bioRxiv DOI: 10.1101/2020.04.13.038687.
- Taki M., Tagami T., Fukushige K., Ozeki T. Fabrication of nanocomposite particles using a two-solution mixing-type spray nozzle for use in an inhaled curcumin formulation. Int. J. Pharm. 2016;511:104–110. doi: 10.1016/j.ijpharm.2016.06.134. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Yoshiya T., Yoshizawa-Kumagaye K., Sugiyama T. Nicotianamine is a novel angiotensin-converting enzyme 2 inhibitor in soybean. Biomed. Res J. 2015;36:219–224. doi: 10.2220/biomedres.36.219. [DOI] [PubMed] [Google Scholar]
- Tomasoni D., Italia L., Adamo M., Inciardi R.M., Lombardi C.M., Solomon S.D., Metra M. COVID 19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur. J. Heart Fail. 2020;10:1002. doi: 10.1002/ejhf.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N., Dash D., Singh R. Curcumin inhibits paraquat induced lung inflammation and fibrosis by extracellular matrix modifications in mouse model. Inflammopharmacology. 2016;24:335–345. doi: 10.1007/s10787-016-0286-z. [DOI] [PubMed] [Google Scholar]
- Utsunomiya T., Kobayashi M., Pollard R.B., Suzuki F. Glycyrrhizin, an active component of licorice roots, reduces morbidity and mortality of mice infected with lethal doses of influenza virus. Antimicrob Agents Chemother. 1997;41:551–556. doi: 10.1128/aac.41.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankadari N., Wilce J.A. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9:601‐604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B., Ganesan S., Venugopal A., Venkatesan D., Ganesan H., Rajagopalan K., Rahman P.K.S.M., Cho N.S., Kumar N.S., Subramaniam M.D. COVID-19: A promising cure for the global panic. Sci Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281‐292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yang P., Liu K., Feng G., Yanli Z., Gongyi Z., Chengyu J. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.R., Gao Y.D., Ma C.H., Zhang X.J., Huang J.F., Zheng Y.T. Mangiferin, an anti-HIV-1 agent targeting protease and effective against resistant strains. Molecules. 2011;16:4264–4277. doi: 10.3390/molecules16054264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K., Chen, W., Zhou, Y.S., Lian, J.Q., Zhang, Z., Du, P., Wang, B., 2020. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. BioRxiv 10.1101/2020.03.14.988345.
- Wang Z.Y., Li Y.Q., Guo Z.W., Zhou X.H., Lu M.D., Xue T.C., Gao B. ERK1/2-HNF4α axis is involved in epigallocatechin-3-gallate inhibition of HBV replication. Acta Pharmacol Sin. 2020;41:278–285. doi: 10.1038/s41401-019-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Li L.F., Wang Q.Y., Shang L.Q., Shi P.Y., Yin Z. Anti-dengue-virus activity and structure-activity relationship studies of lycorine derivatives. Chem Med Chem. 2014;9:1522–1533. doi: 10.1002/cmdc.201300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C.C., Kuo Y.H., Jan J.T., Liang P.H., Wang S.Y., Liu H.G., Lee C.K., Chang S.T., Kuo C.J., Lee S.S., Hou C.C., Hsiao P.W., Chien S.C., Shyur L.F., Yang N.S. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavi-rus. J. Med. Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- Weng J.R., Lin C.S., Lai H.C., Lin Y.P., Wang C.Y., Tsai Y.C., Lin C.W. Antiviral activity of Sambucus FormosanaNakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus Res. 2019;273 doi: 10.1016/j.virusres.2019.197767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.P., Christopher M.E., Viswanathan S., Schnell G., Dai X., Van Loon D., Stephen E.R. Aerosol and nasal delivery of vaccines and antiviral drugs against seasonal and pandemic influenza. Expert Rev Respir Med. 2010;4:171–177. doi: 10.1586/ers.10.15. [DOI] [PubMed] [Google Scholar]
- Xiong H.R., Luo J., Hou W., Xiao H., Yang Z.Q. The effect of emodin, an anthraquinone derivative extracted from the roots of Rheum tanguticum, against herpes simplex virus in vitro and in vivo. J Ethnopharmacol. 2011;133:718–723. doi: 10.1016/j.jep.2010.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Wang C., Zhang R., Xu M., Liu B., Wei D., Wang G., Tian S. Carnosine markedly ameliorates H9N2 swine influenza virus-induced acute lung injury. J Gen Virol. 2015;96:2939–2950. doi: 10.1099/jgv.0.000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.Q., Fu Y.J., Wu S., Qin H.Q., Zhen X., Song B.M., Weng Y.S., Wang P.C., Chen X.Y., Jiang Z.Y. Anti-influenza activity of berberine improves prognosis by reducing viral replication in mice. Phytother Res. 2018;32:2560–2567. doi: 10.1002/ptr.6196. [DOI] [PubMed] [Google Scholar]
- Yang Y., Xiu J., Zhang L., Qin C., Liu J. Antiviral activity of punicalagin toward human enterovirus 71 in vitro and in vivo. Phytomedicine. 2012;20:67–70. doi: 10.1016/j.phymed.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., Chen L. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.S., Tseng C.K., Lin C.K., Hsu Y.C., Wu Y.H., Hsieh C.L., Lee J.C. Celastrol inhibits dengue virus replication via up-regulating type I interferon and downstream interferon-stimulated responses. Antiviral Res. 2017;137:49–57. doi: 10.1016/j.antiviral.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.D., Zheng-Li S. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535‐538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Wang X., Chang Q., Xu J., Huang Y., Guo Q., Zhang S., Wang W., Chen X., Wang J. Neferine, a bisbenzylisoquinline alkaloid attenuates bleomycin-induced pulmonary fibrosis. Eur J Pharmacol. 2010;627:304–312. doi: 10.1016/j.ejphar.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586‐590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.H., Wang Y.F., Liu X.J., Lu J.H., Qian C.W., Wan Z.Y., Li J.X. Antiviral activity of cepharanthine against severe acute respiratory syndrome coronavirus in vitro. Chin. Med. J. 2005;118:493–496. [PubMed] [Google Scholar]
- Zhao H., Wald J., Palmer M., Han Y. Hydroxychloroquine-induced cardiomyopathy and heart failure in twins. J. Thorac. Dis. 2018;10:E70–E73. doi: 10.21037/jtd.2017.12.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen H., Fang F., Ye D.Y., Shu S.N., Zhou Y.F., Dong Y.S., Nie X.C., Li G. Experimental study on the action of allitridin against human cytomegalovirus in vitro: Inhibitory effects on immediate-early genes. Antiviral Res. 2006;72:68–74. doi: 10.1016/j.antiviral.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Zhi H., Jin X., Zhu H., Li H., Zhang Y.Y., Yan L.D., Chen F. Exploring the effective materials of flavonoids-enriched extract from Scutellaria baicalensis roots based on the metabolic activation in influenza A virus induced acute lung injury. J Pharm Biomed Anal. 2020;177 doi: 10.1016/j.jpba.2019.112876. [DOI] [PubMed] [Google Scholar]
- Zhi H.J., Zhu H.Y., Zhang Y.Y., Lu Y., Li H., Chen D.F. In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine. 2019;57:105–116. doi: 10.1016/j.phymed.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Zhu J., Van de Ven W., Vermorken A. Polyphenols with indirect proprotein convertase inhibitory activity. Int. J. Oncol. 2013;43:947–955. doi: 10.3892/ijo.2013.2009. [DOI] [PubMed] [Google Scholar]
- Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses—drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]