Abstract
Objective
The COVID-19 pandemic raises a major concern about its severity in pregnancy, maternal-fetal outcomes, and risk of vertical transmission. We report a retrospective descriptive study of the clinical course and maternal-fetal outcomes of pregnant women with COVID-19.
Methods
This is a single-centre, retrospective study performed in a tertiary care hospital for pregnant women with COVID-19 in India. The medical records of all women who delivered in the COVID19 facility from May 5, 2020, to June 5, 2020, were reviewed independently. Data extracted from the records included demographic characteristics, obstetric details, comorbidities, disease severity, investigations, management, and information on neonates (birthweight, Apgar score, and perinatal complications).
Results
Among 348 women tested for SARS-CoV-2, 57 women (16.3%) were confirmed as positive based on quantitative reverse transcriptase polymerase chain reaction of the nasopharyngeal specimen. Most women (45; 78.9%) had a mild infection with favourable maternal-fetal outcomes. Three maternal deaths were associated with comorbidities. Five neonates tested positive for SARS-CoV-2, remained hemodynamically stable, and were subsequently discharged.
Conclusions
A majority of pregnant women with COVID-19 had mild disease and recovered with good perinatal outcomes. Women with comorbidities may have an increased risk of severe morbidity and mortality. The cycle threshold signifying the viral load and degree of infectivity can modify management during pregnancy. Long-term outcomes and the potential mother-to-child vertical/horizontal transmission need further study.
Keywords: coronavirus infections, pregnancy, reverse transcriptase polymerase chain reaction, viral load, vertical transmission, maternal mortality
RÉSUMÉ
Objectif
La pandémie de COVID-19 soulève une préoccupation importante quant à la sévérité de la maladie pendant la grossesse, au devenir fœto-maternel et aux risques de transmission verticale. Nous présentons une étude descriptive rétrospective de l’évolution clinique et du devenir fœto-maternel chez des femmes enceintes atteintes de la COVID-19.
Méthodologie
Il s'agit d'une étude rétrospective monocentrique menée en Inde dans un hôpital de soins tertiaires auprès de femmes enceintes atteintes de la COVID-19. Les auteurs ont examiné de façon indépendante les dossiers médicaux de toutes les femmes qui ont accouché entre le 5 mai et le 5 juin 2020 dans l'unité réservée aux patients atteints de la COVID-19. Les données extraites des dossiers comprenaient les caractéristiques démographiques, les détails obstétricaux, les comorbidités, la sévérité de l'atteinte, les analyses, la prise en charge et les données néonatales (poids à la naissance, indice d'Apgar et complications périnatales).
Résultats
Parmi les 348 femmes qui ont subi un test de dépistage du SARS-CoV-2, 57 (16,3 %) ont reçu un résultat positif confirmé par la RT-PCR quantitative de l’échantillon nasopharyngé. La plupart d'entre elles (45; 78,9 %) ont eu une atteinte légère avec des issues fœto-maternelles favorables. Trois cas de mortalité maternelle ont été associés à des comorbidités. Quant aux 5 nouveau-nés ayant obtenu un résultat positif au test de dépistage du SARS-CoV-2, leur état hémodynamique est demeuré stable et ils ont par la suite obtenu leur congé.
Conclusions
Une majorité de femmes enceintes ayant contracté la COVID-19 ont éprouvé une atteinte légère et se sont rétablies avec des issues périnatales favorables. Les femmes ayant des comorbidités peuvent présenter un risque accru de morbidité grave et de mortalité. Le seuil de cycle qui indique la charge virale et le degré d'infectiosité peut modifier la prise en charge pendant la grossesse. D'autres études sont nécessaires pour déterminer les issues à long terme et le potentiel de transmission verticale ou horizontale de la mère à l'enfant.

S. Bachani
INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first reported in Wuhan, China, infects host cells through angiotensin-converting enzyme 2 receptors present predominantly within type II alveolar cells of the lung and across the aerodigestive tract.1 , 2 Most infections are mild or asymptomatic (80%), 15% are severe and necessitate supplemental oxygen, and 5% are critical, requiring mechanical ventilation.3 The first case in Delhi, India, was documented on January 30, 2020. At the time of writing, India has seen 440 215 cases, 248 189 recoveries, and 19 011 deaths.4
Physiologic changes in the immune and cardiopulmonary systems render pregnant women more susceptible to respiratory pathogens.5 However, pregnant women seemingly have experienced fewer adverse events related to COVID-19 than were reported for Severe acute respiratory syndrome and Middle East respiratory syndrome.6
As the pandemic unfolds, prevention and control of infection among pregnant women and the potential risk of vertical transmission have become a major concern. The first study describing the clinical characteristics and investigating the possibility of vertical transmission of SARS-CoV-2 in nine pregnant women with laboratory-confirmed COVID-19 infection demonstrated that the severity in pregnant women was similar to that in nonpregnant adults. There was no evidence of vertical transmission: SARS-CoV-2 was not detected in amniotic fluid, cord blood, or neonatal throat swab samples in six cases.7 Most recently, two neonates born to mothers with COVID-19 have been reported to have tested positive for SARS-CoV-2 shortly after delivery, raising concerns about the possibility of vertical transmission.8 , 9 The increasing number of cases and shortage of hospital beds necessitates the use of the cycle threshold (Ct) to indicate viral load. A progressive decrease in viral load was observed in all studies of persons with COVID-19; however, viral load remained detectable until 17–21 days after symptom onset.10 , 11
To substantiate the sparse research on pregnant patients with COVID-19 in developing nations, we report a retrospective descriptive study of the clinical course, Ct values, and maternal-fetal outcomes of pregnant women with COVID-19.
METHODS
Study Design and Participants
This was a single-centre, retrospective study performed in a medical college–affiliated tertiary care hospital in the epicenter of the outbreak in India. The annual delivery rate at this hospital is approximately 23 000, including the level III intensive care unit (ICU). The medical records of all women who delivered in the COVID-19 facility from May 5, 2020, to June 5, 2020, were reviewed independently by two authors. A laboratory-confirmed case of COVID-19 was defined as a positive result on quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) assay of nasopharyngeal swab specimens for adults and neonates. The kit for qRT-PCR, the LabGun COVID-19 RT-PCR kit, was designed by the National Institute of Virology Pune (India) and conformed to the national guidelines from the Indian Council of Medical Research (ICMR). The kit runs primer probes through an initial screening for the E (envelope) gene specific to the Sarbeco subgenus. Samples were confirmed as positive if either of the two SARS-CoV-2–specific genes, vide RDRp (RNA-dependent RNA polymerase) or ORF-1bnsp14b, were detected.12
Data extracted from the patient records included demographic characteristics, comorbidities, symptomatology, pregnancy outcomes, and information on neonates (including birthweight, Apgar score, and perinatal complications). Also noted were the laboratory test results, Ct values, drug therapy, and length of hospital stay. Maternal deaths in this cohort were scrutinized. The study was approved by the institutional ethics committee.
Outcomes and Definitions
The following outcomes and definitions were used:
-
1.
A demographic profile of the pregnant women. Clusters in which SARS-CoV-2 affected more than four households were designated containment by the government authorities.
-
2.
Testing criteria: As per ICMR guidelines, only symptomatic women, high-risk contacts of confirmed cases, and pregnant women from containment areas were tested for SARS-CoV-2 infection.
-
3.
Discharge criteria: Women who were asymptomatic for 10 days after diagnosis, including 3 days after resolution of symptoms, were discharged. In consideration of the benefits of early discharge, repeat testing was not done; this was consistent with current operational guidelines for COVID-19 management with home isolation.
-
4.
The Ct was the number of cycles that had to be run on the PCR machine to detect the SARS-CoV-2–specific gene. The threshold value for infectivity was taken as 35 cycles or less for the E,RDRp and ORF genes. A higher Ct value was suggestive of a lower viral load.
-
5.
The severity of disease was classified as mild, moderate, severe, or critical as per the World Health Organization country and technical guidance.3
-
6.
Maternal mortality comprised maternal deaths occurring during pregnancy or within 6 weeks of abortion/delivery.
Statistical Analysis
Statistical analysis was performed using SPSS for Windows, version 17.0 (SPSS, Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are presented as absolute numbers and percentages. Data were checked for normality before statistical analysis. Normally distributed continuous variables were compared using the unpaired t test, whereas the Mann-Whitney U test was used for variables that were not normally distributed. For all statistical tests, a P value <0.05 was taken to indicate a significant difference.
RESULTS
Patient Characteristics
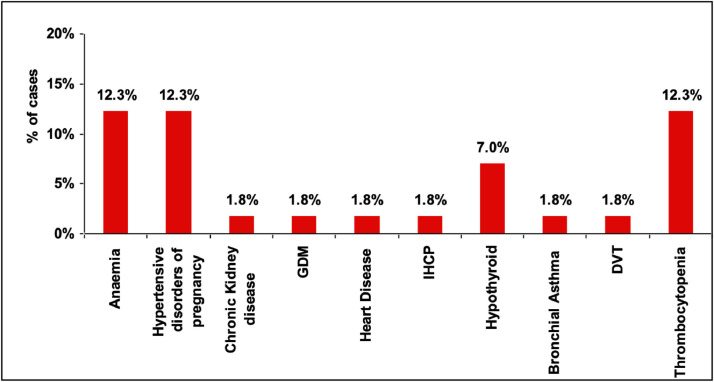
A total 1625 deliveries occurred during the study period. Among them, 348 women were tested for SARS-CoV-2; 57 (16.3%) were confirmed positive, and their data were analyzed. Among these, 15 (26.3%) were residents of containment areas. The mean ± SD age of the cohort was 26.71 ± 4.54 years. Most women (45; 78.9%) had a mild infection with one or two spikes of low-grade fever, cough, and/or diarrhea that resolved in 2–3 days. Three (5.2%) women had moderate symptoms (fever and breathlessness). Among women with spontaneous onset of labour, 13 (22.8%) experienced preterm delivery (Table 1 ). Common comorbidities were anemia, hypertensive disorders of pregnancy, and thrombocytopenia (Figure 1 ). Five (8.7%) women were admitted to the ICU, two of whom recovered.
Table 1.
Demographic, clinical, and hematologic characteristics (n = 57)
| Characteristics | No. (%)a |
|---|---|
| Age, mean ± SD, y | 26.7 ± 4.5 |
| Containment area | 15 (26.3) |
| Noncontainment area | 42 (73.7) |
| Term delivery | 44 (77.2) |
| Preterm delivery | 13 (22.8) |
| Asymptomatic | 10 (17.5) |
| Fever | 45 (78.9) |
| Cough | 3 (5.3) |
| Diarrhea | 2 (3.5) |
| Respiratory distress | 3 (5.3) |
| Hemoglobin, mean ± SD | 10.25 ± 1.98 |
| TLC, median (IQR) | 9700 (5325) |
| Platelets, median (IQR) | 16 570 (92 500) |
IQR: interquartile range; TLC: total leukocyte count.
Unless indicated otherwise.
Figure 1.
Comorbid conditions in pregnant women with COVID 19.
The common comorbidities were Anaemia, Hypertensive disorders of pregnancy and Thrombocytopenia.
Investigations
The hematologic profile in the cohort was within the normal range (Table 2 ). The mean Ct value was 31.45 in asymptomatic women, 25.84 in women with fever, and 28.91 in women with diarrhoea or respiratory distress. The association between clinical symptoms and Ct values was not statistically significant (P = 0.086; Table 2).
Table 2.
Mean Ct values of mothers and SARS-CoV-2–positive neonates
| Ct values of mothers (n = 57) |
Ct values of COVID-19–positive neonates (n = 5) |
|||
|---|---|---|---|---|
| na | Mean ± SD | nb | Mean ± SD | |
| Asymptomatic | 10 | 31.5 ± 6.7b | 1 | 35.3 ± 0 |
| Fever | 45 | 25.8 ± 7.2b | 4 | 25.1 ± 8.9 |
| Diarhoea | 2 | 28.9 ± 10.7 | 1 | 29.8 ± 0 |
| Respiratory distress | 3 | 28.9 ± 10.7 | 1 | 29.8 ± 0 |
| Ct values of available data | Ct values and symptomatology (P = 0.086) | |||
Ct: cycle threshold.
Number of women with specific symptom/asymptomatic with Cycle threshold values for Sarbeco subgenus.
Number of neonates(4/5 asymptomatic)with Cycle threshold values for Sarbeco subgenus.
Pregnancy Outcomes
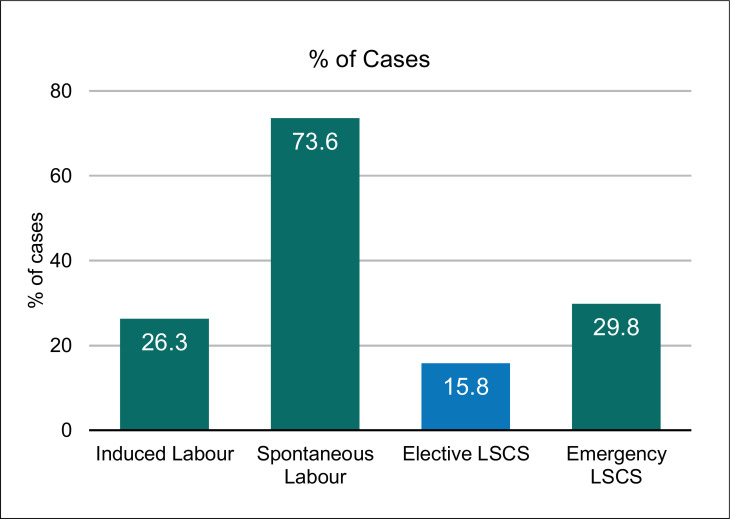
Labour was spontaneous in 42 (73.6%) women and induced with prostaglandins for obstetric indications in 15 (26.3%). Elective cesarean delivery was performed in nine (15.8%), and 17 (29.8%) underwent emergency cesarean delivery for various maternal-fetal indications, the most common being fetal distress (Figure 2 ). COVID-19–positive status did not influence mode of termination. Average length of hospital stay was 10–12 days. Only one woman who developed deep vein thrombosis and surgical site infection was discharged after 4 weeks.
Figure 2.
Labour outcomes of pregnant women with COVID-19.
Labour was spontaneous in 73.6% women. Vaginal delivery occurred in 54.3% while 29.8% women underwent emergency cesarean section.
Maternal Mortality
Three maternal mortalities (5.2%) were individually analyzed (Table 3 ).
Table 3.
Maternal deaths (n = 3)
| Maternal deaths |
|||
|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | |
| Age, y | 26 | 32 | 25 |
| Obstetric history | Primigravida | G3P3L2E 2 previous LSCS |
P3L3 |
| Presentation | In labour | Admitted for safe confinement at 32 wk | Immediate postoperative state |
| Comorbidity | Anemia, thrombocytopenia, hypothyroidism |
Chronic hypertension with medical renal disease, hypothyroidism, IHCP |
Referred after emergency hysterectomy at district hospital |
| Labour, delivery, or intervention | Emergency LSCS | LSCS (semi-emergency) for renal condition | Chemotherapy, uterine artery embolization |
| Length of stay | 4 d | 2 wk | 3 d |
| Cause of death | Pulmonary embolism | Acute on chronic renal failure | Septic shock |
| Neonatal outcome | Baby positive | Baby negative | Baby home delivered, not tested |
| Ct value of mother | E gene: 20.4 RDRp: 21.3 |
E gene: 38.9 RDRp: 36.5 |
E gene: 36.6 RDRp: 36.5 |
| Neutrophil-lymphocyte ratio | 1.5 | 2.3 | 3.5 |
LSCS: lower segment cesarean section; RDRp: RNA-dependent RNA polymerase.
Patient 1
The 26-year-old patient was primigravida with term pregnancy and moderate symptoms of COVID-19, confirmed by qRT-PCR. She presented in labour. She had hypothyroidism, moderate anemia, and thrombocytopenia. Ct values indicated moderate viral load. She was transfused necessary blood products and underwent emergency cesarean delivery for fetal distress. On postoperative day (POD) 1 she became disoriented, agitated, and confused and refused to feed her baby. She was put on non-invasive ventilation (FiO2 0.5) in the ICU and was administered third-generation antibiotics (piperacillin, clindamycin, and azithromycin) and prophylactic low-molecular-weight heparin. However, she died suddenly on POD3, preceded by severe breathlessness and cardiac arrest. Cause of death was pulmonary embolism (a complication of COVID-19).
Patient 2
The second patient was G3, P2, L2, 32 years old, and had previous two cesarean deliveries. Her pregnancy was at 325 weeks gestation with severe fetal growth restriction, chronic hypertension, stage 4 medical renal disease, hypothyroidism, and deranged liver enzymes. She was asymptomatic and was diagnosed as positive for SARS-CoV-2 on testing, which she underwent because she resided in a containment zone. Ct values were in the noninfective category, renal and liver function was deranged, and chest X-ray was normal. However, because of deteriorating renal function, after hemodialysis she underwent cesarean delivery under subarachnoid block on day 10 of diagnosis. She was administered renal disease–modifying drugs, antibiotic piperacillin and monitored for fluid balance. She developed severe metabolic/lactic acidosis on POD1. Despite fluid and electrolyte corrections and mechanical ventilation, she experienced cardiac arrest and died on day 11 of COVID-19–positive status. The cause of death was severe metabolic acidosis due to acute or chronic renal failure (COVID-19 related death).
Patient 3
Patient 3 was P3L3 and 25 years old (5 weeks postpartum). She underwent subtotal hysterectomy for gestational trophoblastic disease at a peripheral district hospital and was referred on POD1 because her surgery was complicated by severe hemorrhage. She was febrile and tested positive for SARS-CoV-2 on the day of admission. The Ct values were just above the cut off of 35. She underwent single-cycle chemotherapy (methotrexate) and uterine artery embolization. Multiple hemotransfusions were given, and she remained on ventilatory support and vasopressors in the postoperative period with an FiO2 of 0.7 and Positive end-expiratory pressure of 6. She was administered third-/fourth-generation antibiotics (meropenem, clindamycin, azithromycin, metrogyl) and prophylactic low-molecular-weight heparin. Her total leucocyte count was 18 200/mm3 and serum creatnin was 1.5 mg/L. Chest X-ray revealed bilateral basal infiltrates. She developed severe hypoxia and hypotension on POD7 (day 8 of COVID-19–positive status) followed by cardiac arrest and died. The cause of death was acute respiratory distress and septic shock (complications of COVID-19).
Neonatal Outcomes
Mean ± SD gestational age and birthweight were 36.72 ± 2.15 weeks and 2910 ± 663.12 g, respectively. Approximately one-fourth of the cohort was born preterm (13 of 56; 22.8%); 41% were female. Among 56 neonates tested, five (8.9%) were confirmed positive for SARS-CoV-2. One neonate was symptomatic and received respiratory support for 48 hours, and four were asymptomatic (Table 4 ). Two neonates (3.4%) were detected as positive for SARS-CoV-2 within 24 hours of birth. Three neonates had been separated from their mother and still tested positive (Table 5 ). Hence, in total, four neonates among the five who tested positive (80%) were either separated from their mother at birth or tested within the first 24 hours of birth. Viral load as assessed by the Ct of qRT-PCR was highest for neonates 3 and 4 (15.82 and 15.33). A neonate who tested positive on day 14 had a Ct above the detection level (36.37). All five neonates remained hemodynamically stable during their hospital stay and were discharged. One neonate's mother (Patient 1) died of COVID-19.
Table 4.
Neonatal outcomes (N = 56)
| Variables | No. (%) |
|---|---|
| Positive for SARS-Cov-2 | 5 (8.9) |
| Symptomatic | 1 (1.8) |
| Asymptomatic | 4 (7.1) |
| Mode of delivery (LSCS) | 26 (46.4) |
| Female sex | 23 (41.0) |
| Preterm (<37 wk) | 13 (22.8) |
| Birthweight, mean ± SD, g | 2910.00 ± 663.17 |
| Deaths (% of total) | 2 (3.5) |
| SARS-CoV-2–negative death | 2 |
LSCS: lower segment cesarean section.
Table 5.
Details of SARS-CoV2–positive neonates (n = 5)
| Neonate 1 | Neonate 2 | Neonate 3 | Neonate 4 | Neonate 5 | |
|---|---|---|---|---|---|
| Sex | Female | Female | Female | Male | Male |
| Gestational age, wk | 375 | 32 | 356 | 40 | 38 |
| Birthweight, g | 2680 | 1160 | 1800 | 3370 | 2750 |
| Mode of delivery | NVD | LSCS | NVD | NVD | NVD |
| Day of maternal positivity | At delivery | Positive twice (13 and 4 d before delivery), negative on day of LSCS | Day 2 postpartum |
Before delivery | Day 2 postpartum |
| Neonate age at testing | Within 24 h of birth | Day 14 of life | Day 4 of life | Day 1 of life | Day 4 of life |
| Intrauterine growth status | AGA | SGA | SGA | AGA | AGA |
| Onset of symptoms | Asymptomatic | Asymptomatic | Asymptomatic | Asymptomatic | Since birth |
| Isolation status | Yes (mother sick) | Yes (baby admitted to NICU) | No | No | Yes (baby had tachypnea) |
| Breastfeeding | No (mother in ICU) | Yes (EBM) | Yes | Yes | EBM |
| Management | Supportive | Supportive | Supportive | Supportive | CPAP |
| Duration of hospital stay, d | 8 | 15 | 10 | 8 | 10 |
| Timing of maternal symptom onset | COVID-19–positive before delivery; died on day 3 | Fever 7 d before delivery | Fever on day 2, mild symptoms | COVID-19–positive at delivery | Fever on day 2 |
AGA: appropriate for gestational age; CPAP: continuous positive airway pressure therapy; EBM: expressed breast milk; ICU: intensive care unit; LSCS: lower segment cesarean section; NICU: neonatal intensive care unit; NVD: normal vaginal delivery; SGA: small for gestational age.
DISCUSSION
The current study summarizes the maternal and neonatal profiles for 56 mother and baby dyads and one postpartum woman. The majority of the women were from the noncontainment zone and had undetected infection owing to the ICMR guideline of no universal testing in the general population. The mean maternal age (26 years) was less than in other studies—36 years by Smith et al.12 and 29.7 years by Breslin et al13—possibly because India has a large youth population. In the current study, 73.6% (inclusive of all preterm deliveries) presented in spontaneous labour, and 26.3% were induced; hence, vaginal delivery rates (45.6%) were marginally higher than cesarean delivery. A systematic review of 33 studies described the outcomes of 385 pregnant women with COVID-19 with gestational age at birth ranging from 30 to 41 weeks and a preterm birth rate of 15.2%.14 Although many of the preterm deliveries were iatrogenic (maternal indications and fetal distress), indications were not clear in others.14 The majority of cesarean deliveriess in the current study were performed for fetal distress (42.5%), which was similar to other studies.7 , 15 , 16 This could suggest an underlying pathology of placental insufficiency/hypoxia leading to fetal distress in COVID-19 infection.
The most common presenting symptom in the current study was low-grade fever in 78.9%, similar to other studies.1 , 5 , 17 , 16 Asymptomatic women (10 of 57; 17.4%) were incidentally detected because they resided in a containment zone. Three women (5.2%) had moderate infection, one of whom progressed to the severe category and died. Early reports suggest that the severity category proportions in the pregnant population are similar to those described for non-pregnant adults with COVID-19 infection (approximately 80% mild, 15% severe, and 5% critical disease).12 Other symptoms, including nasal congestion, rash, sputum production, headache, malaise, and loss of appetite, were reported in less than 5% of cases.14 It has been suggested that the limited data currently available do not indicate that pregnant individuals are at an increased risk of infection, severe morbidity, or mortality compared with non-pregnant individuals in the general population.18
In the current cohort, five women (8.7%) who were admitted to the ICU had comorbidities of moderate anemia, severe thrombocytopenia, chronic hypertension with medical renal disease, and malignancy. Whether COVID-19 increases the risk of severe morbidity and mortality in high-risk pregnancies is a question to be answered. A review of 108 pregnancies did not report any maternal deaths.17 However, Breslin et al.13 reported severe morbidity in mothers with COVID-19 who had a high body mass index and complicated medical history. Hantoushzadeh et al.19 reported a case series of nine pregnant women diagnosed with severe COVID-19 in their second or third trimester. They reported seven deaths; one patient remained critically ill and ventilator-dependent, and one recovered after prolonged hospitalization. Five of the women who died were over 35, older than current study population. Most of the women in the study19 had comorbidities such as obesity, gestational diabetes, and hypothyroidism, and all deaths were due to severe COVID-19. The authors also reported that the outcomes of these women were more severe than the outcomes of high- and low-risk familial/household members.19 Association with comorbidities appears to worsen pregnancy outcomes in women with COVID-19.
La Scola et al.10 reported a strong correlation between Ct value and sample infectivity in a cell culture model in 183 samples. They concluded that patients with Ct values ≥34 do not excrete infectious viral particles.10 In the current study, the cycle threshold value of <35 was interpreted as positive for SARS-CoV-2 RNA. However, no significant association was found between clinical symptoms and Ct values (P = 0.08), likely because the majority of patients had mild symptoms. Wang et al.20 reported mean Ct values for different body compartments to be more than 30, except for nasal swabs, which had a mean of 24.3, indicating high viral load in these specimens.20
In the current study, none of the SARS-CoV-2–positive neonates developed severe manifestations of the disease, and they were subsequently discharged. This suggests a good neonatal outcome in pregnant women with SARS-CoV-2 infection. Zaigham et al.17 reported one positive neonate among 75 tested for SARS-CoV-2 who was clinically well but had transient lymphocytopenia and deranged liver function. Fan et al.21 reported two neonates with mild lymphocytopenia and radiologic findings of pneumonia, although both were clinically well and recovered fully. Thus, we cannot exclude the possibility that fetuses and newborns might show a response, often subclinical, to maternal infection; hence, vertical maternal-fetal transmission cannot be ruled out. This view has been seconded by a recently published study22 that reported three infants born by cesarean delivery who tested positive for SARS-CoV-2 2 days after birth. However, Schwartz et al.,23 in their analysis of 38 infected pregnancies, did not find any evidence for intrauterine transmission. Furthermore, neonates can acquire SARS-CoV-2 in the postpartum period (horizontal transmission), similar to adults. Positive neonate 3 who tested positive for infection beyond 48 hours and after maternal infection was confirmed points towards this route of transmission. The viral load of the three neonates in the present study was comparable with the Ct reported for adults, thus raising the possibility that despite having a viral load similar to that in adults, manifestations are milder in neonates.24
Strength of Study
This was a single-centre, descriptive study with no selection bias. RT-PCR was used for confirming positive results, which is the gold standard. The study substantiates observations of the Ct for specific SARS-CoV-2 viral loads, which makes it more robust. The same diagnostic criteria have been used for neonates. This study can contribute important new information regarding viral load and infectivity. It adds to the sparse data presently available regarding COVID-19 in pregnant patients in developing countries.
Limitations
This is a retrospective study; hence, we could not obtain additional hematologic and radiologic data to correlate with the severity of infection. We were unable to test samples of the placenta, amniotic fluid, and neonatal blood for antibodies for evidence of vertical transmission.
CONCLUSION
The majority of pregnant women with COVID-19 had mild disease and recovered with good perinatal outcomes. Pregnant women with comorbidities and COVID-19 may have an increased risk of severe morbidity and mortality. Fetal distress, which was the most common indicator for cesarean delivery, raises a possible area of research on placental insufficiency in pregnancy with COVID-19. The Ct for SARS-CoV-2 signifies the viral load and can be used to determine the degree of infectivity. The maternal, fetal, and neonatal outcomes of those pregnant women infected in late pregnancy were achieved with intensive, active management, which might be the best practice in the absence of more accurate data. Long-term outcomes and potential mother-to-child vertical/horizontal transmission need further research.
Footnotes
Disclosures: The authors declare they have nothing to disclose.
All authors have indicated they meet the journal's requirements for authorship.
REFERENCES
- 1.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu H, Zhong L, Deng J. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization; Geneva: 2020. Country & technical guidance – coronavirus disease (COVID-19)https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance.mohfw.gov.in Available at: [Google Scholar]
- 4.Ministry of Health and Family Welfare. Available at: mohfw.gov.in. Accessed on June 8, 2020.
- 5.Yan J, Guo J, Fan C. Coronavirus disease 2019 (COVID-19) in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223:111. doi: 10.1016/j.ajog.2020.04.014. e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiao J. What are the risks of COVID 19 infection in pregnant women. Lancet. 2020;395:760–762. doi: 10.1016/S0140-6736(20)30365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Guo J, Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy S. Newborn baby tests positive for coronavirus in London. Available at: https://www.theguardian.com/world/2020/mar/14/newborn-baby-tests-positive-for-coronavirusin-london. Accessed on March 15, 2020.
- 9.Li Y, Zhao R, Zheng S. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020;26:1335–1336. doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Scola B, Le Bideau M, Andreani J. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020;39:1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alagarasu K, Choudhary ML, Lole KS. Evaluation of RdRp& ORF-1b-nsp14- based real-time RT-PCR assays for confirmation of SARS-CoV-2 infection: an observational study. Indian J Med Res. 2020;151:483–485. doi: 10.4103/ijmr.IJMR_1256_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith V, Seo D, Warty R. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breslin N, Baptiste C, Miller R. COVID-19 in pregnancy: early lessons. Am J Obstet Gynecol. 2020;2:100–111. doi: 10.1016/j.ajogmf.2020.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elshafeey F, Magdi R, Hindi N. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int J Gynecol Obstet. 2020;150:47–52. doi: 10.1002/ijgo.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Liu F, Li J. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect. 2020;80:e7–e13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang I, Jiang Y, Wei M. Analysis of pregnancy outcomes of pregnant women during the epidemic of new coronavirus pneumonia in Hubei. Zhonghua Fu Chan KeZaZhi. 2020;55:166–171. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 17.Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99:823–829. doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan GA, Purandare NC, McAuliffe FM. Clinical update on COVID-19 in pregnancy: a review article. J Obstet Gynaecol Res. 2020;46:1235–1245. doi: 10.1111/jog.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hantoushzadeh S, Shamshirsaz AA, Aleyasin A. Maternal death due to COVID-19. Am J Obstet Gynecol. 2020;223:109. doi: 10.1016/j.ajog.2020.04.030. e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan C, Lei D, Fang C. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin Infect Dis. 2020;ciaa226 doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng L, Xia S, Yuan W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174:722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes [e-pub ahead of print]. Arch Pathol Lab Med doi:10.5858/arpa.2020-0901-SA, accessed November 8, 2020. [DOI] [PubMed]
- 24.Xu T, Chen C, Zhu Z. Clinical features and dynamics of viral load in imported and nonimported patients with COVID-19. Int J Infect Dis. 2020;94:68–71. doi: 10.1016/j.ijid.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]