Abstract
Objectives
To analyze the cerebrospinal fluid (CSF) of patients with SARS-CoV-2 infection and neurological manifestations to provide evidence for the understanding of mechanisms associated with central nervous system (CNS) involvement in COVID-19.
Methods
Patients (n = 58) were grouped according to their main neurological presentation: headache (n = 14); encephalopathy (n = 24); inflammatory neurological diseases, including meningoencephalitis (n = 4), acute myelitis (n = 3), meningitis (n = 2), acute disseminated encephalomyelitis (ADEM) (n = 2), encephalitis (n = 2), and neuromyelitis optica (n = 1); and Guillain-Barré syndrome (n = 6). Data regarding age, sex, cerebrovascular disease, and intracranial pressure were evaluated in combination with CSF profiles defined by cell counts, total protein and glucose levels, concentration of total Tau and neurofilament light chain (NfL) proteins, oligoclonal band patterns, and detection of SARS-CoV-2 RNA.
Results
CSF of patients with inflammatory neurological diseases was characterized by pleocytosis and elevated total protein and NfL levels. Patients with encephalopathy were mostly older men (mean age of 61.0 ± 17.6 years) with evidence of cerebrovascular disease. SARS-CoV-2 RNA in CSF was detected in 2 of 58 cases: a patient with refractory headache, and another patient who developed ADEM four days after onset of COVID-19 symptoms. Three patients presented intrathecal IgG synthesis, and four had identical oligoclonal bands in CSF and serum, indicating systemic inflammation.
Conclusion
Patients with neurological manifestations associated with COVID-19 had diverse CSF profiles, even within the same clinical condition. Our findings indicate a possible contribution of viral replication on triggering CNS infiltration by immune cells and the subsequent inflammation promoting neuronal injury.
Keywords: COVID-19, SARS-CoV-2, Cerebrospinal fluid, Neurofilament light protein, Total Tau protein, Oligoclonal bands
Introduction
A plethora of respiratory viruses constantly circulate among human populations worldwide, and they are associated with clinical pictures ranging from common colds to severe pneumonia requiring hospitalization. Occasionally, the emergence of new respiratory viruses causes epidemics or pandemics (Sloots et al., 2008), as several of them have neuroinvasive capacities (Desforges et al., 2019), although they are rarely associated with isolated central nervous system (CNS) infection, except during outbreaks (Bookstaver et al., 2017).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen of coronavirus disease 2019 (COVID-19), which is clinically characterized by fever, myalgia, diarrhea, and respiratory illness (Huang et al., 2020, Mao et al., 2020), has been associated with neurological syndromes such as meningoencephalitis, ischemic stroke, encephalopathy, Guillain-Barré syndrome (GBS), acute necrotizing encephalopathy, and acute disseminated encephalomyelitis (ADEM) (Helms et al., 2020, Moriguchi et al., 2020, Oxley et al., 2020, Parsons et al., 2020, Poyiadji et al., 2020, Toscano et al., 2020). Possible mechanisms implicated in these neurological conditions are neuronal injury associated with direct virus infection, hyperinflammation syndrome associated with cytokine storm, a para- or post-infectious inflammatory disease, an immune-mediated disease, or a secondary process due to severe effects of a systemic disorder (sepsis, hyperpyrexia, hypoxia, hypercoagulability, critical illness) (Gris et al., 2020, Mehta et al., 2020b, Zubair et al., 2020). Several biomarkers of COVID-19 severity have been identified in blood, including C-reactive protein, d-dimer, lactate dehydrogenase, interleukin-6, and ferritin (Ciaccio and Agnello, 2020), but less evidence is available for cerebrospinal fluid (CSF) biomarkers.
In this study, we report the analysis of the CSF of 58 consecutive patients with distinct neurological conditions associated with COVID-19, including acute inflammatory disorders, highlighting the relationship of neurological severity with inflammatory CSF patterns and the levels of biomarkers of neuronal death, total Tau protein, and neurofilament light chain (NfL).
Methods
Study population and CSF analysis
This study represents a case series of patients with COVID-19 admitted between April and June 2020 in reference hospitals in the cities of Rio de Janeiro (Hospital Federal dos Servidores do Estado and Instituto Nacional de Infectologia Evandro Chagas) and Niterói (Complexo Hospitalar Niterói), Brazil, who presented with neurological manifestations (n = 60). All the included patients had COVID-19 confirmed by detection of SARS-CoV-2 RNA in nasopharyngeal swabs by quantitative reverse transcription PCR (RT-qPCR). The patients underwent lumbar puncture, and CSF was immediately processed for determining cell counts, total protein, and glucose levels. CSF samples were also aliquoted and stored at −80 °C for further use.
CSF was investigated for the presence of SARS-CoV-2 RNA using the Biomanguinhos (E + P1) RT-qPCR kit (FIOCRUZ, Brazil) and the XGEN Master COVID-19 (Mobius, Brazil), and evaluated by PCR for other neuropathogens, including arboviruses (Zika, Dengue and Chikungunya viruses), human herpesviruses (HSV-1/2, VZV, EBV, CMV and HHV-6) and those detected by the XGEN Viral Meningitis Panel (Mobius, Brazil) or the FilmArray® Meningitis/Encephalitis Panel (bioMérieux, Brazil). Two patients had CSF positive for pathogens other than SARS-CoV-2, one for herpes simplex virus and another for Cryptococcus sp., and therefore, they were excluded from the study.
The detection of oligoclonal bands (OCB) in the CSF was performed by isoelectric focusing on the comparison between paired CSF and serum samples, as described elsewhere (Andersson et al., 1994). OCB patterns were defined as follows: type 1, no OCB in CSF and serum; type 2, oligoclonal IgG bands only in CSF; type 3, OCB in CSF and serum with additional bands in CSF; type 4, identical OCB in CSF and serum; and type 5, monoclonal bands in CSF and serum. Types 2 and 3 indicate intrathecal IgG synthesis, type 4 shows a systemic, ongoing inflammatory process, and type 5 shows systemic paraproteinemia.
Quantification of total Tau protein and NfL in CSF
Enzyme-linked immunosorbent assays (ELISA) were used to determine the CSF concentration of total Tau protein (Human Tau Total ELISA kit, Invitrogen, Austria) and NfL (Human NEFL ELISA kit, FineTest, China), according to the manufacturer’s instructions.
Statistical analysis
The analysis was performed with data from 58 patients using GraphPad Prism v.5 and R software v.3.6.1. Descriptive statistics included the frequency of categorical variables and summary measures of quantitative variables: mean, median, and interquartile range (IQR). Patients were distributed into four clinical groups to overcome the small number of cases: (1) headache; (2) encephalopathy; (3) inflammatory neurological diseases (IND), which included ADEM, encephalitis, meningitis, meningoencephalitis, myelitis, and neuromyelitis optica; and (4) GBS. The chi-square test was used to estimate the differences in sex, intracranial opening pressure, pleocytosis, and increased CSF total protein levels between these groups. Differences in the frequency of qualitative variables between two groups were determined by Fisher’s exact test. Kolmogorov–Smirnov test was used to determine the normal distribution of quantitative data. Comparative analysis of data with normal distribution was performed using ANOVA with Bonferroni post-test for multiple comparisons, and nonparametric variables were evaluated with the Kruskal–Wallis test. Posthoc analysis was performed with Dunn’s test with Bonferroni correction for multiple comparisons, where indicated. Spearman’s rank correlation coefficients were calculated to determine associations between variables. P value was considered significant at 0.05. The contribution of qualitative and quantitative parameters, including age, sex, evidence of cerebrovascular disease (CVD), CSF cell counts, total protein, glucose, total Tau and NfL levels in explaining neurological outcomes was evaluated by factor analysis of mixed data (FAMD) using the FactoMineR package of R software.
Results
Neurological diseases associated with COVID-19 and differences related to age and sex
Enrolled individuals had a mean age of 51.6 ± 18.0 years, and this population comprised 33 women (56.9%) and 25 men (43.1%) (Table 1 ). Encephalopathy was the most frequent clinical condition, representing 41.4% (n = 24) of the cases, followed by refractory headache (n = 14; 24.1%), GBS (n = 6; 10.3%), meningoencephalitis (n = 4; 6.9%), and myelitis (n = 3; 5.2%). ADEM, encephalitis, and meningitis accounted for 3.5% (n = 2) of the cases, and neuromyelitis optica was reported in only one patient (1.7%). Seven out of 58 individuals showed CVD in brain MRI (12.1%), six with intracranial hemorrhage and one with a transient ischemic attack, and in five of them, encephalopathy was also diagnosed. Among patients who developed GBS (n = 6), two had a clinical picture compatible with Miller-Fisher syndrome.
Table 1.
Clinical data and CSF profile of patients with COVID-19 and neurological alterations.
| Neurological manifestations |
||||||
|---|---|---|---|---|---|---|
| Characteristicsa | Total (n = 58) | Headache (n = 14) |
Encephalopathy (n = 24) |
Inflammatory neurological diseasesf (n = 14) |
GBS (n = 6) |
P valueg |
| Age (years)b | 51.6 ± 18.0 | 43.3 ± 12.1 | 61.0 ± 17.6 | 45.4 ± 15.3 | 47.5 ± 23.1 | 0.006 |
| Male :Female proportion | 1:1.3 | 1:3.7 | 2:1 | 1:3.7 | 1:1 | 0.012 |
| ICP (mmH2O) | 20 | 21 | 19 | 20 | 23 | 0.225 |
| [15–28] | [17.5–30] | [13–22.5] | [15–33] | [18.3–32.5] | ||
| Intracranial hypertensionc | 16/52 (30.8) | 5/13 (38.5) | 4/22 (18.2) | 4/11 (36.4) | 3/6 (50.0) | 0.362 |
| CSF analysis | ||||||
| Cell counts (cells/mm3) | 2 | 1 | 2 | 16 | 1 | 0.002 |
| [1–4] | [1–2] | [1–3] | [2–57] | [1–2.5] | ||
| Pleocytosisd | 10/58 (17.2) | 0/14 (0.0) | 1/24 (4.2) | 9/14 (64.3) | 0/6 (0.0) | <0.0001 |
| Total protein (mg/dL) | 35.0 | 26.5 | 35.5 | 40.5 | 51.0 | 0.037 |
| [25.8–48.0] | [21.3–36.0] | [25.5–47.8] | [31.0–63.5] | [29.8–76.3] | ||
| Increased protein levele | 16/58 (27.6) | 1/14 (7.1) | 7/24 (29.2) | 5/14 (35.7) | 3/6 (50.0) | 0.177 |
| Glucose (mg/dL) | 58.5 | 58.5 | 64.5 | 52.5 | 60.0 | 0.149 |
| [51.0–76.3] | [52.5–62.3] | [52.0–92.8] | [45.3–59.3] | [47.5–77.8] | ||
| Total Tau (pg/mL) | 318.3 | 305.8 | 371.1 | 322.8 | 156.0 | 0.101 |
| [173.0–457.4] | [214.0–381.0] | [273.5–576.9] | [172.4–445.5] | [103.6–261.5] | ||
| NfL (pg/mL) | 1694 | 1531 | 1714 | 3068 | 1192 | 0.253 |
| [1091–3358] | [1209–2198] | [974–2776] | [1410–6846] | [984–2972] | ||
Notes. CSF, cerebrospinal fluid; ICP, intracranial pressure; NfL, neurofilament light chain; ADEM, acute disseminated encephalomyelitis; GBS, Guillain-Barré syndrome.
Median values and interquartile ranges (inside brackets) are shown for CSF cell counts, total protein, glucose, total Tau, and NfL. Statistical analysis was performed with the Kruskal-Wallis test, and posthoc analysis was performed with Dunn’s test with Bonferroni correction for multiple comparisons. Differences in the proportion of males:females between groups, in addition to differences in the frequency (inside parenthesis) of intracranial hypertension, pleocytosis, and increased CSF protein levels were determined by the chi-square test.
Mean ± standard deviation values are shown. Statistical analysis was performed using ANOVA with Bonferroni post-test for multiple comparisons.
Intracranial hypertension was considered for patients with ICP ≥ 25 mmH2O.
Pleocytosis was considered when CSF cell counts were >5 cells/mm3.
CSF total protein levels were considered increased when values were >45 mg/dL.
Inflammatory neurological diseases included ADEM (n = 2), encephalitis (n = 2), meningitis (n = 2), meningoencephalitis (n = 4), myelitis (n = 3), and neuromyelitis optica (n = 1).
P value was considered significant at 0.05 (in bold).
Patients with encephalopathy were older than the other clinical groups, with a mean age of 61.0 ± 17.6 years (Table 1). Patients with COVID-19 and distinct neurological outcomes also presented differences in the distribution of cases according to sex (Table 1). Females were 3.7-times more frequent among patients with headache and IND, while men were twice more frequent in the group with encephalopathy. No difference according to sex was observed among patients with GBS (Table 1).
CSF profile of patients with SARS-CoV-2 infection presenting with neurological manifestations
Data regarding intracranial pressure (ICP) were available for 52 individuals. No difference was observed in ICP median values between the four groups with distinct patterns of neurological manifestations, as shown in Table 1. In most cases, ICP ranged between normal and slightly elevated values. However, one-third of patients showed high ICP (≥25 mmH2O), although this characteristic was not associated with a specific neurological picture (Table 1). Two patients with ICP ≥ 30 mmH2O, one with ADEM and another with persistent headache as the main neurological condition, had detectable SARS-CoV-2 RNA levels in CSF. However, no significant changes were present in their CSF findings.
Conversely, cases of IND were associated with pleocytosis, reflecting the elevated CSF cell counts as compared to the other groups (Table 1). These patients showed a median of 16 leukocytes/mm3 (IQR: 2–57 cells/mm3), predominantly constituted by mononuclear cells (>80%), and those with meningoencephalitis (n = 4) and meningitis (n = 2) accounted for the highest cell counts, ranging from 8 to 396 cells/mm3.
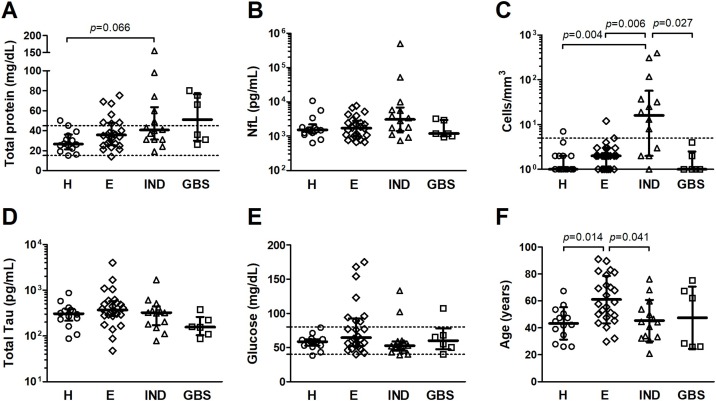
None of the patients presented CSF glucose levels <40 mg/dL, and no difference was observed between the groups (Table 1). Increased protein levels (>45 mg/dL) were detected in 7.1% of the patients with headache; 29.2% and 35.7% of individuals with encephalopathy and IND, respectively; and in half of patients with GBS (Table 1). Although a moderate difference was observed in median total protein levels in CSF (Table 1, p = 0.037), particularly between patients with IND and headache, this was ruled out after correction for multiple comparisons (Figure 1a, Dunn’s test with Bonferroni correction, p = 0.066).
Figure 1.
CSF analysis of patients with COVID-19 and neurological manifestations. Patients were divided into four groups: headache (H); encephalopathy (E); inflammatory neurological diseases (IND), including acute disseminated encephalomyelitis, encephalitis, meningoencephalitis, meningitis, myelitis, and neuromyelitis optica; and Guillain-Barré syndrome (GBS). Comparison analysis was performed for (A) total protein, (B) neurofilament light chain (NfL), (C) cell counts, (D) total Tau protein, (E) glucose, and (F) age. Median values (line) and interquartile range (bars) are shown in (A, B, C, D, and E), and statistical analysis was performed with Dunn’s test with Bonferroni correction for multiple comparisons. Mean values (line) and standard deviation (bars) are shown in (F) and statistical analysis was performed with Bonferroni test for multiple comparisons. Differences with p-value < 0.05 were considered significant. Dashed lines indicate the reference values for (A) total protein (15–45 mg/dL), (C) CSF cell counts (<5 cells/mm3), and (E) glucose (40–80 mg/dL).
Quantification of CSF total Tau protein and NfL was used to determine the extension of neuronal injury. No difference was observed in their concentration between the groups (Table 1). However, patients with IND showed distinct degrees of neuronal damage, as illustrated by the broad variation in NfL levels, ranging from 740 to 500,000 pg/mL (Figure 1b). Analysis of this group according to low and high NfL levels by using the median concentration in this group as a cut-off, demonstrated that no specific condition was associated with high NfL levels in the CSF. Instead, high NfL levels among these patients were associated with intracranial hypertension (Fisher’s exact test, p = 0.015). All patients with relatively low NfL concentration showed ICP ≤20 mmH2O, whereas 4 in 5 patients with high NfL levels and available ICP data presented with intracranial hypertension (ICP ≥ 25 mmH2O).
Analysis of demographic and CSF data in the outcome of neurological diseases in COVID-19
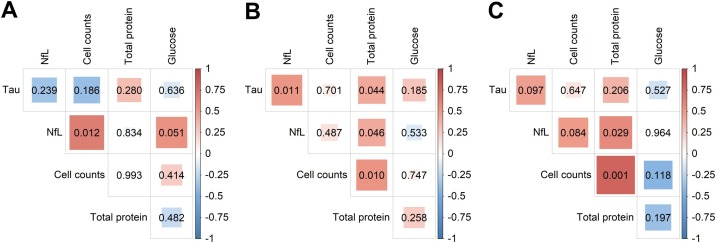
Correlation analysis showed that increased CSF cell counts were associated with higher NfL levels in patients with headache (Figure 2 a, r = 0.647; p = 0.012). In the group with encephalopathy, increased CSF total protein levels were followed by higher levels of total Tau protein (r = 0.414; p = 0.044) and NfL (r = 0.412; p = 0.046), in addition to cell counts (r = 0.515; p = 0.001) (Figure 2b), although the CSF of these patients did not exhibit pleocytosis (Figure 1c). Moreover, Tau and NfL levels concurrently increased in the CSF of patients with encephalopathy (Figure 2b, r = 0.515; p = 0.011). Among patients with IND, higher NfL levels in the CSF was associated with higher ICP (r = 0.817; p = 0.002) and total protein (r = 0.581; p = 0.030), and increased protein levels were in turn associated with pleocytosis (r = 0.800; p = 0.001) (Figure 2c). Unfortunately, the small number of patients with GBS hindered the correlation analysis in this group.
Figure 2.
Correlation analysis of CSF data from patients with COVID-19. Correlation between CSF cell counts, total protein, glucose, Tau protein, and neurofilament light chain (NfL) levels was evaluated with Spearman’s rank of correlation in patients with SARS-CoV-2 infection and presenting with (A) headache (n = 14), (B) encephalopathy (n = 24), and (C) inflammatory neurological diseases (n = 14). Spearman’s rank correlation coefficient in bivariate analyses is represented by the color intensity of squares, which are shown in red for positive correlation and in blue for an inverse correlation. The size of squares corresponds to the level of statistical significance of the correlation (shown inside the squares), and associations with p-values < 0.05 were considered significant.
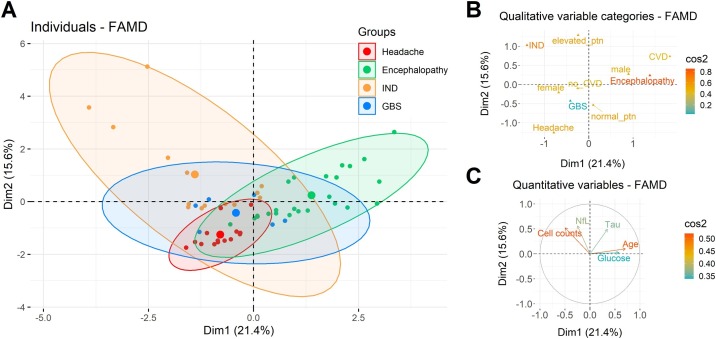
Thus, a mixed analysis of demographic data and CSF profile showed that 36.2% (n = 21) of patients with COVID-19 and well-defined neurological diseases presented similar characteristics, as shown by intersection between the groups (Figure 3 a). On the other hand, patients with IND and encephalopathy displayed distinct profiles, in which elevated CSF total protein levels, cell counts, and NfL contributed for the definition of IND cases. At the same time, age, CVD, and male sex characterized encephalopathy cases (Figure 3b and c).
Figure 3.
Characterization of neurological diseases associated with COVID-19. Qualitative parameters, such as age, sex, cerebrovascular disease (CVD), and elevated cerebrospinal fluid (CSF) total protein, and quantitative variables, including CSF cell counts, glucose, total Tau protein and neurofilament light chain (NfL) levels were evaluated by factor analysis of mixed data (FAMD) method. (A) Individuals (dots) were distributed into four main groups discriminated by colors according to their neurological outcome: headache, encephalopathy, inflammatory neurological diseases (IND), and Guillain-Barré syndrome (GBS). Correlation circles of quantitative variables (ellipses) and central points for the distribution of cases in a category (large dots) are shown for each group. The contribution of (B) qualitative and (C) quantitative variables to explain the neurological diseases associated with COVID-19 are shown.
The local B-cell response accompanying CNS inflammation was evaluated by investigating oligoclonal bands (OCB) in 38 of 58 patients. Most patients (n = 31; 81.6%) had no OCB in CSF and serum (type 1); four individuals (10.5%) showed a type 4 pattern, with identical OCB in CSF and serum, and three (7.9%) were identified with a type 2 pattern, with OCB restricted to the CSF, indicating intrathecal IgG synthesis. All six patients with GBS had no OCB in CSF or serum (type 1), and patients with OCB type 2 and 4 patterns were not restricted to a specific clinical picture. Patients with intrathecal IgG synthesis had normal CSF findings, including normal ICP, CSF cell counts, protein, and glucose levels. This group comprised one patient with encephalopathy, who presented a cerebrovascular accident, one with refractory headache, and the third one with myelitis. The group of four patients showing evidence of systemic immune activation (OCB type 4) included two patients with encephalopathy, one with headache and another with myelitis. They also exhibited normal CSF cell counts, protein, and glucose levels, except for a patient, who had intracranial hypertension (ICP = 26 mmH2O), increased CSF glucose levels (123 mg/dL), and high concentration of Tau (1684 pg/mL) and NfL (2889 pg/mL).
Discussion
As of July 31, 2020, more than 17 million cases of COVID-19 were confirmed worldwide (Dong et al., 2020). Hence, the spectrum of neurological diseases associated with SARS-CoV-2 infection is expanding, including ADEM (Parsons et al., 2020, Zanin et al., 2020), meningoencephalitis (Bernard-Valnet et al., 2020, Dogan et al., 2020, Moriguchi et al., 2020), encephalitis (Pilotto et al., 2020, Ye et al., 2020), GBS (Alberti et al., 2020, Coen et al., 2020, Juliao Caamaño and Alonso Beato, 2020, Ottaviani et al., 2020, Toscano et al., 2020, Zhao et al., 2020), and encephalopathies (Garg et al., 2020). However, conducting clinical studies including CSF sampling and the collection of detailed clinical and laboratory data in the setting of COVID-19 pandemic has been challenging. Here, we evaluated the CSF findings in patients with distinct neurological diseases in the context of SARS-CoV-2 infection.
Individuals with encephalopathy and those with an inflammatory nervous system syndrome had the most distinct profiles. Encephalopathy is characterized by diffuse brain dysfunction, which typically manifests with altered consciousness, and may be followed by seizures, headache, or pyramidal signs. Among patients with encephalopathy, elevated CSF total protein positively correlated with total Tau levels, a biomarker of death of cortical nonmyelinated neurons (Trojanowski et al., 1989). This was likely associated with the increased frequency of CVD, which has been shown in COVID-19 (Li et al., 2020, Mao et al., 2020), or other events leading to encephalopathy, which can originate from hypoxia, sepsis, CNS inflammation, or even from the inability of the body to maintain normal brain activity following multiple organ dysfunction. In our study, encephalopathy was more frequent among the elderly and men. In a recent review, encephalopathy in COVID-19 was shown to be more common among individuals with more than 50 years of age (Garg et al., 2020), which corroborates our data.
Patients with COVID-19 and IND showed a broad spectrum of clinical syndromes, such as encephalitis, meningitis, meningoencephalitis, ADEM, myelitis, and neuromyelitis optica. The shared inflammatory origin of these manifestations and the involvement of distinct CNS structures (brain, meninges, spinal cord) lead to a great diversity in CSF profiles. Pleocytosis with predominant mononuclear cells was frequent, particularly in cases with meningeal involvement. A mild increase in total protein concentration and high NfL levels, which is a biomarker for injury of myelinated axons (Khalil et al., 2018), were present mainly in cases with intracranial hypertension, as a sign of an active and exacerbated inflammatory process.
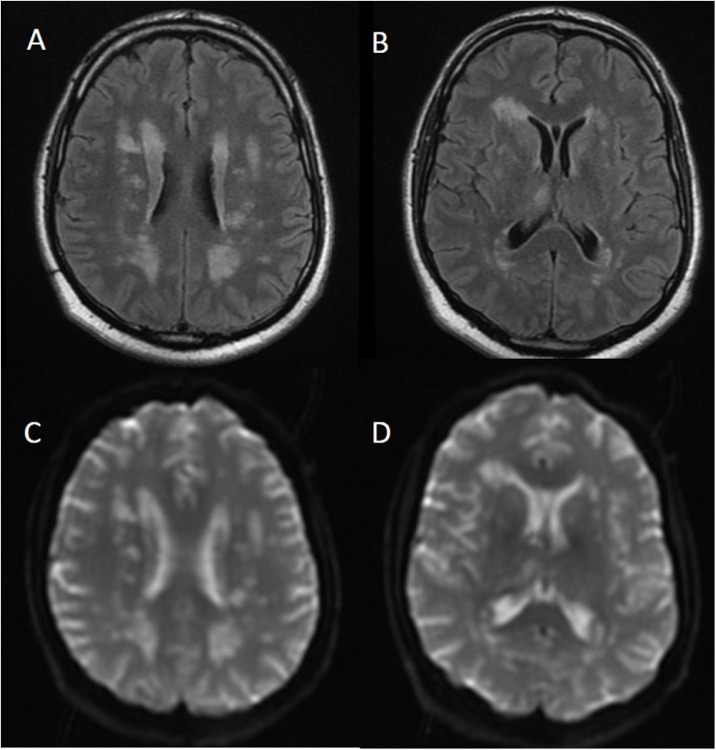
Other aspects of ADEM associated with SARS-CoV-2 infection in addition to CSF findings indicate possible dynamics of neurological disease development in COVID-19. ADEM is usually associated with pleocytosis, but OCB are infrequent (Scolding, 2001). A case of ADEM with normal CSF cell counts and protein levels, mild glucose elevation, with neurological presentation preceding mild respiratory symptoms was recently reported (Abdi et al., 2020). Here, one case of ADEM was also associated with normal CSF findings but with detectable SARS-CoV-2 RNA, although close to the limit of detection of the RT-qPCR. Four days after COVID-19 symptoms, this patient presented with acute confusion state, dysarthria, and intracranial hypertension. Brain MRI findings revealed diffuse, hyperintense lesions both in the deep hemispheric and periventricular white matter (Figure 4 ). High Tau and NfL levels corroborated with CNS lesions. This patient was treated with hypertonic saline and methylprednisolone 1 g IV daily for five days and discharged after 17 days with complete recovery.
Figure 4.
Brain MRI of a patient with ADEM and detectable SARS-CoV-2 RNA in the cerebrospinal fluid. Hyperintense lesions on white matter substance in the deep hemispheric and periventricular areas both on (A, B) fluid-attenuated inversion recovery (FLAIR) and (C, D) apparent diffusion coefficient (ADC) map. Brain imaging was performed on hospital admission (day 1), four days after the onset of COVID-19 typical symptoms.
ADEM is an immune-mediated demyelinating monophasic disorder that affects the brain and occasionally the spinal cord typically arising between 2 and 4 weeks after viral infections or immunizations (Jacob and Weinshenker, 2008, Gray and Gorelick, 2016). Thus, the temporal relationship between COVID-19 symptoms and the onset of ADEM seems to be shorter than the classical picture described for other viral infections. Another patient with ADEM in our study presented a mild increase in CSF cell counts and total protein, and elevated Tau and NfL concentration, compatible with demyelination. Although ADEM is more common among pediatric patients, especially under 10 years of age, it can occur at any age (Tenembaum et al. 2007). However, cases of ADEM associated with SARS-CoV-2 infection to date, including two in this study, have been described in adults (Abdi et al., 2020, Demirci Otluoglu et al., 2020, Parsons et al., 2020, Zanin et al., 2020). This discrepancy may reflect the fact that children with COVID-19 are more likely to be asymptomatic or have mild-to-moderate illness (Mehta et al., 2020a, Rajapakse and Dixit, 2020).
CSF studies are essential to elucidate the etiology of myelitis. The most common viral agents of acute myelitis are human herpesviruses and enteroviruses (Jacob and Weinshenker, 2008), and CSF analysis usually shows mononuclear pleocytosis and total protein elevation; OCB may be detected (Jacob and Weinshenker, 2008), although they are absent in most cases (Scolding, 2001). In our study, two of the three cases of SARS-CoV-2-associated myelitis presented OCB in CSF. In one case, identical OCB patterns were observed between CSF and serum (type 4), while the other case showed intrathecal IgG production (type 2). Type 2 and 4 OCB patterns are observed in CNS infection/inflammation and systemic inflammatory conditions with CNS involvement, respectively, as part of a disease (Haertle et al., 2014). In addition to myelitis, OCB were observed in the CSF of two patients with encephalopathy, one with hemorrhagic CVD, and two cases of refractory headache. Although these CSF findings suggest the participation of immune-mediated processes in the resulting neurological damage, more data are needed to understand the mechanisms underlying these events.
Before the COVID-19 pandemic, few cases of GBS, an acute immune-mediated neurological disorder, were associated with infection by human coronaviruses (Kim et al., 2017, Sharma et al., 2019). In two-thirds of cases, GBS is triggered by a preceding infection, frequently respiratory or gastrointestinal (Wakerley and Yuki, 2013). Cases of GBS have been described in COVID-19, with symptoms starting at a median of 7 days after respiratory or systemic illness (Ellul et al., 2020). However, atypical cases have been reported, such as a patient with normal CSF cell counts and high protein levels, presenting with tetraplegia. However, fever and respiratory symptoms developed only seven days after GBS onset (Zhao et al., 2020). Therefore, CNS infection by SARS-CoV-2 may occur in parallel or independently of acute respiratory involvement. GBS presentation seems to overlap with infection, suggesting that GBS associated with SARS-CoV-2 infection follows a para-infectious rather than a post-infectious syndrome, as previously described for Zika virus (Araujo et al., 2016, Brasil et al., 2016).
Human coronaviruses have been associated with neurological complications, and their presence in brain tissue was demonstrated for HCoV-229E and OC43 strains in autopsy samples (Arbour et al., 2000), and more recently for SARS-CoV-2 as well (Puelles et al., 2020). The olfactory route has been clearly shown as the main route of neuroinvasion used by HCoV-OC43 in susceptible mice, in which neurons infected in the periphery actively transport virus particles using the axonal transport machinery (Desforges et al., 2014, Dubé et al., 2018), and it is the suggested equivalent pathway in humans. The fact that SARS-CoV-2 infects and damages olfactory sensory neurons of hamsters (Zhang et al., 2020a) and can also infect human neural progenitor cells and brain organoids (Zhang et al., 2020b) strongly supports this hypothesis. In the present study, we detected SARS-CoV-2 RNA in only two of 58 cases (3.4%). Previously, we have reported that patients with COVID-19 and neurological alterations have undetectable or extremely low levels of SARS-CoV-2 RNA in the CSF (Espíndola O de et al., 2020). Thus, although human coronaviruses are known for their neurological tropism, detecting their genomic RNA in CSF seems difficult. This is in line with findings showing that SARS-CoV-2 RNA was present in approximately one-third (8 out of 22) of brain autopsy tissue samples, with lower SARS-CoV-2 RNA copies per cell than in lungs and pharynx tissues (Puelles et al., 2020). Expression of angiotensin converting enzyme 2 (ACE2), the cell entry receptor for SARS-CoV-2, is also distinct between human organs. ACE2 is highly expressed in airway epithelia, kidney cells, small intestine, and lung parenchyma (Zubair et al., 2020), and not surprisingly, this correlates with high viral loads demonstrated in some of these tissues (Puelles et al., 2020). In the CNS, ACE2 is expressed in neurons, astrocytes, and oligodendrocytes, and it is concentrated in the substantia nigra, ventricles, middle temporal gyrus, posterior cingulate cortex, and the olfactory bulb, although at lower levels than that in lungs (Chen et al., 2020). These findings in addition to the spontaneous recovery of neurological alterations described in most of cases of COVID-19 in the literature support the idea of transient and/or limited SARS-CoV-2 dissemination in the CNS. Although our study has some limitations, such as small sample size, our data indicate that rather than direct damage promoted by SARS-CoV-2 replication in the CNS, neurological injury is likely a result of misdirected immune responses either associated with autoimmunity or systemic inflammation in response to limited viral replication.
Conclusion
Overall, patients with COVID-19 showing neurological manifestations presented a great diversity of CSF profiles, even within the same neurological condition. Our findings are consistent with a possible contribution of viral infection on triggering the infiltration of immune cells into the CNS and the stimulation of host inflammatory responses involved in subsequent CNS injury, shown by increased levels of total Tau and NfL proteins. However, more data on this issue are pivotal to a better understanding of the mechanisms leading to neurological damage in SARS-CoV-2 infection.
Funding
This work was supported by the Fundação Oswaldo Cruz (FIOCRUZ).
Ethical statement
This study was approved by the Brazilian National Committee of Ethics in Research (CAAE: 30611720.6.0000.5262), and written informed consent was obtained from all participants.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank the technical team of the Laboratório Neurolife for performing CSF analysis. We are grateful to all health professionals who provided supportive care to patients included in this study.
References
- Abdi S., Ghorbani A., Fatehi F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J Neurol Sci. 2020;416(June):117001. doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti P., Beretta S., Piatti M., Karantzoulis A., Piatti M.L., Santoro P. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(4) doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M., Alvarez-Cermeño J., Bernardi G., Cogato I., Fredman P., Frederiksen J. Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry. 1994;57(August (8)):897–902. doi: 10.1136/jnnp.57.8.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo A.Q.C., Silva M.T.T., Araujo A.P.Q.C. Zika virus-associated neurological disorders: a review. Brain. 2016;139(August (Pt 8)):2122–2130. doi: 10.1093/brain/aww158. [DOI] [PubMed] [Google Scholar]
- Arbour N., Day R., Newcombe J., Talbot P.J. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74(October (19)):8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Valnet R., Pizzarotti B., Anichini A., Demars Y., Russo E., Schmidhauser M. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol. 2020;(May) doi: 10.1111/ene.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstaver P.B., Mohorn P.L., Shah A., Tesh L.D., Quidley A.M., Kothari R. Management of viral central nervous system infections: a primer for clinicians. J Cent Nerv Syst Dis. 2017;9 doi: 10.1177/1179573517703342. 1179573517703342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P., Sequeira P.C., Freitas A.D., Zogbi H.E., Calvet G.A., de Souza R.V. Guillain-Barré syndrome associated with Zika virus infection. Lancet. 2016;387(April (10026)):1482. doi: 10.1016/S0140-6736(16)30058-7. [DOI] [PubMed] [Google Scholar]
- Chen R., Wang K., Yu J., Howard D., French L., Chen Z. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain [Internet] Neuroscience. 2020;(April) doi: 10.3389/fneur.2020.573095. http://biorxiv.org/lookup/doi/10.1101/2020.04.07.030650 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaccio M., Agnello L. Biochemical biomarkers alterations in Coronavirus Disease 2019 (COVID-19) Diagnosis (Berl) 2020;(June) doi: 10.1515/dx-2020-0057. [DOI] [PubMed] [Google Scholar]
- Coen M., Jeanson G., Culebras Almeida L.A., Hübers A., Stierlin F., Najjar I. Guillain-Barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav Immun. 2020;87:111–112. doi: 10.1016/j.bbi.2020.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci Otluoglu G., Yener U., Demir M.K., Yilmaz B. Encephalomyelitis associated with Covid-19 infection: case report. Br J Neurosurg. 2020;(July):1–3. doi: 10.1080/02688697.2020.1787342. [DOI] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Stodola J.K., Meessen-Pinard M., Talbot P.J. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194(December):145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1) doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan L., Kaya D., Sarikaya T., Zengin R., Dincer A., Akinci I.O. Plasmapheresis treatment in COVID-19-related autoimmune meningoencephalitis: case series. Brain Behav Immun. 2020;87:155–158. doi: 10.1016/j.bbi.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé M., Le Coupanec A., Wong A.H.M., Rini J.M., Desforges M., Talbot P.J. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol. 2018;92(17) doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A. Neurological associations of COVID-19. Lancet Neurol. 2020;(July) doi: 10.1016/S1474-4422(20)30221-0. S1474442220302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espíndola O de M., Siqueira M., Soares C.N., Lima M.A.S.D de, Leite A.C.C.B., Araujo A.Q.C. Patients with COVID-19 and neurological manifestations show undetectable SARS-CoV-2 RNA levels in the cerebrospinal fluid. Int J Infect Dis. 2020;96(July):567–569. doi: 10.1016/j.ijid.2020.05.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R.K., Paliwal V.K., Gupta A. Encephalopathy in patients with COVID-19: a review. J Med Virol. 2020;(June) doi: 10.1002/jmv.26207. [DOI] [PubMed] [Google Scholar]
- Gray M.P., Gorelick M.H. Acute disseminated encephalomyelitis. Pediatr Emerg Care. 2016;32(June (6)):395–400. doi: 10.1097/PEC.0000000000000825. [DOI] [PubMed] [Google Scholar]
- Gris J.-C., Perez-Martin A., Quéré I., Sotto A. COVID-19 associated coagulopathy: the crowning glory of thrombo-inflammation concept. Anaesth Crit Care Pain Med. 2020;39(3):381–382. doi: 10.1016/j.accpm.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haertle M., Kallweit U., Weller M., Linnebank M. The presence of oligoclonal IgG bands in human CSF during the course of neurological diseases. J Neurol. 2014;261(March (3)):554–560. doi: 10.1007/s00415-013-7234-2. [DOI] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A., Weinshenker B.G. An approach to the diagnosis of acute transverse myelitis. Semin Neurol. 2008;28(February (1)):105–120. doi: 10.1055/s-2007-1019132. [DOI] [PubMed] [Google Scholar]
- Juliao Caamaño D.S., Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain-Barré Syndrome as a rare neurological complication of SARS-CoV-2. J Clin Neurosci. 2020;77(July):230–232. doi: 10.1016/j.jocn.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil M., Teunissen C.E., Otto M., Piehl F., Sormani M.P., Gattringer T. Neurofilaments as biomarkers in neurological disorders. Nature Rev Neurol. 2018;14(October (10)):577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. 2017;13(July (3)):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li M., Wang M., Zhou Y., Chang J., Xian Y. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;(July) doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;(April) doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N.S., Mytton O.T., Mullins E.W.S., Fowler T.A., Falconer C.L., Murphy O.B. SARS-CoV-2 (COVID-19): what do we know about children? A systematic review. Clin Infect Dis. 2020;(May) doi: 10.1093/cid/ciaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94(May):55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani D., Boso F., Tranquillini E., Gapeni I., Pedrotti G., Cozzio S. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. 2020;41(June (6)):1351–1354. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P. Large-Vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J Neurol. 2020;267(October (10)):2799–2802. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Odolini S., Masciocchi S., Comelli A., Volonghi I., Gazzina S. Steroid-Responsive encephalitis in coronavirus disease 2019. Ann Neurol. 2020;(May) doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(August (6)):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse N., Dixit D. Human and novel coronavirus infections in children: a review. Paediatr Int Child Health. 2020;(June):1–20. doi: 10.1080/20469047.2020.1781356. [DOI] [PubMed] [Google Scholar]
- Scolding N. The differential diagnosis of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001;71 Suppl 2(December) doi: 10.1136/jnnp.71.suppl_2.ii9. ii9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K., Tengsupakul S., Sanchez O., Phaltas R., Maertens P. Guillain-Barré syndrome with unilateral peripheral facial and bulbar palsy in a child: a case report. SAGE Open Med Case Rep. 2019;7 doi: 10.1177/2050313X19838750. 2050313X19838750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloots T.P., Whiley D.M., Lambert S.B., Nissen M.D. Emerging respiratory agents: new viruses for old diseases? J Clin Virol. 2008;42(July (3)):233–243. doi: 10.1016/j.jcv.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenembaum S., Chitnis T., Ness J., Hahn J.S., International Pediatric MS Study Group Acute disseminated encephalomyelitis. Neurology. 2007;68(April (16 Suppl 2)):S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski J.Q., Schuck T., Schmidt M.L., Lee V.M. Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem. 1989;37(February (2)):209–215. doi: 10.1177/37.2.2492045. [DOI] [PubMed] [Google Scholar]
- Wakerley B.R., Yuki N. Infectious and noninfectious triggers in Guillain-Barré syndrome. Expert Rev Clin Immunol. 2013;9(July (7)):627–639. doi: 10.1586/1744666X.2013.811119. [DOI] [PubMed] [Google Scholar]
- Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020;(April) doi: 10.1016/j.bbi.2020.04.017. S0889159120304657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien) 2020;162(July (7)):1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A.J., Lee A.C.-Y., Chu H., Chan J.F.-W., Fan Z., Li C. SARS-CoV-2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin Infect Dis. 2020;(July) doi: 10.1093/cid/ciaa995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.-Z., Chu H., Han S., Shu H., Deng J., Hu Y.-F. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30(10):928–931. doi: 10.1038/s41422-020-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;(May) doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]