Introduction
Juvenile myelomonocytic leukemia (JMML) is a rare leukemia affecting children. It is known to be associated with multiple juvenile xanthogranulomas (JXG) and neurofibromatosis type 1 and usually presents before the self-resolution of the JXG. Skin rashes may frequently occur with JMML but are most commonly maculopapular type. Leukemia cutis presenting as a Sweet syndrome–like rash has rarely been reported. We present an interesting and atypical case of JMML in a child with a history of resolved JXG, presenting with leukemia cutis as the first sign of JMML. Histology of the skin rash showed atypical leukemic infiltrates.
Case report
A 15-month-old Chinese boy presented with multiple JXG. His monozygotic twin also had multiple JXG.1 The JXG appeared from 6 months of age and were located on the head and neck. They involuted spontaneously after 2 years, but he re-presented at 4 years of age for fever and a recurrent, nonpruritic, erythematous, Sweet syndrome–like rash of 1-month duration. On examination, the child was febrile and pale. There were multiple bright red, erythematous, indurated papules and plaques on his face, trunk, limbs, hands, and feet (Figs 1 and 2). Some lesions took on an annular configuration, and blistering was seen over scattered lesions (Fig 3). There was hepatosplenomegaly and lymphadenopathy.
Fig 1.
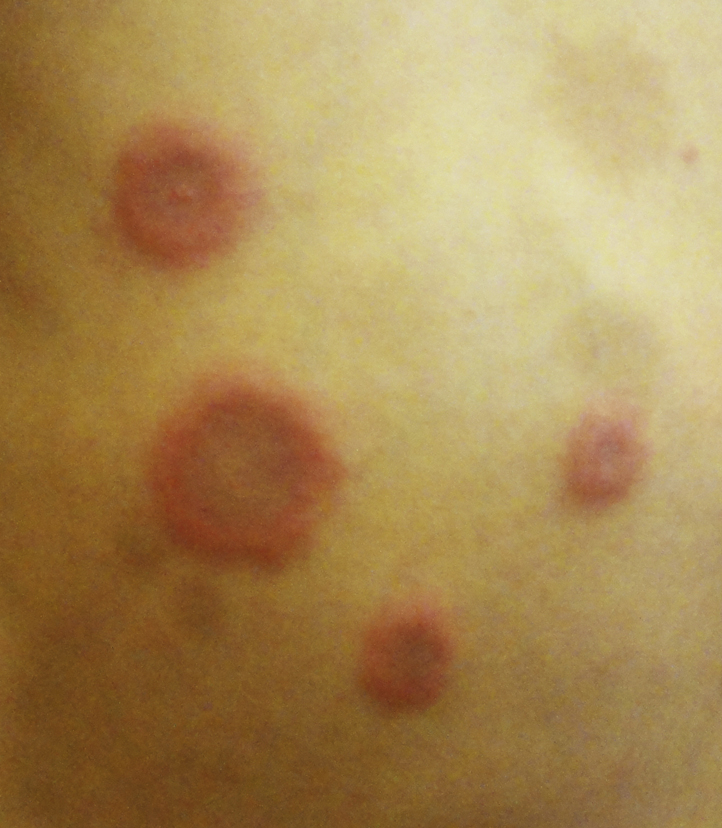
Sweet syndrome–like eruption on the patient's trunk.
Fig 2.
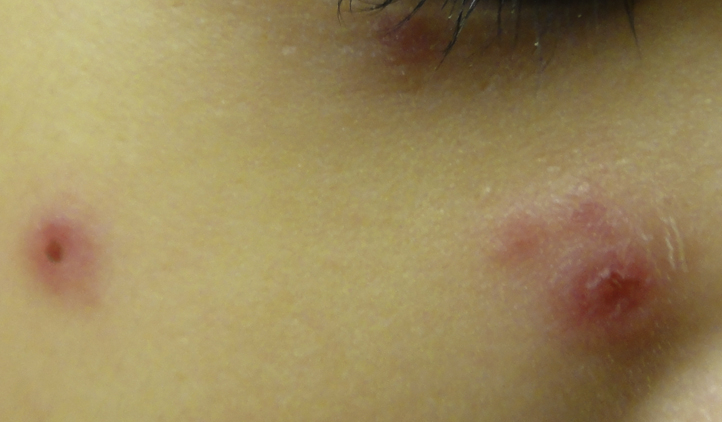
Sweet syndrome–like eruption on the patient's face.
Fig 3.
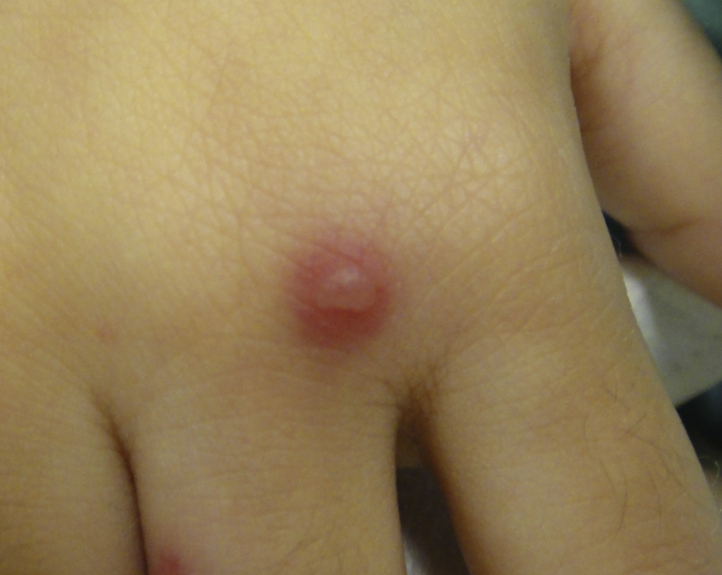
Bright erythematous papule with overlying vesicle on the patient's hand.
Full blood count showed anemia of 5.6 g/dL (normal, 11.0-14.0 g/dl), marked neutrophilia of 80 × 109/L (1.50-8.00 × 109/L) with monocytosis, and thrombocytopenia of 78 × 109/L (200-490 × 109/L). Blast cells were present on peripheral blood film.
A punch biopsy of the Sweet-like skin lesions found prominent superficial and deep perieccrine, periadnexal, and perivascular mononuclear infiltrates. The mononuclear cells were small to medium-sized, with irregular, deep-staining nuclei and scant cytoplasm. These cells were seen dissecting between dermal collagen bundles (Fig 4). There were admixed larger cells with large and pale staining nuclei and more abundant eosinophilic cytoplasm. The atypical cells stained positively for CD3, CD4, CD43, myeloperoxidase, and lysozyme immunohistochemical stains. Bone marrow biopsy showed hypercellular marrow with myeloid hyperplasia and left shift maturation.
Fig 4.
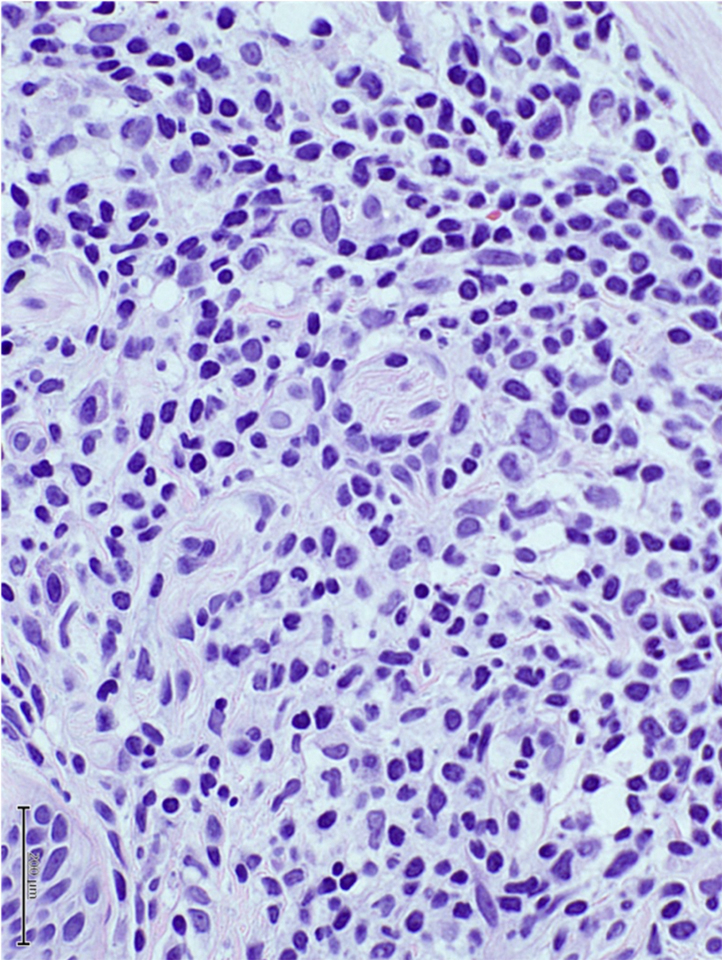
Punch biopsy shows atypical mononuclear cells with deep staining nuclei. (Hematoxylin-eosin stain; original magnification: ×400.)
Direct sequencing of unfractionated bone marrow cells found a somatic heterozygous variant in the PTPN11 gene (c.1508 G>C; p.[503 G>A]). This variant had been reported as a JMML-associated PTPN11 pathogenic variant. No variants were found in NF1, KRAS, NRAS, and CBL genes. JMML and leukemia cutis were diagnosed, and the patient underwent a haploidentical bone marrow transplant.
Discussion
Skin manifestations of leukemia in children are rare and comprise both specific leukemic infiltrates, termed leukemia cutis or nonspecific reaction patterns, like Sweet syndrome, characterized clinically by multiple, tender, red-to-violaceous, indurated papules and plaques, and histologically by dense dermal neutrophilic infiltrates. Only rare reports exist of Sweet syndrome occurring in children with leukemia, most commonly acute myelogenous leukemia, and are even rarer in JMML.2, 3, 4 Leukemia cutis in children is extremely uncommon and is most often associated with acute myeloid leukemia followed by acute lymphoblastic leukemia. The median age at presentation is approximately 2 years, and lesions present commonly as erythematous-to-violaceous nodules or papules occurring on the head and lower limbs.5 In our case, although the patient's clinical presentation was consistent with Sweet syndrome, there was absence of dense neutrophilic infiltrates within the dermis. Conversely, the infiltrate comprised atypical mononuclear cells more typical of infiltrates seen in JMML presenting with leukemia cutis. Most previous case reports of leukemia cutis occurring in JMML have also described a dermal infiltrate of mononuclear cells that resemble mature and immature monocytes in a perivascular and periadnexal distribution within the dermis.6
JMML is a rare, chronic, malignant, myeloproliferative/myelodysplastic disorder of early childhood. It represents 2% to 3% of pediatric leukemias and is characterized clinically by hepatosplenomegaly, fever, leukocytosis with monocytosis, thrombocytopenia, and raised fetal hemoglobin. JMML is characterized by the constitutive activation of the RAS signaling pathway, and about 90% are caused by germline or somatic mutations in CBL, NRAS, KRAS, NF1, or PTPN11 genes.7 PTPN11 mutations have been found in approximately 35% of JMML patients. Most patients will require allogeneic stem cell transplant for remission. However, spontaneous resolution has been reported in 15% of patients. Recently, DNA methylation patterns seen in JMML can be predictive of outcome and can identify patients who may experience spontaneous resolution.8
JXG is a common pediatric skin tumor that is benign and self-limiting. JXG is postulated to originate from dermal dendritic histiocytes. It commonly presents in the early months of life and can increase in number and size until 1 or 2 years of age. Most lesions undergo spontaneous regression, causing hyperpigmentation and atrophy of the residual skin. The exact etiology and pathogenesis of JXG is unknown, although a disordered macrophage response to nonspecific injury has been proposed. Multiple JXG have been associated with JMML. Multiple JXG have also been associated with Langerhans cell histiocytosis (LCH). Most cases of JMML associated with multiple JXG occur while the JXG have not spontaneously regressed. The occurrence of JMML a few years after resolution of multiple JXG, as occurred in our patient, is unusual. It is important to impress on parents of children with multiple JXG, that there is still a residual risk of JMML occurring even after spontaneous regression of the JXG.
Unlike in JMML and LCH, genetic aberrations in cases of JXG has not been as well established. Recently, studies by whole-exome sequencing have provided support for a role for MAPK signaling in JXG, with mutations identified in NRAS, KRAS, ARAF, BRAF, and MAP2K1.9 Other than the RAS pathway, the PI3K pathway has also been identified to possibly be involved in the pathogenesis of JXG.10
We presented a rare case of a young child presenting with Sweet syndrome–like leukemia cutis as a first presentation of JMML. The JMML had unusually presented more than 1 year after the resolution of his multiple JXG. With this report, we emphasize the importance of surveillance for JMML in young children with multiple JXG, even after spontaneous resolution. The appearance of a Sweet syndrome–like rash in these patients should prompt urgent investigations to exclude JMML.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Tan K., Koh M., Tay Y. Juvenile xanthogranuloma in monozygotic twins. Pediatr Dermatol. 2010;27:666–667. doi: 10.1111/j.1525-1470.2010.01332.x. [DOI] [PubMed] [Google Scholar]
- 2.Hospach T., von den Driesch P., Dannecker G.E. Acute febrile neutrophilic dermatosis (Sweet's syndrome) in childhood and adolescence: two new patients and review of the literature on associated diseases. Eur J Pediatr. 2008;168:1–9. doi: 10.1007/s00431-008-0812-0. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura H., Kaneko T., Nakano H., Terui K., Ito E., Sawamura D. Juvenile myelomonocytic leukemia presenting multiple painful erythematous lesions diagnosed as Sweet's syndrome. J Dermatol. 2008;35:368–370. doi: 10.1111/j.1346-8138.2008.00486.x. [DOI] [PubMed] [Google Scholar]
- 4.Del Puerto Troncoso C., Curi Tuma M., González Bombardiere S., Silva-Valenzuela S. Neutrophilic figurate erythema of infancy associated with juvenile myelomonocytic leukemia. Actas Dermosifiliogr. 2015;106:431–433. doi: 10.1016/j.ad.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Andriescu E.C., Coughlin C.C., Cheng C.E. Pediatric leukemia cutis: a case series. Pediatr Dermatol. 2019;36:658–663. doi: 10.1111/pde.13864. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R.K., Qureshi A., Choi J.K. Histologic findings in skin biopsy in a JMML rash: a case report and review of literature. Pediatr Dev Pathol. 2014;17:130–133. doi: 10.2350/12-12-1283-CR.1. [DOI] [PubMed] [Google Scholar]
- 7.Niemeyer C.M., Flotho C. Juvenile myelomonocytic leukemia: who's the driver at the wheel? Blood. 2019;133:1060–1070. doi: 10.1182/blood-2018-11-844688. [DOI] [PubMed] [Google Scholar]
- 8.Stieglitz E., Mazor T., Olshen A. Genome-wide DNA methylation is predictive of outcome in juvenile myelomonocytic leukemia. Nat Commun. 2017;8:2127. doi: 10.1038/s41467-017-02178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond E.L., Durham B.H., Haroche J. Diverse and targetable kinase alterations drive histiocytic neoplasms. Cancer Discov. 2016;6:154–165. doi: 10.1158/2159-8290.CD-15-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakraborty R., Hampton O.A., Shen X. Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood. 2014;124:3007–3015. doi: 10.1182/blood-2014-05-577825. [DOI] [PMC free article] [PubMed] [Google Scholar]


