Abstract
Nicotinic acetylcholine receptors are an important class of excitatory receptors in the central nervous system of arthropods. In the ticks Ixodes ricinus, the functional and pharmacological properties of nicotinic receptors located in their neurons are still unknown. The objective of this study was to characterize the pharmacological properties of tick nicotinic receptors using membrane microtransplantation in Xenopus laevis oocytes and two-electrodes voltage clamp method. The membranes microtransplanted were extracted from the tick synganglion. We found that oocytes microtransplanted with tick synganglion membranes expressed nicotinic acetylcholine receptor subtypes which were activated by acetylcholine (1 mM) and nicotine (1 mM). Currents induced by pressure application of acetylcholine and nicotine were diminished by 10 nM α-bungarotoxin and methyllycaconitine, suggesting that they expressed two subtypes of nicotinic receptors, α-bungarotoxin-sensitive and -insensitive, respectively. In addition, we found that nicotine receptors expressed in the synganglion membranes were poorly sensitive to the neonicotinoid insecticides clothianidin (CLT), imidacloprid (IMI), acetamiprid (ACE) and thiamethoxam (TMX), in agreement with their lack of activity as acaricides. Interestingly, current amplitudes were strongly potentialized in the presence of 1 μM PNU-120596. CLT was more active as an agonist than IMI, TMX and ACE. Finally, we demonstrated that microtransplantation of purified membrane from the tick synganglion can be a valuable tool for the development and screening of compounds targeting tick nicotinic acetylcholine receptor subtypes.
Keywords: Tick, Ixodes ricinus, Synganglion membrane, Microtransplantation, Nicotinic acetylcholine receptors
Graphical abstract
1. Introduction
Ticks are known as harmful hematophagous ectoparasites and vectors of many pathogenic viruses, bacteria, and protozoa of medical and veterinary importance (Lindquist, 2008). They constitute major parasites for animal health through their role as vectors of various vector-borne-diseases (theileriosis and babesiosis, cowdriosis, …) or through blood depletion. Ten percent of the currently known tick species act as pathogenic agents causing a broad range of animal and human diseases (Jongejan and Uilenberg, 2004). Some tick species such as the forest tick, Ixodes ricinus, transmit a number of tick-borne diseases to human, e.g. Lyme borreliosis and tick-borne encephalitis (Barbour and Benach, 2019). These diseases can lead to different health issues including neurodegenerative problems, cardiac disorder or arthritic pain (Cardenas-de la Garza et al., 2019). The increase over the last decades in the number of newly discovered tick-borne agents, and the absence of anti-tick vaccines, has prompted the search of new strategies to limit tick-borne diseases and biting. Thus, one strategy is understanding the pharmacological properties of tick ion channels, in particular nicotinic acetylcholine receptors (nAChRs), which are members of the cys-loop ligand gated ion channel superfamily that mediates the rapid neurotransmission. In insects and mammals, pharmacological approaches using electrophysiological studies indicated the existence of different nAChR subtypes. Functional nAChRs exist as hetero- or homopentamer subtypes, with diverse pharmacological profiles and kinetic properties. Evidence suggest some similarities between vertebrate nAChR subtypes containing the α7-subunit and insect α-bungarotoxin (α-Bgt)-sensitive receptors (Thany et al., 2007). Moreover, in contrast to their poor agonist activity on mammalian nAChRs (Tomizawa et al., 2003), neonicotinoid insecticides differently activated insect nAChR subtypes or binding sites (Thany, 2009, 2011; Taillebois et al., 2018; Houchat et al., 2019). Transcriptomic analyses on the tick Rhipicephalus sanguineus and Dermacentor variabilis synganglia have shown that they expressed cDNAs encoding for nAChR subunits (Lees et al., 2010; Bissinger et al., 2011). Recently, Lees et al., demonstrated that R. sanguineus α1 subunit co-expressed with the chicken β2 subunit, in the Xenopus oocytes, evoked acetylcholine (ACh) and nicotine currents, but the expressed hybrid receptor was insensitive to IMI (Lees et al., 2014). We propose that different nAChR subtypes exist in the tick nervous system and account for the binding of neonicotinoid insecticides. Thus, in the present study, we aimed to characterize the functional properties of tick native nAChRs expressed in the synganglion, in particular, if they are similarly activated by ACh and nicotine, sensitive to neonicotinoid insecticides and blocked by nAChR antagonists, as found with other arthropods (Tan et al., 2007; Thany et al., 2007).
The Xenopus laevis oocyte as proven to be an efficient system for the heterologous functional expression of ligand-gated and voltage-gated ion channels (Eusebi et al., 2009; Millar and Gotti, 2009). Here, we used a method that is well known with mammalian model but has been rarely used for arthropods, the microtransplantation of purified membranes from neuronal tissues into Xenopus oocytes (Miledi et al., 2002; Palma et al., 2003; Eusebi et al., 2009; Murenzi et al., 2017). Microtransplantation of oocytes with membranes prepared from rat pituitary GH (4)C1 cells, stably expressing human neuronal α7 nAChRs or human embryonic kidney cell line (HEK293) expressing α4β2 receptors, led to functional receptors with native properties of the corresponding cell lines (Palma et al., 2003). In particular, dose-response curve relationships, agonist and antagonist sensitivity. Indeed, ACh currents from GH (4)C1−α7 gave a linear current-voltage curve at hyperpolarized membrane potentials, with ACh-evoked currents blocked by the α7 nicotinic antagonist methyllycaconitine (MLA). In contrast, ACh currents through HEK-α4β2 cells were inhibited by the competitive blocker dihydro-β-erythroidine (DHβE) and the ACh dose-response curve was bimodal (Palma et al., 2003). Recently, microtransplantation was also used with success for an insect model, the aphid Acyrthosiphon pisum, where extracted membranes were microtransplanted into Xenopus oocytes (Crespin et al., 2016). Electrophysiological recordings demonstrated that aphid membranes expressed nAChR subtypes activated by ACh and nicotine (Crespin et al., 2016). More broadly, this method allowed the characterisation of the functional properties of transmembrane proteins in their native environment (Eusebi et al., 2009). Thus, we applied this approach to study the pharmacological profile of Ixodes ricinus neuronal nAChR subtypes, by extracting cell membranes from the synganglion.
2. Materials and methods
2.1. Ticks breeding and sample collection
Female adult Ixodes ricinus were collected in the state forest of Chize, next to the CEBC laboratory (CNRS UMR 7372, Université de La Rochelle, Villiers-en-Bois, France) and provided by the BIOEPAR laboratory (INRAE, ONIRIS, Nantes, France). The synganglia of female ticks were extracted in PBS (8.10 mM Na2HPO4-12 H2O, 1.44 mM NaH2PO4–H20, 137 mM NaCl) and conserved at −80 °C before membrane extraction.
2.2. Membrane preparation
A pool of 150 synganglia was prepared, as described in Crespin et al. (2016), with the following modifications. Synganglia were mechanically dissociated in 500 μl of extraction buffer (50 mM Tris/HCl, 1 mM EGTA, 3 mM EDTA, supplemented with protease inhibitors) using a pellet Argos mixer (World Precision Instruments, Hertfordshire, UK). All experiments were conducted at 4 °C. The solution was first centrifuged 1 min at 1500 rpm. The supernatant was collected in a new tube with 5 ml of extraction buffer, then homogenized and centrifuged at 15,000 rpm for 70 min. The pellet containing synganglion membranes was further concentrated using a sucrose gradient (7 ml of 20% sucrose and 5 ml of 50% sucrose, respectively), and centrifuged at 26,200 rpm, for 2 h 45 min. The membrane fraction located between 20% and 50% sucrose phase was supplemented with 20 ml of extraction buffer, then centrifuged at 26,200 rpm, for 50 min. The pellet was resuspended in 5 ml of glycine buffer, centrifuged 45 min at 26,200 rpm. Then, collected in 100 μl of glycine buffer and stored at −80 °C.
2.3. Protein quantification
Protein quantification was carried out with the Bradford test method (Hammond and Kruger, 1988). For each solution, 5 μl were deposited on a 96-well microplate. In each well, 250 μL of Bradford reagent (Bio-Rad Laboratories, Inc.) were added. After 10 min incubation at room temperature, the plate was read at 595 nm with a microplate reader (μQuant, Bio-Tek Instrument) (Hammond and Kruger, 1988). Results were calculated from a standard range of BSA (0–2000 μg/mL). Concentration of membrane proteins was around 2495 μg/ml.
2.4. Xenopus oocyte preparation
Xenopus laevis oocytes were provided by CRB (University of Rennes 1, Rennes, France). The CRB Xenope is a French national platform dedicated to X. laevis breeding for experimental research. Oocytes were defolliculated according to Cartereau et al. (2018), and were incubated in a standard oocyte saline (SOS) solution (100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2 and 5 mM HEPES, pH 7.5) supplemented with antibiotics (50 mg/ml gentamycin, 100 U/ml penicillin, 100 mg/ml streptomycin and 2.5 mM pyruvate). Defolliculated oocytes were microinjected with 36.8 nl of the tick membrane solution using a nanoliter injector (Nanoliter, 2010; World Precision Instruments, Hertfordshire, UK) and were incubated in the SOS solution at 18 °C during 24 h before electrophysiological measurements.
2.5. Electrophysiological recording
A two-electrodes voltage clamp technique was used to record neuronal receptor responses from I. ricinus synganglion membranes microtransplanted in oocytes. Electrodes were prepared with a glass puller (model P-97, Sutter Instrument), filled with a 3 M KCl solution and the resistance was controlled to be between 0.1 and 3 MΩ. After incubation, oocytes were placed in a recording chamber at room temperature (20–22 °C), with a continuous flow of SOS supplemented with atropine (0.5 mM) or not (addition of atropine allows the inhibition of muscarinic acetylcholine receptors (mAChRs) responses and thus, to selectively record the nAChR responses). All compounds were diluted at 1 mM with SOS or SOS with atropine. Recordings were made using a Digidata-1322 A (Axon Instruments) A/D converter and analyzed with pCLAMP 10 (Molecular Devices, Union City, CA, USA). The oocyte membrane potential was hold at −60 mV and perfusion for each molecule tested was performed during 20 s. After the application of one molecule, oocytes were washed with a continuous flow of SOS or SOS with atropine, during 5 min.
2.6. Fluorescence labelling of tick synganglion nAChRs
To visualize by fluorescence the presence of α-Bgt-sensitive nAChRs on the tick synganglion membranes, we used α-Bgt conjugated with a fluorescent probe, Alexa Fluor-488 (Thermo Fisher Scientific, France). 20 μl of tick membrane solution was mixed with 100 μl PBS (0.25% BSA) and 10 μl Alexa Fluor-488-α-Bgt solution (100 μg/ml), at room temperature during 4 h. The mix was centrifugated at 14,000 rpm during 20 min, at 4 °C. The pellet was collected in 500 μl PBS (0.25% BSA) and centrifuged at 10,000 rpm during 20 min, at 4 °C. Then, stored in 2–5 μl PBS (0.25 BSA). The fluorescence was visualized on glass slip (glycerol/PBS) with an inverted microscope, Olympus IX3 (Olympus, France).
2.7. Drugs
ACh, nicotine, atropine, PNU-120956, MLA, α-Bgt, mecamylamine, clothianidin (CLT), IMI, acetamiprid (ACE) and thiamethoxam (TMX) were purchased from Sigma (St Quentin, France). They were dissolved first in 100% DMSO and then diluted in SOS at a final concentration of 0.01% DMSO.
2.8. Statistical analysis
Currents were normalized using the mean current amplitudes under the same control conditions (I/Imean). Data were shown as mean ± S.E.M and analyzed using Prism 7 (GraphPad Software, La Jolla, CA, USA). Differences between normalized currents from control and experimental groups were analyzed using one-way Anova and Wilcoxon paired test (samples < 30), using Prism 7. The EC50 values for ACh and nicotine were determined using nonlinear regression on normalized data (1 mM ACh as maximal response) using Prism 7. The dose–response curves were derived from the fitted curve following the equation: Y = Imin + (Imax – Imin)/(1 + 10(log(EC50–X)H) where Y is the normalized response, Imax and Imin are the maximum and minimum responses, H is the Hill coefficient, EC50 is the concentration giving half the maximum response and X is the logarithm of the compound concentration. In all experiments, ‘n’ represents the number of tested oocytes.
3. Results
3.1. Expression of tick native nicotinic acetylcholine receptors in the synganglion membranes
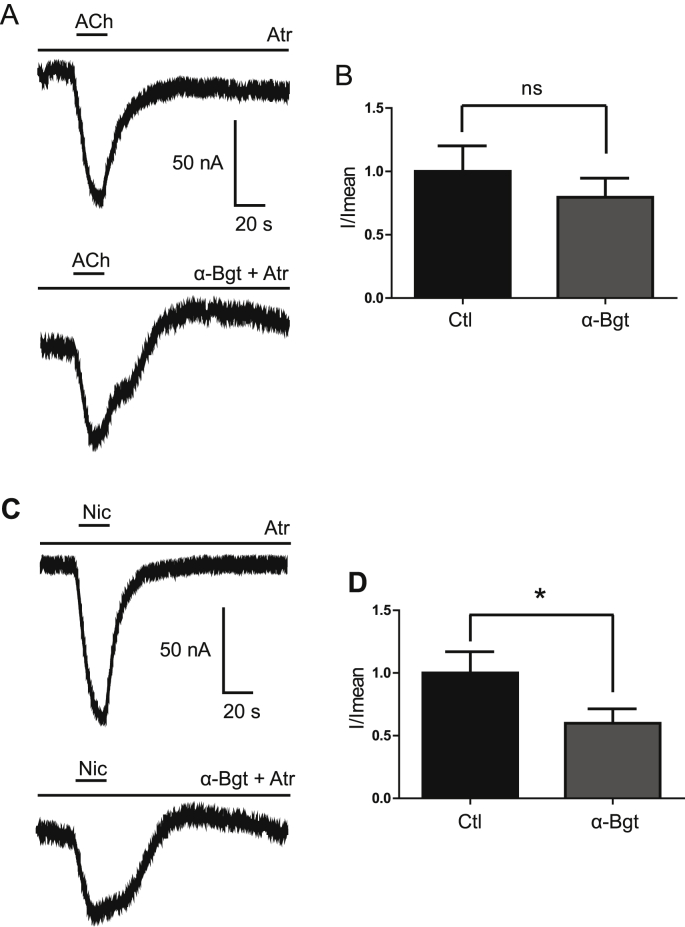
The oocytes microtransplanted with tick synganglion membranes expressed nAChR subtypes which were highly variables in terms of currents stability (data not shown). Indeed, application of 1 mM ACh or nicotine evoked inward currents which were unstable over the time (Fig. 1). This suggested that ACh and nicotine currents are mediated by complex responses including muscarinic ACh receptors. Thus, to establish the conditions for measuring stable ACh- and nicotine-induced currents, we added in the bath 0.5 μM atropine, to block muscarinic acetylcholine receptors (mAChRs). In this condition, application of 1 mM ACh, in the presence of 0.5 μM atropine, evoked a stable current without affecting the current amplitudes (Fig. 1A and B). Similar currents were detected with 1 mM nicotine (Fig. 1C and D). Currents were also slowed with a slow rate of desensitization, but with no change in the current amplitudes (Fig. 1C and D). Subsequently, in all experiments, 0.5 μM atropine was added in the bath to study nAChRs and block the ionic currents resulting from mAChRs activation. Thus, in the presence of 0.5 μM atropine, 20 s application of ACh and nicotine evoked stable inward currents with peak amplitudes ranging from −75 ± 10 nA to −96 ± 13 nA at 1 mM ACh and nicotine, respectively (n = 20). Concentration-response relationships performed with ACh and nicotine revealed an EC50 values of 115.4 μM and 216.2 μM (n = 20, Fig. 1E), respectively. We also observed that application of 1 mM muscarine (Mus) resulted in an inward current of −105 ± 14 nA but did not induce variable current amplitude as found with ACh and nicotine. Currents were completely blocked by 0.5 μM atropine, confirming that ACh- and nicotine-evoked currents were affected by the presence in the injected membranes of muscarinic ACh receptors (Fig. 1F and G). In order to characterize nAChR subtypes involved in ACh- and nicotine-elicited currents, the nAChR antagonist, α-bungarotoxin (α-Bgt) was bath applied during 15 min. The currents mediated by 1 mM ACh were neither blocked or diminished by 10 nM α-Bgt but nicotine-induced currents were partially inhibited by 10 nM α-Bgt (n = 10, p < 0.05, Fig. 2), suggesting that α-Bgt-sensitive receptors are present in the synganglion membranes. To confirm this hypothesis, membranes were treated with bright and photostable Alexa fluor 488-α-Bgt to visualize α-Bgt-sensitive receptors. As it can be seen in Fig. 3A, specific fluorescence spots were observed in synganglion membranes preparation and no fluorescence spots were found in the control solutions. These data confirmed that α-Bgt-sensitive receptors were expressed in the synganglion membranes expressed in the oocytes.
Fig. 1.
Properties of acetylcholine and nicotine currents in oocytes microtransplanted with tick synganglion membranes. (A and B) Currents elicited by 1 mM acetylcholine (ACh). Top current without atropine (control, Ctl) and below with 0.5 μM atropine (Atr). Horizontal bars above each response indicate the compound application. The perfusion time was 20 s. Histograms illustrate the comparison between ACh without atropine (Ctl) or with 0.5 μM atropine (Atr) (p > 0.05, n = 16, ns = no significant). (C and D) Currents elicited by 1 mM nicotine (Nic) compared in control condition (Ctl: without atropine) and under bath application of 0.5 μM atropine (Atr). Histograms show that there are no significant changes in the current amplitude (p > 0.05, n = 23, ns = no significant). (E) ACh and nicotine concentration-current response relationships from n = 12, in the presence of 0.5 μM atropine. Each point represents peak currents normalized to Imean. (F and G) Currents elicited by 1 mM muscarine. Top current represents 1 mM muscarine (Mus)-induced current, and below the blocking activity induced by 0.5 μM atropine. Histograms illustrate the current amplitudes between muscarine without atropine (control condition, Ctl) and with 0.5 μM atropine (Atr). n = 8, *p < 0.05. In each histogram, data are mean ± S.E.M.
Fig. 2.
Effect of α-Bgt on ACh- and nicotine-evoked currents in oocyte microtransplanted with tick synganglion membranes. (A and B) Currents elicited by 1 mM ACh. Top current without α-Bgt and below with 10 nM α-Bgt. Horizontal bars above each response indicate the compound application, and the perfusion time was 20 s. Histograms illustrate the comparison between ACh without 10 nM α-Bgt (Ctl) or with 10 nM α-Bgt. P > 0.05, n = 9, ns = no significant. (C and D) Currents elicited by 1 mM nicotine (Nic), without and under bath application of 10 nM α-Bgt. Histograms illustrating current amplitudes when α-Bgt is added in the bath. Each histogram represents n = 10, *p < 0.05.
Fig. 3.
Fluorescent labelling of tick synganglion membranes showing the presence of nAChRs α-Bgt -sensitive subtypes, using fluorescence microscopy. (A) Synganglion membrane stained with Alexa Fluor 488-α-Bgt complex. (B) The same preparation as A, visualized with transmitted light. (C) Synganglion membrane as a control preparation, without Alexa Fluor 488-α-Bgt complex. (D) The same preparation as C, visualized with transmitted light. Scale bar = 200 μm.
3.2. Effect of PNU-120596 on ACh and nicotine responses
PNU-120596 (PNU) is a positive allosteric modulator of nAChRs (Kalappa and Uteshev, 2013). Here, we were interested to study its effect on ACh and nicotine currents. In the presence of bath application of 1 μM PNU, ACh and nicotine current amplitudes were not significantly different (p > 0.05, n = 12, Fig. 4). Note that, application of PNU on no injected oocytes did not induce currents (data not shown). Interestingly, bath application of 1 μM PNU with 0.5 μM atropine, strongly potentiated ACh and nicotine responses (Fig. 5). Currents induced by 1 mM ACh were increased from −96 nA ± 5 nA to −175 ± 20 nA, in presence of 1 μM PNU and 0.5 μM atropine (n = 11, p < 0.05, Fig. 5A and B). Similarly, nicotine-induced currents were increased from −105 nA ± 0.5 nA to −241 nA ± 4.5 nA (n = 23, p < 0.05, Fig. 5C and D). Based on these results, oocytes microtransplanted with tick synganglion membranes were incubated with 10 nM α-Bgt in the presence of both 0.5 μM atropine and 1 μM PNU (Fig. 6). ACh- and nicotine-induced currents were significantly reduced by respectively ~33% (n = 7, p < 0.05, Fig. 6A and B) and ~47% (n = 13, p < 0.05, Fig. 6C and D). Moreover, we examined the effect of the nAChR antagonists, mecamylamine and the selective α-Bgt-sensitive nAChRs, methyllycaconitine (MLA) (Davies et al., 1999), on ACh- and nicotine-evoked currents. Mecamylamine and MLA did not inhibit ACh-evoked currents when applied at concentrations of 1 μM for mecamylamine (p > 0.05, n = 12, Fig. 7A and B) and 10 nM for MLA (p > 0.05, n = 7, Fig. 7C and D), respectively. Using the same conditions, we found that currents induced by 1 mM nicotine were not affected by 1 μM mecamylamine (p > 0.05, n = 9, Fig. 8A and B), but they were diminished by 33% when 10 nM MLA was added in the bath (p < 0.05, n = 12, Fig. 8C and D), confirming the presence of α-Bgt-sensitive receptors.
Fig. 4.
Modulatory effect of PNU-120596 (PNU) on ACh- and nicotine-evoked currents in oocytes microtransplanted with tick synganglion membranes. (A and B) Currents elicited by 1 mM ACh. Top current without PNU-120596 (PNU) and below with 1 μM PNU-120596. Each horizontal bars indicates when the compound us added. Histograms illustrate the comparison between ACh without PNU-120596 (Ctl) or with 1 μM PNU-120596. P > 0.05, n = 8, ns = no significant. (C and D) Currents elicited by 1 mM nicotine and under bath application of 1 μM PNU-120596. Histograms show that there is no significant difference in the current amplitude when PNU-120596 was added in the bath. P > 0,05, n = 9, ns = no significant. Each histogram represents mean ± S.E.M, and the perfusion time was 20 s.
Fig. 5.
Modulatory effect of combined application of atropine and PNU-120596 on ACh- and nicotine-evoked currents in oocyte microtransplanted with tick synganglion membranes. (A and B) In top: currents elicited by 1 mM ACh in presence of 0.5 μM atropine (Atr). Below: in the presence of 0.5 μM atropine (Atr) and 1 μM PNU-120596 (PNU). Horizontal bars indicate the compound application. Histograms illustrate the mean current amplitudes and represent, n = 11. (C and D) Currents induced by 1 mM nicotine under 0.5 μM atropine (Atr) and bath application of 0.5 μM atropine and 1 μM PNU (PNU). Histograms illustrate current amplitudes and show that there is a significant difference. Each histogram represents n = 23. Note that in all case, the perfusion time for ACh and nicotine was 20 s, histogram are mean ± S.E.M and *p < 0.05.
Fig. 6.
Effect of α-Bgt under bath application of 0.5 μM atropine (Atr) and 1 μM PNU-120596. (A and B) Currents elicited by 1 mM ACh without 10 nM α-Bgt, top current, and with 10 nM α-Bgt (below). Horizontal bars indicate the compound application. Histograms illustrate currents recorded in the same conditions, and represent n = 7. (C and D) Currents elicited by 1 mM nicotine (Nic) without α-Bgt and under bath application of 10 nM α-Bgt. Histograms show that there is a significant difference in the current amplitude when 10 nM α-Bgt is added in the bath. Each histograms represent n = 13. In all case, each histogram represent mean ± S.E.M and * p < 0.05.
Fig. 7.
Effect of mecamylamine and methyllycaconitine on ACh-evoked currents. 1 mM ACh (20 s, horizontal bar) currents were recorded under bath application of 0.5 μM atropine (Atr) and 1 μM PNU-120596 (PNU). (A and B) As shown, 1 μM mecamylamine (Meca) has no effect on 1 mM ACh-induced currents. Each histogram represents mean ± S.E.M of n = 12. ns = no significant and p > 0.05. (C and D) Similarly, 10 nM MLA has no effect on ACh currents. Histograms illustrate the current amplitudes and represents mean ± S.E.M of n = 7, p > 0.05. Ctl: represents application of ACh without 1 μM mecamylamine or 10 nM MLA.
Fig. 8.
Effect of mecamylamine and methyllycaconitine on nicotine-evoked currents. All currents were recorded under bath application of 0.5 μM atropine (Atr) and 1 μM PNU-120596 (PNU). (A and B) Currents elicited by 1 mM nicotine (Nic) are not affected by 1 μM mecamylamine (Meca). Each histogram represents mean ± S.E.M of n = 9, ns = no significant, p > 0.05. (C and D) Currents elicited by 1 mM nicotine (Nic, 20 s, horizontal bar) are diminished by 10 nM MLA. Histograms illustrate the effect of 10 nM MLA, and represent mean ± S.E.M of n = 12, *p < 0.05. Ctl: represents application of 1 mM nicotine without 1 μM mecamylamine or 10 nM MLA.
3.3. Effect of neonicotinoid insecticides on tick synganglion membranes expressed in Xenopus oocytes
The ability of neonicotinoid insecticides to act as agonists of the tick nAChRs expressed in the synganglion was examined by recording the currents induced by 1 mM CLT, IMI, TMX and ACE. The four neonicotinoids were poor activators of tick nAChRs. The mean current amplitude induced by each compound was less than −15 nA (Fig. 9) whereas previous studies demonstrated that they can activate insect nAChRs as agonists (Tan et al., 2007; Thany, 2009). We then evaluated the agonist effect of the four neonicotinoid insecticides in the presence of 1 μM PNU-120596. CLT appeared the more effective, with a mean current amplitude of −258 ± 38 nA (p < 0.05, n = 9, Fig. 9A and B), demonstrating that CLT-induced responses were potentiated by 32 fold. When IMI, TMX and ACE were applied, responses were potentiated by 6.42, 4.6 and 5.5, respectively (Fig. 9C–H). We demonstrated that PNU-120596 significantly increased the current amplitudes induced by neonicotinoid insecticides, and that it had similar action on ACh, nicotine and neonicotinoids.
Fig. 9.
Effect of PNU-120596 on current evoked by the neonicotinoid compounds, clothianidin (CLT), imidacloprid (IMI), thiamethoxam (TMX) and acetamiprid (ACE), in oocyte microtransplanted with tick synganglion membranes. In all case, we found that, current induced by 1 mM of each neonicotinoid are significantly increased under bath application of 1 μM PNU-120596. (A and B) Currents and histograms under 1 mM CLT (n = 9, *p < 0.05). (C and D) Currents induced by 1 mM IMI and the corresponding histograms (n = 19, *p < 0.05). (E and F) Currents and histograms representing currents elicited by 1 mM TMX (n = 14, *p < 0.05). (G and H) Currents elicited by 1 mM ACE and Histograms illustrating the increase under bath application of PNU-120596 (n = 20, *p < 0.05). In all case, histograms are mean ± S.E.M. All compounds were applied during 20 s (black bars). Ctl: represents application of each compound without 1 μM PNU in the bath.
4. Discussion
In the present study, we demonstrated for the first time that microtransplantation of ticks synganglion membranes into X. laevis oocytes allows a functional characterisation of nAChRs of this arthropod taxa of major importance for human and animal health. We found that the functional expression of nAChRs could be studied when mAChRs were blocked by atropine. The presence of muscarinic and nicotinic receptors in the synganglia was previously demonstrated by transcriptomic analyses in the female synganglion of the black-legged tick Ixodes scapularis (Egekwu et al., 2014; Gulia-Nuss et al., 2016) and of the brown dog tick, Rhipicephalus sanguineus (Lees et al., 2010). Here, we injected X. laevis oocytes with tick synganglion membranes containing native nAChRs to investigate their expression and pharmacological properties. We found that both ACh- and nicotine-induced currents had slow kinetic currents. Indeed, using the two-electrodes voltage clamp method, we found that ACh and nicotine mediated substantially equivalent currents, with rapid desensitization The reasons for the difference of responses induced when using microtransplanted oocytes with membrane preparations were unknown. In previous studies it was found that application of AMPA to HEK-GLUR1 cells elicited inward currents which decayed faster in oocytes than in HEK cells (Palma et al., 2003). It was proposed that this difference could be due to internal cell signaling between membrane preparations in the oocytes and the recording of nicotinic receptors on native cells. Using R. sanguineus nAChR α1 subunit, it was found that its expression into X. laevis oocytes failed to function as a homomer. When co-expressed with chicken β2 subunit, the chimeric α1β2 nAChR evoked inward currents with a slow activation and deactivation kinetics, in response to ACh and nicotine (Lees et al., 2014). We however argued that the functional properties of expressed receptors could be influenced by the host heterologous cell expression system, in terms of activation and deactivation currents induced by agonists. Despite this kinetic difference of currents, we demonstrated that synganglion membranes injected in the oocytes expressed functional receptors. We found also that they were sensitive to the nAChR antagonists, suggesting that tick synganglion membranes expressed α-Bgt-sensitive and -insensitive nAChR subtypes. Note that, because two types of mAChRs are present in several arthropods (Collin et al., 2013), we suggest that atropine could block type A receptors instead of type B. Moreover, under PNU-120596 application, we demonstrated that ACh and nicotine-evoked currents were significantly increased and diminished by MLA, confirming that (i) ACh and nicotine currents were potentiated by PNU-120596 and (ii) the presence of α-Bgt-sensitive receptors in the tick synganglia. Moreover, similar increase of currents was also confirmed with the four neonicotinoid insecticides, IMI, CLT, TMX and ACE. We found that neonicotinoids are poor activators of tick nAChRs and neonicotinoid elicited currents were strongly increased by PNU-120596. CLT was more active as an agonist than IMI, TMX and ACE.
Pending on the characterization of tick Ixodes ricinus native functional receptors in X. laevis oocytes, we propose that expression of tick membranes could be used for further studies on the interactions between acaricide compounds and tick nAChRs. Indeed, in the present study, receptors retained their original functional properties and their dynamic regulation by specific receptor associated proteins or phosphorylation mechanism known to modulate insect nAChR function (Courjaret et al., 2003). The initial microtransplantation study was developed injecting AChRs and chloride channels from the electric organ of Torpedo into X. laevis oocytes (Marsal et al., 1995). Then, the same approach was applied by injecting vertebrate brain tissues, demonstrating that this method is very useful for screening and drug assay (Eusebi et al., 2009, Palma, 2011). Recently, enriched-nAChR membranes from the pea aphid Acyrthosiphon pisum was expressed in the X. laevis oocytes which allowed the electrophysiological recordings of nicotine-, CLT- and TMX-evoked currents (Crespin et al., 2016).
5. Conclusion
Altogether, these data indicate that the microtransplantation of tick synganglion membranes is a powerful approach for studying nAChR pharmacology, and may constitute a first step in the development and testing of new acaricides.
Declaration of competing interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The contents of this review are solely the responsibility of the authors.
Acknowledgements
This work was granted in part by the Institut Carnot France Futur Elevage “Xenobio-Tick” and the “Screen-Robot” project (APR IA Région Centre Val de Loire). A. Le Mauff received a Ph.D grant from the French Department of Army (DGA: Direction Générale de l’Armement). H. Chouick received his master of science internship grant from the ANR Xenobio-Tick project. We are grateful to Ms. Guillot-Combe for her support. We are thankful for the help of G. Capron, A. Agoulon, N. de la Cotte, for the collect and the selection of ticks from the field, L. Šimo for the training on tick dissections.
References
- Barbour A.G., Benach J.L. Discovery of the lyme disease agent. mBio. 2019;10 doi: 10.1128/mBio.02166-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissinger B.W., Donohue K.V., Khalil S.M., Grozinger C.M., Sonenshine D.E., Zhu J., Roe R.M. Synganglion transcriptome and developmental global gene expression in adult females of the American dog tick, Dermacentor variabilis (Acari: Ixodidae) Insect Mol. Biol. 2011;20:465–491. doi: 10.1111/j.1365-2583.2011.01086.x. [DOI] [PubMed] [Google Scholar]
- Cardenas-de la Garza J.A., De la Cruz-Valadez E., Ocampo-Candiani J., Welsh O. Clinical spectrum of Lyme disease. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:201–208. doi: 10.1007/s10096-018-3417-1. [DOI] [PubMed] [Google Scholar]
- Cartereau A., Martin C., Thany S.H. Neonicotinoid insecticides differently modulate acetycholine-induced currents on mammalian alpha 7 nicotinic acetylcholine receptors. Br. J. Pharmacol. 2018;175:1987–1998. doi: 10.1111/bph.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C., Hauser F., Gonzalez de Valdivia E., Li S., Reisenberger J., Carlsen E.M., Khan Z., Hansen N.O., Puhm F., Sondergaard L., Niemiec J., Heninger M., Ren G.R., Grimmelikhuijzen C.J. Two types of muscarinic acetylcholine receptors in Drosophila and other arthropods. Cell. Mol. Life Sci. 2013;70:3231–3242. doi: 10.1007/s00018-013-1334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courjaret R., Grolleau F., Lapied B. Two distinct calcium-sensitive and -insensitive PKC up- and down-regulate an alpha-bungarotoxin-resistant nAChR1 in insect neurosecretory cells (DUM neurons) Eur. J. Neurosci. 2003;17:2023–2034. doi: 10.1046/j.1460-9568.2003.02644.x. [DOI] [PubMed] [Google Scholar]
- Crespin L., Legros C., List O., Tricoire-Leignel H., Mattei C. Injection of insect membrane in Xenopus oocyte: an original method for the pharmacological characterization of neonicotinoid insecticides. J. Pharmacol. Toxicol. Methods. 2016;77:10–16. doi: 10.1016/j.vascn.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Davies A.R., Hardick D.J., Blagbrough I.S., Potter B.V., Wolstenholme A.J., Wonnacott S. Characterisation of the binding of [3H]methyllycaconitine: a new radioligand for labelling alpha 7-type neuronal nicotinic acetylcholine receptors. Neuropharmacology. 1999;38:679–690. doi: 10.1016/s0028-3908(98)00221-4. [DOI] [PubMed] [Google Scholar]
- Egekwu N., Sonenshine D.E., Bissinger B.W., Roe R.M. Transcriptome of the female synganglion of the black-legged tick Ixodes scapularis (Acari: Ixodidae) with comparison between Illumina and 454 systems. PloS One. 2014;9 doi: 10.1371/journal.pone.0102667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebi F., Palma E., Amici M., Miledi R. Microtransplantation of ligand-gated receptor-channels from fresh or frozen nervous tissue into Xenopus oocytes: a potent tool for expanding functional information. Prog. Neurobiol. 2009;88:32–40. doi: 10.1016/j.pneurobio.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Gulia-Nuss M., Nuss A.B., Meyer J.M., Sonenshine D.E., Roe R.M., Waterhouse R.M., Sattelle D.B., de la Fuente J., Ribeiro J.M., Megy K., Thimmapuram J., Miller J.R., Walenz B.P., Koren S., Hostetler J.B., Thiagarajan M., Joardar V.S., Hannick L.I., Bidwell S., Hammond M.P., Young S., Zeng Q., Abrudan J.L., Almeida F.C., Ayllon N., Bhide K., Bissinger B.W., Bonzon-Kulichenko E., Buckingham S.D., Caffrey D.R., Caimano M.J., Croset V., Driscoll T., Gilbert D., Gillespie J.J., Giraldo-Calderon G.I., Grabowski J.M., Jiang D., Khalil S.M.S., Kim D., Kocan K.M., Koci J., Kuhn R.J., Kurtti T.J., Lees K., Lang E.G., Kennedy R.C., Kwon H., Perera R., Qi Y., Radolf J.D., Sakamoto J.M., Sanchez-Gracia A., Severo M.S., Silverman N., Simo L., Tojo M., Tornador C., Van Zee J.P., Vazquez J., Vieira F.G., Villar M., Wespiser A.R., Yang Y., Zhu J., Arensburger P., Pietrantonio P.V., Barker S.C., Shao R., Zdobnov E.M., Hauser F., Grimmelikhuijzen C.J.P., Park Y., Rozas J., Benton R., Pedra J.H.F., Nelson D.R., Unger M.F., Tubio J.M.C., Tu Z., Robertson H.M., Shumway M., Sutton G., Wortman J.R., Lawson D., Wikel S.K., Nene V.M., Fraser C.M., Collins F.H., Birren B., Nelson K.E., Caler E., Hill C.A. Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Commun. 2016;7:10507. doi: 10.1038/ncomms10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J.B., Kruger N.J. The bradford method for protein quantitation. Methods Mol. Biol. 1988;3:25–32. doi: 10.1385/0-89603-126-8:25. [DOI] [PubMed] [Google Scholar]
- Houchat J.N., Dissanamossi B.M., Landagaray E., Mathé-Allainmat M., Cartereau A., Graton J., Lebreton J., Le Questel J.Y., Thany S.H. Mode of action of sulfoxaflor on α-bungarotoxin-insensitive nAChR1 and nAChR 2 subtypes: Inhibitory effect of imidacloprid. Neurotoxicology. 2019;74:132–138. doi: 10.1016/j.neuro.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Jongejan F., Uilenberg G. The global importance of ticks. Parasitology. 2004;129(Suppl. l):S3–S14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- Kalappa B.I., Uteshev V.V. The dual effect of PNU-120596 on α7 nicotinic acetylcholine receptor channels. Eur. J. Pharmacol. 2013;718:226–234. doi: 10.1016/j.ejphar.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees K., Jones A.K., Matsuda K., Akamatsu M., Sattelle D.B., Woods D.J., Bowman A.S. Functional characterisation of a nicotinic acetylcholine receptor alpha subunit from the brown dog tick, Rhipicephalus sanguineus. Int. J. Parasitol. 2014;44:75–81. doi: 10.1016/j.ijpara.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees K., Woods D.J., Bowman A.S. Transcriptome analysis of the synganglion from the brown dog tick, Rhipicephalus sanguineus. Insect Mol. Biol. 2010;19:273–282. doi: 10.1111/j.1365-2583.2009.00968.x. [DOI] [PubMed] [Google Scholar]
- Lindquist L. Tick-borne encephalitis (TBE) in childhood. Acta Paediatr. 2008;97:532–534. doi: 10.1111/j.1651-2227.2008.00761.x. [DOI] [PubMed] [Google Scholar]
- Marsal J., Tigyi G., Miledi R. Incorporation of acetylcholine receptors and Cl- channels in Xenopus oocytes injected with Torpedo electroplaque membranes. Proc. Natl. Acad. Sci. U. S. A. 1995;92:5224–5228. doi: 10.1073/pnas.92.11.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Eusebi F., Martinez-Torres A., Palma E., Trettel F. Expression of functional neurotransmitter receptors in Xenopus oocytes after injection of human brain membranes. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13238–13242. doi: 10.1073/pnas.192445299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar N.S., Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Murenzi E., Toltin A.C., Symington S.B., Morgan M.M., Clark J.M. Evaluation of microtransplantation of rat brain neurolemma into Xenopus laevis oocytes as a technique to study the effect of neurotoxicants on endogenous voltage-sensitive ion channels. Neurotoxicology. 2017;60:260–273. doi: 10.1016/j.neuro.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Palma E., Trettel F., Fucile S., Renzi M., Miledi R., Eusebi F. Microtransplantation of membranes from cultured cells to Xenopus oocytes: a method to study neurotransmitter receptors embedded in native lipids. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2896–2900. doi: 10.1073/pnas.0438006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillebois E., Cartereau A., Jones A.K., Thany S.H. Neonicotinoid insecticides mode of action on insect nicotinic acetylcholine receptors using binding studies. Pestic. Biochem. Physiol. 2018;151:59–66. doi: 10.1016/j.pestbp.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Tan J., Galligan J.J., Hollingworth R.M. Agonist actions of neonicotinoids on nicotinic acetylcholine rececptors expressed by cockroach neurons. Neurotoxicology. 2007;28:829–842. doi: 10.1016/j.neuro.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Thany S.H. Agonist actions of clothianidin on synaptic and extrasynaptic nicotinic acetylcholine receptors expressed on cockroach sixth abdominal ganglion. Neurotoxicology. 2009;30:1045–1052. doi: 10.1016/j.neuro.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Thany S.H. Thiamethoxam, a poor agonist of nicotinic acetylcholine receptors expressed on isolated cell bodies, acts as a full agonist at cockroach cercal afferent/giant interneuron synapses. Neuropharmacology. 2011;60:587–592. doi: 10.1016/j.neuropharm.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Thany S.H., Lenaers G., Raymond-Delpech V., Sattelle D.B., Lapied B. Exploring the pharmacological properties of insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2007;28:14–22. doi: 10.1016/j.tips.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Tomizawa M., Zhang N., Durkin K.A., Olmstead M.M., Casida J.E. The neonicotinoid electronegative pharmacophore plays the crucial role in the high affinity and selectivity for the Drosophila nicotinic receptor: an anomaly for the nicotinoid cation--pi interaction model. Biochemistry. 2003;42:7819–7827. doi: 10.1021/bi0300130. [DOI] [PubMed] [Google Scholar]