Introduction
Leprosy is a chronic granulomatous infection caused by Mycobacterium leprae with varied clinical presentations. Owing to its low incidence, together with its myriad clinical presentations, leprosy poses a diagnostic challenge and can easily be confused with other infective and noninfective granulomatous dermatoses.1 Dermoscopy is a simple and non-invasive technique that is widely used for the diagnosis and monitoring of melanoma and nonmelanoma skin cancers and many inflammatory disorders. However, the utility of this technique for the diagnosis of granulomatous diseases has only recently been demonstrated.2 Although dermoscopy shows diagnostically useful patterns in infections and inflammatory diseases with granulomatous presentations, the dermoscopic features of leprosy are poorly documented in the literature.
Case report
A 45-year-old Moroccan man who lived in Spain for many years, but frequently visited his country of origin, was seen at the dermatology service for skin lesions on the trunk and upper and lower extremities. The lesions, which appeared 5 years earlier, were progressive in nature, caused intense itching, and were treated with topical corticosteroids and oral antihistamines without improvement. The patient had undergone analyses 3 years earlier, but no diagnosis had been established: 2 biopsies revealed superficial and deep perivascular and periadnexal dermatitis, raising clinical suspicion of lupus tumidus and granuloma annulare, respectively, whereas multiple laboratory and serological tests showed no relevant alterations.
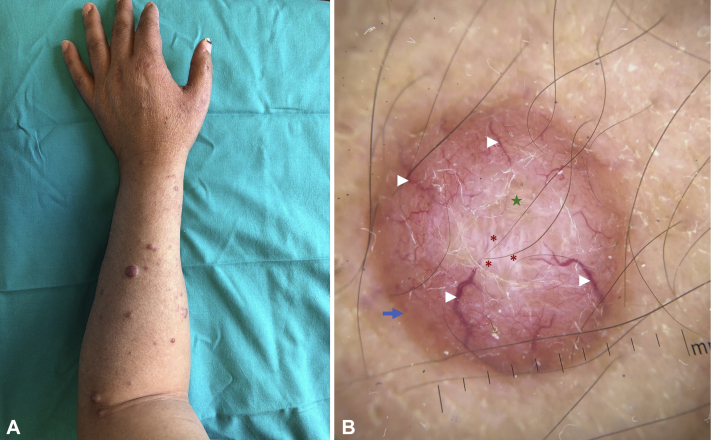
Physical examination found multiple dome-shaped, erythematous to skin-colored infiltrated papules and nodules on the upper and lower extremities and to a lesser extent on the trunk (Fig 1, A). Dermoscopy was performed using a hand-held DermLite II pro HR (3 Gen, Inc., San Juan Capistrano CA) dermatoscope at 10× magnification in polarized contact mode, and photographs were captured with an Apple iPhone X (Apple Inc., Cupertino, CA). Dermoscopy of many lesions found crown vessels and linear branching vessels, yellowish-orange structureless areas, shiny white structures, and peripheral hyperpigmentation (Fig 1, B). The differential diagnosis included sarcoidosis and mycobacterial infection.
Fig 1.
A, Multiple dome-shaped, erythematous to skin-colored infiltrated papules and nodules on the forearm. B, Dermoscopic image of a forearm lesion shows crown vessels (arrowhead), a central yellowish structureless area (star), shiny white structures (asterisk), and peripheral hyperpigmentation (blue arrow). (Original magnification: ×10.)
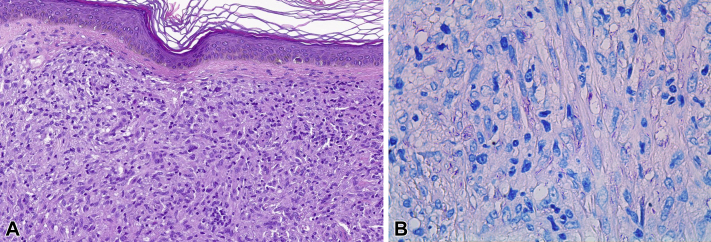
Skin biopsy found diffuse pseudotumoral infiltrate in the dermis and hypodermis that consisted of foamy cells admixed with lymphocytes and plasma cells and mimicked a fibrohistiocytic tumor. Immunohistochemistry for S100 and CD1a was negative, ruling out histiocytosis. Ziehl-Neelsen and Fite-Faraco staining found the presence of acid-alcohol–resistant bacilli. Grocott methenamine silver and periodic acid–Schiff (PAS) staining resulted in strong immunolabelling and clearer visualization of the bacilli, which were arranged in a “cigar bundle” pattern (Fig 2).
Fig 2.
A, Punch biopsy shows a dense histiocytic infiltrate in the dermis with Grenz zone mimicking a fibrohistiocytic neoplasm. B, Fite-Faraco staining shows the presence of bacilli (pink color). (A, Hematoxylin-eosin stain; B, Fite-Faraco stain; original magnifications: A, ×20; B, ×63.)
Based on the clinical and pathologic findings, a diagnosis of granulomatous dermatitis suggestive of histoid leprosy (HL) was established. The diagnosis was confirmed on detection by polymerase chain reaction of M leprae DNA at the Fontilles Foundation, the reference center for leprosy in Spain.
Based on the findings, the 2 biopsies previously taken from the patient were reassessed. Fite-Faraco, Grocott, and PAS staining found small foci of acid-alcohol–resistant bacilli.
The patient's family history was positive for leprosy among family members in his country of origin. The patient began triple therapy with oral dapsone (100 mg/d), rifampin (600 mg/mo) and clofazimine (50 mg/d and 300 mg/mo). Because HL lesions contain abundant bacilli and therefore constitute an important reservoir of infection, individuals cohabitating with the patient were examined periodically.
Discussion
HL is a rare variant of lepromatous leprosy that accounts for only 2.9% of all leprosy cases.3 Clinically, it manifests as dome-shaped papules and nodules on apparently normal skin. Histology shows spindle-shaped histiocytes arranged in varying patterns.4 This form of leprosy is highly bacilliferous and therefore requires early diagnosis and management.5
A recent study correlated the dermoscopic features of different forms of leprosy with clinical and histopathologic findings.6 HL is distinguished by the presence of dome-shaped yellowish-brown areas with few crown vessels over the margins. In a recent clinicodermoscopic study of HL patients,7 linear branching vessels and shiny white structures were the most common findings in all cases analyzed. The background color of the lesions varied from yellowish-brown to pink, and perilesional brown pigmentation was observed. Structureless yellowish-orange areas are thought to correspond to underlying dermal granulomas, and the prominence of vascular structures is attributed to the upward displacement of the dilated vessels by the underlying granulomas.6 Crown vessels are variants of linear branching vessels that originate in the periphery and never fully cross the lesion. In line with previous reports,6, 7, 8 this prominent dermoscopic feature of HL was present in our patient. Furthermore, in our patient, polarized light dermoscopy revealed the presence of shiny white structures. These structures may correspond to the fibrous tissue strands observed in histology.7 Finally, the perilesional brown pigmentation may simply reflect the phototype of the patient.9
Because dermoscopy of HL reveals patterns characteristic of a granulomatous skin condition, including yellow-orange structures together with linear branching vessels, granulomatous skin diseases should be the primary differential diagnosis. Cutaneous sarcoidosis, lupus vulgaris, and necrobiosis lipoidica may manifest with linear branching vessels, but shiny white structures are not included in the dermoscopic features reported to date for these conditions.2 However, linear branching vessels in conjunction with shiny white structures can be observed in basal cell carcinoma. In such cases, it is important to consider the clinical findings, including the number and size of the lesions and other dermoscopic features. Finally, although crown vessels are frequently observed in dermoscopy of sebaceous hyperplasia and molluscum contagiosum, the white-yellow multilobular center characteristic of these conditions is absent in HL lesions.
Histologically, this variant of leprosy should be distinguished from fibrohistiocytic neoplasms, as both have a nodular pseudotumoral growth pattern. Histiocytic cells vary from spindle-shaped to polygonal with foamy cytoplasm. Classical Fite-Faraco and Ziehl-Neelsen staining find numerous acid-fast bacilli, although these are harder to observe in other variants of leprosy in which bacilli are less abundant. PAS and Grocott stains can also be useful to detect M leprae.10 Detection of M leprae DNA is required to confirm diagnosis in doubtful cases.
Linear branching vessels, especially crown vessels, and shiny white structures are prominent dermoscopic features of HL that may be useful for early detection and management of this highly bacilliferous form of leprosy.
Acknowledgments
We thank the Fontilles Foundation, for its invaluable help with diagnostic confirmation and treatment provision and Owen Howard for his writing assistance.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
IRB approval: not applicable.
References
- 1.Maymone M.B.C., Laughter M., Venkatesh S. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020;83(1):1–14. doi: 10.1016/j.jaad.2019.12.080. [DOI] [PubMed] [Google Scholar]
- 2.Errichetti E., Stinco G. Dermatoscopy of granulomatous disorders. Dermatol Clin. 2018;36(4):369–375. doi: 10.1016/j.det.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Mathur M., Jha A., Joshi R., Wagle R. Histoid leprosy: a retrospective clinicopathological study from central Nepal. Int J Dermatol. 2017;56(6):664–668. doi: 10.1111/ijd.13593. [DOI] [PubMed] [Google Scholar]
- 4.Kaur I., Dogra S., De D., Saikia U.N. Histoid leprosy: a retrospective study of 40 cases from India. Br J Dermatol. 2009;160(2):305–310. doi: 10.1111/j.1365-2133.2008.08899.x. [DOI] [PubMed] [Google Scholar]
- 5.Palit A., Inamdar A.C. Histoid leposy as reservoir of the disease: a challenge to leprosy elimination. Lepr Rev. 2007;78(1):47–49. [PubMed] [Google Scholar]
- 6.Vinay K., Kamat D., Chatterjee D., Narang T., Dogra S. Dermatoscopy in leprosy and its correlation with clinical spectrum and histopathology: a prospective observational study. J Eur Acad Dermatol Venereol. 2019;33(10):1947–1951. doi: 10.1111/jdv.15635. [DOI] [PubMed] [Google Scholar]
- 7.Acharya P., Mathur M.C. Clinicodermoscopic study of histoid leprosy: a case series. Int J Dermatol. 2020;59(3):365–368. doi: 10.1111/ijd.14731. [DOI] [PubMed] [Google Scholar]
- 8.Mathur M., Acharya P., Karki A. Visual dermatology: crown vessels in dermoscopy of histoid leprosy. J Cutan Med Surg. 2019;23(3):333. doi: 10.1177/1203475419825759. [DOI] [PubMed] [Google Scholar]
- 9.Ankad B.S., Sakhare P.S. Dermoscopy of histoid leprosy: a case report. Dermatol Pract Concept. 2017;7(2):63–65. doi: 10.5826/dpc.0702a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xavier-Júnior J.C., Ocanha J.P., Marques M.E., Marques S.A. Mycobacterium leprae is usually positive to periodic acid-Schiff and Grocott stains. Am J Dermatopathol. 2016;38(4):322–324. doi: 10.1097/DAD.0000000000000360. [DOI] [PubMed] [Google Scholar]