Abstract
Background Internal carotid artery (ICA) injury is a rare but potentially catastrophic complication of transsphenoidal resection (TSR) of pituitary tumors, potentially resulting in a host of deficits due to the risk of hemorrhage, ischemia, or even death. The endoscopic endonasal approach (EEA) has gained considerable popularity in the modern era, with few busy neurosurgeons remaining committed to practicing transnasal pituitary microsurgery. Our objective was therefore to characterize the overall incidence of ICA injury in a large, longitudinal, single-surgeon microscopic TSR series conducted during the modern EEA era.
Methods Retrospective case series.
Results Overall TSR volume by the senior author (F.B.M.) was 817 pituitary tumors during the study period, 2002 to 2017. Within that cohort, two instances of ICA injury were identified (0.2%), including one each with Cushing's disease and acromegaly, both of whom ultimately recovered without residual neurologic deficit. No pediatric injuries were identified.
Conclusion Vascular injury is an exceedingly rare complication of transsphenoidal pituitary surgery. Adjuncts to prevent this complication include careful review of the coronal magnetic resonance imaging, identification of the midline, as needed use of the Doppler, and initial caudal opening of the sellar dura. Although potentially disastrous, good neurologic outcomes may be obtained, with immediate judicious packing followed by immediate digital subtraction angiography to assess vessel patency and secondary complications such as pseudoaneurysm.
Keywords: transsphenoidal resection, internal carotid artery, vascular injury, complications
Introduction
Injury of the internal carotid artery (ICA) is perhaps the most feared and potentially morbid complication of transsphenoidal resection (TSR) for pituitary adenoma. Historically, a large number of surgeon-related factors have been discussed as predisposing to inadvertent ICA injury during tumor removal via microsurgery (MS) including failure to correctly identify the midline, not appreciating medial deviations of the carotid artery on preoperative imaging, injury during aggressive bony dissection or excessive lateral excursion during tumor resection, or general inexperience of the operator.
As the endoscopic endonasal approach (EEA) to sellar tumors has gained popularity and become the dominant modality for pituitary adenoma resection, much attention has been drawn to both the contrast between MS and EEA, as well technological learning curve associated with EEA, and the significantly increased risk of operative complications (including ICA injury) during the period of early adoption. A large number of publications have explored the relationship between the techniques, including single-surgeon experiences with both, intersurgeon or interinstitution comparisons, multicenter prospective registries, and systematic reviews, among others—the net results of which have been equivocal and heterogeneous regarding the differential risks of complications.
As the neurosurgical community has increasingly embraced EEA, the number of publications reporting transnasal MS outcomes in a contemporary cohort has plummeted, with only three comprehensive series documenting outcomes after MS in more than 250 patients published in the past 15 years. Correspondingly, we sought to report a large, single-surgeon, single-institution experience with MS in a modern cohort of pituitary adenoma patients, with particular attention to the risk of ICA injury. Furthermore, surgical techniques to decrease this risk are discussed, as is the importance of maintaining a MS skillset within the armamentarium of a versatile pituitary adenoma surgeon.
Methods
A prospectively maintained neurosurgical database was retrospectively reviewed for all MS TSR operations performed by the senior author (F.B.M.) during the study period, 2002 to 2017. The study period was limited due to constraints pertinent to our electronic medical record, which is incomplete prior to that time; correspondingly, the total volume of >2,000 transsphenoidal pituitary adenoma resections by the senior author could not be included. Current Procedural Terminology codes were used to screen for TSR, followed by pathology site and diagnosis code screening to confirm pituitary adenoma. The primary study outcome was intraoperative ICA injury, which was identified via keyword search for “carotid” in all pertinent operative reports and postoperative clinical notes; positively screening subjects were subsequently chart reviewed by two study staff (L.P.C. and C.S.G.) to confirm intraoperative findings consistent with ICA injury, and to capture secondary data points, including age and demographics, tumor secretory status and pathologic staining profile, injury morphology, mechanism, and treatment, and clinical outcome, including neurologic disability and vital status at last follow-up. Pertinent study components were approved and overseen by our Institutional Review Board (protocol #15-003098).
Results
Retrospective review identified more than 1,200 transnasal MS TSR records, of which 817 were confirmed pituitary adenoma operations. Repeat resections accounted for 5.5% overall ( n = 45). Median age at the time of surgery was 49 years (range: 5–83), including six pediatric patients (median age: 10 years; range: 5–16). Overall, 54% of patients were female ( n = 441). Screening identified 311 operative reports in which the carotid was explicitly mentioned (38.1%); secondary review confirmed vascular injury in two cases (0.2%). Both injuries occurred in adult patients, one during a repeat resection in a patient with refractory Cushing's disease and one during a primary resection for acromegaly; disease-specific injury rates were 0.7 and 0.9%, respectively ( Table 1 ). Further details from the patient histories are reviewed later and summarized in Table 2 .
Table 1. ICA injury incidence by tumor pathology and patient age.
| Total patients ( n ) | Carotid injuries ( n ) | Incidence (%) | |
|---|---|---|---|
| All transsphenoidal pituitary tumor resections | 817 | 2 | 0.2 |
| Adult pituitary tumors | 810 | 2 | 0.2 |
| Cushing's disease | 135 | 1 | 0.7 |
| Acromegaly | 111 | 1 | 0.9 |
| Pediatric pituitary tumors | 6 | 0 | 0.0 |
Abbreviation: ICA, internal carotid artery.
Table 2. Detailed description of series ICA injuries.
| Tumor type | Risk factors | ICA segment | Injury mechanism | Acute management | ICA outcome | Neuro outcome |
|---|---|---|---|---|---|---|
| Cushing's disease | Multiple resections; SRS | Parasellar | Damaged by ring curette during tumor resection | Simple packing, endovascular assessment | Patent; normal morphology | Intact |
| Acromegaly | Adjacent dural AVF | Parasellar | Sharp puncture during dural opening | Packing, fat graft, inflatable balloon, endovascular assessment | Killed due to progressive pseudoaneurysm | Intact |
Abbreviations: AVF, arteriovenous fistula; ICA, internal carotid artery; SRS, stereotactic radiosurgery.
Case 1
A 60-year-old man with a past medical history of endocarditis, open aortic and pulmonary valve replacements, coronary artery disease status postbypass grafting, and continuous positive airway pressure–dependent obstructive sleep apnea was diagnosed with acromegaly in the work-up for ventricular tachycardia at an outside institution. Pituitary macroadenoma was identified on magnetic resonance imaging (MRI) of the brain, for which he was referred to our practice ( Fig. 1A ). Preoperative ophthalmologic evaluation did not reveal a visual field deficit, initial insulin-like growth factor 1 (IGF-1) was 1,493 ng/mL (reference range: 50–317 ng/mL), and studies of all other pituitary axis end hormones including cortisol, free thyroxine, and testosterone were within normal limits. MS TSR with our experienced multidisciplinary ENT neurosurgery team was recommended as initial therapy, and the patient agreed to proceed with surgery.
Fig. 1.
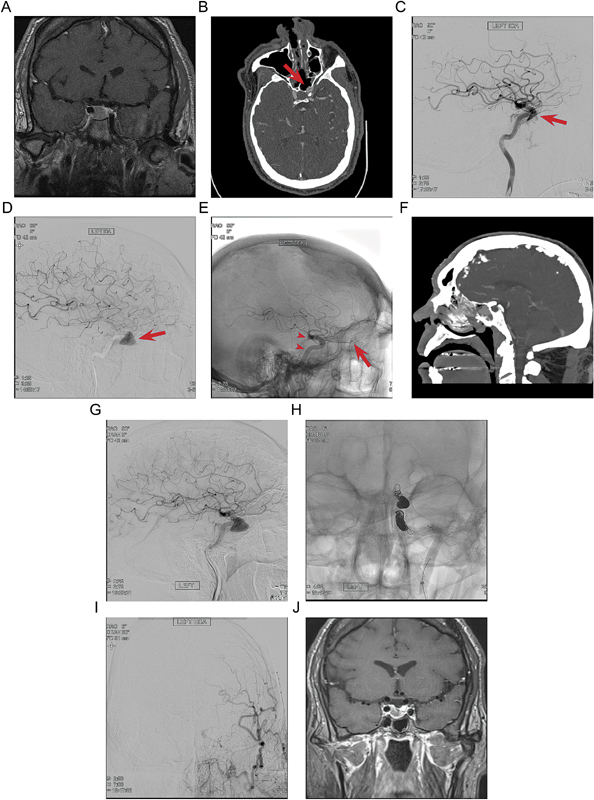
( A ) Preoperative contrast-enhanced MRI in the coronal plane demonstrates an enhancing sellar mass. ( B ) Postoperative axial CT angiography demonstrates a bulge of the left ICA in the sphenoid sinus through a bony defect (red arrow). ( C ) Postoperative lateral angiogram of the left ICA shows opacification of the cavernous sinus in the mid-arterial phase, indicating a direct cavernous carotid fistula (red arrow). ( D ) Three-day postoperative lateral left ICA angiogram shows growth of the pseudoaneurysm (red arrow) and diminution of the direct cavernous carotid fistula. ( E ) Magnified unsubtracted lateral angiogram on postoperative day 3 shows the placement of two flow diversion embolization devices across the pseudoaneurysm (red arrowheads), with persistent flow through the ophthalmic artery (red arrow ) . ( F ) Sagittal CT angiogram on postoperative day 5 shows continued filling of the large pseudoaneurysm in the sphenoid sinus. ( G ) Lateral angiogram on postoperative day 6 shows persistent filling of the large pseudoaneurysm in the sphenoid sinus. ( H ) Unsubtracted AP angiogram shows successful platinum coil embolization of the ICA pseudoaneurysm. ( I ) ECA injection angiogram in AP projection on postoperative day 22, taken following after an episode of epistaxis, demonstrates prominent ECA flow via ophthalmic collaterals without filling of the pseudoaneurysm. ( J ) Two-month follow-up contrast-enhanced MRI in the coronal plane demonstrates reduction in the size of the pituitary adenoma. CT, computed tomography; ECA, external carotid artery; ICA, internal carotid artery; MRI, magnetic resonance imaging.
Intraoperatively, marked osteoarticular hypertrophy attributable to severe prolonged acromegaly was encountered, which rendered definitive discrimination of the bony sella by direct visualization effectively impossible. Fluoroscopy was used for anatomic localization, and removal of the bony sellar floor proceeded uneventfully. Hypervascular-appearing dura was encountered, coagulated, and open sharply, resulting in brisk arterial bleeding. Temporary control was established using compressed gelatin sponge and cotton patties, which were replaced by an abdominal fat graft, supported using an inflatable nasal Fogarty balloon catheter.
Once reliable hemostasis was established, the operation was aborted, and the patient was taken for emergent computed tomography (CT) angiography followed by DSA, which demonstrated a new left ICA pseudoaneurysm ( Fig. 1B, C ). The patient was admitted to neurosciences intensive care unit and kept intubated overnight, after which sedation was weaned to extubation; throughout the early postoperative period, the neurologic examination remained consistently at baseline. Repeat CT angiogram on postoperative day (POD)3 demonstrated expansion of the pseudoaneurysm; correspondingly, a balloon occlusion test was conducted ( Fig. 1D ). This was well tolerated for more than 15 minutes without a neurologic change. Given the rapid pseudoaneurysm expansion and low risk of further complications given the successful occlusion test, flow diversion was offered as a final attempt to salvage the vessel.
The patient was reintubated and, under general anesthesia, 4.75 × 18 mm and 4.75 × 16 mm Pipeline Embolization Devices (Medtronic, Minneapolis, Minnesota, United States) were placed in the left ICA, spanning the pseudoaneurysm ( Fig. 1E ). Subsequent DSA on POD5 showed further progressive pseudoaneurysm growth, and after extensive discussion with the patient and family, endovascular sacrifice of the left ICA was recommended ( Fig. 1F, G ). This was performed on POD6 using platinum coils, and the procedure was tolerated without evidence of a new neurologic deficit, after which he was ultimately discharged home on POD10 ( Fig. 1H ).
On POD22, the patient returned to the emergency department with severe epistaxis, prompting urgent packing followed by cerebral DSA, which demonstrated hypertrophic external carotid artery vascularity, but no recurrence of the pseudoaneurysm, treatment failure, or contrast extravasation from the ICA system ( Fig. 1I, J ). In follow-up at 2 months, consideration was given to primary stereotactic radiosurgery; however, pretreatment laboratory evaluation demonstrated normal IGF-1 with a marked reduction adenoma size as compared with baseline imaging, presumably due to decreased arterial supply to the tumor. Treatment was deferred at that time and the patient has been followed up conservatively; at 1-year postinjury, the patient's weight was decreased by 50 lbs, yet he continued to report disabling fatigue, in spite of persistent normal pituitary function across all hormonal axes.
Case 2
A patient with Cushing's disease presented with recurrent, symptomatic hypercortisolemia and microadenoma, status post three prior resections, and stereotactic radiosurgery at an outside institution. Correspondingly, the intraoperative dissection was markedly difficult due to the heavily scarred and fibrotic operative field, within which few reliable anatomic landmarks were identifiable to guide exposure of the sella. After careful dissection, the sella was safely identified, and the dura was opened sharply.
Exploration of the sella proceeded in the usual fashion, adenoma tissue was identified in the lateral compartment, and tumor resection was initiated with blunt ring curettes. As tumor was gently elevated off the medial wall of the cavernous sinus, bright red, high-pressure bleeding was encountered from an obvious arterial source. This was subsequently identified to be the anterior genu of the left ICA, which was minimally prolapsed toward the sella, most likely due to the prior operations. Hemorrhage was quickly controlled via primary packing with compressed gelatin sponge, and the patient was taken directly for digital subtraction angiography (DSA), which confirmed patency of the ICA and all its branches. The patient awoke from anesthesia with an incomplete left third nerve palsy, which spontaneously resolved in follow-up. Multiple repeat DSA and MR angiography studies confirmed no delayed development of pseudoaneurysm, thrombosis, or other injury sequela. With respect to her endocrine status, the patient remained persistently hypercortisolemic and was offered bilateral adrenalectomy after recovering from her pituitary operation. This resulted in durable resolution of her symptoms, and subsequent clinical assessments and MRI studies demonstrated no evidence of Nelson–Salassa's syndrome over 8 years of follow-up.
Discussion
We present the second longest series of transnasal MS TSR for pituitary adenoma reporting incidence of ICA injury during the modern endoscopic era, with an ICA injury incidence of 0.2% in 817 pituitary adenoma operations. Our results provide an essential counterpoint to the literature suggesting a safety advantage with EEA in shorter series, compared against historic or same surgeon MS controls, or in meta-analysis. 1 2 3 4 5 6 7 8 9 Based on this, we emphasize the importance of surgeon experience as the most important single variable contributing to risk of this potentially catastrophic complication, independent of surgical approach.
Comparison with Comparable Preceding Analyses
Although an ICA injury incidence of 0.2% is by no means trivial, it is among the lowest reported, with most prior series of either MS or EEA TSR for pituitary adenoma describing 0.5 to 1.7% incidences. 1 2 3 4 5 6 7 10 11 12 13 Perhaps more importantly, our results accord with comparably expansive series in both MS and EEA practices, including the three preceding analyses explicitly reporting ICA injuries in larger cohorts of pituitary adenoma patients. 6 9 14 The only larger preceding MS TSR series documenting ICA outcomes was reported by Mortini et al in 2005, with zero injury in 1,140 TSR operations for pituitary adenoma. 9 Saliently, they also stratified 15 preceding analyses reporting complications in any MS TSR operations, and noted that the incidence of ICA injuries in cohorts of <200, 200 to 500, and >500 patients were 1.4, 0.6, and 0.4%, respectively.
Critically, two of the lowest incidence series reviewed were published by Laws and Wilson and Dempsey, by far the most experienced and forward-thinking practitioners of transsphenoidal surgery in their generation. 8 9 15 That their results remained so impressive in a mixed-pathology series that included higher risk lesions such as craniopharyngioma, and that encompassed portions of their personal learning curves and the development of transsphenoidal surgery at large, compellingly reinforces our hypothesis that surgeon experience may be the most significant protective factor, with respect to general complication avoidance, and reduction of vascular injury in particular.
Two other large MS TSR series reported prior to the study period by Raymond et al in 1997 and Fukushima and Maroon in 1998 documented incidences of 0.9 and 0.4%, in population of ∼1,800 and 1,600 operations, respectively. Recently, two comparably large TSR series reporting ICA injury outcomes described extensive single-center EEA experiences. Kalinin et al recorded four ICA injuries in more than 3,000 EEA TSR operations, for a cumulative incidence of 0.1%, the lowest rate reported in any positive series. 14 With respect to morphology, they attributed the injuries to incorrect assessment of the midline in two cases, a medially displaced ICA in one and aggressive dissection along the cavernous sinus wall in the other; two mortalities resulted from the injuries, while the other individual patients remained neurologically stable.
Gardner et al reported seven ICA injuries in a series of 2015 EEA operations for mixed pathologies, of which two occurred in 660 pituitary adenoma resections (0.3%). 6 The first case involved a rongeur injury during bony exposure that require clip occlusion for intraoperative stabilization, followed by endovascular ICA sacrifice; unfortunately, the patient died within 36 hours of surgery, attributed to postoperative cardiac ischemia. 6 The second case was attributed to avulsion of an ICA perforator during tumor resection; a pseudoaneurysm developed acutely, was successfully treated via endovascular stenting, and the patient developed no neurologic deficits.
Taken together with our own results, these series compellingly demonstrate how, even in exceedingly experienced hands, ICA injury is a formidable complication with potentially grave consequences; notwithstanding, in many circumstances, the experienced surgeon is also able to triage swiftly and effectively, avoiding an adverse outcome in the majority of cases.
MS versus EEA: Are We Asking the Right Question?
As more data have accumulated on EEA and experience has grown with the technique, a remarkable number of comparative analyses have been designed to compare the efficacy of MS and EEA for pituitary TSR, with broadly equivocal results. Of particular interest, several meta-analyses and national database-derived publications have attempted to reconcile the conflicting conclusions, several of which have specifically addressed the question of differential risk of vascular injury.
Ammirati et al reviewed combined MS and EEA cohorts of 3,023 and 1,887 patients in a comprehensive 2013 meta-analysis focused on perioperative complications. 11 They identified a small but statistically significant increase in vascular complications associated with EEA TSR, with an overall incidence of 1.58% (95% confidence interval [CI]: 1.07–2.19%), as compared with MS at 0.50% (95% CI: 0.28–0.78%). Although their results are quite interesting, their methodology was subject to criticism from multiple sides: by excluding studies that did not very clearly separate complications and pathologies, several large, landmark series from highly experienced surgeons were excluded; the minimum cohort size for inclusion was 10, meaning that many early EEA publications during the “learning curve” period were included; nearly half of the EEA complications were attributable to a single study; and, in spite of their general rigor, ICA injuries were not parsed separately from intracerebral hemorrhages, infarcts, and other miscellaneous vascular complications. 16 17 The authors state in their discussion how the majority of the complications localized to suprasellar distributions, and could therefore be inferred as attributable to intraoperative injury; however, given the narrow margin between the CIs, the potential for an exclusion error to impact on significance is not negligible ( Table 3 ).
Table 3. ICA injury incidence in series with comparable TSR experience.
| Author | Year | ICA injury incidence | Operative approach |
|---|---|---|---|
| Mortini | 2005 | 0/1,140 (0.0%) | MS |
| Gardner | 2013 | 2/660 (0.3%) | EEA |
| Kalinin | 2013 | 4/3,000 (0.1%) | EEA |
| Meyer | 2017 | 2/817 (0.2%) | MS |
Abbreviations: EEA, endoscopic endonasal approach; ICA, internal carotid artery; MS, microsurgery; TSR, transsphenoidal resection.
Note : Both EEA and MS groups included, given total and MS experiences both >250 cases.
Almost all other meta-analyses or national database studies comparing MS and EEA in pituitary TSR either did not report explicitly on ICA injury rates, or found no significant difference, as in the recent publications from Asemota et al and Goudakos et al, both of which noted incidences from 0.0 to 0.5% using either approach. 1 18 The noteworthy exception was published by Esquenazi et al in 2017, in which studies of recurrent disease demonstrated a marginally significant advantage after MS (0.7 vs. 0.9%, p = 0.01). 19 Perhaps most interestingly, two other recent publications by Rowan et al in 2017 reported survey results from practicing skull base surgeons at national courses, and documented more than 20% ICA injuries during the preceding 12 months in a population of 134 operators, suggesting that the rates published in the literature significantly underestimate the true incidence of this complication. 20 21 When considered in light of data from multiple sources highlighting the predictive value of experience in complication avoidance during transsphenoidal pituitary surgery, the argument for discouraging amateurism galvanizes, as does the importance of encouraging all practicing neurosurgeons to report their complications honestly and in detail—particularly for rare entities. 4 8 9 14 22 23 24 25
Ultimately, it is our view that both operative techniques have inherent strengths and weaknesses ( Table 4 ). While MS through a transnasal approach offers a narrower field, depth perception is truly three dimensional, as compared with the wide-angle two-dimensional EEA vista. MS cases can be quickly set up and completed within 60 minutes, whereas EEA requires much more apparatus, and is prone to a more tedious intraoperative course. Although MS instrumentation is relatively modest and simple, the EEA apparatus is at once limiting in the technological demands placed on the staff, but also empowering, with angled lenses able to deliver views that are impossible to achieve with the microscope. Given the emphasis our results and those of our predecessors place on experience, and the established learning curve for developing a safe EEA practice, MS has the advantage of a manual skillset that overlaps with general cranial neurosurgery; by contrast, although transnasal endoscopy requires discrete training, it also gives its advanced practitioners access to another set of tools and techniques that may prove helpful in other applications. 16 26 27 28 29 That the MS operator is obliged to operate independently is at once beneficial and limiting: two hands cannot hold as many instruments as three or four, and an extra suction can be invaluable during heavy bleeding, yet in the setting of a potentially catastrophic situation such as an ICA injury, the advantages of perfect coordination without dependence on verbal communication may facilitate a highly efficient repair, potentially decreasing ischemia time. It is important underscore that this publication is in no way advocating that one approach is superior, or carries higher risk of carotid artery injury. Carotid injury is thankfully a rare occurrence in transsphenoidal operations, and in our experienced surgeon's cohort, MS does not lead to higher rates than have been published with endoscopic techniques.
Table 4. Subjective comparison of techniques, based on the authors' experience.
| Microscopic TSR | Endoscopic TSR |
|---|---|
| Narrow microscopic field, but with depth perception | Wide endoscopic field, but without depth perception |
| Shorter operative and turn-over times; may require two setup if ENT prefers endoscopic approach | Longer operative and turn-over times; congruent instrumentation with endoscopic ENT practice |
| Simple, more foolproof instrumentation | Modern instrumentation, more fail-prone |
| Microscope-and-speculum system imposes a streamlined working corridor; may decrease risk of inadvertent ICA exposure, or lateral excursion into vessel | Wider exposure with broad visualization may allow for more definitive identification of small, laterally placed anatomic structures (e.g., vidian canal as marker for petrous ICA) |
| Congruent manual skillset with cranial microsurgery | Novel skill developed, but with learning curve |
| One-surgeon two-hands technique highly efficient; minimizes opportunity for coordination error | Two-surgeon three-/four-hands technique highly versatile; supports additional instrument, but requires expert coordination and communication |
Abbreviations: ICA, internal carotid artery; TSR, transsphenoidal resection.
With these concepts in mind, our ultimate assessment is that each approach has a critical and still evolving role in the overarching treatment paradigm for pituitary disease. Further, it seems most likely that the inherent differences between the techniques, as well as the associated patient selection biases, are the key drivers behind the divergent results that our predecessors have reported in trials and analyses pitting the techniques head-to-head.
Lessons: Injury Prevention
Technical considerations for ICA injury in either MS or EEA TSR can be conceptualized as falling into two categories: prevention strategies and postinjury actions. Prevention is rooted in careful review of all preoperative imaging studies, with careful attention paid to the anatomic position of the carotid siphon, where the anterior genus may be medially deviated in what has been colloquially referred to as “kissing carotids.” 10 30 31 If significant medial deviation of either carotid is noted, or the aperture between the carotids is <4 to 5 mm at the sellar floor, consideration should be given to a day-of-surgery CT angiogram for stereotactic guidance. However, even with stereotaxis, a particularly small window may render sellar and suprasellar access quite difficult. Intraoperatively, as the bony sellar floor is exposed and removed, definitive anatomic orientation must be maintained at all times, particularly with respect to midline structures. Again, careful review of preoperative imaging is critical, potentially supplemented by stereotaxis in challenging cases; however, deliberate, verbal confirmation of the rostrum, keel, septum, and other pertinent structures must be carried together with the exposing ENT surgeon before any hand-offs are performed, or bony decompression is initiated. Bone removal begins with a small central fracturing of the central sellar floor, which can be outwardly expanded using a small punch or rongeur to take modest, stepwise bites. Twisting motions intended to remove larger fragments should be avoided, as the fracture lines will be unpredictable, and the distal ends of the bone may be sharp and can potentially lacerate the ICA. Acromegalic patients present a particular set of challenges due to their propensity for atypical patterns of osteoarticular hypertrophy, and extreme care should be taken during bone removal, particularly at the lateral sellar margins.
With the bone work carefully completed, the dural incision is another critical point for avoidance of error. Beginning with a sharp, limited, caudal incision at the midline has several benefits in addition to avoidance of a medially deviated ICA, including preservation of the underlying gland, decreased risk of cerebrospinal fluid leak from an excessively rostral durotomy, and a more stable environment to establish control in the event of any unexpected complications. With a small incision safely opened, blunt dissectors can carefully expand the plane between dura and gland, allowing the dural opening to be more safely carried laterally and superiorly, completing the exposure.
At any point during the operation, ambiguity with regard to the ICA position may be resolved by the use of a micro Doppler—particularly prior to the dural opening, or if there is any suspicion for atypical or medialized positioning of the carotids. Finally, in special cases, such as the most severe acromegalics, or pediatric patients without a well-pneumatized sella, the sublabial incision may provide a wider and more direct midline corridor, which facilitates safe use of a high-speed drill in coordination with the MS technique.
Lessons: Injury Management
In the unlikely event of an intraoperative ICA injury, above all, the neurosurgeon must remain calm and clear headed, to facilitate rapid and effective communication with the surgical team. 32 Immediate temporary control of bleeding can almost always be established with compressed gelatin sponge and cottonoid patties, and once tamponade has been established, the anesthesiologist and circulating nurse should be apprised of the situation. Unless the bleeding is torrential and poorly controlled, the injury site should be carefully inspected, to verify that the carotid was injured and not a venous sinus. Rarely, the source of bleeding may be a cavernous carotid dural branch which can be difficult to differentiate from a true carotid injury. As needed an abdominal fat or muscle graft can be used to buttress the Gelfoam packing as a prelude to angiography. This can be supported with an inflatable Fogarty balloon placed transnasal to reside in the sphenoid sinus. Of course, the trick is to pack enough to stop the bleeding but to avoid severe stenosis or occlusion of the carotid artery.
Once the patient is stabilized and the injury packed, immediate imaging is required, ideally via DSA or, if a neurointerventional suite is not available, CT angiogram. 33 34 If a large pseudoaneurysm, dissection flap, or other acute arterial injury is confirmed on DSA, management options include observation with short interval repeat imaging, endoluminal reconstruction techniques (e.g., stenting, flow diversion), or test balloon occlusion prior to ICA sacrifice via coil embolization (with or without stent assistance). 22 35 36 As in Case 1, we recommend interval observation and balloon occlusion testing prior to carotid sacrifice, if at all possible.
Study Limitations
The present study is subject to several key limitations, in addition to all the well-described issues with retrospective reviews, such as selection bias, and confounders. Chiefly, due to the nature of a referral practice and the relative youth of our EEA cohort, a direct comparison between MS and EEA could not be completed at this time using our institutional experience. It is unfortunate that a substantial number of the senior surgeon's cohort cases were not able to be included prior to 2002 due to incomplete medical record availability, and we cannot rule out that additional cases of carotid injury occurred during that time frame. We also cannot exclude the possibility that our search criteria of “carotid” did not leave out patients where operative and postoperative clinical notes only used “vessel” or “blood vessel”; however, we think it is highly unlikely that an adverse event as serious as a carotid injury would not have had the term “carotid” used a single time during formal documentation, and in particular, formal catheter angiography reports would also indicate which vessels were studied. Additionally, as the study cohort was derived from a single surgeon's practice, the results are not strictly generalizable; however, this feature is also a strength of the analysis, given that intersurgeon variability is removed as a confounder, and we are therefore able to present an important data point for the incidence of ICA injury in a busy, experienced, and exclusively microsurgical pituitary practice during the modern era.
Conclusion
The use of the operative microscope during transnasal TSR of pituitary lesions facilitates a quick operation with excellent results at exceedingly low risk. Techniques to avoid ICA injury include attentive and deliberate review of preoperative imaging, identification of the midline before removal of the sellar floor, initiating dural opening with a limited caudal incision, and intraoperative ICA localization via Doppler ultrasound and/or stereotaxis. When compared with other landmark series and meta-analyses, our experience with MS confirms that in experienced hands, it remains a safe, effective, and essential component of the pituitary armamentarium.
Conflict of Interest Jenna Meyer, MS, Avital Perry, MD, Christopher S. Graffeo, MD, Lucas P. Carlstrom, MD, Christopher R. Marcellino, Anthony Burrows, MD, Irina Bancos, MD, and Colin Driscoll, MD, declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Presentation
Components of this work were submitted as an abstract to the North American Skull Base Society, 2018.
References
- 1.Asemota A O, Ishii M, Brem H, Gallia G L. Comparison of complications, trends, and costs in endoscopic vs microscopic pituitary surgery: analysis from a US Health claims database. Neurosurgery. 2017;81(03):458–472. doi: 10.1093/neuros/nyx350. [DOI] [PubMed] [Google Scholar]
- 2.Cappabianca P, Cavallo L M, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002;97(02):293–298. doi: 10.3171/jns.2002.97.2.0293. [DOI] [PubMed] [Google Scholar]
- 3.Cavallo L M, Briganti F, Cappabianca P et al. Hemorrhagic vascular complications of endoscopic transsphenoidal surgery. Minim Invasive Neurosurg. 2004;47(03):145–150. doi: 10.1055/s-2004-818489. [DOI] [PubMed] [Google Scholar]
- 4.Dusick J R, Esposito F, Malkasian D, Kelly D F.Avoidance of carotid artery injuries in transsphenoidal surgery with the Doppler probe and micro-hook blades Neurosurgery 2007600402322–328., discussion 328–329 [DOI] [PubMed] [Google Scholar]
- 5.Fukushima T, Maroon J C. Repair of carotid artery perforations during transsphenoidal surgery. Surg Neurol. 1998;50(02):174–177. doi: 10.1016/s0090-3019(96)00416-8. [DOI] [PubMed] [Google Scholar]
- 6.Gardner P A, Tormenti M J, Pant H, Fernandez-Miranda J C, Snyderman C H, Horowitz M B.Carotid artery injury during endoscopic endonasal skull base surgery: incidence and outcomesNeurosurgery 2013; 73(2, Suppl Operative):ons261–ons269, discussion ons269–ons270 [DOI] [PubMed]
- 7.Gondim J A, Almeida J P, Albuquerque L A et al. Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary. 2011;14(02):174–183. doi: 10.1007/s11102-010-0280-1. [DOI] [PubMed] [Google Scholar]
- 8.Laws E R., Jr Vascular complications of transsphenoidal surgery. Pituitary. 1999;2(02):163–170. doi: 10.1023/a:1009951917649. [DOI] [PubMed] [Google Scholar]
- 9.Mortini P, Losa M, Barzaghi R, Boari N, Giovanelli M.Results of transsphenoidal surgery in a large series of patients with pituitary adenoma Neurosurgery 200556061222–1233., discussion 1233 [DOI] [PubMed] [Google Scholar]
- 10.Ahuja A, Guterman L R, Hopkins L N.Carotid cavernous fistula and false aneurysm of the cavernous carotid artery: complications of transsphenoidal surgery Neurosurgery 19923104774–778., discussion 778–779 [DOI] [PubMed] [Google Scholar]
- 11.Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84(08):843–849. doi: 10.1136/jnnp-2012-303194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciric I, Ragin A, Baumgartner C, Pierce D.Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience Neurosurgery 19974002225–236., discussion 236–237 [DOI] [PubMed] [Google Scholar]
- 13.Kassam A B, Prevedello D M, Carrau R L et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors' initial 800 patients. J Neurosurg. 2011;114(06):1544–1568. doi: 10.3171/2010.10.JNS09406. [DOI] [PubMed] [Google Scholar]
- 14.Kalinin P, Sharipov O, Shkarubo A.Damage to the cavernous segment of the internal carotid artery upon transsphenoidal endoscopic removal of pituitary adenomas (report of four cases) Zh Vopr Neirokhir Im N N Burdenko 2013770628–37.; discussion 38 [PubMed] [Google Scholar]
- 15.Wilson C B, Dempsey L C. Transsphenoidal microsurgical removal of 250 pituitary adenomas. J Neurosurg. 1978;48(01):13–22. doi: 10.3171/jns.1978.48.1.0013. [DOI] [PubMed] [Google Scholar]
- 16.Graffeo C S, Dietrich A R, Grobelny B et al. A panoramic view of the skull base: systematic review of open and endoscopic endonasal approaches to four tumors. Pituitary. 2014;17(04):349–356. doi: 10.1007/s11102-013-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laws E R. Complications of transsphenoidal surgery: the shortcomings of meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84(08):829. doi: 10.1136/jnnp-2012-304541. [DOI] [PubMed] [Google Scholar]
- 18.Goudakos J K, Markou K D, Georgalas C. Endoscopic versus microscopic trans-sphenoidal pituitary surgery: a systematic review and meta-analysis. Clin Otolaryngol. 2011;36(03):212–220. doi: 10.1111/j.1749-4486.2011.02331.x. [DOI] [PubMed] [Google Scholar]
- 19.Esquenazi Y, Essayed W I, Singh H et al. Endoscopic endonasal versus microscopic transsphenoidal surgery for recurrent and/or residual pituitary adenomas. World Neurosurg. 2017;101:186–195. doi: 10.1016/j.wneu.2017.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowan N R, Turner M T, Valappil B, Fernandez-Miranda J C, Wang E W, Gardner P A. Injury of the carotid artery during endoscopic endonasal surgery: surveys of skull base surgeons. J Neurol Surg B Skull Base. 2018;79(03):302–308. doi: 10.1055/s-0037-1607314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowan N R, Turner M T, Wang E W, Fernandez-Miranda J, Gardner P A, Snyderman C H. A skull base course participants' experience with endoscopic endonasal carotid artery injuries. J Neurol Surg B Skull Base. 2017;78:A081. [Google Scholar]
- 22.Brinjikji W, Lanzino G, Cloft H J. Cerebrovascular complications and utilization of endovascular techniques following transsphenoidal resection of pituitary adenomas: a study of the Nationwide Inpatient Sample 2001-2010. Pituitary. 2014;17(05):430–435. doi: 10.1007/s11102-013-0521-1. [DOI] [PubMed] [Google Scholar]
- 23.Graffeo C S, Perry A, Carlstrom L P, Copeland W R, III, Van Abel K M, Link M J. Small stories: in defense of the humble case report. World Neurosurg. 2017;105:1009–1010. doi: 10.1016/j.wneu.2017.05.150. [DOI] [PubMed] [Google Scholar]
- 24.Graffeo C S, Perry A, Carlstrom L P et al. Characterizing and predicting the Nelson-Salassa syndrome. J Neurosurg. 2017;127(06):1277–1287. doi: 10.3171/2016.9.JNS161163. [DOI] [PubMed] [Google Scholar]
- 25.Perry A, Graffeo C S, Copeland W R, III et al. Delayed cerebrospinal fluid rhinorrhea after Gamma Knife radiosurgery with or without preceding transsphenoidal resection for pituitary pathology. World Neurosurg. 2017;100:201–207. doi: 10.1016/j.wneu.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Barr J, Graffeo C S. Procedural experience and confidence among graduating medical students. J Surg Educ. 2016;73(03):466–473. doi: 10.1016/j.jsurg.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Koc K, Anik I, Ozdamar D, Cabuk B, Keskin G, Ceylan S.The learning curve in endoscopic pituitary surgery and our experience Neurosurg Rev 20062904298–305., discussion 305 [DOI] [PubMed] [Google Scholar]
- 28.O'Malley B W, Jr, Grady M S, Gabel B C et al. Comparison of endoscopic and microscopic removal of pituitary adenomas: single-surgeon experience and the learning curve. Neurosurg Focus. 2008;25(06):E10. doi: 10.3171/FOC.2008.25.12.E10. [DOI] [PubMed] [Google Scholar]
- 29.Qureshi T, Chaus F, Fogg L, Dasgupta M, Straus D, Byrne R W. Learning curve for the transsphenoidal endoscopic endonasal approach to pituitary tumors. Br J Neurosurg. 2016;30(06):637–642. doi: 10.1080/02688697.2016.1199786. [DOI] [PubMed] [Google Scholar]
- 30.Fujii K, Chambers S M, Rhoton A L., Jr Neurovascular relationships of the sphenoid sinus. A microsurgical study. J Neurosurg. 1979;50(01):31–39. doi: 10.3171/jns.1979.50.1.0031. [DOI] [PubMed] [Google Scholar]
- 31.Renn W H, Rhoton A L., Jr Microsurgical anatomy of the sellar region. J Neurosurg. 1975;43(03):288–298. doi: 10.3171/jns.1975.43.3.0288. [DOI] [PubMed] [Google Scholar]
- 32.Cinar C, Bozkaya H, Parildar M, Oran I. Endovascular management of vascular injury during transsphenoidal surgery. Interv Neuroradiol. 2013;19(01):102–109. doi: 10.1177/159101991301900116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciceri E F, Regna-Gladin C, Erbetta A et al. Iatrogenic intracranial pseudoaneurysms: neuroradiological and therapeutical considerations, including endovascular options. Neurol Sci. 2006;27(05):317–322. doi: 10.1007/s10072-006-0703-y. [DOI] [PubMed] [Google Scholar]
- 34.Raymond J, Hardy J, Czepko R, Roy D. Arterial injuries in transsphenoidal surgery for pituitary adenoma; the role of angiography and endovascular treatment. AJNR Am J Neuroradiol. 1997;18(04):655–665. [PMC free article] [PubMed] [Google Scholar]
- 35.Kadyrov N A, Friedman J A, Nichols D A, Cohen-Gadol A A, Link M J, Piepgras D G. Endovascular treatment of an internal carotid artery pseudoaneurysm following transsphenoidal surgery. Case report. J Neurosurg. 2002;96(03):624–627. doi: 10.3171/jns.2002.96.3.0624. [DOI] [PubMed] [Google Scholar]
- 36.Sylvester P T, Moran C J, Derdeyn C P et al. Endovascular management of internal carotid artery injuries secondary to endonasal surgery: case series and review of the literature. J Neurosurg. 2016;125(05):1256–1276. doi: 10.3171/2015.6.JNS142483. [DOI] [PubMed] [Google Scholar]


