Abstract
Objective This study was aimed to evaluate health-related quality of life in patients undergoing anterolateral craniofacial resection (AL-CFR) with orbital exenteration (OE) for malignant skull base tumors and to investigate the effects of early psychiatric intervention.
Design Present study is a prospective, observational study.
Setting The study took place at the hospital department.
Participants Twenty-six consecutive patients were selected who underwent AL-CFR with OE at our hospital between 2005 and 2015.
Main Outcome Measures Health-related quality of life was assessed preoperatively and 3, 6, 12, and 24 months after surgery using the Hospital Anxiety and Depression Scale (HADS) and medical outcomes study 8-items Short Form health survey (SF-8). In all cases, psychiatric intervention was organized by the consultation liaison psychiatry team preoperatively and postoperatively.
Results Ten (38.0%) of the 26 patients died and 16 (62.0%) were alive and disease-free at the end of the study. The 3-year overall and disease-free survival rates were 64.9% and 53.3%, respectively. Twenty-one patients (80.8%) developed psychiatric complications after surgery and needed treatment with psychotropic medication. Before surgery, 28% of patients had HADS scores ≥8 for anxiety and 20% had scores ≥8 for depression. Seven of the eight items in the SF-8 were significantly lower than those for the general Japanese population. However, scores for all the SF-8 items gradually improved during postoperative follow-up, reaching approximately 50 points, which is the national standard value, at 2 years after surgery.
Conclusions Craniofacial resection with OE was feasible and well tolerated in patients with malignant skull base tumors who received early psychiatric intervention to decrease the considerable psychological impact of this procedure.
Keywords: anterolateral craniofacial resection, orbital exenteration, quality of life, skull base malignant tumor, early psychiatric intervention, hospital anxiety and depression scale, medical outcomes study 8-items short form health survey
Introduction
Craniofacial resection allows a skull base tumor to be resected en bloc with negative margins and is considered the gold standard treatment for malignant tumors at this site. After the first report by Ketcham et al in 1963, craniofacial resection was initially considered a challenging operation that occasionally had severe complications. 1 However, craniofacial resection has become safe and feasible because of improvements in diagnostic modalities (computed tomography, magnetic resonance imaging, and 18F-fluorodeoxyglucose-positron emission tomography) and surgical techniques, for example, free flap reconstruction and navigation. 2 3 4 5 Moreover, recent advances in technology, such as preoperative surgical simulation with three-dimensional virtual imaging, have enhanced surgeons' understanding of the operative anatomy of individual patients in three-dimensions, thereby contributing to training for surgeons. 6 At our hospital, we have performed craniofacial resection and reported favorable prognoses and acceptable complication rates in patients with locally advanced malignant skull base tumors. 5 6 7 8 9
The management of orbital invasion of malignant tumors, such as sinonasal carcinoma or skin cancer, remains controversial because of the considerable psychological impact on patients. It is well known that intraperiosteal orbital involvement is an important prognostic factor for malignant skull base tumors. 10 11 The prognosis is negatively affected in patients with malignant maxillary sinus tumors when there is tumor invasion beyond the orbital periosteum. A clear decrease in 5-year overall survival (OS) from 49% in patients with invasion of the bony orbital wall and involvement of the orbital periosteum to 17% in those with involvement of the orbital soft tissues has been reported. 12 Most authorities agree that, if possible, wide en bloc surgical resection of the tumor with negative margins is the most important goal. Several reports have shown that a positive surgical margin is also a risk factor for poor survival in patients with malignant skull base tumors. 13 14 Surgical resection with negative margins is essential when these tumors show widespread orbital invasion, respond poorly to radiotherapy and chemotherapy, or recur after radiotherapy. 15 However, the choice of extended surgical resection with orbital exenteration (OE) is often difficult for patients because of the negative cosmetic impact. Extended surgery with OE has a marked effect on physical appearance and function, and tends to cause anxiety, depression, and deterioration in quality of life (QoL). 16 17 The indirect negative effect of psychiatric disorders, such as depression, on the outcomes of cancer treatment is also well known. 18 19 A recent prospective clinical cohort study in patients with head and neck cancer showed an association between depression and mortality and that decreasing psychological distress was important with regard to the outcome of treatment and improving QoL. 20
Anterolateral craniofacial resection (AL-CFR) with OE is an effective treatment for patients with locally advanced malignant skull base tumors, particularly locally advanced sinonasal carcinoma. However, AL-CFR with OE is a highly invasive surgery, so it is important to manage psychological distress in patients undergoing this procedure. To the best of our knowledge, there has been no research on anxiety, depression, and health-related QoL in patients who undergo AL-CFR with OE. The aim of this study was to assess the effect of AL-CFR with OE on health-related QoL in patients with malignant skull base tumors and to investigate the effects of early psychiatric intervention.
Materials and Methods
Study Design
Twenty-six consecutive patients with anterior to middle malignant skull base tumors who were treated at our hospital between 2005 and 2015 were enrolled in this prospective observational study. All patients underwent AL-CFR with OE. Patients with a cognitive disorder, schizophrenia, or another psychotic disorder were excluded. Fig. 1 shows the psychiatric assessment and intervention protocol for patients who undergo AL-CFR with OE at our hospital. Health-related QoL is assessed using the Hospital Anxiety and Depression Scale (HADS) and medical outcomes study 8-items Short Form health survey (SF-8). These brief self-reported questionnaires are completed by each patient preoperatively and 3, 6, 12, and 24 months after surgery.
Fig. 1.

Psychiatric assessment and intervention for patients who have undergone anterolateral craniofacial resection with orbital exenteration at our hospital. HADS, hospital anxiety and depression scale; SF-8, medical outcomes study 8-items Short Form health survey.
The study was approved under the guidelines for epidemiological studies by the Ethics Review Committee of Nagoya University Graduate School of Medicine and Nagoya University Hospital (approval no. 2007–0543). Informed consent was obtained from each patient by the attending psychiatrist before enrolment in the study.
Treatment Strategy
The surgical procedure used at our institution has already been described in detail. 6 8 In brief, using a Weber–Fergusson incision, we remove the tumor en bloc with the surrounding tissues, including the orbit, hard palate, oral mucosa, and affected mucosa of the nasal septum. A frontotemporal craniotomy is performed, and the anterior and middle cranial bases are exposed epidurally. After en bloc resection of the tumor, the defect in the cranial base is covered using a myocutaneous rectus abdominis free flap and occasionally a temporoparietal-galeal flap. The upper and lower eyelids are preserved unless the tumor has directly invaded the eyelids. Orbital bony reconstruction with a free flap and orbital floor reconstruction with titanium mesh are not performed.
The tumor margins are evaluated in all cases, and it is recommended that postoperative radiotherapy (50–60 Gy in 2-Gy fractions at five fractions per week to the tumor bed) be administered within 8 weeks of tumor resection. We recommend cisplatin-based chemoradiotherapy as adjuvant treatment for patients with positive margins or extranodal spread. After these treatments, the patients are followed up routinely at 3-month intervals in the outpatient clinic.
Psychiatric Assessment and Intervention
In this study, all patients underwent psychiatric intervention organized by the consultation liaison psychiatric team preoperatively and postoperatively, as described previously by Adachi et al. 21 Psychiatrists assessed the mental condition of each patient preoperatively and arranged active intervention by the psychiatric liaison team for patients with treated psychiatric disorders. All psychiatric diagnoses were made by psychiatrists based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). 22 Baseline demographic and medical data were obtained by interview or from the medical records. During periods when patients were at high risk for delirium, for example, stay in the intensive care unit or the first postoperative week, haloperidol 2.5 to 5.0 mg was infused intravenously because of swallowing difficulties. Thereafter, depending on the patient's postoperative mental state, psychopharmacological agents, for example, risperidone 0.5 to 1.0 mg for delirium, mirtazapine 15 to 30 mg for depression, and brotizolam 0.25 mg for insomnia, were more likely to be administered orally.
Hospital Anxiety and Depression Scale Questionnaire
The Japanese version of the HADS questionnaire was used to assess the severity of depression in our patients. 23 This instrument is often used in cancer studies and consists of two subscales, that is, anxiety and depression, with seven items each and rated on a four-point (0–3) Likert's scale. The scores for each subscale range from 0 to 21, with higher scores reflecting more severe symptoms. A threshold value of 8 is recommended to identify all potential cases of anxiety and depression. 24
Short Form-8 Questionnaire
The SF-8 was used to assess health-related QoL in our patients. The SF-8 is a generic questionnaire that is derived from the longer 36-item Short Form health survey (SF-36). 25 The original instrument was developed in English and was subsequently translated into Japanese. Importantly, results obtained from the SF-8 demonstrate a high correlation with the SF-36, and the reliability and validity of this instrument has been confirmed in the general population of Japan. 26 Administration of the SF-8 generates a health profile of eight domains, including general health, physical function, role physical, bodily pain, vitality, social functioning, mental health, and role emotional. The SF-8 also provides two higher order summary scores, that is, the physical component summary (PCS) and the mental component summary (MCS). Scores for each summary ranges from 0 to 100. A higher score represents better health-related QoL. The QoL scores are assessed using the norm-based scoring method outlined in the manual of the original version of the SF-8. 24 This norm-based scoring method can be used to compare the results of the SF-8 and SF-36.
Statistical Methods
Descriptive statistics and figures were obtained using GraphPad Prism (Version 6.0c, GraphPad Software Inc., La Jolla, California, United States). The Kaplan–Meier method was used to estimate survival, defined as the interval between surgery and a target event or last contact. The target events included survival (overall [OS]) and (disease-free [DFS]). Adverse events that occurred within 30 days postoperatively were considered to be surgical complications. Cerebrospinal fluid (CSF) leak was classified as major if it lasted for more than 1 week or required operative intervention. Loss of all or part of the flap used in the reconstruction and the need for surgical treatment were considered to be major wound complications.
The mean scores on the SF-8 subscales and two SF-8 composite scores before and 3, 6, 12, and 24 months after surgery were compared with 1,000 published sample scores for the general Japanese population. We performed a homogeneity test with all the data and applied the Student's t -test when equal variance was assumed and Welch's t -test when equal variance was not assumed. Further analysis of both of the HADS scores and SF-8 scores was performed for the patients who were alive and disease-free at the end of the study (survivors) and for the patients who had died (nonsurvivors). Differences in scores between survivors and nonsurvivors were analyzed using the Mann–Whitney U -test. A p -value < 0.05 was considered statistically significant.
Results
Patient Characteristics
Table 1 shows the characteristics and histological diagnoses for the 26 patients (21 men, 5 women; median age at the time of surgery 61.5 years [range, 36–76 years]). Nine patients (34.6%) were younger than 60 years of age and 17 (65.4%) were aged 60 years or older. Sixteen patients (61.5%) had T4a disease and 10 (38.5%) had T4b disease. The tumor was located in the maxillary sinus in 19 patients (73.1%), ethmoid sinus in four (15.4%), frontal sinus in one (3.8%), and facial skin in two (7.7%). The histological diagnosis was squamous cell carcinoma in 21 patients (80.8%), adenoid cystic carcinoma in two (7.7%), anaplastic carcinoma in one (3.8%), adenocarcinoma in one (3.8%), and myoepithelial carcinoma in one (3.8%). Twelve patients (46.2%) developed surgical complications after craniofacial resection comprising minor CSF leak in two cases (7.7%), major CSF leak in four (15.4%), a minor wound in five (19.2%), a major wound in three (11.5%), and cerebral infarction in one (3.8%). Twelve (46.2%) of the 26 patients experienced tumor recurrences with local recurrence in six cases (23.1%), regional recurrence in seven (26.9%), and distant metastasis in two (7.7%). The questionnaire response rates for survivors were 95% preoperatively and 69, 54, 52, and 80% at 3, 6, 12, and 24 months, respectively, after surgery.
Table 1. Patient characteristics.
| Variable | n (%) |
|---|---|
| Sex | |
| Male | 21 (81) |
| Female | 5 (19) |
| Primary location | |
| Maxillary sinus | 19 (73) |
| Ethmoid sinus | 4 (15) |
| Frontal sinus | 1 (4) |
| Facial skin | 2 (8) |
| T classification | |
| T4a | 16 (62) |
| T4b | 10 (38) |
| Complication | |
| No | 14 (54) |
| Yes | 12 (46) |
| CSF leak, minor | 2 (8) |
| CSF leak, major | 4 (15) |
| Wound, minor | 5 (19) |
| Wound, major | 3 (12) |
| Cerebral infarction | 1 (4) |
| Recurrence | |
| No | 14 (54) |
| Yes | 12 (46) |
| Local recurrence | 6 (23) |
| Regional recurrence | 7 (27) |
| Distant metastasis | 2 (8) |
| Final outcome | |
| Alive without disease | 16 (62) |
| Deceased | 10 (38) |
Abbreviation: CSF, cerebrospinal fluid.
Survival Outcome
Ten (38.0%) of the patients died and 16 (62.0%) were alive and disease-free at the end of the study. The Kaplan–Meier curves for OS and DFS in the 26 patients are shown in Fig. 2 . The 3-year OS and DFS rates were 64.9% and 53.3%, respectively.
Fig. 2.
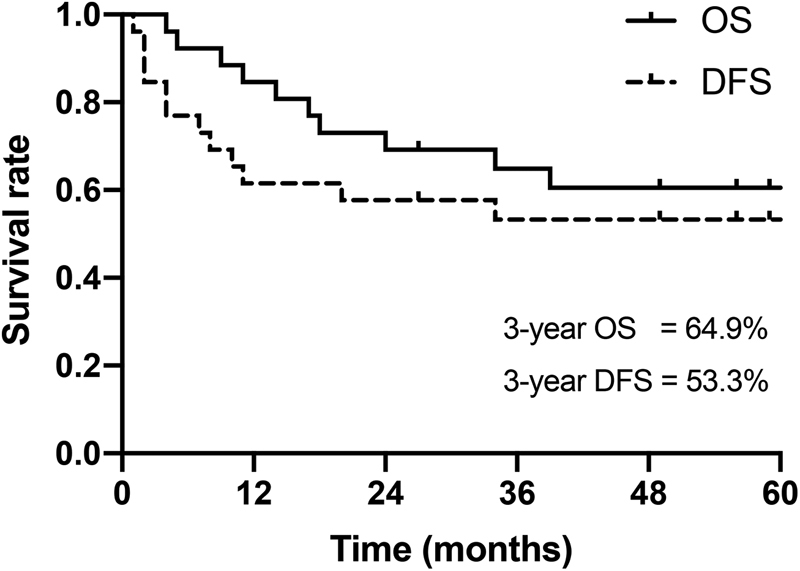
Kaplan–Meier survival curves for overall survival (OS) and disease-free survival (DFS) in 26 cases.
Psychiatric Assessment and Intervention
Twenty-one (80.8%) of the 26 patients developed the following psychiatric complications after surgery: delirium ( n = 13), adjustment disorder ( n = 7), insomnia ( n = 5), and anxiety disorder ( n = 1). In total, 80.8% of patients received psychotropic medication, that is, minor tranquilizers ( n = 20), major tranquilizers ( n = 13), and antidepressants ( n = 1). Supportive psychotherapy was provided concomitantly to all patients by the psychiatrists and psychologists. The mean duration of psychiatric treatment was 139 days. The remission rate was 61.5, 69.2, 69.2, and 80.8% at 3, 6, 12, and 24 months, respectively, after surgery. None of our patients attempted suicide during the study period.
Hospital Anxiety and Depression Scale Scores during Follow-up
The changes in the HADS score during follow-up are shown in Fig. 3A and B . The mean anxiety score was 5.1 preoperatively, 4.6, 3.8, 4.6, and 3.3 at 3, 6, 12, and 24 months, respectively, after surgery; the respective mean depression scores were 5.1, 7.4, 5.8, 5.6, and 4.2. The proportions of patients with HADS anxiety and depression scores ≥ 8 (indicating all potential cases) are shown in Fig. 3C and D . Preoperatively, 28% of patients were identified as potentially having anxiety and 20% as possibly having depression. The proportion of patients with anxiety at the follow-up assessments was not higher than that before surgery; however, possible cases of depression increased to 60% at 3 months after surgery but trended downwards thereafter.
Fig. 3.

Changes in the Hospital Anxiety and Depression Scale (HADS) score during follow-up. Dotted line: HADS anxiety and depression scores of 8 (indicating all potential cases).
Short Form-8 Scores during Follow-Up
Table 2 shows the mean scores for all eight components of the SF-8 during follow-up and compares them with those in the general Japanese population. Before surgery, the mean scores on the SF-8 were 44.4 for physical function, 37.7 for role physical, 46.0 for bodily pain, 45.3 for general health, 48.4 for vitality, 40.1 for social functioning, 42.6 for role emotional, and 48.4 for mental health. All scores for the SF-8 items, except for vitality, were significantly lower than those in the general Japanese population. As time passed, all scores gradually improved through to 24 months postoperatively, and finally improved to around 50 points which is the national standard value.
Table 2. Comparison of SF-8 scores in patients who underwent anterolateral craniofacial resection with orbital exenteration and those in the general Japanese population.
| SF-8 item | Assessment time points | General Japanese population |
||||
|---|---|---|---|---|---|---|
| Preoperatively | 3 mo | 6 mo | 12 mo | 24 mo | ||
| PF | 44.4 b | 43.8 b | 45.8 | 45.9 a | 47.8 c | 50.7 |
| RP | 37.7 a | 40.6 b | 45.2 | 44.7 c | 49.2 | 50.9 |
| BP | 46.0 b | 47.9 | 48.5 | 50.2 | 52.9 | 51.7 |
| GH | 45.3 b | 49.6 | 50.4 | 50.5 | 52.3 | 51.2 |
| VT | 48.4 | 48.0 c | 53.6 | 52.2 | 52.7 | 51.7 |
| SF | 40.1 a | 41.8 a | 45.9 | 45.7 c | 46.9 | 50.0 |
| RE | 42.6 b | 41.1 b | 47.8 | 46.4 c | 51.6 | 50.9 |
| MH | 48.4 c | 50.0 | 51.4 | 50.1 | 49.2 | 51.0 |
Abbreviations: BP, bodily pain; GH, general health; MH, mental health; PF, physical function; RE: role emotional; RP, role physical; SF, social functioning; SF-8, medical outcomes study 8-items Short Form health survey; VT, vitality.
p < 0.001.
p < 0.01.
p < 0.05.
Fig. 4 shows the time courses for the PCS and MCS scores on the SF-8 and compares them with those for the general Japanese population. Before surgery, the mean scores were 41.6 for the PCS and 46.1 for the MCS, both of which were significantly lower than the general Japanese population scores. Although the PCS and MCS scores remained significantly lower at 3 months after surgery when compared with the scores in the general population, both scores finally improved to around 50 points and no significant differences were found at 24 months after surgery.
Fig. 4.
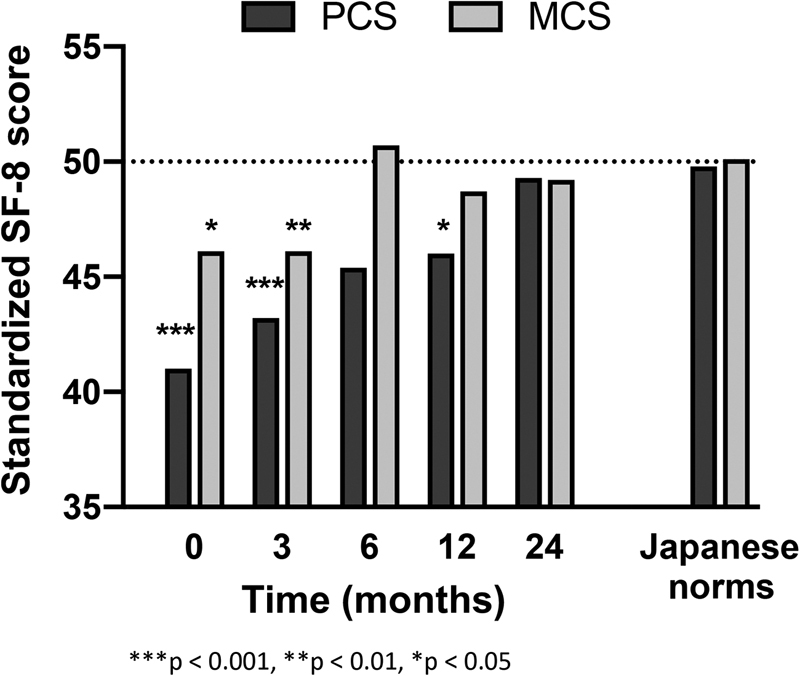
Physical component summary (PCS) and mental component summary (MCS) scores during follow-up: A comparison with Japanese population norms.
Health-Related Quality of Life in Survivors and Nonsurvivors
Fig. 5 shows further analysis of the HADS anxiety and depression scores and the SF-8 PCS and MCS scores in 16 survivors and 10 nonsurvivors, which was not performed at 24 months after surgery because of small number of nonsurvivors. No significant differences in HADS scores and SF-8 scores were found between survivors and nonsurvivors preoperatively, and at 3 and 6 months after surgery. However, at 12 months, the mean HADS anxiety score was significantly lower in survivors than in nonsurvivors (3.5 vs. 7.7, p < 0.05; Fig. 5A ) and the mean SF-8 MCS score was significantly higher in survivors than in nonsurvivors (50.7 vs. 39.9, p < 0.05; Fig. 5D ).
Fig. 5.

Comparison of hospital anxiety and depression scale (HADS) anxiety and depression scores and medical outcomes study 8-items Short Form health survey (SF-8), physical component summary (PCS) and mental component summary (MCS) scores between survivors and non-survivors during follow-up. * denotes p < 0.05.
In survivors, the HADS anxiety and depression scores indicated a transient increase at 3 months after surgery that trended downwards, thereafter, until the risk was no greater than that in the total study population ( Fig. 5A and B ). Similarly, in the survivors, the SF-8 PCS and MCS scores indicated a gradual improvement to around 50 points by 12 months postoperatively ( Fig. 5C and D ).
Discussion
The present study is the first prospective investigation of psychiatric conditions in patients with malignant skull base tumors who undergo AL-CFR with OE. Given that AL-CFR is a lengthy procedure and involves considerable intraoperative blood loss, admission to the intensive care unit (ICU), tracheostomy, and insertion of a feeding tube, there is a high risk of postoperative delirium and depression. In our study, 20 to 30% of patients were suspected to have anxiety and depression, and scores for 7 of the 8 items in the SF-8 were significantly lower than those in the general Japanese population before surgery. Moreover, 81% of the patients developed psychiatric complications after surgery and required treatment with psychotropic medication. Recent studies have indicated that early psychiatric intervention is essential for patients with cancer and could improve their QoL and prevent subsequent psychological distress. 27 28 Considering the high levels of anxiety about AL-CFR and prolonged treatment in patients with malignant skull base tumors, early psychiatric intervention before surgery is important.
Persistent, recurrent, and late depression are associated with worse survival in patients with head and neck cancer, and those with depression postoperatively are at higher risk of premature death. 20 The psychological impact of OE is considerable and continues for a prolonged period after discharge from hospital. Rasmussen et al reported that OE had a marked negative influence on employment, socioeconomic, and marital status. 17 At 1 year after surgery, 30% of the patients in our study had required psychiatric treatment, and the scores on four of the SF-8 items were significantly lower than those in the general population. Similarly, Abergel et al evaluated 39 patients who had undergone anterior skull base surgery and showed that the QoL score deteriorated in most patients in the 6 months following surgery but had improved by 12 months postoperatively. 29 Therefore, active intervention by a psychiatric liaison team should be continued for at least 1 year after surgery in patients who undergo AL-CFR with OE. Furthermore, patients with head and neck cancer have high-suicide rates and a high prevalence of major depressive disorder because the cancer may affect communication and functioning and the surgical treatment required may cause severe facial disfigurement. 30 It is noteworthy that none of our patients attempted suicide during the study period. A multidisciplinary approach with psychiatrists, psychologists, nurses, and skull base surgeons is important for patients who undergo AL-CFR with OE, and interventions can be life-saving when suicidal ideation is present.
QoL is a multidimensional concept, and its measurement should ideally encompass physical, social, psychological, and functional domains. In skull base surgery, measuring QoL is challenging because of the variety of characteristics of tumors involving the skull base. Although many tools have been developed for measurement of QoL, there is no gold standard test for this concept. 31 In this study, we used the HADS to assess anxiety and depression and the SF-8 to evaluate health-related QoL. The HADS is used worldwide to evaluate anxiety and depression. We have previously reported that preoperative evaluation of the severity of depression before surgery using the HADS can identify patients who develop depression after surgery. 21 The SF-8 has often been used to evaluate the multidimensional concept of QoL. 32 33 Using a combination of the HADS and the SF-8, we were able to assess health-related QoL and psychological status accurately over an extended period in patients treated by AL-CFR with OE.
The surgical strategy used and whether the orbit should be removed or preserved depends on the degree of orbital involvement. There is a present trend of preserving the orbit in patients with minimal invasion of the periosteum and limited periorbital involvement. Recent reports have shown that orbit-sparing surgery in selected cases can have an acceptable oncological outcome without functional compromise. 34 35 However, there is no high-quality evidence that a conservative approach is beneficial even when there is minimal orbital invasion. 36 Moreover, Safi et al reported that OE was associated with a significantly better 5-year OS rate (66%) than preservation of the orbit plus radiotherapy (14%) in 52 patients with sinonasal malignancies invading the orbit beyond the orbital periosteum. 37 Despite the fact that only patients with advanced (T4a and T4b) head and neck cancer were included in the present study, we achieved a relatively good result (3-year OS of 64.9% and DFS of 53.3%) in comparison with that in the international collaborative study. 2 Our data indicate that craniofacial resection with OE for locally advanced head and neck cancer is feasible and well tolerated if patients receive early psychiatric intervention.
Our study has some limitations, particularly the small sample size and relatively large number of dropouts during the course of the study. Therefore, our present findings need to be confirmed in a larger patient population, hopefully with a higher questionnaire response rate.
Conclusions
Craniofacial resection with OE is feasible and well tolerated in patients with malignant skull base tumors in whom early psychiatric intervention is implemented to lessen the psychological impact of the procedure.
Conflict of Interest The authors have no conflicts of interest to disclose.
These authors contributed equally to this work.
References
- 1.Ketcham A S, Wilkins R H, Vanburen J M, Smith R R. A combined intracranial facial approach to the paranasal sinuses. Am J Surg. 1963;106:698–703. doi: 10.1016/0002-9610(63)90387-8. [DOI] [PubMed] [Google Scholar]
- 2.Ganly I, Patel S G, Singh B et al. Craniofacial resection for malignant paranasal sinus tumors: report of an International Collaborative Study. Head Neck. 2005;27(07):575–584. doi: 10.1002/hed.20165. [DOI] [PubMed] [Google Scholar]
- 3.Bilsky M H, Bentz B, Vitaz T, Shah J, Kraus D.Craniofacial resection for cranial base malignancies involving the infratemporal fossaNeurosurgery 2005;57(4, Suppl.):339–347, discussion 339–347 [DOI] [PMC free article] [PubMed]
- 4.Cantu G, Solero C L, Riccio S et al. Surgery for malignant maxillary tumors involving the middle cranial fossa. Skull Base. 2010;20(02):55–60. doi: 10.1055/s-0029-1234021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito K, Fukuta K, Takahashi M, Tachibana E, Yoshida J. Management of the cavernous sinus in en bloc resections of malignant skull base tumors. Head Neck. 1999;21(08):734–742. doi: 10.1002/(sici)1097-0347(199912)21:8<734::aid-hed9>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Nishio N, Fujii M, Hayashi Y et al. Preoperative surgical simulation and validation of the line of resection in anterolateral craniofacial resection of advanced sinonasal sinus carcinoma. Head Neck. 2017;39(03):512–519. doi: 10.1002/hed.24653. [DOI] [PubMed] [Google Scholar]
- 7.Nishio N, Fujimoto Y, Fujii M et al. Craniofacial resection for T4 maxillary sinus carcinoma: managing cases with involvement of the skull base. Otolaryngol Head Neck Surg. 2015;153(02):231–238. doi: 10.1177/0194599815586770. [DOI] [PubMed] [Google Scholar]
- 8.Heo Y H, Yagi S, Toriyama K et al. Relationship between BMI and postoperative complications with free flap in anterolateral craniofacial reconstruction. Plast Reconstr Surg Glob Open. 2016;4(03):e636. doi: 10.1097/GOX.0000000000000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwami K, Fujii M, Kishida Y et al. Role of transcranial sphenoidotomy in skull base surgery: classification of surgical techniques based on the surgical anatomy of the sphenoid sinus. J Neurosurg. 2018;1:1–10. doi: 10.3171/2018.6.JNS181013. [DOI] [PubMed] [Google Scholar]
- 10.Howard D J, Lund V J, Wei W I. Craniofacial resection for tumors of the nasal cavity and paranasal sinuses: a 25-year experience. Head Neck. 2006;28(10):867–873. doi: 10.1002/hed.20432. [DOI] [PubMed] [Google Scholar]
- 11.Neel G S, Nagel T H, Hoxworth J M, Lal D. Management of orbital involvement in sinonasal and ventral skull base malignancies. Otolaryngol Clin North Am. 2017;50(02):347–364. doi: 10.1016/j.otc.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Nazar G, Rodrigo J P, Llorente J L, Baragaño L, Suárez C. Prognostic factors of maxillary sinus malignancies. Am J Rhinol. 2004;18(04):233–238. [PubMed] [Google Scholar]
- 13.Patel S G, Singh B, Polluri A et al. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003;98(06):1179–1187. doi: 10.1002/cncr.11630. [DOI] [PubMed] [Google Scholar]
- 14.Bentz B G, Bilsky M H, Shah J P, Kraus D. Anterior skull base surgery for malignant tumors: a multivariate analysis of 27 years of experience. Head Neck. 2003;25(07):515–520. doi: 10.1002/hed.10250. [DOI] [PubMed] [Google Scholar]
- 15.Ma C, Li J, Shen Y, Wu Y, Shi R, Sun J. Is there a role for craniofacial surgery in the treatment of extensive or recurrent head and neck tumors involving the cranial base? J Oral Maxillofac Surg. 2017;75(09):2006–2019. doi: 10.1016/j.joms.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Liu D, Guo Q, Shen B. Quality of life in advanced maxillary sinus cancer after radical versus conservative maxillectomy. J Craniofac Surg. 2013;24(04):1368–1372. doi: 10.1097/SCS.0b013e31828601d6. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen M LR, Ekholm O, Prause J U, Toft P B. Quality of life of eye amputated patients. Acta Ophthalmol. 2012;90(05):435–440. doi: 10.1111/j.1755-3768.2010.02092.x. [DOI] [PubMed] [Google Scholar]
- 18.Prieto J M, Atala J, Blanch J et al. Role of depression as a predictor of mortality among cancer patients after stem-cell transplantation. J Clin Oncol. 2005;23(25):6063–6071. doi: 10.1200/JCO.2005.05.751. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton J B, Deal A M, Moore A D, Best N C, Galbraith K V, Muss H. Psychosocial predictors of depression among older African American patients with cancer. Oncol Nurs Forum. 2013;40(04):394–402. doi: 10.1188/13.ONF.394-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen F, Verdonck-de Leeuw I M, Cuijpers P et al. Depressive symptoms in relation to overall survival in people with head and neck cancer: a longitudinal cohort study. Psychooncology. 2018;27(09):2245–2256. doi: 10.1002/pon.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adachi Y, Kimura H, Sato N et al. Preoperative level of depression is a predictor of postoperative levels of depression in patients with head and neck cancer. Jpn J Clin Oncol. 2014;44(04):311–317. doi: 10.1093/jjco/hyu002. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders, 5th ed Arlington, VA: American Psychiatric Publishing; 2013 [Google Scholar]
- 23.Kugaya A, Akechi T, Okuyama T, Okamura H, Uchitomi Y. Screening for psychological distress in Japanese cancer patients. Jpn J Clin Oncol. 1998;28(05):333–338. doi: 10.1093/jjco/28.5.333. [DOI] [PubMed] [Google Scholar]
- 24.Zigmond A S, Snaith R P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(06):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Ware J E, Kosinski M, Dewey J E, Gandek B.How to Score and Interpret Single-Item Health Status Measures: A Manual for Users of the SF-8 Health SurveyQuality Metric Inc., Lincoln, RI; 2001
- 26.Fukuhara S, Suzukamo Y.Manual of the SF-8 Japanese version Kyoto: Institute for Health Outcomes and Process Evaluation Research; 2004(in Japanese) [Google Scholar]
- 27.Sugimoto H, Kawashima H, Ohno E et al. The prognostic factors and trajectory of HRQOL in patients with pancreatic cancer who received psychiatric intervention. J Gastroenterol Hepatol. 2016;31(03):685–690. doi: 10.1111/jgh.13172. [DOI] [PubMed] [Google Scholar]
- 28.Galway K, Black A, Cantwell M, Cardwell C R, Mills M, Donnelly M. Psychosocial interventions to improve quality of life and emotional wellbeing for recently diagnosed cancer patients. Cochrane Database Syst Rev. 2012;11:CD007064. doi: 10.1002/14651858.CD007064.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abergel A, Fliss D M, Margalit N, Gil Z. A prospective evaluation of short-term health-related quality of life in patients undergoing anterior skull base surgery. Skull Base. 2010;20(01):27–33. doi: 10.1055/s-0029-1242982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kam D, Salib A, Gorgy G et al. Incidence of suicide in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1075–1081. doi: 10.1001/jamaoto.2015.2480. [DOI] [PubMed] [Google Scholar]
- 31.de Almeida J R, Witterick I J, Gullane P J et al. Quality of life instruments for skull base pathology: systematic review and methodologic appraisal. Head Neck. 2013;35(09):1221–1231. doi: 10.1002/hed.23120. [DOI] [PubMed] [Google Scholar]
- 32.Kaji M, Fujiwara Y, Shiba M et al. Prevalence of overlaps between GERD, FD and IBS and impact on health-related quality of life. J Gastroenterol Hepatol. 2010;25(06):1151–1156. doi: 10.1111/j.1440-1746.2010.06249.x. [DOI] [PubMed] [Google Scholar]
- 33.Leonhardt F D, Quon H, Abrahão M, O'Malley B W, Jr, Weinstein G S. Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient-reported quality of life and function. Head Neck. 2012;34(02):146–154. doi: 10.1002/hed.21688. [DOI] [PubMed] [Google Scholar]
- 34.Vartanian J G, Toledo R N, Bueno T, Kowalski L P. Orbital exenteration for sinonasal malignancies: indications, rehabilitation and oncologic outcomes. Curr Opin Otolaryngol Head Neck Surg. 2018;26(02):122–126. doi: 10.1097/MOO.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 35.Muscatello L, Fortunato S, Seccia V, Marchetti M, Lenzi R. The implications of orbital invasion in sinonasal tract malignancies. Orbit. 2016;35(05):278–284. doi: 10.1080/01676830.2016.1193532. [DOI] [PubMed] [Google Scholar]
- 36.Reyes C, Mason E, Solares C A, Bush C, Carrau R. To preserve or not to preserve the orbit in paranasal sinus neoplasms: a meta-analysis. J Neurol Surg B Skull Base. 2015;76(02):122–128. doi: 10.1055/s-0034-1390403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safi A-F, Behn L, Rothamel D et al. Therapy of sinonasal malignancies invading the orbit-orbital exenteration versus preservation of the orbit plus radiotherapy. J Craniomaxillofac Surg. 2017;45(02):258–261. doi: 10.1016/j.jcms.2016.11.013. [DOI] [PubMed] [Google Scholar]


