Abstract
Background Lesions affecting sphenoid sinus lateral recess (SSLR) are difficult to visualize and manipulate through the transnasal routes, especially when the sinus is highly pneumatized. External approaches to this area involve extensive surgery and are associated with significant morbidity. The aims of this study are to present our experience with the endoscopic transpterygoid approach as a method for approaching lesions of the SSLR and to evaluate the outcomes of this procedure.
Methods Clinical charts of patients who had lesions in the SSLR and who were treated at our institution from September 1998 to June 2018 were retrospectively reviewed. All these patients were managed by the endoscopic endonasal transpterygoid approach.
Results Thirty-nine patients were identified. No cerebrospinal fluid leak recurrences were observed during follow-up (range: 1–19.7 years; median: 2.3 years). Hypoesthesia (temporary, 1; persistent, 4) in the region innervated by the maxillary branch of the trigeminal nerve was detected in five (12.8%) patients, while symptoms due to the Vidian nerve damage (dry eye, 3; dry nasal mucosa, 1) were present in four (10%) patients.
Conclusions Although the endoscopic endonasal transpterygoid approach is an excellent corridor for dealing with lesions of the SSLR, limited rate of neurologic and lacrimal complications was observed. Potential morbidity of the intervention should be discussed during preoperative counselling.
Keywords: skull base, endoscopic transpterygoid approach, sphenoid sinus lateral recess, cerebrospinal fluid leak, meningoencephalocele
Introduction
The lateral recess of the sphenoid sinus (SSLR) is defined as the space which is limited laterally to the Vidian canal to foramen rotundum line (V-R line). 1 This space, although not always present, can be quite extensive involving both the greater wing and pterygoid process of the sphenoid bone, resulting in the thinning of the middle fossa floor ( Fig. 1 ). 2 3 Spontaneous cerebrospinal fluid (CSF) leaks have been observed frequently in the SSLR 4 and a widely accepted theory suggests that development of such leaks in the SSLR can be the result of extensive lateral recess pneumatization, attenuated sphenoid sinus recess roof and skull base, and the development of arachnoid pits from underlying intracranial hypertension (ICH). 3 However, they can also occur in the absence of lateral pneumatization of the sphenoid as a result of precipitation of an inciting factor. 5 In adulthood, SSLR defects may present as an incidental neuroimaging finding of meningoencephalocele (MEC) or symptomatically with CSF rhinorrhea and meningitis. Less commonly, they can occur as a result of trauma, iatrogenic injury, or skull base erosion from inflammatory or neoplastic disorders.
Fig. 1.
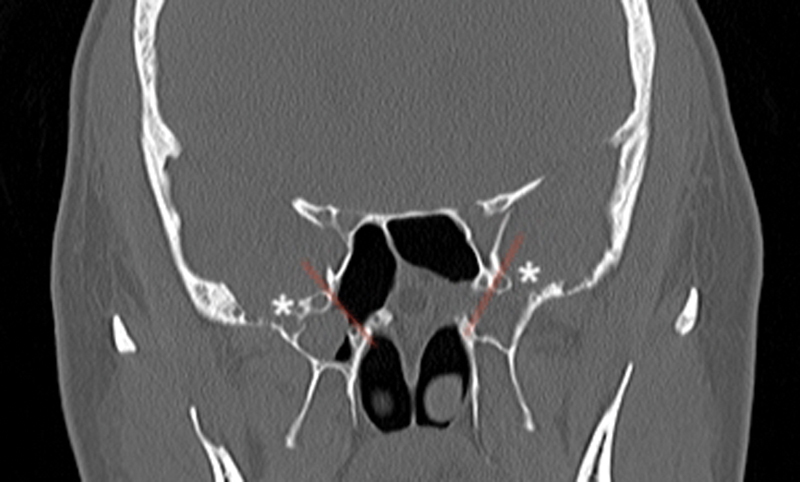
The V-R line (red line) which connects the medial edge of the foramen rotundum and Vidian canal is defined as the demarcation between the sphenoid body and lateral parts of sphenoid bone (greater wings superiorly and pterygoid process inferiorly). The sinus extends laterally into both the greater wing and the pterygoid process bilaterally. Note the bilateral defects (*) in the middle fossa floor lateral to the V-R line with a soft tissue density mass filling the sphenoid sinus suggesting encephalocele. V-R, Vidian-Rotundum.
Historically, the sphenoid skull base defects were approached transcranially (through the pterional route or through the middle cranial fossa); 6 7 8 other reported routes included the transethmoidal, transpalatal, and sublabial routes. 9 10 The transcranial approach incurs the morbidity associated with temporal lobe retraction and edema, secondary hemorrhages risk, as well as yielding suboptimal working angles around SSLR. 6 10 The availability of angled endoscopes and the development of customized instrumentations for skull base surgery together with the experience gained during endoscopic sinus surgery have facilitated endoscopic transnasal procedures, which were very difficult to perform in the past. 11
Due to the adjacent cranial nerves and critical vasculature, transnasal obliteration of the sphenoid sinus without directly repairing the skull base defect is performed by most centers. 12 13 14 Increasingly, the endoscopic endonasal transpterygoid approach (EETPA) is utilized as a direct surgical corridor allowing multilayer reconstruction and creation of a large sphenoidotomy to allow for a more physiological outflow from the sphenoid rather than obliteration. 15 16
In the present study, we evaluate the management and outcomes of CSF leaks located in the SSLR, repaired via endoscopic transpterygoid methods in our 20-year experience.
Material and Methods
All consecutive cases of CSF leak with or without MEC of the SSLR operated on through an EETPA and repaired in a multilayer fashion from September 1998 to June 2018 were included in the study. Patients with CSF leaks treated with different techniques (i.e., sinus obliteration) were excluded from the study. The study met the approval of the institutional Board of Ethics. Presenting symptoms and signs, duration of symptoms at diagnosis, previous procedures and type (if performed), body mass index (BMI), estimated size of the defect, graft material, length of hospitalization, recurrence, and complications were all collected and analyzed. The diagnosis of a sinonasal CSF leak was based on symptoms (e.g., monolateral watery rhinorrhea), preoperative b2-transferrin test on nasal secretions and confirmed using proper imaging, such as thin-sliced CT scan (to find out the location of the bony defect) and magnetic resonance imaging (MRI) with T2, T2-fluid-attenuated inversion recovery, three-dimensional constructive interference in steady state sequences and contrast-enhanced MR cisternography obtained with fat-suppressed T1-weighted sequences for demonstrating CSF leak. Indirect signs of ICH (for the patients with spontaneous etiology) were also evaluated with CT and/or MRI. Perioperative variables, including complications and postoperative outcomes, along with neurological morbidity data updated at most recent follow-up, were used as the main outcome measures.
Surgical Notes
Transethmoidal–Pterygoidal–Sphenoidal Approach
A lateral extension of the standard transethmoidal approach is used for reaching defects in this location ( Fig. 2 ). 17 We prefer to use a two-surgeon, four-instrument approach through binostril routes. Resection of sphenoidal rostrum and posterior 1 cm of the nasal septum is undertaken to provide a “cross-court” angle if needed. 18 During the transpterygoid approach to the SSLR, surgery is performed from a midline to a more lateral location, and the last surgical steps are performed with the aid of a 45° telescope, double-angled instruments, and angled drills. This approach is further classified as a “type B” transpterygoid approach, indicating drilling of the anteromedial aspect of the base of the pterygoid process. 19 We tailor the amount of bone removal according to the pathology (i.e., the size of the MEC) by removing the anteromedial aspect of the pterygoid base without the need to remove the more lateral and inferior parts of pterygoid bone. In cases of wide pneumatization of the SSLR, the drilling of the pterygoid base until the foramen rotundum can be enough to expose the defect, which is usually lateral to the maxillary branch of the trigeminal nerve (V2). Conversely, for cases with a poor pneumatization of the lateral recess of sphenoid sinus it may be necessary to drill out completely the upper portion of pterygoid plate with ligation of sphenopalatine artery to gain sufficient working access. As the anatomy and pneumatization varies, retraction of the pterygopalatine fossa (PPF) content to allow exposure of the sphenoid sinus floor and the pterygoid process is not necessary in all patients. We also agree it is not always necessary to remove the pterygoid process completely, for achieving good SSLR exposure. 20 21
Fig. 2.
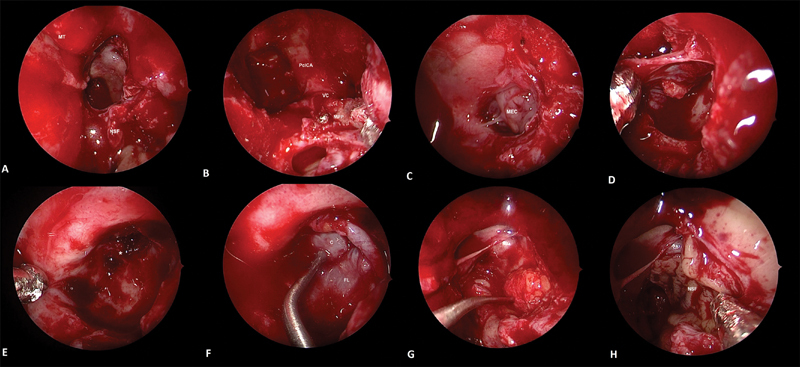
Endoscopic picture showing left SSLR leak and repair of defect using gasket-seal closure. ( A ) Following total sphenoethmoidectomy, middle turbinate is resected (MT indicating its original attachment). Nasoseptal flap stored in the choana during the surgical procedure. ( B ) The sphenoid sinus anterior wall till the lateral end of the recess and anterior aspect of pterygoid bone floor were drilled as lateral as the anterior orifice of the VC with a high-speed diamond drill (Midas Rex; Medtronic, Humacao, Puerto Rico, United States). Paraclival segment of the internal carotid artery and medial pterygoid plate are seen. ( C ) After completion of bone drilling, adequate surgical space was gained to expose the MEC and osteodural defect localized in SSLR. ( D ) MEC was reduced in size by endoscopic bipolar and complete mucosal extirpation from the SSLR was performed. ( E ) Better visualization of osteodural defect (*) by a 45- angled endoscope. ( F ) A piece of fascia lata is fashioned to be larger than the bone defect. The fascia lata is centered over the defect and a piece of cartilage roughly the size of the defect in the cranial base is harvested from the septum. This piece of cartilage is centered over the fascia lata. The cartilage buttress is then wedged in place and the redundant fascia lata is draped around the bone, creating a watertight seal. ( G ) SSLR is obliterated with a free fat graft. ( H ) Nasoseptal flap positioned over the obliterated SSLR. C, cartilage; FL, fascia lata; MEC, meningoencephalocele; MPP, medial pterygoid plate; MT, middle turbinate; NSF, nasoseptal flap; SSLR, sphenoid sinus lateral recess; VC, Vidian canal.
Resection of Encephalocele and Skull Base Reconstruction
The encephalocele is cauterized using an angled bipolar diathermy until it is reduced to the level of the skull base bony defect. Following delineation of the bony margins of the leak, multilayer reconstruction is performed. The reconstruction largely depends on the size of the defect: triple-layer technique (intradural-epidural-extracranial) when the defect is large enough to fit an inlay graft (generally >5 mm) and a double layer one (epidural–extracranial) when it is small. Because CSF leaks in this site are usually spontaneous, multilayered closure with a bone/cartilage graft harvested from septum or middle turbinate and tissue inlay grafts (e.g., dural substitute, fascia lata, temporalis fascia, and pericranium) is generally used. Finally, a mucoperiosteum free graft or a pedicled vascular flap (e.g., nasoseptal flap) is used to cover the extracranial side of the defect. Alternatively, for larger repairs, the fascia layer may be centered over the defect on the sphenoid side of the lesion and then rigid osseous/cartilaginous graft, roughly the same size of the bone defect, is gently countersunk into through the defect. In this so-called gasket-seal closure, 22 the fascia graft is intracranial in its central aspects but covers the circumference of the sinonasal skull base at the defect margins. The PPF fat may also be dissected and transferred to cover the lateral sphenoid recess opening. Fibrin glue and Surgicel (Johnson & Johnson medical, Arlington, Texas, United States) are used to fix the grafting materials and the nasal cavities are packed bilaterally with Merocel 2000 (Medtronic Xomed Surgical Products, Jacksonville, Florida, United States) for ∼48 hours. No Foley balloon catheter is needed to buttress the reconstruction. The sphenoid cavity is not obliterated and is left open to maintain physiological function and allow postoperative examination.
Postoperative Follow-Up
We do not routinely use postoperative lumbar drainage because we believe that the success of procedure depends on the quality of the closure of the skull base defect. As such the presence of a decreased intracranial pressure, via lumbar drain, is not necessary because CSF pressure further compresses the graft to the skull base. We use prophylactic antibiotics for the first 24 to 48 hours.
Long-term follow-up is required following CSF leaks repair as recurrence may occur even after many years. In high-risk patients, weight loss, sodium restriction often with the addition of acetazolamide, must be considered to minimize the long-term failure rates of reconstruction, though more evidence is required to support this treatment protocol. Patients are instructed to avoid blowing the nose and vigorous physical activity for 1 month after surgery. Postoperative endoscopic follow-up for cleaning and decrusting of the sinonasal spaces is performed weekly for the first month, then every 3 months and then at regular 6-month intervals. Information on early and late postoperative outcomes were obtained by either outpatient evaluation or by telephone correspondence, when necessary.
Results
Patient Demographics
A total of 39 patients with CSF leaks/encephaloceles in the SSLR were identified. The mean age (±standard deviation) of the cohort was 53.07 ± 15.21 years (range: 15–77 years); 64% (25 cases) of the patients were female; and 36% (14 cases) were male. Causes of the CSF leaks included previous surgery in 1 patient (2%), trauma in 6 patients (15%), and spontaneous defect in 32 patients (82%). The CSF leak was assessed intraoperatively by means of low-dose intrathecal fluorescein injection in 33 cases. 23 The majority of patients (33 patients, 85%) presented initially with unilateral clear rhinorrhea which exacerbated on leaning forward/coughing. Other clinical manifestations included chronic headache in 10 patients (26%), a history of meningitis in 5 (13%), seizure in 3 (7%), nasal obstruction in 2 (%5), vertigo in 1 (2%), and otalgia in 1 (2%) patient.
In the 32 patients with spontaneous CSF leaks, obesity was present in 14 (43.7%) cases (mean BMI: 30,2 kg/m 2 ; range: 21,6–55,1 kg/m 2 ) and 21 (65.6%) were females. Among these, radiographic signs of prolonged ICH were present in 20 (62.5%) patients with arachnoid pits in 11 (34.3%), anterior cranial fossa skull-base attenuation in 5 (15.6%), and total/partial empty sella in 9 (28.1%) patients. Radiographic analysis of the sphenoid sinuses demonstrated some degree of pterygoid pneumatization in 92% of all patients unilaterally in 63% and bilaterally in 25%. All of the lesions were lateral to V2. Estimated size of the leaks ranged between 2 and 8 mm, with the most under 1 cm in the greater dimension. In five patients, nasoseptal flap was used as the final layer. In seven cases (17.9%), the defect was associated with meningeal and brain tissues herniation, while in 15 (38.4%) only by the meningeal sac with no brain involvement. To note, 35 patients had single defects, while 4 patients had a second, synchronous CSF leaks in the ipsilateral cribriform plate, which were repaired concurrently ( Table 1 ).
Table 1. Patients' demographics and leaks data.
| Number (%) | |
|---|---|
| Etiology | |
| Iatrogenic | 1 (2%) |
| Spontaneous | 32 (82%) |
| Trauma | 6 (15.3%) |
| Multiple defects | |
| Yes | 4 (10.2%) |
| No | 35 (89.7%) |
| Presenting symptoms | |
| Clear rhinorrhea | 33 (85%) |
| Chronic headache | 10 (26%) |
| Meningitis | 5 (13%) |
| Seizure | 3 (7%) |
| Nasal obstruction | 2 (%5) |
| Vertigo | 1 (2%) |
| Otalgia | 1 (2%) |
| Intrathecal fluorescein | 33 (85%) |
| Radiographic signs of prolonged ICH (for spontaneous leaks) | 20 (62.5%) |
| Arachnoid pits | 11 (34.3%) |
| ACF skull-base attenuation | 5 (15.6%) |
| Total/partial empty sella | 9 (28.1%) |
| Obesity (for spontaneous leaks) | 14 (44%) |
| Accompanying MEC/MC | |
| MEC MC |
7 (17.9%) 15 (38.4%) |
| Previous Surgery | |
| TC | 7 (17.9%) |
| TP | 1 (2.5%) |
| Obliterative TS | 6 (15.3%) |
ACF, anterior cranial fossa; ICH, intracranial hypertension; MC, meningocele; MEC, meningoencephalocele; TC, transcranial; TP, transpalatal; TS, trans-sphenoid.
Previous Treatments
Six patients had undergone a total of seven previous middle cranial fossa craniotomies and one transpalatal approach that had failed to control the CSF leak. Conservative management with a subarachnoid lumbar drain was unsuccessfully attempted in two (5%) patients. Six patients underwent unsuccessful packing of the sphenoid; these procedures did not provide access to the lateral recess endoscopically and directly address the defect. One such patient who had his sphenoid sinus packed with abdominal fat developed a temporal lobe abscess 5 months after the first repair and underwent two endoscopic attempts for abscess drainage and defect repair by packing the SSLR before referral to our institution. One patient had a history of a previous surgery within the adjacent sphenoidal area for a cystic neoformation at 6 years of age. Four years after this surgery, she was found to have SSLR MEC with CSF leak and underwent two unsuccessful external approaches (translabyrinthine and transpteryonal) for defect repair.
Outcomes
All of the defects were successfully repaired by a single endoscopic transpterygoid surgery. At the most recent follow-up (range: 1–19.7 years, median: 2.3 years), all reconstructions were intact, based on clinical history and endoscopic examination. All patients were in good clinical conditions with no further episodes of meningitis or seizures. However, one patient developed a new site of CSF leakage at a different site in the left cribriform plate 30 months following her initial repair of right SSLR treated successfully ( Fig. 3 ). Another patient was diagnosed with right SSLR leak 32 months after her successful repairment of left SSLR. One patient had a right SSLR CSF-leak treated successfully and 21 months later a right tegmen tympani MEC was detected. All of these three patients presented with symptoms and signs of ICH and BMI > 25 kg/m 2 .
Fig. 3.
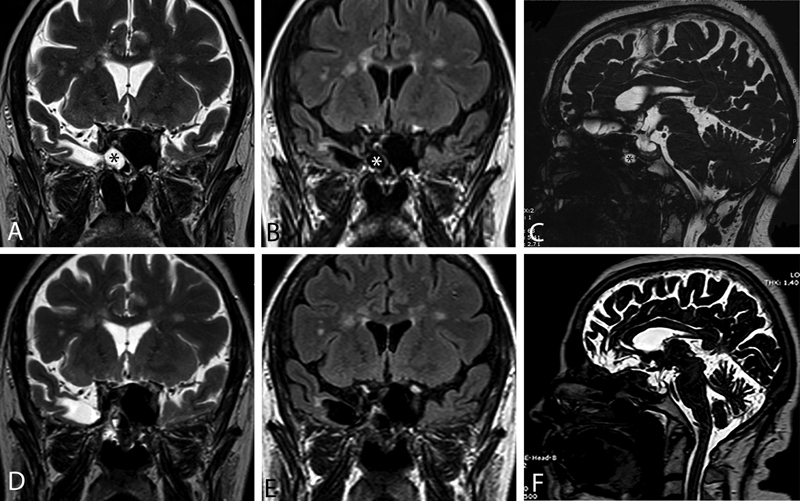
A 67-year-old female patient with a body mass index of 27.0 kg/m 2 had undergone two prior sphenoidotomies with “packing” without identification and repair of the associated encephalocele and skull base defect. She presented to us with clear rhinorrhea with a history of two meningitis attacks. Coronal images, T2-weighted ( A ) and T2 fluid attenuated inversion recovery ( B ), show an encephalocoele raising from the right temporal lobe. Sagittal T2 magnetic resonance imaging. Red arrow shows right sphenoid sinus lateral recess defect accompanying meningoencephalocele, and the asterisk indicates total empty sella ( C ). At 2 years postoperative control, images in the same sequences obtained postoperatively ( D – F ) demonstrated complete healing in the operation site.
After surgery, one (2%) patient complained of paraesthesia in the malar region, while four (10%) patients referred persistent hypoesthesia of the hemipalate on the operated side, in the area mainly supplied by the greater palatine and nasopalatine nerves, both of which are branches of V2. The distress caused by malar paraesthesia and dental hypoesthesia was mild in all cases, and none of the patients had previously mentioned the problem during follow-up visits, as it was not deemed relevant. Three patients suffered from ipsilateral regressive ocular/nasal dryness due to the Vidian nerve (VN) damage; however, one patient operated consecutively for both sides was still suffering from bilateral eye dryness at the time of writing following 2.5 years after the last surgery ( Table 2 ). No intraoperative complication was observed in any of the patients. Mean hospitalization time was 6.2 days (range: 3–27 days) and all patients were discharged with nasal irrigation and systemic antibiotic therapy.
Table 2. Analysis of complications.
| Temporary | Persistent | |
|---|---|---|
| Numbness/pain in the ipsilateral cheek/hard palate (V2 damage) | 1 (2%) | 4 (10%) |
| Dry eye (Vidian nerve damage) | 2 (5%) | 1 (2%) |
| Dry nasal mucosa (Vidian nerve damage) | 1 (2%) | 0 |
V2, Maxillary branch of the trigeminal nerve.
Discussion
The optimal treatment of SSLR CSF leaks and MECs is controversial. Although repairing the defect of SSLR region endoscopically is less invasive than the external (transcranial or extracranial) approaches, it can be more challenging to perform. 24 Therefore, in some centers, the transcranial/extracranial approaches are still thought to be the best choice in providing a controlled CSF leak repair and resection of the SSLR encephalocele. 6 25 26 27 In the literature, there are also patients reported with lateral sphenoid encephaloceles which had undergone unsuccessful transnasal endoscopic attempts (mostly attributable to the lack of access to the SSLR) and underwent transcranial/transfacial middle fossa approaches for treatment. 6 10 26 27 In contrary, so many prior unsuccessful open approaches in the management of SSLR defects have been successfully treated using the endoscopic endonasal transpterygoid techniques, as described in this study and reported in the literature by others. 12 28 The outcomes of the present case-series together with other results of success rates higher than 90% 15 16 28 remain superior to previous series involving external approaches with a high rate (up to 40%) of failure. 6 26 29 Moreover, our complication rate was very low (1%) and well below the morbidity for open procedures. 30 To the best of our knowledge, this is the largest group of patients reported in the literature with SSLR CSF leaks that have been treated with endoscopic transpterygoid approach.
Three endoscopic endonasal approaches are described in dealing with skull base defects of the sphenoid sinus: the endoscopic endonasal trans-sphenoid approach (EETSA), the endoscopic endonasal transethmoidal-sphenoidal approach (EETESA), and the EETPA. For central defects in sphenoid sinus medial to the V-R line, the EETSA and EETESA are utilized; neither the trans-septal nor the transethmoidal approach provides adequate access to pathology in a sphenoid sinus with extensive pneumatization into the pterygoid process, and for this reason, the EETPA is the surgical corridor of choice in such cases ( Fig. 4 ). 3 17 20 31 Most failures experienced after the primary endoscopic repair are due to the challenges of adequately addressing CSF leaks that involve lateral extensions of the sphenoid sinus. 3 32 The problem of access is further amplified by the desire to limit the size of the sinusotomy to prevent extrusion of the graft in the obliteration process. However, EETPA allows reaching the SSLR through the posterior wall of the maxillary sinus and the PPF. With the aid of angled scopes and instruments, a panoramic view of the lateral skull base can be obtained, and thus multilayer reconstruction can be achieved. In our technique, we drill the base of the pterygoid process and we go on laterally until a complete marsupialization of the lateral recess of the sphenoid sinus is performed. The Vidian canal represents the medial boundary of transpterygoid approach, and drilling the base of the pterygoid plates may pose a risk to the VN and less commonly, the more lateral V2. VN trauma or resection of the sphenopalatine ganglia may result in eye dryness. 33 34 In our study, we had one patient with permanent dry-eye symptoms operated on both sides for MEC; we did not formally assess dry eye with Shirmer test to compare it to the preoperative status. The reported dry-eye symptoms in series of VN endoscopic neurectomy tend to be temporary. 35 36 It is likely that accessory neural pathways for tear production exist that maintain basal tear production. However, as preservation of VN function is highly recommended for those patients with preoperative dry-eye symptoms or V1 deficits, 37 techniques for VN preservation and transposition for an EETPA have been described previously. 38 Damage to the V2 and its branches may result in deficit of sensation around the lateral cheek, superior lip, and hard palate. 31 In our study, we had four patients with persistent numbness over the ipsilateral cheek/hard palate following at least 2.5 years after surgery, so far. However, one patient with temporary numbness in the ipsilateral hard palate was free of this symptom 3 years after the surgery. Other studies using the same technique 15 16 39 reported a 100% success rate without any reported significant nerve injuries.
Fig. 4.
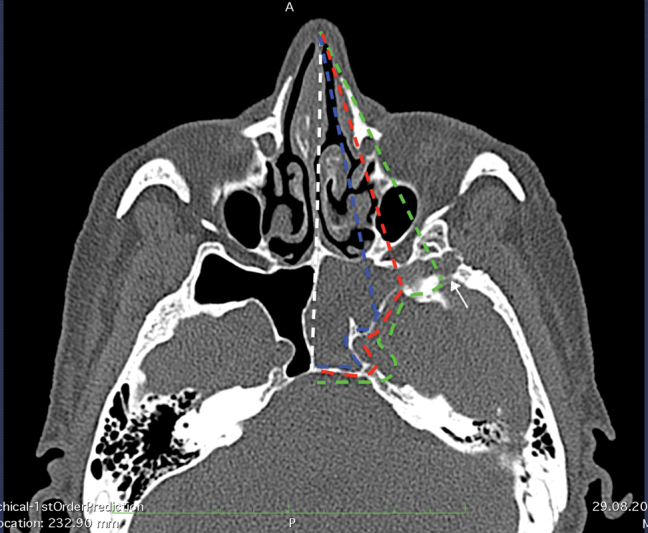
Surgical trajectories demonstrating the direct access attained with EETSA, EETESA, and EETPA approaches. Blue dotted line, access attained with EETSA; red dotted line, access attained with EETESA; green dotted line, the enhanced lateral extent afforded with EETPA; white dotted line, midline. The axial computed tomography scan demonstrates how this skull base defect is inaccessible using the transethmoidal endoscopic technique owing to the far lateral and inferior location of the defect in the hyperpneumatizated SSLR (encephalocele) (arrow). EETESA, endoscopic endonasal transethmoidal-sphenoidal approach; EETPA, endoscopic endonasal transpterygoid approach; EETSA, endoscopic endonasal trans-sphenoid approach; SSLR, sphenoid sinus lateral recess.
Modified techniques for transpterygoid approach have been described aiming at mobilization of the PPF contents laterally to preserve the neurovascular structures. 31 40 In the study of Bolger, 31 inferior aspect of the lateral sphenoid recess is accessed by creating a transpterygoid sphenoidotomy lateral to the region of the sphenopalatine foramen and VN and well below the infraorbital nerve following lateralization of the PPF contents. The sphenoidotomy is created with drill which is then used to remove a portion of the lateral pterygoid plate. Our transpterygoid approach differs from that described by Bolger as we remain more superior and medial to the approach proposed by them. In their follow-up of eight patients, one patient had anesthesia over the left cheek, hard palate mucosa, and posterior molar region areas due to partial laceration of V2 during resection of a large encephalocele that required sphenopalatine ganglia resection. Other studies using the same technique 12 28 reported a success rate of higher than 90% without any significant nerve injuries. No alternation in the tear product in long-term follow-up was mentioned. In the study of Schmidt et al, 40 the contents of the PPF are retracted laterally, the sphenoid process of the palatine bone is removed, followed by the removal of the medial pterygoid plate from the inferior portion up to the junction of the medial pterygoid plate and floor of the sphenoid sinus where the VN may be identified and preserved. Postoperatively, all of the four patients were reported to be neurologically intact.
Differently, El-Tarabishi et al 41 reported successful repair of seven cases of CSF leaks involving the SSLR using an endoscopic endonasal retrograde trans-sphenoidal approach by aid of 45° lens. In their approach, they extended the sphenoidotomy laterally to the medial orbital wall and lateral wall of the sphenoid sinus. The removal of posterior meatal wall, middle meatal antrostomy, and entering in the PPF is not encountered as in the EETPA. The effectiveness of their technique appears limited to defects in superior wall of SSLR as it is necessary to drill out the upper portion of the pterygoid plate to expose and access the lateral wall of the sphenoid sinus (i.e., the lateral end of pterygoid recess) and more precise multilayered closure.
Obliteration of the sphenoid sinus, alone or with an overlay graft/flap, is still commonly used for the repair of a CSF leaks of the sphenoid sinus, particularly in cases with limited visualization at the lateral wall of the sinus. 13 14 This technique aims to create a sphenoid sinusotomy of sufficient size to provide access to the site of CSF leak while maintaining a sphenoid rostrum adequate to hold graft material in place. Alternatively, the lateral sphenoid can be obliterated by placing a fascial and/or muscle graft between the foramen rotundum and Vidian canal to compartmentalize the lateral recess from the sphenoid sinus after ensuring that the mucosa could be completely extirpated from the SSLR. 28 31 These kinds of lesions usually arise in very pneumatized sphenoid sinuses. Among our first cases which were treated with a direct transnasal trans-sphenoid approach with fat obliteration, in one patient, a large arachnoid cyst was observed under the temporal lobe very close to the bone defect and the V2 canal. In revision, the defect was managed with transpterygoidal approach with multilayer repair. After this rare complication, we abandon this practice of sinus obliteration.
A variety of materials have been described for CSF leak repair, including synthetic materials, autologous fascia, bone, fat, and cartilage. Reconstruction was performed mostly using autologous materials. In the first years of our experience (until 2005), DuraGen (Integra Life Sciences, Plainsboro, New Jersey, United States) was often used as an epidural underlay graft in various types of anterior skull base defects. However, we observed one case of infection and extrusion of this material some years after surgery followed by radiotherapy. We have considered this condition a very rare complication of synthetic material. Therefore, after this patient, we changed our minds and started to use autologous materials only. Reconstructions with single or double layers, with or without rigid support, have been described, with success rates of 90%, regardless of the materials, and surgical techniques used. 42 43 Although methods and materials for skull base reconstruction continue to be controversial, we are convinced that multilayer reconstruction with rigid autologous tissue is the most reliable way to obtain a watertight closure. Since increased intracranial pressure may be the underlying cause of spontaneous leaks, robust reconstruction has a better chance of resisting against an increased intracranial pressure. 44 For these reasons, we routinely use rigid material such as cartilage or bone, which is placed between the fascia and bone defect on the intracranial side, providing support and creating a second layer of the seal. Other authors also utilize cartilage or bone for reconstruction in repairing such defects. 3 12 28
As largely described in literature, the introduction of the nasoseptal flap in 2006 has improved the success rate of endoscopic skull base reconstruction. Since its introduction, we have used such technique in selected cases of SSLR CSF leaks, especially in case of large defect size, high flow CSF leak, and whenever technically feasible. In revision cases, where the nasal septum is damaged, the nasoseptal flap has not been performed. Conversely, we have had cases where the defects were located far-laterally in hyperpneumatizated lateral recess which may prevent proper placement of the graft. In such cases, complete mucosal extirpation from the lateral recess was followed by placement of a gasket seal closure with an additional layer of a pedicled septal flap for definitive closure of the leak. Although not necessary for all cases, this technique provides secure material to obtain a tight repair in patients with high risk for a recurrent CSF leak, especially those who present with high BMI with spontaneous etiology.
Conclusion
The EETPA provides a wide corridor for access to defects in the SSLR, permits a direct repair, and maintains sphenoid sinus patency. Our experience provides compelling evidence that the EETPA, regardless of their etiology, represents a safe and effective methods for repairing such laterally placed defects. Nonetheless, potential morbidities of the procedure, although rare, should be addressed preoperatively during informed consent discussion.
Acknowledgments
None.
Conflicts of Interest There are no conflicts of interest.
Author contributions
Concept - G.B., M.T.Z., P.C.; Design - G.B., M.T.Z., P.C.; Supervision - G.B., M.T.Z., H.A.E., P.C., D.L.; Resource - E.C., F.R., S.G.; Materials - E.C., F.R., S.G., J.Z., D.L.; Data Collection &/or Processing - E.C., F.R., S.G., J.Z.; Analysis &/or Interpretation - G.B., M.T.Z., H.A.E., D.L., M.B., P.C.; Literature Search - G.B., M.T.Z., M.B., J.Z., P.C.; Writing - G.B., M.T.Z., H.A.E., P.C.; Critical Reviews - G.B., M.T.Z., M.B., P.C.
All authors have read and approved the final version of the article.
Financial disclosures
GB gratefully acknowledges support from the Turkish ENT Society for support of the Research Fellowship. The authors have no other funding, financial relationships, or conflicts of interest to disclose related to this article.
References
- 1.Vaezi A, Cardenas E, Pinheiro-Neto C. Classification of sphenoid sinus pneumatization: relevance for endoscopic skull base surgery. Laryngoscope. 2015;125(03):577–581. doi: 10.1002/lary.24989. [DOI] [PubMed] [Google Scholar]
- 2.Martínez-Capoccioni G, Serramito-García R, Huertas-Pardo B, García-Allut A, Martín-Martín C. Spontaneous cerebrospinal fluid leaks in the anterior skull base: a surgical challenge. J Laryngol Otol. 2015;129(04):358–364. doi: 10.1017/S0022215115000584. [DOI] [PubMed] [Google Scholar]
- 3.Lai S Y, Kennedy D W, Bolger W E. Sphenoid encephaloceles: disease management and identification of lesions within the lateral recess of the sphenoid sinus. Laryngoscope. 2002;112(10):1800–1805. doi: 10.1097/00005537-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Woodworth B A, Prince A, Chiu A G. Spontaneous CSF leaks: a paradigm for definitive repair and management of intracranial hypertension. Otolaryngol Head Neck Surg. 2008;138(06):715–720. doi: 10.1016/j.otohns.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal V, Nair P, Shivhare P. A case of evolving bilateral sphenoidal meningoencephaloceles: case report and review of the literature. World Neurosurg. 2017;100:7.08E13–7.08E19. doi: 10.1016/j.wneu.2017.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Landreneau F E, Mickey B, Coimbra C.Surgical treatment of cerebrospinal fluid fistulae involving lateral extension of the sphenoid sinus Neurosurgery 199842051101–1104., discussion 1104–1105 [DOI] [PubMed] [Google Scholar]
- 7.Schick B, Brors D, Prescher A. Sternberg's canal--cause of congenital sphenoidal meningocele. Eur Arch Otorhinolaryngol. 2000;257(08):430–432. doi: 10.1007/s004050000235. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman B, Nulsen F E, Weiss M H, Brodkey J S, White R J, Sykora G F. Acquired spontaneous, nontraumatic normal-pressure cerebrospinal fluid fistulas originating from the middle fossa. Radiology. 1977;122(02):379–387. doi: 10.1148/122.2.379. [DOI] [PubMed] [Google Scholar]
- 9.Cakmak O, Shohet M R, Kern E B. Isolated sphenoid sinus lesions. Am J Rhinol. 2000;14(01):13–19. doi: 10.2500/105065800781602993. [DOI] [PubMed] [Google Scholar]
- 10.Mortuaire G, Louis E, Pellerin P. Sphenoidal cerebrospinal fluid rhinorrhea: an original surgical approach. J Craniofac Surg. 2004;15(03):458–463. doi: 10.1097/00001665-200405000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Donald P J. Sphenoid marsupialization for chronic sphenoidal sinusitis. Laryngoscope. 2000;110(08):1349–1352. doi: 10.1097/00005537-200008000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Forer B, Sethi D S. Endoscopic repair of cerebrospinal fluid leaks in the lateral sphenoid sinus recess. J Neurosurg. 2010;112(02):444–448. doi: 10.3171/2009.7.JNS09306. [DOI] [PubMed] [Google Scholar]
- 13.Fyrmpas G, Konstantinidis I, Selviaridis P, Constantinidis J. Management of spontaneous cerebrospinal fluid leaks of the sphenoid sinus: our experience. J Laryngol Otol. 2014;128(09):797–802. doi: 10.1017/S0022215114001698. [DOI] [PubMed] [Google Scholar]
- 14.Tosun F, Carrau R L, Snyderman C H, Kassam A, Celin S, Schaitkin B. Endonasal endoscopic repair of cerebrospinal fluid leaks of the sphenoid sinus. Arch Otolaryngol Head Neck Surg. 2003;129(05):576–580. doi: 10.1001/archotol.129.5.576. [DOI] [PubMed] [Google Scholar]
- 15.Janakiram T N, Subramaniam V, Parekh P. Endoscopic endonasal repair of sphenoid sinus cerebrospinal fluid leaks: our experience. Indian J Otolaryngol Head Neck Surg. 2015;67(04):412–416. doi: 10.1007/s12070-015-0924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulu M O, Aydin S, Kayhan A. Surgical management of sphenoid sinus lateral recess cerebrospinal fluid leaks: a single neurosurgical center analysis of endoscopic endonasal minimal transpterygoid approach. World Neurosurg. 2018;118:e473–e482. doi: 10.1016/j.wneu.2018.06.219. [DOI] [PubMed] [Google Scholar]
- 17.Castelnuovo P, Dallan I, Pistochini A, Battaglia P, Locatelli D, Bignami M. Endonasal endoscopic repair of Sternberg's canal cerebrospinal fluid leaks. Laryngoscope. 2007;117(02):345–349. doi: 10.1097/01.mlg.0000251452.90657.3a. [DOI] [PubMed] [Google Scholar]
- 18.Castelnuovo P, Pistochini A, Locatelli D. Different surgical approaches to the sellar region: focusing on the “two nostrils four hands technique”. Rhinology. 2006;44(01):2–7. [PubMed] [Google Scholar]
- 19.Kasemsiri P, Solares C A, Carrau R L. Endoscopic endonasal transpterygoid approaches: anatomical landmarks for planning the surgical corridor. Laryngoscope. 2013;123(04):811–815. doi: 10.1002/lary.23697. [DOI] [PubMed] [Google Scholar]
- 20.Pasquini E, Sciarretta V, Farneti G, Mazzatenta D, Modugno G C, Frank G. Endoscopic treatment of encephaloceles of the lateral wall of the sphenoid sinus. Minim Invasive Neurosurg. 2004;47(04):209–213. doi: 10.1055/s-2004-818522. [DOI] [PubMed] [Google Scholar]
- 21.Bachmann-Harildstad G, Kloster R, Bajic R. Transpterygoid transsphenoid approach to the lateral extension of the sphenoid sinus to repair a spontaneous CSF leak. Skull Base. 2006;16(04):207–212. doi: 10.1055/s-2006-950389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leng L Z, Brown S, Anand V K, Schwartz T H.“Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery Neurosurgery 2008620502E342–E343., discussion E343 [DOI] [PubMed] [Google Scholar]
- 23.Tabaee A, Placantonakis D G, Schwartz T H, Anand V K. Intrathecal fluorescein in endoscopic skull base surgery. Otolaryngol Head Neck Surg. 2007;137(02):316–320. doi: 10.1016/j.otohns.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Tomazic P V, Stammberger H. Spontaneous CSF-leaks and meningoencephaloceles in sphenoid sinus by persisting Sternberg's canal. Rhinology. 2009;47(04):369–374. doi: 10.4193/Rhin08.236. [DOI] [PubMed] [Google Scholar]
- 25.Kwon J E, Kim E. Middle fossa approach to a temporosphenoidal encephalocele -technical note- Neurol Med Chir (Tokyo) 2010;50(05):434–438. doi: 10.2176/nmc.50.434. [DOI] [PubMed] [Google Scholar]
- 26.Gürkanlar D, Akyüz M, Açıkbaş C, Ermol C, Tuncer R. Difficulties in treatment of CSF leakage associated with a temporal meningocele. Acta Neurochir (Wien) 2007;149(12):1239–1242. doi: 10.1007/s00701-007-1273-3. [DOI] [PubMed] [Google Scholar]
- 27.Hammer A, Baer I, Geletneky K, Steiner H H. Cerebrospinal fluid rhinorrhea and seizure caused by temporo-sphenoidal encephalocele. J Korean Neurosurg Soc. 2015;57(04):298–302. doi: 10.3340/jkns.2015.57.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander N S, Chaaban M R, Riley K O, Woodworth B A. Treatment strategies for lateral sphenoid sinus recess cerebrospinal fluid leaks. Arch Otolaryngol Head Neck Surg. 2012;138(05):471–478. doi: 10.1001/archoto.2012.614. [DOI] [PubMed] [Google Scholar]
- 29.Spetzler R F, Wilson C B. Management of recurrent CSF rhinorrhea of the middle and posterior fossa. J Neurosurg. 1978;49(03):393–397. doi: 10.3171/jns.1978.49.3.0393. [DOI] [PubMed] [Google Scholar]
- 30.Hubbard J L, McDonald T J, Pearson B W, Laws E R., Jr Spontaneous cerebrospinal fluid rhinorrhea: evolving concepts in diagnosis and surgical management based on the Mayo Clinic experience from 1970 through 1981. Neurosurgery. 1985;16(03):314–321. doi: 10.1227/00006123-198503000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Bolger W E. Endoscopic transpterygoid approach to the lateral sphenoid recess: surgical approach and clinical experience. Otolaryngol Head Neck Surg. 2005;133(01):20–26. doi: 10.1016/j.otohns.2005.03.063. [DOI] [PubMed] [Google Scholar]
- 32.Marston A P, Van Gompel J J, Carlson M L, O'Brien E K. A unique case of bilateral recurrent sphenoid sinus cerebrospinal fluid leaks: primary acquired leak within the lateral sphenoid sinus recess, followed by a leak via Sternberg's canal. Ann Otol Rhinol Laryngol. 2015;124(08):593–597. doi: 10.1177/0003489415570936. [DOI] [PubMed] [Google Scholar]
- 33.Zanation A M, Snyderman C H, Carrau R L, Gardner P A, Prevedello D M, Kassam A B. Endoscopic endonasal surgery for petrous apex lesions. Laryngoscope. 2009;119(01):19–25. doi: 10.1002/lary.20027. [DOI] [PubMed] [Google Scholar]
- 34.Kassam A B, Prevedello D M, Carrau R L.The front door to meckel's cave: an anteromedial corridor via expanded endoscopic endonasal approach- technical considerations and clinical seriesNeurosurgery 2009;64(3, Suppl):ons71–ons82, discussion ons82–ons83 [DOI] [PubMed]
- 35.Jang T Y, Kim Y H, Shin S H. Long-term effectiveness and safety of endoscopic vidian neurectomy for the treatment of intractable rhinitis. Clin Exp Otorhinolaryngol. 2010;3(04):212–216. doi: 10.3342/ceo.2010.3.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan G, Ma Y, Li H, Li W, Wang J. Long-term results of bilateral endoscopic vidian neurectomy in the management of moderate to severe persistent allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2012;138(05):492–497. doi: 10.1001/archoto.2012.284. [DOI] [PubMed] [Google Scholar]
- 37.Lin P Y, Cheng C Y, Wu C C. Bilateral neurotrophic keratopathy complicating Vidian neurectomy. Am J Ophthalmol. 2001;132(01):106–108. doi: 10.1016/s0002-9394(00)00958-2. [DOI] [PubMed] [Google Scholar]
- 38.Prevedello D M, Pinheiro-Neto C D, Fernandez-Miranda J C.Vidian nerve transposition for endoscopic endonasal middle fossa approaches Neurosurgery 201067(2, Suppl Operative):478–484. [DOI] [PubMed] [Google Scholar]
- 39.Al-Nashar I S, Carrau R L, Herrera A, Snyderman C H. Endoscopic transnasal transpterygopalatine fossa approach to the lateral recess of the sphenoid sinus. Laryngoscope. 2004;114(03):528–532. doi: 10.1097/00005537-200403000-00026. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt R F, Choudhry O J, Raviv J. Surgical nuances for the endoscopic endonasal transpterygoid approach to lateral sphenoid sinus encephaloceles. Neurosurg Focus. 2012;32(06):E5. doi: 10.3171/2012.3.FOCUS1267. [DOI] [PubMed] [Google Scholar]
- 41.El-Tarabishi M N, Fawaz S A, Sabri S M, El-Sharnobi M M, Sweed A. A modification of endoscopic endonasal approach for management of encephaloceles in sphenoid sinus lateral recess. Eur Arch Otorhinolaryngol. 2016;273(12):4305–4314. doi: 10.1007/s00405-016-4125-z. [DOI] [PubMed] [Google Scholar]
- 42.Carrau R L, Snyderman C H, Kassam A B. The management of cerebrospinal fluid leaks in patients at risk for high-pressure hydrocephalus. Laryngoscope. 2005;115(02):205–212. doi: 10.1097/01.mlg.0000154719.62668.70. [DOI] [PubMed] [Google Scholar]
- 43.Hegazy H M, Carrau R L, Snyderman C H, Kassam A, Zweig J. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta-analysis. Laryngoscope. 2000;110(07):1166–1172. doi: 10.1097/00005537-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Sautter N B, Batra P S, Citardi M J. Endoscopic management of sphenoid sinus cerebrospinal fluid leaks. Ann Otol Rhinol Laryngol. 2008;117(01):32–39. doi: 10.1177/000348940811700108. [DOI] [PubMed] [Google Scholar]


