Abstract
Introduction In pediatric patients, endoscopic transnasal surgery (ETNS) poses challenges because of the small size of the developing skull and narrow endonasal corridors.
Objective This study aimed to evaluate the efficacy of ETNS in children by assessing our experience of endoscopic skull base surgery.
Materials and Methods All pediatric patients ( n = 54) who were eligible for surgery using only the endonasal endoscopic approach at our tertiary center between 2012 and 2018 were included in this study. The surgeries were performed simultaneously by an endoscopic skull base team of neurosurgeons and otolaryngologists. Hormonal analyses were conducted before and after surgery in all patients with sellar/parasellar lesions. Patients older than 8 years underwent smell and visual testing.
Results In the 54 patients aged 1 to 17 years who underwent surgery, craniopharyngioma was the most common pathology (29.6%), followed by pituitary adenoma (22.2%). Gross total resection was achieved in 33 (76.7%) of 41 patients who underwent surgery because of the presence of tumors. All visual deficits improved, although one patient sustained olfactory deterioration. Sixteen (29.6%) patients presented with complications such as transient diabetes insipidus and temporary visual loss.
Conclusions Despite anatomy-related challenges in children, adequate results can be achieved with high rates of success, and the functional and anatomical integrity of the developing skull and nose of children can be preserved. In pediatric patients, ETNS is a safe and effective option for addressing various lesions along the skull base.
Keywords: endoscopic, pediatric, skull base, surgery, endonasal, transsphenoidal
Introduction
In the past 20 years, endoscopic transnasal surgery (ETNS) has developed considerably in terms of advances in surgical devices and techniques. 1 2 3 4 Experience gained in performing these procedures in adults was subsequently utilized for pediatric patients. Nevertheless, due to the rarity of sellar tumors in children, these techniques were less frequently utilized. 5 6 7 Moreover, unfavorable anatomical structures, such as small nostrils and narrow sinonasal cavities, in children lead to its occasional use rather than the lack of dedicated surgical instrumentation for this approach. 8 9 Although anatomical structures such as small vestibule and reduced pneumatization of the sphenoid sinus must be considered, some series on pediatric endoscopic surgery are still reported. 2 3 5 6 7 10 11 12 Although skull base tumors in children are typically benign, resulting hormonal problems, mass effect exerted by the lesions, visual loss, and growth retardation may cause significant morbidity. 5 6 7 13 14 15 16 As most skull base lesions in children are located in the midline, ETNS is an advantageous approach in pediatric cases. 17 18 19 20 21 One of the primary concerns when planning surgery for a pediatric patient is to prevent disturbance in the development of the craniofacial bone structure. ETNSs are minimally invasive in nature. Further, the integrity of the bony structure of the skull and hypothalamic and pituitary axis are primarily protected during ETNS. 5 6 7 8 11 13 16
In this study, data, including age, gender, follow-up duration, complications during treatment, leakage of cerebrospinal fluid (CSF) during or after surgery, hormone levels, results of visual analysis before and after surgery, and extent of surgical excision, were assessed. Furthermore, we report for the first-time smell analysis in pediatric patients with skull base pathologies who underwent surgery using the endonasal endoscopic approach.
This study aimed to assess whether the endoscopic endonasal transsphenoidal approach is a viable option for pediatric patients with skull base pathologies.
Methods
All pediatric patients with skull base pathology who were eligible for surgery using the endonasal endoscopic approach in Ankara University Medical School between 2012 and 2018 were included. All surgeries were performed by a team of neurosurgeons and otolaryngologists who are members of the endoscopic skull base group.
All patients were assessed at the pediatrics department for eligibility for surgery and hormonal medications preoperatively. Postoperative care was provided in the pediatric neurosurgical intensive care unit, and patients were assessed in the pediatrics department if necessary.
Preoperative magnetic resonance imaging (MRI) and computed tomography (CT) were performed for all patients. Tumor size, access routes, bony defects, sinuses, neural structures, and normal anatomical landmarks as well as variations were evaluated by the multidisciplinary surgical team. In most cases, a neuronavigation device was used to identify structures during surgery, particularly in complex conditions and whenever necessary. Before surgery, a 1-mm thickness CT or 1-mm thickness MRI was conducted and merged for navigation. Neurophysiological monitorization was performed during surgery for visual-evoked potentials (VEPs) in patients whose pathology was correlated to optic nerves and tracts. The surgical equipment included an 18-cm long rigid endoscope (diameters: 4 mm and 2.7 mm) with 0° optics and a multiangle 4-mm endoscope (Endocameleon Storz, Tuttlingen, Germany), as well as micro neurosurgical and endoscopic instrumentarium and endonasal drill and Doppler systems. Transnasal transsphenoidal is the most common and best surgical route to approach the sphenoid sinus and other areas, and it was selected based on tumor extension or lesion site. The “two nostrils four hands technique” was used in all surgeries. During the different stages of surgery, the primary surgeon was exchanged with either an otolaryngologist or neurosurgeon according to the primary dissection areas below and above the dura mater accordingly.
Due to anatomical requirements, including the small nostrils and unpneumatizated sphenoid sinuses ( Fig. 1 ), particularly in younger children, appropriate instrumentation and techniques were utilized. Tools smaller in size and appropriate atraumatic techniques were used for the small nostrils. Bone drilling was performed for resculpturing and creating artificial sphenoid sinuses in unpneumatizated samples and surgical steps, as shown in the illustrations ( Fig. 2 ). For patients who underwent surgery for CSF rhinorrhea and/or meningocele diagnosed based on a comprehensive algorithm, a preoperative intrathecal sodium fluorescein injection was administered from the lumbar site the night before surgery for intraoperative CSF tracking. 22 23
Fig. 1.
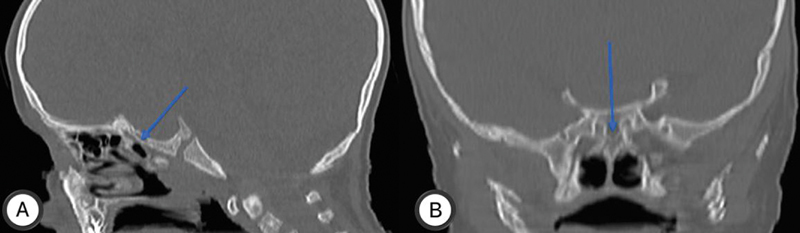
Unpneumatized sphenoid sinuses in a 2-year-old boy with craniopharyngioma, in sagittal ( A ) and coronal ( B ) of bone window paranasal sinuses CT (blue arrows).
Fig. 2.
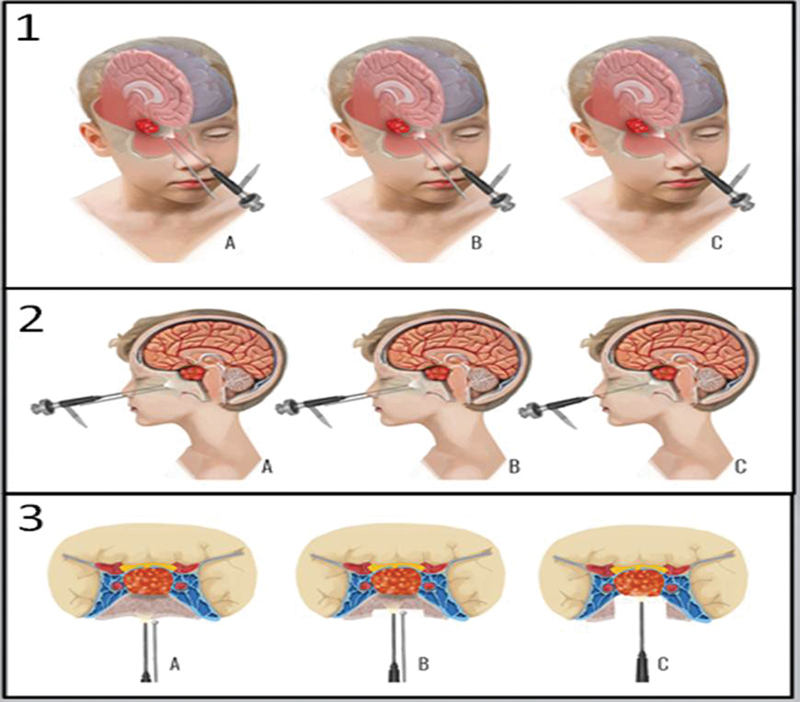
(1) Three-dimensional illustration of a pediatric patient with unpneumatized sphenoid sinuses ( A ). Drilling of the unpneumatized sphenoid sinus ( B ), and after drilling, an artificial sphenoid sinus was created and the tumor was reached ( C ). (2) Image of a pediatric patient with unpneumatized sphenoid sinus ( A ). Drilling of the unpneumatized sphenoid sinus ( B ), and after drilling, an artificial sphenoid sinus was created and the tumor was reached ( C ) on sagittal plane. (3) Image of a pediatric patient with unpneumatized sphenoid sinus ( A ). Drilling of the unpneumatized sphenoid sinus ( B ), and after drilling, an artificial sphenoid sinus was created and the tumor was reached ( C ) on axial–coronal plane.
The hormone levels of all patients diagnosed with tumors before surgery were assessed. Additionally, the sense of smell of all patients aged between 8 and 17 years was pre- and postoperatively evaluated. A supra threshold smell test with eight different odor samples was conducted before and 1 month after surgery to assess olfactory functions. Pre- and postoperative visual acuity and visual field were assessed via computerized visual field examination at the ophthalmology clinic if the patients' lesion was correlated to optic nerves and tracts. As pediatric patients are not cooperative, those younger than 8 years were not included for subjective smell and visual tests. Follow-ups were conducted in neurosurgical and otorhinolaryngological examination rooms for control of both the lesion and nasal functions. Primarily MRI and occasionally CT were performed for radiological evaluation of the lesions, and nasal endoscopy was conducted to assess nasal status. In patients with tumors, the level of resection was determined by comparing postoperative MRI results with preoperative lesion sizes. Considering blood hormone and electrolyte levels, pediatric endocrinology consultation was conducted. Ophthalmology consultation was conducted during the preoperative and postoperative periods to determine visual acuity.
Results
Only the endonasal endoscopic approach was used in a series of 54 pediatric patients between 2012 and 2018 at Ankara University Medical School by our multidisciplinary endoscopic skull base surgery group.
The mean age of the patients was 10.4 (range: 1–17) years ( Table 1 ). Of the patients, 33 (61.1%) were men and 21 (38.8%) were women ( Table 1 ).
Table 1. Demographic information of the patients.
| Patient characteristics | Value |
|---|---|
| Total number of patients | 54 |
| Male | 33 (61.1%) |
| Female | 21 (38.8%) |
| Age at surgery (years) | |
| Mean | 10.4 |
| Range | 1–17 |
The mean hospital stay of the patients was 4.4 days. All patients are admitted to intensive care unit for at least 1 day after surgery. The mean intensive care unit stay was 1.4 days. After discharge from the hospital, all patients underwent follow-up as previously described. The mean follow-up duration was 17 (range: 10–71) months.
Most lesions were benign in nature. Pathological analysis revealed the following: 16 (29.6%) craniopharyngiomas, 12 (22.2%) hypophyseal adenomas, 5 (9.2%) meningocele, 2 (3.7%) hypophysitis, 2 (3.7%) malignant tumors (small blue round cells), 2 (3.7%) pilocytic astrocytoma, 1 (1.8%) germinoma, 1 (1.8) embryonal tumor, 24 1 (1.8%) hemangiopericytoma, 1 (1.8%) capillary hemangioma, 1 (grade II, 1.8%) neurocytoma, 1 (1.8%) dermoid cyst, 25 1 (1.8%) chordoma, 1 (1.8%) hamartoma, 1 (1.8%) fibrous dysplasia, and 1 (1.8%) abscess. Four (7.4%) patients underwent surgery for traumatic rhinorrhea, and one (1.8%) patient underwent endoscopic endonasal odontoid resection 26 because of basilar invagination ( Table 2 ) ( Figs. 3 4 5 6 ).
Table 2. Pathology of the patients who underwent surgery.
| Craniopharyngioma | 16 |
| Hypophyseal adenoma | 12 |
| Meningocele | 5 |
| Traumatic rhinorrhea | 4 |
| Germinoma | 2 |
| Hypophysitis | 2 |
| Malignant tumor (small blue round cell) | 2 |
| Pilocytic astrocytoma | 2 |
| Basilar invagination | 1 |
| Capillary hemangioma | 1 |
| Hemangiopericytoma | 1 |
| Dermoid cyst | 1 |
| Fibrous dysplasia | 1 |
| Chordoma | 1 |
| Hamartoma | 1 |
| Neurocytoma (grade II) | 1 |
| Abscesses | 1 |
Fig. 3.
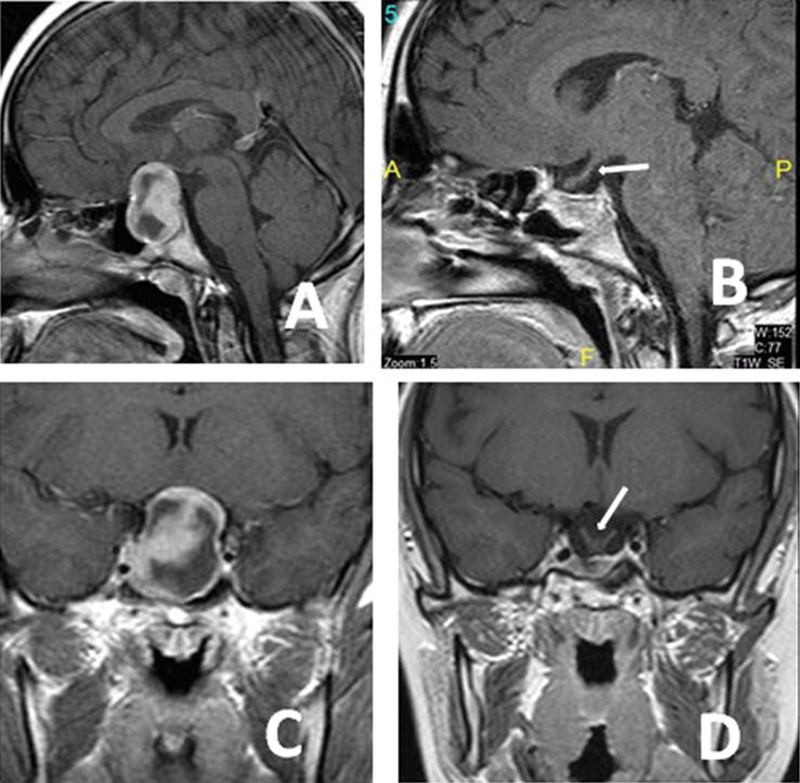
An 8-year-old girl presented with endocrinological and visual problems. Preoperative axial ( A ), preoperative sagittal ( B ), postoperative axial ( C ), and postoperative sagittal ( D ) magnetic resonance imaging sections showing that the tumors were completely removed. The pathology was hypophyseal adenoma. The arrow is showing the decompressed optic chiasma ( D ).
Fig. 4.
An 8-year-old girl presented with endocrinological and visual problems. Preoperative axial ( A ), preoperative sagittal ( B ), postoperative axial ( C ), and postoperative sagittal ( D ) magnetic resonance imaging sections showing that the tumors were completely removed. The pathology was craniopharyngioma.
Fig. 5.
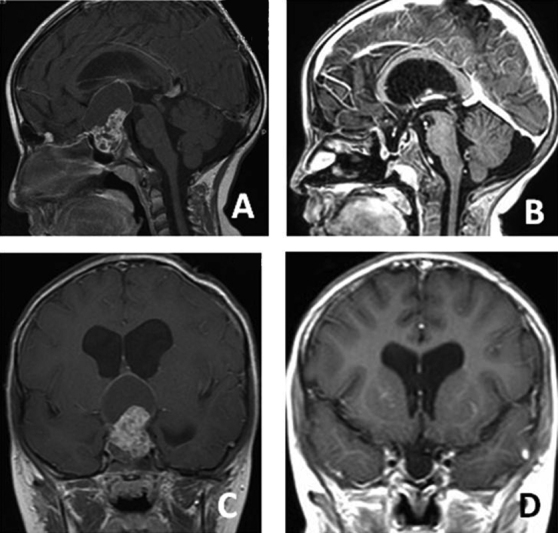
A 5-year-old boy presented with visual problems. Preoperative axial ( A ), preoperative sagittal ( B ), postoperative axial ( C ), and postoperative sagittal ( D ) magnetic resonance imaging sections. The pathology was craniopharyngioma. A semipneumatized sphenoid sinus was observed in this patient. Drilling was then required.
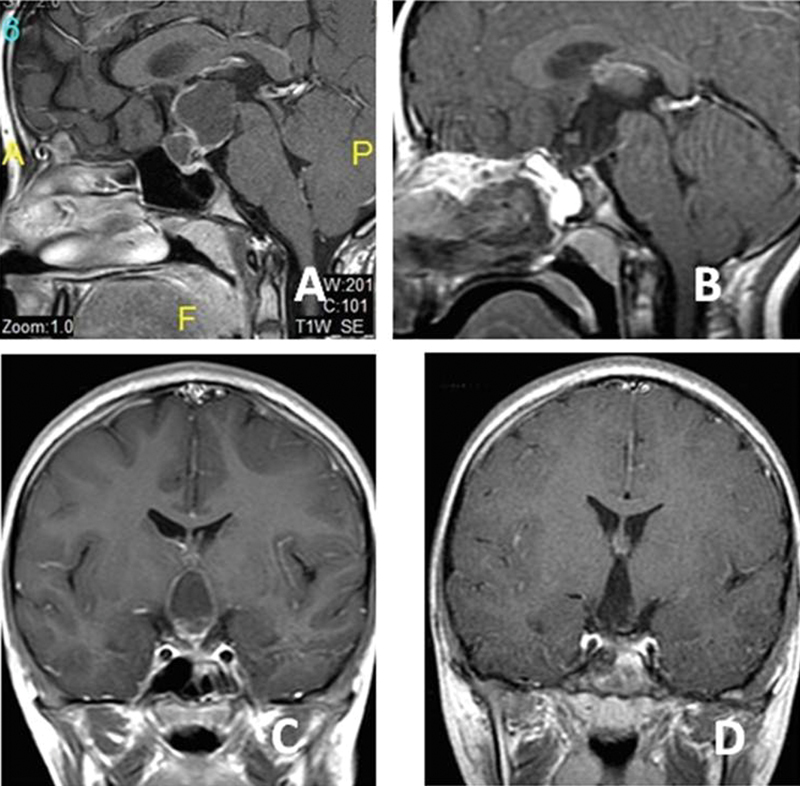
Fig. 6.
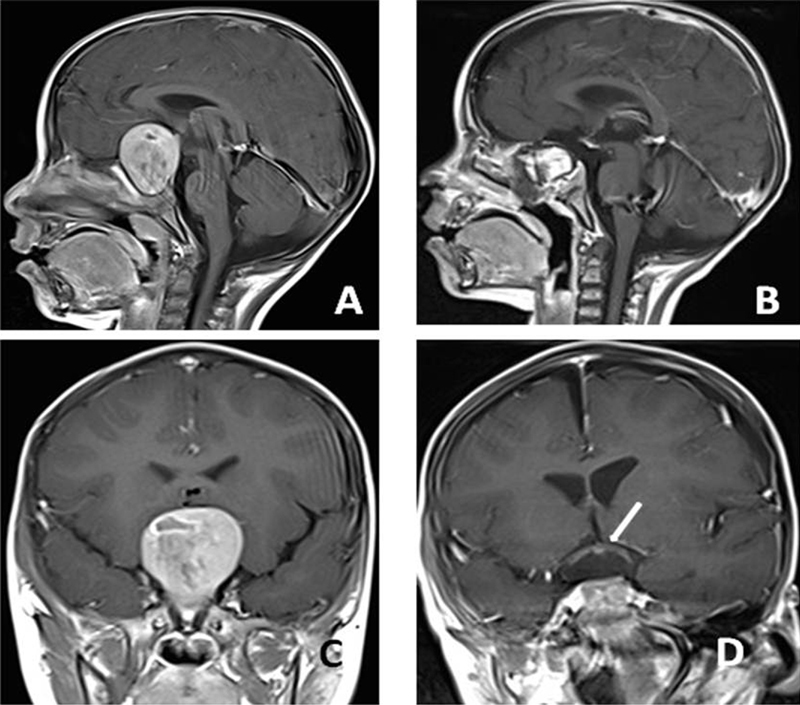
A 2-year-old boy presented with amaurosis (the same patient in Fig. 1 ). Preoperative axial ( A ), preoperative sagittal ( B ), postoperative axial ( C ), and postoperative sagittal ( D ) magnetic resonance imaging sections. The pathology was craniopharyngioma. No pneumatized sphenoid sinus was observed in this patient. After drilling the bone and creating artificial sphenoid sinuses, it was possible to reach the tumor. Postoperative sections ( C and D ) showed the total removal of the tumor and decompression of the optic chiasma (C-arrows).
The presentation of cases was according to the following symptoms: visual problems in 19 (35.1%) patients, headache in 18 (33.3%) patients, endocrinological problems (growth retardation, diabetes insipidus, obesity, gynecomastia, etc.) in 17 (31.4%) patients, rhinorrhea in 6 (11.1%) patients, nasal obstruction in 6 (11.1%) patients, seizures in 3 (5.5%) patients, and nausea in 2 (3.7%) patients ( Table 3 ).
Table 3. Signs and symptoms of the patient upon presentation.
| Symptoms and signs | Number of patients |
|---|---|
| Visual loss/blindness | 19 (35.1%) |
| Headache | 18 (33.3%) |
| Growth retardation | 8 (14.8%) |
| Rhinorrhea | 6 (11.1%) |
| Nasal obstruction | 6 (11.1%) |
| Obesity | 5 (9.2%) |
| Polydipsia/polyuria | 3 (5.5%) |
| Epilepsy/seizures | 3 (5.5%) |
| Nausea | 2 (3.7%) |
| Exophthalmos | 1 (1.8%) |
| Dysphagia | 1 (1.8%) |
| Gynecomastia | 1 (1.8%) |
A total of 43 (79.6%) patients underwent surgery because of the presence of tumors. In three (6.9%) patients, the primary goal was to conduct biopsy for histopathologic examination. Furthermore, subtotal and gross total resections were achieved in 7 (16.2%) and 33 (76.7%) patients, respectively ( Table 4 ).
Table 4. Resection rates of the tumors in the series.
| Resection | Number of lesions |
|---|---|
| Gross total | 33 (76.7%) |
| Subtotal | 7 (16.2%) |
| Biopsy | 3 (6.9%) |
| Total | 43 |
Sixteen (29.6%) patients presented with either perioperative or postoperative complications. In the intensive care unit, 11 patients had postoperative transient diabetes insipidus (TDI). Those patients had increased urine output (>2 mL/kg/h), low urine density, and elevated serum sodium levels. TDI was treated with oral medications (desmopressin) and appropriate fluid replacement. All patients with TDI who were treated recovered 2 days after surgery. None of the patients required constant treatment with desmopressin. The indication for surgery for such patients was either craniopharyngioma or hypophyseal adenoma. Two patients with craniopharyngioma had temporary loss of vision that completely improved within a week after surgery. Nevertheless, one patient who underwent surgery for odontoid resection because of basilar invagination and who did not present with CSF leak during surgery and had an uneventful postoperative period got lethargic after a powerful sneeze 10 days after discharge, and his CT scan revealed a pneumocephalus. Following application of fluorescein to monitor any CSF leak, this patient underwent a second surgery the next day of last admission to locate the source of the pneumocephalus. Although no CSF was observed even under blue light filter to detect the presence of fluorescein, a nasoseptal flap was raised and laid to the nasopharyngeal operation field, which was originally left for secondary healing, and this is always performed in adults when this transnasal approach is used. After surgery, the neurological condition of the patient improved, and the patient was discharged and remained stable during follow-ups. Our experience with this case has already been published, and in the pediatric population, nasoseptal flap is a reliable barrier even if no apparent dural defect is observed. 26 In terms of other complications, a patient with craniopharyngioma had panhypopituitarism after surgery. This patient was assessed in the pediatric endocrinology department for hormone replacement therapy. Regarding perioperative complications, one patient had relatively intense cavernous sinus hemorrhage that required two units of erythrocyte suspension replacement during surgery. Hemorrhage was controlled using an absorbable hemostat made from oxidized cellulose polymer and hemostatic matrix made from human thrombin ( Table 5 ).
Table 5. Number of patients with complications and various complications.
| Complications | Number of patients |
|---|---|
| Transient diabetes insipidus | 11 |
| Temporary loss of vision | 2 |
| Pneumocephalus/rhinorrhea | 1 |
| Panhypopituitarism | 1 |
| Cavernous sinus hemorrhage | 1 |
| Total | 16 (29.6%) |
Regarding the endocrinological status of the group whose lesions were at the sella and of patients with endocrinological problems before surgery, six patients had hypophyseal failure (four with craniopharyngioma, one with hypophyseal adenoma, and one with hypophysitis patients), five patients had high prolactin levels (three with hypophyseal adenoma, one with craniopharyngioma, and one with hypophysitis presenting with high prolactin), and four patients had increased blood cortisone levels (two with craniopharyngioma and two with hypophyseal adenoma). One patient with hypophyseal adenoma had lower insulin-like growth factor-1 levels. The hormone levels of all these patients returned to normal 1 month after surgery. The remaining patients who had tumors around the sella had normal hormone levels before surgery. After surgery, two patients with craniopharyngioma required permanent hormone replacement. A consultation regarding hormone therapy was conducted with the pediatric endocrinology department.
Moreover, patients with tumors were assessed for chemoradiotherapy at the pediatric oncology department if their disease required further treatment. Two patients with malignant tumor (small blue round cells) received additional chemotherapy, seven patients received additional radiotherapy (five with craniopharyngioma, one with neurocytoma, and one with germinoma), and three patients received chemoradiotherapy (two with pilocytic astrocytoma and one with embryonal tumor). Patients were followed-up while they were on adjuvant therapy.
A total of 19 (35.1%) patients had visual acuity loss before surgery. All these patients had improved visual acuity after surgery because of the decompression of the optic nerves and optic chiasm ( Fig. 7 ). Two patients had transient visual reduction after the early period of surgery, which improved to preoperative levels after 2 weeks. None of the patients had permanent visual loss after surgery ( Table 6 ).
Fig. 7.
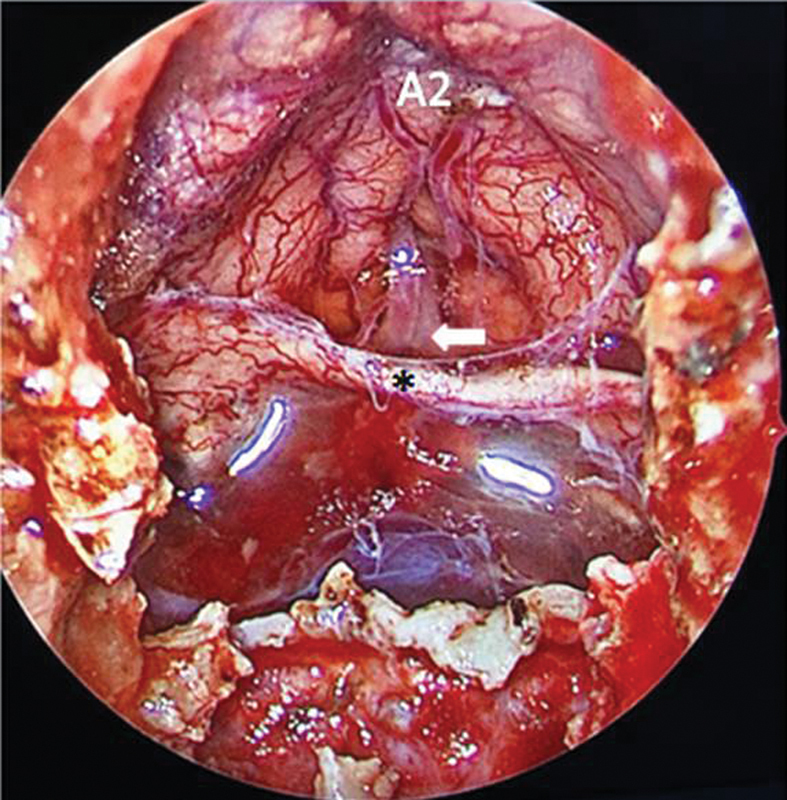
Perioperative endoscopic view of the patients in Fig. 6 after removal of the tumor. Decompressed optic chiasm (*), anterior communicating artery (arrow), and A2.
Table 6. Visual and olfactory outcomes in the series.
| Visual improvement or no change | 100% |
| Olfactory function improvement or no change (first 6 months postoperatively) | 68.5% |
| Olfactory function improvement or no change (after 6 months postoperatively) | 97.1% |
A total of 35 patients aged 8 to 18 years were subjected to smell tests before and after surgery. Based on the suprathreshold test, which includes identification of eight different odors, 11 (31.4%) of 35 patients had a slight loss of olfactory functions after surgery. The loss was scored 1 of 8 points in all patients except one, who recovered completely 6 months after surgery. These patients include six craniopharyngiomas, three macroadenomas, and two meningoceles. No changes or improvements in olfactory functions were observed in the remaining 24 (68.5%) patients ( Table 6 ).
Furthermore, an intraoperative CSF leakage was observed in 27 (50%) patients during surgery. These patients include 16 craniopharyngiomas, 4 macroadenomas, 4 meningoceles, 2 pilocytic astrocytoma, and 1 germinoma. However, only one patient had rhinorrhea after the postoperative period, and repair was performed during the second surgery. 26 For patients who underwent surgery for CSF rhinorrhea and/or meningocele, preoperative intrathecal sodium fluorescein administration helped in the identification of the CSF leak site or detecting multiple leak sites as well as in intraoperative verification of watertight sealing in each case during surgery. Apart from these conditions, other CSF leaks or oozings that occurred because of tumor resections were immediately closed at the end of surgery using a multilayer reconstruction technique. As the last layer of the reconstruction, a pedicled nasoseptal flap was used in all patients with CSF leak, considering faster healing and lesser compliance to postoperative instructions, such as “no heavy lifting or no positive Valsalva pressure application,” in the pediatric population. A nasoseptal flap was used in the following pathologies: 16 craniopharyngiomas, 4 macroadenomas, 4 meningoceles, 1 odontoidectomy, and 1 germinoma ( Table 7 ). No lumbar drainage was used in any case. Watertight healing was achieved in all patients controlled with postoperative objective measures. 22 23 27
Table 7. CSF leakage in the patients.
| CSF leakage | Number of patients |
|---|---|
| Perioperative CSF leakage | 27 (50%) |
| Postoperative CSF leakage | 1 (1.8%) |
Abbreviation: CSF, cerebrospinal fluid.
No mortality was recorded during the perioperative period. One patient with craniopharyngioma died from endocrinological failure and electrolyte imbalance on the 15th day after surgery.
Discussion
ETNS is a widely used and accepted surgical approach for targeting skull base pathologies in all age populations. Several studies reported pediatric patients who underwent surgery using this approach. 2 3 4 5 6 7 The present study had the second largest cohort with a series of 54 pediatric patients who underwent surgery using the ETNS approach, and the experiences of our multidisciplinary skull base workgroup were presented.
In 2014, Chivukula et al conducted a study with the highest number of cases to date and presented the experience of the Pittsburg Group. 2 In that study, the most common complication reported was headache (19.5%), followed by diabetes insipidus (15%), which is primarily attributed to the manipulation of the infundibulum during surgery. If a patient is closely monitored, such complications can be easily diagnosed. In our series, 11 (20.3%) patients had postoperative TDI. Those patients were treated with appropriate fluid therapy, desmopressin, and adequate hormone replacement. Headache was not a major complication during the postoperative period in our study. Thus, the authors did not focus and measure such symptoms with a visual analog scale.
Rhinorrhea is one of the most important complications of ETNS in the pediatric group. 28 29 In this study, one patient had postoperative rhinorrhea. CSF leakage and pneumocephalus required emergent surgery. In the second surgery, a Hadad flap (vascularized nasoseptal flap) was used to repair the skull base defect. The medical condition of the patient subsequently improved. 26 Hadad flap is advised not only for adults but also for children. 30 31 This reconstruction technique is extremely effective, and it is a safe technique for defects in the anterior skull base 17 19 27 30 and is extremely advantageous for the open approach technique in children. 31 32 33
One of the indications for ETNS is meningoencephalocele/cephalocele. Cephalocele refers to the congenital herniation of the intracranial contents through a bone defect. This pathology is described as an encephalocele when the sac contains neural elements and as a meningocele when no recognizable brain tissue is present within the sac. 34 35 Patients with a history of rhinorrhea or meningitis were selected for surgery. Before surgery, CT was performed to determine the bony defect and MRI was conducted to identify the contents and size of the meningocele. The goal of the endoscopic endonasal approach is to expose the ventral aspect of the cephalocele, reduce the sac, resect the meningocele, and repair the resulting CSF leak. Endoscopic repair of the meningocele defect is a minimally invasive procedure and is advised for successful treatment of the lesions. 34 35 36 Keshri et al reported six patients with meningocele who underwent surgery using the transnasal endoscopic approach. 35 Ma et al reported a series of 23 patients who underwent surgery for CSF leak with or without encephalocele, 28 and Marfatia et al reported a series of 19 pediatric patients with meningoencephaloceles who underwent surgery. 37 A high success rate was observed in those studies, which is similar to the success rate in our study.
Locatelli et al reported 27 pediatric patients who underwent surgery for sellar lesions. 5 Similar to our study, endoscopic surgeries were performed by a team of neurosurgeons and otolaryngologists. The “two nostrils four hands technique” is extremely useful as one hand holds the endoscope and the other holds the aspirator, and the remaining two hands can perform the necessary surgical procedures. Rigante et al reported 21 patients who underwent surgery for sellar lesions using the endoscopic and microscopic approach. 21 Six patients required chronic hormone replacements after surgery in that series. In our series, two patients with craniopharyngioma required permanent hormone replacement. Giovannetti et al reported 44 patients who underwent surgery using the endoscopic endonasal technique for skull base pathologies. They observed that two patients presented with CSF leak and cerebral abscess, and three patients had diabetes insipidus. In our series, one patient had rhinorrhea 26 and no abscess was observed during the postoperative period. Craniopharyngiomas and hypophyseal adenomas are the most common pathologies in the sellar region in children, 2 3 4 5 6 7 and approximately half of our patients presented with these two pathologies. The other pathologies were meningocele, Rathke's cleft cyst, germinoma, fibrous dysplasia, chordoma, and hypophysitis in this area. 2 3 4 5 6 7 16 17 33 38 However, extremely rare pathologies, such as pontine cavernoma, dermoid cyst, and embryonal tumor, can be observed in this area and can operated using the ETNS technique in children. 7 24 25 Some studies compared the endonasal approach with other techniques, and results showed that this approach is better than other approaches. 18 20 21
In our study, neurophysiologic monitoring was conducted in all patients during surgery. As sellar lesions are at close proximity to visual pathways, VEP analysis can be extremely helpful in preventing recurrence. While the patient is being positioned for surgery, an optical stimulation device is placed over the closed eyelids, and electrodes are planted on the occipital scalp. This permits intraoperative monitoring of visual functions during surgery. Additionally, electromyography, somatosensory-evoked potential, and motor-evoked potential monitoring are helpful in selected cases. In a series of 129 patients who underwent 159 surgeries, Elangovan et al 39 stated that intraoperative neurophysiological monitoring is helpful during pediatric transnasal transsphenoidal surgery.
The olfactory functions of patients aged between 8 and 17 years were preoperatively assessed in our study. Patients younger than 8 years were excluded for the smell test as they are insufficiently cooperative. Patients were subjected to the test before and 1 and 6 months after surgery. In total 32 patients were subjected to the smell test. Eleven (31.4%) patients experienced loss of the sense of smell, whereas the remaining 24 (68.5%) patients did not present with changes or improvements in olfactory functions. All patients except one with conditions that worsened after surgery recovered completely 6 months after surgery. Our team previously published a study on the differences in olfactory functions after transsphenoidal pituitary surgery using endoscopic and microscopic approaches. 40 To the best of our knowledge, this is the only study that conducted a smell analysis on pediatric patients who underwent ETNS surgery.
In our series, visual analysis was performed in all patients aged between 8 and 17 years. A total of 27 patients were subjected to visual tests. An ophthalmology consultation was conducted for the visual field test before and after surgery. The optical functions of 19 (35.1%) patients were diminished before surgery. Postoperative analysis after 1 month revealed that all patients had good visual function after surgery. Only one (3.7%) patient had temporary loss of vision immediately after surgery. ETNS previously achieved good visual outcomes not only for adults but also for children, 7 41 42 43 which is similar to our results.
Tan et al reported a series of three cases in 2014. One of the cases involved a 3-year-old boy with posterior fossa rhabdomyosarcoma. 44 Using the ETNS approach, a wide range of surgical fields from the crista galli to the odontoid process can be achieved. Moreover, various surgeries can be performed by experienced surgical teams using this technique. 3 4 7 8
Limits of Study
Fifty-four patients are included in this study. Larger number of cases would improve the outcome of this study. A larger number of patients would yield more significant results.
Furthermore, mean follow-up duration is 17 months. This might seem like a not so long term. A longer follow-up duration would yield a more accurate analysis of postoperative period (e.g., smell function, tumor recurrence rates by time, long term hormone levels…).
During this study, a single endonasal endoscopy surgical team performed all operations. A multicenter study including data from various sources would yield more significant results.
Conclusion
ETNS is a safe and effective surgical approach that can be used as an option in managing various skull base lesions in children. In relation to the relatively small sinonasal cavity of the pediatric age group, it provides adequate exposure of the surgical field, resulting in a high rate of tumor removal and improvement in visual and endocrinological conditions without affecting nasal functions and the sense of smell. Moreover, its complication rates are comparable or even lower than those of other conventional approaches. In particular, after the use of the nasoseptal flap, the most common complication is postoperative CSF leakage, and safety issues were then considered. Nevertheless, due to the specific requirements of ETNS in this age group, this surgery should be conducted in specific multidisciplinary centers with adequate experience in adults and with appropriate technology and specific instrumentation for dealing with pediatric populations.
Acknowledgment
The authors report no financial interests or potential conflicts of interest. This paper has not been previously published, in whole or in part, or submitted elsewhere for review.
The results of the first study were presented at the 27 th Annual Meeting North America Skull Base Society, New Orleans, 2017.
Conflict of Interest None declared.
These authors contributed equally to this work.
References
- 1.Fliss D M. Recent advances in pediatric skull base surgery. J Neurol Surg B Skull Base. 2018;79(01):1–2. doi: 10.1055/s-0038-1624584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chivukula S, Koutourousiou M, Snyderman C H, Fernandez-Miranda J C, Gardner P A, Tyler-Kabara E C. Endoscopic endonasal skull base surgery in the pediatric population. J Neurosurg Pediatr. 2013;11(03):227–241. doi: 10.3171/2012.10.PEDS12160. [DOI] [PubMed] [Google Scholar]
- 3.Kassam A, Thomas A J, Snyderman C.Fully endoscopic expanded endonasal approach treating skull base lesions in pediatric patients J Neurosurg 2007106(2, Suppl):75–86. [DOI] [PubMed] [Google Scholar]
- 4.Khalili S, Palmer J N, Adappa N D. The expanded endonasal approach for the treatment of intracranial skull base disease in the pediatric population. Curr Opin Otolaryngol Head Neck Surg. 2015;23(01):65–70. doi: 10.1097/MOO.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 5.Locatelli D, Massimi L, Rigante M. Endoscopic endonasal transsphenoidal surgery for sellar tumors in children. Int J Pediatr Otorhinolaryngol. 2010;74(11):1298–1302. doi: 10.1016/j.ijporl.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Zhan R, Xin T, Li X, Li W, Li X. Endonasal endoscopic transsphenoidal approach to lesions of the sellar region in pediatric patients. J Craniofac Surg. 2015;26(06):1818–1822. doi: 10.1097/SCS.0000000000001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannetti F, Mussa F, Priore P. Endoscopic endonasal skull base surgery in pediatric patients. A single center experience. J Craniomaxillofac Surg. 2018;46(12):2017–2021. doi: 10.1016/j.jcms.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Banu M A, Rathman A, Patel K S.Corridor-based endonasal endoscopic surgery for pediatric skull base pathology with detailed radioanatomic measurements Neurosurgery 20141002273–293., discussion 293 [DOI] [PubMed] [Google Scholar]
- 9.Holzmann D, Bozinov O, Krayenbühl N. Is there a place for the endoscope in skull base surgery in children less than 5 years? J Neurol Surg A Cent Eur Neurosurg. 2014;75(02):133–139. doi: 10.1055/s-0032-1327442. [DOI] [PubMed] [Google Scholar]
- 10.Youssef C A, Smotherman C R, Kraemer D F, Aldana P R. Predicting the limits of the endoscopic endonasal approach in children: a radiological anatomical study. J Neurosurg Pediatr. 2016;17(04):510–515. doi: 10.3171/2015.6.PEDS14695. [DOI] [PubMed] [Google Scholar]
- 11.Tatreau J R, Patel M R, Shah R N. Anatomical considerations for endoscopic endonasal skull base surgery in pediatric patients. Laryngoscope. 2010;120(09):1730–1737. doi: 10.1002/lary.20964. [DOI] [PubMed] [Google Scholar]
- 12.Kahilogullari G, Meco C, Beton S, Zaimoglu M, Basak H, Unlu A. Endoscopic endonasal skull base surgery for 38 pediatric cases: Ankara University experience. J Neurol Surg B Skull Base. 2017;78(01):S1–S156. [Google Scholar]
- 13.Koutourousiou M, Gardner P A, Fernandez-Miranda J C, Tyler-Kabara E C, Wang E W, Snyderman C H. Endoscopic endonasal surgery for craniopharyngiomas: surgical outcome in 64 patients. J Neurosurg. 2013;119(05):1194–1207. doi: 10.3171/2013.6.JNS122259. [DOI] [PubMed] [Google Scholar]
- 14.Bakhsheshian J, Jin D L, Chang K E. Risk factors associated with the surgical management of craniopharyngiomas in pediatric patients: analysis of 1961 patients from a national registry database. Neurosurg Focus. 2016;41(06):E8. doi: 10.3171/2016.8.FOCUS16268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jane J A, Jr, Prevedello D M, Alden T D, Laws E R., Jr The transsphenoidal resection of pediatric craniopharyngiomas: a case series. J Neurosurg Pediatr. 2010;5(01):49–60. doi: 10.3171/2009.7.PEDS09252. [DOI] [PubMed] [Google Scholar]
- 16.Pandey P, Ojha B K, Mahapatra A K. Pediatric pituitary adenoma: a series of 42 patients. J Clin Neurosci. 2005;12(02):124–127. doi: 10.1016/j.jocn.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Komotar R J, Starke R M, Raper D M, Anand V K, Schwartz T H. Endoscopic endonasal versus open repair of anterior skull base CSF leak, meningocele, and encephalocele: a systematic review of outcomes. J Neurol Surg A Cent Eur Neurosurg. 2013;74(04):239–250. doi: 10.1055/s-0032-1325636. [DOI] [PubMed] [Google Scholar]
- 18.Elliott R E, Jane J A, Jr, Wisoff J H.Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches Neurosurgery 20116903630–643., discussion 643 [DOI] [PubMed] [Google Scholar]
- 19.Di Rocco F, Couloigner V, Dastoli P, Sainte-Rose C, Zerah M, Roger G. Treatment of anterior skull base defects by a transnasal endoscopic approach in children. J Neurosurg Pediatr. 2010;6(05):459–463. doi: 10.3171/2010.8.PEDS09325. [DOI] [PubMed] [Google Scholar]
- 20.Massimi L, Rigante M, D'Angelo L. Quality of postoperative course in children: endoscopic endonasal surgery versus sublabial microsurgery. Acta Neurochir (Wien) 2011;153(04):843–849. doi: 10.1007/s00701-010-0929-6. [DOI] [PubMed] [Google Scholar]
- 21.Rigante M, Massimi L, Parrilla C. Endoscopic transsphenoidal approach versus microscopic approach in children. Int J Pediatr Otorhinolaryngol. 2011;75(09):1132–1136. doi: 10.1016/j.ijporl.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Meco C, Oberascher G. Comprehensive algorithm for skull base dural lesion and cerebrospinal fluid fistula diagnosis. Laryngoscope. 2004;114(06):991–999. doi: 10.1097/00005537-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Meco C, Arrer E, Oberascher G. Efficacy of cerebrospinal fluid fistula repair: sensitive quality control using the beta-trace protein test. Am J Rhinol. 2007;21(06):729–736. doi: 10.2500/ajr.2007.21.3105. [DOI] [PubMed] [Google Scholar]
- 24.Yakar F, Doğan İ, Meco C, Heper A O, Kahilogullari G. Sellar embryonal tumor: a case report and review of the literature. Asian J Neurosurg. 2018;13(04):1197–1201. doi: 10.4103/ajns.AJNS_30_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahilogullari G, Yakar F, Bayatli E, Erden E, Meco C, Unlu A. Endoscopic removal of a suprasellar dermoid cyst in a pediatric patient: a case report and review of the literature. Childs Nerv Syst. 2018;34(08):1583–1587. doi: 10.1007/s00381-018-3777-y. [DOI] [PubMed] [Google Scholar]
- 26.Kahilogullari G, Meco C, Zaimoglu M. Pneumocephalus after endoscopic odontoidectomy in a pediatric patient: the lesson learned. Childs Nerv Syst. 2015;31(09):1595–1599. doi: 10.1007/s00381-015-2740-4. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Ari O, Wengier A, Ringel B. Nasoseptal flap for skull base reconstruction in children. J Neurol Surg B Skull Base. 2018;79(01):37–41. doi: 10.1055/s-0037-1617435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma J, Huang Q, Li X. Endoscopic transnasal repair of cerebrospinal fluid leaks with and without an encephalocele in pediatric patients: from infants to children. Childs Nerv Syst. 2015;31(09):1493–1498. doi: 10.1007/s00381-015-2746-y. [DOI] [PubMed] [Google Scholar]
- 29.Stapleton A L, Tyler-Kabara E C, Gardner P A, Snyderman C H, Wang E W. Risk factors for cerebrospinal fluid leak in pediatric patients undergoing endoscopic endonasal skull base surgery. Int J Pediatr Otorhinolaryngol. 2017;93:163–166. doi: 10.1016/j.ijporl.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Gump W C. Endoscopic endonasal repair of congenital defects of anterior skull base: developmental considerations and surgical outcomes. J Neurol Surg B Skull Base. 2015;76(04):291–295. doi: 10.1055/s-0034-1544120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh A, Hatten K, Learned K O. Pediatric nasoseptal flap reconstruction for suprasellar approaches. Laryngoscope. 2015;125(11):2451–2456. doi: 10.1002/lary.25395. [DOI] [PubMed] [Google Scholar]
- 32.Wasserzug O, DeRowe A, Ringel B, Fishman G, Fliss D M. Open approaches to the anterior skull base in children: review of the literature. J Neurol Surg B Skull Base. 2018;79(01):42–46. doi: 10.1055/s-0037-1621739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duek I, Pener-Tessler A, Yanko-Arzi R. Skull base reconstruction in the pediatric patient. J Neurol Surg B Skull Base. 2018;79(01):81–90. doi: 10.1055/s-0037-1615806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen G, Zhang Q, Ling F. An endoscopic endonasal approach for the surgical repair of transsphenoidal cephalocele in children. J Clin Neurosci. 2011;18(05):723–724. doi: 10.1016/j.jocn.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 35.Keshri A K, Shah S R, Patadia S D, Sahu R N, Behari S. Transnasal endoscopic repair of pediatric meningoencephalocele. J Pediatr Neurosci. 2016;11(01):42–45. doi: 10.4103/1817-1745.181249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeinalizadeh M, Sadrehosseini S M, Habibi Z, Nejat F, Silva H B, Singh H. Endonasal management of pediatric congenital transsphenoidal encephaloceles: nuances of a modified reconstruction technique. Technical note and report of 3 cases. J Neurosurg Pediatr. 2017;19(03):312–318. doi: 10.3171/2016.10.PEDS16270. [DOI] [PubMed] [Google Scholar]
- 37.Marfatia H K, Parelkar K A, Chakraborty A, Mishra S. Pediatric meningoencephaloceles endoscopic repair: our experience. Allergy Rhinol (Providence) 2018;9:2.152656718802408E15. doi: 10.1177/2152656718802408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stapleton A L, Tyler-Kabara E C, Gardner P A, Snyderman C H. Endoscopic endonasal surgery for benign fibro-osseous lesions of the pediatric skull base. Laryngoscope. 2015;125(09):2199–2203. doi: 10.1002/lary.25070. [DOI] [PubMed] [Google Scholar]
- 39.Elangovan C, Singh S P, Gardner P. Intraoperative neurophysiological monitoring during endoscopic endonasal surgery for pediatric skull base tumors. J Neurosurg Pediatr. 2016;17(02):147–155. doi: 10.3171/2015.7.PEDS14403. [DOI] [PubMed] [Google Scholar]
- 40.Kahilogullari G, Beton S, Al-Beyati E S. Olfactory functions after transsphenoidal pituitary surgery: endoscopic versus microscopic approach. Laryngoscope. 2013;123(09):2112–2119. doi: 10.1002/lary.24037. [DOI] [PubMed] [Google Scholar]
- 41.Stefko S T, Snyderman C, Fernandez-Miranda J. Visual outcomes after endoscopic endonasal approach for craniopharyngioma: the Pittsburgh experience. J Neurol Surg B Skull Base. 2016;77(04):326–332. doi: 10.1055/s-0036-1571333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel V S, Thamboo A, Quon J. Outcomes after endoscopic endonasal resection of craniopharyngiomas in the pediatric population. World Neurosurg. 2017;108:6–14. doi: 10.1016/j.wneu.2017.08.058. [DOI] [PubMed] [Google Scholar]
- 43.Fredes F, Undurraga G, Rojas P. Visual outcomes after pituitary surgery in patients presenting with preoperative visual deficits. J Neurol Surg B Skull Base. 2017;78(06):461–465. doi: 10.1055/s-0037-1604169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan S H, Ganesan D, Prepageran N, Waran V. A minimally invasive endoscopic transnasal approach to the craniovertebral junction in the paediatric population. Eur Arch Otorhinolaryngol. 2014;271(11):3101–3105. doi: 10.1007/s00405-014-3149-5. [DOI] [PubMed] [Google Scholar]


