Abstract
Our aim was to assess the effect of perioperative interventions targeting psychological distress on clinical outcome after total knee arthroplasty (TKA). We searched studies on the effect of perioperative interventions focused on psychological distress used in conjunction with TKA on pain, function, and quality of life (QoL) on PubMed, Embase.com, PsycINFO/OVID, CENTRAL, the Cochrane Database of Systematic Reviews, Scopus, and Web of Science. We included 40 studies (22 RCTs, ten cohort studies, and eight quasi-experimental studies) with a total of 3846 patients. We graded the quality of evidence as low for pain and function and as moderate for QoL. Patients receiving music, education, cognitive behavioural therapy, guided imagery, pain coping skills training, Reiki, occupational therapy with self-monitoring, and biofeedback-assisted progressive muscles relaxing training had lower pain scores or declined opioid prescriptions after TKA. Pain coping skills training, audio recording-guided imagery scripts, video promoting self-confidence, psychological therapies by video, Reiki, music, occupational therapy with self-monitoring, education, and psychotherapy improved postoperative functional outcome. Education through an app improved QoL after TKA. The studies in our systematic review show that perioperative interventions targeting psychological distress for patients receiving TKA seem to have a positive effect on postoperative pain, function, and QoL. RCTs with strict methodological safeguards are still needed to determine if perioperative interventions focused on psychological distress should be used in conjunction with TKA. These studies should also assess which type of intervention will be most effective in improving patient-reported outcome measures and declining opioid prescriptions.
Electronic supplementary material
The online version of this article (10.1007/s00296-020-04644-y) contains supplementary material, which is available to authorized users.
Keywords: Total knee arthroplasty, Psychological distress, Pain, Function, Quality of life
Introduction
Total knee arthroplasty (TKA) is the treatment of choice for medically operable patients with end-stage osteoarthritis (OA) of the knee joint if non-surgical therapies fail to obtain adequate pain relief and functional improvement [1]. TKA proved to be a cost-effective procedure with excellent postoperative implant-related outcomes, such as radiographic appearance and implant features [2]. Nevertheless, a significant number of patients report pain (8.0–26.5%) on long-term follow-up after TKA [3] and as many as 11–19% of the patients are not satisfied with their procedure [4, 5]. Persistent pain after TKA is commonly treated with opioids after surgery [6]. Currently, increasing misuse and addiction to opioids are a rapidly evolving public health issue [7]. Improving pain scores after surgery by understanding factors influencing postoperative pain may help prevent further expansion of this opioid crisis [7].
Unfavourable outcome after TKA is related to age, gender, level of education, pre-operative function and pain [8], comorbidities [9], social support [9], Body Mass Index (BMI) [10], and surgical factors [11–13]. Preoperative psychological factors such as mental health status, symptoms of anxiety and depression, and poor coping skills have also been examined [13–15]. Systematic reviews [16–18] and meta-analyses [19, 20] on this subject reported that psychological distress might affect the postoperative outcome (pain and function) after TKA. Perioperative interventions targeting these psychological factors may improve clinical outcome after surgery. Previous studies have examined the effect of interventions influencing psychological factors to improve postoperative clinical outcome after TKA [21–24]. We found three previous systematic reviews on psychological interventions in conjunction to orthopaedic surgeries [25–27]. The systematic review of Bay et al. [25] did not support the effectiveness of psychological interventions in improving patient-reported joint outcomes after TKA as the interventions explored by studies were found to be ineffective at the latest follow-up. The results of Szeverenyi et al. [26] and Tong et al. [27] indicated that psychological interventions might improve postoperative outcome of orthopaedic surgery. These previous reviews included several types of orthopaedic procedures (among which TKA, total hip arthroplasty (THA) and spinal procedures) and did not focus on TKA. Besides, the most up-to-date search was performed in January 2018 [27].
To our knowledge, focused systematic reviews of studies on TKA patients with wide search and inclusion criteria investigating the effect of interventions targeting psychological distress on patient-reported outcome measures pain, function and/or quality of life (QoL) after surgery have not yet been reported. The aim of our systematic review was to assess the effect of perioperative interventions focused on psychological distress on pain, function and QoL after primary TKA for OA of the knee.
Methods
Search strategy and study selection
We registered our review protocol at PROSPERO international prospective register of systematic reviews (https://www.crd.york.ac.uk/PROSPERO/) with reference number CRD42016052466 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42016052466). We performed this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement criteria [28].
We performed the literature search according to the guidance of Gasparyan et al. [29]. A professional medical librarian (CdH) identified therapeutic studies (published articles and abstracts of major conferences) exploring the influence of any type of perioperative (before TKA, during surgery, or during postoperative rehabilitation) interventions targeting psychological distress on postoperative outcome (pain, function, and/or QoL) after TKA by searching PubMed, Embase.com, PsycINFO/OVID, CENTRAL, the Cochrane Database of Systematic Reviews, Scopus and Web of Science from inception up to May 26, 2020.
The following terms, including synonyms and closely related words, were used as index terms or free-text words: ‘total knee arthroplasty’ and ‘psychological intervention’. Full search strategies for all the databases are available in Supplementary Appendix 1. Duplicate articles were excluded.
Selection of articles was limited to adults > 18 years who had undergone a primary total knee replacement for osteoarthritis of the knee. We included different study designs (RCTs, cohorts, quasi-experimental studies) investigating the effect of any intervention targeting psychological distress on postoperative pain, function and/or QoL. Minimum duration of follow-up was not an inclusion criterion with the aim to create a complete overview of all studies that have investigated the effect of perioperative interventions focused on psychological distress on pain, function and/or QoL. Perioperative interventions influencing psychological factors of patients had to be clearly defined. Full-text availability was required. There were no restrictions with respect to language, age, or publication source of the paper.
Exclusion criteria were studies not meeting domain, determinant, or outcome, case reports, descriptive studies (in which there was no control group), non-primary literature studies (letter to the editor, reviews, thesis, expert opinions) and articles with no separated results of patients after TKA and total hip arthroplasty (THA) or other types of surgery if various surgical procedures were analysed.
Main outcome variables
Two authors (JS & GO) independently screened articles for title and abstract and thereafter full text if the abstract potentially met the inclusion criteria. Subsequently, the authors (JS & GO) individually extracted information regarding study design, baseline patient characteristics, baseline clinical findings, follow-up, number of patients initially included in the study, the number of patients available for follow-up and data regarding the primary outcomes of the systematic review. When there was disagreement with respect to data extraction, a third author (AH or RP) could make the final decision.
Quality assessment
We assessed the risk of bias of the included studies using Cochrane Collaboration’s tool for assessing the risk of bias [30]. Using this tool, two authors (JS & GO) independently scored six types of bias (selection bias, performance bias, detection bias, attrition bias, reporting bias, and other types of bias) as low, high, or unclear on potential risk of bias [30].
We used the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) approach to qualify the overall level of evidence of outcome measures pain, function and/or QoL (https://www.gradeworkinggroup.org/). Using the GRADEpro software (McMaster University, 2015, available from www.gradepro.org), we graded the quality of evidence as high, moderate, low, or very low [31].
Data analysis
We arranged the studies according to the type of perioperative intervention (music, education, psychotherapy, and remaining) and collected data of the effect of perioperative interventions targeting psychological distress on postoperative clinical outcome measures pain, function, and QoL. Initially, our intention was to pool data to perform a meta-analysis.
Results
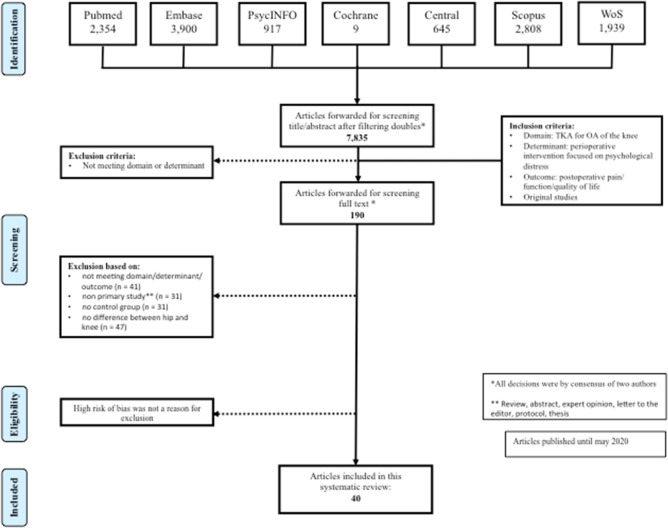
The search strategy and article selection of articles published from 1964 to 26 May 2020 are shown in the flowchart (Fig. 1). Out of 7835 articles remaining after deduplication, we included 40 studies of which 22 RCTs (one randomised controlled pilot study), 10 cohort studies, and 8 quasi-experimental studies with a total number of 3846 patients.
Fig. 1.
Search strategy and article selection
Interventions
A description of the interventions in the experimental and the control groups and the time at which the interventions were applied are presented in Table 1.
Table 1.
Overview of included studies
| Type of intervention | Study | Description of intervention | When was the intervention applied? |
|---|---|---|---|
| Music |
Allred [32] Prospective cohort |
I: Easy-listening music with headphones for 20 min | Before and after their first ambulation at the first postoperative day |
| C: 20-min quiet rest period | |||
| Aris [33] RCT | I: Additional relaxing music therapy during recovery (< 60 beats per minute) | During recovery | |
| C: Usual care | |||
| Chen [34] RCT | I: Five compositions of 30 min soothing piano and Chinese violin music (60–80 beats per minute) | Ward before surgery, in the waiting area of the surgical room and twice during postoperative recovery | |
| C: No music | |||
| Hsu [35] Prospective cohort | I: Slow relaxing music with slow tempo, low tone and soft melody | Once a day at the 10 a.m. continuous passive motion (CPM) session on the first and second postoperative day | |
| C: No music, required to rest in bed | |||
| Hsu [36] Single-group QES | I: Music for 10 min before receiving CPM until the end of the CPM session | During CMP the first and second days after surgery | |
| C: Rest in bed for 10 min before CPM began | |||
| Keshmiri [37] RCT | I1: Isolation of noice by soundproof headphones in conjunction to disposable earplugs | During surgery, after the effect of sedative (Propofol) was applied | |
| I2: Music of patients' choice with headphones | |||
| C: No isolation of noise or music | |||
| Leonard [38] RCT | I: Co-treatment session that used live music to support exercise | Postsurgery, after admission to the inpatient rehabilitation unit | |
| C: Physiotherapy without music | |||
|
Santhna [39] QES |
I: Music for five days post-operatively and analgesics | 5 days postoperatively | |
| C: No music, only pharmacological intervention | |||
| Simcock [40] RCT | I: Music of patients' choice with headphones | During surgery, after a spinal-epidural anaesthesia and sedation with propofol | |
| C: White noise emanating from the headphones | |||
| Education | Atabaki [41] RCT | I: Educational intervention presented as a combination of lecture, group discussion, individual education, questions and answers | Four perioperative stages (one day before surgery, 24 h and 48 h later, upon discharge from the hospital) |
| C: Usual care | |||
| Aytekin [42] Prospective cohort | I: Education (about OA, joint protection, home safety, and TKA) and home-based exercise | During 12 weeks before the operation | |
| C: No additional training program, usual care | |||
| Chen [43] QES | I: Cognitive-behavioural educational intervention (pamphlet, CD and oral instructions) | Before surgery after hospitalisation and 1 days postsurgery | |
| C Routine care and usual instructions delivered orally | |||
| Huang [44] RCT | I: 40-min preoperative home rehabilitation education program by a physiotherapist | 2–4 weeks prior to admission | |
| C: No education program | |||
| Huang [45] RCT | I: Traditional education, telephone education and mobile education | Following surgery | |
| C: Traditional face-to-face and telephone education | |||
| Lee [46] RCPS | I1: Psychoeducation on CPSP and prerecorded hypnotic intervention using audiotapes | One delivered before and another delivered at least 24 h after surgery | |
| I2: Psychoeducation on CPSP and diaphragmatic breathing relaxation exercise | |||
| C: Usual care | |||
| Lin [47] QES | I: One-to-one less than 30 min preadmission preoperative teaching* | Preadmission preoperative | |
| C: Postadmission preoperative teaching and no video | |||
| Louw [48] CCTWAA | I: Education program and an additional 30-min group pain neuroscience education session | Before surgery | |
| C: Only education program | |||
| Malletschek [49] RCT | I: Additional pain psychoeducation over at least 45 min | 3–6 days after TKA | |
| C: Usual care | |||
| Moulton [50] Prospective cohort | I: Joint school by members of a multidisciplinary group explaining the process of the surgery | Preoperative for 2 h | |
| C: No joint school | |||
| Piva [51] RCT | I: Interactive education to promote physical activity and healthy eating | During 3 months postoperative: 2 lectures during the first postoperative week and mini-sessions of physical activity promotion in the subsequent weeks | |
| C: No education | |||
| Reslan [52] QES | I: One to one intervention (30–40 min) including education and exercise training by a nurse | Prior to surgery | |
| C: Standard hospital care | |||
| Timmers [53] RCT | I: Day-to-day postoperative care information related to topics such as pain, physiotherapy exercises, wound care, and daily self-care activities through an application | During the 28-day period after discharge | |
| C: Only weekly, basic information | |||
| Wilson [54] RCT | I: Usual teaching and preoperative educational intervention** | Teaching session and booklet within 4 weeks prior to surgery Phone call during a week before surgery | |
| C: Usual teaching | |||
| Yajnik [55] Retrospective cohort | I: Pain management educational card*** | Prior to peripheral nerve block placement on the day of surgery, at the time of ward admission by the bedside nurse and once daily during rounds | |
| C: Before implementation of pain management educational card | |||
| Psychotherapy | Birch [56] RCT | I: CBT based pain education of approximately 45 min delivered by 2 physiotherapists | 3 sessions preoperatively and 4 sessions postoperatively (2 weeks before surgery until 3 months after surgery) |
| C: Usual care | |||
| Cai [57] RCT | I: CBT | After TKA | |
| C: No CBT | |||
| Cai [58] RCT | I: Individually tailored CBT by a physiotherapist and a psychologist | During 4 weeks after surgery | |
| C: No CBT | |||
| Das Nair [59] RCT | I: 10 sessions of CBT during hour-long sessions by one or two psychologists | During waiting time for surgery | |
| C: No CBT | |||
| Harnirattisai [60] QES | I: 25-min sessions of nurse-patient interaction and discussion**** | At the fourth postoperative day and two weeks after surgery | |
| C: No behavioural change intervention | |||
| Jacobson [61] RCT | I: 19- to 21- minute audio recordings of guided imagery$ scripts designed for TKA patients | Every day for two weeks before surgery and three weeks after surgery | |
| C: Commercially available 17- to 21-min audio recordings | |||
| Riddle [62] QES | I: Intervention delivered by trained psychologists# | During 8 weekly sessions from approximately one month prior to surgery to one month after surgery | |
| C: No intervention | |||
| Riddle [63] RCT | I1: Eight 50-min sessions of 1-on-1 pain coping skills training | Approximately 2 weeks preoperatively to approximately 6 weeks postoperatively | |
| I2: Eight 50-min sessions of 1-on-1 arthritis education by registered nurses | |||
| C: Usual care | |||
| Russo [64] RCT | I: Video according to the Videoinsight Methods^ principles | Three times a week during the first 3 months after surgery | |
| C: No video | |||
| Tristaino [23] Prospective cohort | I: Four psychologist-patient sessions of 30 min focusing on defining the psychological themes and concepts on which to focus the activity | One before surgery, two during postoperative hospital stay and one during rehabilitation | |
| C: Standard of care | |||
| Remaining | Baldwin [66] RCT | I1: Three or four 30-min Reiki treatments provided by three expert Reiki professionals | During the hospital stay |
| I2: Standard of care and three or four sham Reiki session delivered by non-trained people | |||
| C: Standard of care and sessions of “quiet time” | |||
| Christiansen [67] RCT | I: Standard of care rehabilitation plus weight baring biofeedback training | On the morning before surgery (20 min) and after admission to the post anaesthesia care unit (30 min) and 20 min at the first, second and third postoperative day | |
| C:Standard of care rehabilitation alone | |||
| Hiraga [68] NRCT | I: Occupational therapy & self-monitoring using a diary | From 1 to 2 weeks postoperatively | |
| C: Occupational therapy only | |||
| Koo [69] RCT | I: Enhanced reality analgesia | Shortly after physiotherapy for 5 times a week, for 2 weeks | |
| C: No enhanced reality analgesia | Shortly after physiotherapy for 5 times a week, for 1 week | ||
| Notte [70] Prospective cohort | I: Weight bearing (WB) biofeedback-assisted progressive muscle relaxation training sessions using a Nintendo Wii fit Plus game and associated Wii balance board | Twice weekly at home for 6 weeks after surgery | |
| C: Standard of care | |||
| Wang [71] QES | I: CPM therapy and 30-min biofeedback relaxation training | One day before surgery and twice a day on the five first postoperative days, concurrent with CPM therapy | |
| C: Only CPM therapy |
I intervention group, C control group, RCT randomised controlled trial, CPM continuous passive motion, QES quasi-experimental study, OA osteoarthritis, TKA total knee arthroplasty, CD compact disk, RCPS randomized controlled pilot study, CPSP chronic postsurgical pain, CCTWAA controlled clinical trial with alternating allocation, CBT cognitive behavioural therapy, NRCT non-randomised controlled trial
*Preoperative education about care pathway, knee surgery, pain management, expected discharge goals and in-patient and out-patient arthroplasty rehabilitation by an educational nurse and a booklet
**Preadmission preoperative teaching with an instruction booklet during a preoperative outpatient clinic visit. Upon admission to the hospital, they were presented with an educational videotape
***A booklet containing symptom management after TKA, an individual teaching session, and a follow-up support call by the principal investigator
****25-Min sessions of nurse-patient interaction and discussion regarding specific exercises and physical activity, self-monitoring, goal setting, family support and encouragement, and information prompting
$Guided imagery is a widely used mind–body intervention by the generation of self- or practitioner-guided positive sensory and affective mental images to promote health changes in the body, reducing anxiety and stress, and evoking psychological and physiologic relaxation [61]
#Intervention addressed to the recovery of physical function, the concerns during the recovery period and strategies for coping with pain after the operation delivered by trained therapists
^The video was established to produce positive and therapeutic insight, according to the Videoinsight Methods principles [65]
Music
Nine studies examined the effect of perioperative listening to music on postoperative outcome. Eight of these studies [32–34, 36–40] assessed the effect of music on pain and three [35, 36, 40] on function. Music was offered at different time points and different types of music were provided.
Education
The effect of education on postoperative outcome was investigated in fifteen studies in which the time of education varied from 12 weeks before surgery to 3 months after surgery (Table 1).
Psychotherapy
Psychological therapies provided with direct support from a professional were examined by eight studies. The patients in the RCTs of Jacobson et al. [61] and Russo et al. [64], who also received psychological therapy, received their psychological intervention by audio recordings, or watching a video instead of direct contact with a health care professional.
Other/remaining interventions
Four remaining interventions (Reiki, biofeedback relaxing training and enhanced reality analgesia, self-monitoring using a diary), applied to six studies, could not be allocated to the music, educational, or psychological therapy intervention groups and were, therefore, classified as remaining interventions (Table 1).
Outcomes
Outcome measures pain, function, and/or QoL were assessed in 22 RCTs (one randomised controlled pilot study), 10 cohort studies, and 8 quasi-experimental studies. Mean age of the patients ranged from 61.7 to 74.1 years and duration of follow-up ranged between 60 min and 2 years. Due to the heterogeneity of the type of studies, interventions, outcome measures and follow-up there was no possibility to pool data to perform a meta-analysis.
Pain
34 studies examined the influence of a perioperative intervention targeting psychological distress on clinical outcome pain after the TKA. Many different scoring systems were used to score postoperative pain and eight studies assessed pain medication use as an outcome measure for pain (Table 2).
Table 2.
The influence of perioperative interventions targeting psychological distress on pain after the TKA
| Type of intervention | Study | Nr TKA | Females (%) | Age mean ± SD |
Follow-up | Outcome score (pain) | I score ± SD |
C score ± SD |
Statistically significance at latest follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|
| Music | Allred 2010 | T | 56 | 31 (55.4) | 63.9 (64-84)* | 6 hours | VAS | 41.2 ± 25.8 | 45.1 ± 31.2 | P = 0.337 |
| I | 28 | MPQ | 15.9 ± 10.6 | 14.9 ± 12.3 | P = na, no statistical analysis between groups | |||||
| C | 28 | Opioid use (morphine or dilaudid) | na | na | P = 0.388 and P = 0.152 (regarding which oral medication) | |||||
| Aris 2019 | T | 56 | 60 minutes | VAS |
0 (24.39 ** |
1.5 (32.61)** | P = 0.045 | |||
| I | 28 | 19 (67.9) | 63.71±11.005 | |||||||
| C | 28 | 19 (67.9) | 64.50±8.851 | |||||||
| Chen 2015 | T | 30 | 20 (66.7) | 68 (53-85)* | Postoperative days | VAS (recovery) | 3.22 ± 0.22*** | 3.00 ± 0.25*** | P = 0.50 | |
| I | 15 | VAS (ward) | 3.07 ± 0.26*** | 2.87 ± 0.18*** | P = 0.53 | |||||
| C | 15 | Opioid use (parenteral morphine, meperidine, fentanyl in PO recovery) | 7.39 ± 2.66 | 6.86 ± 2.29 | P = 0.57 | |||||
| Opioid use (parenteral morphine, meperidine, fentanyl in the ward) | 12.04 ± 14.43 | 12.90 ± 8.05 | P = 0.89 | |||||||
| Hsu 2019 | T | 49 | 34 (69.4) | 73.9 ± 7.5 | 2 days | NRS | 0.06 ± 0.24 | 2.14 ± 1.10 | P < 0.01 | |
| I | 49 | |||||||||
| C | 49 | |||||||||
| Keshmiri 2014 | T | 83 | 52 (62.7) | 68.7 ± 0.96 | 2-7 days | VAS (day 1-3) | 1.33 ± 0.11 (I1) & 1.44 ± 0.13 (I2) | 1.49 ± 0.13 | P = 0.718 | |
| I1 | 28 | VAS (day 4-7) | 0.9 ± 0.15 (I1) & 0.81 ± 0.13 (I2) | 1.23 ± 0.19 | P = 0.330 | |||||
| I2 | 27 | VAS (day 17) | 1.09 ± 0.12 (I1) & 1.08 ± 0.11 (I2) | 1.34 ± 0.14 | P = 0.435 | |||||
| C | 28 | Days of pain catheter duration (type of pain medication na) | 3.43 ± 0.11 (I1) 7 3.48 ± 0.12 (I2) | 3.36 ± 0.19 | P = 0.452 | |||||
| Leonard 2019 | T | 32 | Postoperative days | NRS | 5.44 ± 3.2 | 5.56 ± 2.52 | "No significant difference" | |||
| I | 16 | 11 (68.8) | 67.9 (45-87)* | Observational coding for pain | 3.06 ± 3.13 | 2.31 ± 2.36 | P = 0.02 | |||
| C | 16 | 12 (75) | 67.6 (53-80)* | |||||||
| Santhna 2015 | T | 40 |
14 (70) 18 (90) |
63.80±5.64 64.90±6.94 |
5 (days) | PRI | 11.78^ | 29.23^ | P = 0.00 | |
| I | 20 | |||||||||
| C | 20 | VAS | 14.20^ | 26.80^ | P = 0.00 | |||||
| PPI | 15.00^ | 26.00^ | P = 0.001 | |||||||
| Paracetamol | 16000mg^^ |
17000 mg^^ |
P > 0.05 | |||||||
| Celecoxib | 600 mg^^ |
1600 mg^^ |
P > 0.05 | |||||||
| Tramadol |
125 mg^^ |
225 mg^^ |
P > 0.05 | |||||||
| Simcock 2008 | T | 30 | 18 (60) | 67.3±9.1 | 24 hours | VAS (3 hours PO) | 3.87 ± 3.44 | 1.47 ± 1.39 | P = 0.01 | |
| I | 15 | VAS (6 hours PO) | 5.26 ± 3.04 | 3.38 ± 2.48 | P = 0.075 | |||||
| C | 15 | VAS (24 hours PO) | 4.03 ± 2.89 | 2.41 ± 1.67 | P = 0.04 | |||||
| Education | Atabaki 2019 | T | 56 | 6 (weeks) | WOMAC | 40.47 ± 10.47 | 57.29 ± 7.51 | P = 0.001 | ||
| I | 48 | 46 (95.8) | 65.39 ± 5.08 | |||||||
| C | 48 | 41 (85.4) | 63.83 ± 5.14 | |||||||
| Aytekin 2019 | T | 44 | 6 months | VASpr | 0.4 ± 0.9 | 0.8 ± 1.1 | "no significant difference between groups" | |||
| I | 23 | 18 (78.3) | 67.8 ± 6.3 | VASpa | 1.5 ± 1.5 | 2.3 ± 2.3 | "no significant difference between groups" | |||
| C | 21 | 18(85.7) | 69.7 ± 6.4 | KOOSpain | 87.9 ± 15.4 | 92.7 ± 8.3 | "no significant difference between groups" | |||
| Chen 2014 | T | 92 | 63 (68.5) | 69.26 ± 9.025 | 5 days | NRS (worst pain) | 4.89 ± 2.82 | 5.57 ± 2.84 | P = 0.308 | |
| I | 42 | NRS (average pain) | 2.38 ± 1.97 | 2.43 ± 2.03 | P = 0.916 | |||||
| C | 50 | NRS (current pain) | 2.46 ± 2.31 | 2.57 ± 2.26 | P = 0.836 | |||||
| Huang 2011 | T | 242 | 174 (71.6) | 70.2 ± 7.3 | 5 days | VAS | 2.4 ± 0.7 | 2.5 ± 0.6 | P = 0.686 | |
| I | 125 | |||||||||
| C | 117 | |||||||||
| Louw 2019 | T | 103 | 6 (months) | NRS | na | na | P = 0.386 | |||
| I | 49 | 32 (65.3%) | 74.1 ± 9.5 | Morfine | 2601.62 ± 1103.90 | 2734.02 ± 1324.60 | P = 0.635 | |||
| C | 54 | 23 (51.9) | 69.6 ± 10.6 | |||||||
| Mallet-schek 2019 | T | 75 | 47 (62.7) | 59-78* | 3 months | KOOSpain | na | na | P = 0.01 | |
| I | 37 | |||||||||
| C | 38 | |||||||||
|
Lee 2019 |
T | 24 | 6 months | NRS | 1.40 ± 0.89 (I1) & 1.73 ± 1.40 (I2) | 2.23 ± 1.41 |
HYP vs. control: P = 0.134 and P = 0.038 (when controlled for covariates) MET vs. Control: PP = 0.975 |
|||
| I1 | 8 | 7 (87.5) | 65.63 ± 9.27 | |||||||
| I2 | 8 | 7 (87.5) | 56.25 ± 11.22 | |||||||
| C | 8 | 8 (100) | 67.88 ± 10.38 | |||||||
| Moulton 2017 | T | 563 | na | 70.1 ± na | 2 years | OKS (6 months PO) | 28.71 ± na | 31.60 ± na | P = 0.251 | |
| I | 503 | OKS (2 years PO) | 30.17 ± na | 33.26 ± na | P= 0.440 | |||||
| C | 60 | |||||||||
| Piva 2017 | T | 44 | 31 (70.5) | 6 months | WOMAC pain | min 1.7 (95% CI -3.0,-4.0) ^^^ | min 0.3 (95% CI - 1.5, 1.0) ^^^ | P = 0.035 | ||
| I | 22 | 68.1 ± 7.5 | ||||||||
| C | 22 | 68.3 ± 5.5 | ||||||||
| Reslan 2018 | T | 60 | na | 4 weeks | HSSpain | 22.83 ± 4.78 | 19.18 ± 5.14 | PP = 0.001 | ||
| I | 30 | 19 (63.6) | ||||||||
| C | 30 | 17 (56.7) | ||||||||
| Timmers 2019 | T | 213 | 4 weeks after discharge | NRS at rest | 3.45^ | 4.59^ | PP = 0.001 | |||
| I | 114 | 74 (64.9) | 64.74 ± 7.57 | NRS activity | 3.99^ | 5.08^ | P < 0.001 | |||
| C | 99 | 60 (60.6) | 65.63 ± 7.90 | NRS at night | 4.18^ | 5.21^ | P = 0.003 | |||
| Wilson 2016 | T | 143 | 89 (62.6) | 3 days | BPI-I | 24.4 ± 14.4 | 22.4 ± 15.1 | PP = 0.45 | ||
| I | 73 | 67 ± 8 | NRS (rest) | 2.8 ± 2.5 | 2.8 ± 2.7 | P = 0.70 | ||||
| C | 70 | 66 ± 8 | NRS (moving) | 5.4 ± 3.0 | 6.1 ± 2.5 | P = 0.20 | ||||
| NRS worst pain last 24 hours) | 7.0 ± 2.4 | 7.0 ± 2.3 | P = 0.87 | |||||||
| Opioid use (morphine, hydroporphone, oxycodon, codeine) | 40 (45)*^ |
40 (42)*^ |
"no difference between groups in daily 24-hours opioid administration" | |||||||
| Yajnik 2018 | T | 40 | 3 (7.5) | 68 (46-80)* | 2 days | Opioid use (morphine, MME PO day 1 and 2) | 38 (1-117)* | 72 (32-285)* | P = 0.001 | |
| I | 20 | Minimum pain (patients’ verbal rating 0–10) 1 day PO | 0 (0 - 3)* | 0 (0 - 6)* | P = 0.151 | |||||
| C | 20 | Maximum pain (patients’ verbal rating 0–10) 1 day PO | 4 (2 - 9)* | 8 (1 - 10)* | P = 0.114 | |||||
| Psycho-therapy | Birch 2019 | T | 60 | 1 (year) | VAS activity | 12 (5-18)^^^ | 9 (3-15) ^^^ | P = NS | ||
| I | 31 | 22 (33) | 66 ± 9 | VAS rest | 7 (1–12)^^^ | 6 (1–12) ^^^ | P = NS | |||
| C | 29 | 18 (27) | 66 ± 10 | |||||||
| Cai 2017 | T | 108 | 6 months | KSS | 82.61 ± 6.38 | 73.30 ± 8.45 | P < 0.01 | |||
| I | 54 | 31 (57.4) | 62.42 ± 6.59 | |||||||
| C | 54 | 34 (63.0) | 63.94 ± 6.58 | |||||||
| Cai 2018 | T | 100 | 62 (55.9) | 6 months | NRS | 5.63 ± 0.73 | 6.27 ± 0.86 | time effects: P < .001); group effects: P = 0.003); group-by-time interaction: P = 0.080 | ||
| I | 50 | 65.26 ± 8.30 | ||||||||
| C | 50 | 66.18 ± 7.04 | ||||||||
| Das Nair 2018 | T | 50 | 23 (46) | WOMAC pain | 6.5 ± 3.6 | 7.5 ± 2.3 | P = 0.40 | |||
| I | 25 | 65.7 ± 8.6 | ICOAP constant pain (item 1-5) | 6.4 ± 4.4 | 6.2 ± 3.2 | P = 0.99 | ||||
| C | 25 | 66.7 ± 9.9 | ICOAP constant pain (item 1, 3, 4, 5) | 4.8 ± 3.7 | 5.1 ± 3.0 | P = 0.82 | ||||
| ICOAP constant pain (converted rasch score item 1, 3, 4, 5) | 5.5 ± 4.1 | 6.0 ± 3.2 | P = 0.75 | |||||||
| ICOAP intermittent pain (item 6-11) | 8.5 ± 5.6 | 10.2 ± 4.5 | P = 0.43 | |||||||
| ICOAP intermittent pain (item 6, 7, 10, 11) | 5.7 ± 3.8 | 7.1 ± 3.3 | P = 0.33 | |||||||
| ICOAP intermittent pain (converted rasch score item 6, 7, 10 11) | 5.5 ± 3.4 | 6.7 ± 3.0 | P = 0.34 | |||||||
| Jacobson 2016 | T | 58 | 51 (62.2) | 65 (41-81)* | 6 months | WOMAC pain | 2.7 ± 3.1 | 3.5 ± 3.3 | P < 0.001 | |
| I | 29 | VAS daily pain | na | na | P not available at 6 months postoperatively | |||||
| C | 29 | |||||||||
| Riddle 2011 | T | 63 | 45 (71.4) | 2 months | WOMAC pain | 6.0 ± 4.1 | 8.6 ± 3.7 | P = 0.017 | ||
| I | 18 | 63.8 ± 11.5 | ||||||||
| C | 45 | 60.8 ± 9.9 | ||||||||
| Riddle 2019 | T | 402 | 12 months | WOMAC pain | 3.3 (95% CI 2.5, 4.2) (I1) & 3.0 (95% CI 2.1, 3.8) (I2)^^^ | 2.9 (95% CI 2.0, 3.8)^^^ | P= 0.60 | |||
| I1 | 130 | 94 (72.3) | 62.6 ± 7.9 | NRS | 1.8 (95% CI 1.2, 2.4) (I1) & 2.0 (95% CI 1.3,2.6) (I2) ^^^ | 1.7 (95% CI 1.1, 2.2) ^^^ | P = na | |||
| I2 | 135 | 85 (63.0) | 64.2 ± 8.5 | |||||||
| C | 137 | 88 (64.2) | 62.7 ± 7.7 | |||||||
| Tristaino 2015 | T | 64 | 44 (62.0) | 4 months | SF-36 bodily pain | 70.1 ± 21.5 | 67.8 ± 26.8 | P = 0.715 | ||
| I | 33 | 64.2 ± 8.6 | ||||||||
| C | 31 | 66.1 ± 6.6 | ||||||||
| Remaining | Baldwin 2017 | T | 56 | na | na | 72 hours | VAS | na | na | "Reiki significant pain reduction (P = 0.003), Sham Reiki and SOC no significant reduction" |
| I1 | 25 | Opioid use (oxycontin, oxycodone, morphine) | na | na | P = na, not mentioned in significant results) | |||||
| I2 | 12 | |||||||||
| C | 19 | |||||||||
| Hiraga 2019 | T | 41 | 4 weeks | NRS rest | 1.3 ± 0.4 | 1.2 ± 0.4 | P = 0.965 | |||
| I | 20 | 16 (80) | 76.4 ± 7.1 | NRS walk | 1.3 ± 0.2 | 3.2 ± 0.6 | P= 0.017 | |||
| C | 21 | 19 (90.4) | 76.6 ± 5.5 | |||||||
| Koo 2018 | T | 120 | 5 weeks | VAS | na (figure) | na (figure) | "No signicance was found in VAS analyses between the groups" | |||
| I | 60 | 17 (28.3) | 65.00 ± 6.97 | |||||||
| C | 60 | 15 (25) | 63.71 ± 5.09 | |||||||
| Notte 2016 | T | 43 | na | na | 3 days postoperatively | NRS | na | na | P = 0.000 (1, 2, 3 days PO) | |
| I | 23 | Opioid use (type of opioid na) | na | na | P = 0.92 | |||||
| C | 20 | |||||||||
| Wang 2015 | T | 66 | 23 (34.9) | 73.5 ± 9.5 | 5 days | NRS | 3.36 ± 1.47 | 4.23 ± 1.67 | P < 0.001 | |
| I | 33 | Opioid use (pethidine PO day 5) | 1 (3.2) | 0 (0.0) | P = 0.49 | |||||
| C | 33 | PMU (Acetaminophen or COX-2 inhibitor + pethidine or tramadol PO day 5) | 24 (77.4) | 21 (63.6) | P = 0.27 | |||||
Nr number; TKA total knee arthroplasty; SD standard deviation; I intervention group; C control group; T total study group; VAS visual analog scale; P P value; MPQ short form McGill pain questionnaire; na: not available; PO postoperative; NRS numeric rating score; PRI Pain Rating intensity; PPI Present Pain Intensity; mg milligram; WOMAC Western Ontario and McMaster universities osteoarthritis index; VASpr visual analog scale pain resting; VASpa visual analog scale pain acitivity; KOOSpain pain subscale of the knee injury and osteoarthritis outcome score; HYP hypnotic intervention; MET minimal-effect treatment; OKS Oxford knee score; 95% CI 95% confidence interval; HSS hospital for special surgery; BPI-I Brief Pain Inventory interference; MME Morphine Milligram Equivalents; NS not significant; KSS knee society score; ICOAP Intermittent and Constant Osteoarthritis Pain scale; SF-36 Short Form-36; SOC stand of care; PMU pain medication use; COX-2 cyclooxygenase-2
Instead of mean and SD: *median (range), **median and mean rank, ***mean and standard error, ^mean rank only, ^^median only, ^^^mean estimate with the 95% CI in parentheses, *^median (interquartile range) instead of mean and SD
As shown in Table 2, patients in the intervention groups had significant better postoperative pain scores or declined prescriptions of opioids in 20 studies. Therapies applied in these studies were music during surgery [40] or after surgery [33, 36, 38, 39], education [41, 49, 51–53, 55], cognitive behavioural therapy [57, 58], guided imagery [61], pain coping skills training [62], Reiki therapy [66, 70], occupational therapy in combination with self-monitoring using a diary [68], weight-bearing biofeedback training [67] and biofeedback-assisted progressive muscle relaxing training [71]. The remaining 14 studies did not show a significant effect on any of the pain-related outcome measures or pain medication use at the latest follow-up when using a perioperative intervention focused on psychological distress in conjunction to TKA.
Function
A total of 29 studies examined the effect of an intervention targeting psychological distress on function after the TKA (Table 3).
Table 3.
The influence of perioperative interventions targeting psychological distress on function after the TKA
| Type of intervention | Study | Nr TKA | Females (%) | Age mean ± SD | Follow-up | Outcome score (function) | I score ± SD | C score ± SD | Statistically significance at latest follow-up | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Music | Hsu [35] | T | 91 | 67 (73.6) | 2 days | CPM angles 1 day PO | 24.29 ± 5.00 | 12.98 ± 4.43 | P < 0.01 | ||
| I | 49 | 73.9 ± 7.5 | CPM angles 2 days PO | 21.22 ± 2.98 | 16.07 ± 4.49 | P < 0.01 | |||||
| C | 42 | 71.33 ± 8.45 | Active knee flexion ROM 2 days PO | 106.22 ± 6.17 | 95.00 ± 6.80 | P < 0.01 | |||||
| Hsu [36] | T | 49 | 2 days | Increased degree of knee flexion during CPM | 21.22 ± 2.98 | 10.02 ± 3.03 | P < 0.01 | ||||
| I | 49 | 34 (69.4) | 73.9 ± 7.5 | ||||||||
| C | 49 | 34 (69.4) | 73.9 ± 7.5 | ||||||||
| Leonard [38] | T | 32 | Postoperative days | Observational coding for pedalling adherence | 7.81 ± 0.40 | 7.44 ± 1.21 | "No significant difference" | ||||
| I | 16 | 11 (68.8) | 67.9 (45–87)* | ||||||||
| C | 16 | 12 (75) | 67.6 (53–80)* | ||||||||
| Education | Atabaki [41] | T | 96 | 6 weeks | WOMAC stiffness | 19.53 ± 12.34 | 41.66 ± 10.09 | P = 0.001 | |||
| I | 48 | 46 (95.8) | 65.39 ± 5.08 | WOMAC performance difficulty | 43.48 ± 7.96 | 55.82 ± 4.30 | P = 0.001 | ||||
| C | 48 | 41 (85.4) | 63.83 ± 5.14 | ||||||||
| Aytekin [42] | T | 44 | 6 months | KOOS total | 82.2 ± 16.1 | 85.5 ± 9.5 | "No significant difference between groups" | ||||
| I | 23 | 18 (78.3) | 67.8 ± 6.3 | KOOSdaily living activities | 87.2 ± 18.3 | 91.1 ± 9.2 | "No significant difference between groups" | ||||
| C | 21 | 18(85.7) | 69.7 ± 6.4 | KOOSsports | 52.8 ± 24.4 | 56.1 ± 13.1 | "No significant difference between groups" | ||||
| Chen [43] | T | 92 | 63 (68.5) | 69.26 ± 9.025 | 5 days | Overall rating of nine physical function items | 12.38 ± 2.806 | 12.05 ± 3.682 | P = 0.625 | ||
| I | 42 | Ankle pumping | 1.55 ± 0.39 | 1.54 ± 0.44 | P = 0.927 | ||||||
| C | 50 | Quadriceps setting | 0.17 ± 0.39 | 0.23 ± 0.43 | P = 0.518 | ||||||
| Knee flexion/extension | 0.44 ± 0.53 | 0.69 ± 0.66 | P = 0.062 | ||||||||
| Straight-leg raises | 1.22 ± 2.58 | 0.64 ± 0.56 | P = 0.000 | ||||||||
| MPOAL | 3.71 ± 0.622 | 3.08 ± 1.090 | P = 0.004 | ||||||||
| Huang 2011 | T | 242 | 174 (71.6) | 70.2 ± 7.3 | 5 days | Ability to walk during discharge | 85.7 ± na | 81.2 ± na | P = 0.343 | ||
| I | 125 | ROM | 76 ± 22 | 74 ± 20 | P = 0.582 | ||||||
| C | 117 | ||||||||||
| Huang [45] | T | 150 | 102 (68.0) | 3 months |
ROM ITT ROM PP |
110.6 ± 6.68 110.0 ± 6.33 |
105.00 ± 8.82 103.26 ± 7.57 |
P < 0.001 P < 0.001 |
|||
| I | 75 | 62.42 ± 6.59 | |||||||||
| C | 75 | 63.94 ± 6.58 | |||||||||
| Lin [47] | T | 60 | 31 (51.7) | 68.6 ± na | EPC | 14.93 ± na | 8.87 ± na | P < 0.05 | |||
| I | 30 | Knee flexion | 77.84 ± na | 70.16 ± na | P = 0.013 | ||||||
| C | 30 | Ambulation ability | na | na | "The differences between groups were not significant" | ||||||
| Louw [48] | T | 101 | 6 months | WOMAC | na | na | P = 0.222 | ||||
| I | 49 | 32 (65.3) | 74.1 ± 9.5 | ||||||||
| C | 54 | 23 (51.9) | 69.6 ± 10.6 | ||||||||
| Malletschek 2019 | T | 75 | 47 (62.7) | 59 – 78** | 3 months | KSS | na | na | P = 0.08 | ||
| I | 37 | ||||||||||
| C | 38 | ||||||||||
| Moulton [50] | T | 563 | na | 70.1 ± na | 2 years | OKS (6 months PO) | 28.71 ± na | 31.60 ± na | P = 0.251 (6 months) | ||
| I | 503 | ||||||||||
| C | 60 | ||||||||||
| OKS (2 years PO) | 30.17 ± na | 33.26 ± na | P = 0.440 (2 years) | ||||||||
| Piva [51] | T | 44 | 31 (70.5) | 6 months | SF-36 PF | 76.7 ± 16.1 | 70.3 ± 24.2 | P = 0.017 | |||
| I | 22 | 68.1 ± 7.5 | Single-leg stance test | 16.1 ± 9.6 | 17.4 ± 9.8 | P = 0.037 | |||||
| C | 22 | 68.3 ± 5.5 | WOMAC PF | 11.8 ± 6.7 | 12.8 ± 10.8 | P = 0.558 | |||||
| Stair-climb | 14.3 ± 4.1 | 15.6 ± 7.4 | P = 0.054 | ||||||||
| Chair-stand | 12.2 ± 2.8 | 13.7 ± 7.5 | P = 0.149 | ||||||||
| 6-Min walk | 472.6 ± 86.5 | 518.0 ± 103.3 | P = 0.638 | ||||||||
| Gait speed | 1.14 ± 0.16 | 1.18 ± 0.24 | P = 0.790 | ||||||||
| Daily activity | 152.5 ± 93.3 | 174.9 ± 126.1 | P = 0.279 | ||||||||
| Reslan [52] | T | 60 | 4 weeks | HSSfunction | 15.73 ± 3.49 | 13.92 ± 3.35 | P = 0.026 | ||||
| I | 30 | 19 (63.6) | na | HSSrom | 17.04 ± 2.55 | 16.53 ± 4.20 | P = NS | ||||
| C | 30 | 17 (56.7) | na | HSSquadriceps muscle strength | 9.13 ± 3.81 | 8.47 ± 2.93 | P = NS | ||||
| HSSflexion deformity | 10.02 ± 1.21 | 8.47 ± 1.93 | P = 0.007 | ||||||||
| HSSinstability | 9.89 ± 3.41 | 8.27 ± 2.89 | P = 0.049 | ||||||||
| LEFS | 60.35 ± 11.22 | 53.83 ± 12.98 | P = 0.048 | ||||||||
| Timmers [53] | T | 213 | 4 weeks after discharge | KOOS | 37.61 ± 10.17 | 43.08 ± 12.96 | P < 0.001 | ||||
| I | 114 | 74 (64.9) | 64.74 (7.57) | Ability to perform physiotherapy | 7.50*** | 6.88*** | P = 0.03 | ||||
| C | 99 | 60 (60.6) | 65.63 (7.90) | Ability to perform self-care activities | 8.32*** | 7.64*** | P = 0.004 | ||||
| Yajnik 2018 | T | 40 | 3 (7.5) | 68 (46–80)* | 2 days | Maximum ambulation 1 day PO | 20 (0–59)^ | 12 (0–30) ^ | P = 0.069 (POD 1) | ||
| I | 20 | Maximum ambulation 2 days PO | 46 (6–67)^ | 38 (0–61) ^ | P = 0.141 (POD 2) | ||||||
| C | 20 | ||||||||||
| Psycho-herapy | Birch 2019 | T | 60 | 1 year | OKS | 33 (29, 27)^^ | 37 (33, 41)^^ | P = NS | |||
| I | 31 | 22 (33) | 66 (9) | 6-Min walk | 441 (402,480)^^ | 406 (367, 446)^^ | P = NS | ||||
| C | 29 | 18 (27) | 66 (10) | Sit to stand | 12 (11, 14) ^^ | 11 (95% CI 10,13) ^^ | P = NS | ||||
| Cai [57] | T | 108 | 6 months | KSS | 82.61 ± 6.38 | 73.30 ± 8.45 | P < 0.01 | ||||
| I | 54 | 31 (57.4) | 62.42 ± 6.59 | First time out of bed (hours) | 22.13 ± 4.18 | 36.41 ± 7.31 | P = < 0.001 | ||||
| C | 54 | 34 (63.0) | 63.94 ± 6.58 | ||||||||
| Cai [58] | T | 100 | 62 (55.9) | 6 months | HSS function | 80.68 ± 8.02 | 68.98 ± 8.64 | P < 0.001 (time interaction), P < 0.001 (group interaction), P = 0.003 (group-by-time interaction) | |||
| I | 50 | 65.26 ± 8.30 | |||||||||
| C | 50 | 66.18 ± 7.04 | |||||||||
| Das Nair [59] | T | 50 | 23 (46.0) | 6 months | WOMAC function | 20.9 ± 12.7 | 32.0 ± 4.8 | P = 0.009 | |||
| I | 25 | 65.7 ± 8.6 | WOMAC stiffness | 3.2 ± 1.9 | 4.2 ± 0.9 | P = 0.11 | |||||
| C | 25 | 66.7 ± 9.9 | |||||||||
| Harnirattisai [60] | T | 63 | 59 (93.7) | 67.88 (60–85)* | 6 weeks | PTT total | 8.86 ± 1.89 | 6.43 ± 1.66 | P = na | ||
| I | 42 | PPT standing balance | Δ 2.00 ± 1.22^^^ | Δ 1.09 ± 1.22^^^ | P = 0.016 | ||||||
| C | 21 | PPT walking speed | Δ 1.55 ± 1.02^^^ | Δ 0.76 ± 0.83^^^ | P = 0.004 | ||||||
| PPT chair-stand | Δ 2.36 ± 1.05^^^ | Δ 1.33 ± 1.02^^^ | P < 0.001 | ||||||||
| ADL and daily requirements exercise activity | na | na | "There were no significant differences in ADL participation" | ||||||||
| Jacobson [61] | T | 58 | 51 (62.2) | 65 (41–81)* | 6 months | SF-36 physical | 50.4 ± 6.0 | 47.3 ± 7.5 | P = na | ||
| I | 29 | WOMAC stiffness | 1.9 ± 1.4 | 2.1 ± 1.9 | P = na | ||||||
| C | 29 | WOMAC function | 7.2 ± 7.1 | 10.2 ± 10.5 | P = na | ||||||
| Gait velocity | na | na | P = 0.0154 (group-by-imaging ability interaction) | ||||||||
| Timed walk in seconds | 7.4 ± 2.2 | 8,5 ± 2.3 | P = na | ||||||||
| Riddle [62] | T | 63 | 45 (71.4) | 2 months | WOMAC disability | 18.3 ± 12.2 | 24.1 ± 10.9 | P = 0.023 (for differences among discharge scores for the 2 groups after adjusting for baseline differences) | |||
| I | 18 | 63.8 ± 11.5 | |||||||||
| C | 45 | 60.8 ± 9.9 | |||||||||
| Riddle [63] | T | 402 | 12 months | WOMACfunction | 11.7 (8.6, 14.9) (I1) & 12.2 (9.0, 15.4) (I2)^^ | 10.5 (7.4, 13.6)^^ | P > 0.05 | ||||
| I1 | 130 | 94 (72.3) | 62.6 ± 7.9 | SPPB | 8.0 (7.2, 8.7) (I1) & 8.4 (7.6, 9.1) (I2)^^ | 8.6 (95% CI 7.8, 9.4)^^ | P > 0.05 | ||||
| I2 | 135 | 85 (63.0) | 64.2 ± 8.5 | ||||||||
| C | 137 | 88 (64.2) | 62.7 ± 7.7 | ||||||||
| Russo 2016 | T | 110 | na | 69.1 ± na | 3 months | SF-36 physical | 45.6 ± 8.3 | 46.2 ± 9.9 | P > 0.01 | ||
| I | 55 | KSS | 87.8 ± 9.6 | 78.3 ± 8.2 | P = < 0.005 | ||||||
| C | 55 | WOMAC | 79.9 ± 13.0 | 69.7 ± 9.5 | P = < 0.005 | ||||||
| VAS functional score | 2.8 ± 1.6 | 4.0 ± 1.5 | P = < 0.005 | ||||||||
| Tristaino 2015 | T | 44 | 44 (62.0) | 4 months | SF-36 PCS | 49.5 ± 6.6 | 50.9 ± 9.8 | P = 0.5114 | |||
| I | 33 | 64.2 ± 8.6 | Days until physiotherapy objective reached | 8.1 ± 2.4 | 8.8 ± 2.3 | P = 0.2424 | |||||
| C | 31 | 66.1 ± 6.6 | |||||||||
| Remaining | Chistriansen 2015 | T | 26 | 13 (50) | 26 weeks | FTSST | 9.5 ± 2.4 | 9.6 ± 1.6 | P = 0.21 | ||
| I | 13 | 68.2 ± 8.6 | Hip moment (Nm/kg) during FTSST | 0.65 ± 0.24 | 0.63 ± 0.20 | P = 0.686 | |||||
| C | 13 | 66.6 ± 8.1 | Knee moment (Nm/kg) during FTSST | 1.03 ± 0.22 | 0.97 ± 0.11 | P = 0.434 | |||||
| Ankle moment (Nm/kg) during FTSST | 0.17 ± 0.16 | 0.24 ± 0.14 | P = 0.227 | ||||||||
| Walking speed (m/s) | 1.29 ± 0.25 | 1.24 ± 0.13 | P = 0.68 | ||||||||
| Hip moment during walking | 0.28 ± 0.19 | 0.36 ± 0.22 | P = 0.160 | ||||||||
| Knee extension moment during walking | 0.61 ± 0.25 | 0.42 ± 0.44 | P = 0.008 | ||||||||
| Ankle moment during walking | 0.09 ± 0.29 | 0.01 ± 0.19 | P = 0.877 | ||||||||
| Hiraga [68] | T | 41 | 4 weeks | Daily step count | 3580.5 ± 1545.2 | 2088.4 ± 2008.3 | P = 0.041 | ||||
| I | 20 | 16 (80) | 76.4 ± 7.1 | Psychical activity time | 1741.4 ± 551.3 | 731.8 ± 321.1 | P = 0.000 | ||||
| C | 21 | 19 (90.4) | 76.6 ± 5.5 | ||||||||
| Koo [69] | T | 120 | 5 weeks | WOMAC | 14.59 ± 9.14 | 10.86 ± 10.84 | P = 0.398 | ||||
| I | 60 | 17 (28.3) | 65.00 ± 6.97 | Graded ambulation distance | na | na | P = na | ||||
| C | 60 | 15 (25) | 63.71 ± 5.09 | 6-Min walk test | 407.00 ± 83.62 | 353.35 ± 82.35 | P = 0.163 | ||||
| Timed-stand test | 19.29 ± 2.80 | 19.00 ± 6.16 | P = 0.967 | ||||||||
Nr Number, TKA total knee arthroplasty, SD standard deviation, I intervention group, C control group, T total study group, CPM continuous passive motion, PO postoperative, P P value, ROM range of motion, WOMAC Western Ontario and McMaster Universities osteoarthritis index, KOOS Knee Injury and Osteoarthritis Outcome Score, MPOAL muscle power of the affected leg, ITT intention to treat, PP per protocol, na not available, EPC exercises performance checklist, KSS Knee Society Score, OKS Oxford knee score, SF-36 PF Short Form-36 physical functioning, HSS hospital for special surgery knee score, NS not significant, LEFS lower extremity functional scale, POD postoperative day, PPT physical performance test, ADL activities of daily living, SPPB short physical performance battery, VAS visual analog scale, PCS physical component scale, FTSST five-time sit-to-stand test, Nm/kg Newtonmeter/kilogram, m/s metre per second
Instead of mean and SD
*Mean (range)
**Range only
***Mean only
^Median (10th–90th percentiles)
^^Mean estimate with the 95% CI parentheses
^^^Mean change score baseline—6 weeks postoperative
As shown in Table 3, function was significantly improved by perioperative interventions in 18 studies. Pain coping skills training [62], audiorecording guided imagery scripts [61], video promoting self-confidence and psychological support [64], music [35, 36], occupational therapy in combination with self-monitoring using a diary [68], various types of education [41, 43, 45, 47, 51–53], weight-bearing biofeedback training [67], and psychological therapies (behavioural change intervention [60] and cognitive behavioural therapy [57–59]) positively affected any, but not all, of the functional outcome measures after TKA. In the most recent study by Riddle et al. [63], patients receiving pain coping skills training did not have significantly better scores on WOMAC function and the short physical performance battery. Other types of education [42, 44, 48–50, 55], music during physiotherapy [38], enhanced reality analgesia [69], cognitive behavioural therapy delivered by physiotherapists [56], and psychological support from a professional psychologist [23] did also not affect any of the functional outcome measures after TKA.
QoL
Two recent studies [49, 53] examined the effect a perioperative intervention on QoL (Table 4). Patients receiving postoperative day-to-day education through an app seemed to report significantly better QoL compared to patients who received usual care [53]. Additional psychoeducation did not significantly improve QoL [49].
Table 4.
The influence of perioperative interventions targeting psychological distress on QoL after the TKA
| Type of intervention | Study | Nr TKA | Females (%) | Age mean ± SD | Follow-up | Outcome score (QoL) | I score ± SD I score ± SD | C score ± SD | Statistically significance at latest follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|
| Education | Malletscheck 2019 | T | 75 | 47 (62.7) | 59–78* | 3 months | KOOS QoL | na | na | P = NS |
| I | 37 | |||||||||
| C | 38 | |||||||||
| Timmers 2019 | T | 213 | 4 weeks after discharge | EQ-5D | 0.76 ± 0.16 | 0.67 ± 0.25 | P < 0.001 | |||
| I | 114 | 74 (64.9) | 64.74 ± 7.57 | |||||||
| C | 99 | 60 (60.6) | 65.63 ± 7.90 | |||||||
Nr Number, TKA total knee arthroplasty, SD standard deviation, QoL quality of life, I intervention group, C control group, T total study group, KOOS Knee Injury and Osteoarthritis Outcome Score, na not available, P P value, NS not significant, EQ-5D EuroQOL Five-Dimensional Questionnaire
Instead of mean and SD
*Range
Quality assessment
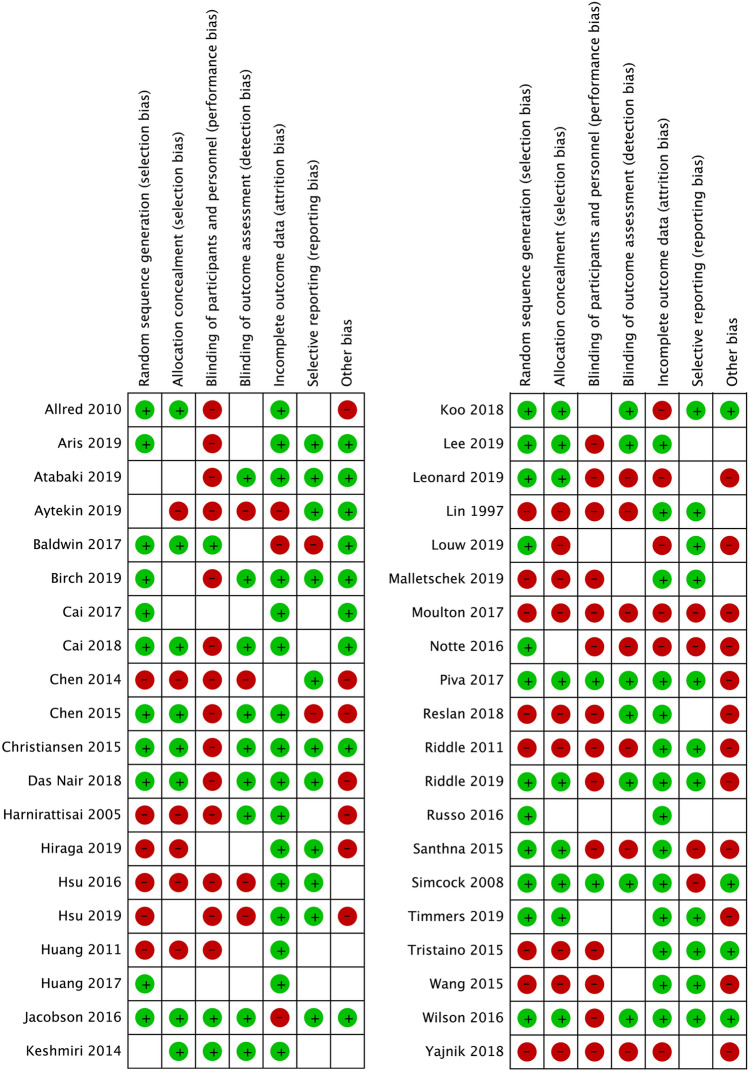
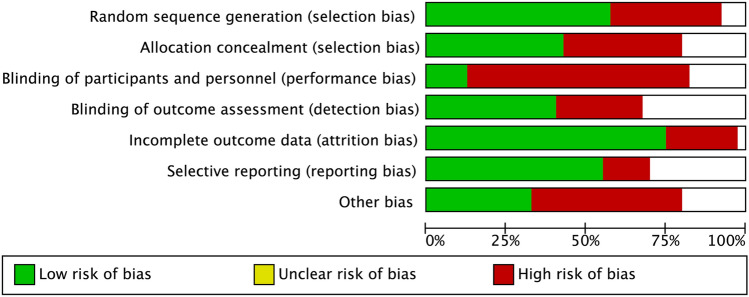
Figure 2 shows our risk of bias assessment of the included studies. Figure 3 represents our judgement about each risk of bias item presented as percentages across all studies. The most prevalent shortcomings regarding the risk of bias were inadequate blinding participants and/or personnel during the study (performance bias) and “other types of bias”. Bias due to inadequate generation of a randomisation sequence or inadequate allocation concealment prior to assignment (selection bias) also caused high scores on the risk of bias (Fig. 3).
Fig. 2.
Risk of bias summary. Authors' judgements about each risk of bias item for each included study. Green: low risk of bias. Red: high risk of bias. No fill: unclear risk of bias
Fig. 3.
Risk of bias graph. Authors' judgements about each risk of bias item presented as percentages across all included studies. Green: low risk of bias. Red: high risk of bias. No fill: unclear risk of bias
The overall level of evidence of the studies using the GRADE approach was qualified as low for pain and for function and as moderate for QoL. Serious uncertainty in the assessment of the risk of bias, inconsistency, and indirectness were the main reasons for downgrading the overall level of evidence (Table 5).
Table 5.
The overall level of evidence using the GRADE approach
| Certainty assessment | No of patients | Certainty | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | ITPD | No ITPD | |||||||
| Pain (follow up: range 60 min to 6 months; assessed with: Various outcome measures) | |||||||||||||||
| 34 | 19 randomised trials and 15 remaining* | Serious | Serious | Serious | Not serious | all plausible residual confounding would suggest spurious effect, while no effect was observed | 1618 | 996 | ⨁⨁◯◯ low | ||||||
| Function (follow up: range 2 days to 2 years; assessed with: Various outcome measures) | |||||||||||||||
| 29 | 16 randomised trials and 13 remaining** | Serious | Serious | Serious | Not serious | all plausible residual confounding would suggest spurious effect, while no effect was observed | 1580 | 1003 | ⨁⨁◯◯ low | ||||||
| QoL (follow up: range 24 weeks to 3 months; assessed with: Various outcome measures) | |||||||||||||||
| 2 | 1 randomised trial and one non-randomised trial | Serious | Serious | Not serious | Not serious | all plausible residual confounding would suggest spurious effect, while no effect was observed | 151 | 137 | ⨁⨁⨁◯ moderate | ||||||
GRADE grading of recommendation, assessment, development, and evaluation, № number, ITPD intervention targeting psychological distress, QoL quality of life
*8 prospective cohort studies, 6 quasi-experimental studies, 1 retrospective cohort study
**6 prospective cohort studies, 6 quasi-experimental studies, 1 retrospective cohort study
Discussion
In this systematic review, we give an overview of studies that assessed the effect of perioperative interventions targeting psychological distress on pain, function, and QoL applied to patients undergoing TKA for primary OA of the knee. Perioperative music [33, 36, 38–40], education [41, 49, 51–53, 55], cognitive behavioural therapy [57, 58], pain coping skills training [62], guided imagery [61], perioperative Reiki therapy [66, 70], occupational therapy in combination with self-monitoring using a diary [68], and biofeedback-assisted progressive muscle relaxing training [71] seem to improve postoperative pain or to decline opioid prescriptions after TKA. For function, pain coping skills training [62], audiorecording guided imagery scripts [61], video promoting self-confidence and psychological support [64], music [35, 36], occupational therapy in combination with self-monitoring using a diary [68], various types of education [41, 43, 45, 47, 51–53], weight-bearing biofeedback training [67], psychological therapies (behavioural change intervention [60] and cognitive behavioural therapy [57–59]) seem to significantly improve at least one postoperative functional outcome measure. Day-to-day education after TKA using an app might improve postoperative QoL.
This is a methodologically well-conducted systematic review for which a professional medical librarian (CdH) has developed the search strategy to conduct a comprehensive search in several databases to identify eligible studies. Two authors (JS & GO) performed the screening, data extraction, risk of bias assessment, and overall level of evidence grading independently. We have created a complete overview of all studies by minimizing our exclusion criteria regarding study design, minimum follow-up, and language. Studies without significant results on the effect of an intervention are often refused for publication. Due to the heterogeneity of the outcome measures of the included studies, it was not possible to conduct a funnel plot to assess this type of bias (publication bias) in our systematic review. However, we included multiple studies [32–34, 38, 39, 42, 46, 55, 56, 68] with small sample sizes (smaller than 30 patients) with no significant results on both outcome measures pain and function. Therefore we assume the risk of publication bias to be low.
Unfortunately, drawing meaningful conclusions from the included studies was hampered. First of all, there was a substantial heterogeneity with respect to study design, analysis, domain, interventions, and outcome measures, which precluded pooling for a meta-analysis. Second, according to the GRADE approach, we have graded the quality of evidence as low for outcome measures pain and function. Therefore, the true effect of the interventions targeting psychological distress on postoperative pain and function may be different from our estimate of the effect.
The previous systematic reviews of Szeverenyi et al. [26] and Tong et al. [27] concluded that psychological interventions seem to reduce postoperative side effects and anxiety and to improve recovery and mental components of quality of life after orthopaedic surgeries. However, Szeverenyi et al. [Sweverenyi] did not clarify the type of orthopaedic procedures (only joint replacement or no joint replacement) and Tong et al. [27] included several orthopaedic procedures (THA, TKA, and spinal procedures) of which only two studies [61, 63] represented separated data of patients undergoing TKA. The findings of our review do not support the earlier systematic review of Bay et al. [25], in which most interventions explored by the included studies were found to be ineffective on patient-reported outcome after THA and TKA. Only three studies with patients receiving TKA were included by Bay et al. [25]. Compared to that review, we included fifteen additional RCTs [33, 34, 37, 38, 41, 44, 45, 49, 53, 54, 56–58, 58, 63]. Second, due to the current lack of RCTs on one specific type of intervention focused on psychological distress (for example only pain coping skills training) applied to patients undergoing TKA, we have decided to also include a wider range of study designs to create a complete overview of the perioperative interventions focused on psychological distress that have been used to decrease pain and improve function and/or QoL after surgery. Besides, ten studies [32, 34, 37, 39, 48, 54, 55, 66, 70, 71] in our systematic review evaluated the degree of postoperative pain not only by measuring pain scores, but also by assessing postoperative prescription of opioids or other types of pain medication. Investigating alternative nonpharmacologic methods to reduce postoperative pain and opioid use may help prevent further expansion of opioid misuse and addiction, which is currently a rapidly evolving public health crisis [7].
To the best of our knowledge, except for the mentioned systematic reviews [25, 26], no other systematic reviews or meta-analysis with comparable objectives have been published. Therefore, this is the first systematic review with wide search and inclusion criteria focused on TKA patients investigating the effect of interventions focused on psychological distress on patient-reported outcome measures pain, function, and QoL after surgery. Unfortunately, our review also highlighted the limitations of current literature on this subject. To avoid heterogeneity of outcome measures between studies, we would discourage the use of different questionnaires to assess patient-reported outcome measures (PROMs) in orthopaedic research. The reliability and reproducibility of the EuroQOL Five-Dimensional Questionnaire (EQ-5D) and the responsiveness of the Patient-Reported Outcomes Measurement Information System (PROMIS) Global Health survey have been well validated for patients undergoing TKA [72]. We would, therefore, recommend the use of the EQ-5D and PROMIS to allow tracking and evaluation of the effectiveness of perioperative interventions for psychological distress in conjunction with TKA in the following studies [72].
Conclusions
The studies included in our systematic review show the positive effect of multiple perioperative interventions targeting psychological distress for patients receiving TKA to improve postoperative pain (or to decline prescriptions of opioids), function, and QoL. RCTs with strict methodological safeguards (such as long-term follow-up, large number of patients participating in the study, low risk of bias) prospectively comparing outcome for patients with and without perioperative support are still needed to determine if perioperative interventions targeting psychological distress should be used in conjunction with primary TKA for OA of the knee. These studies should also assess which type of intervention will be most effective in improving patient-reported outcome measures and declining opioid prescriptions in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
"We are pleased to acknowledge Qiukui Hao, Chinese associate professor from the West China Hospital, Sichuan University, China, for the performance of the data-extraction and quality assessment of two Chinese articles [45, 57]."
Compliance with ethical standard
Conflict of interest
Author Juliette Caroline Sorel, Geke Marianne Overvliet, Maaike Gerarda Johanna Gademan, Chantal den Haan declare that they have no conflicts of interest. Author Adriaan Honig reports personal fees from NOV-Dutch Orthopaedic Society, grants from The Netherlands Organisation for Health Research and Development (in Dutch: ZonMw), other from LINK/LIMA, other from Stryker, personal fees from LINK, personal fees from BMJ, non-financial support from LINK, grants from Achmea Healthcare Foundation (in Dutch Stichting Achmea Gezonheidszorg fonds), grants from Dutch health insurances (Zorgverzekeraars Nederland), grants from Foundation of medical research OLVG, Amsterdam, the Netherlands, grants from Van Rens Foundation, grants from Reuma Nederland, other from McMaster University, outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Skou ST, Roos EM, Laursen MB, et al. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373:1597–1606. doi: 10.1056/NEJMoa1505467. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins PJ, Clement ND, Hamilton DF, Gaston P, Patton JT, Howie CR. Predicting the cost-effectiveness of total hip and knee replacement: a health economic analysis. Bone Joint J. 2013;95:115–121. doi: 10.1302/0301-620X.95B1.29835. [DOI] [PubMed] [Google Scholar]
- 3.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012 doi: 10.1136/bmjopen-2011-000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker P, van der Meulen J, Lewsey J, Gregg P. Nation joint registry for England and Wales. The role of pain and function in determining patient satisfaction after total knee replacement. Data from the National Joint Registry for England and Wales. J Bone Joint Surg Br. 2007;89:893–900. doi: 10.1302/0301-620X.89B7.19091. [DOI] [PubMed] [Google Scholar]
- 5.Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Red. 2010;468:57–63. doi: 10.1007/s11999-009-1119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedard NA, Pugely AJ, Westermann RW, Duchman KR, Glass NA, Callaghan JJ. Opioid use after total knee arthroplasty: trends and risk factors for prolonged use. J Arthroplasty. 2017;32:2390–2394. doi: 10.1016/j.arth.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Volkow ND, Collins FS. The Role of science in addressing the opioid crisis. N Engl J Med. 2017;377:391–394. doi: 10.1056/NEJMsr1706626. [DOI] [PubMed] [Google Scholar]
- 8.Alattas SA, Smith T, Bhatti M, Wilson-Nunn D, Donell S. Greater pre-operative anxiety, pain and poorer function predict a worse outcome of a total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017;25:3403–3410. doi: 10.1007/s00167-016-4314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harmelink KEM, Zeegers AVCM, Hullegie W, Hoogeboom TJ, Nijhuis-van der Sanden MWG, Staal JB. Are there prognostic factors for one-year outcome after total knee arthroplasty? a systematic review. J Arthroplasty. 2017;32:3840–3853. doi: 10.1016/j.arth.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Sun K, Li H. Body mass index as a predictor of outcome in total knee replace: A systemic review and meta-analysis. Knee. 2017;24:917–924. doi: 10.1016/j.knee.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Núñez M, Núñez E, del Val JL, et al. Health-related quality of life in patients with osteoarthritis after total knee replacement: factors influencing outcomes at 36 months of follow-up. Osteoarthritis Cartilage. 2007;15:1001–1007. doi: 10.1016/j.joca.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Papakostidou I, Dailiana ZH, Papapolychroniou T, et al. Factors affecting the quality of life after total knee arthroplasties: a prospective study. BMC Musculoskelet Discord. 2012;13:116. doi: 10.1186/1471-2474-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heck DA, Robinson RL, Partridge CM, Lubitz RM, Freund DA. Patient outcomes after knee replacement. Clin Orthop Relat Res. 1998;356:93–110. doi: 10.1097/00003086-199811000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Lingard EA, Katz JN, Wright EA, Sledge CB. Kinemax Outcomes Group. Predicting the outcome of total knee arthroplasty. J Bone Joint Surg Am. 2004;86:2179–2186. doi: 10.2106/00004623-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Roth JS, Buehler KC, Shen J, Naughton M. Patient factors predict functional outcomes after cruciate retaining TKA: a 2-year follow-up analysis. J Arthroplasty. 2013;28:1321–1326. doi: 10.1016/j.arth.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Khatib Y, Madan A, Naylor JM, Harris IA. Do psychological factors predict poor outcome in patients undergoing TKA? A systematic review. Clin Orthop Relat Res. 2015;473:2630–2638. doi: 10.1007/s11999-015-4234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns LC, Ritvo SE, Ferguson MK, Clarke H, Seltzer Z, Katz J. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: a systematic review. J Pain Res. 2015;8:21–32. doi: 10.2147/JPR.S64730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández C, Díaz-Heredia J, Berraquero ML, Crespo P, Loza E, Ruiz Ibán MÁ. Pre-operative predictive factors of post-operative pain in patients with hip or knee arthroplasty: a systematic review. Reumatol Clin. 2015;11:361–380. doi: 10.1016/j.reuma.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: systematic review and meta-analysis. Br J Anaesth. 2015;114:551–561. doi: 10.1093/bja/aeu441. [DOI] [PubMed] [Google Scholar]
- 20.Sorel JC, Veltman ES, Honig A, Poolman RW. The influence of preoperative psychological distress on pain and function after total knee arthroplasty: a systematic review and meta-analysis. Bone Joint J. 2019 doi: 10.1302/0301-620X.101B1.BJJ-2018-0672.R1. [DOI] [PubMed] [Google Scholar]
- 21.Clarke HD, Timm VL, Goldberg BR, Hattrup SJ. Preoperative patient education reduces in-hospital falls after total knee arthroplasty. Clin Orthop Relat Res. 2012;470:244–249. doi: 10.1007/s11999-011-1951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon D, Malhas A, Goubran A, Subramanian P, Messer C, Houlihan-Burne D. Implementing the rapid recovery program in primary hip and knee arthroplasty in a UK state run hospital. Eur J Orthop Surg Traumatol. 2011;21:151–158. doi: 10.1007/s00590-010-0690-9. [DOI] [Google Scholar]
- 23.Tristaino V, Lantieri F, Tornago S, Gramazio M, Carriere E, Camera A. Effectiveness of psychological support in patients undergoing primary total hip or knee arthroplasty: a controlled cohort study. J Orthopaed Traumatol. 2016;17:137–147. doi: 10.1007/s10195-015-0368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White L, Stockwell T, Hartnell N, Henessy M, Mullan J. Factors preventing kneeling in a group of pre-educated patients post total knee arthroplasty. J Orthopaed Traumatol. 2016;17:333–338. doi: 10.1007/s10195-016-0411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bay S, Kuster L, McLean N, Byrnes M, Kuster MS. A systematic review of psychological interventions in total hip and knee arthroplasty. BMC Musculoskeletal Disorders. 2018;19:201. doi: 10.1186/s12891-018-2121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szeverenyi C, Kekecs Z, Johnson A, Elkins G, Csernatony Z, Varga K. The use of adjunct psychosocial interventions can decrease postoperative pain and improve the quality of clinical care in orthopedic surgery: A systematic review and meta-analysis of randomized controlled trials. J Pain. 2018;19(11):1231–1252. doi: 10.1016/j.jpain.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Tong F, Dannaway J, Enke O, Eslick G. Effect of preoperative psychological interventions on elective orthopaedic surgery outcomes: a systematic review and meta-analysis. ANZ J Surg. 2020;90(3):230–236. doi: 10.1111/ans.15332. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31(11):1409–1417. doi: 10.1007/s00296-011-1999-3. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org
- 31.Guyatt G, Oxman AD, Aki EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Allred KD, Byers JF, Sole ML. The effect of music on postoperative pain and anxiety. Pain Manag Nurs. 2010;11:15–25. doi: 10.1016/j.pmn.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Aris A, Sulaiman S, Che Hasan MK. The influence of music therapy on mental well-being among postoperative patients of total knee arthroplasty (TKA) Enferm Clin. 2019;29(2):16–23. doi: 10.1016/j.enfcli.2019.04.004. [DOI] [Google Scholar]
- 34.Chen HJ, Chen TY, Huang CY, Hsieh YM, Lai HL. Effects of music on psychophysiological responses and opioid dosage in patients undergoing total knee replacement surgery. Jpn J Nurs Sci. 2015;12:309–319. doi: 10.1111/jjns.12070. [DOI] [PubMed] [Google Scholar]
- 35.Hsu CC, Chen WM, Chen SR, Tseng YT, Lin PC. Effectiveness of music listening in patients with total knee replacement during CPM rehabilitation. Biol Res Nurs. 2016;18:68–75. doi: 10.1177/1099800415572147. [DOI] [PubMed] [Google Scholar]
- 36.Hsu CC, Chen SR, Lee PH, Lin PC. The effect of music listening on pain, heart rate variability, and range of motion in older adults after total knee replacement. Clin Nurs Res. 2019;28(5):529–547. doi: 10.1177/1054773817749108. [DOI] [PubMed] [Google Scholar]
- 37.Keshmiri A, Wolf T, Wiech O, Benditz A, Grifka J, Springorum H. Einfluss der intraoperativen Schallprotektion auf postoperative Schmerzen. Schmerz. 2014;28:82–89. doi: 10.1007/s00482-013-1368-0. [DOI] [PubMed] [Google Scholar]
- 38.Leonard H. Live music therapy during rehabilitation after total knee arthroplasty: a randomized controlled trial. J Music Ther. 2019;56:61–89. doi: 10.1093/jmt/thy022. [DOI] [PubMed] [Google Scholar]
- 39.Santhna LP, Norhamdan MY, Damrudi M. The effectiveness of music therapy for post-operative pain control among total knee replacement patients. Med & Health. 2015;10(1):66–79. [Google Scholar]
- 40.Simcock XC, Yoon RS, Chalmers P, Geller JA, Kiernan HA, Macaulay W. Intraoperative music reduces perceived pain after total knee arthroplasty: a blinded, prospective, randomized, placebo-controlled clinical trial. J Knee Surg. 2008;21:275–278. doi: 10.1055/s-0030-1247831. [DOI] [PubMed] [Google Scholar]
- 41.Atabaki S, Farahani MA, Haghani S. Effect of rehabilitation education on pain, knee stiffness and performance difficulty in patients undergoing knee replacement surgery: A randomized clinical trial. J Acute Dis. 2019;8(6):233–238. doi: 10.4103/2221-6189.272854. [DOI] [Google Scholar]
- 42.Aytekin E, Sukur E, Oz N, et al. The effect of a 12 week prehabilitation program on pain and function for patients undergoing total knee arthroplasty: A prospective controlled study. J Clin Orthop Trauma. 2019;10:345–349. doi: 10.1016/j.jcot.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen SR, Chen CS, Lin PC. The effect of educational intervention on the pain and rehabilitation performance of patients who undergo a total knee replacement. J Clin Nurs. 2014;23:279–287. doi: 10.1111/jocn.12466. [DOI] [PubMed] [Google Scholar]
- 44.Huang S-W, Chen P-H, Chou Y-H. Effects of a preoperative simplified home rehabilitation education program on length of stay of total knee arthroplasty patients. Orthop Traumat Surgery Res. 2012;98:259–264. doi: 10.1016/j.otsr.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Huang P, He J, Zhang YM. The mobile application of patient management in education and follow-up for patients following total knee arthroplasty. Zhonghua Yi Xue Za Zhi. 2017;97:1592–1595. doi: 10.3760/cma.j.issn.0376-2491.2017.20.019. [DOI] [PubMed] [Google Scholar]
- 46.Lee JK, Zubaidah JO, Fadhilah ISI, Normala I, Jensen MP. Prerecorded hypnotic peri-surgical intervention to alleviate risk of chronic postsurgical pain in total knee replacement: a randomized controlled pilot study. Int J Clin Exp Hypn. 2019;67:217–245. doi: 10.1080/00207144.2019.1580975. [DOI] [PubMed] [Google Scholar]
- 47.Lin PC, Lin LC, Lin JJ. Comparing the effectiveness of different educational programs for patients with total knee arthroplasty. Orthop Nurs. 1997;16(5):43–49. doi: 10.1097/00006416-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Louw A, Puentedura EJ, Reed J, Zimney K, Grimm D, Landers MR. A controlled clinical trial of preoperative pain neuroscience education for patients about to undergo total knee arthroplasty. Clin Rehabil. 2019;33(11):1722–1731. doi: 10.1177/0269215519857782. [DOI] [PubMed] [Google Scholar]
- 49.Malletschek A, Tiller D, Wohlrab D. Psychoedukation bei Patienten mit Knieendoprothese : Erweitertes Schmerzmanagement [Psychoeducation in knee arthroplasty patients : Additional pain management] Orthopade. 2020;49(1):26–31. doi: 10.1007/s00132-019-03749-y. [DOI] [PubMed] [Google Scholar]
- 50.Moulton LS, Evans PA, Starks I, Smith T. Preoperative education prior to elective knee arthroplasty surgery does not change patient outcomes. Musculoskeletal Care. 2017;15:341–344. doi: 10.1002/msc.1177. [DOI] [PubMed] [Google Scholar]
- 51.Piva SR, Almeida GJ, Gil AB, DiGioia AM, Helsel DL, Sowa GA. Effect of comprehensive behavioral and exercise intervention on physical function and activity participation after total knee replacement: a pilot randomized study. Arthritis Care Res (Hoboken) 2017;69(12):1855–1862. doi: 10.1002/acr.23227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reslan HA, Moustafa SM, Saghieh S, Sharaza ES, Badr LK. Does intervention improve the outcomes of patients after total knee replacement surgery? Int J Ortho Trauma Nurs. 2018;31:26–31. doi: 10.1016/j.ijotn.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Timmers T, Janssen L, van der Weegen W, Das D, Marijnissen WJ, Hannink G, van der Zwaard BC, Plat A, Thomassen B, Swen JW, Kool RB, Lambers Heerspink FO. the effect of an app for day-to-day postoperative care education on patients with total knee replacement: randomized controlled trial. JMIR Mhealth Uhealth. 2019;7(10):e15323. doi: 10.2196/15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson RA, Watt-Watson J, Hodnett E, Tranmer J. A randomized controlled trial of an individualized preoperative education intervention for symptom management after total knee arthroplasty. Orthop Nurs. 2016;35:20–29. doi: 10.1097/NOR.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 55.Yajnik M, Hill JN, Hunter OO, et al. Patient education and engagement in postoperative pain management decreases opioid use following knee replacement surgery. Patient Educ Couns. 2019;102:383–387. doi: 10.1016/j.pec.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Birch S, Stilling M, Mechlenburg I, Hansen TB. No effect of cognitive behavioral patient education for patients with pain catastrophizing before total knee arthroplasty: a randomized controlled trial. Acta Orthop. 2020;91(1):98–103. doi: 10.1080/17453674.2019.1694312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai LB, Liu YJ, Zhao H, Xu HP, Gao HH, Dong YZ. Cognitive behavior therapy alleviates kinesiophobia after total knee arthroplasty. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:3658–3663. [Google Scholar]
- 58.Cai L, Gao H, Xu H, Wang Y, Lyu P, Liu Y. Does a program based on cognitive behavioral therapy affect kinesiophobia in patients following total knee arthroplasty? a randomized, controlled trial with a 6-month follow-up. J Arthroplasty. 2018;33:704–710. doi: 10.1016/j.arth.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 59.Das Nair R, Mhizha-Murira JR, Anderson P, et al. Home-based pre-surgical psychological intervention for knee osteoarthritis (HAPPiKNEES): a feasibility randomized controlled trial. Clin Rehabil. 2018;32:777–789. doi: 10.1177/0269215518755426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harnirattisai T, Johnson RA. Effectiveness of a behavioral change intervention in Thai elders after knee replacement. Nurs Res. 2005;54:97–107. doi: 10.1097/00006199-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Jacobson AF, Umberger WA, Palmieri PA, Alexander TS, Myerscough RP, Draucker CB, Steudte-Schmiedgen S, Kirschbaum C. Guided imagery for total knee replacement: a randomized, placebo-controlled pilot study. J Altern Complement Med. 2016;22:563–575. doi: 10.1089/acm.2016.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riddle DL, Keefe FJ, Nay WT, McKee D, Attarian DE, Jensen MP. Pain coping skills training for patients with elevated pain catastrophizing who are scheduled for knee arthroplasty: a quasi-experimental study. Arch Phys Med Rehabil. 2011;92:859–865. doi: 10.1016/j.apmr.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riddle DL, Keefe FJ, Ang DC, et al. Pain coping skills training for patients who catastrophize about pain prior to knee arthroplasty: a multisite randomized clinical trial. J Bone Joint Surg Am. 2019;6(101):218–227. doi: 10.2106/JBJS.18.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russo LR, Benedetti MG, Mariani E, Roberti di Sarsina T, Zaffagnini S. The videoinsight® method: improving early results following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017;25:2967–2971. doi: 10.1007/s00167-016-4118-x. [DOI] [PubMed] [Google Scholar]
- 65.Zaffagnini S, Russo LR, Marcheggiani Muccioli GM, Marcacci M. The Videoinsight® method: improving rehabilitation following anterior cruciate ligament reconstruction—a preliminary study. Knee Surg Sports Traumatol Arthrosc. 2013;21:851–858. doi: 10.1007/s00167-013-2392-4. [DOI] [PubMed] [Google Scholar]
- 66.Baldwin AL, Vitale A, Brownell E, Kryak E, Rand W. Effects of reiki on pain, anxiety, and blood pressure in patients undergoing knee replacement: a pilot study. Holist Nurs Pract. 2017;31:80–89. doi: 10.1097/HNP.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 67.Christiansen CL, Bade MJ, Davidson BJ, Dayton MR, Stevens-Lapsley JE. Effects of weight-bearing biofeedback training on functional movement patterns following total knee arthroplasty: a randomized controlled trial. J Orthop Sports Phys Ther. 2015;45:647–655. doi: 10.2519/jospt.2015.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hiraga Y, Hisano S, Nomiyama K, Hirakawa Y. Effects of using activity diary for goal setting in occupational therapy on reducing pain and improving psychological and physical performance in patients after total knee arthroplasty: A non-randomised controlled study. Hong Kong J Occup Ther. 2019;32(1):53–61. doi: 10.1177/1569186119849117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koo K, Park DK, Youm YS, Cho SD, Hwang CH. Enhanced reality showing long-lasting analgesia after total knee arthroplasty: prospective. Random Clin Trial Sci Rep. 2018;8:2343. doi: 10.1038/s41598-018-20260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Notte BB, Fazzine C, Mooney RA. Reiki's effect on patients with total knee arthroplasty: A pilot study. Nursing. 2016;46:17–23. doi: 10.1097/01.NURSE.0000476246.16717.65. [DOI] [PubMed] [Google Scholar]
- 71.Wang TJ, Chang CF, Lou MF, et al. Biofeedback relaxation for pain associated with continuous passive motion in Taiwanese patients after total knee arthroplasty. Res Nurs Health. 2015;38:39–50. doi: 10.1002/nur.21633. [DOI] [PubMed] [Google Scholar]
- 72.Shim J, Hamilton DF. Comparative responsiveness of the PROMIS-10 Global Health and EQ-5D questionnaires in patients undergoing total knee arthroplasty. Bone Joint J. 2019;101-B:832–837. doi: 10.1302/0301-620X.101B7.BJJ-2018-1543.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.