Abstract
Anxiety leads to a global decline in quality of life and increase in social burden. However, treatments are limited, because the molecular mechanisms underlying complex emotional disorders are poorly understood. We explored the anxiolytic effects of 8-O-acetyl shanzhiside methylester (8-OaS), an active component in Lamiophlomis rotata (L. rotata; Benth.) or Kudo, a traditional herb that has been shown to be effective in the clinical treatment of chronic pain syndromes in China. Two mouse anxiety models were used: forced swimming stress (FSS)–induced anxiety and complete Freund’s adjuvant (CFA)–induced chronic inflammatory pain. All animal behaviors were analyzed on the elevated plus maze and in the open-field test. 8-OaS significantly ameliorated anxiety-like behaviors in both anxiety models and inhibited the translation enhancement of GluN2A, GluN2B, and PSD95. Moreover, a reduction in GABA receptors disrupted the excitatory/inhibitory (E/I) balance in the basolateral amygdala (BLA), indicated by increased excitatory and decreased inhibitory presynaptic release. 8-OaS also blocked microglia activation and reduced the phosphorylation of p38, c-Jun N-terminal kinase (JNK), NF-κB p65, and tumor necrosis factor alpha (TNF-α) in the BLA of anxiety mice. 8-OaS exhibits obvious anxiolytic effects by regulating the excitatory/inhibitory (E/I) synaptic transmission and attenuating inflammatory responses in the BLA.
Keywords: Anxiety, 8-O-Acetyl shanzhiside methylester, Excitatory/inhibitory balance, Basolateral amygdala, Inflammatory response
Introduction
Anxiety disorder is highly common worldwide, with an estimated prevalence of 15% in developed countries, and seriously affects people’s life and work (Wu et al. 2008). Anxiety disorder is a chronic and functional disability with high psychological pressure, characterized by overwhelming stress, attention difficulties, and physiological symptoms such as muscle tension and insomnia (Beery and Kaufer 2015; Du et al. 2019). Antidepressants and benzodiazepines are clinically useful for the treatment of anxiety, but can lead to considerable side effects such as liability of physical dependence, addiction, excessive sedation, and abuse (Locke et al. 2015). Better anxiolytic drugs with fewer side effects are therefore needed.
In clinical experiments, acute stress, chronic inflammation, and pain cause anxiety behaviors. Stress is an established and pivotal precipitating factor for several neuropsychiatric diseases, especially for anxiety and mood disorders (Thakare et al. 2017). Animal studies have shown that stress can induce various alterations of neurotransmission systems, and hyperexcitation can promote anxiety-like behavior in the basolateral amygdala (BLA), because of enhanced excitatory glutamate or reduced inhibitory GABA transmission (Liu et al. 2012; Stanika et al. 2009). The imbalance between excitation and inhibition in neural circuits is therefore highly relevant to the occurrence and development of anxiety. Additionally, neuroimaging studies show that an increase in inflammation is associated with enhanced threat- and anxiety-related brain circuitry, particularly activation of the amygdala (Tasan et al. 2010). Moreover, animal studies have demonstrated an increase in anxiety-like behaviors during experimentally induced systemic inflammatory responses (Gallagher et al. 2019). Regulating the excitatory and inhibitory transmission balance as well as inflammation may thus be an effective method for treating anxiety disorders.
Lamiophlomis rotata (L. rotata) Kudo (“Duyiwei” in Chinese) is a Chinese folk medicinal plant from Xi-zang (Tibet), which is traditionally used to relieve pain, detumescence, and hemostasis; reinforce marrow; and promote blood circulation to remove blood stasis (Jiang et al. 2010; Yi et al. 1997). The active ingredients of L. rotata are iridoid glycosides, mainly shanzhiside methylester (SM) and 8-O-acetyl-SM (8-OaS) (La et al. 2015; Shang et al. 2011). Emerging studies suggest that 8-OaS has neuroprotective effects on hypoxia and glucose deficiency by suppressing inflammatory and apoptosis-related cascade reactions (Jiang et al. 2010; Jiang et al. 2011). In addition, the main mechanism of 8-OaS analgesia is the regulation of nociceptive information transmission (related to the deactivation of the NMDAR/PKC and NO/cGMP/PKG pathways) and spinal neuroinflammatory responses (decrease in TNF-α and IL-1 β, increase in IL-10 production) (Zheng et al. 2011). 8-OaS inhibits the production of TNF-α and other pro-inflammatory cytokines and reduces the phosphorylation of P38 MAPK and NF-κB in neuropathic pain models (Fan et al. 2016; Ji et al. 2014; Xu et al. 2006; Zhu et al. 2014). These effects of 8-OaS are correlated with the inhibition of the inflammatory response. However, its anxiolytic effects in a model of acute stress and inflammation as well as the involved mechanisms remain unclear.
This study thus aimed to investigate the roles and mechanism of 8-OaS in the modulation of anxiety-like behaviors, in two animal models of anxiety, an acute forced swimming stress (FSS) model and a chronic CFA-induced inflammatory pain model.
We found that 8-OaS exerted anxiolytic-like effects in the open-field test (OFT) and the elevated plus maze (EPM) anxiety tests in both models. The correlative proteins involved in the balance between excitation and inhibition, including GluN2A, GluN2B, PSD95, and subunits of GABAA receptors (GABAAα2 and GABAAγ2 in FSS-induced mice, and inflammatory signaling proteins including phospho-JNK, phospho-P38, IL-6, TNF-α, and NF-κB p65) were sequentially examined following 8-OaS administration in CFA-injected mice. Our results clarify the role of 8-OaS in stress- and CFA-induced mood disorders and suggest 8-OaS as a potential therapeutic target for the treatment of clinical anxiety.
Materials and Methods
Animals
Six- and 8-week-old C57BL/6 male mice were provided by the Laboratory Animal Center of Fourth Military Medical University (FMMU). The animals were housed in a colony room at 24 ± 2 °C and 50–60% humidity and under a 12-h light/dark cycle (light on at 7:00 AM) with food and water available ad libitum. All behavioral tests were conducted during the light period. Mice were adapted to the laboratory conditions for at least 1 week before testing. All procedures were approved by the FMMU Animal Care Committee.
Chronic Inflammatory Pain Model (Model 1)
As in a previous study (Wang et al. 2015), the left hind paw plantar of test mice was injected with CFA (10 μl, 50% in saline) to induce persistent inflammatory pain. The same volume of 0.9% saline was injected into the left hind paw plantar of control mice.
Forced Swimming Test (Model 2)
The forced swimming test was conducted in an open cylindrical container (diameter 10 cm, height 25 cm) filled with water at 23–25 °C for 15 min. Mice were placed in separate containers and forced to swim without their tails touching the bottom. The animals were forced to swim for 15 min a day for 2 consecutive days. At the end of each session, the mice were taken out of the water and dried immediately.
Experimental Design and Drug Treatment
8-OaS (purity > 98%) was purchased from Shanghai Pure One Biotechnology (Shanghai, China), dissolved in 0.9% saline containing 10% dimethylsulfoxide (DMSO), and administered intraperitoneally (i.p.) at different doses (0.02, 0.2, 2 mg/kg) after the CFA insult. The 8-OaS doses used here were based on earlier research (Zhang et al. 2018). Mice in the control group were treated with saline for 2 weeks (from day 1 to day 14). Mice in the chronic inflammatory pain model groups (n = 6 per group) were continuously injected with 8-OaS or saline for 2 weeks (from day 1 to day 14), and all behavior tests were conducted on day 15 after CFA injection. In the acute stress model groups, mice were continuously injected with 8-OaS or saline for 3 days (from day 1 to day 3), and behavior tests were conducted on day 4. After the behavior tests, all animals were sacrificed, and the BLA was collected to detect the molecular mechanism underlying the effects of 8-OaS administration during phases of anxiety.
Open-field Test
The open-field test (OFT) was performed as depicted previously (Yang et al. 2016). An individual animal was placed in the center area of the box for 15 min. Exploratory behaviors were recorded using a camera fixed above the floor and analyzed with a video-tracking system. The OFT test was performed before the EPM test but on the same day.
Elevated Plus Maze Test
To assess anxiety-like behaviors, EPM tests were also performed as detailed in a previous report (Sun et al. 2016). The apparatus contained two opposing open arms (25 × 8 × 0.5 cm3) and two closed arms (25 × 8 × 12 cm3) extending from a common central zone (8 × 8 cm2). The EPM was located 50 cm above the floor. For each test, an individual animal was initially placed in the central area facing an open arm and allowed to move freely for 5 min while being recorded with a camera fixed above the maze.
Immunohistochemistry Staining
After the behavior tests, the brain slices for immunohistochemistry staining were prepared from the intracardially perfused brains, and staining procedures were used as described previously (Sun et al. 2018). All frozen brain sections were washed with 0.3% Triton X-100 PBS and then blocked (10% goat serum, 0.1% Triton X-100 in PBS) for 2 h at 4 °C. Slices were incubated with mouse goat anti-Iba-1 (1:1000) in blocking solution for 24 h at 4 °C. Next, the sections were rinsed with PBS and incubated with donkey anti-goat IgG Alexa Fluor 594 (1:200) in PBS for 1 h at room temperature. All antibodies were diluted in PBS with 0.1% Triton X-100 and 2% bovine serum albumin. Nuclei were counter-stained with Hoechst33258 (1:100). The brain slices were then moved to slides, coverslipped with 50% glycerin, and photographed with a FluoView FV100 microscope (Olympus).
Enzyme-Linked Immunosorbent Assay
After the behavior tests, the BLA tissue samples were removed from the brains of the dissected mice. According to the manufacturer’s instructions (R&D Systems, Minneapolis, MN), levels of the inflammatory cytokines interleukin 1β (IL-1β), interleukin 6 (IL-6), and TNF-α were detected in the BLA using a double-antibody sandwich method.
Determination of GABA and Glutamate
After treatment with 8-OaS for 3 days, GABA and glutamate were detected using high-performance liquid chromatography (HPLC; Agilent Technologies 1260 Infinity, Agilent Technologies, Wilmington, DE), according to previously described methods (Wang et al. 2017). Before derivatization, the samples were dissolved with boracic acid buffer (pH 9.0) and centrifuged for 15 min at 3000 r/min at 4 °C. Samples were mixed with 2,4-dinitrofluorobenzene (DNFB) and 0.5 mol/L NaHPO3 buffer for 1 h at 60 °C; then, a phosphate buffer solution (pH 7) was added to stop the reaction. Samples were analyzed using a UV detector (360 nm, Agilent Technologies 1260 Infinity). The mobile phase was KH2PO4 buffer (pH 6.0), acetonitrile, and ddH2O (84:8:8, v/v/v) at a flow rate of 1.0 mL/min. A Themo TC-C18 column (250 × 4.6 mm2; particle size 5 mm) was used. The concentrations were obtained using the LC solution software (Shimadzu) based on standard substances.
Western Blot Analysis
Samples from BLA tissues were harvested after various treatments according to our previously reported method (Yang et al. 2016). The western blot analysis determined the expression levels of GluA1 (1:1000; Abcam, ab31232), phosphorylated forms of GluA1 including phospho-GluA1-831 (1:1000; Abcam, ab5847) and phospho-GluA1-845 (1:1000; Abcam, ab5849), GluN2A (1:1000; Abcam, ab133265), GluN2B (1:1000; Millipore, Billerica, MA; MAB5780), PSD95 (1:1000; Abcam; ab2723), phosphorylated forms of JNK (1:1000; Cell Signaling Technology; Danvers, MA) and P38 (1:1000; Cell Signaling Technology), TNF-α (1:1000; Cell Signaling Technology), and p65 (1:1000; Cell Signaling Technology); β-actin (1:10,000; Sigma, St. Louis, MO) served as a loading control. The target protein signal was detected and digitized using ECL solution and ImageJ software (NIH, Bethesda, MD).
Statistical Analysis
Data were analyzed with SPSS 19.0 and expressed as means ± SEM. Comparisons between the two groups were conducted using independent sample t tests. Results of multiple groups were analyzed using one-way ANOVA followed by least significant difference (LSD) tests. In all cases, statistical significance was accepted at p < 0.05.
Results
8-OaS Relieves Anxiety-Like Behaviors Induced by Forced Swimming Stress
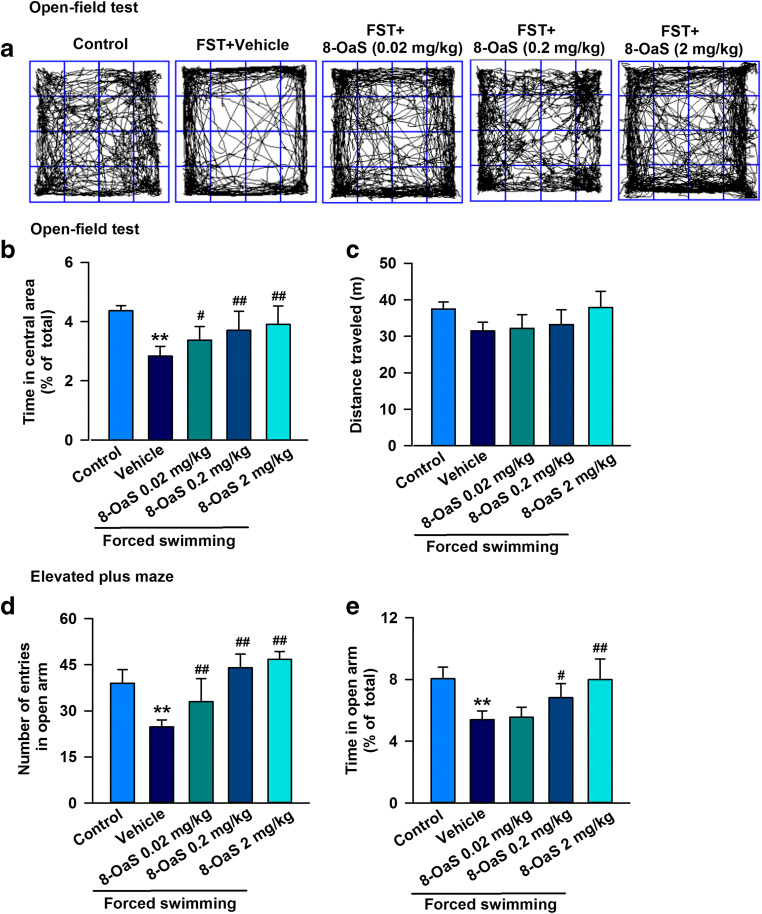
The FSS model was used to investigate the importance of 8-OaS in acute stress–induced anxiety-like behaviors. Anxiety-like behaviors were assessed with the EPM and OFT, which are widely used for this purpose in rodents. In the OFT, compared with the control group, the stress group displayed an obvious decrease in the time spent in the central area, while the total distance traveled showed no change (Fig. 1a, b). In the EPM test, the stressed group showed a remarkable decrease in the number of entries and the time spent in the open arms (Fig. 1a, b), although the total distance traveled showed no significant change compared with the vehicle group (Fig. 1c). Administration of 8-OaS for 3 days remarkably improved anxiety-like behaviors in these stressed mice, as our results show that the number of entries into and the time spent in the open arms in the EPM test increased (Fig. 1d, e) and that the mice spent more time in the central square in the OFT (Fig. 1b). These results suggest that 8-OaS is involved in anxiolytic responses that reverse anxiety-like behaviors after acute stress.
Fig. 1.
8-OaS relieved anxiety-like behaviors in FSS-induced mice. Representative traces in the OFT during a period of 15 min. Behavioral tests were performed on day 14. a, c In the OFT, 8-OaS administration (0.02, 0.2, 2 mg/kg) for 14 days significantly increased b the time spent in the central area but had no effect on c the total distance traveled. In the EPM test, 8-OaS treatment reversed d the number of entries into and e time spent in the open and closed arms. Each value represents the mean ± SEM of three independent experiments (n = 12, *p < 0.05, **p < 0.01 versus the control group; #p < 0.05, ##p < 0.01 versus the FSS group)
8-OaS Attenuates CFA-Induced Anxiety-Like Behaviors in the BLA
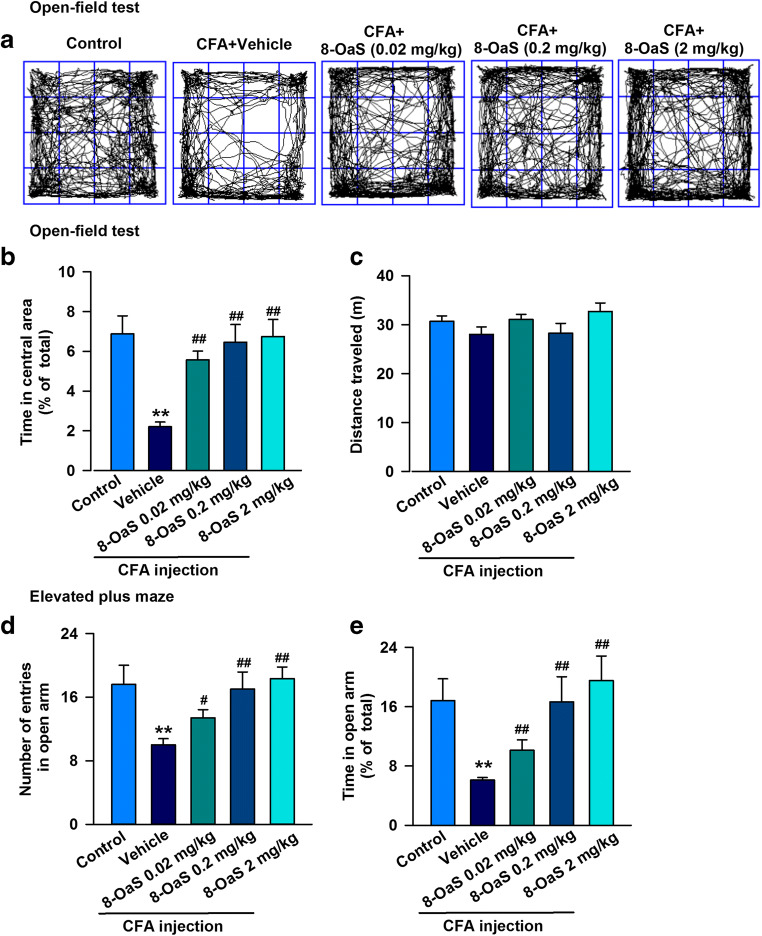
CFA was injected to induce chronic inflammation–mediated anxiety in mice. Different behaviors were assessed using the OFT, and we found that the time spent in the central zone was reduced in CFA-injected mice compared with that in the vehicle group (Fig. 2a, b). However, there were no obvious changes in the total distance traveled (Fig. 2c). In the EPM, our data shows that CFA induced anxiety-like behaviors and that mice made less entries into the open arms (Fig. 2d) and spent less time in the open arms (Fig. 2e) at 14 days after CFA injection, compared with the control group. Treatment with 8-OaS for 2 weeks significantly ameliorated the CFA-induced anxiety-like behaviors in mice and increased the number of entries and the time spent in the open arms in the EPM test (Fig. 2d, e) as well as the time spent in the central square in the OFT (Fig. 2b). These observations further confirm that 8-OaS treatment leads to anxiolytic effects on CFA-induced inflammatory pain.
Fig. 2.
8-OaS attenuated anxiety-like behaviors in CFA-injected mice. a Representative traces in the OFT during a period of 15 min. Behavioral tests were performed on day 3. b In the OFT, 8-OaS treatment (0.02, 0.2, 2 mg/kg) for 3 days remarkably reversed the time in the center area in CFA-injected mice; however, no difference in c the total distance traveled was observed in either group in the OFT. d In the EPM test, 8-OaS (0.02, 0.2, 2 mg/kg) increased the number of entries into and time spent in the open arms in CFA-injected mice. Each value represents the mean ± SEM of three independent experiments (n = 12, *p < 0.05, **p < 0.01 versus the control group; #p < 0.05, ##p < 0.01 versus the CFA-injected group)
8-OaS Maintains GABAergic and Glutamatergic Transmission Balance
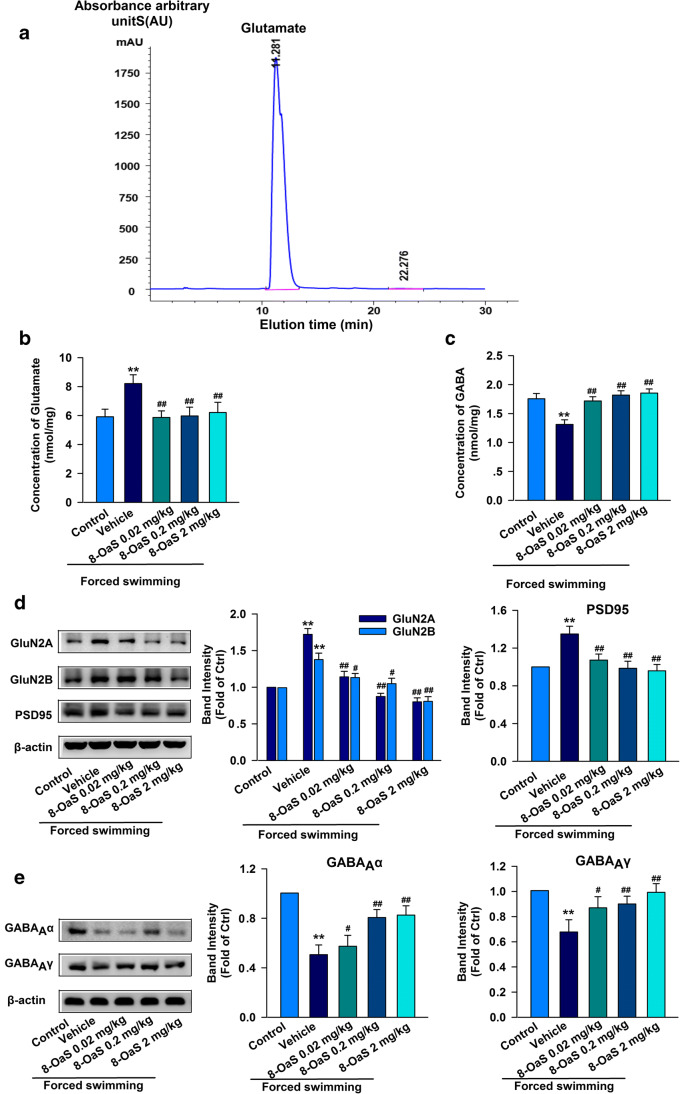
The excitatory/inhibitory (E/I) balance of neural activities is necessary for all central physiological functions. An imbalance in E/I signaling leads to seizures, schizophrenia, autism, and anxiety (Lucchetti et al. 2013). To investigate the levels of neurotransmitters in the BLA, we specifically checked glutamate and GABA concentrations using HPLC. Chromatograms of glutamate and GABA yielded peaks at 11.28 min and 22.27 min, respectively (Fig. 3a). The FSS group showed higher glutamate (8.21 ± 0.60 nmol/mg) and lower GABA (1.31 ± 0.08 nmol/mg) content in the BLA than in the vehicle group (glutamate 5.91 ± 0.52 nmol/mg; GABA 1.75 ± 0.09 nmol/mg) (Fig. 3b, c). Glutamate levels were lower after 8-OaS treatment (Fig. 3b). In contrast, GABA levels were enhanced after 8-OaS administration compared with those seen in control group mice (Fig. 3c). These data indicate that treatment with 8-OaS reversed the abnormal release of neurotransmitters in the BLA that had been induced by acute stress.
Postsynaptic receptors also play a vital role in the excitation/inhibition of signal transduction between neurons. Excitatory postsynaptic receptors include NMDA and AMPA receptors. Excitatory postsynaptic receptors are mainly GABAA receptors. Thus, we determined the expression changes in receptor subunits in the BLA after acute stress. The levels of GluN2A, GluN2B, and PSD95 (Fig. 3d) were remarkably increased in the BLA. Administration of 8-OaS for 3 days obviously decreased the elevation of GluN2A, GluN2B, and PSD95 (Fig. 3d) in the BLA of acutely stressed mice. Next, two lowly expressed subunits of GABAA receptors—GABAAα2 and GABAAγ2—were examined in the FSS group, compared with that in the vehicle group (Fig. 3e), and were found to be enhanced after 8-OaS administration for 3 days (Fig. 3e). These results suggest that 8-OaS treatment ameliorated the imbalance between GABAergic and glutamatergic transmission in the BLA of FSS mice.
Anti-inflammatory Effects of 8-OaS in the Mouse BLA
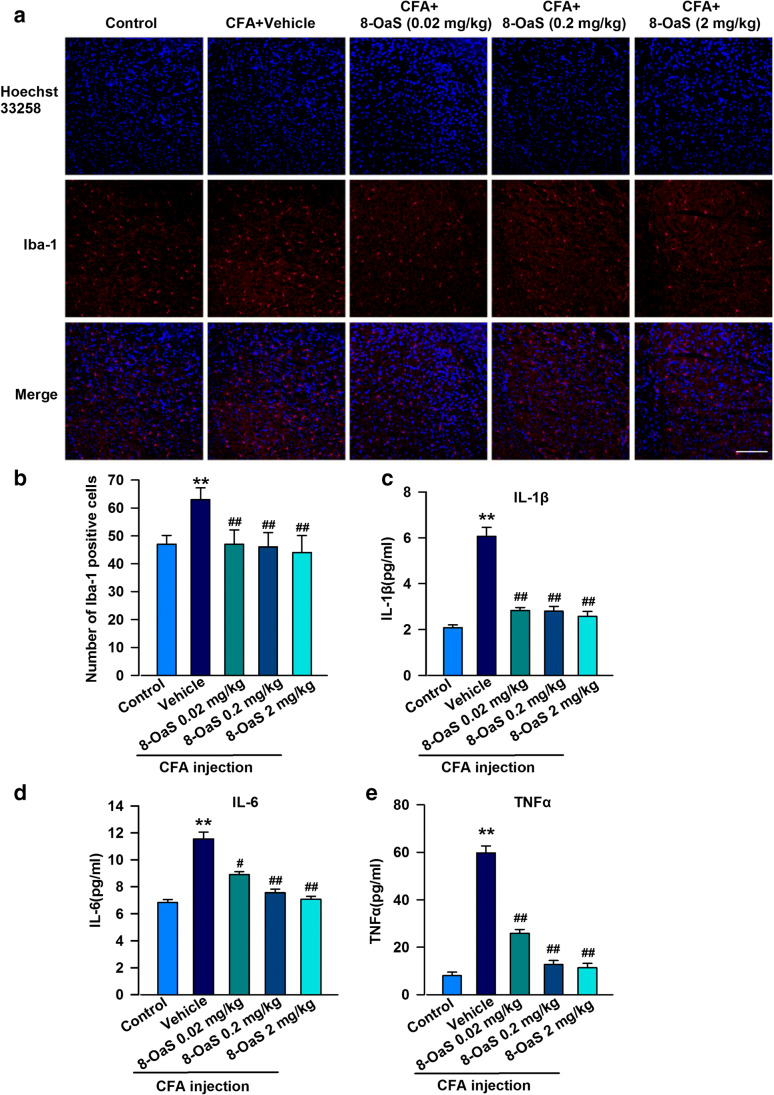
Microglia are responsible for immune responses upon inflammatory stimulation via the production of cytokines and chemokines. The chronic inflammation caused by CFA injection can activate the microglia in the BLA area. The morphological features of the activated microglia are enlargement and roundness, and the expression ratio of the specific marker Iba-1 increases (Fig. 4a, b), which can then drive the secretion of cytokines that mediate inflammatory responses in the brain. The fluorescence intensity of Iba-1 was markedly decreased after treatment with 8-OaS for 14 days. This finding suggests that 8-OaS reduced the activation of immune cells in the brain after CFA challenge.
Fig. 4.
The effects of 8-OaS on inflammation in the mouse BLA during CFA-induced chronic inflammatory pain. a After 14 days, the brain slices containing BLA were immunostained with microglial marker Iba-1 antibody (red), and nuclei were stained with Hoechst33258 (blue). Scale bar = 20 μm. b 8-OaS inhibited the activation of microglia in the BLA of CFA-injected mice and had a dose-dependent effect. 8-OaS decreased the elevated levels of c IL-1β, d IL-6, and e TNF-a in the BLA on day 14 after CFA injection. Each value represents the mean ± SEM of three independent experiments (n = 6, *p < 0.05, **p < 0.01 versus the control group; #p < 0.05, ##p < 0.01 versus the CFA-injected group)
To further identify the roles of 8-OaS on inflammation, the concentrations of inflammatory cytokines in the BLA were analyzed by an enzyme-linked immunosorbent assay. Concentrations of pro-inflammatory cytokines, such as IL-1β (6.065 ± 0.353 pg/mL), IL-6 (11.55 ± 0.504 pg/mL), and TNF-α (59.722 ± 2.945 pg/mL), were upregulated after CFA injection. However, 8-OaS treatment reversed these results, as our data for IL-1β (4.826 ± 0.121, 3.820 ± 0.203, 3.101 ± 0.214 pg/mL), IL-6 (9.573 ± 0.221, 7.516 ± 0.279, 7.426 ± 0.223 pg/mL), and TNF-a (30.833 ± 1.734, 16.611 ± 1.732, 15.278 ± 1.904 pg/mL) show (Fig. 4c, d). These data suggest that 8-OaS plays an effective role in anti-inflammation, which may be one of the ways in which it reduces chronic inflammatory pain.
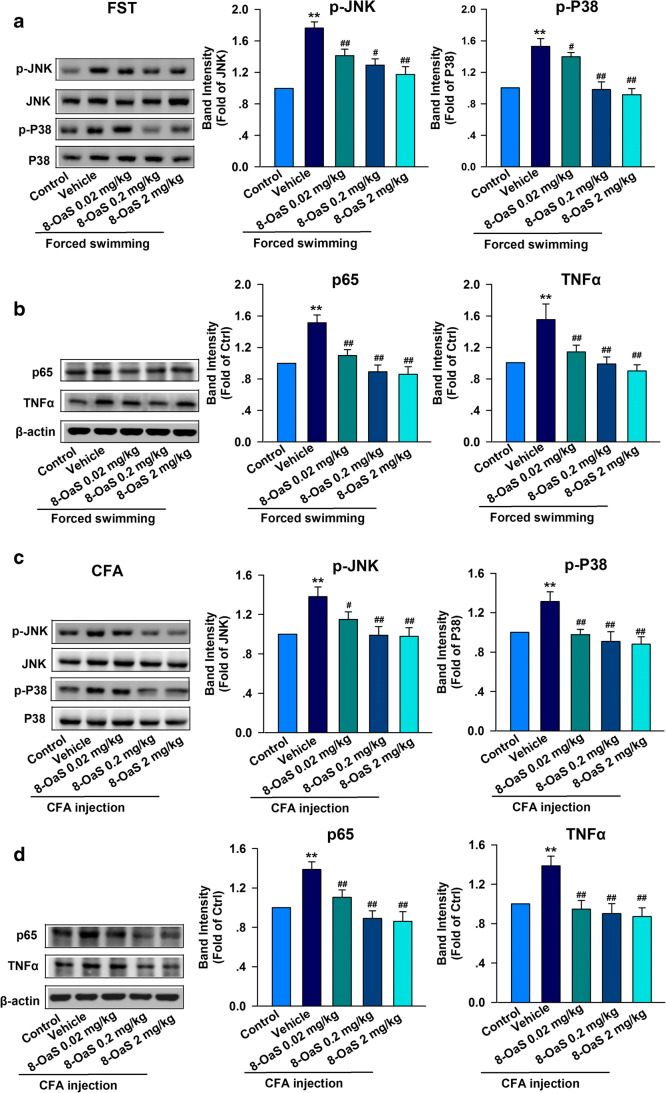
Effect of 8-OaS on Inflammation Signaling Pathways in Different Anxiety Models
The inflammation signaling pathway was activated upon CFA stimulation and acute stress, and we further studied which cytokines were responsible for this process. Western blotting was used to determine the expression levels of p-JNK, p-P38, p65, and TNF-α in the BLA. In the FSS model, the expression levels of p-JNK, p-P38 (Fig. 5a), p65, and TNF-α (Fig. 5b) were significantly enhanced in the BLA of mice after acute stress, compared with those in the control group. 8-OaS treatment resulted in the upregulation of p-JNK, p-P38 (Fig. 5a), p65, and TNF-α (Fig. 5b) expression.
In the CFA model, compared with the control group, hind paw CFA injection resulted in the significant induction of p-JNK, p-P38 (Fig. 5c), p65, and TNF-α (Fig. 5d) proteins in the BLA, while 8-OaS administration for 2 weeks reduced the levels of p-JNK, p-P38 (Fig. 5c), p65, and TNF-α (Fig. 5d) relative to the control group. This suggests that 8-OaS is effective against inflammation, which here resulted in the alleviation of different anxiety models, including a CFA-induced chronic inflammatory pain model and an FSS-induced acute pain model.
Discussion
In this study, we show that 8-OaS exhibits anxiolytic effects by using acute stress–induced and chronic inflammatory pain–induced models. Glutamatergic/GABAergic synaptic dysfunction caused by forced swimming was eliminated by 8-OaS. In addition, pro-inflammatory cytokine release and microglia activation were significantly attenuated by 8-OaS administration after CFA injection. Finally, p-JNK and p-P38 were involved in both the acute stress–induced and the chronic inflammatory pain–induced model. This suggests that the anxiolytic effect of 8-OaS was likely mediated by inhibiting the JNK/P38 MAPK pathway.
The L. rotata aqueous extract for oral administration was approved by the Food and Drug Administration of China (Beijing) in 1989 for pain therapy, and 8-OaS was considered to be the main effective ingredient, partially blocking formalin-induced tonic hyperalgesia as well as peripheral nerve injury–induced and bone cancer–induced mechanical allodynia (Zhu et al. 2014). Recently, it has been further demonstrated that 8-OaS alleviates neuropathic pain by inhibiting inflammatory responses in the spinal dorsal horn (Zhang et al. 2018). Numerous studies have provided evidence that the impact of a variety of inflammatory stimuli is related to mood and anxiety-related disorders. Clinical research also revealed that biomarkers of inflammation, such as inflammatory cytokines and acute-phase proteins, are reliably increased in a proportion of patients with major depressive disorder (MDD), bipolar disorder, anxiety disorders, and posttraumatic stress disorder (PTSD) (Felger 2018). Similar to that in analgesic experiments, we found that 8-OaS alleviated CFA-induced chronic inflammatory pain accompanying anxiety-like behaviors in mice. The activation of glial cells leads to the occurrence of neuroinflammation, which is mainly due to the early activation of microglia and the long-term activation of astrocytes. In addition, microglial injury may lead to depression, and drugs that inhibit microglial activation, such as minocycline and tumor necrosis factor alpha (TNF-α) inhibitors, are also considered effective antidepressants (Miller and Raison 2015; Yirmiya et al. 2015). In addition, microglia release and respond to several cytokines, including IL-1, IL-6, TNF-a, and IFN-c, which contribute to the maintenance of persistent pain states in autoimmune inflammation of the nervous system (Lee 2020; Santos et al. 2019). In this study, we observed that the upregulation of Iba-1 expression and the release of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) in the BLA after CFA injection were decreased by 8-OaS, suggesting that the anxiolytic actions of 8-OaS are linked to the inactivation of microglia.
Anxiety is a general neurobehavioral correlate of various stressors, and both acute and chronic stress exposure could precipitate anxiety disorders (Chrousos 2009). In our study, forced swimming was used as acute stress to induce anxiety-like behaviors, which were reflected in behavioral changes in the OFT and EPM. Such stress responses can influence the immune system via microglial elimination/repopulation (Ray et al. 2017). Additionally, complex neurotransmitter networks provide a possible link between anxiogenesis and immunomodulation during stress. Our group previously demonstrated that glutamatergic and GABAergic systems were disrupted in a stress-induced model (Tian et al. 2013), where we used HPLC to examine glutamate and GABA concentrations and found increased glutamate and decreased GABA content in the BLA of FSS-treated mice, which was however reversed by 2 mg/kg 8-OaS. The excitatory and inhibitory (E/I) transmission balance is crucial for normal functioning of the brain, and enhanced excitatory or reduced inhibitory transmission can result in hyperexcitation and promote anxiety-like behaviors (Wu et al. 2008). It has also been reported that tonic GABAergic reduction is induced by social isolation stress (Matsumoto et al. 2007; Tian et al. 2013). Additionally, NMDA receptors, which are critical excitatory postsynaptic receptors, consist of three subunits: GluN1, GluN2, and GluN3 (Chen et al. 2019; Jeyifous et al. 2016). GluN2A and GluN2B are the most common NMDAR subtypes and play a significant role in the mammalian CNS, showing enhanced activity attributable to neurotransmitter hyperexcitability, a condition that is related to increased anxiety (Prager et al. 2014). PSD95 is a postsynaptic anchor protein that binds to NMDA and AMPA receptors (Jeyifous et al. 2016). Among the three subtypes of GABA receptors (GABAA, GABAB, and GABAC subtypes), GABAA receptors are typical ligand-gated ion channels that play the most important role in GABAergic inhibitory function, whereas subunits of GABAA receptors—GABAAα2 and GABAAγ2—mediate anxiety in the BLA (Jeyifous et al. 2016; Möhler 2012). Consistent with these earlier findings, we found that the levels of the crucial postsynaptic proteins GluN2A, GluN2B, and PSD95 were enhanced, whereas the expression of GABAA-α2 and GABAA-γ2 receptors decreased in the BLA of mice with stress-induced anxiety. Furthermore, the increase in these receptors was reversed in the BLA of stressed animals after administration of 2 mg/kg 8-OaS. After FSS exposure, both neurotransmitters and postsynaptic receptors showed changes in excitation/inhibition signaling. This shows that, under acute stress, 8-OaS can improve anxiety-like behaviors by modifying the excitation/inhibition balance between neuronal signals.
TNF-α is a proinflammatory cytokine and a well-characterized indicator of neuroimmune inflammation. Besides, TNF-α regulates the trafficking of the AMPA receptor (AMPAR) on the synapse, by driving the rapid exocytosis on hippocampal pyramidal cells (Beattie et al. 2002; Ogoshi et al. 2005) and the endocytosis on the GABAergic MSNs of the striatum (Lewitus et al. 2014). Subsequent work has revealed that TNF-α can drive the simultaneous endocytosis of GABA-A receptors (GABARs), leading to a substantial shift in the excitation-to-inhibition (E/I) balance (Stellwagen et al. 2005). In our two anxiety models, TNF-α expression in the BLA was increased in both the forced swimming and the CFA injection condition. This suggests that there is a similar anxiety mechanism underlying the imbalance between neuroinflammation and synaptic transmission (Renna et al. 2018; ML et al. 2016; Ren and Dubner 2008; Kim et al. 2019; Zheng et al. 2011). Other studies have reported that L. rotata can promote microglia to secrete β-endorphin, with p38 MAPK signaling as a key linkage (Fan et al. 2016; Zhu et al. 2014). Here, we also found that the phosphorylation levels of p38 and JNK, part of the mitogen-activated protein kinase (MAPK) family, were markedly enhanced after chronic inflammation pain or acute stress. Fittingly, 8-OaS inhibited this signaling pathway in both anxiety models, resulting in the decreased expression of NF-κB p65 and TNF-α. Therefore, 8-OaS exhibited anxiolytic effects by inhibiting the JNK/P38 MAPK pathway, reducing the inflammatory response mediated by microglia activation, and altering synaptic function due to changes in neurotransmitter levels. However, the relationship between these processes is not clear, and further study is needed for clarification.
In conclusion, our findings demonstrate that 8-OaS alleviates anxiety-like behaviors in animal models of acute and chronic anxiety by regulating MAPK signaling pathways involved in anti-inflammatory activities and restoring the E/I balance. This study describes a new pharmacological effect of 8-OaS, thereby providing a theoretical basis for further clinical research on anxiolytic. However, other pathways may be involved in the anxiolytic activities of 8-OaS, and further experiments are needed to verify and expand the current findings.
Authors’ Contributions
ST, YQ, and ZMG designed the study. TQQ, WWJ, and LQQ carried out the experiments, and ST and LL analyzed the data. YL and ZK drafted the manuscript and verified the important intellectual content. All authors critically read and revised the manuscript and approved the final version of the manuscript for submission.
Funding Information
This work was supported in part by research grants from the National Natural Science Foundation of China (no. 81801079 to TS, no. 81870893 to QY) and from the National Postdoctoral Program for Innovative Talents (no. BX20160025 to QY).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
The experimental protocols were approved by the Animal Care Committee of the Fourth Military Medical University (FMMU). This article does not contain any studies with human participants performed by any of the authors.
Disclaimer
The funding sources played no role in the study design, the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ting Sun and Li Luo contributed equally to this work.
Contributor Information
Ming-Gao Zhao, Email: minggao@fmmu.edu.cn.
Qi Yang, Email: yangqifmmu@126.com.
References
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859295/5563/2282. [DOI] [PubMed] [Google Scholar]
- Beery AK, Kaufer D. Stress, social behavior, and resilience: insights from rodents. Neurobiol Stress. 2015;1:116–127. doi: 10.1016/j.ynstr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen S-R, Chen H, Zhang J, Pan H-L. Increased α2δ-1-NMDA receptor coupling potentiates glutamatergic input to spinal dorsal horn neurons in chemotherapy-induced neuropathic pain. J Neurochem. 2019;148:252–274. doi: 10.1111/jnc.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Du K, Lu W, Sun Y, Feng J, Wang JH. mRNA and miRNA profiles in the nucleus accumbens are related to fear memory and anxiety induced by physical or psychological stress. J Psychiatr Res. 2019;118:44–65. doi: 10.1016/j.jpsychires.2019.08.013. [DOI] [PubMed] [Google Scholar]
- Fan H, Li TF, Gong N, Wang YX. Shanzhiside methylester, the principle effective iridoid glycoside from the analgesic herb Lamiophlomis rotata, reduces neuropathic pain by stimulating spinal microglial beta-endorphin expression. Neuropharmacology. 2016;101:98–109. doi: 10.1016/j.neuropharm.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Felger JC. Imaging the role of inflammation in mood and anxiety-related disorders. Curr Neuropharmacol. 2018;16:533–558. doi: 10.2174/1570159X15666171123201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D, Siddiqui F, Fish J, Charlat M, Chaudry E, et al. Mesenchymal stromal cells modulate peripheral stress-induced innate immune activation indirectly limiting the emergence of neuroinflammation-driven depressive and anxiety-like behaviors. Biol Psychiatry. 2019;86:712–724. doi: 10.1016/j.biopsych.2019.07.015. [DOI] [PubMed] [Google Scholar]
- Jeyifous O, Lin EI, Chen X, Antinone SE, Mastro R, et al. Palmitoylation regulates glutamate receptor distributions in postsynaptic densities through control of PSD95 conformation and orientation. Proc Natl Acad Sci U S A. 2016;113:E8482–E8491. doi: 10.1073/pnas.1612963113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang WL, Fu FH, Zheng SG, Zhang DL, Zhu HB, Jian H. 8-O-acetyl shanzhiside methylester attenuates apoptosis and ameliorates mitochondrial energy metabolism in rat cortical neurons exposed to oxygen-glucose deprivation. Eur J Pharmacol. 2010;629:20–24. doi: 10.1016/j.ejphar.2009.11.065. [DOI] [PubMed] [Google Scholar]
- Jiang WL, Zhang SP, Zhu HB, Hou J. Effect of 8-O-acetyl shanzhiside methylester increases angiogenesis and improves functional recovery after stroke. Basic Clin Pharmacol Toxicol. 2011;108:21–27. doi: 10.1111/j.1742-7843.2010.00620.x. [DOI] [PubMed] [Google Scholar]
- Kim YK, Amidfar M, Won E. A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;91:103–112. doi: 10.1016/j.pnpbp.2018.06.008. [DOI] [PubMed] [Google Scholar]
- La M, Zhang F, Gao S, Liu X, Wu Z, et al. Constituent analysis and quality control of Lamiophlomis rotata by LC-TOF/MS and HPLC-UV. J Pharm Biomed Anal. 2015;102:366–376. doi: 10.1016/j.jpba.2014.09.038. [DOI] [PubMed] [Google Scholar]
- Lee STH. Inflammation, depression, and anxiety disorder: a population-based study examining the association between Interleukin-6 and the experiencing of depressive and anxiety symptoms. Psychiatry Res. 2020;285:112809. doi: 10.1016/j.psychres.2020.112809. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Pribiag H, Duseja R, St-Hilaire M, Stellwagen D. An adaptive role of TNFalpha in the regulation of striatal synapses. J Neurosci. 2014;34:6146–6155. doi: 10.1523/JNEUROSCI.3481-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S-b, Zhang N, Guo Y-y, Zhao R, Shi T-y, et al. G-protein-coupled receptor 30 mediates rapid neuroprotective effects of estrogen via depression of NR2B-containing NMDA receptors. J Neurosci Off J Soc Neurosci. 2012;32:4887–4900. doi: 10.1523/JNEUROSCI.5828-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91:617–624. [PubMed] [Google Scholar]
- Lucchetti G, Peres MF, Lucchetti AL, Mercante JP, Guendler VZ, Zukerman E. Generalized anxiety disorder, subthreshold anxiety and anxiety symptoms in primary headache. Psychiatry Clin Neurosci. 2013;67:41–49. doi: 10.1111/j.1440-1819.2012.02405.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Puia G, Dong E, Pinna G. GABA(A) receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress. 2007;10:3–12. doi: 10.1080/10253890701200997. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL. Are anti-inflammatory therapies viable treatments for psychiatric disorders?: where the rubber meets the road. JAMA psychiatry. 2015;72:527–528. doi: 10.1001/jamapsychiatry.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ML W, A I, MD L, CA M, L L, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21:797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- Ogoshi F, Yin HZ, Kuppumbatti Y, Song B, Amindari S, Weiss JH. Tumor necrosis-factor-alpha (TNF-alpha) induces rapid insertion of Ca2+-permeable alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate (Ca-A/K) channels in a subset of hippocampal pyramidal neurons. Exp Neurol. 2005;193:384–393. doi: 10.1016/j.expneurol.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Prager EM, Pidoplichko VI, Aroniadou-Anderjaska V, Apland JP, Braga MFM. Pathophysiological mechanisms underlying increased anxiety after soman exposure: reduced GABAergic inhibition in the basolateral amygdala. Neurotoxicology. 2014;44:335–343. doi: 10.1016/j.neuro.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Gulati K, Rai N. Stress, anxiety, and immunomodulation: a pharmacological analysis. Vitam Horm. 2017;103:1–25. doi: 10.1016/bs.vh.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna ME, O'Toole MS, Spaeth PE, Lekander M, Mennin DS. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: A systematic review and meta-analysis. Depress Anxiety. 2018;35:1081–1094. doi: 10.1002/da.22790. [DOI] [PubMed] [Google Scholar]
- Santos P, Herrmann AP, Elisabetsky E, Piato A. Anxiolytic properties of compounds that counteract oxidative stress, neuroinflammation, and glutamatergic dysfunction: a review. Braz J Psychiatry. 2019;41:168–178. doi: 10.1590/1516-4446-2018-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang X, Wang J, Li M, Miao X, Pan H, Yang Y, Wang Y. Antinociceptive and anti-inflammatory activities of Phlomis umbrosa Turcz extract. Fitoterapia. 2011;82:716–721. doi: 10.1016/j.fitote.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc Natl Acad Sci U S A. 2009;106:9854–9859. doi: 10.1073/pnas.0903546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci Off J Soc Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Wang J, Li X, Li YJ, Feng D, et al. Gastrodin relieved complete Freund’s adjuvant-induced spontaneous pain by inhibiting inflammatory response. Int Immunopharmacol. 2016;41:66–73. doi: 10.1016/j.intimp.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Sun T, Li YJ, Tian QQ, Wu Q, Feng D, et al. Activation of liver X receptor beta-enhancing neurogenesis ameliorates cognitive impairment induced by chronic cerebral hypoperfusion. Exp Neurol. 2018;304:21–29. doi: 10.1016/j.expneurol.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, et al. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci Off J Soc Neurosci. 2010;30:6282–6290. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakare VN, Dhakane VD, Patel BM. Attenuation of acute restraint stress-induced depressive like behavior and hippocampal alterations with protocatechuic acid treatment in mice. Metab Brain Dis. 2017;32:401–413. doi: 10.1007/s11011-016-9922-y. [DOI] [PubMed] [Google Scholar]
- Tian Z, Wang Y, Zhang N, Guo YY, Feng B, Liu SB, Zhao MG. Estrogen receptor GPR30 exerts anxiolytic effects by maintaining the balance between GABAergic and glutamatergic transmission in the basolateral amygdala of ovariectomized mice after stress. Psychoneuroendocrinology. 2013;38:2218–2233. doi: 10.1016/j.psyneuen.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Wang DS, Tian Z, Guo YY, Guo HL, Kang WB, et al. Anxiolytic-like effects of translocator protein (TSPO) ligand ZBD-2 in an animal model of chronic pain. Mol Pain. 2015;11:16. doi: 10.1186/s12990-015-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang J, Yang L, Zhou SM, Guan SY, et al. Effect of Praeruptorin C on 3-nitropropionic acid induced Huntington’s disease-like symptoms in mice. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2017;86:81–87. doi: 10.1016/j.biopha.2016.11.111. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Kim SS, Zhuo M. Molecular targets of anxiety: from membrane to nucleus. Neurochem Res. 2008;33:1925–1932. doi: 10.1007/s11064-008-9679-8. [DOI] [PubMed] [Google Scholar]
- Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by lumbar 5 ventral root transection in rat. Pain. 2006;123:306–321. doi: 10.1016/j.pain.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang M, Guo YY, Sun T, Li YJ et al (2016) Systemic inflammation induces anxiety disorder through CXCL12/CXCR4 pathway. Brain Behav Immun. 10.1016/j.bbi.2016.03.001 [DOI] [PubMed]
- Yi JH, Huang XP, Chen Y, Luo ZY, Zhong CC. Studies on the iridoid glucosides of the root of Lamiophlomis rotata (benth.) kudo, a medicinal plant in Xi zang (Tibet) Yao xue xue bao = Acta Pharm Sin. 1997;32:357–360. [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–658. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Zhang W, Bai Y, Qiao Y, Wang J, Li MY, et al. 8-O-acetyl shanzhiside methylester from Lamiophlomis rotata reduces neuropathic pain by inhibiting the ERK/TNF-alpha pathway in spinal astrocytes. Front Cell Neurosci. 2018;12:54. doi: 10.3389/fncel.2018.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Ouyang H, Zheng X, Liu S, Mata M, Fink DJ, Hao S. Glial TNFalpha in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Mol Pain. 2011;7:40. doi: 10.1186/1744-8069-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Gong N, Fan H, Peng CS, Ding XJ, Jiang Y, Wang YX. Lamiophlomis rotata, an orally available Tibetan herbal painkiller, specifically reduces pain hypersensitivity states through the activation of spinal glucagon-like peptide-1 receptors. Anesthesiology. 2014;121:835–851. doi: 10.1097/aln.0000000000000320. [DOI] [PubMed] [Google Scholar]