Introduction
Medical education must prepare clinicians to care for diverse populations,1,2 which is especially important in dermatology, as cutaneous disorders present differently depending on skin pigmentation.3,4 Patients with nonwhite skin tones are underrepresented in the dermatologic literature and broader educational resources,5, 6, 7 a deficiency that is particularly problematic for rare disorders like autoimmune bullous diseases (AIBD).8 We present a case series of AIBD in patients with nonwhite skin tones, emphasizing differences in disease presentation and pigmentary sequelae.3,4
Case 1
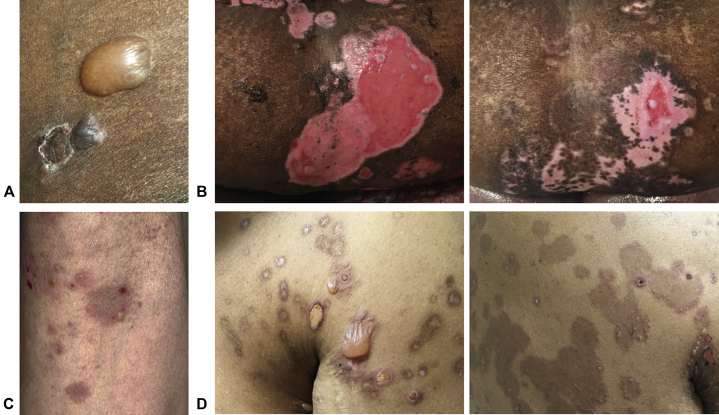
An 82-year-old African-American woman presented with a painful blistering eruption that began on her hands, abdomen, and extremities without mucosal erosions. Physical examination found tense bullae on the extremities and trunk lacking appreciable erythema (Fig 1, A) and large abdominal erosions (Fig 1, B, left). Histopathology, immunofluorescence, and enzyme-linked immunosorbent assay (ELISA) confirmed a diagnosis of bullous pemphigoid (BP) (Table I). The patient was treated with clobetasol cream, a tapering course of prednisone, and mycophenolate. After 2 months, she achieved near-total healing of erosions but had residual hypopigmentation and perifollicular repigmentation (Fig 1, B, right).
Fig 1.
Bullous pemphigoid. A, From case 1, a tense bulla on the thigh lacked the surrounding erythema typically seen in bullous pemphigoid. B, From case 1, (left) blistering of the epidermis on the abdomen produced large pink erosions lacking pigmentation; (right) after 2 months of treatment, the abdominal erosions were nearly fully re-epithelialized, but with significant hypopigmentation and a notable perifollicular pattern of repigmentation. C, From case 2, several urticarial plaques with peripheral erythema, vesiculation, and central hyperpigmentation were noted on the arm. D, From case 3, (left) hyperpigmented patches with central erosions were present on the chest and shoulder along with a few tense, fluid-filled bullae; (right) numerous hyperpigmented patches with peripheral vesiculation were noted on the back.
Table I.
Case summaries
| Case | Age, race & sex | Skin biopsy findings | DIF (location) | IIF (titer) | ELISA∗ | Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 82-year-old African-American woman | Subepidermal blister with eosinophils and neutrophils in the blister cavity and dermal pigment incontinence | Positive (BMZ) | Positive (1:2048) | BP180: 60 BP230: 130 | Bullous pemphigoid |
| 2 | 62-year-old Filipino man | Subepidermal blister with numerous eosinophils in the blister cavity | Positive (BMZ) | Negative | BP180: 197 BP230: 10 | Bullous pemphigoid |
| 3 | 63-year-old African-American man | Subepidermal blister containing eosinophils and neutrophils | Positive (BMZ) | Positive (1:4) | BP180: 182 BP230: 1 | Bullous pemphigoid |
| 4 | 50-year-old African-American man | Subcorneal blister formation with acantholysis | Positive (cell surface) | Positive (1:128) | Dsg1: 144 Dsg3: 104 | Pemphigus foliaceus† |
| 5 | 39-year-old Peruvian woman | Superficial epidermal acantholysis | Positive (cell surface) | Positive (1:32) | Dsg1: 206 Dsg3: 3 | Pemphigus foliaceus |
| 6 | 25-year-old Dominican man | Granular layer epidermal acantholysis and dermal pigment incontinence | Positive (cell surface) | Positive (1:16) | Dsg1: 221 Dsg3: 2 | Pemphigus foliaceus |
| 7 | 58-year-old Indian man | Suprabasal acantholysis and tombstoning of basal keratinocytes | Positive (cell surface) | Positive (1:64) | Dsg1: 162 Dsg3: 103 | Pemphigus vulgaris |
| 8 | 51-year-old Cambodian woman | Suprabasal epidermal acantholysis | Positive (cell surface) | Positive (1:8) | Dsg1: 49 Dsg3: 79 | Pemphigus vulgaris |
| 9 | 62-year-old Indian woman | Suprabasal epidermal acantholysis | Negative | Positive (1:128) | Dsg1: 184 Dsg3: 181 | Pemphigus vulgaris |
BMZ, Basement membrane zone; DIF, direct immunofluorescence; IIF, indirect immunofluorescence.
Positive cutoff is >9 for anti-BP180 and anti-BP230, >36 for anti-Dsg1, >37 for anti-Dsg3.
The patient never had any mucosal erosions, consistent with a clinical diagnosis of pemphigus foliaceus despite positivity for anti-Dsg3 by ELISA.
Case 2
A 62-year-old Filipino man with a hematopoietic stem cell transplant for chronic leukemia presented with painful oral erosions and a pruritic blistering eruption on the trunk and extremities when tapering oral tacrolimus, used for graft-versus-host disease (GVHD) prophylaxis. Physical examination found urticarial plaques on the arms and trunk with peripheral vesicles and central hyperpigmentation (Fig 1, C); shallow erosions were noted on the buccal mucosa. The differential diagnosis included GVHD or coincident AIBD. Biopsy, immunofluorescence, and ELISA were consistent with BP (Table I). The patient was treated with betamethasone ointment, dexamethasone mouthwash, doxycycline, and a tapering course of prednisone with rapid improvement. In consultation with his oncologist, mycophenolate was initiated, which sustained clinical remission, but postinflammatory hyperpigmentation remained after 3 months.
Case 3
A 63-year-old African-American man with psoriasis treated with secukinumab for 3 years presented with a new pruritic blistering eruption, oral erosions, and odynophagia. Physical examination found erosions and tense bullae on the trunk and extremities (Fig 1, D, left); shallow oral erosions were noted on the buccal mucosa. Histopathology, immunofluorescence, and ELISA confirmed a diagnosis of BP (Table I). Secukinumab was halted, and treatment with doxycycline and triamcinolone ointment was initiated but was unsuccessful. Subsequently, the patient was treated with prednisone and mycophenolate, which induced remission. After his erosions healed, postinflammatory hyperpigmentation developed, which lasted more than 1 year (Fig 1, D, right).
Case 4
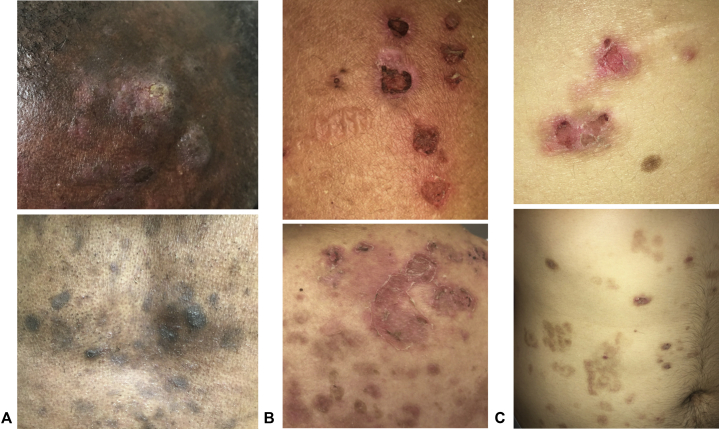
A 50-year-old African-American man with treated chronic hepatitis B virus (HBV) infection and latent tuberculosis presented with painful crusted lesions on the scalp, face, and trunk without mucosal erosions. Physical examination found eroded and crusted plaques and hyperpigmented patches on the scalp, face, trunk, and arms (Fig 2, A, upper); no oral or ocular erosions were noted. Histopathology, immunofluorescence, and ELISA (Table I), along with absent mucosal erosions, were most consistent with pemphigus foliaceus (PF) despite anti-Dsg3 antibodies, which can be nonpathogenic. The patient was treated with triamcinolone ointment, a tapering course of prednisone, and intralesional triamcinolone for recalcitrant scalp lesions. He experienced mild flares every few weeks controlled with triamcinolone. However, he had persistent postinflammatory hyperpigmentation at sites of healed lesions (Fig 2, A, lower).
Fig 2.
Pemphigus foliaceus. A, From case 4, (top) several hypo- and hyperpigmented plaques were noted on the temple and scalp, some with superficial erosion and crusting; (bottom) after treatment, many hyperpigmented patches and a few crusted papules were noted on the chest. B, From case 5, several eroded and crusted erythematous papules and flaccid bullae were noted on the arm; (bottom) several erythematous patches with superficial desquamation were present on the upper back along with many hyperpigmented patches. C, From case 6, (top) eroded and crusted papules with peripheral hyperpigmentation were noted on the back; (bottom) many hyperpigmented patches were noted on the abdomen at sites of healed erosions.
Case 5
A 39-year-old Peruvian woman with hypothyroidism and pernicious anemia presented with painful, pruritic erosions and crusting of the scalp, face, trunk, and arms. She denied mucosal erosions. She had been treated with triamcinolone ointment and several 2-week courses of prednisone with improvement, but her eruption recurred with prednisone discontinuation. Physical examination found extensive scalp scaling and eroded and crusted papules on the face, trunk, and arms with peripheral hyperpigmentation and flaccid superficial bullae (Fig 2, B); no oral or ocular erosions were noted. Skin biopsy, immunofluorescence, and ELISA confirmed a diagnosis of PF (Table I). The patient re-started prednisone along with triamcinolone ointment with rapid improvement of erosions, but extensive postinflammatory hyperpigmentation developed. Because of the recalcitrant disease, she was treated with rituximab.
Case 6
A 25-year-old Dominican man presented with a pruritic peeling rash involving his scalp, face, trunk, and arms without mucosal erosions. Physical examination found crusted papules on the trunk and extremities with peripheral hyperpigmentation (Fig 2, C, upper); no oral or ocular erosions were found. Skin biopsy, immunofluorescence, and ELISA confirmed a diagnosis of PF (Table I). Despite multiple tapering courses of prednisone with minocycline and mycophenolate, his disease persisted. Treatment with rituximab induced remission off all other therapies. However, the patient had persistent postinflammatory hyperpigmentation (Fig 2, C, lower). The patient's disease recurred after 6 months and was successfully re-treated with rituximab.
Case 7
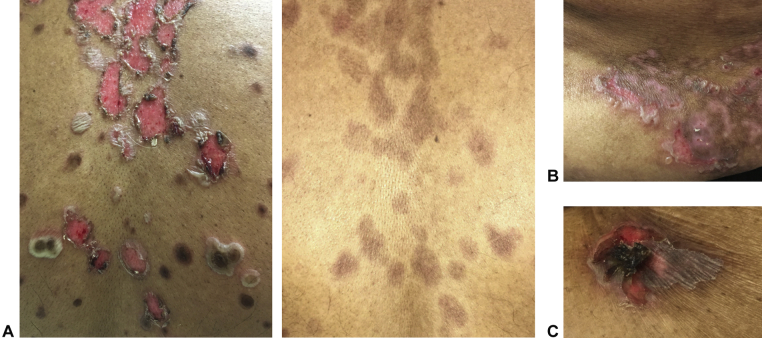
A 58-year-old Indian man presented with painful oral ulcers and a blistering eruption involving the head, neck, trunk, and extremities. Physical examination found flaccid bullae and large erosions with surrounding hyperpigmentation on the trunk (Fig 3, A, left) and shallow palatal erosions. Biopsy, immunofluorescence, and ELISA confirmed a diagnosis of pemphigus vulgaris (PV) (Table I). The patient was treated with betamethasone ointment and prednisone along with rituximab. He achieved clinical remission off prednisone, but had notable hyperpigmentation at sites of prior lesions (Fig 3, A, right). After 6 months, his disease recurred and re-treatment with rituximab again induced remission.
Fig 3.
Pemphigus vulgaris. A, From case 7, (left) several tense and flaccid bullae were noted on the back along with many erosions with a pink base and peripheral hyperpigmentation; (right) after treatment, all erosions on the back were fully healed, but significant hyperpigmented patches remained. B, From case 8, several tense and flaccid bullae and erosions were noted on the abdomen along with numerous hypo- and hyperpigmented patches. C, From case 9, a hyperpigmented plaque with erosion and hemorrhagic crusting was noted on the abdomen.
Case 8
A 51-year-old Cambodian woman with chronic HBV infection presented with blisters and erosions of the scalp, trunk, and extremities with painful oral erosions and odynophagia. Physical examination found flaccid bullae on the trunk and arms with peripheral hypo- and hyperpigmentation (Fig 3, B); erosions of the tongue were noted. Biopsy, immunofluorescence, and ELISA established a diagnosis of PV (Table I). The patient was initially treated with doxycycline and prednisone. Because of disease recurrence with prednisone tapering, she was treated with rituximab (plus entecavir prophylaxis for HBV) and achieved remission. Two years later, she had widespread erosions of the skin and tongue. She was treated with a tapering course of prednisone and rituximab, leading to healed erosions but persistent postinflammatory hypo- and hyperpigmentation.
Case 9
A 62-year-old Indian woman presented with oral erosions diagnosed clinically as oral lichen planus initially controlled with dexamethasone mouthwash and clobetasol gel. After several months, she noted new hyperpigmented plaques on her trunk and extremities, some of which had blistering and erosions. She also attributed a 25-pound weight loss to odynophagia. Physical examination found several eroded hyperpigmented plaques on the extremities and trunk (Fig 3, C); shallow erosions were present on the palate, buccal mucosa, and tongue. Biopsy, immunofluorescence, and ELISA confirmed a diagnosis of PV (Table I); given her weight loss, rat bladder indirect immunofluorescence was performed and did not find paraneoplastic pemphigus antibodies. The patient was treated with a tapering course of prednisone and rituximab. After a 2-year remission, new erosions developed on the skin and oral and vaginal mucosae. Re-treatment with rituximab induced remission, but she had postinflammatory hyperpigmentation at sites of healed erosions.
Discussion
Differences in pigmentation can affect the appearance of dermatologic diseases and contribute to diagnostic delay.3,4 In diseases like BP, most classic clinical images feature less pigmented skin and depict intense erythema within urticarial lesions or surrounding tense bullae. In cases 1 and 3, both African-Americans with BP, erythema was not visibly appreciated despite the typical eosinophil-rich infiltrate noted on pathology. Hyperpigmentation or a purplish hue of inflammatory lesions in darker skin tones can also be mistaken for diseases causing interface dermatitis, such as GVHD as in case 2 or lichen planus as in case 9.
Healing of erosions in AIBD can lead to long-lasting pigmentary changes despite achieving control of the acute blistering process, as seen in several of our cases. Deeper erosions as in case 1 can lead to loss of pigment, which may return in a perifollicular pattern as was described in a BP case series from India.9 Our patients with BP tended to have more dramatic pigmentary sequelae than with PV or PF, which may reflect the deeper level of blistering. Postinflammatory pigmentary changes in AIBD can be a source of distress for patients as a reminder of their disease and can take months or years to resolve, although advances have been made in treating dyspigmentation.10
Therapy for bullous diseases almost universally includes topical steroids; in fact, in one trial, aggressive use of topical corticosteroids achieved better disease control than oral steroids in BP.11 Importantly, potent corticosteroids can induce hypopigmentation, a side effect that can be more apparent in darker skin tones.12, 13, 14 As well, because many cases of AIBD involve the scalp, dermatologists must understand the diversity of hair types and hair care practices in patients of color, which affect topical vehicle preference.15
To ensure physicians and other clinicians are competent to diagnose and treat conditions in people with skin of color, increased diversity in clinical images used for training and continuing medical education is essential. Representing the full spectrum of skin tones can be challenging for rare disorders like AIBD. In using images such as those presented here, we advocate for preparing trainees and clinicians to care for patients of all skin tones to avoid deepening existing health care disparities in dermatology.
Footnotes
Funding sources: Dr Simpson is supported by grant NIHK08-AR075846.
Conflicts of interest: None disclosed.
IRB approval: not applicable.
References
- 1.Taylor S.C., Heath C. Cultural competence and unique concerns in patients with ethnic skin. J Drugs Dermatol. 2012;11(4):460–465. [PubMed] [Google Scholar]
- 2.McKesey J., Berger T.G., Lim H.W. Cultural competence for the 21st century dermatologist practicing in the United States. J Am Acad Dermatol. 2017;77(6):1159–1169. doi: 10.1016/j.jaad.2017.07.057. [DOI] [PubMed] [Google Scholar]
- 3.Kundu R.V., Patterson S. Dermatologic conditions in skin of color: part I. Special considerations for common skin disorders. Am Fam Physician. 2013;87(12):850–856. [PubMed] [Google Scholar]
- 4.Hadi A., Elbuluk N. Common conditions in skin of color. Semin Cutan Med Surg. 2016;35(4):184–190. doi: 10.12788/j.sder.2016.065. [DOI] [PubMed] [Google Scholar]
- 5.Ebede T., Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55(4):687–690. doi: 10.1016/j.jaad.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 6.Adelekun A., Onyekaba G., Lipoff J.B. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.04.084. [DOI] [PubMed] [Google Scholar]
- 7.Louie P., Wilkes R. Representations of race and skin tone in medical textbook imagery. Soc Sci Med. 2018;202:38–42. doi: 10.1016/j.socscimed.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Alpsoy E., Akman-Karakas A., Uzun S. Geographic variations in epidemiology of two autoimmune bullous diseases: pemphigus and bullous pemphigoid. Arch Dermatol Res. 2015;307(4):291–298. doi: 10.1007/s00403-014-1531-1. [DOI] [PubMed] [Google Scholar]
- 9.Kumaresan M., Srinivas C.R. Perifollicular pigmentation in bullous pemphigoid: a diagnostic sign. Indian Dermatol Online J. 2011;2(1):36–37. doi: 10.4103/2229-5178.79862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis E.C., Callender V.D. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3(7):20–31. [PMC free article] [PubMed] [Google Scholar]
- 11.Joly P., Roujeau J.C., Benichou J. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. 2002;346(5):321–327. doi: 10.1056/NEJMoa011592. [DOI] [PubMed] [Google Scholar]
- 12.Magri F., Iacovino C., Vittori J. Linear cutaneous hypopigmentation and atrophy associated with intralesional steroid injection:a rarely described adverse reaction. Dermatologic Therapy. 2019;32(9):e12941. doi: 10.1111/dth.12941. [DOI] [PubMed] [Google Scholar]
- 13.Fisher D.A. Adverse effects of topical corticosteroid use. West J Med. 1995;162(2):123–126. [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon H.H., Suh D.H. Linear extensions of hypopigmentation as a side effect of topical corticosteroid application. Int J Dermatol. 2016;55(5):e315–e317. doi: 10.1111/ijd.13154. [DOI] [PubMed] [Google Scholar]
- 15.Fisher E.J., Adams B.B. African American and Caucasian patients' vehicle preference for the scalp. J Am Acad Dermatol. 2008;58(2 Suppl):S46–S47. doi: 10.1016/j.jaad.2006.05.020. [DOI] [PubMed] [Google Scholar]