Highlights
-
•
Dose escalation to MRI-defined prostate lesions using SABR is feasible and safe.
-
•
After an extended follow up, rates of Grade 2 + GU and GI toxicities were low.
-
•
No biochemical relapse was observed.
-
•
10 minor dose variations were reported out of 80 ideal dose constraints.
Keywords: Intermediate- and high-risk, Prostate cancer, Radiotherapy, Stereotactic ablative body radiotherapy
Abstract
Introduction
Dose escalation to dominant intraprostatic lesions (DILs) is a novel method to increase the therapeutic ratio in localised prostate cancer. The Stereotactic Prostate Augmented Radiotherapy with Cyberknife (SPARC) trial was designed to determine the feasibility of a focal boost defined with multiparametric magnetic resonance imaging (mpMRI) using stereotactic ablative body radiotherapy (SABR).
Materials and methods
Patients were included with newly diagnosed intermediate to high risk prostate cancer with at least one of: Gleason score 4 + 3, stage T3a, or PSA > 20 ng/ml. Visible disease on mpMRI was mandatory and up to 2 separate nodules were allowed. All patients received androgen deprivation. Patients received 36.25 Gy in 5 fractions using CyberKnife® and the DIL received a simultaneous boost to a maximum of 47.5 Gy, as allowed by OAR constraints. Genitourinary (GU) and gastrointestinal (GI) toxicity was reported using the RTOG scoring criteria. International Index of Erectile Function (IIEF) and EQ-5D global health scores were regularly captured.
Results
An interim safety analysis was performed on the first 8 patients, recruited between July 2013 and December 2015. Median follow up was 56 months (range 50–74). Median D95 values for the prostate PTV and boost volume were 36.55 Gy (range 35.87–36.99) and 46.62 Gy (range 44.85–48.25) respectively. Of the dose constraints, 10/80 were not achieved but all were minor dose variations. Grade 2+ acute GU and GI toxicities were 37.5% respectively while grade 2+ late GU and GI toxicities were 12.5% and 0% respectively. IIEF and quality of life scores recovered over time and all patients remain in biochemical remission.
Conclusion
The first patients have been successfully treated with prostate SABR and focal boost on the SPARC trial, with excellent adherence to the planning protocol. Toxicity and efficacy results are promising and further recruitment is underway.
1. Introduction
In the treatment of localised prostate cancer, dose-escalated radiation therapy improves biochemical control at the expense of increased toxicity [1]. Stereotactic ablative body radiotherapy (SABR) is a technique that enables dose escalation and early randomised evidence suggests excellent tolerability [2]. The treatment of prostate cancer using extreme hypofractionation is guided by the likelihood of an improved therapeutic ratio between prostate cancer cell death and late rectal complications. Based on radiobiological and patient data, there is strong evidence that the alpha/beta ratio of prostate cancer may be as low as 1.4 Gy [3] while the alpha/beta ratio of the rectum is around 3 Gy [4]. Extreme hypofractionation may have a proportionally larger effect on prostate cancer cells than normal surrounding tissues.
The region at highest risk of recurrence has been shown to correspond to the dominant intraprostatic lesion (DIL) both clinically and radiologically [5], [6]. As this nodule often exhibits the most aggressive biological behaviour in multifocal disease, it can dictate the overall clinical prognosis [7]. Data supporting dose escalation to the DIL is growing in both external beam and brachytherapy literature [8], with randomised studies currently underway.
The Stereotactic Prostate Augmented Radiotherapy with Cyberknife (SPARC) trial was designed following an initial planning study at our institution [9]. The primary objective is to assess acute genitourinary (GU) toxicity. Secondary endpoints include acute gastrointestinal (GI) toxicity, late toxicity, dosimetric feasibility, quality of life and biochemical control. Here, we report an unplanned interim safety analysis for the first 8 patients treated on the SPARC trial, prior to further patient enrolment.
2. Materials and methods
In July 2013, we began a phase 2 study that was approved by the local Research Ethics Committee (Clinical Trials.gov ID: NCT02145494).
2.1. Patient eligibility
Patients were included with newly diagnosed and previously untreated prostate cancer, confirmed with a minimum of 10 biopsy cores. Patients must have at least one of: Gleason score 4 + 3, American Joint Committee on Cancer stage T3a, or prostate specific antigen (PSA) >20 ng/ml. The DIL was defined as any intraprostatic lesion likely to harbour clinically significant cancer on multiparametric magnetic resonance imaging (mpMRI). The likelihood of the presence of prostate cancer was determined based on an overall combination of the findings from T2WI, DWI, and DCE-MRI using a Likert scale between 1 and 5 (1, very low level of suspicion; 5, definitely cancer) [10]. Up to 2 separate nodules was allowed. All patients required a bone scan to exclude metastatic disease within 3 months of their initial staging CT and mpMRI. Patients were excluded if there were>2 DILs on mpMRI, disease visible in > 50% of the prostate volume on any axial slice, PSA > 40 ng/ml, T3b disease or greater, presence of nodal or distant metastases, contraindications to MRI, fiducials or ADT, any previous treatments for prostate cancer or any previous radiotherapy to the pelvis. Once enrolled, patients were commenced on androgen deprivation therapy (ADT), which included 4 weeks of cyproterone acetate followed by subcutaneous injections of a luteinizing hormone-releasing hormone (LHRH) analogue. The duration of ADT was at the discretion of the treating clinician. Arrangements were made for planning once the PSA dropped below 4 ng/ml.
2.2. Planning
Three to four gold seed fiducials were implanted at least 1 week prior to planning CT under antibiotic cover. A urinary catheter was inserted at time of planning and all patients had standardised bladder filling (300 mls at 45 min prior to planning CT scan). To adequately prepare the bowel, patients were prescribed Micolette® enemas for 2 days prior to and on the day of planning CT. The standardised 3 T mpMRI performed at enrolment (prior to ADT and fiducial insertion) was fused with the planning CT. Fusion was prioritised at the region of the prostate containing the DIL. Boost volumes were defined jointly by an oncologist and radiologist, with particular reference to the T2, diffusion (DWI) and perfusion (DCE) images. The MultiPlan® treatment planning system (Accuray inc, Sunnyvale, CA) was employed.
Our institutional margins for prostate SABR match those used in the current randomised PACE trial [11]:
GTV (n) = dominant nodule (s) on MRI
PTV (n) = GTV (no margin)
GTV(p) = Prostate and proximal 2/3 seminal vesicles (adapted at clinician discretion). This volume always entirely includes the GTV(n).
PTV (p) = CTV + 5 mm/3mm posteriorly
Constraints for organs at risk (OAR) were based on those currently used at our institution for standard (non-boost) prostate SABR and previously validated in a boost setting [9]. To ensure isotoxic dose escalation, OAR constraints were prioritised over boost volume coverage. These include:
-
•
Rectum: V18.1 Gy < 50%, V29 Gy < 20%, V36 Gy < 1 cc (optimal) and < 2 cc (mandatory).
-
•
Bladder V18.1 Gy < 40%, V37 Gy < 5 cc (optimal) and < 10 cc (mandatory)
-
•
Prostatic urethra: V42Gy < 50%, V45.6 Gy < 10%
-
•
Femoral head V14.5 Gy < 5%
-
•
Penile Bulb: V29.5 Gy < 50%
-
•
Bowel: V30 Gy < 1 cc, V18.1 Gy < 5 cc
Our planned cohort size is 20 patients, which confers 80% power to rule out >50% grade 2 + GU toxicity over the follow up period, assuming a true rate of toxicity <20% [12]. Statistical analysis to identify correlations between dosimetric and patient variables was unable to be performed in this interim analysis due to small sample size. A Gaussian distribution could not be assumed of any variable and hence descriptive statistics are presented as median and range where appropriate.
2.3. Treatment and follow up
All patients were followed prospectively. SABR was delivered using the CyberKnife® in 5 fractions delivered on alternate days. Patients received 36.25 Gy (7.25 Gy/fraction) and the DIL received a simultaneous isotoxic boost to a maximum of 47.5 Gy, as allowed by OAR constraints. Using an α/β ratio of 1.5 Gy, this corresponds to bioequivalent does of 212 Gy to the whole prostate and 348 Gy to the visible cancer. Patients could start treatment on any day of the week and all treatment was completed within 14 days of commencement. Dose was prescribed to cover ≥ 95% of PTV(p) and ≥ 95% of PTV(n). In addition, the prostate GTV was ideally covered by 40 Gy in 5 fractions such that GTV V40Gy ≥ 95%. 30–60 s intra-fraction monitoring was employed.
Patients were seen twice in the first month, every 3 months in the first two years, and 6 monthly thereafter. GU and GI toxicity was reported at each follow up visit using the Radiation Therapy Oncology Group (RTOG) scoring criteria and International Prostate Symptom Score (IPSS), along with PSA. International Index of Erectile Function (IIEF) and EQ-5D global health score [13] were captured at 6 month intervals. Biochemical failure was determined using the Phoenix definition [14].
3. Results
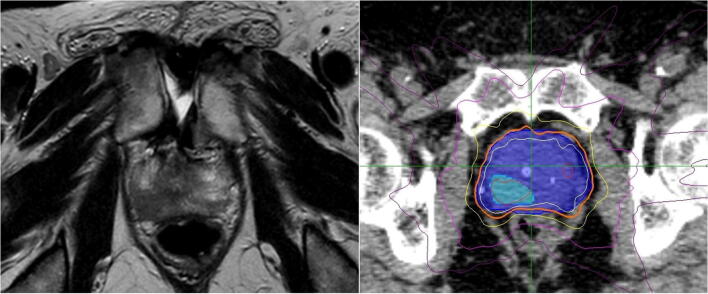
Between July 2013 and December 2015, 8 patients were recruited. Median follow up was 56 months (range 50–74) and median patient age was 75 (range 62–80). All but one patient had T3a disease and the majority of DILs were in the peripheral zone. Patients had either Gleason 3 + 4 or 4 + 3 disease (4 patients each). Median duration of ADT was 6 months (range 6–18). Patient characteristics are outlined in Table 1. Median predicted treatment time was 44 min (range 36–56). Median beam number was 209 (range 165–222) and median node number was 79 (range 45–88). An example of an MRI-defined DIL and corresponding radiotherapy plan is shown in Fig. 1.
Table 1.
Patient characteristics. AJCC = American Joint Committee on Cancer, PSA = prostate specific antigen, DIL = dominant intraprostatic lesion, GTV = gross tumour volume, PTV = planning target volume.
| Characteristic | Value (range) |
|---|---|
| Median age | 75 (62–80) |
| Median follow up | 56 months (50–74) |
| Gleason Score | |
| 3 + 4 | 4 |
| 4 + 3 | 4 |
| AJCC stage | |
| T2c N0 | 1 |
| T3a N0 | 7 |
| Median PSA | 7.4 ng/ml (4.7–10.8) |
| Median prostate volume (GTVp) | 40.7 cc (16.0–66.0) |
| DIL location | |
| Peripheral Zone | 5 |
| Transition Zone | 2 |
| Central Zone | 1 |
| Median DIL volume (GTVn) | 0.6 cc (0.3–3.5) |
| Median PTV and rectum overlap volume | 1.8 cc (1.3–2.9) |
| Median DIL distance to rectum | 3.6 mm (1.5–29.9) |
| Median DIL distance to urethra | 3.5 mm (1.2–15.2) |
| Median DIL distance to bladder neck | 17.7 mm (0.7–28.1) |
Fig. 1.
(a) T2-weighted MRI showing localized, low signal abnormality in the right peripheral zone. (b) PTVp shown as shaded dark blue and PTVn as shaded light blue. Isodose lines: 47.5 Gy (red), 40 Gy (white), 36.25 Gy (orange), 29 Gy (yellow), 18.1 Gy (magenta), 10 Gy (purple). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.1. Toxicity
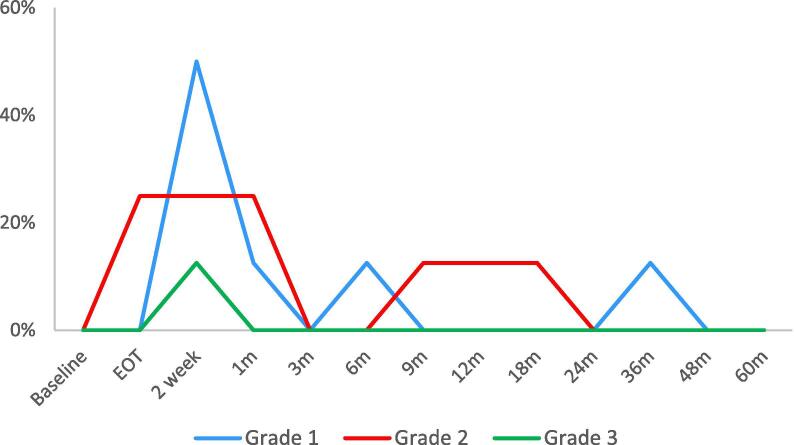
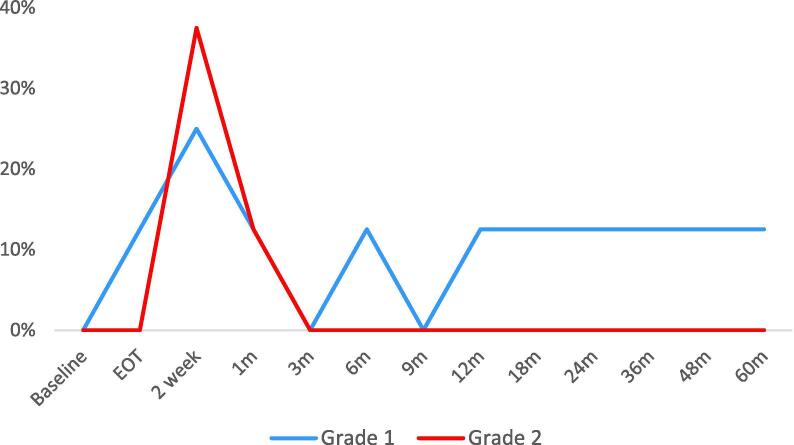
The median IPSS before treatment was 10.5 which increased in the acute phase, peaking at 16.5 2 weeks post treatment. IPSS returned to baseline after approximately 2 months and continued to drop below baseline values during follow up. GU toxicity corresponded with IPSS with a peak at 2 weeks post treatment (Fig. 2). The rate of grade 2+ acute GU toxicity was 37.5%. The patient who experienced grade 3 cystitis (haematuria) was referred for cystoscopy but no intervention was required. All acute GU toxicities resolved by 3 months. The rate of grade 2+ late GU toxicities was 12.5%. Similar to GU toxicity, the rates of GI toxicity peaked at 2 weeks post treatment (Fig. 3). 37.5% developed grade 2+ toxicities which were predominantly increased stool frequency. Only 1 patient developed late grade 1 toxicities. No grade 2+ late GI events were observed.
Fig. 2.
Genitourinary (GU) toxicity. EOT = end of treatment.
Fig. 3.
Gastrointestinal (GI) toxicity. EOT = end of treatment.
Only a small variation in erectile function was observed. Median baseline IIEF was 11.5 and nadired to 8 at 6 months post treatment. Interestingly, the median value recovered to pre-treatment levels at 12 months, with a slow decline thereafter. This decline could be a combination of treatment and age-related changes. No large impact on quality of life was observed via the EQ-5D global health score. At 3 months, the median health score had dropped from 90 to 80 but recovered to baseline values at 12 months. There was no obvious change in EQ-5D score during follow up.
3.2. PSA response
Median baseline PSA was 7.35 ng/ml (range 4.7–10.8). PSA dropped immediately due to the introduction of ADT, to a median value of 0.035 (range 0–1.8) at 12 months. All median PSA values remained below 0.2 after 6 months of follow up. No biochemical relapse was observed. A small rise in median PSA was observed at 18 months (0.105, range 0–1.1).
3.3. Dosimetry
Median prescription isodose was 70%. Median conformity index (the ratio of volume encompassed by the prescription isodose to the PTV volume) was 1.08. The 50% gradient index (the ratio of volume encompassed by the 50% isodose to the PTV volume) ranged between 3.22 and 4.65. Complete PTVp coverage was achieved in 6 patients (75%) while GTVp coverage was achievable in only 3 patients (37.5%). As expected, PTVn coverage was more difficult to achieve within the predefined OAR constraints. Only 3 plans (37.5%) achieved a D95 > 47.5 Gy. A summary of target volume coverage and OAR constraints is outlined in Table 2.
Table 2.
Treatment planning goals and achieved dosimetry, GTV = gross tumour volume, PTV = planning target volume.
| Structure | Criteria | Goal | Median delivered (range) |
|---|---|---|---|
| GTVp | D95 | 40 Gy | 39.16 Gy (37.32–40.69) |
| PTVp | D95 | 36.25 Gy | 36.55 Gy (35.87–36.99) |
| GTVn = PTVn | D95 | 47.5 Gy | 46.62 Gy (44.85–48.25) |
| Rectum | V18.1 Gy | <50% | 29.65% (25.0–36.7) |
| V29Gy | <20% | 12.10% (10.2–13.8) | |
| V36Gy | <1cc | 0.93 cc (0.6–1.96) | |
| Bladder | V18.1 Gy | <40% | 22.70% (9.7–43.2) |
| V37Gy | <5cc | 4.44 cc (1.74–6.98) | |
| Prostatic urethra | V42Gy | <50% | 30.5% (0.7–42.9) |
| V45.6 Gy | <10% | 0% (0–9) | |
| Left Femoral Head | V14.5 Gy | <5% | 0% (0–0.1) |
| Right Femoral Head | V14.5 Gy | <5% | 2.15% (0–15.2) |
| Penile Bulb | V29.5 Gy | <50% | 0% (0–7.7) |
| Bowel | V30Gy | <1cc | 0% (0–0.13) |
| V18.1 Gy | <5cc | 0% (0–2.98) |
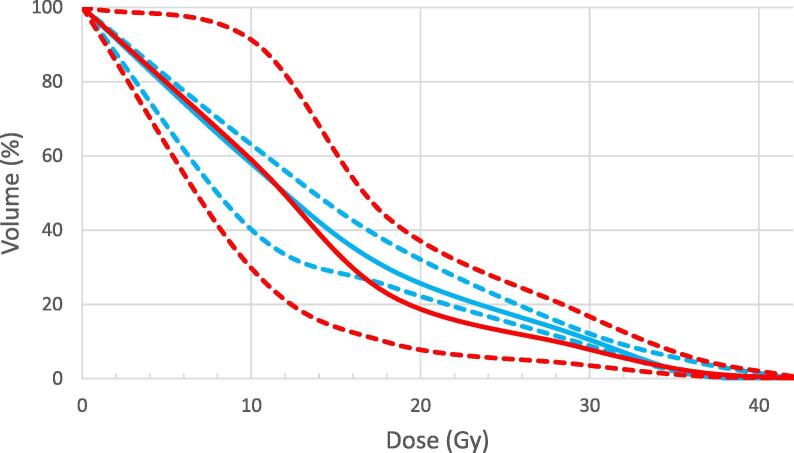
10/80 optimal OAR goals were unable to be met. 3 plans (patients 1, 2 and 5) had minor variations in maximum rectal dose (i.e. V36 = 1–2 cc). Factors possibly associated with rectal DVH variations, such as prostate volume, boost volume, PTV/rectum overlap and boost distance to rectum were investigated but no obvious association was found. Similarly, 3 plans (patients 1, 2 and 8) had minor protocol variations in maximum bladder dose (V37 = 5–10 cc) and no obvious correlative factors. Fig. 4 shows the median rectal and bladder dose-volume histogram (DVH) and ranges. The right femoral head constraint was exceeded in 3 patients (patients 1, 6 and 7). All urethral, penile and bowel constraints were achieved.
Fig. 4.
Dose-volume histogram for rectum (blue) and bladder (red). Median values shown as solid lines and range values as dotted lines. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The use of SABR to treat prostate cancer can be considered as an alternative to standard fractionation in patients with low risk prostate cancer [15] however the role of SABR in higher risk disease is not well defined. The feasibility of incorporating a simultaneous boost into SABR plans has been confirmed using both CyberKnife® and gantry-based linear accelerator platforms [16], [17], [18], [19]. These studies utilise robust image guidance to safely model DIL boosts of up to 55 Gy in 5 fractions within established OAR constraints. Multi-parametric MRI is the most common imaging modality used to guide focal boosts as it can detect high risk lesions in approximately 90% of cases when compared to prostatectomy specimens [20]. PSMA PET has also shown promising results in the evaluation of intraprostatic tumour burden, although the combination of both modalities seems to provide the most accurate localization [21]. Evidence to support dose escalation to the DIL is increasing, with the hypo-FLAME trial recently reporting favourable results [22]. A recent systematic review identified 22 trials of prostate radiation therapy with focal dose escalation to the intraprostatic dominant nodule [8], a term used interchangeably with DIL. Biochemical control was achieved in 80–100% of cases.
4.1. Toxicity in published studies
A systematic review of 2036 prostate SABR patients showed pooled late grade 3+ GU and GI toxicity to be 2% and 1% respectively [23]. Studies incorporating a DIL boost report slightly higher grade 3+ toxicity; between 2 and 6% for IMRT and brachytherapy boost techniques, and up to 10% for SABR boosts [8] but long term follow up is lacking.
Only a small number of centres have reported toxicity data for prostate cancer treated with SABR plus a DIL boost. Aluwini et al. [24] treated 50 patients with low and intermediate risk prostate cancer to a dose of 38 Gy in 4 daily fractions with an simultaneous boost to 44 Gy using CyberKnife®. EORTC/RTOG grade 2+ acute GU toxicity was 23% and grade 2+ acute GI toxicity was 14%. Herrera et al published dose-finding study of DIL boost whilst treating the whole prostate to 36.35 Gy in 5 fractions using CyberKnife® in intermediate and high risk prostate cancer [25]. Only 1 patient received concurrent ADT and all patients had rectal SpaceOAR insertion. Dose escalation to 50 Gy was found to be feasible with 25% grade 2+ acute GU toxicity and 5% grade 2+ acute GI toxicity. Toxicity results of the 5STAR trial was recently reported by Alayed et al [26]. 30 patients with intermediate or high risk prostate cancer received 35 Gy to the prostate, 25 Gy to the pelvis, and a DIL boost up to 50 Gy in 5 fractions. All patients received 12–18 months ADT. Grade 2+ acute GU toxicity was 66.7% and grade 2+ acute GI toxicity was 16.7%. Finally, the hypo-FLAME trial [22] treated 100 patients with intermediate and high risk prostate cancer with an isotoxic DIL boost to 50 Gy in 5 fractions. After a median follow up of 18 months, no grade 3+ toxicities have been reported.
Our median follow up is among the largest in current SABR boost literature and it is encouraging that the results still compare favourably in GU, GI, sexual and quality of life domains. Unfortunately, recruitment was slow due to the restricted commissioning of SABR on the NHS and direct comparison with other groups is difficult.
4.2. Dosimetry
Prior to institutional ethics approval, a feasibility study of 15 patients with DIL who had been treated with IMRT was performed at our institution [9]. Both Cyberknife® and RapidArc® plans were generated. Clinically acceptable boost plans of 47.5 Gy in 5 fractions were produced using both planning systems and the number of missed constraints was identical for both (11/75). The ideal rectal constraint of 1 cc < 36 Gy was most difficult to meet, as is the case in our initial patient experience. Similarly, maximum bladder dose (V37 Gy) was also difficult to achieve. All urethral, penile bulb and bowel constraints were met. Our first 2 patients had the most DVH protocol variations between them, which may reflect the learning curve with the new technique. Patient 1 had challenging anatomy with a small bladder fill (131 ml) as well as the biggest boost volume (3.5 cc) and rectum/PTV overlap (2.9 cc). In contrast, the majority of planning parameters for patient 2 were close to median. There were no obvious associations with target coverage or OAR goals. As expected, the majority of DIL locations were in the peripheral zone and so plan optimisation was often limited by proximity to anterior rectum and urethra.
Aluwini et al restricted 80% of the prescription dose to <1.5 cc for both bladder and rectum. Minor variations (80% PD to 1.5–2 cc) for both bladder and rectum were accepted in 30% of patients which was similar to our experience. Dosimetry reported by Macdonald et al. [27] showed excellent PTV and boost volume coverage within slightly more permissive planning goals (rectum and bladder D1cc < 38.06 Gy). Planning goals published by Herrera et al. [25] were similar to our study.
4.3. PSA response
No biochemical recurrence has been detected at this stage of follow up. The immediate and significant PSA drop can be attributed to the introduction of ADT however these levels were maintained after cessation of ADT. A small rise in median PSA was observed at 18 months but this did not meet the definition of PSA bounce. Benign PSA bounces are described in up to 30% of patients undergoing prostate SABR and are defined as a PSA rise of at least 0.2 ng/mL above its previous nadir with a subsequent decline to that nadir or lower [28]. This phenomenon can occur up to 30 months from treatment. In our cohort, the transient PSA rise could be due to the recovery of testosterone levels.
Given the novel approach of SABR boost, the majority of current evidence is tailored towards feasibility and toxicity rather than PSA response. However, early results suggest a 2 yr biochemical disease-free survival between 95 and 100% [24], [29].
5. Conclusion
The first patients have been successfully treated with prostate SABR and focal boost on the SPARC trial, with excellent adherence to the planning protocol. Target volume coverage was satisfactory whilst respecting the same OAR dose constraints used for conventional SABR at our institution. Maximum rectal and bladder doses were the most difficult constraint to achieve. Toxicity and efficacy results are promising and further recruitment is underway.
Author contributions
-
•
Guarantor of the integrity of the entire study: Nicholas van As
-
•
Study concept and design: Daniel Henderson, Alison Tree, Nicholas van As
-
•
Literature research: Luke Nicholls, Daniel Henderson, Alison Tree
-
•
Clinical studies: Luke Nicholls, Yae-eun Suh, Ewan Chapman, Daniel Henderson, Caroline Jones, Kirsty Morrison, Aslam Sohaib, Helen Taylor, Alison Tree, Nicholas van As
-
•
Data analysis: Luke Nicholls, Yae-eun Suh, Kirsty Morrison
-
•
Manuscript preparation: Luke Nicholls, Yae-eun Suh, Nicholas van As
-
•
Manuscript editing: Luke Nicholls, Yae-eun Suh, Ewan Chapman, Daniel Henderson, Caroline Jones, Kirsty Morrison, Aslam Sohaib, Helen Taylor, Alison Tree, Nicholas van As
Declaration of Competing Interest
DH reports personal fees and non-financial support from Accuray, outside the submitted work. AT reports grants from Accuray, outside the submitted work; and grants from Elekta and Varian. Personal fees and non-financial support from Elekta, Janssen, Astellas, Genesis Care, Bayer and Ferring. NVA reports grants and personal fees from Accuray, outside the submitted work.
Acknowledgement
This work was supported by the Royal Marsden NHS Foundation trust via an internal research fund.
References
- 1.Zaorsky N.G., Keith S.W., Shaikh T., Nguyen P.L., Horwitz E.M., Dicker A.P. Impact of radiation therapy dose escalation on prostate cancer outcomes and toxicities. Am J Clin Oncol. 2018;41(4):409–415. doi: 10.1097/COC.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand D.H., Tree A.C., Ostler P., van der Voet H., Loblaw A., Chu W. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019;20(11):1531–1543. doi: 10.1016/S1470-2045(19)30569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miralbell R., Roberts S.A., Zubizarreta E., Hendry J.H. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17–e24. doi: 10.1016/j.ijrobp.2010.10.075. [DOI] [PubMed] [Google Scholar]
- 4.Marzi S., Saracino B., Petrongari M.G., Arcangeli S., Gomellini S., Arcangeli G. Modeling of alpha/beta for late rectal toxicity from a randomized phase II study: conventional versus hypofractionated scheme for localized prostate cancer. J Exp Clin Cancer Res. 2009;28:117. doi: 10.1186/1756-9966-28-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cellini N., Morganti A.G., Mattiucci G.C., Valentini V., Leone M., Luzi S. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: implications for conformal therapy planning. Int J Radiat Oncol Biol Phys. 2002;53(3):595–599. doi: 10.1016/s0360-3016(02)02795-5. [DOI] [PubMed] [Google Scholar]
- 6.Pucar D., Hricak H., Shukla-Dave A., Kuroiwa K., Drobnjak M., Eastham J. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007;69(1):62–69. doi: 10.1016/j.ijrobp.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 7.Huang C.C., Deng F.M., Kong M.X., Ren Q., Melamed J., Zhou M. Re-evaluating the concept of “dominant/index tumor nodule” in multifocal prostate cancer. Virchows Arch. 2014;464(5):589–594. doi: 10.1007/s00428-014-1557-y. [DOI] [PubMed] [Google Scholar]
- 8.Feutren T., Herrera F.G. Prostate irradiation with focal dose escalation to the intraprostatic dominant nodule: a systematic review. Prostate Int. 2018;6(3):75–87. doi: 10.1016/j.prnil.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tree A., Jones C., Sohaib A., Khoo V., van As N. Prostate stereotactic body radiotherapy with simultaneous integrated boost: which is the best planning method? Radiat Oncol. 2013;8:228. doi: 10.1186/1748-717X-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickinson L., Ahmed H.U., Allen C., Barentsz J.O., Carey B., Futterer J.J. Scoring systems used for the interpretation and reporting of multiparametric MRI for prostate cancer detection, localization, and characterization: could standardization lead to improved utilization of imaging within the diagnostic pathway? J Magn Reson Imaging. 2013;37(1):48–58. doi: 10.1002/jmri.23689. [DOI] [PubMed] [Google Scholar]
- 11.Prostate Advances in Comparative Evidence (PACE) [updated 2015. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01584258.
- 12.A'Hern R.P. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20(6):859–866. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
- 13.EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. [DOI] [PubMed]
- 14.Roach M., 3rd, Hanks G., Thames H., Jr., Schellhammer P., Shipley W.U., Sokol G.H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Morgan S.C., Hoffman K., Loblaw D.A., Buyyounouski M.K., Patton C., Barocas D. Hypofractionated radiation therapy for localized prostate cancer: an ASTRO, ASCO, and AUA evidence-based guideline. J Clin Oncol. 2018;36(34):3411–3430. doi: 10.1200/JCO.18.01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Roover R., Crijns W., Michiels S., Draulans C., Poels K., Haustermans K. PO-0929 Focal boost dose escalated prostate SBRT on the Halcyon fast-rotating O-ring linac. Radiother Oncol. 2019;133:S499–S500. [Google Scholar]
- 17.Kwee S., Wang H., Kuang Y., Wu L., Hirata E. Proceedings of the world molecular imaging congress 2016, New York, New York, September 7–10, 2016: general abstracts. Mol Imag Biol. 2016;18(2):1–1278. doi: 10.1007/s11307-016-1031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray L., Lilley J., Thompson C.M., Sykes J.R., Franks K.N., Sebag-Montefiore D. Isotoxic simultaneous integrated boost to dominant intraprostatic lesions using stereotactic ablative radiation therapy and volumetric modulated arc therapy. Int J Radiat Oncol • Biol • Phys. 2013;87(2):S14–S15. doi: 10.1016/j.ijrobp.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang H.F., Cheng J., Alshammari M., Lee C., Bloch N.B., Keohan S. Dosimetric validation of robotic prostate SBRT With simultaneous integrated dose escalation to dominant intraprostatic lesion using a magnetic resonance-based 3D-printed prostate model in an anthropomorphic pelvis phantom. Int J Radiat Oncol • Biol • Phys. 2016;96(2):E613. [Google Scholar]
- 20.Monni F., Fontanella P., Grasso A., Wiklund P., Ou Y.C., Randazzo M. Magnetic resonance imaging in prostate cancer detection and management: a systematic review. Minerva Urol Nefrol. 2017;69(6):567–578. doi: 10.23736/S0393-2249.17.02819-3. [DOI] [PubMed] [Google Scholar]
- 21.Zamboglou C., Drendel V., Jilg C.A., Rischke H.C., Beck T.I., Schultze-Seemann W. Comparison of (68)Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics. 2017;7(1):228–237. doi: 10.7150/thno.16638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Draulans C, van der Heide UA, Haustermans K, Pos FJ, van der Voort van Zyp J, De Boer H, et al. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiother Oncol. 2020;147:92-8. [DOI] [PubMed]
- 23.Cushman T.R., Verma V., Khairnar R., Levy J., Simone C.B., 2nd, Mishra M.V. Stereotactic body radiation therapy for prostate cancer: systematic review and meta-analysis of prospective trials. Oncotarget. 2019;10(54):5660–5668. doi: 10.18632/oncotarget.27177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aluwini S., van Rooij P., Hoogeman M., Kirkels W., Kolkman-Deurloo I.K., Bangma C. Stereotactic body radiotherapy with a focal boost to the MRI-visible tumor as monotherapy for low- and intermediate-risk prostate cancer: early results. Radiat Oncol. 2013;8:84. doi: 10.1186/1748-717X-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrera F.G., Valerio M., Berthold D., Tawadros T., Meuwly J.Y., Vallet V. 50-Gy stereotactic body radiation therapy to the dominant intraprostatic nodule: results from a phase 1a/b trial. Int J Radiat Oncol Biol Phys. 2019;103(2):320–334. doi: 10.1016/j.ijrobp.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Alayed Y., Davidson M., Liu S., Chu W., Tseng C.L., Cheung P. Evaluating the tolerability of a simultaneous focal boost to the gross tumour in prostate SABR: a toxicity and quality of life comparison of two prospective trials. Int J Radiat Oncol Biol Phys. 2020 doi: 10.1016/j.ijrobp.2019.12.044. [DOI] [PubMed] [Google Scholar]
- 27.McDonald A.M., Dobelbower M.C., Yang E.S., Clark G.M., Jacob R., Kim R.Y. Prostate stereotactic body radiation therapy with a focal simultaneous integrated boost: acute toxicity and dosimetry results from a prospective trial. Adv Radiat Oncol. 2019;4(1):90–95. doi: 10.1016/j.adro.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Draulans C., De Roover R., van der Heide U.A., Haustermans K., Pos F., Smeenk R.J. Stereotactic body radiation therapy with optional focal lesion ablative microboost in prostate cancer: Topical review and multicenter consensus. Radiother Oncol. 2019;140:131–142. doi: 10.1016/j.radonc.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 29.Kotecha R., Djemil T., Tendulkar R.D., Reddy C.A., Thousand R.A., Vassil A. Dose-escalated stereotactic body radiation therapy for patients with intermediate- and high-risk prostate cancer: initial dosimetry analysis and patient outcomes. Int J Radiat Oncol Biol Phys. 2016;95(3):960–964. doi: 10.1016/j.ijrobp.2016.02.009. [DOI] [PubMed] [Google Scholar]