Abstract
Introduction
The learning curves analysed to date for robot-assisted laparoscopic prostatectomy are based on arbitrary cut-offs of the total cases.
Methods
We analysed a large dataset of robot-assisted laparoscopic prostatectomies from a single centre between 2008 and 2019 for assessment of the learning curve for perioperative outcomes with respect to time and individual cases.
Results
A total of 1,406 patients were evaluated, with mean operative time 198.08 minutes and mean console time 161.05 minutes. A plot of operative time and console time showed an initial decline followed by a near-constant phase. The inflection points were detected at 1,398 days (308th case) for operative time and 1,470 days (324th case) for console time, with a declining trend of 8.83 minutes and 7.07 minutes, respectively, per quarter-year (p<0.001). Mean estimated blood loss showed a 70.04% reduction between the start (214.76ml) and end (64.35ml) (p<0.001). The complication rate did not vary with respect to time (p=0.188) or the number of procedures (p=0.354). There was insufficient evidence to claim that the number of operations (p=0.326), D’Amico classification (p=0.114 for intermediate versus low; p=0.158 for high versus low) or time (p=0.114) was associated with the odds of positive surgical margins.
Conclusions
It takes about 300 cases and nearly 4 years to standardise operative and console times, with a requirement of around 80 cases per annum for a single surgical team in the initial years to optimise the outcomes of robot-assisted laparoscopic prostatectomy.
Keywords: Learning curve, Robot-assisted laparoscopic prostatectomy, Perioperative outcomes, Perioperative complications
Introduction
The surgical learning curve – the period of time in which the surgeon’s perspective for a particular procedure changes from novice to competent – is an arbitrary timeline in which the overall efficiency may be less and there may be higher rates of complications until the surgeon has adequate experience.1,2 The field of minimally invasive surgery has paved the way to higher and higher advances, with the ultimate goal of best possible patient outcomes. Prostate cancer is not an exception. The outcomes are not limited to the trifecta of cancer control, urinary continence and recovery of sexual function; complication rates and negative surgical margins have been added as the two parameters for the pentafecta.3 Various groups have discussed the learning curve for radical prostatectomy (open/laparoscopic/robotic), but these are limited by arbitrary cut-offs and separate evaluation of different outcomes.4–10
The process of gaining proficiency generally has four phases. Unconscious incompetence is the first step, when the surgeon does not know their limitations. This is followed by conscious incompetence and conscious competence. The last phase, unconscious competence, is achieved when the skill becomes an automated process par excellence. The overall process of learning is attributed to various aspects, including surgeon-related factors, patient-related factors and overall team experience. Surgeon-related factors include experience in open and minimally invasive procedures, formal fellowship training, and ongoing commitment to learning. Patient-related factors include disease group and stage (D’Amico).
We describe our outcomes for the learning curve for 1,406 robot-assisted laparoscopic prostatectomies for a single surgical team at a single institution over the past 11 years.
Methods
We retrospectively analysed the outcomes of 1,406 consecutive robot-assisted laparoscopic prostatectomies undertaken between 27 November 2008 and 24 April 2019 at a single high-volume United Kingdom National Health Service (NHS) centre. The procedures were performed by a single surgical team with previous backgrounds of open and laparoscopic surgery at the beginning of data collection. All cases were operated by a da Vinci Si® (Intuitive Surgical, Sunnyvale, CA, USA) system with a dual console. Parameters used for the assessment of the learning curve were intraoperative factors (console time, total operative time, estimated blood loss and blood transfusion rates) and postoperative parameters (length of stay, days for catheter removal, complications and positive surgical margin status).
For outcomes with a measure that is continuous in nature, plots of the outcomes over time and number of operations were examined to determine the shape of the model required. Because the operations were carried out by a number of surgeons, each of whom operated multiple times, multilevel analysis was used to account for the lack of independence between observations.
For outcomes whose learning curves showed two phases (initial decline over time or number of operations followed by a near-constant level), we used a multilevel model that reflected this pattern. A number of models with different inflection points were fitted; the best fit to the data was determined by the minimum Akaike information criterion. For outcomes where no inflection point existed in the plot, we used a multilevel model allowing for a trend over time or number of operations. Model errors were checked for normality and heterogeneity. Where appropriate, a transformation of the outcome variable was used.
For outcomes measured in a discrete manner, plots of the observations over time and number of operations were examined and summary proportions calculated. Multilevel binary logistic regression models were used to examine the trend in rates over time or number of operations.
Results
We evaluated a total of 1,406 patients, with a mean age of 62.14 years (range 39–84 years) and a mean prostate-specific antigen level of 8.53ng/ml (range 0.22–82ng/ml) (Table 1). The distribution of patients based on the D’Amico classification was 18.6% (n = 261) low risk, 66.2% (n = 931) intermediate risk and 15.2% (n = 214) high risk. The mean operative time was 198.08 minutes, and the mean console time 161.05 minutes (Table 1).
Table 1.
Demographic profile
| Number of patients (n) | 1,406 |
| Age (years) | 62.14 (39–84) |
| Prostate-specific antigen (n) | 8.53 (0.22–82.00) |
| Clinical stage (n) | |
| T1 | 13 (0.95%) |
| T1a | 2 (0.1%) |
| T1b | 2 (0.1%) |
| T1c | 335 (24.2%) |
| T2 | 57 (4.1%) |
| T2a | 317 (22.9%) |
| T2b | 173 (12.5%) |
| T2c | 385 (27.8%) |
| T3 | 30 (2.2%) |
| T3a | 68 (4.9%) |
| T3b | 2 (0.1%) |
| T3c | 1 (0.1%) |
| 4 | 1 (0.1%) |
| Gleason score (n) | |
| 6 | 466 (33.4%) |
| 7 | 836 (59.9%) |
| 8 | 80 (5.7%) |
| 9 | 13 (0.9%) |
| 10 | 1 (0.1%) |
| D’Amico classification | |
| Low | 261 (18.6%) |
| Intermediate | 931 (66.2%) |
| High | 214 (15.2%) |
| Intra- and postoperative | |
| Operation duration (min) | 198.08 (60–522) |
| Console time (min) | 161.05 (10–450) |
| Mean estimated blood loss (ml) | |
| ≤49ml | 152 (11.5%) |
| 50–99ml | 51 (3.9%) |
| 100–199ml | 364 (27.6%) |
| 200–499ml | 292 (22.1%) |
| ≥500ml | 460 (34.9%) |
| Blood transfusion (n) | 10 (0.7%) |
| Complications (Clavien) (n) | |
| 1 | 50 (4.4%) |
| 2 | 23 (2.0%) |
| 3a | 13 (1.1%) |
| 3b | 35 (3.1%) |
| 4a | 2 (0.2%) |
| 4b | 4 (0.4%) |
| Positive surgical margin (n) | 289 (20.7%) |
| Catheter time (days) | 10.95 (0–14) |
| Pathological features | |
| Pathological stage (n) | |
| pT0 | 3 (0.2%) |
| pT1a | 1 (0.1%) |
| pT1b | 0 (0.0%) |
| pT1c | 7 (0.5%) |
| pT2 | 14 (1.0%) |
| pT2+ | 54 (3.9%) |
| pT2a | 97 (6.9%) |
| pT2b | 28 (2.0%) |
| pT2c | 840 (60.2%) |
| pT3a | 274 (19.6%) |
| pT3b | 75 (5.4%) |
| pT4 | 2 (0.1%) |
| pT4a | 1 (0.1%) |
| Gleason score (n) | |
| 6 | 298 (21.4%) |
| 7 | 967 (69.5%) |
| 8 | 102 (7.3%) |
| 9 | 22 (1.6%) |
| 10 | 2 (0.1%) |
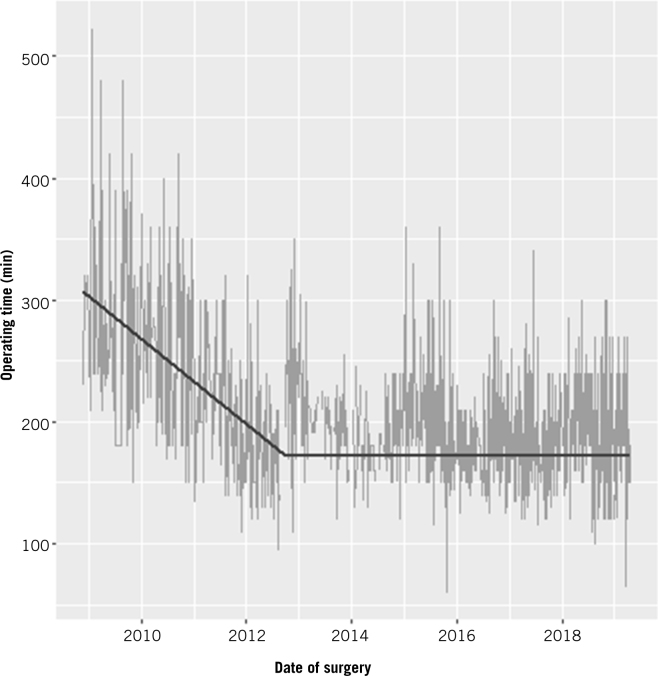
A plot of total operating time over time showed an initial decline followed by a near-constant phase. The inflection point was detected at 1,398 days after the first operation, or following the 308th operation if we modelled total operating time in terms of the number of operations instead of time. The declining slope of the initial phase was 8.83 minutes per quarter-year (95% confidence interval (CI) 7.72–9.94 minutes; p<0.001) or 11.05 minutes per 25 operations (95% CI 9.70–12.41; p<0.001). The trend after the inflection point was not significantly different from zero (p=0.901 in terms of time; p=0.880 in terms of number of operations) (Table 2). The difference in operating times between the start and end of data collection was estimated to be 96.04 minutes (95% CI 80.52–111.55 minutes; p<0.001) for the time model, or 88.07 minutes (95% CI 75.86–100.28 minutes; p<0.001) for the number of operations model (Fig 1).
Table 2.
Statistical correlations for the learning curve for perioperative outcomes
| Parameter | Pattern | Rate of decrease | Inflection point | |
|---|---|---|---|---|
| Operating time | Decline followed by constant level | In terms of time | 8.83 minutes per quarter-year(95% CI 7.72–9.94 minutes; p<0.001) | 1,398 days |
| In terms of number of operations | 11.05 minutes per 25 operations(95% CI 9.70–12.41 minutes; p<0.001) | 308 operations | ||
| Console time | Decline followed by constant level | In terms of time | 7.07 minutes per quarter-year(95% CI 6.07–8.08 minutes; p<0.001) | 1,470 days |
| In terms of number of operations | 9.08 minutes per 25 operations(95% CI 7.82–10.33 minutes; p<0.001) | 324 operations | ||
| Blood loss | Steady decline | In terms of time | 2.93% per quarter year(95% CI 2.49–3.38%; p<0.001) | n/a |
| In terms of number of operations | 1.99% per 25 operations(95% CI 1.68–2.30%; p<0.001) | n/a | ||
| Transfusion rate | Insufficient evidence of trend | In terms of time: p=0.233 | ||
| In terms of number of operations: p=0.092 | ||||
| Complication rate | Insufficient evidence of trend | In terms of time: p=0.188 | ||
| In terms of number of operations: p=0.354 | ||||
| Major complication rate | Insufficient evidence of trend | In terms of number of operations: p=0.107 | ||
| Positive surgical margin rate | Insufficient evidence of trend | In terms of number of operations: p=0.326 | ||
| In terms of time: p=0.114 | ||||
| In terms of pT stage: <pT3: p=0.871 ≥pT3: p=0.303 |
||||
| In terms of number of D’Amico classification: Intermediate versus low: p=0.114 High versus low: p=0.158 |
||||
| In terms of gland size <30g: p=0.477 >80g: p=0.216 |
||||
| In terms of location of margin positivity: Apical: p=0.359 Circumferential: p=0.725 Basal: p=0.535 |
||||
Figure 1.
Timewise progression of learning curve for total operative time
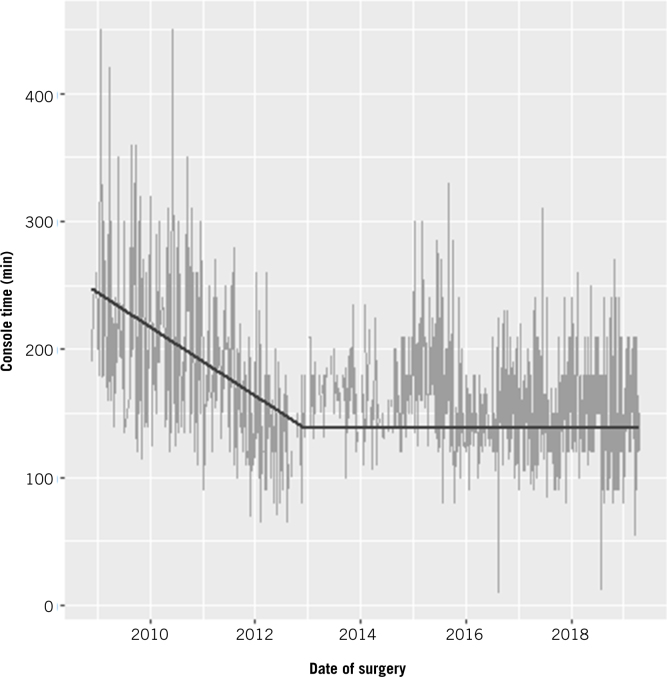
A plot of console time over time showed an initial decline followed by a phase of shallower decline. The inflection point was detected at 1,470 days after the first operation, or following the 324th operation if we modelled total operating time in terms of the number of operations. The declining slope of the initial phase was 7.07 minutes per quarter-year (95% CI 6.07–8.08 minutes; p<0.001), or 9.08 minutes per 25 operations (95% CI 7.82–10.33 minutes; p<0.001). The trend after the inflection point was 0.54 minutes per quarter-year (95% CI 0.16–0.92 minutes, p = 0.005), or 0.28 minutes per 25 operations (95% CI 0.07–0.49 minutes; p=0.010) (Table 2). The difference in console times between the start and end of data collection was estimated to be 56.51 minutes (95% CI 41.17–71.85 minutes; p<0.001) for the time model, or 57.36 minutes (95% CI 45.51–69.20 minutes; p<0.001) for the number of operations model (Fig 2).
Figure 2.
Timewise progression of learning curve for console time
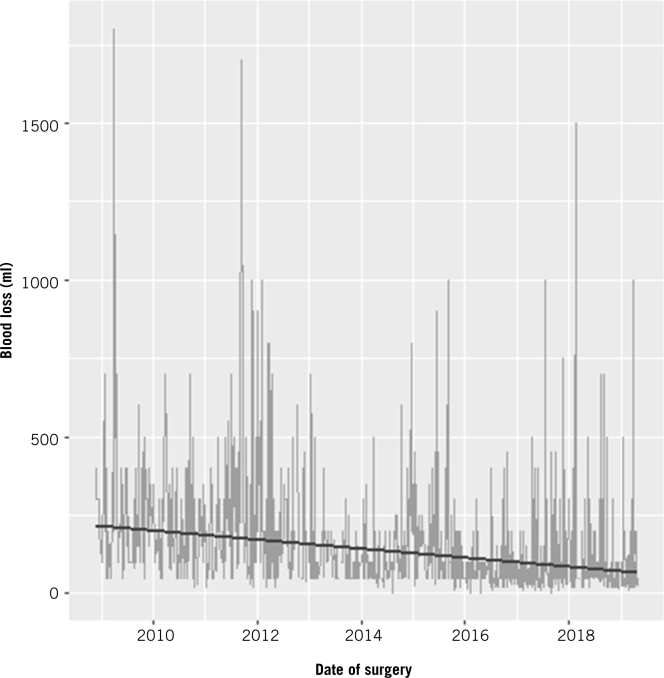
A plot of blood loss over time or number of operations showed an overall decline with a steady trend. The estimated decline in blood loss was 2.93% per quarter-year (95% CI 2.49–3.38%; p<0.001) for the time model, or 1.99% per 25 operations (95% CI 1.68–2.30%; p<0.001) for the number of operations model. Overall, the estimated blood loss decreased from 214.76ml (95% CI 153.29–300.88ml) at the start of data collection to 64.35ml (95% CI 45.75–90.52ml) at the end of data collection for the time model, a reduction of 70.04% (95% CI 64.11–74.98%; p<0.001). For the number of operations model, the estimated blood loss decreased from 182.89ml (95% CI 124.80–268.00ml) to 60.34ml (95% CI 41.17–88.43ml), a reduction of 67.01% (95% CI 60.69–72.16%; p<0.001) (Fig 3). Blood transfusion was required in 0.7% of patients.
Figure 3.
Timewise progression of learning curve for estimated blood loss
An overall positive surgical margin was seen in 20.7% of cases. For the model involving time, there was insufficient evidence to claim that either time (p=0.114) or D’Amico classification (p=0.090 for intermediate versus low; p=0.135 for high versus low) was associated with the odds of positive surgical margins. The odds of positive surgical margins were increased by 148% for classification pT3/4 compared with pT0/1/2 (95% CI 85.2–232.9%, p<0.001). For the number of operations model, there was insufficient evidence to claim that either the number of operations (p=0.326) or D’Amico classification (p=0.114 for intermediate versus low; p=0.158 for high versus low) was associated with the odds of positive surgical margins.
The vast majority of patients (92.12%) had a length of stay of 1 day (39.51%), 2 days (40.60%) or 3 days (12.01%). Only 2.29% of patients had a stay of more than 5 days. There is no evidence of a trend over time or number of operations.
The vast majority of patients (92.19%) had catheters for 7 days (38.86%), 10 days (23.88%) or 14 days (29.45%). There is no evidence of a trend over time or number of operations. The mean time for catheter removal was 10.95 days.
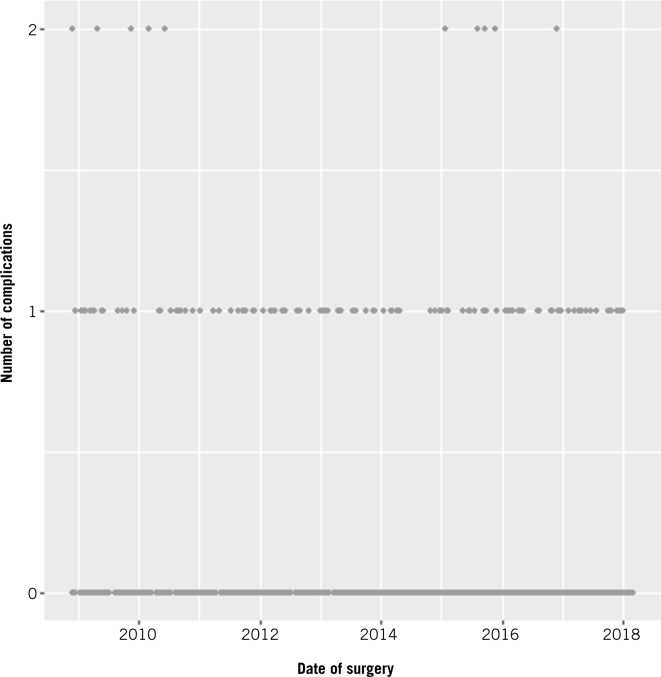
Most patients (87.94%) had no postoperative complications, 11.18% had one complication, and 0.88% had two complications. Minor complications by Clavien Dindo grade (I and II) were 6.6%; major grade complications (>II) were 4.8%. A multilevel binary logistic regression was carried out to see whether the rate of having any complications changed over time or number of operations. There was insufficient evidence of a trend over time (p=0.188) or number of operations (p=0.354) (Fig 4).
Figure 4.
Timewise progression of learning curve for postoperative complications
Discussion
This study represents the experience of a high-volume centre in robot-assisted laparoscopic prostatectomy, with specific consideration of the learning curve based on case-and time-wise progression. The learning curve is not an arbitrary judgement based on a certain level of cut-offs for the number of times a particular procedure is performed. We believe that learning ability improves with every case in the initial stages, and hence it is a curve rather than a fixed interval based on cut-offs. Ideally, as a surgeon travels the path from novice, to advanced beginner, to proficient and then to expert, there is a significant improvement in the functionality, with certain definite inflection points. We have tried to correlate the surgical outcomes along these lines to estimate the number of cases and the time required to achieve a level of proficiency in robot-assisted laparoscopic prostatectomy, the most commonly performed robotic procedure worldwide.
If we consider the total operative time, every quarter-year leads to an improvement of about 8.83 minutes, with a significant inflection at 1,398 days after the start time to qualify as the maximum achievable reduction in time. This maximal reduction stands as high as 96.04 minutes, allowing for a multitude of benefits, including reduced anaesthetic times and reduced burden on the healthcare system, especially important in the NHS where, according to a report of theatre productivity metrics based on a national dataset, every extra minute of theatre time translates into £20 of expenditure.11
Similar figures for the console time are quite promising, amounting to a reduction of 56.51 minutes, with a significant inflection point at 1,470 days, and a gradual decline of 7.07 minutes every quarter-year. The inflection points for operative time and console time correspond to the 308th and 324th cases, respectively. Considering the time and number of cases required before standardisation of operative times, beyond which there can be no further significant improvement in operative or console time, we can say that about 80 cases per annum would be necessary for optimisation in the initial learning phase. This point stands as an important highlight, as the majority of data do not consider time and number of cases simultaneously.12
Abboudi and colleagues systematically reviewed learning curves for different urological procedures.12 They stressed that learning curves for surgeons performing 5 prostatectomies a year for 25 years cannot be compared with those for surgeons performing 2 cases a week. The estimated number of cases for completion of learning curves for operative times varies between 40 and 750.12–18 Our paper stands as one of the only audits that narrows down the estimation of requirement to 80 cases for a single surgical team in the initial years. The reductions in operative and console times per quarter-year are, respectively, nearly 8.8 minutes and 7 minutes, or 11 minutes and 9 minutes per 25 cases.
These observations reinforce the fact that learning improves with every case initially, until a level of expertise is achieved beyond which operative timings are never of concern. This would enable a novice to reassess their timings every quarter-year or every 25 cases to audit their own improvement. This would be of more significance for improvement rather than arbitrarily mentioning the numbers for optimisation of timings for robot-assisted laparoscopic prostatectomy.
Considering the final pathological T-stage, it would be reasonable to have operated on more organ-confined prostates (<pT3) than non-organ-confined glands (≥pT3). In our cohort, subgroup analysis showed the inflection point was detected at the 63rd operation on patients with ≥pT3 disease. The declining slope of the initial phase was 50.71 minutes per 25 operations on patients with ≥pT3 disease (95% CI 36.91–64.50 minutes; p<0.001). The trend after the inflection point was not significantly different from zero (p=0.783). The numbers for console time matched exactly with operative time, and the inflection point was found to be the 63rd case. Before achieving these inflections in non-organ-confined glands, 231 organ-confined prostates were operated on. These numbers help us understand the initial strategy of gaining adequate experience in organ-confined prostates before non-organ-confined prostates.
Positive surgical margin, the most important surgical outcome after robot-assisted laparoscopic prostatectomy, has variations with respect to the number of cases performed. The established literature in this context is heterogeneous, with varying numbers given for the learning curve for optimising positive surgical margin rates – from as low as 50 case to as high as 1600, based on different centres and inclusion criteria.12–18 In our cohort, however, the positive surgical margin rate did not vary significantly with number of cases or time. To interrogate these data further, we analysed positive surgical margin rates with respect to different subgroups. We considered pathological T-stage in light of having potentially different outcomes in non-organ-confined prostates, D’Amico stratification, gland size (<30 or >80g) and location of margin positivity. Positive surgical margin rates did not vary with the number of cases for organ-confined glands (<pT3) (p=0.871) or non-organ-confined glands (≥pT3) (p=0.303).
One paper has reported that positive margin rates do improve with respect to time in both ≤pT2 and >pT3 groups, but the analysis was limited to an initial experience of 100 cases divided into arbitrary groups of 33, 33 and 34 each.6 Similarly, in a dataset involving 500 patients (250 in each group), positive surgical margin rates were different only in the pT3 group.19
One of the series comparing robot-assisted laparoscopic prostatectomy with open retropubic radical prostatectomy mentioned in a subgroup analysis that positive surgical margin rates improved with number of cases of robot-assisted laparoscopic prostatectomy, considering the pT2 and pT3 groups separately, after 95 and 60 cases, respectively.16 The authors note that at the end of analysis, the positive surgical margin rate in the pT3 group was higher in robot-assisted laparoscopic prostatectomy than retropubic radical prostatectomy, mentioning the fact that the learning curve for robot-assisted laparoscopic prostatectomy for the pT3 group may not have been completed until the time when the analysis was performed.16
In contrast, similar to our series, some authors have concluded that the overall positive surgical margin rates considering pT stage did not vary with the number of cases or surgeon.20
We believe that if we consider a larger horizon and analyse the results on a case-by-case basis rather than as arbitrary demarcations in different groups, the overall positive surgical margin rate shows steady improvement, which may not be statistically significantly similar to our results.
Similarly, there was insufficient evidence to say that positive surgical margin rates varied with D’Amico stratification (low risk, p=0.268; intermediate risk, p=0.048; high risk, p=0.420). This subgroup analysis was performed considering cases only for the individual group based on D’Amico stratification for decision-making in choosing cases in the early part of a career. Additionally, there have been variations in positive surgical margin rates with respect to gland size, and generally, there is an inverse proportion to gland size, with particularly higher rates for glands smaller than 30g.21,22 In subgroup analysis there was insufficient evidence to claim that the number of operations was associated with the odds of positive surgical margin (p=0.477) when considering only patients with gland size below 30g (n=54) or over 80g (n=194) (p=0.216). For similar reasons, apart from the potential difficulty in dealing with smaller or larger glands, positive surgical margin rate is not a concern in the initial phases for choosing a particular patient. Pertaining to the location of margin positivity, there was no difference with respect to location as per the number of cases (apical, p=0.359; circumferential, p=0.725; basal, p=0.535).
The majority of data described to date with respect to the learning curve are compared different robot-assisted laparoscopic prostatectomy techniques,8 non-robotic-trained surgeons and robotic-trained surgeons,23 and robot-assisted laparoscopic prostatectomy and retropubic radical prostatectomy.24 We believe strongly that in a dedicated high-volume centre, these positive surgical margin rates with respect to location do not vary significantly along the learning curve, provided adequate vigilance and periodic evaluations are performed for quality control.
Although overall complication rates are less with robot-assisted laparoscopic prostatectomy, it is important to assess whether there is improvement in the complication rates Neither the overall (p=0.354) nor major (grade 3 onwards) (p=0.107) complications vary with the number of cases if the plot is considered on a case-by-case basis. Similarly, in the initial phase, comparison between retropubic radical prostatectomy and robot-assisted laparoscopic prostatectomy shows similar outcomes for complication rates.24 Evaluation of 500 robot-assisted laparoscopic prostatectomies by a single surgeon showed a decreasing trend of complications after dividing into groups 1–250 and 251–500, but this was not significant (p=0.129).19 On the contrary, major complication rates plateau after the initial 250 cases in robot-assisted laparoscopic prostatectomy series, but this stands as the arbitrary cut-off.25
A certain element of bias would always exist in evaluation of the learning curve by dividing into fixed groups in order to have statistical significance. Ideal outcomes should assess these parameters based on time and individual cases with similar outcomes without any major alterations. The same is true for estimated blood loss, as we could conclude that estimated blood loss reduces steadily over time until the last case, without any cut-off, which has been seen in certain studies.25
Although the data are entered in real time to avoid any recall bias, this is a retrospective evaluation, which stands as a limitation. Additionally, the data are the outcomes of a single surgical team in a high-volume centre rather than of an individual surgeon. To counter this, appropriate statistical measures have been taken. We believe strongly in a team approach for a robust oncology programme, and we think the learning curve should be assessed for the entire team to achieve the best possible results. Dedicated pathologists have reported the outcomes, which eliminates bias due to variations in reporting, especially for positive surgical margin rates. Long-term oncological or functional outcomes for continence and potency have not been considered for assessment of the learning curve, as these vary for individual surgeons. Additionally, these functional outcomes are subject to multiple patient-and procedure-related factors, which would create bias in the evaluation. Hence, we have not considered these parameters for evaluation.
This is a rare evaluation of perioperative data for assessment of the learning curve for robot-assisted laparoscopic prostatectomy, which considers results by plotting all cases in real time as the learning curve progresses with time. These data give an opportunity for a novice to consider the outcomes every 25 cases or every quarter-year. It also gives a correlation of the approximate volumes required to overcome the learning curve for a team in the initial years for optimisation of timing and theatre use.
We would like to reinforce that the established literature, which essentially divides the number of procedures in arbitrary cut-offs, would have biases due to statistical adjustments for deciding the level of cut-offs and hence should be taken with caution.
Nearly 1,398 days (308 cases) are required to overcome the initial learning curve for optimising the operative timings for robot-assisted laparoscopic prostatectomy. Estimated blood loss shows a steady and significant decline over time. Length of stay or duration of catheterisation does not have larger variations with respect to time. There is insufficient evidence to say that positive surgical margin rates or complication rates (overall or major) improve along the learning curve when all cases are considered individually along with the timeline. Substratification for analysing positive margin rates does not show any significance with respect to pT stage, gland size or D’Amico classification with respect to the number of cases. Location of margin positivity is not altered along the learning curve.
Conclusions
It takes about 300 cases and nearly 4 years to standardise the operative and console times, with around 80 cases per annum required for a single surgical team in the initial years to optimise their perioperative outcomes of robot-assisted laparoscopic prostatectomy. Robot-assisted laparoscopic prostatectomy is a safe procedure without any major alterations in complication rates or positive margin rates along the learning curve. Perioperative outcomes of any procedure for assessment of the learning curve should be evaluated by considering both the timeline and individual cases for realistic assessment.
References
- 1.Herrell SD, Smith JA Jr. Robotic-assisted laparoscopic prostatectomy: what is the learning curve?. Urology 2005; : 105–107. [DOI] [PubMed] [Google Scholar]
- 2.Artibani W, Novara G. Cancer-related outcome and learning curve in retropubic radical prostatectomy: ‘if you need an operation, the most important step is to choose the right surgeon’. Eur Urol 2008; : 874–876. [DOI] [PubMed] [Google Scholar]
- 3.Patel VR, Sivaraman A, Coelho RF et al. Pentafecta: a new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol 2011; : 702–707. [DOI] [PubMed] [Google Scholar]
- 4.Vickers AJ, Bianco FJ, Serio AM et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst 2007; : 1171–1177. [DOI] [PubMed] [Google Scholar]
- 5.Vickers AJ, Savage CJ, Hruza M et al. The surgical learning curve for laparoscopic radical prostatectomy: a retrospective cohort study. Lancet Oncol 2009; : 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atug F, Castle EP, Srivastav SK et al. Positive surgical margins in robotic assisted radical prostatectomy: impact of learning curve on oncologic outcomes. Eur Urol 2006; : 866–871. [DOI] [PubMed] [Google Scholar]
- 7.Zorn KC, Wille MA, Thong AE et al. Continued improvement of perioperative, pathological and continence outcomes during 700 robot assisted radical prostatectomies. Can J Urol 2009; : 4742–4749. [PubMed] [Google Scholar]
- 8.Samadi DB, Muntner P, Nabizada Pace F et al. Improvements in robot assisted prostatectomy: the effect of surgeon experience and technical changes on oncologic and functional outcomes. J Endourol 2010; : 1105–1110. [DOI] [PubMed] [Google Scholar]
- 9.Ou YC, Yang CR, Wang J et al. The learning curve for reducing complications of robotic assisted laparoscopic radical prostatectomy by a single surgeon. BJU Int 2011; : 420–425. [DOI] [PubMed] [Google Scholar]
- 10.Sharma NL, Papadopoulos A, Lee D et al. First 500 cases of robotic assisted laparoscopic radical prostatectomy from a single UK centre: learning curves of two surgeons. BJU Int 2011; : 739–747. [DOI] [PubMed] [Google Scholar]
- 11.NHS Improvement. Operating theatres: opportunities to reduce waiting lists. London: NHS Improvement; 2019. [Google Scholar]
- 12.Abboudi H, Khan MS, Guru KA et al. Learning curves for urological procedures: a systematic review. BJU Int 2014; : 617–629. [DOI] [PubMed] [Google Scholar]
- 13.O’Malley PJ, Van Appledorn S, Bouchier-Hayes DM, et al. Robotic radical prostatectomy in Australia: initial experience. World J Urol 2006; : 165–170. [DOI] [PubMed] [Google Scholar]
- 14.Gyomber D, Bolton D, Webb D et al. An analysis of variability of learning curve, margin status and early postoperative outcomes in 1200 robot assisted laparoscopic prostatectomies in a multi-user centre. J Urol 2010; : e723. [Google Scholar]
- 15.Sooriakumaran P, John M, Leung R et al. A multi-institutional study of 3794 patients undergoing robotic-assisted laparoscopic radical prostatectomy shows the learning curve is not as short as previously thought. Eur Urol Suppl 2011; : 1569–9056. [Google Scholar]
- 16.Doumerc N, Yuen C, Savdie R et al. Should experienced open prostatic surgeons convert to robotic surgery? The real learning curve for one surgeon over 3 years. BJU Int 2010; : 378–384. [DOI] [PubMed] [Google Scholar]
- 17.Tabata K, Iwamura M, Satoh T et al. The learning curve for laparoscopic radical prostatectomy: 200 cases of a single surgeon. J Endourol 2011; : A230–A231. [Google Scholar]
- 18.Kim IY, Kang D, Jeong J et al. Learning curve effects on the functional and oncologic outcomes of robot-assisted radical prostatectomy by a single surgeon: an analysis of 200 consecutive patients. J Endourol 2010; : A248. [Google Scholar]
- 19.Ou YC, Yang CK, Chang KS et al. The surgical learning curve for robotic-assisted laparoscopic radical prostatectomy: experience of a single surgeon with 500 cases in Taiwan, China. Asian J Androl 2014; : 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arslan ME, Canda AE, Atmaca AF et al. Learning curve of robotic radical prostatectomy. EMJ Urol 2015; : 50–55. [Google Scholar]
- 21.Zorn KC, Orvieto MA, Mikhail AA et al. Effect of prostate weight on operative and postoperative outcomes of robotic-assisted laparoscopic prostatectomy. Urology 2007; : 300–305. [DOI] [PubMed] [Google Scholar]
- 22.Allaparthi SB, Hoang T, Dhanani NN et al. Significance of prostate weight on peri and postoperative outcomes of robot assisted laparoscopic extraperitoneal radical prostatectomy. Can J Urol 2010; : 5383–5389. [PubMed] [Google Scholar]
- 23.Kwon EO, Bautista TC, Jung H et al. Impact of robotic training on surgical and pathologic outcomes during robot-assisted laparoscopic radical prostatectomy. Urology 2010; : 363–368. [DOI] [PubMed] [Google Scholar]
- 24.Philippou P, Waine E, Rowe E. Robot-assisted laparoscopic prostatectomy versus open: comparison of the learning curve of a single surgeon. J Endourol 2012; : 1002–1008. [DOI] [PubMed] [Google Scholar]
- 25.Good DW, Stewart GD, Laird A et al. A critical analysis of the learning curve and post learning curve outcomes of two experience- and volume-matched surgeons for laparoscopic and robot-assisted radical prostatectomy. J Endourol 2015; : 939–947. [DOI] [PubMed] [Google Scholar]