Abstract
Females of the brine shrimp Artemia franciscana produce either free-swimming nauplii via ovoviviparous pathway of reproduction or encysted embryos, known as cysts, via oviparous pathway, in which biological processes are arrested. While previous study has shown a crucial role of ATP-dependent molecular chaperone, heat shock protein 70 (Hsp70) in protecting A. franciscana nauplii against various abiotic and abiotic stressors, the function of this protein in diapausing embryos and cyst development, however, remains unknown. RNA interference (RNAi) was applied in this study to examine the role of Hsp70 in cyst development and stress tolerance, with the latter performed by desiccation and freezing, a common method used for diapause termination in Artemia cysts. Hsp70 knockdown was apparent in cysts released from females that were injected with Hsp70 dsRNA. The loss of Hsp70 affected neither the development nor morphology of the cysts. The time between fertilization and cyst release from Artemia females injected with Hsp70 dsRNA was delayed slightly, but the differences were not significant when compared to the controls. However, the hatching percentage of cysts which lacks Hsp70 were reduced following desiccation and freezing. Taken together, these results indicated that Hsp70 possibly plays a role in the stress tolerance but not in the development of diapause-destined embryos of Artemia. This research makes fundamental contributions to our understanding of the role molecular chaperone Hsp70 plays in Artemia, an excellent model organism for diapause studies of the crustaceans.
Keywords: Hsp70, Artemia franciscana, Cysts, RNA interference (RNAi)
Introduction
Brine shrimps Artemia are termed as “extremophiles” due to their ability to withstand a wide range of environmental stresses (Chen et al. 2007). Under favorable conditions, live nauplii emerge from the egg sac of Artemia females, which then undergo a series of molts before reaching adulthood (Liang and MacRae 1999). Alternatively, in anticipation of unfavorable or extreme conditions, females release oviparous-developing encysted embryos which enter diapause. Once diapause is terminated, cysts hatch into nauplii if conditions are favorable or they enter quiescence where they remain dormant until conditions suitable for development occur (King 2013).
Diapause, a physiological state of developmental delay, is characterized by dormancy, greatly reduced metabolic activity, and enhanced stress tolerance in organisms ranging from crustaceans to mammals (Clegg 1997; Ptak et al. 2012). Diapause ensures survival under adverse environmental conditions. As one example, encysted embryos of A. franciscana can withstand repeated heat, desiccation, freezing, and thawing for years (Clegg 2007), with metabolic activity halted during diapause (Clegg 2011). Hsps play significant roles during diapause of many organisms, including Artemia. Hsps have been documented to prevent protein damage and protect against a wide range of environmental perturbations during diapause. In the event of stress, more than one Hsp is induced, likely because molecular chaperones like Hsps function together in networks (Haslbeck 2002). Hsp90, Hsp70, Hsp60, and small heat shock proteins (sHsps) are synthesized during diapause (MacRae 2010) and they may co-operate in protein refolding after diapause termination. sHsps bind denatured proteins through hydrophobic interactions and deliver them usually via co-factors to Hsp70 which play an important role in ATP-dependent protein refolding. Hsp90 interacts with Hsp70, increasing protein folding efficiency, and both chaperones may sequester proteins in the absence of ATP. p26, an abundant diapause sHsp, plays a major role in embryo development, diapause maintenance, and stress tolerance in Artemia cysts (King and MacRae 2012).
To date, little is known about the function of Hsp70 during diapause. It has been shown that Hsp70 and several sHsps in insects such as flesh fly S. crassipalpis (Li et al. 2007), Colorado potato beetle L. decemlineata (Yocum 2001), solitary bee M. rotundata (Yocum et al. 2005), silkworm B. mori (Hwang et al. 2005), onion maggot D. antiqua (Chen et al. 2006), and rice stem borer C. suppressalis (Sonoda et al. 2006) are induced prior to diapause, and the accumulation persists throughout the event. The role of Hsp70 in the diapause stage of Artemia was rarely addressed. In our previous study, by using RNAi, Hsp70 was shown to be an essential protein in boosting the tolerance of A. franciscana nauplii against various abiotic and biotic stressors. The survival of nauplii lacking Hsp70 was reduced approximately 41% by heat stress and 34% upon Vibrio campbellii infection when compared to control animals (Iryani et al. 2017). Knockdown of Hsp70 with this method was shown to be transient, as the protein which was initially undetected in nauplii stage was expressed in juvenile and adult stages of Artemia. The Hsp70-knockdown nauplii developed normally, with their morphology similar to those accumulating normal amounts of the protein (Iryani et al. 2020). Here, we continue this study to unravel the function of Hsp70 in A. franciscana during diapause with the aim to first demonstrate the presence of Hsp70 in the cysts. Knockdown of Hsp70 was performed with RNAi and the development, viability, and stress tolerance of Artemia cysts lacking Hsp70 were investigated.
Materials and methods
Artemia culture
The cysts of A. franciscana (INVE Aquaculture, Belgium) were hatched in seawater (30 mg/L salinity) under vigorous aeration and constant illumination for 24 to 48 h. Nauplii were collected with a 200-μm plankton net and subsequently transferred to a 120-L fiber glass culture tank. Nauplii were fed daily with PKC Nutri+®, a formulated Artemia feed developed at Universiti Malaysia Terengganu (UMT) for use in biomass production. Nauplii were grown to adult stage at 28 °C under constant aeration. Adult Artemia collected after 28-day culture were used for microinjection.
Preparation of Hsp70 dsRNA
pRSET-C plasmid (Invitrogen, Burlington, ON., Canada) containing Artemia Hsp70 cDNA was extracted from the overnight cultures of E. coli with a miniprep kit (PureLink™ Quick Plasmid Miniprep Kit, Invitrogen, USA). Forward (5′-TAATACGACTCACTATAGGGATTCTCAAAGACAAGC-3′) and reverse (5′-TAATAC GACTCACTATAGGGCATAGAGCTTGGTAAT-3′) primers, specific for Hsp70, were used for cDNA amplification by PCR using ImmoMix™ (Bioline, UK) at the manufacturer’s suggested concentration. The PCR product was used as template to generate Hsp70 dsRNA using MEGAscript® RNAi kit (Ambion Applied Biosystems, USA) and green fluorescence protein (GFP) dsRNA was used as control solution (Iryani et al. 2017; Iryani et al. 2020).
Injection of A. franciscana females with dsRNA and observation of embryo development
Adult A. franciscana females with unfertilized egg sacs were chosen for microinjection. Artemia females destined to produce cysts possess a brown shell gland positioned in between the two egg sacs (Liang and MacRae 1999). Females were placed on cold agarose, and approximately 100 ng of Hsp70 dsRNA was injected into the egg sac with a micromanipulator (InjectMan®, Eppendorf, Germany). Males were introduced to females 24 h after injection. Mating pairs were fed daily with microalgae Chlorella vulgaris and females were monitored for fertilization (Iryani et al. 2017; Iryani et al. 2020). The time from fertilization to release of cysts from females was determined.
Detection of Hsp70 mRNA and protein in A. franciscana cysts
Cysts were incubated in filtered, autoclaved seawater at room temperature for at least 10 days to determine if they hatched and failed to enter diapause in the absence of Hsp70. Total RNA from cysts was extracted with TRIsure™ reagent (Bioline, UK) by following the manufacturer’s instructions with minor modification. Twenty cysts released from females injected with either Hsp70 or GFP dsRNA were homogenized in 100 μl of TRIsure. Twenty microliters of chloroform was added to the samples, vortex vigorously, and incubated for 2 min at room temperature. The mixture was centrifuged and colorless aqueous phase was transferred to a new tube prior to RNA isolation. Single-stranded cDNA was produced with Tetro cDNA Synthesis Kit (Bioline, UK) and then amplified by PCR and visualized in a Gel Doc.
For detection of Hsp70 by SDS-PAGE and western immunoblotting, proteins were extracted from 40 cysts as described in the previous study with minor modification. Fifteen microliters of each sample was applied per lane of 10% SDS gels prior to electrophoresis and then blotted to PVDF membrane (BioRadImmun-Blot™ PVDF, USA) for antibody probing. PVDF membrane was probed in monoclonal antibody that recognize Hsp70 (SMC-164D) (StressMarq, Canada), followed by polyclonal HRP-conjugated goat anti-mouse IgG (Bioreagent-SAB-100J) (Stressgen, Canada) prior to the detection of antibody-reactive proteins (Iryani et al. 2020).
Phenotypic modification and metabolic activity of cysts
Microscopic observation was employed to determine if cysts released from females injected with Hsp70 dsRNA were morphologically normal compared to the control cysts. Cyst diameter was determined with Nikon Profile Projector V-12B and Nikon Digital Counter SC-212. The experiment was done 3 times, each with 30 cysts.
Metabolic activity was evaluated by phenol red assay to determine if cysts were viable upon release from females (King 2013). Ten cysts, either with or without Hsp70, were incubated immediately after release from females in 100 μl of test solution consisting of seawater containing 0.03% phenol red, 1000 U penicillin, and 100 μg/ml streptomycin sulfate (pH 8.5) in 96-well plates. Other wells contained test solutions with commercially produced cysts (INVE Aquaculture, Belgium) killed by heating in a boiling water bath or test solution only. Changes in test solution absorbance which indicates metabolic activity were measured at 553 nm. The experiment was done twice, each with three independent cyst samples.
Assessment of cyst stress tolerance
Cysts collected from Artemia females injected with dsRNA for Hsp70 or GFP were incubated in seawater for 10 days at room temperature to allow diapause entry. For diapause termination, cysts were dried in a desiccator over Dryrite for 4 weeks and then frozen at − 20 °C for 8 weeks (King and MacRae 2012). Cyst stress tolerance was determined by a hatching assay, i.e., cysts were incubated in seawater at room temperature for at least 10 days, during which nauplii were counted and removed on a daily basis. Experiments were terminated 5 days after last hatching was observed. The experiment was done twice in triplicate, each with 30 cysts.
Statistical analysis
Significant differences between treatments were determined by performing one-way analysis of variance (ANOVA) at a significance level of 0.05. All data were expressed as the mean ± standard deviation of these measurements. All statistical analyses were performed with software SPSS® version 20.0 for Windows®.
Results and discussion
RNAi was previously used to examine sHsps function in cyst stress tolerance and embryo development in A. franciscana (King and MacRae 2012; King et al. 2014). These studies indicated that various Hsps occur in Artemia and their function may differ across different life stages of development. It is therefore interesting to determine if Hsp70 has a functional role in the cysts and early life stages of Artemia. In this study, we used RNAi to examine function of Hsp70 in A. franciscana during diapause. Following injection of Artemia females with Hsp70 dsRNA, two approaches were used to test for Hsp70 knockdown in cysts; the detection of Hsp70 mRNA and protein by RT-PCR and Western immunoblotting. Electrophoresis of RT-PCR products yielded a band in agarose gels stained with ethidium bromide when mRNA from cysts produced by Artemia females injected with control solution was used for amplification. However, PCR product of the cyst mRNA generated in females injected with Hsp70 dsRNA was greatly diminished (Fig. 1a). While immunoprobing of Western blots revealed that Hsp70 occurred in cysts released from females injected with GFP dsRNA, the protein was undetectable in cysts from females receiving Hsp70 dsRNA (Fig. 1b). These results proved that the effectiveness of RNAi in knocking down a specific protein in A. franciscana and the expression of Hsp70 were obscured both at gene and protein level, findings similar as seen upon injection of the A. franciscana with dsRNA for p26 (King and MacRae 2012).
Fig. 1.
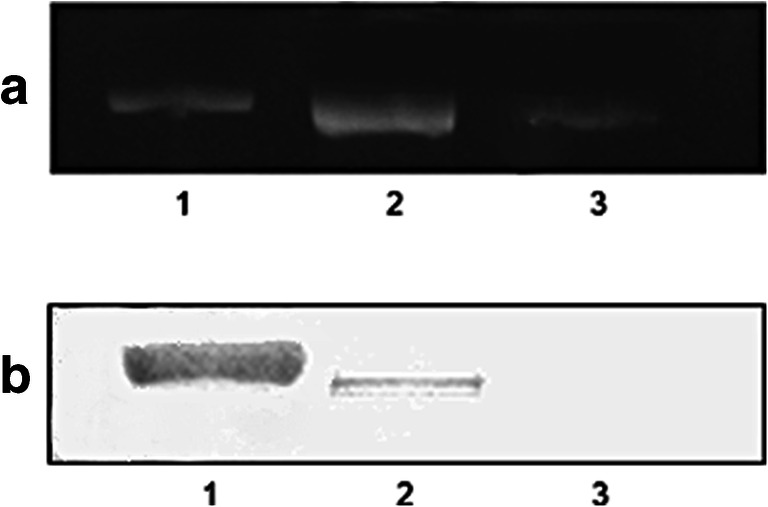
Knockdown of Hsp70 mRNA and protein in cysts. a RT-PCR amplification of Hsp70 mRNA in A. franciscana cysts. PCR products were resolved in 1.7% agarose gels by electrophoresis. b Western blot containing Artemia protein extract probed with antibody specific to Hsp70. Antibody reactive protein was detected by DAB reagent. 1, markers; 2, cysts from females injected with GFP dsRNA; 3, cysts from females injected with Hsp70 dsRNA
Next, we determined whether reduced amounts of Hsp70 affect the development and morphology of diapause-destined embryos. Twenty-four hours after injection, we transferred one male to each female for mating and egg fertilization. The time from fertilization to release of cysts was then recorded. Females injected with GFP dsRNA required at least 5 days to release cysts, while those that were injected with Hsp70 dsRNA required approximately 6 days, but the time difference was insignificant when compared to the control (p > 0.05). These similar results indicated a minor or non-essential role for Hsp70 in the development of A. franciscana cyst. In addition, an early finding by us revealed that silencing an Hsp70 isoform, out of the five known isoforms in A. franciscana (Junprung et al. 2019), did not affect embryo development as the time to release of nauplii from females was within the normal time frame (Iryani et al. 2017). To compare, knockdown of p26 delayed cysts release from A. franciscana females. p26 prevented protein aggregation during encystment, diapause, and quiescence with the RNAi effect prolonged to four successive broods released from the same female (King and MacRae 2012). Also, the loss of artemin, a sHsp, increased the time for complete brood release in A. franciscana (King et al. 2014).
Having determined the time to post-fertilization liberation of cysts, we then examined the cysts under a profile projector to compare the morphology of cysts released from females injected with Hsp70 dsRNA to the control cysts. Diameter of cysts was also measured and displayed on a digital counter attached to the projector. From our observation, cyst morphology was similar in both treatments. They were spherical in shape and brown in color, typical characteristics of Artemia cysts. The average diameter of cysts was 0.25 ± 0.014 mm and 0.24 ± 0.015 mm, respectively, for cysts containing and lacking Hsp70. These results showed that knockdown of Hsp70 had no apparent effect on the cyst morphology. Likewise, knockdown of a type 1 J-domain protein, ArHsp40, and type 2 J-domain protein, ArHsp40–2, had no apparent effect on brood size and time required to release cysts and nauplii in A. franciscana (Rowarth and MacRae 2018).
To determine if cysts were viable upon release from females, we examined their metabolic activity by phenol red assay. Groups of ten cysts containing Hsp70, cysts lacking Hsp70, and commercial cysts killed by heating were incubated in seawater containing 0.03% phenol red for 24 h. We found that cysts containing or lacking Hsp70 produced similar color changes in the test solution, indicating that they were metabolically active and thus considered as alive. Color changes of phenol red were due to acidification of the test solution by carbon dioxide released from cysts.
In the last experiment, we investigated cyst tolerance against stress associated with diapause termination. Diapause was terminated in laboratory produced cysts by 4 weeks of desiccation followed by 8 weeks at − 20 °C, procedures required to break diapause and served as stressors. Following desiccation and freezing, 71% of cysts containing Hsp70 survived whereas for cysts with reduced amounts of Hsp70, 50% survived as indicated by hatching (Fig. 2). The result demonstrated that Hsp70 knockdown decreased cyst tolerance against stress related to diapause termination (p < 0.05). The hatching percentage obtained was higher than those with their Hsp40 reduced by RNAi. Approximately 20 and 40% of cysts hatched, respectively, upon ArHsp40 and ArHsp40–2 knockdown (Rowarth and MacRae 2018). In another case, only 5 to 6% of cysts hatched upon p26 and group 1 LEA protein knockdown under the same experimental condition as used in this study (King and MacRae 2012; Toxopeus et al. 2014). The significant reduction in hatching upon Hsp70 knockdown could be due to several factors. Hsp70 binds substrate proteins in the absence of ATP to reduce the occurrence of non-functional protein formation (King and MacRae 2015) and suppressing Hsp70 expression may disrupt the normal cellular function during diapause termination. It is reported that during stress, ArHsp40 and ArHsp40–2 deliver substrates to Hsp70, providing protection against desiccation, heat, and oxidation (Rowarth and MacRae 2018). Hsp70 may be a part of Hsp110–Hsp70–Hsp40 protein disaggregation system (Kityk et al. 2018), and with cooperation of sHsp p26, they protect partially denatured proteins from irreversible denaturation (King and MacRae 2012). These molecular chaperones function together as they promote protein folding to rescue damaged proteins during stress. In this case, cysts without Hsp70 may not function as efficiently as when the protein is present and therefore experience reduced survival upon diapause termination.
Fig. 2.
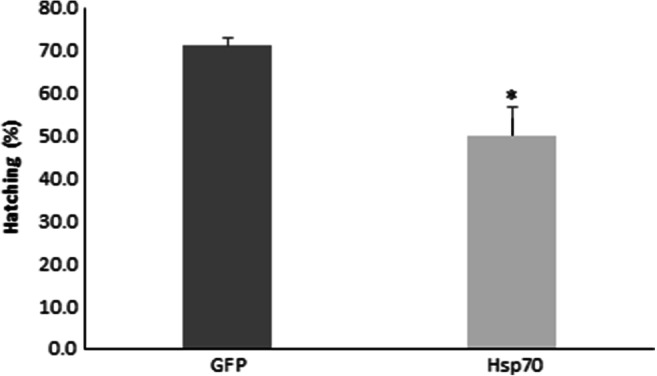
Cysts lacking of Hsp70 exhibited reduced stress tolerance. Artemia cysts either containing or lacking Hsp70 were exposed to desiccation and freezing to terminate diapause. Cysts then rehydrated in seawater at room temperature and monitored for hatching. The bars in the graph represent hatching percentages and the error bars represent the corresponding SD from three replicates. Asterisks denote statistically significant differences between treatments with (P < 0.05)
It is also possible that Hsp70 knockdown reduces the ability of Artemia to withstand freezing, as part of the process coupled with desiccation to break diapause. In fact, numerous studies revealed that Hsp70 are required for protection of crustaceans and insects against cold stress. For example, suppressing the synthesis of Hsp70 in the Flesh fly, S. crassipalpis reduced cold tolerance during overwintering pupal diapause (Rinehart et al. 2007). In another example, injection of PaHsp70 dsRNA reduced adult Linden bug, P. apterus tolerance to cold stress with severe obstruction to the repair of chilling injuries (Koštál and Tollarová-Borovanska 2009). How Hsp70 protects Artemia and these insects against cold tolerance and/or freezing remained unknown and in this context, it is of great importance to determine the mechanistic action(s) involved in low temperatures adaptation and the functional role(s) this Hsp family plays during diapause.
Regardless of the outcomes, this study represents the first to reveal the effect of Hsp70 knockdown on the development, morphology, and stress tolerance in cysts of the brine shrimp Artemia. The role of cysts Hsp70 may not be as major as those observed with sHsps but information generated from this study provided a clear indication that Hsp70 occurs in cysts and that they confer stress tolerance during diapause. Findings herein are novel and fundamentally important for further investigation on the role of Hsp70 during the diapause of Artemia, and perhaps other crustacean species.
Acknowledgments
This paper is dedicated to the late Prof. Dr. Thomas H. MacRae from Dalhousie University, Canada, for his tireless efforts in developing the method to knockdown Hsp70 in Artemia. MTMI was supported by MyBrain 15 Scholarship from the Ministry of Higher Education, Malaysia.
Funding information
This work was partially supported by the Fundamental Research Grant Scheme (FRGS) No. 59331 and No. 59578 from the Ministry of Higher Education, Malaysia to YYS, as well as the Sultan Mizan Professiorial Chair Research Fund No. 53264 to PS.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Chen B, Kayukawa T, Monteiro A, Ishikawa Y. Cloning and characterization of the Hsp70 gene, and its expression in response to diapauses and thermal stress in the onion maggot, Delia antiqua. J Bioch Mol Biol. 2006;39:749–758. doi: 10.5483/bmbrep.2006.39.6.749. [DOI] [PubMed] [Google Scholar]
- Chen T, Villeneuve TS, Garant KA, Amons R, MacRae TH. Functional characterization of artemin, a ferritin homolog synthesized in Artemia embryos during encystment and diapause. FEBS J. 2007;274:1093–1101. doi: 10.1111/j.1742-4658.2007.05659.x. [DOI] [PubMed] [Google Scholar]
- Clegg JS. Embryos of Artemia franciscana survive four years of continuous anoxia: the case for complete metabolic rate depression. J Exp Biol. 1997;200:467–475. doi: 10.1242/jeb.200.3.467. [DOI] [PubMed] [Google Scholar]
- Clegg JS. Protein stability in Artemia embryos during prolonged anoxia. Biol Bull. 2007;212:74–81. doi: 10.2307/25066582. [DOI] [PubMed] [Google Scholar]
- Clegg JS. Stress-related proteins compared in diapause and in activated, anoxic encysted embryos of the animal extremophile, Artemia franciscana. J Insect Physiol. 2011;57:660–664. doi: 10.1016/j.jinsphys.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Haslbeck M. sHsps and their role in the chaperone network. Cell Mol Life Sci. 2002;59:1649–1657. doi: 10.1007/PL00012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JS, Go HJ, Goo TW, Yun EY, Choi KH, Seong SI. The analysis of differentially expressed novel transcripts in diapausing and diapause-activated eggs of Bombyx mori. Arch Insect Biochem Physiol. 2005;59:197–201. doi: 10.1002/arch.20057. [DOI] [PubMed] [Google Scholar]
- Iryani MTM, MacRae TH, Panchakshari S, Tan J, Bossier P, Wahid MEA. Knockdown of heat shock protein 70 (Hsp70) by RNAi reduces the tolerance of Artemia franciscana nauplii to heat and bacterial infection. J Exp Mar Biol Ecol. 2017;487:106–112. doi: 10.1016/j.jembe.2016.12.004. [DOI] [Google Scholar]
- Iryani MTM, MacRae TH, Sorgeloos P, Tengku-Muhammad TS, Danish-Daniel M, Min-Pau T, Satyantini WH, Abd-Wahid ME, Sun J, Lv A, Sung YY. RNA interference of Hsp70 in Artemia franciscana nauplii and its effect on morphology, growth, survival and immune response. Aquaculture. 2020;520(2020):735012. doi: 10.1016/j.aquaculture.2020.735012. [DOI] [Google Scholar]
- Junprung W, Norouzitallab P, De Vos S, Tassanakajon A, Viet DN, Van Stappen G, Bossier P. Sequence and expression analysis of HSP70 family genes in Artemia franciscana. Sci Rep. 2019;9:839. doi: 10.1038/s41598-019-44884-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AM. Elucidating the functions of proteins up-regulated during diapause in Artemia franciscana using RNAi. FEBS J. 2013;280:4761–4772. doi: 10.1111/febs.12442. [DOI] [PubMed] [Google Scholar]
- King AM, MacRae TH. The small heat shock protein p26 aids development of encysting artemia embryos, prevents spontaneous diapause termination and protects against stress. PLoS One. 2012;7(8):e43723. doi: 10.1371/journal.pone.0043723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AM, MacRae TH. Insect heat shock proteins during stress and diapause. Annu Rev Entomol. 2015;60:59–75. doi: 10.1146/annurev-ento-011613-162107. [DOI] [PubMed] [Google Scholar]
- King AM, Toxopeus J, MacRae TH. Artemin, a diapause-specific chaperone, contributes to the stress tolerance of Artemia cysts and influences their release from females. J Exp Biol. 2014;217:1719–1724. doi: 10.1242/jeb.100081. [DOI] [PubMed] [Google Scholar]
- Kityk R, Kopp J, Mayer MP. Molecular mechanism of J-domain triggered ATP hydrolysis by Hsp70 chaperones. Mol Cell. 2018;69:227–237. doi: 10.1016/j.molcel.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Koštál V, Tollarová-Borovanská M. The 70 kDa heat shock protein assists during the repair of chilling injury in the insect, Pyrrhocoris apterus. PLoS One. 2009;4(2):e4546. doi: 10.1371/journal.pone.0004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DF, Zhang MC, Yang HJ, Zhu YB, Xu X. Beta-integrin mediates WSSV infection. Virology. 2007;368:122–132. doi: 10.1016/j.virol.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Liang P, MacRae TH. The synthesis of a small heat shock/a-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev Biol. 1999;207:445–456. doi: 10.1006/dbio.1998.9138. [DOI] [PubMed] [Google Scholar]
- MacRae TH. Gene expression, metabolic regulation and stress tolerance during diapause. J Cell Mol Life Sci. 2010;67:2405–2424. doi: 10.1007/s00018-010-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak GE, Tacconi E, Czernik M, Toschi P, Modlinski JA, Loi P. Embryonic diapause is conserved across mammals. PLoS One. 2012;7(3):e33027. doi: 10.1371/journal.pone.0033027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart JP, Li A, Yocum GD, Robich RM, Hayward SAL, Denlinger DL. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. PNAS. 2007;104:11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowarth NM, MacRae TH. ArHsp40 and ArHsp40-2 contribute to stress tolerance and longevity in Artemia franciscana, but only ArHsp40 influences diapause entry. J Exp Biol. 2018;221:jeb189001. doi: 10.1242/jeb.189001. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Fukumoto K, Izumi Y, Yoshida H, Tsumuki H. Cloning of heat shock protein genes (Hsp90 and Hsc70) and their expression during larval diapause and cold tolerance acquisition in the rice stem borer Chilo suppressalis (Walker) Arch Insect Biochem Physiol. 2006;63:36–47. doi: 10.1002/arch.20138. [DOI] [PubMed] [Google Scholar]
- Toxopeus J, Warner AH, MacRae TH. Group 1 LEA proteins contribute to the desiccation and freeze tolerance of Artemia franciscana embryos during diapause. Cell Stress Chaperones. 2014;19:939–948. doi: 10.1007/s12192-014-0518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum GD. Differential expression of two Hsp70 transcripts in response to cold shock, thermoperiod, and adult diapause in the Colorado potato beetle. J Insect Physiol. 2001;47:1139–1145. doi: 10.1016/S0022-1910(01)00095-6. [DOI] [PubMed] [Google Scholar]
- Yocum GD, Kemp WP, Bosch J, Knoblett JN. Temporal variation in overwintering gene expression and respiration in the solitary bee Megachile rotundata. J Insect Physiol. 2005;51:621–629. doi: 10.1016/j.jinsphys.2004.11.008. [DOI] [PubMed] [Google Scholar]


