Abstract
The adoptive transfer of donor-derived virus-specific T cells (VSTs) is an effective treatment for infections following allogeneic hematopoietic cell transplantation. Acute exercise mobilizes effector lymphocytes and VSTs to the circulation and augments the ex vivo manufacture of VSTs. This study determined if β2 adrenergic receptor (AR) signaling precipitated the VST response to acute exercise. Healthy participants (n = 12) completed 30 min of steady-state cycling exercise after ingesting a placebo, a β1 + 2 AR antagonist (nadolol) or a β1 AR antagonist (bisoprolol). Circulating VSTs to cytomegalovirus (CMV), Epstein–Barr virus (EBV), and adenovirus (AdV) antigens were enumerated before and after exercise, and peripheral blood mononuclear cells were cultured with viral peptides for 8 days to expand multi-VSTs. Compared with placebo, nadolol blunted the exercise-induced mobilization of CMV-VSTs (Δ VSTs/100,000 CD3+ T cells = 93 ± 104 vs. 22 ± 91 for placebo and nadolol, respectively; p = 0.036), while bisoprolol did not, despite both drugs evoking similar reductions in exercising heart rate and blood pressure. Circulating AdV and EBV VSTs (VSTs/mL blood) only increased after exercise with placebo. Although not significant, nadolol partially mitigated exercise-induced increases in multi-VST expansion, particularly in participants that demonstrated an exercise-induced increase in VST expansion. We conclude that exercise-induced enhancements in VST mobilization and expansion are at least partially β2 AR mediated, thus highlighting a role for the β2 AR in targeted therapy for the augmentation of VST immune cell therapeutics in the allogeneic adoptive transfer setting. Moreover, long-term regular exercise may provide additional viral protection in the host through frequent β2 AR-dependent mobilization and redistribution of VSTs cumulated with each bout of exercise.
Keywords: Adoptive transfer immunotherapy, Catecholamines, Effector lymphocytes, Exercise immunology, Viral immunity, Stem cell transplantation
Introduction
Allogeneic hematopoietic cell transplantation (HCT) for the treatment of hematologic malignancies and nonmalignant disorders of the bone marrow and immune system provides prolonged, disease-free survival for recipients (Gyurkocza et al. 2010; Simmons et al. 2019); however, the period required for immune reconstitution following HCT leaves recipients immunocompromised and susceptible to viral infections (Bollard and Heslop 2016; Houghtelin and Bollard 2017; Leen et al. 2014), and patients with severe acute or chronic graft versus host disease are at a higher risk of viral infections (Miller et al. 2017). In the first 100 days after allogeneic HCT, 16% of all transplant-related fatalities are related to infections (D'Souza et al. 2017; Simmons et al. 2019), of which cytomegalovirus (CMV), adenovirus (AdV), and Epstein–Barr virus (EBV) are three of the most common (Kaeuferle et al. 2019; Leen et al. 2013; Naik et al. 2016). The adoptive transfer of donor-derived virus-specific T cells (VSTs) for both the treatment and prevention of these viral infections appears to be a safe and effective alternative to antivirals, which are often associated with significant toxicities and do not provide prolonged protection without T-cell restoration (Bollard and Heslop 2016; Keller and Bollard 2020; Leen et al. 2014; Naik et al. 2016). While VSTs have been shown to be effective in treating viral infections, rapidly generating sufficient numbers of VSTs for infusion remains a challenge (Bollard and Heslop 2016). Direct capture of VSTs from blood using activated T-cell or multimer selection rapidly isolates VSTs but generates limited numbers of cells for purity testing, infusion, and additional doses (Bollard and Heslop 2016; Hanley 2018; Leen et al. 2014; Lindemans et al. 2010). In contrast, the selective amplification of VSTs ex vivo generates large numbers of VSTs for infusion (Bollard and Heslop 2016; Leen et al. 2014; Lindemans et al. 2010). With advancements in the manufacture of VSTs, multi-virus VST populations can be generated in as few as 9–11 days, but the success of these techniques relies on sufficient numbers of VSTs in the peripheral blood to generate multi-VSTs that recognize a broad range of viruses and viral epitopes (Bollard and Heslop 2016; Gerdemann et al. 2012; Keller et al. 2019; Papadopoulou et al. 2014).
A single bout of dynamic exercise appears to be a safe and effective method for improving current VST manufacturing protocols (Heinemann et al. 2020; Kunz et al. 2018; Spielmann et al. 2016). Acute exercise elicits a transient but robust lymphocytosis, during which lymphocyte subsets exhibiting greater cytotoxic and effector functions are preferentially mobilized into the peripheral blood (Anane et al. 2009; Campbell et al. 2009; Simpson et al. 2010; Simpson et al. 2007), and the numbers of circulating functional VSTs specific for both CMV and AdV are elevated in response to exercise (Kunz et al. 2018; Spielmann et al. 2014). In addition, acute exercise augments the rapid manufacture (Gerdemann et al. 2012) of VSTs to AdV (Kunz et al. 2018) and the concomitant expansion of VSTs to CMV and EBV (Spielmann et al. 2016), while regular exercise/physical fitness appears to provide protection against latent viral reactivation and improvements in viral control (Agha et al. 2020; Simpson et al. 2017b). Therefore, exercise appears to be a simple but effective adjuvant for both the direct capture and the ex vivo manufacture of VSTs for a range of viruses.
In the present study, we sought to determine if the mobilization of VSTs and the augmented ex vivo expansion of multi-VSTs with exercise is β2 AR mediated. We did this by administering a non-preferential β1 + 2 AR antagonist to healthy humans in an attempt to block β2 AR-mediated VST mobilization and augmented expansion in response to exercise. We also administered a preferential β1 AR antagonist to control for non-β2 AR-dependent reductions in hemodynamic forces (e.g., heart rate and blood pressure) during exercise. We found that β1 + 2 AR but not β1 AR blockade blunted the mobilization of VSTs to CMV to the peripheral blood and tended to impair the enhancing effects of exercise on VST expansion. We contend that the β2 AR is a potential therapeutic target to augment the mobilization and ex vivo manufacture of VSTs from healthy donors in the allogeneic adoptive transfer immunotherapy setting. Moreover, regular exercise may provide additional viral protection in the host through the frequent β2 AR-dependent mobilization and redistribution of VSTs cumulated with each bout of exercise.
Materials and methods
Subjects
Twelve (three female) healthy, non-smoking subjects aged 22–43 years (mean age 31 years) with positive CMV and EBV IgG antibody serology participated in the study. CMV and EBV serostatus was determined using commercially available ELISA kits (BioCheck Inc., CA, USA and GenWay Biotech, CA, USA, respectively). Potential participants were screened for cardiovascular risk factors and contraindications to exercise via the American Heart Association/American College of Sports Medicine Health/Fitness Facility Preparticipation Screening Questionnaire (Balady et al. 1998). Height and weight were recorded, and the average body mass index of participants was 26.7 ± 4.06 kg/m2. All participants reported activity patterns equating to a score of ≥ 5 on a 0–7-point activity scale (corresponding to regular vigorous activity) (Jackson et al. 1990) for the month preceding study participation. Subjects were excluded if they had any contraindications to β-blocker administration, were pregnant or breast feeding, exhibited symptoms of asthma, or were taking any medications known to affect the immune system. Subjects were asked to refrain from alcohol consumption and exercise 48 h prior to each laboratory visit. After both verbal and written information regarding the risks and demands of the study was provided, written consent was obtained from each participant. All study procedures were reviewed and approved by the Committee for the Protection of Human Subjects at the University of Houston.
Study design and exercise trials
Following screening, subjects reported to the Laboratory of Integrated Physiology at the University of Houston for four subsequent visits, at the same time of day, separated by at least 1 week to ensure drug washout, but not more than 2 weeks. The first visit consisted of a discontinuous, incremental cycling protocol on a stationary indoor cycle ergometer (Velotron; Quarq Technology, Spearfish, SD, USA) to determine the individual blood lactate threshold, as previously described (Kunz et al. 2015). Briefly, capillary blood samples were collected from the earlobe at rest and after each 3-min cycling stage of increasing workload for the measurement of blood lactate concentration via an automated lactate analyzer (Analox P-GM7 Micro Stat; Analox, UK). The test was terminated one incremental stage after attaining a blood lactate concentration of > 4 mM. Following the test, the lactate threshold was determined visually using the breakpoint method, as described by Weltman (1995), in which the threshold is defined as the power output (W) corresponding to the first rise in blood lactate concentration over resting values.
The subsequent three visits consisted of 30 min of continuous steady-state cycling at a power output corresponding to 10% above the workload at the individual lactate threshold, selected to elicit a robust catecholamine response (Podolin et al. 1991) while still allowing subjects to complete the prescribed 30 min of steady-state exercise at a fixed workload with reduced heart rate and blood pressure. Three hours prior to each exercise trial, a baseline blood sample was collected from an antecubital vein following 5 min of seated rest (PRE) and subjects were administered a single dose of one of three medications: a placebo, a β1 preferential antagonist (bisoprolol 10 mg), or a β1 + 2 non-selective antagonist (nadolol 80 mg). As there are no currently available “selective” β2-AR antagonists for human use, a direct comparison of preferential β1-AR and β2-AR antagonists was not feasible. Therefore, we utilized the β1 + 2 non-selective antagonist nadolol, which has an ~23-fold β2-AR preference over the β1-AR (Baker 2005), and the β1 preferential antagonist bisoprolol. Importantly, we have shown that the standardized doses used in the present study reduce hemodynamic responses to exercise by a similar magnitude in the study population (~ 70 to ~ 110 kg) (Agha et al. 2018), allowing for the comparison of the effects of β1-AR antagonism and β1 + 2 non-selective antagonism under similar hemodynamic shear stress conditions. Therefore, differences observed between nadolol and placebo, but not between bisoprolol and placebo, could be attributed to the effects of β2-AR engagement and not the effects of changes in hemodynamic shear stress. Drugs were administered in a randomized, double-blind, counterbalanced fashion 3 h prior to exercise as peak serum concentrations of both nadolol and bisoprolol occur 2–4 h following oral consumption (Borchard 1998). During exercise, ECG (Quark C12x; COSMED, Pavona di Albano Laziale, Italy), heart rate, minute ventilation, and oxygen uptake (Quark CPET; COSMED) were measured continuously. The rating of perceived exertion (Borg 1973) was reported every 5 min, and blood lactate and blood pressure were measured every 10 min. Immediately following exercise, venous blood was collected from an antecubital vein (POST). Frozen serum samples from blood obtained PRE and POST were analyzed for epinephrine, norepinephrine, and cortisol levels using commercially available ELISA kits (2-CAT ELISA Fast Track and Cortisol ELISA; LDN, Nordhorn, Germany).
Blood processing
All blood samples were processed within 2 h of collection. Complete blood counts, including a white blood cell differential, were obtained from EDTA anti-coagulated blood via an automated hematology analyzer (Mindray BC3200; Mindray, Shenzhen, China). Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood using standard density gradient centrifugation procedures. Phenotyping and VST expansions were performed on freshly isolated PBMCs.
Multi-virus T-cell expansions
The expansion of CMV, EBV, and AdV-specific T cells was performed as previously described (Gerdemann et al. 2012; Spielmann et al. 2016). From the ~ 40 mL of blood collected before drug administration and immediately after exercise, 10 × 106 PBMCs could be consistently isolated for VST expansion. The isolated PBMCs (10 × 106) were pelleted in a 15-mL conical tube and pulsed for 1 h at 37 °C with commercially available peptide mixtures (PepMix™ Peptide Pools) of 15mers (overlapping by 11aa) spanning the CMV-pp65, CMV-IE-1, EBV-LMP2, EBV-BMLF1, AdV-hexon, and AdV-penton proteins (JPT Peptide Technologies, Berlin, Germany). Each 10 × 106 PBMC cell pellet was simultaneously pulsed with 400 ng of each peptide. Pulsed cells were resuspended in 10 mL of l-glutamine-enriched (2 mmol) RPMI 1640 media (Mediatech, Manassas, VA, USA) supplemented with 10% FBS. Media was also supplemented with IL-4 (1666 UI/mL; eBioscience, San Diego, CA, USA) and IL-7 (10 ng/mL, eBioscience), which were shown to selectively promote the expansion of CD4+ and CD8+ Th1-polarized cells (Gerdemann et al. 2012). Cells were then cultured at 37 °C in a gas-permeable G-Rex10 cell culture flask (Wilson-Wolf Manufacturing, New Brighton, MN, USA). Following the previously described protocols, cytokines were refreshed at day 5 of the culture. Briefly, cells were washed once with PBS and resuspended in fresh complete media. In addition to the 10 ng/mL of IL-7 and 1666 UI/mL IL-4, 5 ng/mL of IL-15 (eBioscience) was added, as IL-15 promotes cell growth, but when added at day 0 supports NK-cell outgrowth (Gerdemann et al. 2012). To prevent the need for splitting expanded cells and introducing variability, cells were harvested at day 8 of culture for subsequent analysis, as previously described (Spielmann et al. 2016).
VST enumeration
Enzyme-linked immunospot (ELISPOT) assays (eBioscience) were used to determine the number of functional VSTs producing IFN-γ in response to virus-specific peptide stimulation, as previously described (Spielmann et al. 2016). Freshly isolated PBMCs (100,000 cells/well) and the expanded cell populations (20,000 cells/well) were stimulated with 1 μg/mL of CMV-pp65, CMV-IE-1, EBV-LMP2, EBV-BMLF1, AdV-hexon, or AdV-penton peptide mixtures (JPT) on prepared IFN-γ ELISPOT multiscreen IP plates (EMD Millipore, Darmstadt, Germany) and incubated for 12 h at 37 °C. The response to each peptide was measured in triplicate. Cells stimulated with PHA (2 μg/mL; Sigma-Aldrich) served as positive controls, and unstimulated cells served as negative controls. The number of IFN-γ spot-forming cells (SFCs) was evaluated by Zellnet Consulting (Fort Lee, NJ, USA). The frequency of VSTs per 100,000 T cells (% of PBMCs positive for CD3, as determined by flow cytometry) was calculated by adjusting the number of IFN-γ SFCs by the number of input T cells, (SFC/input T cells × 105). The total number of VSTs put into culture on day 0 and generated following the 8-day expansion was calculated by accounting for the total number of T cells in culture (SFC per T cell × total T-cell number), and the total number of generated VSTs per VST stimulated at day 0 was calculated from these values (total VSTs on day 8/total VSTs in culture). On day 0, the total number of VSTs in whole blood was calculated by multiplying the number of SFCs per T cell by the total blood T-cell count (SFCs per T cell × total T cells per μL of blood). For each measure, the number of CMV-pp65-specific, CMV-IE-1-specific, EBV-LMP2-specific, EBV-BMLF1-specific, AdV-hexon-specific, and AdV-penton-specific T cells was determined. The number of VSTs specific for each virus was calculated by combining the number of cells specific for the corresponding individual antigens (e.g., pp65-specific and IE-1-specific T-cell numbers were combined to generate the total number of CMV-specific T cells). The total number of VSTs was determined by combining the number of cells specific for all individual peptides.
Phenotype analysis
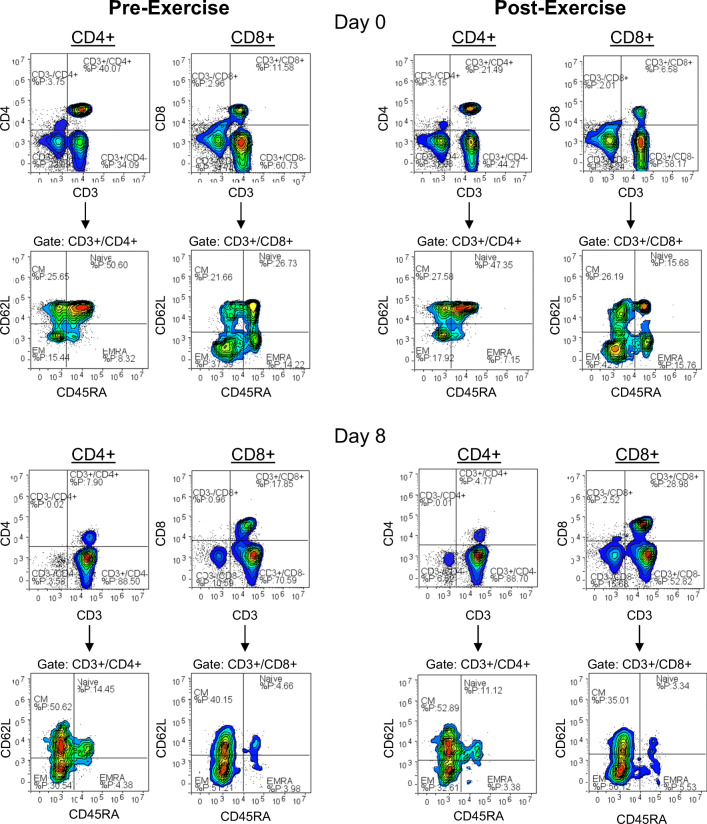
Flow cytometry was performed to determine the phenotypic characteristics of freshly isolated PBMCs on day 0 and of the expanded cells on day 8. Cells were labeled with FITC-conjugated anti-CD45RA, PE-conjugated anti-CD62L, PerCP Cy5.5-conjugated anti-CD4 or anti-CD8, PECy5.5 anti-CD56, and APC-conjugated anti-CD3 (eBioscience) in a four-color direct immunofluorescence assay. Cells (100,000) were incubated for 30 min in the dark at room temperature with pre-diluted monoclonal antibodies and phenotypes were assessed using a four-color Accuri C6 flow cytometer (Accuri BD, Ann Arbor, MI, USA). For phenotypic analysis, the lymphocyte population was gated using forward and side-scatter characteristics. NK cells were identified as CD56+/CD3− cells within the lymphocyte gate. T cells were identified using side-scatter against APC fluorescence-identified CD3+ cells. Within the CD3+ cells, the expression of CD4 and CD8 was determined. Subsequently, the expression of CD45RA/CD62L on CD3+CD4+ and CD3+CD8+ cells was examined to determine the proportions of naïve (CD45RA+/CD62L+), central memory (CM; CD45RA−/CD62L+), effector memory (EM; CD45RA−/CD62L−), and CD45RA+ effector memory (EMRA; CD45RA+/CD62L−) subsets within the CD4+ and CD8+ T cell populations (Fig. 1) (Appay et al. 2008; Sallusto et al. 2004). The total number of T cells and NK cells was calculated by multiplying the corresponding percentages of positively stained cells in the lymphocyte gate by the total lymphocyte count obtained from the hematology analyzer. The total numbers of CD4+ and CD8+ T cells were calculated from the total number of T cells, and the numbers of T cells in each subset were calculated from the total numbers of CD4+ and CD8+ T cells.
Fig. 1.
Gating strategy and representative flow cytometry contour plots for identifying CD4+ and CD8+ T-cell subset populations. The phenotypic characteristics of freshly isolated PBMCs (day 0) and expanded cell products (day 8) from blood obtained pre- and post-exercise were assessed in a four-color direct immunofluorometric assay. Within the CD3+/CD4+ and CD3+/CD8+ T-cell populations, the proportions of naïve, central memory (CM), effector memory (EM), and CD45RA+ effector memory (EMRA) cells were assessed. Representative flow cytometry contour plots for pre- and post-exercise freshly isolated PBMCs and expanded cell products are displayed
The phenotypes of the manufactured VSTs were determined from cryopreserved expanded cell products (CMV n = 6; EBV n = 5, AdV n = 5). VSTs in the manufactured cells were identified using a commercially available IFN-γ Secretion Assay Kit (Miltenyi Biotech, Bergisch, Gladbach, Germany), and the phenotypes of IFN-γ expressing VSTs were resolved via a MACSQuant Analyzer 10 Flow Cytometer (Miltenyi Biotech, Bergisch, Gladbach, Germany). Briefly, thawed cells were resuspended and incubated in RPMI + 5% human serum (1 × 106 cells/mL) and supplemented with 200 IU/mL IL-2 overnight. Subsequently, cells were resuspended in RPMI + 5% human serum and plated in a U-bottom 96-well plate (1 × 107 cells/mL). Cells were incubated with peptides specific to CMV (IE1 and pp65 at 50 μg/mL each), ADV (hexon and penton at 35.7 and 38.5 μg/mL, respectively), and EBV (consensus peptides at 38.8 μg/mL) for 3 h at 37 °C. Pre-exercise cells pooled from multiple trials were unstimulated or stimulated with Cytostim to serve as the negative and positive controls, respectively. Following incubation, cells were washed and incubated with IFN-γ capture reagent for 45 min at 37 °C. The cells were subsequently washed and incubated for 30 min in the dark with anti-CD45, anti-CD8, anti-CD4, and IFN-γ detection antibody (Miltenyi Biotech, Bergisch, Gladbach, Germany). Propidium iodide was added to identify viable cells. Forward and side light scatter and side scatter against CD45 were used to identify lymphocyte populations. Within the lymphocytes, the VSTs were identified as IFN-γ-expressing cells using side scatter against the IFN-γ detection antibody, and CD4 and CD8 expression was evaluated within this population.
Statistical analysis
Data were tested for the assumptions of normality prior to statistical testing. Variables with non-normal distributions were logarithmically transformed. Variables failing to meet the assumption of normality following transformation were analyzed using non-parametric statistical tests. All data are presented in their original metrics. To examine the effects of exercise under each condition, differences from PRE to POST within each trial were tested using paired t tests or Wilcoxon rank-sum tests (non-parametric) with Bonferroni corrections for multiple comparisons. Paired t tests or Wilcoxon rank-sum tests with Bonferroni corrections were also utilized to determine the efficacy of the expansion (day 0 to day 8). One-way repeated measures analyses of variance (ANOVAs) or Friedman’s tests (non-parametric) were performed to determine the effects of bisoprolol (β1 AR blockade) and nadolol (β1 + 2 AR blockade) on the physiological response to exercise. Absolute and percent changes from PRE to POST were calculated for the VST populations, and repeated measures ANOVAs or Friedman’s tests were performed to examine differences between the three drug trials. Planned Bonferroni-corrected post hoc contrasts between each of the trials were performed (paired t tests or Wilcoxon rank-sum tests). The effects of drug administration on exercise responses were examined with two-way repeated measures ANOVAs, testing the main effects of exercise (two levels—PRE vs. POST) and drug condition (3 levels—placebo, nadolol, and bisoprolol) and the interaction between exercise and drug condition. Mauchly’s test was used to test the assumption of sphericity, and when data did not meet this assumption, the Greenhouse–Geisser values are reported. Planned Bonferroni-corrected post hoc contrasts between PRE and POST exercise were performed for all trials, regardless of the main effects of exercise. Statistical significance was set a priori at 0.05 for all statistical tests, and all data analysis was performed using “Statistical Package for the Social Sciences” (SPSS) version 25 software (IBM, Chicago, IL, USA).
Results
β1 and β1 + 2 AR blockade are comparable at lowering exercising heart rate and blood pressure
All participants successfully completed the three exercise trials at + 10% of lactate threshold, and the physiological responses to exercise are presented in Table 1. Bisoprolol (β1 AR preferential antagonist) and nadolol (β1 + 2 AR non-selective antagonist) similarly reduced exercising heart rate and systolic blood pressure (p < 0.001) compared with placebo. Subjects’ ratings of perceived exertion (RPE) were higher while exercising on bisoprolol (p = 0.036) and there was a trend for RPE to be higher on nadolol compared with placebo (p = 0.069). Oxygen consumption (VO2) and ventilation during exercise were unaffected by drug administration. In all three trials, blood lactate, epinephrine, and norepinephrine were significantly elevated POST (p < 0.05), while cortisol was only significantly elevated POST in the nadolol trial (Table 2).
Table 1.
Physiological responses to exercise with or without adrenergic receptor blockade
| Trial F (p value) | ||||
|---|---|---|---|---|
| Placebo | Bisoprolol | Nadolol | ||
| Cycling power (W) | 140.0 (34.7) | 140.0 (34.7) | 140.0 (34.7) | ND |
| Exercising heart rate (bpm) | 158.4 (9.97) | 121.8 (6.81)* | 117.8 (8.75)* | 120.056 (< 0.001) |
| Systolic blood pressure (mmHg) | 161.2 (13.1) | 146.1 (12.7)* | 138.9 (13.1)* | 20.765 (< 0.001) |
| Diastolic blood pressure (mmHg) | 77.3 (6.1) | 75.9 (12.1) | 74.6 (6.4) | 0.500 (0.535) |
| VO2 (mL/kg/min) | 31.0 (6.3) | 30.2 (6.7) | 29.7 (5.3) | 1.464 (0.253) |
| Minute ventilation (L/min) | 70.3 (18.9) | 68.5 (20.2) | 68.0 (16.8) | 0.570 (0.574) |
| RPE (Borg (1973) 6–20 Scale)a | 14 (2.8) | 16 (4.5)* | 16 (5.0) | (0.008) |
Heart rate, VO2, and ventilation were measured continuously during a 30-min bout of cycling exercise performed 3 h after the ingestion of a single dose of one of three medications: a placebo, a β1 preferential antagonist (bisoprolol 10 mg), or a β1 + 2 non-selective antagonist (nadolol 80 mg). RPE was recorded every 5 min, and blood pressure was measured every 10 min. For each parameter, the averages during the last 20 min of exercise were calculated for each subject. n = 12 for all parameters. Data are expressed as mean (SD) for normally distributed data, and median (IQR) for non-normal data on which non-parametric tests were performed
BPM beats per minute, BP blood pressure, RPE rating of perceived exertion
aNon-parametric tests were performed and data are presented as median (IQR)
*Significantly different from placebo (p < 0.05)
Table 2.
Catecholamine, lactate, and cortisol responses to exercise with or without adrenergic receptor blockade
| Pre-Ex | Post-Ex | Time effect t (p value) | ||
|---|---|---|---|---|
| Epinephrine (ng/mL) | Placebo | 0.049 (0.038) | 0.142 (0.101)* | − 5.092 (< 0.001) |
| n = 12 | Bisoprolol | 0.048 (0.035) | 0.146 (0.053)* | − 6.099 (< 0.001) |
| Nadolol | 0.046 (0.037) | 0.255 (0.184)* | − 7.743 (< 0.001) | |
| Norepinephrine (ng/mL) | Placebo | 0.725 (0.357) | 1.748 (0.936)* | − 6.358 (< 0.001) |
| n = 12 | Bisoprolol | 0.687 (0.296) | 1.879 (0.750)* | − 7.892 (< 0.001) |
| Nadolol | 0.824 (0.466) | 1.629 (0.745)* | − 8.450 (< 0.001) | |
| Lactate (mmol) | Placebo | 0.57 (0.30) | 2.91 (0.94)* | − 7.957 (< 0.001) |
| n = 12 | Bisoprolol | 0.76 (0.31) | 3.73 (1.28)* | − 7.408 (< 0.001) |
| Nadolol | 0.62 (0.24) | 2.82 (0.81)* | − 8.644 (< 0.001) | |
| Cortisol (ng/mL)a | Placebo | 165 (184) | 181 (74) | − 0.863 (0.999) |
| n = 12 | Bisoprolol | 206 (132) | 255 (90) | − 0.471 (0.999) |
| Nadolol | 168 (122) | 231 (110)* | − 2.432 (0.045) |
Catecholamine, cortisol, and lactate were measured at rest and after a 30-min bout of cycling exercise performed 3 h after the ingestion of a single dose of one of three medications: a placebo, a β1 preferential antagonist (bisoprolol 10 mg), or a β1 + 2 non-selective antagonist (nadolol 80 mg). Plasma cortisol and catecholamine levels were measured in plasma obtained prior to drug administration and immediately post-exercise. Blood lactate was measured before exercise and every 10 min during exercise. Post values represent the average blood lactate during exercise. Data are expressed as mean (SD) for normally distributed data, and median (IQR) for non-normal data on which non-parametric tests were performed. Bold values indicate a Bonferroni-corrected significant difference (p < 0.05) between pre- and post-exercise within a trial
aNon-parametric tests were performed and data are presented as median (IQR)
*Significantly different from pre-exercise (p < 0.05)
β1 + 2 but not β1 AR blockade blunts the mobilization of exercise-responsive leukocyte subtypes to the blood compartment
The effects of exercise on the numbers of leukocytes, lymphocytes, and lymphocyte subsets for each drug trial are presented in Table 3. A significant (p < 0.05) leukocytosis and lymphocytosis following exercise was observed for all three drug trials. Further, regardless of drug condition, the numbers of NK cells, CD3+ T cells, CD8+ T cells, and CM and EM CD8+ T cells increased POST; however, the number of the highly differentiated EMRA CD8+ T cells more than doubled in the placebo (percent increase of 147%; p = 0.030) and bisoprolol trials (percent increase of 106%, p = 0.090), while only marginal increases were observed POST in the nadolol trial (percent increase of 37%, p = 0.234), indicating a β2 AR-mediated reduction in the mobilization of EMRA cells.
Table 3.
Leukocyte and lymphocyte responses to exercise with or without adrenergic receptor blockade
| Pre-Ex | Post-Ex | Time effect F(p value) | ||
|---|---|---|---|---|
| WBC (×103/μL)a | Placebo | 5.6 (1.3) | 9.0 (2.4)* | 3.059 (0.006) |
| n = 12 | Bisoprolol | 5.5 (0.8) | 9.8 (2.2)* | 3.061 (0.006) |
| Nadolol | 5.8 (1.6) | 8.0 (2.1)* | 3.059 (0.006) | |
| Lymphocytes (×103/μL) | Placebo | 2.2 (0.4) | 3.3 (0.5)* | − 7.970 (< 0.001) |
| n = 12 | Bisoprolol | 2.1 (0.4) | 3.5 (0.3)* | − 8.602 (< 0.001) |
| Nadolol | 2.0 (0.4) | 2.8 (0.8)* | − 4.736 (0.001) | |
| NK cells (cells/μL) | Placebo | 199 (93) | 727 (173)* | − 11.288 (< 0.001) |
| n = 10 | Bisoprolol | 235 (101) | 912 (290)* | − 6.982 (< 0.001) |
| Nadolol | 187 (85) | 484 (163)* | − 6.475 (< 0.001) | |
| CD3 T cells (×103/μL) | Placebo | 1.6 (0.4) | 2.1 (0.5)* | − 3.741 (0.009) |
| n = 12 | Bisoprolol | 1.6 (0.4) | 2.2 (0.3)* | − 5.809 (< 0.001) |
| Nadolol | 1.5 (0.4) | 2.0 (0.6)* | − 3.382 (0.018) | |
| CD4+ T cells (cells/μL) | Placebo | 854 (339) | 904 (316) | − 0.725 (0.999) |
| n = 12 | Bisoprolol | 790 (288) | 910 (347) | − 2.089 (0.183) |
| Nadolol | 790 (329) | 968 (489) | − 1.954 (0.231) | |
| Naïve (cells/μL) | Placebo | 400 (234) | 366 (190) | 0.484 (0.999) |
| n = 10 | Bisoprolol | 364 (151) | 324 (115) | 1.313 (0.666) |
| Nadolol | 388 (280) | 442 (346) | − 1.013 (0.999) | |
| CM (cells/μL) | Placebo | 252 (46) | 302 (69) | − 2.675 (0.075) |
| n = 10 | Bisoprolol | 219 (57) | 271 (77)* | − 3.186 (0.033) |
| Nadolol | 235 (66) | 329 (96) | − 2.932 (0.051) | |
| EM (cells/μL)a | Placebo | 120 (101) | 146 (100) | 1.478 (0.417) |
| n = 10 | Bisoprolol | 137 (81) | 164 (107) | 2.293 (0.066) |
| Nadolol | 110 (47) | 155 (90) | 1.682 (0.279) | |
| EMRA (cells/μL) | Placebo | 15 (11) | 26 (31) | − 0.340 (0.999) |
| n = 10 | Bisoprolol | 15 (12) | 24 (23) | − 1.187 (0.798) |
| Nadolol | 15 (13) | 13 (14) | 1.403 (0.582) | |
| CD8+ T cells (cells/μL) | Placebo | 532 (210) | 773 (300)* | − 4.542 (0.003) |
| Bisoprolol | 531 (180) | 815 (325)* | − 5.988 (< 0.001) | |
| Nadolol | 463 (180) | 628 (254)* | − 4.964 (< 0.001) | |
| Naïve (cells/μL) | Placebo | 280 (170) | 318 (169) | − 1.366 (0.615) |
| n = 10 | Bisoprolol | 268 (155) | 296 (152) | − 1.747 (0.345) |
| Nadolol | 240 (145) | 308 (204) | − 1.895 (0.273) | |
| CM (cells/μL) | Placebo | 37 (11) | 70 (36)* | − 3.608 (0.018) |
| n = 10 | Bisoprolol | 37 (10) | 78 (34)* | − 4.939 (0.003) |
| Nadolol | 33 (13) | 64 (31)* | − 4.638 (0.003) | |
| EM (cells/μL) | Placebo | 94 (37) | 162 (80)* | − 3.568 (0.018) |
| n = 10 | Bisoprolol | 104 (40) | 188 (48)* | − 7.212 (< 0.001) |
| Nadolol | 89 (39) | 139 (56)* | − 4.386 (0.006) | |
| EMRA (cells/μL) | Placebo | 86 (58) | 213 (166)* | − 3.268 (0.030) |
| n = 10 | Bisoprolol | 103 (65) | 212 (172) | − 2.572 (0.090) |
| Nadolol | 78 (47) | 107 (73) | − 1.986 (0.234) |
The effects of an acute bout of exercise on the total number (per μL of whole blood) of white blood cells, lymphocytes, and lymphocyte subsets in healthy participants after ingesting a placebo, a β1 AR antagonist (bisoprolol, 10 mg), or a β1 + 2 AR antagonist (nadolol, 80 mg) 3 h prior to exercise. Blood was collected prior to medication ingestion and immediately following 30 min of steady-state cycling exercise. WBC and lymphocyte counts were quantified using an automated hematology analyzer, and the lymphocyte subset proportions identified by flow cytometry were used to quantify lymphocyte subset numbers. WBC = white blood cell; naïve = CD45RA+/CD62L+; CM = central memory, CD45RA−/CD62L+; EM = effector memory, CD45RA−/CD62L−; EMRA = CD45RA+ effector memory, CD45RA+/CD62L−. Data are expressed as mean (SD) for normally distributed data and median (IQR) for non-normal data on which non-parametric tests were performed. Bold values indicate a Bonferroni-corrected significant difference (p < 0.05) between pre- and post-exercise within a trial
aNon-parametric tests were performed and data are presented as median (IQR)
*Significantly different from pre-exercise (p < 0.05)
Exercise mobilizes VSTs to the blood under the influence of β AR signaling
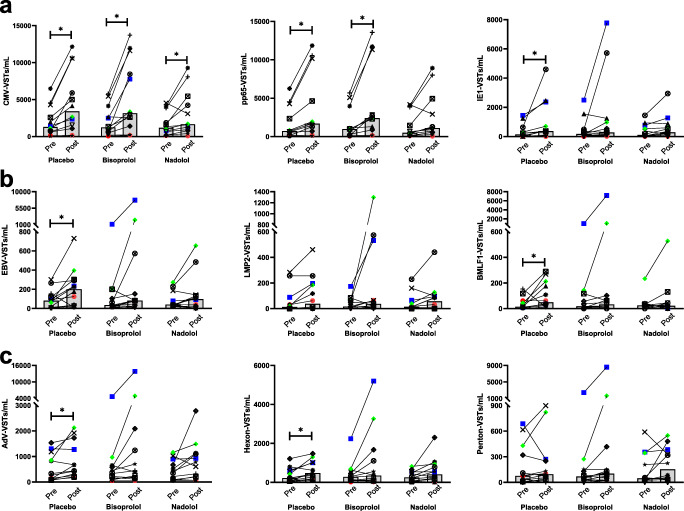
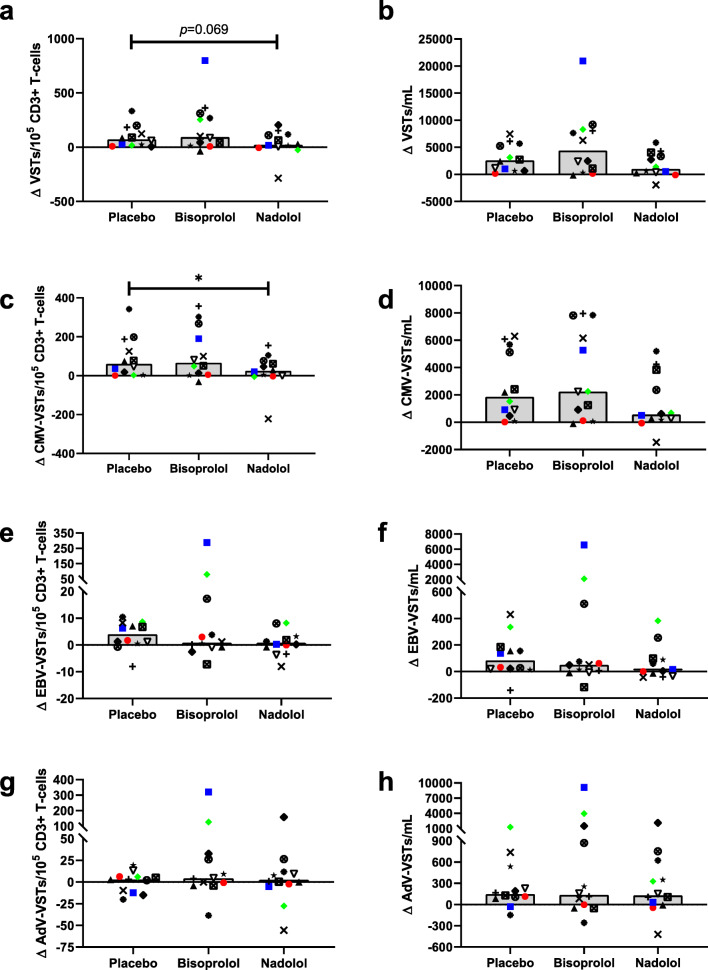
The effects of exercise with and without β AR blockade on the number (VST/mL) and frequency (VSTs/100,000 CD3+ T cells) of the individual peptide-specific T cells are presented in Fig. 2 and Table 4, respectively. Interestingly, the effects of exercise on the individual peptides for each virus were sometimes divergent within a subject; therefore, for subsequent analyses, we combined the two peptides for each virus and examined the virus-specific response rather than the peptide-specific response to exercise and β AR blockade. The number of circulating total VSTs (VSTs/mL) (Table 4) and the number of VSTs to CMV (Fig. 2a) increased following exercise in all three drug trials. In contrast, the frequency of VSTs (VSTs/100,000 CD3+ T cells) was only significantly (p < 0.05) greater POST in the placebo and bisoprolol trials, indicating an attenuation in the preferential mobilization of these cells following β1 + 2 AR blockade (nadolol) (Table 4). A significant exercise-induced mobilization of circulating VSTs specific to EBV and AdV was only observed in the placebo trial (Fig. 2b, c). Therefore, the mobilization of these cells may be either β1 AR mediated or the result of increased hemodynamic forces associated with exercise, as both nadolol and bisoprolol lowered exercising heart rate and blood pressure to the same degree. The effects of placebo, β1 + 2 AR blockade (nadolol), and β1 AR blockade (bisoprolol) on the exercise-induced mobilization of VSTs were examined by comparing the absolute changes from pre- to post-exercise in the numbers and frequency of circulating VSTs (Fig. 3). Significant differences in the mobilization of total VSTs per 100,000 CD3+ T cells were observed between the three drug trials (main effects of drug, p = 0.039), with nadolol showing the smallest increase in the frequency of VSTs (VSTs per 100,000 CD3+ T cells) following exercise (Fig. 3a), although the pairwise comparison between nadolol and placebo was not statistically significantly different after controlling for the multiple comparisons (p = 0.069). The total VST response was driven largely by the number of CMV-specific VSTs. Compared with placebo, nadolol significantly (p = 0.036) attenuated the mobilization of CMV-specific VSTs per 100,000 CD3+ T cells (Fig. 3c) and the influx of CMV-specific VSTs into circulation after exercise was smallest for nadolol (Fig. 3d). An exercise-induced increase in the proportion of EBV- and AdV-specific VSTs within the CD3+ T-cell pool (VSTs/100,000 CD3+ T cells) was not observed in the present study (Table 4), and neither bisoprolol nor nadolol administration statistically significantly affected their mobilization (Fig. 3e–h).
Fig. 2.
The frequency of virus-specific T cells in peripheral blood pre- and post-exercise. The number of circulating T cells specific for (a) CMV, (b) EBV, and (c) AdV was assessed in blood samples obtained from healthy participants after ingesting a placebo, a β1 AR antagonist (bisoprolol, 10 mg), or a β1 + 2 AR antagonist (nadolol, 80 mg) 3 h prior to exercise (pre) and immediately following 30 min of steady-state cycling exercise. The number of VSTs specific for each individual viral peptide were determined by IFN-γ ELISPOT, (a) the number of VSTs to CMV was determined by combining the number of VSTs to IE-1 and pp65, (b) the number of EBV-specific T cells was determined by combining the number of VSTs to LMP2 and BMLF1, and (c) the number of AdV-specific T cells was determined by combining the number of VSTs to hexon and penton. Bars represent the median value. n = 12 for all graphs excluding pp65 (n = 11). *Represents a significant (p < 0.05) Bonferroni-corrected p values comparing pre- with post-exercise. CMV = cytomegalovirus; EBV = Epstein-Barr virus; AdV = adenovirus
Table 4.
VST mobilization in response to acute exercise with or without adrenergic receptor blockade
| Pre-Ex | Post-Ex |
Time effect statistic (p value) |
||
|---|---|---|---|---|
| Total VSTs | ||||
| /100,000 CD3+ | Placebo (n = 12) | 192 (182) | 288 (262)* | − 3.780 (0.009) |
| Bisoprolol (n = 12) | 208 (195) | 395 (401)* | − 3.991 (0.006) | |
| Nadolol (n = 12) | 180 (171) | 214 (172) | − 1.300 (0.660) | |
| /mL blood | Placebo (n = 12) | 2726 (2235) | 5764 (4463)* | − 6.319 (< 0.001) |
| Bisoprolol (n = 12) | 2966 (2621) | 8529 (8201)* | − 5.668 (< 0.001) | |
| Nadolol (n = 12) | 2245 (1739) | 4033 (3120)* | − 3.044 (0.033) | |
| VSTs to CMV | ||||
| /100,000 CD3+ | Placebo (n = 12) | 144 (161) | 237 (253)* | − 3.759 (0.009) |
| pp65 (n = 11) | 134 (169) | 210 (269)* | − 3.064 (0.036) | |
| IE1a (n = 12) | 9 (28) | 20 (89)* | − 2.934 (0.009) | |
| Bisoprolol (n = 12) | 145 (143) | 260 (243)* | − 3.509 (0.015) | |
| pp65 (n = 11) | 122 (148) | 215 (258)* | − 3.085 (0.036) | |
| IE1a (n = 12) | 16 (22) | 14 (51) | − 0.941 (0.999) | |
| Nadolol (n = 12) | 138 (152) | 160 (168) | − 1.270 (0.690) | |
| pp65 (n = 11) | 125 (160) | 143 (177) | − 1.341 (0.630) | |
| IE1a (n = 12) | 7 (45) | 16 (51) | − 1.334 (0.546) | |
| VSTs to EBV | ||||
| /100,000 CD3+ | Placeboa (n = 12) | 5.6 (6.9) | 9.9 (12) | − 2.197 (0.084) |
| LMP2a (n = 12) | 0.9 (4.6) | 2.0 (9.7) | − 1.260 (0.624) | |
| BMLF1a (n = 12) | 1.1 (3.0) | 2.8 (6.7) | − 2.118 (0.102) | |
| Bisoprolola (n = 12) | 2.2 (9.1) | 4.0 (15) | − 1.334 (0.546) | |
| LMP2a (n = 12) | 1.1 (4.3) | 1.6 (15) | − 1.376 (0.507) | |
| BMLF1a (n = 12) | 1.3 (5.6) | 1.5 (3.3) | − 0.178 (0.999) | |
| Nadolola (n = 12) | 3.6 (7.6) | 4.3 (7.2) | − 0.549 (0.999) | |
| LMP2a (n = 12) | 0.8 (3.7) | 2.5 (5.8) | − 1.632 (0.519) | |
| BMLF1a (n = 12) | 1.6 (2.4) | 1.2 (2.6) | − 0.471 (0.999) | |
| VSTs to AdV | ||||
| /100,000 CD3+ | Placebo (n = 12) | 41 (43) | 41 (34) | − 2.030 (0.201) |
| Hexon (n = 12) | 27 (31) | 31 (26) | − 2.449 (0.096) | |
| Pentona (n = 12) | 3.8 (29) | 4.5 (13) | − 1.255 (0.627) | |
| Bisoprolol (n = 12) | 52 (95) | 92 (189) | − 0.909 (0.999) | |
| Hexon (n = 12) | 32 (46) | 48 (19) | − 0.528 (0.999) | |
| Pentona (n = 12) | 3.8 (7.8) | 4.6 (12) | − 13,804 (0.213) | |
| Nadolol (n = 12) | 36 (34) | 46 (56) | − 0.834 (0.999) | |
| Hexon (n = 12) | 25 (42) | 35 (34) | − 0.495 (0.999) | |
| Pentona (n = 12) | 3.0 (17) | 6.9 (18) | − 0.533 (0.594) | |
The effects of an acute bout of exercise on the frequency (per 100,000 CD3+ T cells) and the total number (per mL of whole blood) of VSTs in healthy participants after ingesting a placebo, a β1 AR antagonist (bisoprolol, 10 mg), or a β1 + 2 AR antagonist (nadolol, 80 mg) 3 h prior to exercise. Blood was collected prior to medication ingestion and immediately following 30 min of steady-state cycling exercise. The number of VSTs specific for each individual viral peptide was determined by IFN-γ ELISPOT. The number of VSTs to CMV was determined by combining the number of VSTs to IE-1 and pp65, the number of EBV-specific T cells was determined by combining the number of VSTs to LMP2 and BMLF1, and the number of AdV-specific T cells was determined by combining the number of VSTs to hexon and penton. Total VSTs were calculated by combining the number of VSTs to CMV, EBV, and AdV. Data are expressed as mean (SD) for normally distributed data and median (IQR) when non-parametric tests were used for non-normal data. Bold values indicate a Bonferroni-corrected significant difference (p < 0.05) between pre- and post-exercise within a trial
VST virus-specific T cell, CMV cytomegalovirus, EBV Epstein–Barr virus, AdV adenovirus
aNon-parametric tests were performed and data are presented as median (IQR)
*Significantly different from pre-exercise (p < 0.05)
Fig. 3.
The effects of exercise and β AR blockade on the frequency and number of circulating VSTs. The absolute change from pre- to post-exercise in the frequency (per 100,000 CD3+ T cells) and number (per mL of whole blood) of VSTs in healthy participants (n = 12) after ingesting a placebo, a β1 AR antagonist (bisoprolol, 10 mg), or a β1 + 2 AR antagonist (nadolol, 80 mg) 3 h prior to exercise. Blood samples were obtained prior to medication ingestion and immediately following exercise. The number of VSTs specific for each virus was determined by IFN-γ ELISPOT. The change in the frequency of VSTs (left) and the number of circulating VSTs (right) for each drug trial are presented for (a, b) the total number of VSTs, determined by combining the number of VSTs to CMV, EBV, and AdV; (c, d) the number of VSTs to CMV, determined by combining the number of VSTs to IE-1 and pp65; (e, f) the number of EBV-specific T cells, determined by combining the number of VSTs to LMP2 and BMLF1 and (g, h) the number of AdV-specific T cells, determined by combining the number of VSTs to hexon and penton. Bars represent the median value. Bonferroni-corrected pair-wise comparison p values are presented. *Represents a significant (p < 0.05) pairwise difference between two conditions. VST = virus-specific T cell; CMV = cytomegalovirus; EBV = Epstein–Barr virus; AdV = adenovirus
Exercise-induced increases in the expansion of VSTs are mitigated by β1 + 2 AR blockade prior to exercise
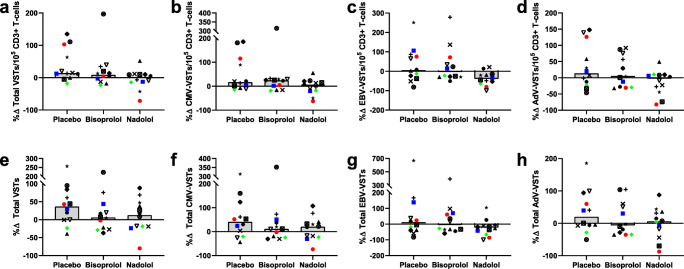
VSTs specific to CMV, EBV, and AdV were successfully generated under all conditions (Table 5). In the placebo trial, exercise increased the frequency of expanded cells that were specific to CMV, EBV, or AdV (expressed as total VSTs/100,000 CD3+ T cells at day 8; p = 0.021; Table 6). The total number of VSTs generated after the 8-day expansion was greater post-exercise compared with pre-exercise in the placebo condition, but this was not statistically significant (p = 0.057). Exercise-induced increases in generated VSTs were not observed in either the bisoprolol or nadolol trials (Table 6). Although under placebo conditions exercise did not enhance the manufacture of VSTs specific to EBV, in the nadolol trial a significant reduction in the frequency of manufactured cells that were specific to EBV was observed following exercise (p = 0.012; Table 6). To examine the mediating effects of β AR blockade on VST expansion, the percent change from pre- to post-exercise in the frequency and number of manufactured VSTs was calculated (Fig. 4). The administration of nadolol and bisoprolol did not significantly affect the exercise-induced changes in the manufacture of VSTs. The lack of statistical significance is likely partially due to the small sample size and large variability; however, in those individuals in which a robust exercise response was observed in the placebo condition, β AR blockade, and β1 + 2 AR blockade (nadolol) in particular, may have impaired exercise-induced increases in expanded VST number and frequency (Fig. 4). Although not statistically significant, for the majority of subjects in which exercise augmented VST expansion, nadolol attenuated the pre- to post-exercise increases in the frequency of manufactured cells that were VSTs (p = 0.150; 9/10 subjects with positive percent change values in the placebo condition; Fig. 4a), VSTs to CMV (p = 0.123; 8/9 subjects; Fig. 4b), VSTs to EBV (p = 0.150; 6/7 subjects; Fig. 4c), and VSTs to AdV (p = 0.519; 7/7 subjects; Fig. 4d). A similar pattern was observed in the total number of generated VSTs. In those subjects who saw a positive percent change in the placebo trial, the percent change in the nadolol trial was lower than that in the placebo trial in 6/8 subjects for total generated multi-VSTs (Fig. 4e) and 8/9 subjects for total generated VSTs to CMV (Fig. 4f). The number of VSTs generated on a per-cell basis (VSTs generated at day 8/VSTs cultured at day 0) was unaffected by exercise or β AR blockade (data not shown).
Table 5.
Stimulation of peripheral blood mononuclear cells with viral peptides and growth cytokines increases VSTs
| Day 0 | Day 8 | t statistic (p value) | ||
|---|---|---|---|---|
| Total VSTs (×103) | ||||
| Pre-Exercise | Placebo | 14.0 (12.9) | 208.3 (165.0)* | − 9.967 (< 0.001) |
| n = 12 | Bisoprolol | 15.4 (14.0) | 276.2 (249.0)* | − 17.670 (< 0.001) |
| Nadolol | 12.9 (12.1) | 240.2 (244.4)* | − 10.680 (< 0.001) | |
| Post-Exercise | Placebo | 17.9 (14.6) | 283.9 (211.8)* | − 9.431 (< 0.001) |
| n = 12 | Bisoprolol | 24.1 (23.4) | 302.4 (267.0)* | − 12.205 (< 0.001) |
| Nadolol | 14.6 (11.2) | 264.6 (251.6)* | − 10.172 (< 0.001) | |
| VSTs to CMV (×103) | ||||
| Pre-Exercise | Placebo | 10.5 (11.3) | 159.3 (139.8)* | − 8.135 (< 0.001) |
| n = 12 | Bisoprolol | 10.8 (10.8) | 204.1 (205.9)* | − 17.149 (< 0.001) |
| Nadolol | 9.8 (10.8) | 168.9 (199.3)* | − 9.541 (< 0.001) | |
| Post-Exercise | Placebo | 14.6 (14.1) | 220.2 (177.6)* | − 7.077 (< 0.001) |
| n = 12 | Bisoprolol | 15.9 (14.2) | 236.6 (220.2)* | − 12.184 (< 0.001) |
| Nadolol | 10.9 (10.9) | 182.3 (189.9)* | − 9.554 (< 0.001) | |
| VSTs to EBV (×103) | ||||
| Pre-Exercise | Placebo | 0.5 (0.5) | 10.2 (8.0)* | − 11.654 (< 0.001) |
| n = 12 | Bisoprolol | 0.8 (1.6) | 13.0 (12.1)* | − 6.9541 (< 0.001) |
| Nadolol | 0.5 (0.5) | 10.4 (8.3)* | − 7.351 (< 0.001) | |
| Post-Exercise | Placebo | 0.6 (0.6) | 9.5 (4.9)* | − 6.899 (< 0.001) |
| n = 12 | Bisoprolol | 2.6 (6.3) | 10.4 (6.9)* | − 6.212 (< 0.001) |
| Nadolol | 0.5 (0.6) | 10.2 (13.1)* | − 6.174 (< 0.001) | |
| VSTs to AdV (×103) | ||||
| Pre-Exercise | Placebo | 3.0 (3.2) | 38.8 (40.9)* | − 8.093 (< 0.001) |
| n = 12 | Bisoprolol | 3.8 (6.6) | 59.1 (77.4)* | − 12.827 (< 0.001) |
| Nadolol | 2.7 (2.5) | 60.9 (95.6)* | − 8.954 (< 0.001) | |
| Post-Exercise | Placebo | 2.6 (2.2) | 54.2 (59.9)* | − 8.411 (< 0.001) |
| n = 12 | Bisoprolol | 5.6 (11.2) | 55.4 (64.2)* | − 8.938 (< 0.001) |
| Nadolol | 3.2 (4.0) | 72.1 (114.3)* | − 7.588 (< 0.001) | |
Healthy participants performed a 30-min bout of cycling exercise after ingesting a placebo, a β1 AR antagonist (bisoprolol, 10 mg), or a β1 + 2 AR antagonist (nadolol, 80 mg) 3 h prior to exercise. Peripheral blood mononuclear cells (PBMCs) were isolated from blood obtained prior to medication ingestion (pre) and immediately after exercise (post). Ten million PBMCs from each condition were cultured with peptides spanning CMV, EBV, and AdV proteins and growth cytokines for 8 days, and the number of VSTs specific to CMV, EBV, and AdV stimulated at day 0 and generated at day 8 were quantified by IFN-γ ELISPOT. Data are expressed as mean (SD). Bonferroni-corrected p values comparing the number of VSTs stimulated at day 0 with the number generated at day 8 for each condition are presented. The total number of VSTs was determined by combining the number of VSTs to CMV, EBV, and AdV
VST virus-specific T cell, CMV cytomegalovirus, EBV Epstein–Barr virus, AdV adenovirus
*Significantly different from day 0 (p < 0.05)
Table 6.
The effects of exercise and β AR blockade on the number and frequency of manufactured VSTs
| Main effects | |||||||
|---|---|---|---|---|---|---|---|
| Pre | Post |
Exercise
tstatistics (pvalue) |
ExerciseFstatistics (p value) |
DrugFstatistics (pvalue) |
Interaction Fstatistics (pvalue) |
||
| Total VSTs (n = 12) | |||||||
| Total generated (×103) | Placebo | 208.3 (165.0) | 283.9 (211.8) | − 2.126 (0.057) |
1.543 (0.240) |
1.286 (0.296) |
1.317 (0.288) |
| Bisoprolol | 276.2 (249.0) | 302.4 (267.0) | − 0.730 (0.481) | ||||
| Nadolol | 240.2 (244.4) | 264.6 (251.6) | 0.073 (0.943) | ||||
| Per 100,000 CD3+ | Placebo | 1883.8 (1100.4) | 2348.8 (1235.2) | − 2.696 (0.021)* |
3.544 (0.086) |
1.390 (0.270) |
2.052 (0.152) |
| Bisoprolol | 2202.0 (1195.2) | 2558.9 (1485.9) | − 1.313 (0.216) | ||||
| Nadolol | 1912.6 (932.0) | 1878.9 (1066.2) | 0.228 (0.824) | ||||
| VSTs to CMV (n = 12) | |||||||
| Total generated (×103) | Placebo | 159.3 (139.8) | 220.2 (177.6) | − 2.010 (0.070) |
2.290 (0.158) |
1.330 (0.285) |
1.310 (0.290) |
| Bisoprolol | 204.1 (205.9) | 236.6 (220.2) | − 1.070 (0.307) | ||||
| Nadolol | 168.9 (199.3) | 182.3 (189.9) | − 0.132 (0.898) | ||||
| Per 100,000 CD3+ | Placebo | 1436.5 (1041.2) | 1827.0 (1195.2) | − 2.113 (0.058) |
3.737 (0.079) |
2.176 (0.137) |
1.690 (0.208) |
| Bisoprolol | 1655.7 (1234.2) | 2016.9 (1480.8) | − 1.530 (0.154) | ||||
| Nadolol | 1265.6 (826.8) | 1307.2 (1015.4) | − 0.406 (0.693) | ||||
| VSTs to EBV (n = 12) | |||||||
| Total generated (×103) | Placebo | 10.2 (8.0) | 9.5 (4.9) | − 0.210 (0.837) |
0.365 (0.559) |
0.604 (0.556) |
0.930 (0.411) |
| Bisoprolol | 13.0 (12.1) | 10.4 (6.9) | − 0.273 (0.790) | ||||
| Nadolol | 10.4 (8.3) | 10.2 (13.1) | 1.701 (0.120) | ||||
| Per 100,000 CD3+ | Placebo | 98.7 (74.5) | 86.4 (27.9) | − 0.189 (0.853) |
1.954 (0.192) |
0.293 (0.749) |
2.105 (0.148) |
| Bisoprolol | 128.6 (122.5) | 120.7 (118.5) | − 0.563 (0.584) | ||||
| Nadolol | 141.0 (161.1) | 97.6 (138.6) | 3.057 (0.012)* | ||||
| VSTs to AdV (n = 12) | |||||||
| Total generated (×103) | Placebo | 38.8 (40.9) | 54.2 (59.9) | − 1.384 (0.194) |
0.028 (0.869) |
1.474 (0.251) |
1.402 (0.267) |
| Bisoprolol | 59.1 (77.4) | 55.4 (64.2) | − 0.372 (0.717) | ||||
| Nadolol | 60.9 (95.6) | 72.1 (114.3) | 0.869 (0.403) | ||||
| Per 100,000 CD3+ | Placebo | 348.6 (278.6) | 435.4 (357.4) | − 1.723 (0.113) |
0.454 (0.514) |
0.838 (0.446) |
1.483 (0.249) |
| Bisoprolol | 417.7 (296.2) | 421.3 (222.9) | − 0.076 (0.941) | ||||
| Nadolol | 506.0 (457.4) | 474.0 (453.2) | 0.616 (0.551) | ||||
Healthy subjects performed 30 min of cycling exercise after ingesting a placebo, a β1 AR antagonist (bisoprolol, 10 mg), or a β1 + 2 AR antagonist (nadolol, 80 mg) 3 h prior to exercise. VSTs were manufactured by stimulating peripheral blood mononuclear cells (PBMCs) collected prior to medication ingestion (pre) and immediately after exercise (post) with viral peptides and growth cytokines for 8 days. The total number of VSTs generated and the frequency of VSTs (VSTs per 100,000 CD3+ T cells) following the 8-day expansion were quantified. Data are expressed as mean (SD). The main effects of exercise on the number of generated VSTs and the main effects of each drug condition were evaluated. The interaction between exercise and the drug conditions was analyzed to determine the mediating effects of β AR blockade on the effects of exercise. Planned Bonferroni-corrected comparisons between pre- and post-exercise for each drug trial are also presented (exercise t statistics) and significant differences between pre- and post-exercise within a trial are denoted by bold text. The total number of VSTs was determined by combining the number of VSTs to CMV, EBV, and AdV
VST virus-specific T cell, CMV cytomegalovirus, EBV Epstein–Barr virus, AdV adenovirus
*Significantly different from pre-exercise (p < 0.05)
Fig. 4.
The effects of exercise and β AR blockade on the frequency and number of manufactured VSTs. Three bouts of exercise were performed by healthy participants (n = 12) after ingesting a placebo, a β1 AR antagonist (bisoprolol, 10 mg), or a β1 + 2 AR antagonist (nadolol, 80 mg) 3 h prior to exercise. VSTs were generated from cells obtained prior to drug ingestion and immediately following exercise. The percent change from pre- to post-exercise in the frequency of VSTs in the expanded cell populations (a–d) and in the total number of VSTs generated (e–h) for each drug trial are presented for (a, e) the total number of VSTs, determined by combining the number of VSTs to CMV, EBV, and AdV; (b, f) the number of VSTs to CMV, determined by combining the number of VSTs to IE-1 and pp65; (c, g) the number of EBV-specific T cells, determined by combining the number of VSTs to LMP2 and BMLF1, and (d, h) the number of AdV-specific T cells, determined by combining the number of VSTs to hexon and penton. Bars represent the median value. No significant differences were observed. VST = virus-specific T cell; CMV = cytomegalovirus; EBV = Epstein–Barr virus; AdV = adenovirus
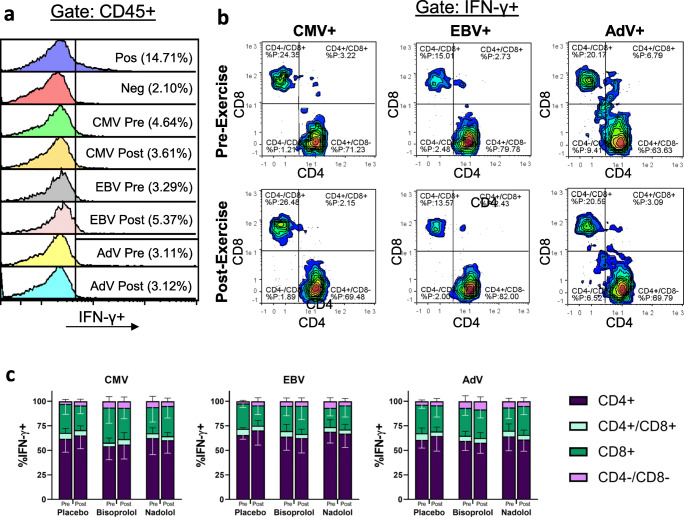
Exercise alters the phenotypes of the expanded cell products without altering the composition of CD4+ and CD8+ T cells among the manufactured VSTs
Neither exercise nor the administration of β AR antagonists prior to exercise affected the total number of cells generated (Table 7). Many of the effects of exercise on the proportions of lymphocyte populations observed at day 0 were also evident in the expanded cell products. Exercise decreased the proportions of CD3+ and CD4+ T cells and increased NK-cell proportions in the expanded cell products, and these alterations were unaffected by nadolol or bisoprolol administration. Overall, exercise decreased the proportion of total CD4+ T cells (p = 0.018) and increased the proportion of total CD8+ T cells (p = 0.012) in the expanded cells, and post hoc comparisons indicated that these changes were significant only in the placebo and bisoprolol trials. Similarly, exercise reduced the proportion of naïve CD8+ T cells (p = 0.016), but this reduction was not significant in the nadolol trial. Exercise also increased the proportion of EMRA CD8+ T cells (p = 0.014), particularly in the bisoprolol trial (p = 0.003). Together, these results indicate that nadolol may blunt some of the observed exercise-induced population shifts in the expanded cell products. While exercise and β AR blockade impacted the overall expanded cell products, phenotypic characterization of the manufactured VSTs identified using an IFN-γ capture assay revealed no effects of exercise or β AR antagonism on the proportions of manufactured VSTs that were CD4+ or CD8+ (Fig. 5).
Table 7.
The effects of exercise and β AR blockade on the proportions of lymphocyte subsets in expanded cell products
| Placebo | Bisoprolol | Nadolol | Main effects |
Interaction F (p value) |
|||
|---|---|---|---|---|---|---|---|
|
Exercise F (p value) |
Drug F (p value) |
||||||
| Total cells (×106) | Pre | 10.4 (5.4) | 12.7 (7.5) | 12.5 (8.3) | 1.030 (0.332) | 0.404 | 0.979 |
| n = 12 | Post | 12.1 (5.4) | 12.9 (8.1) | 12.8 (7.7) | (0.672) | (0.366) | |
| CD3+ cells (%) | Pre | 91.2 (5.0) | 91.4 (3.3) | 91.9 (4.5) | 35.893 | 1.219 | 2.101 |
| n = 12 | Post | 87.7 (6.7)* | 87.2 (7.1)* | 89.9 (4.7)* | (< 0.001) | (0.315) | (0.165) |
| CD4+ T cells (%) | Pre | 46.7 (15.9) | 48.1 (17.0) | 46.9 (19.3) | 7.666 (0.018) | 0.677 | 2.470 |
| n = 12 | Post | 41.0 (17.2)* | 42.3 (18.9)* | 41.0 (11.4) | (0.519) | (0.108) | |
| Naïve (%) | Pre | 26.1 (9.8) | 29.0 (16.3) | 26.6 (15.1) | 23.425 | 0.189 | 1.311 |
| n = 10 | Post | 23.4 (10.5) | 24.5 (14.7)* | 23.8 (13.9)* | (0.001) | (0.829) | (0.294) |
| CM (%) | Pre | 46.8 (9.9) | 46.1 (13.9) | 49.4 (12.4) | 12.819 | 0.887 | 0.373 |
| n = 10 | Post | 49.6 (13.7) | 50.5 (14.9)* | 52.6 (12.6)* | (0.006) | (0.429) | (0.694) |
| EM (%) | Pre | 23.4 (8.1) | 21.5 (10.9) | 21.2 (9.3) | 1.436 | 0.451 | 0.081 |
| n = 10 | Post | 22.4 (7.3) | 20.9 (8.7) | 20.9 (6.1) | (0.261) | (0.644) | (0.923) |
| EMRA (%) | Pre | 3.7 (3.2) | 3.4 (2.1) | 2.7 (1.4) | 0.784 | 0.429 | 0.859 |
| n = 10 | Post | 4.6 (5.4) | 4.1 (2.6) | 2.8 (1.5) | (0.399) | (0.657) | (0.440) |
| CD8+ T cells (%) | Pre | 41.0 (11.4) | 39.2 (12.8) | 42.4 (8.9) | 9.091 (0.012) | 0.287 | 1.416 |
| n = 12 | Post | 47.1 (16.3)* | 44.4 (18.3)* | 44.6 (12.7) | (0.753) | (0.264) | |
| Naïve (%) | Pre | 31.5 (19.8) | 38.5 (23.1) | 34.0 (24.4) | 8.787 | 0.369 | 3.429 |
| n = 10 | Post | 25.1 (19.6)* | 27.8 (19.5)* | 29.7 (22.6) | (0.016) | (0.697) | (0.087) |
| CM (%) | Pre | 34.3 (19.5) | 33.3 (22.1) | 34.3 (22.7) | 1.588 | 0.116 | 1.463 |
| n = 10 | Post | 35.1 (25.4) | 39.2 (21.6) | 36.2 (22.8) | (0.239) | (0.891) | (0.258) |
| EM (%) | Pre | 24.0 (10.8) | 21.9 (9.6) | 23.3 (10.3) | 1.005 | 0.430 | 0.049 |
| n = 10 | Post | 25.6 (14.6) | 23.0 (9.3) | 24.6 (11.6) | (0.342) | (0.569) | (0.952) |
| EMRA (%) | Pre | 10.2 (11.1) | 6.3 (3.4) | 8.4 (5.3) | 9.284 | 0.590 | 3.739 |
| n = 10 | Post | 14.2 (12.1) | 9.9 (5.3)* | 9.6 (6.9) | (0.014) | (0.564) | (0.073) |
| NK cells (%) | Pre | 2.9 (1.7) | 3.3 (2.0) | 2.9 (2.1) | 31.049 | 1.798 | 4.141 |
| n = 12 | Post | 6.4 (4.2)* | 6.6 (3.4)* | 4.7 (3.0)* | (< 0.001) | (0.189) | (0.054) |
Healthy participants completed a 30-min bout cycling exercise after ingesting a placebo, a β1 AR antagonist (bisoprolol, 10 mg), or a β1 + 2 AR antagonist (nadolol, 80 mg) 3 h prior to exercise. Peripheral blood mononuclear cells isolated from blood obtained prior to drug ingestion and immediately after exercise were grown in the presence of viral peptides and growth cytokines for 8 days and the lymphocyte subset proportions in the expanded cell populations were identified by flow cytometry. The main effects of exercise on the number of generated VSTs and the main effects of each drug condition were evaluated. The interaction between exercise and the drug conditions was analyzed to determine the mediating effects of β AR blockade on the effects of exercise. WBC = white blood cell; naïve = CD45RA+/CD62L+; CM = central memory, CD45RA−/CD62L+; EM = effector memory, CD45RA−/CD62L−; EMRA = CD45RA+ effector memory CD45RA+/CD62L−). Data are expressed as mean (SD)
*Significantly different (Bonferroni-corrected p < 0.05) from pre-exercise within a trial
Fig. 5.
The effects of exercise and β AR blockade on the phenotypes of expanded VSTs. Healthy participants completed a 30-min bout of cycling exercise after ingesting a placebo, a β1 AR antagonist (bisoprolol, 10 mg), or a β1 + 2 AR antagonist (nadolol, 80 mg) 3 h prior to exercise. Peripheral blood mononuclear cells (PBMCs) isolated from blood obtained prior to drug ingestion (pre) and immediately after exercise (post) were grown in the presence of viral peptides and growth cytokines for 8 days then cryopreserved until analysis. Expanded cells were subsequently thawed and received no stimulation (negative control), stimulated with Cytostim (positive control), or stimulated with viral peptides specific for CMV, EBV, or AdV. (a) Following stimulation, virus-specific T cells (VSTs) were identified as IFN-γ + cells within the CD45+ lymphocyte population. Representative flow cytometry plots demonstrating the proportions of IFN-γ + VSTs within the CD45+ cell population generated from pre-exercise or post-exercise PBMCs are presented. (b) Within the IFN-γ + VST populations, the proportions of CD4 and CD8 cell populations were identified by flow cytometry, and representative flow plots for each virus pre- and post-exercise are shown. (c) Mean ± SD proportions of CD4+, CD8+, CD4+/CD8+ double positive, and CD4−/CD8− double negative VSTs specific for CMV (n = 6), EBV (n = 5), and AdV (n = 5). No significant differences from pre- to post-exercise were observed for any condition or VST population. VST = virus-specific T cell; CMV = cytomegalovirus; EBV = Epstein–Barr virus; AdV = adenovirus
Discussion
The aim of this study was to determine the effects of β2 AR blockade on the exercise-induced mobilization of VSTs and on the enhancing effects of exercise on the ex vivo expansion of VSTs. Here, we report that β1 + 2 AR blockade, but not β1 AR blockade, blunted the mobilization of VSTs, and CMV-VSTs in particular. Although exercise only moderately enhanced the expansion of VSTs in this study, the administration of a β1 + 2 AR antagonist prior to exercise partially abrogated the exercise-induced augmentation in the expansion of VSTs. The implications of these findings could have clinical relevance, as either exercise or the infusion of β2 AR agonists during blood donations could significantly enhance the isolation of VSTs for adoptive transfer immunotherapy for the treatment of viral infections following alloHCT. Taken together, these findings lend support to the idea that systemic β AR activation can serve as a suitable and economical adjuvant for the mobilization and manufacture of various cell products for therapeutic purposes (Simpson et al. 2017a; Baker et al. 2019).
Corroborating previous findings (Kunz et al. 2018; Spielmann et al. 2014), a 30-min bout of steady-state cycling exercise significantly increased the total number of circulating VSTs to CMV, EBV, and AdV. Further, a preferential mobilization of VSTs to CMV was observed, as evidenced by the increased frequency of these cells following exercise. Demargination of leukocytes from the endothelium as a result of increased sheer stress on blood vessels and capillary structures during exercise likely contributes to the increase in circulating VSTs (Walsh et al. 2011). However, nadolol, a non-preferential β1 + 2 AR antagonist, mitigated the mobilization of CMV-VSTs, while bisoprolol, a β1 AR preferential antagonist, did not inhibit CMV-VST mobilization despite reducing exercising heart rate and systolic blood pressure to a similar extent as nadolol. Therefore, the preferential mobilization of CMV-VSTs appears to be β2 AR mediated. In contrast, no preferential mobilization of EBV- or AdV-VSTs was observed, and the effects of β AR antagonism were minimal. The differences in responsiveness to exercise and β AR antagonism are likely mediated by differences in the cellular density of high-affinity β2 ARs (Maisel et al. 1990). CD8+ T cells exhibit a greater number of β2 ARs than CD4+ T cells (Anane et al. 2009; Fan and Wang 2009), and as T cells move through stages of activation and differentiation, β2 AR density increases (Slota et al. 2015). Concomitantly, cells known to have a greater number of β2 ARs are also the most responsive to exercise, and their mobilization is inhibited upon β2 AR antagonism (Campbell et al. 2009; Graff et al. 2018). Although we did not phenotype the circulating VSTs, CMV-specific T cells are typically highly differentiated CD8+ T cells, while EBV-specific T cells are predominantly CD8+ T cells exhibiting a central memory phenotype (Appay et al. 2002; Appay et al. 2008). The phenotypes of AdV-specific T cells are less well characterized, but CD4+ AdV-specific T cells have been shown to play a large role in the immune response to AdV infection (Geyeregger et al. 2013; Hutnick et al. 2010; Lindemans et al. 2010). Therefore, the observed differences between VSTs in the responses to exercise and β1 + 2 AR antagonism likely reflect the relative densities of β2 ARs on the VSTs.
In addition to an exercise-induced mobilization of VSTS, our laboratory has shown that an acute bout of exercise can augment the rapid ex vivo expansion of VSTs against CMV, EBV, and AdV antigens (Kunz et al. 2018; Spielmann et al. 2016). Therefore, we also sought to determine if the observed exercise-induced enhancements in VST manufacture were in fact β2 AR mediated. In a majority of the subjects, acute exercise augmented the total number of expanded multi-VSTs (8/12 subjects) and CMV-VSTs in particular (9/12 subjects), and the administration of nadolol prior to exercise tended to abrogate the exercise response, indicating that mechanisms involving β2 AR engagement may play a role in the exercise-induced augmentation in VST manufacture. As with the mobilization of VSTs, the effects of exercise and β2 AR blockade on the expansion of VSTs were variable for each virus. A higher density of β2 ARs on the CMV-specific T cells may have enhanced their response to exercise and β2 AR blockade, but it is also possible that the expansion of EBV- and AdV-specific T cells may have been impaired by antigenic competition in culture (Harris et al. 2019; Keller and Bollard 2020), particularly post-exercise. The CMV-pp65 antigen is known to be immunodominant (Tischer et al. 2014), and CMV-specific T cells tend to exhibit higher antigen avidity (Price et al. 2005) and may therefore inhibit the access of other T cells to antigen-presenting cells (Kedl et al. 2000). Not only did the numbers of CMV-specific T cells tend to be greater at rest but they were also mobilized to a greater extent than EBV- and AdV-specific T cells with exercise. Therefore, post-exercise expansions may have been further polarized toward CMV-specific T cells.
Neither exercise nor β AR blockade appears to affect the function of the VSTs at the individual cell level. The expansion of VSTs on a per-cell basis was not influenced by exercise or β AR antagonism; therefore, the β2 AR-mediated exercise-induced increase in VST numbers likely accounts for the exercise-induced enhancement in VST expansion. In support of this contention, many of the exercise-induced alterations in T-cell memory subset phenotypes in the cultured PBMC fractions were preserved in the manufactured cell products following the 8-day culture, indicative of the maintenance of exercise-induced T-cell compositional shifts throughout the culture period. Importantly, neither exercise nor β2 AR blockade affected the proportions of CD4+ and CD8+ T cells in the expanded VSTs. VST populations consisting of both CD4+ and CD8+ T cells appear to be beneficial for adoptive transfer immunotherapy, as CD4+ VSTs have been shown to play a vital role in promoting the persistence of CD8+ T cells in vivo (Houghtelin and Bollard 2017; Novy et al. 2007; Pourgheysari et al. 2009). Although we did not test the function of the VSTs in the present study, previous work from our laboratory has demonstrated that VSTs manufactured from blood obtained post-exercise maintain their antigen-specific and MHC-restricted cytotoxic effector function against autologous peptide-pulsed target cells in vitro (Kunz et al. 2018; Spielmann et al. 2016).
The present data suggests that the exercise-induced mobilization of VSTs into circulation is mediated by β2 AR-dependent mechanisms. These findings may have implications for both the direct capture and the ex vivo manufacture of these cells for adoptive transfer immunotherapy. Despite a lower exercising heart rate compared with placebo, exercise-induced VST mobilization was not significantly impaired by bisoprolol administration; therefore, β2 AR engagement with only moderate elevations in heart rate may be sufficient to trigger this response. While the prescribed exercise is likely safe for a large proportion of donors, β2 AR agonist infusion (e.g., with isoproterenol) may be more easily applied in the clinical setting. This might help overcome the limitation of low circulating cell numbers that hinder current direct VST isolation techniques using MHC class I multimers or cytokine capture and ex vivo expansion methods using overlapping peptide pools (Bollard and Heslop 2016). These findings could also have implications for anti-viral immunity outside of the transplant setting. For instance, the frequent mobilization and redistribution of antigen-responsive VSTs with each bout of exercise could increase anti-viral immunosurveillance to improve latent viral control (Duggal et al. 2019; Simpson et al. 2016). This enhanced immunosurveillance may persist, as Heinemann et al. (2020) recently reported increased numbers of functionally active circulating VSTs 24 h after acute exercise. Indeed, higher levels of physical activity and cardiorespiratory fitness are associated with lower antibody titers against several latent herpesviruses, including CMV, EBV, and HSV-1, and have been shown to reduce the incidence of latent herpesvirus reactivations in astronauts (Agha et al. 2020; Simpson et al. 2017b).
We acknowledge several limitations of our study. The small sample size coupled with the large observed variability hindered our ability to detect significant effects of exercise and β2 AR blockade on VST mobilization and expansion. Interestingly, the greatest variability in the VST responses to exercise was observed in the bisoprolol trial. That the variability was lower in the nadolol trial may indicate that β2 AR antagonism more consistently inhibits exercise responses, while the individual response to β1 AR may vary more between individuals. Unfortunately, the high variability in the bisoprolol trial introduced significant heterogeneity of variance and likely impacted our ability to detect significant differences in our analyses. Many other factors may contribute to the inter- and intra-subject variability. Antigen selection may explain some of the inter-subject variability and the variability in response to exercise, as individuals vary in immune responses to specific antigens (Tischer et al. 2014). This may also explain the sometimes divergent responses to exercise in the individual antigens from the same virus. Further, even short delays in blood collection following exercise may have impacted the exercise response, as lymphocytes rapidly egress the peripheral blood following cessation of exercise (Rooney et al. 2018). Day-to-day variability in cell numbers and expansions was also observed, independent of the drug condition. Many fluctuating factors, including stress and disruptions in sleep patterns, can contribute to leukocyte number and function and may have contributed to the observed intrasubject variability (Besedovsky et al. 2012; Sanders 2012; Sanders and Straub 2002).
In conclusion, we have provided the first evidence that β2 AR engagement drives the exercise-induced mobilization of VSTs, and CMV-VSTs in particular. We also suggest that β2 AR engagement may partially explain the enhanced ex vivo expansion of VSTs following acute exercise, as β1 + 2 AR blockade tended to attenuate the exercise-induced enhancements in those individuals for which exercise improved VST manufacture. While these findings have clear implications for improving current VST manufacturing protocols for adoptive transfer immunotherapy, there are also broader implications for the beneficial effects of exercise on the immune system. It has been postulated that, through the cumulative effects of each bout of acute exercise, regular exercise can enhance immune function, curtail immunosenescence, and control the reactivation of CMV and other latent viruses (Simpson 2011; Simpson et al. 2016). We posit that exercise may provide additional viral protection through the frequent mobilization and redistribution of VSTs with an enhanced ability to proliferate in response to cognate antigens. Therefore, the present findings not only have implications for adoptive transfer immunotherapy but may also support a functional role for exercise-induced β2 AR engagement in the anti-viral immune protection that appears to be provided by long-term regular exercise.
Acknowledgments
The authors would like to thank Rod Azadan, Rachel Graff, Bridgette Rooney, Dr. Austin B. Bigley, and Dr. Melissa Markofski for their assistance with this project.
Availability of data and material
Not applicable.
Code availability (software application or custom code)
Not applicable.
Funding information
This work was supported by NASA Grants NNX12AB48G, NNX16AB29G, and NNX16AG02G to R.J.S., NIH Grant R21 CA197527-01A1 to R.J.S. and A.B.B., NIH grant R01 AI110007 to R.A.B., NIH grant P01 CA148600-01A1 to C.M.B., and an American College of Sports Medicine Foundation Doctoral Student Research grant awarded to H.K.
Compliance with ethical standards
Conflicts of interest/competing interests
The authors declare no conflicts of interest.
Ethics approval
All study procedures were reviewed and approved by the Committee for the Protection of Human Subjects at the University of Houston.
Consent to participate
Written consent was obtained from all participants after both verbal and written information regarding the risks and demands of the study was provided.
Consent for publication
Written consent for the publication of the results of this study was obtained from all participants.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agha NH, Baker FL, Kunz HE, Graff R, Azadan R, Dolan C, Laughlin MS, Hosing C, Markofski MM, Bond RA, Bollard CM, Simpson RJ. Vigorous exercise mobilizes CD34+ hematopoietic stem cells to peripheral blood via the beta2-adrenergic receptor. Brain Behav Immun. 2018;68:66–75. doi: 10.1016/j.bbi.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agha NH, Mehta SK, Rooney BV, Laughlin MS, Markofski MM, Pierson DL, Katsanis E, Crucian BE, Simpson RJ. Exercise as a countermeasure for latent viral reactivation during long duration space flight. FASEB J. 2020;34:2869–2881. doi: 10.1096/fj.201902327R. [DOI] [PubMed] [Google Scholar]
- Anane LH, Edwards KM, Burns VE, Drayson MT, Riddell NE, van Zanten JJCSV, Wallace GR, Mills PJ, Bosch JA. Mobilization of gammadelta T lymphocytes in response to psychological stress, exercise, and beta-agonist infusion. Brain Behav Immun. 2009;23:823–829. doi: 10.1016/j.bbi.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GMA, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry Part A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol. 2005;144:317–322. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FL, Bigley AB, Agha NH, Pedlar CR, O'Connor DP, Bond RA, Bollard CM, Katsanis E, Simpson RJ (2019) Systemic β-Adrenergic Receptor Activation Augments the ex vivo Expansion and Anti-Tumor Activity of Vγ9Vδ2 T-Cells. Front Immunol. https://pubmed.ncbi.nlm.nih.gov/32038628/ [DOI] [PMC free article] [PubMed]
- Balady GJ, Chaitman B, Driscoll D, Foster C, Froelicher E, Gordon N, Pate R, Rippe J, Bazzarre T. Recommendations for cardiovascular screening, staffing, and emergency policies at health/fitness facilities. Circulation. 1998;97:2283–2293. doi: 10.1161/01.CIR.97.22.2283. [DOI] [PubMed] [Google Scholar]
- Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard CM, Heslop HE. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood. 2016;127:3331–3340. doi: 10.1182/blood-2016-01-628982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchard U. Pharmacological properties of beta-adrenoceptor blocking drugs. J Clin Basic Cardiol. 1998;1:5–9. [Google Scholar]
- Borg GA. Perceived exertion: a note on “history” and methods. Med Sci Sports. 1973;5:90–93. [PubMed] [Google Scholar]
- Campbell JP, Riddell NE, Burns VE, Turner M, van Zanten JJ, Drayson MT, Bosch JA. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav Immun. 2009;23:767–775. doi: 10.1016/j.bbi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- D'Souza A, Lee S, Zhu X, Pasquini M. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transpl. 2017;23:1417–1421. doi: 10.1016/j.bbmt.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. 2019;19:563–572. doi: 10.1038/s41577-019-0177-9. [DOI] [PubMed] [Google Scholar]
- Fan X, Wang Y. B2 adrenergic receptor on T lymphocytes and its clinical implications. Prog Nat Sci. 2009;19:17–23. doi: 10.1016/j.pnsc.2008.10.001. [DOI] [Google Scholar]
- Gerdemann U, Keirnan JM, Katari UL, Yanagisawa R, Christin AS, Huye LE, Perna SK, Ennamuri S, Gottschalk S, Brenner MK, Heslop HE, Rooney CM, Leen AM. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012;20:1622–1632. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyeregger R, Freimüller C, Stevanovic S, Stemberger J, Mester G, Dmytrus J, Lion T, Rammensee HG, Fischer G, Eiz-Vesper B, Lawitschka A, Matthes S, Fritsch G. Short-term in-vitro expansion improves monitoring and allows affordable generation of virus-specific T-cells against several viruses for a broad clinical application. PLoS One. 2013;8:e59592. doi: 10.1371/journal.pone.0059592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff RM, Kunz HE, Agha NH, Baker FL, Laughlin M, Bigley AB, Markofski MM, LaVoy EC, Katsanis E, Bond RA, Bollard CM, Simpson RJ. beta2-adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav Immun. 2018;74:143–153. doi: 10.1016/j.bbi.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3:285–299. doi: 10.1586/ehm.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley PJ. Build a Bank: off-the-shelf virus-specific T cells. Biol Blood Marrow Transpl. 2018;24:e9–e10. doi: 10.1016/j.bbmt.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Harris KM, Davila BJ, Bollard CM, Keller MD. Virus-specific T cells: current and future use in primary immunodeficiency disorders. J Allergy Clin Immunol Pract. 2019;7:809–818. doi: 10.1016/j.jaip.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann NC, Tischer-Zimmermann S, Wittke TC, Eigendorf J, Kerling A, Framke T, Melk A, Heuft HG, Blasczyk R, Maecker-Kolhoff B, Eiz-Vesper B. High-intensity interval training in allogeneic adoptive T-cell immunotherapy—a big HIT? J Transl Med. 2020;18:148. doi: 10.1186/s12967-020-02301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghtelin A, Bollard CM. Virus-specific T cells for the immunocompromised patient. Front Immunol. 2017;8:1272. doi: 10.3389/fimmu.2017.01272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutnick NA, Carnathan D, Demers K, Makedonas G, Ertl HC, Betts MR. Adenovirus-specific human T cells are pervasive, polyfunctional, and cross-reactive. Vaccine. 2010;28:1932–1941. doi: 10.1016/j.vaccine.2009.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AS, Blair SN, Mahar MT, Wier LT, Ross RM, Stuteville JE. Prediction of functional aerobic capacity without exercise testing. Med Sci Sports Exerc. 1990;22:863–870. doi: 10.1249/00005768-199012000-00021. [DOI] [PubMed] [Google Scholar]
- Kaeuferle T, Krauss R, Blaeschke F, Willier S, Feuchtinger T. Strategies of adoptive T-cell transfer to treat refractory viral infections post allogeneic stem cell transplantation. J Hematol Oncol. 2019;12:13. doi: 10.1186/s13045-019-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MD, Bollard CM. Virus-specific T-cell therapies for patients with primary immune deficiency. Blood. 2020;135:620–628. doi: 10.1182/blood.2019000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MD, Darko S, Lang H, Ransier A, Lazarski CA, Wang Y, Hanley PJ, Davila BJ, Heimall JR, Ambinder RF, Barrett AJ, Rooney CM, Heslop HE, Douek DC, Bollard CM. T-cell receptor sequencing demonstrates persistence of virus-specific T cells after antiviral immunotherapy. Br J Haematol. 2019;187:206–218. doi: 10.1111/bjh.16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz H, Bishop NC, Spielmann G, Pistillo M, Reed J, Ograjsek T, Park Y, Mehta SK, Pierson DL, Simpson RJ. Fitness level impacts salivary antimicrobial protein responses to a single bout of cycling exercise. Eur J Appl Physiol. 2015;115:1015–1027. doi: 10.1007/s00421-014-3082-8. [DOI] [PubMed] [Google Scholar]
- Kunz HE, Spielmann G, Agha NH, O'Connor DP, Bollard CM, Simpson RJ. A single exercise bout augments adenovirus-specific T-cell mobilization and function. Physiol Behav. 2018;194:56–65. doi: 10.1016/j.physbeh.2018.04.035. [DOI] [PubMed] [Google Scholar]
- Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, Kapoor N, Pai SY, Rowley SD, Kebriaei P, Dey BR, Grilley BJ, Gee AP, Brenner MK, Rooney CM, Heslop HE. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121:5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Heslop HE, Brenner MK. Antiviral T-cell therapy. Immunol Rev. 2014;258:12–29. doi: 10.1111/imr.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemans CA, Leen AM, Boelens JJ. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood. 2010;116:5476–5485. doi: 10.1182/blood-2010-04-259291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel AS, Harris T, Rearden CA, Michel MC. Beta-adrenergic receptors in lymphocyte subsets after exercise. Alterations in normal individuals and patients with congestive heart failure. Circulation. 1990;82:2003–2010. doi: 10.1161/01.CIR.82.6.2003. [DOI] [PubMed] [Google Scholar]
- Miller HK, Braun TM, Stillwell T, Harris AC, Choi S, Connelly J, Couriel D, Goldstein S, Kitko CL, Magenau J, Pawarode A, Reddy P, Riwes M, Yanik GA, Levine JE. Infectious risk after allogeneic hematopoietic cell transplantation complicated by acute graft-versus-host disease. Biol Blood Marrow Transpl. 2017;23:522–528. doi: 10.1016/j.bbmt.2016.12.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Nicholas SK, Martinez CA, Leen AM, Hanley PJ, Gottschalk SM, Rooney CM, Hanson IC, Krance RA, Shpall EJ, Cruz CR, Amrolia P, Lucchini G, Bunin N, Heimall J, Klein OR, Gennery AR, Slatter MA, Vickers MA, Orange JS, Heslop HE, Bollard CM, Keller MD. Adoptive immunotherapy for primary immunodeficiency disorders with virus-specific T lymphocytes. J Allergy Clin Immunol. 2016;137(1498–1505):e1491. doi: 10.1016/j.jaci.2015.12.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol. 2007;179:8243–8251. doi: 10.4049/jimmunol.179.12.8243. [DOI] [PubMed] [Google Scholar]
- Papadopoulou A, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med. 2014;6:242ra283. doi: 10.1126/scitranslmed.3008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolin DA, Munger PA, Mazzeo RS. Plasma catecholamine and lactate response during graded exercise with varied glycogen conditions. J Appl Physiol (1985) 1991;71:1427–1433. doi: 10.1152/jappl.1991.71.4.1427. [DOI] [PubMed] [Google Scholar]
- Pourgheysari B, Piper KP, McLarnon A, Arrazi J, Bruton R, Clark F, Cook M, Mahendra P, Craddock C, Moss PAH. Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplant. 2009;43:853–861. doi: 10.1038/bmt.2008.403. [DOI] [PubMed] [Google Scholar]
- Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, Sewell AK, Connors M, Douek DC. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med. 2005;202:1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney BV, Bigley AB, LaVoy EC, Laughlin M, Pedlar C, Simpson RJ. Lymphocytes and monocytes egress peripheral blood within minutes after cessation of steady state exercise: a detailed temporal analysis of leukocyte extravasation. Physiol Behav. 2018;194:260–267. doi: 10.1016/j.physbeh.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav Immun. 2012;26:195–200. doi: 10.1016/j.bbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders VM, Straub RH. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav Immun. 2002;16:290–332. doi: 10.1006/brbi.2001.0639. [DOI] [PubMed] [Google Scholar]
- Simmons HZ, Bazzell AF, Dains JE. Adverse effects of virus-specific T-cell therapy: an integrative review. J Adv Pract Oncol. 2019;10:120–131. [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ. Aging, persistent viral infections, and immunosenescence: can exercise “make space”? Exerc Sport Sci Rev. 2011;39:23–33. doi: 10.1097/JES.0b013e318201f39d. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Florida-James GD, Cosgrove C, Whyte GP, Macrae S, Pircher H, Guy K. High-intensity exercise elicits the mobilization of senescent T lymphocytes into the peripheral blood compartment in human subjects. J Appl Physiol (1985) 2007;103:396–401. doi: 10.1152/japplphysiol.00007.2007. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Cosgrove C, Chee MM, McFarlin B, Bartlett DB, Spielmann G, O'Connor DP, Pircher H, Shiels PG. Senescent phenotypes and telomere lengths of peripheral blood T-cells mobilized by acute exercise in humans. Exerc Immunol Rev. 2010;16:40–55. [PubMed] [Google Scholar]
- Simpson RJ, Bigley AB, Spielmann G, LaVoy EC, Kunz H, Bollard CM. Human cytomegalovirus infection and the immune response to exercise. Exerc Immunol Rev. 2016;22:8–27. [PubMed] [Google Scholar]
- Simpson RJ, Bigley AB, Agha N, Hanley PJ, Bollard CM. Mobilizing immune cells with exercise for cancer immunotherapy. Exerc Sport Sci Rev. 2017;45:163–172. doi: 10.1249/JES.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ, Hussain M, Baker F, Bigley AB, Peek MK, Stowe RP. Cardiorespiratory fitness is associated with better control of latent herpesvirus infections in a large ethnically diverse community sample: evidence from the Texas City stress and health study. Brain Behav Immun. 2017;66:e35. doi: 10.1016/j.bbi.2017.07.128. [DOI] [Google Scholar]
- Slota C, Shi A, Chen G, Bevans M, Weng NP. Norepinephrine preferentially modulates memory CD8 T cell function inducing inflammatory cytokine production and reducing proliferation in response to activation. Brain Behav Immun. 2015;46:168–179. doi: 10.1016/j.bbi.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann G, Bollard CM, Bigley AB, Hanley PJ, Blaney JW, LaVoy ECP, Pircher H, Simpson RJ. The effects of age and latent cytomegalovirus infection on the redeployment of CD8+ T cell subsets in response to acute exercise in humans. Brain Behav Immun. 2014;39:142–151. doi: 10.1016/j.bbi.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Spielmann G, Bollard CM, Kunz H, Hanley PJ, Simpson RJ. A single exercise bout enhances the manufacture of viral-specific T-cells from healthy donors: implications for allogeneic adoptive transfer immunotherapy. Sci Rep. 2016;6:25852. doi: 10.1038/srep25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer S, Dieks D, Sukdolak C, Bunse C, Figueiredo C, Immenschuh S, Borchers S, Stripecke R, Maecker-Kolhoff B, Blasczyk R, Eiz-Vesper B. Evaluation of suitable target antigens and immunoassays for high-accuracy immune monitoring of cytomegalovirus and Epstein–Barr virus-specific T cells as targets of interest in immunotherapeutic approaches. J Immunol Methods. 2014;408:101–113. doi: 10.1016/j.jim.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- Weltman A. The blood lactate response to exercise. Champaign, IL: Human Kinetics; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.