Abstract
Eukaryotic cells respond to hypothermic stress through a series of regulatory mechanisms that preserve energy resources and prolong cell survival. These mechanisms include alterations in gene expression, attenuated global protein synthesis and changes in the lipid composition of the phospholipid bilayer. Cellular responses to hyperthermia, hypoxia, nutrient deprivation and oxidative stress have been comprehensively investigated, but studies of the cellular response to cold stress are more limited. Responses to cold stress are however of great importance both in the wild, where exposure to low and fluctuating environmental temperatures is common, and in medical and biotechnology settings where cells and tissues are frequently exposed to hypothermic stress and cryopreservation. This means that it is vitally important to understand how cells are impacted by low temperatures and by the decreases and subsequent increases in temperature associated with cold stress. Here, we review the ways in which eukaryotic cells respond to hypothermic stress and how these compare to the well-described and highly integrated stress response systems that govern the cellular response to other types of stress.
Keywords: Eukaryotic cells, Hypothermia, Cold stress response, Protein synthesis, mTOR
Introduction
The definition of ‘stress’ can be ambiguous. It is often used to describe the environmental perturbations that interrupt homeostasis in higher organisms, but in single cells it refers to changes in the internal or external environment that compromise cell survival. This means that organisms experience stress at various magnitudes depending on their biological form (Barouki 2007; Karatsoreos 2018). Stress is also more generally described as a range of stimuli that generate a molecular, endocrine, behavioural and cellular response in organisms (Buchanan 2000). As would be expected, living systems have evolved to cope with the impact of environmental stresses with numerous regulatory mechanisms existing to prolong cell survival and attenuate metabolic activity. A core part of many of these regulatory mechanisms are systems that control the level of protein synthesis (Fulda et al. 2010; Hotamisligil and Davis 2016).
Protein synthesis is one of the most energy-consuming cellular processes, requiring approximately 30–40% of accessible energy (Browne et al. 2004; Li et al. 2014). Achieving the correct tertiary and quaternary structure of newly synthesised proteins is a crucial challenge, with the accumulation of misfolded proteins posing a serious risk to cell viability (Balchin et al. 2016). It is therefore unsurprising that global attenuation of transcription and translation occurs in response to hypoxia, oxidative stress and nutrient deprivation (Wengrod and Gardner 2015; Pakos-Zebrucka et al. 2016), and in response to both high and low temperature stress (Sonna et al. 2013; Eskla et al. 2018). For example, both oxidative stress and the impairment, or saturation, of protein maturation systems within the endoplasmic reticulum (ER) induce a series of comparable responses, most notably, the phosphorylation of the eukaryotic initiation factor α (eIFα) subunit by PKR-like endoplasmic reticulum kinase (PERK) (Bi et al. 2005; Pakos-Zebrucka et al. 2016). These stress-induced global reduction in protein synthesis occurs even whilst proteins that are essential to cell survival are selectively upregulated (Liu and Qian 2014; Zhu et al. 2016a). In combination, such changes preserve resources, promote cell survival and can prevent cell death.
It is not however clear the extent to which cellular responses to different stresses are linked and some responses are better understood than others. For example, in comparison to our understanding of other stresses, the cellular response to hypothermia (low temperature stress) is less well understood. Here we detail the cellular response to oxidative stress, hypoxia and nutrient deprivation and to heat stress, contrasting these to the response to moderate and severe hypothermia. This highlights the unique features of the hypothermia response, details the key ways that these responses overlap, and identifies how hypothermia integrates into the general cellular stress response systems.
Oxidative stress, hypoxia and nutrient deprivation
Oxidative damage occurs in all living cells and arises when there is an imbalance between reactive oxygen species (ROS) such as superoxide anions and hydroxyl radicals, and antioxidant defence (Allen et al. 2009; Aitken et al. 2016). Lipids, proteins and DNA are irreversibly damaged by high concentrations of ROS which leads to compromised cell viability. ROS is however a fundamental part of cellular functioning, with mitochondria acting as the main source of ROS production in the cell and accounting for the equivalent of approximately 1–2% of ROS, from oxygen consumption (Filomeni et al. 2010). Through redox signalling, ROS modify proteins by interfering with their sulphur-containing residues and cysteine and methionine molecules, causing changes in the function and structure of the protein. As well as hindering interactions between proteins, these changes prevent post-translational modifications such as phosphorylation and methylation, and subsequently interfere with normal signalling mechanisms and cellular function (Jones 2008; Ghosh et al. 2017). Oxidative stress and ROS are often associated with low oxygen tension and nutrient deprivation although the mechanisms that connect these processes are not fully understood. Stress generally causes unprocessed and unfolded proteins to accumulate within the cells which trigger a network of protective responses that promote cell survival and prevent cell death. Prolonged and severe forms of stress are, however, cytotoxic and ultimately induce apoptosis (Zeeshan et al. 2016).
Hypoxic stress, when cells experience low O2 conditions, can be experienced as a part of normal function and also results from a variety of disease states. For example, the transient pauses in breathing that occur in recurrent sleep apnea lead to chronic intermittent hypoxia during sleep (Basner 2007). Hypoxic stress is also common in tumours as they develop and outgrow their vasculatures, causing regions of cells to be exposed to low oxygen levels. In all such cases, hypoxia results in cells temporarily arresting the cell cycle, whilst proteins that are essential for survival are produced, using ATP generated from anaerobic metabolism (Uniacke et al. 2014). Low oxygen tension also induces an alternative translation initiation scheme known as the hypoxia-inducible-factor response. This system relies on the participation of hypoxia-inducible factor (HIF), a master regulator of hypoxia made up of a unique oxygen-labile α subunit and β subunit (Pereira et al. 2013). Mammals possess three isoforms of the alpha subunit which are HIF1α, the isoform expressed in all cells, and HIF2α and HIF3α, which are selectively expressed in certain tissues (Majmundar et al. 2010). The β subunit has one isoform (HIFβ) and is stable regardless of the oxygen tension, but the α subunit is degraded by the von Hippel-Lindau tumour suppressor protein during normal environmental conditions. When oxygen levels are reduced, HIFα is stabilised and translocated to the nucleus where it forms a functional heterodimer with HIFβ alongside RNA-binding protein RBM4 and eukaryotic initiation factor eIF4E2 (Pereira et al. 2013). The HIF heterodimeric complex binds to the consensus hypoxia-responsive elements (HREs) in the promoter region of a number of target genes to regulate angiogenesis, erythropoiesis as well as glycolysis.
Although HIFα fulfils a significant role in the hypoxic adaptive response, it is not sufficient for implementing all the adaptive changes required for cells to survive under hypoxic conditions (Nallamshetty et al. 2013). Pathways involving mechanistic target of rapamycin (mTOR), ER stress, and the unfolded protein response are also involved in the hypoxia response. Here, the hypoxia-induced inhibition of mTOR occurs independently from the hypoxia-inducible-factor response although HIFs do form part of the network of transcription factors that downregulate mTOR substrates eIF4E binding protein (4E-BP1) and ribosomal S6 kinase (S6K), and act to inhibit eukaryotic initiation factor eIF4F (Koritzinsky et al. 2006; Melanson et al. 2017). The ER kinase (PERK) is also involved in downregulating protein synthesis and is activated as part of the unfolded protein response. It inhibits translation initiation by phosphorylating transcription factor eIF2α and forms part of a set of molecular events, the integrated stress response, which occur during most forms of cellular stress (Koritzinsky et al. 2006; Pakos-Zebrucka et al. 2016).
Similar to hypoxia, nutrient and amino acid deprivation induce a set of responses that coordinate cell survival. A key regulator of these events is energy sensor AMP-activated protein kinase (AMPK), which is activated in response to increased ratios of AMP:ATP or ADP:ATP (Kumar et al. 2020). During normal cellular activity, cells maintain an ATP to ADP ratio of approximately 10:1, but low oxygen and nutrient levels lead to increased AMP concentrations as a result of the adenylate kinase reaction (2ADP ↔ ATP + AMP) (Hardie 2011). When activated, AMPK limits energy-consuming processes, including glycolysis and fatty acid oxidation, in order to preserve energy. AMPK also regulates translation through inhibition of eEF2 and mTOR substrates 4E-BP1 and S6K (Leprivier et al. 2013). Additionally, cells respond to nutrient deprivation when uncharged tRNA molecules bind to General Control Non-Derepressible 2 (GCN2) which in turn deactivates eIF2α. Protein synthesis is subsequently prevented as the α-subunit fails to participate in cap-dependant translation initiation. Conversely, phosphorylated eIF2α is associated with translation of mRNAs containing an upstream ORF cluster such as yeast transcription factor GCN4 and its mammalian orthologue ATF4. When upregulated, ATF4 induces expression of genes involved in amino acid metabolism, including amino acid synthetases and transporters, and amino acyl tRNA synthetases (Ye et al. 2015; Gameiro and Struhl 2018). These proteins promote cellular adaptation to nutrient deprivation by facilitating nutrient availability. Furthermore, the autophagy response serves as a protective mechanism that removes damaged polypeptides and organelles, and reabsorbs intracellular proteins in a vacuole-like structure, whilst retrieving nutrients from the cell (Reiling and Sabatini 2006).
There is significant overlap in the response to nutrient deprivation and hypoxia and to some extent, oxidative stress, because these stresses are directly linked to the rate of respiration. Ultimately, nutrient deprivation and hypoxia lead to the inhibition of mTOR and the phosphorylation of eIF2α, through separate signalling networks. The disruption of pre-initiation complexes in response to mTORC1 inhibition and eIF2α phosphorylation results in the localisation of several proteins including transcription factors, polysomes and mRNA molecules into the cytoplasm where they form stress granules (SGs) (Wengrod and Gardner 2015).
Heat stress
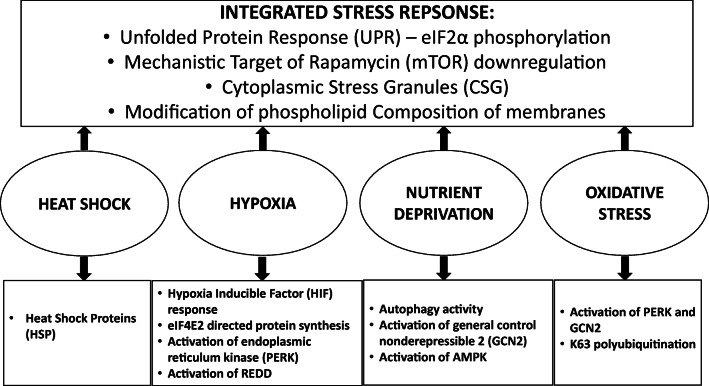
The responses to oxidative stress, hypoxia and nutrient deprivation also overlap with the heat shock response, perhaps the most well studied cellular stress and one that is particularly well conserved between prokaryotic and eukaryotic systems. Although the heat shock response shares similarities with hypoxia and nutrient deprivation and other stresses, it exhibits a unique response as shown in Fig. 1 (Welch and Suhan 1985; Mahat et al. 2016). One of the characteristic responses to heat stress is change in cell form, which normally manifests as shrinkage, caused by modification of the cytoskeleton (Luchetti et al. 2004; Saadeldin et al. 2020). Heat stress also induces a number of morphological changes in organelles, notably the ER and Golgi apparatus which is thought to occur as a result of accumulated unfolded proteins. Furthermore, cells respond to heat stress by forming aggregates of heat stress granules (HSGs) that assemble in the cytoplasm and induce upregulation of genes encoding heat shock proteins (HSPs). These granules play a central role in controlling pre-mRNA processing, with mRNAs preserved in HSGs in the form of eIF2α-GTP-Met-tRNA, along with small ribosomal subunits, initiation factors and RNA-binding proteins. Stress granules with a similar composition are also formed during hypoxia (Kedersha et al. 2005; Timalsina et al. 2018).
Fig. 1.
Eukaryotic response to heat shock, hypoxia, nutrient deprivation and oxidative. These stresses share common adaptive response through eIF2α phosphorylation and mTOR signalling, and also induce specific responses that promote cell survival
HSPs are the most distinctive feature of the heat shock response and have been extensively documented over the last 60 years thus their role is more well understood than many other stress responses (Ritossa 1962). The HSPs are assigned to a particular class according to their molecular weight and sequence homology (Jolly and Morimoto 2000; Richter et al. 2010). The first class contains small HSPs which have a molecular weight of less than 40 kDA, and the second class have a molecular weight of 60–100 kDA. Even under optimal cellular conditions, there is a constant need for these chaperones to bind to and interact with proteins that are not fully synthesised or are inappropriately folded thus they are expressed at low levels under normal conditions. They are upregulated during the heat shock response by heat shock transcription factors (HSF) which bind to the heat shock element (HSE), inducing transcription of heat shock genes (Trinklein et al. 2004; Barna et al. 2018). HSPs are responsible for organelle localisation, minimising the formation of aggregates and targeting aggregated proteins for degradation (Richter et al. 2010; Kuechler et al. 2020). This is achieved through molecular chaperones binding to the hydrophobic regions of specific peptides sequences which prevent intermolecular interactions that ordinarily cause partial folding of proteins and lead to aggregate formation. Their function, however, is not restricted to the maintenance of protein homeostasis. They are known to participate in autocrine and paracrine interactions with adjacent cells, and also promote cell motility (Pereira et al. 2013; Uniacke et al. 2014).
Hypothermia
Hypothermia in humans is generally defined as a core temperature of 35 °C and below (Niazi and Lewis 1958), and temperatures below this point are considered stressful for human cells in culture (Frink et al. 2012). In common with the general response seen more broadly in eukaryotes, human cells respond to hypothermia by altering the lipid composition of the bilayer to withstand the decreased membrane fluidity caused by low temperatures, and by modifying proteins (Hofmann et al. 2012; Young et al. 2013). The changes in protein synthesis involve attenuating global protein synthesis through the downregulation of key initiation and elongation factors, whilst upregulating the expression of a small group of proteins, cold shock proteins (Roobol et al. 2009). Interestingly, the cellular response to mild hypothermia differs to that of more severe hypothermic conditions, supporting the notion that cells continue to grow and proliferate at moderately low temperatures and undergo growth arrest at more severe temperature (Al-Fageeh and Smales 2006a, b). For instance, Saccharomyces cerevisiae respond to mild cold shock (10–18 °C) by upregulating translational machinery and Tip1, Tir1, Tir3 and Nsr1, genes associated with transcription, although global protein production during these conditions are reduced (Homma et al. 2003). More severe forms of cold stress (< 10 °C) cause growth arrest and induce upregulation of an alternative set of proteins that synthesise trehalose to sustain cell viability (Homma et al. 2003; Kandror et al. 2004) (Table 1). A critical factor in the cellular response to low temperatures is the rates of temperature change experienced during both cooling and warming. For instance, mammalian cells respond to cold stress at a quicker rate and at a less drastic temperature reduction (25–35 °C) than most organisms (Roobol et al. 2009). Rapid changes limit the ability of cells to respond with potentially protective changes and instead shift the response such that it is instead focused on the repair and mitigation of damage associated with the temperatures experienced.
Table 1.
Summary of stress granule formation and expression of cold-shock proteins in response to mild cold stress and severe cold stress in mammalian cells and Saccharomyces cerevisiae
| Mammalian cells | Yeast (S. cerevisiae) |
|
|---|---|---|
|
Mild cold stress (25 to 35 °C in mammalian cells) |
No stress granule formation | No stress granule formation |
| (10 to 18 °C in yeast) | RBM3 and CIRP expressed | Tip1, Tir1, Tir2, and Nsr1 expressed |
| Hsp12 and Hsp26 expressed | ||
|
Severe cold stress (0 to 10 °C in mammalian cells) (< 10 °C in yeast) |
Stress granules strongly induced at 10 °C (no stress granule formation at 4 °C) |
Stress granules induced at 10 °C |
| Stress granules strongly induced at 0 °C | ||
| No RBM3 and CIRP expressed | Tps1, Tps2, NTH1 and CCT expressed | |
| Hsp12, Hsp42, Hsp104 and Ssa4 expressed |
Although HSPs were originally discovered during response to heat stress, there is evidence of their expression during warming and recovery from cold stress in several types of cells including SCC12F cells, HeLa cells and rat primary cardiomyocytes (Frink et al. 2012; Hofmann et al. 2012; Young et al. 2013). For example, Holland et al. (1993) studied the changes in translation initiation and HSP expression in SCC12F cells following recovery from exposure to various reduced temperatures (4–20 °C). Increased expression of HSP90 and HSP72 was observed at 15 °C and 4 °C although this increase was modest in comparison to that observed during heat shock. This increase in HSP expression is likely to be dependent on cell type as studies of CHOK1 cells, P19 embryonic carcinoma cells and NIH 3T3 fibroblast cell during cold shock recovery, reported increased synthesis of a number of constitutively expressed HSPs but not of HSP72 or any other HSP involved in the full classical heat shock response (Roobol et al. 2009). Transcription factors that participate in protein folding, metabolic pathways and degrading are also upregulated during the heat shock response.
Cold shock proteins
Two cold shock proteins (CSP) have been identified in mammalian cells. Cold-inducible RNA-binding motif protein 3 (RBM3) and cold-inducible RNA-binding protein (CIRP) are both RNA-binding proteins with conserved glycine rich domains which categorise them as members of the family of glycine-rich proteins (Al-Fageeh and Smales 2006a, b; Zhu et al. 2016b). CIRP was the first CSP to be identified in mammalian cells; however, far less is known about its molecular mechanisms in comparison to RBM3. Both proteins are transcriptionally upregulated during mild cold stress (32 to 34 °C) and function as regulators of translational reprogramming by binding to the 5′-UTR or 3′-UTR of mRNA molecules and facilitating their response to environmental signals (Nishiyama et al. 1997; Fujita et al. 2017). In mammalian cells cultured at 32 °C, mRNA molecules bind to RBM3 and CIRP in the nucleus and cytoplasm and form a complex known as ribonucleoprotein particles (mRNPs) (Reineke and Lloyd 2015). The fate of mRNPs are determined by the RBP composition and some play a crucial role in inducing immediate translation of the attached mRNA whilst others are transported to specific subcellular regions for storage or localised translation (Smart et al. 2007).
Studies by Dresios et al. (Dresios et al. 2005) demonstrate increased RBM3 expression and binding to 60S ribosomal subunits, in cells cultured at 32 °C. This results in elevated levels of 80S polysomes which is consistent with increased association of mRNA and ribosomal subunits, and translation initiation. In addition, RBM3 regulates eIF2α activity during cold stress by inhibiting PERK dependent eIF2α phosphorylation and also by interacting with components within SGs to modulate transcriptional and translational processes (Zhu et al. 2016a). Although RBM3 may facilitate protein synthesis during cold stress, it has a relatively small impact on polysome profiles and its association with only a subset of 60S ribosomal subunits makes it unlikely that its ribosomal binding solely accounts for its effect on translation initiation (Dresios et al. 2005; Logan and Storey 2020). Global protein synthesis remains supressed in cells exposed to cold stress but RBM3 and CIRP positively affect protein synthesis by preventing a more drastic reduction in protein synthesis.
Whilst the exact mechanisms are not fully understood, RBM3 and CIRPS are known to mediate neuroprotection during hypothermia by increasing resistance to neural apoptosis (Chip et al. 2011). In studies of cortical organotypic slice cultures obtained from mice, blocking the RBM3 over-expression that occurs immediately after mild cold stress diminishes the protective effect of hypothermia, and inducing RBM3 expression increases resistance against the induction of apoptosis. RBM3 and CIRP also have an important role in maintaining testicular function in human testis, where temperatures are typically maintained between 2 and 8 °C lower than normal body temperature (Danno et al. 2000). In animal models, surgical induction of cryptorchidism, a condition where the testis fail to descend from the abdominal region to the scrotum, causes disruption of spermatogenesis and infertility (Bergh and Ollesoder 2007). Studies suggest that this occurs because expression of RBM3 and CIRP is restricted to cells subjected to mild cold stress only so although the events are not fully known, RBM3 and CIRP clearly have a positive effect on testicular function and fertility.
Cell membrane adaptation to hypothermia
As well as modulating protein synthesis, cells respond to hypothermia through changes in the lipid composition of their membrane. These alterations occur through signal transduction mechanisms that are transmitted by sensory proteins embedded within the membrane. As temperatures are reduced, the proportion of unsaturated fatty acids in the membrane increases through a regulatory mechanism that controls fatty acid synthesis (Aguilar et al. 1998). This highly conserved adaptive response allows living cells to resists the effect of reduced temperatures on the physical state of their membrane, and also regulates the activity of membrane bound proteins such as the translocators of molecules, ion channels, receptor bound protein kinases and sensory proteins (Los and Murata 2004).
Membrane fluidity refers to the degree of unsaturation within the lipid bilayer, and is an indication of molecular disorder and molecular motion within the cell (Murata and Los 1997). Previous studies have found that even small changes in temperature significantly impact the fatty acid content of the lipid bilayer and prolonged exposure to hypothermia, or dysregulated adaptive responses ultimately lead to cell death (Young et al. 2013). Low external temperatures increase the packing order of lipid molecules within the cell membrane leading to increased membrane saturation. This in turn decreases membrane fluidity as well as the velocity of membrane-associated processes thus, ion permeability, mobility of integral enzymes and exchange between the internal and external environment is significantly reduced (Emara et al. 2012; Panas et al. 2016). Cells respond by triggering a series of events that cause the fluidity of the membrane to increase and upregulate the expression of genes that encode fatty acid desaturases. Fatty acid desaturases catalyse the formation of double bonds in fatty acid molecules within the lipid bilayer causing increased incorporation of saturated fatty acids (SFA) thereby decreasing the fluidity of the membrane (Murata and Los 1997). In mammalian cells, two groups of fatty acid desaturases regulate membrane rigidity: stearoyl-CoA desaturase (SCD) and ∆6D which consists of ∆5D and ∆6D. Four SCD genes (SCD 1–4) have been identified in mice and two (hSDC-1 and hSDC-5) have been characterised in humans (Wang et al. 2005). SCDs are the mammalian analogue of OLE1 in yeast and catalyse the production of ∆9 monounsaturated fatty acids such as oleate, whilst ∆5D and ∆6D are involved in the production of highly polyunsaturated fatty acids (Sampath and Ntambi 2005).
Upon transfer from high to low temperatures, SCDs are expressed in cells through regulated intramembrane proteolysis of transmembrane proteins. This involves cleavage of the ectodomain and intramembrane processing of the transmembrane (Aguilar et al. 1998). The exposed functional domain is translocated to the nucleus where it binds to the promoter region of the SCD gene, leading to its expression. In the case of SCD-1 and SCD-2, transcription is regulated through processing of sterol regulatory element-binding proteins (SREBPs) (Miyazaki et al. 2004). These transmembrane proteins are transcription factors that belong to the basic helix-loop-helix leucine zipper family and are positioned with their NH2-terminal and COOH-terminal domains facing the cytosol and their hydrophilic loop projecting into the lumen of the ER (Hannah et al. 2001). When cells are subjected to hypothermic stress, SREBPs bind to SREBP cleavage-activating protein (SCAP) and form complex SCAP-SREBP which is transported to the Golgi apparatus for cleavage, before it is translocated to the nucleus, where it binds to sterol regulatory elements (SRE) and activates the expression of SCD genes. SREBP has two characterised isoforms, SREBP 1 and SREBP 2, which are expressed by two different genes. SREBP-1 has two different isoforms, SREBP-1a and SREBP-1c, which are derived from a single gene through the use of alternative promoters. These genes also differ in their first exons (Shimomura et al. 1998). SREBP-2 is responsible for transcription of genes involved in cholesterol synthesis and uptake, whereas SREBP-1c targets genes for fatty acid synthesis. SREBP-1a induces production of both cholesterol and fatty acids but is also involved in the expression of ∆5D and ∆6D (Horton et al. 2002; Nakamura and Nara 2002).
SREBP activity is regulated through mTOR and its substrate lipin 1. The gene encoding lipin1 was first identified through positional cloning of the mutant gene underlying liver dystrophia in mice (Péterfy et al. 2001). In mammalian cells, there are four known lipin protein isoforms which are encoded by three different genes. Two lipin-1 protein isoforms are generated from the lpin1 gene whilst lipin-2 and lipin-3 proteins are encoded by LPIN 2 and LPIN 3 respectively (Reue and Zhang 2008). Lipin-2 is predominantly expressed in the liver and brain whereas lipin-3 is present at low levels in most tissues, although abundant in the small intestine and liver (Donkor et al. 2007). Lipin 1 phosphorylation and inhibition is regulated by mTOR1 and directly controls activation or suppression of SREBP transcriptional activity. Studies by Peterson et al. (2011) demonstrate that loss of mTOR1-mediated lipin 1 phosphorylation, promotes its nuclear entry and subsequent downregulation of nuclear SREBP. This interrupts the expression of SCD genes required for the synthesis of fatty acid desaturases. Surprisingly, multiple lipin 1 phosphorylation sites are partially or completely resistant to the effect of mTOR inhibitor rapamycin which is proven through uninterrupted SREBP expression in rapamycin treated cells. On the other hand, ATP-competitive inhibitor of mTOR, Torin1, blocks phosphorylation of all mTOR1 phosphorylation sites regardless of their sensitivity to rapamycin. This suggests that the mechanisms governing mTOR1 and lipin 1 regulation of SREBP are not fully understood and may involve a mechanism that is independent of strict mTOR phosphorylation (Kang et al. 2012). It is therefore clear that the core common responses to multiple stresses, including hypothermia, exclusively involve stress granules formation and the regulation of mTOR.
Stress granule formation and eIF2α phosphorylation
In vitro studies of cellular responses to cold stress confirm the presence of cytosolic SGs which are by-products of translation arrest and polysome disassembly (Kramer et al. 2008). Originally observed in mammalian cells, SGs have been found in numerous eukaryotes including yeast and plants (Kedersha et al. 1999; Weber et al. 2008). It is however not known if they are formed in all eukaryotic cells (Hofmann et al. 2012b). The exact function of SGs remains unclear but it is hypothesised that they play a key role in sequestering proteins and modulating signalling cascades required for cell survival (Aulas et al. 2016). A number of studies have investigated the composition of SGs, with these studies showing that they contain a complex mix of proteins and RNAs as shown in Table 2.
Table 2.
Components of stress granules that form within mammalian and yeast cells upon exposure to moderate hypothermia (25–35 °C). The molecules listed are orthologues of the two eukaryotic organisms and perform interrelated functions
| Mammalian cells: | Yeast: | ||
|---|---|---|---|
| African green monkey COS7 kidney, mouse embryonic fibroblasts, Du145, HeLa, Huh7 | S. cerevisiae | ||
| eIF3B | Eukaryotic initiation factors | eIF4E | Eukaryotic initiation factors |
| eIF4A | eIF4GI | ||
| eIF4E | eIF4GII | ||
| eIF4G | Poly (A) binding protein PABP | ||
| eIF2α | |||
| Poly(A) mRNA | |||
| Poly(A) binding protein PABP | |||
| G3BP | Ras GTPase-activating binding-protein | Pub1 | Yeast orthologue of mammalian TIA 1 |
| HuR | RNA binding proteins | Ngr1 | Yeast orthologue of mammalian TIA R |
| TIA 1 | |||
| TIA R | |||
| Ataxin-2 | Stress granule assembly protein | Pbp1 | Yeast orthologue of mammalian Ataxin-2 |
| 40S ribosomal subunit | Hrp1 | Nuclear cytoplasmic binding-proteins | |
| Gbp2 | |||
| Nrp1 | RNA binding proteins | ||
| Ygr250c | |||
| Eap1 | |||
In mammalian cells, SG formation is initiated by phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2α) (Anderson et al. 2015). In the presence of GTP, eIF2α transports initiator tRNA methionine (tRNAimet) to the site of translation initiation on the 40S small ribosomal subunit, and alongside eIF3 and eIF4, form the pre-initiation complex. Phosphorylation of eIF2α on serine 51 inhibits GTD/GTP exchange by eIF3 and prevents further eIF2-GTP-tRNAimet formation which leads to failure of tRNAimet delivery to the site of translation initiation (Hofmann et al. 2012; Aulas et al. 2016). In mammalian cells, eIF2α phosphorylation occurs through four known kinases, each activated by specific yet overlapping cellular stresses (Li et al. 2006). The double-stranded RNA (ds-RNA) dependent kinase (PKR) is activated by ds-RNA synthesised during viral replication and participates in anti-viral mechanisms through eIFα phosphorylation and suppression of protein synthesis, resulting in reduced viral replication (Reineke and Lloyd 2015). Heme-regulated inhibitor (HRI) is expressed in erythroid cells and facilitates production of suitable quantities of globin, required for erythrocyte formation (Chefalo et al. 1998). PERK responds to unfolded proteins within the ER by inducing reduced protein production thereby reducing the burden of additional protein substrates for ER folding (Yan et al. 2002). PERK activation also occurs under hypothermic or hypoxic culture conditions, or when cellular energy levels are depleted (Bi et al. 2005; Hofmann et al. 2012). Lastly, GCN2 which is activated by uncharged tRNAs during amino acid starvation, induces phosphorylation of eIF2α in response to lack of available substrates (Ye et al. 2015). Despite the core role of eIF2α, poly (A) binding proteins (PABP), small ribosomal subunits, mRNA molecules, and translation initiation factor eIF2, eIF3, eIF4E and eIF4G, also contribute to SG formation during cold stress.
SG formation is not exclusive to cold stress and forms part of an integrated system of mechanisms that are characteristic of cellular response to heat shock, hypoxia, nutrient deprivation, oxidative stress and other stressors. In respect to their composition, size and components, these SGs demonstrate stress and organism-specific differences. For instance, in Saccharomyces cerevisiae, heat shock induces HSGs containing eIF3 whereas nutrient deprivation–derived stress granules do not (Buchan and Parker 2009). Furthermore, SGs induced by nutrient deprivation contain eIF4E and eIF4G, whereas SGs induced by oxidative stress contain other distinct components such as increased abundance of eIF2 and downstream factors (Kedersha and Anderson 2009). Moreover, depending on culture conditions, stress granules harbour additional protein components such as RNA helicases, translation and stability regulators and factors involved in cell signalling. SGs induced by heat stress characteristically include low molecular weight HSPs such as the HSP70-HSP40-HSP110 network in mammalian cells, although similar to cold stress, HSGs emerge in response to phosphorylation of eIF2α (Cherkasov et al. 2013). They also contain many of the components found in cold stress–induced SGs, namely arrested mRNA molecules, eIF4E, eIF4G, poly (A) binding proteins (PABP), the 40S ribosomal subunits and TIA-1 (Buchan and Parker 2009). Studies of oxidative stress demonstrate its ability to induce SG formation, particularly in mammalian cells where treatment with sodium arsenite and hydrogen peroxide results in the formation of various SG types. In comparison to hydrogen peroxide, arsenite-induced SGs are smaller and disassociate at a quicker rate but these differences are structural and evidently due to the fact that SG formation processes differ according to the initiating stressor (Chen and Liu 2017). This notion is supported by the compositional differences between the two treatments; arsenite-induced SG formation requires and contains eIF4E whilst hydrogen peroxide–induced SGs contain significantly reduced amounts of eIF3, eIF4E and eIF4G (Emara et al. 2012). Additionally, arsenite-induced SGs require phosphorylation of eIF2α whereas hydrogen peroxide–induced SGs do not. SGs induced by nutrient deprivation also share some unique features with other stressors including its induction by eIF2 phosphorylation through GCN2 activation. This response is accompanied by disassembly of polysomes and accumulation of stalled ribosomal complexes, although mRNAs bearing 5′-terminal oligopyrimidine tracts (5′TOPs) are selectively packaged into these SGs (Panas et al. 2016). Nutrient deprivation also induces the assembly of granules that contain many of the initiation factors found in other SGs, but lack 40S ribosomal subunits and eIF3 subunits (Emara et al. 2012; Hoyle et al. 2007). In comparison to other stressors, nutrient deprivation causes the most severe stress-induced inhibition of translation initiation and is characterised by brighter, larger and more frequent SGs in cells in comparison to those induced by other forms of stress (Buchan et al. 2008). Evidently, the composition of SGs is comparable, irrespective of the stressor although there are some distinctive, stress-specific differences. All SGs generally contain stalled mRNA molecules, ribosomal subunits and many of the initiation factors involved in translation initiation and elongation; however, the abundance of each molecule is dependent on the type of stress induced. In comparison to other stressors, eukaryotic cells are most sensitive to nutrient deprivation, which leads to the formation of relatively large SGs, but cold stress and heat shock induce upregulation of distinctive proteins that form part of the SGs that stabilise mammalian cells and increase cell survival during fluctuating temperatures.
Mechanistic target of rapamycin and control of translation
mTOR, previously known as mammalian target of rapamycin, is a serine/threonine protein kinase that plays a central role in the regulation of cell growth and proliferation, protein degradation and lipid metabolism (Reiling and Sabatini 2006; Ben-Sahra and Manning 2017). Its yeast orthologue, TOR was first discovered by mutations in Saccharomyces cerevisiae that induced resistance to the growth inhibitory properties of antifungal macrolide rapamycin (Heitman et al. 1991). Since then, its kinase activity has been associated with anabolic pathways such as protein synthesis, ribosome production, lipogenesis and nucleotide synthesis, all of which are crucial to cell and tissue growth (Xie et al. 2016; Peterson et al. 2011). Moreover, mTOR suppresses cellular autophagy by inhibiting its activation and supressing the production of lysosomes, the organelle in which autophagy occurs (Thoreen et al. 2009). The mTOR protein contains numerous sub-domains with highly conserved genetic sequences. In human, mice and rat, mTOR shares a 95% amino acid sequence homology, indicating comparable or similar cellular functions between species (Giles and Albitar 2005). mTOR forms part of the PI3K-related protein kinase family (PIKK) which act as regulatory enzymes during DNA damage, DNA repair and DNA recombination. Its catalytic domain is highly homologous to the lipid kinase domain of phosphoinositide 3-kinase (PI3K) which interacts with several subunits to form two distinct complexes: mTOR1 and mTOR2. The complexes participate in different pathways and recognise distinct substrates through unique mTOR-interacting proteins (Guertin et al. 2017). Both complexes contain the mTOR catalytic subunit, mLST8, also known as GβL, DEPTOR and the Tti1 and/Tel2 complex. The regulatory-associated protein of mTOR (raptor) is exclusive to rapamycin sensitive mTOR1 and functions as both a scaffolding protein and a bridging protein that connects downstream substrate targets to the mTOR kinase domain whilst enhancing its phosphorylation activity (Sampath and Ntambi 2005; Aylett et al. 2016). Germline disruption of raptor in mice results in embryonic lethality at the implantation stage. In fact, the inner cell mass (ICM) and trophoblast of blastocysts obtained from mice deficient in raptor fail to expand in culture and eventually die, highlighting the importance of mTOR and protein synthesis in early embryonic development (Gangloff et al. 2004).
Rapamycin-insensitive companion of mTOR (rictor) and mSin1 are associated with mTOR2 only (Guertin et al. 2017). In eukaryotic organisms, mTORC2 phosphorylates and activates Akt (PKB) which is also involved in cell proliferation, growth, survival, and metabolism in accordance with its environmental cues (Bai and Jiang 2010). When activated by membrane-bound receptors, PI3K catalyses the conversion of phosphatidylinositol (4,5)-biphosphate (PIP2) to phosphatidylinositol (3,4,5)-triphosphate (PIP3) which binds to Akt to induce dimerisation and exposure of it catalytic domain (Shukla et al. 2007). In order be fully active, Akt must be phosphorylated at two critical residues: THR308 and SER473. THR308 is known to be phosphorylated by upstream kinase PDK1, but the identity of the kinase responsible for SER473 phosphorylation has been elusive until recently. Several candidates including PDK1, integrin-linked kinase (ILK) and Akt itself were previously proposed but there is now compelling evidence that rictor phosphorylates Akt at SER473 (Maira et al. 2008). Prolonged treatment of cells with rapamycin inhibits Akt phosphorylation as newly synthesised mTOR components are sequestered. This interferes with the assembly of mTOR2, thereby inhibiting the activity of rictor within the cell (Sarbassov et al. 2005). Activated Akt regulates mTOR1 activity either by direct phosphorylation or through the GTPase activity of Rheb, Tuberous sclerosis complex (TSC1) and (TSC2) (Péterfy et al. 2001; Reue and Zhang 2008).
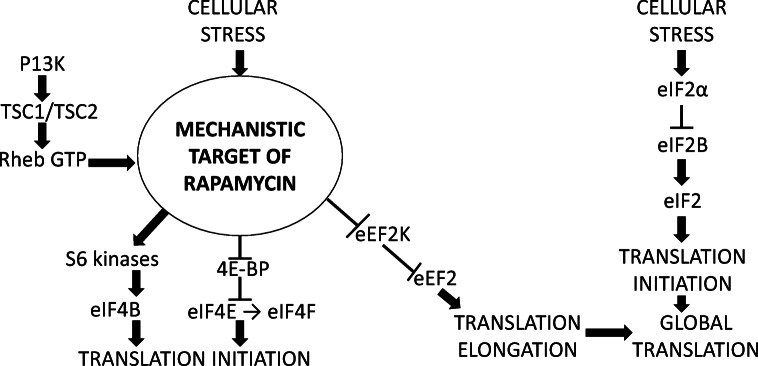
Translation initiation is a rate-determining step involved in protein production and is controlled through two downstream substrates of mTOR1; S6K and 4E-BP, both of which are regulated through rapamycin-sensitive phosphorylation (Fig. 2). Active mRNA molecules possess a 5′terminal 7-methylguanosine cap which is required for translation. This is recognised and bound by cap binding protein eIF4E, which associates with scaffold protein eIF4G and RNA helicase eIF4A to form the eIF4F complex. Changes in the rate of translation are intimately linked with changes in the eIF4F activity and actively growing cells produce higher levels of eIF4F (Giles and Albitar 2005). Alongside pre-initiation complex eIF2α-GTP-tRNAimet and eIF3, eIF4F delivers the mRNA molecule to the 40S ribosomal subunit in preparation of initiation and also interacts with PABP to circularise the mRNA molecule between the 5′terminal and 3′ poly (A) tail (Thoreen et al. 2009). 4E-BP interrupts this process by binding to same region eIF4E as eIF4G, thereby preventing eIF4E and eIF4G interacting and engaging in translation initiation complexes. Although there are three known mammalian variants of 4E-BPs but 4E-BP1 is the most characterised (Wang et al. 2001). Under normal conditions, 4E-BP1 is phosphorylated by mTOR which decreases its affinity for eIF4E, making it available to form part of the eI4F complex (Connolly et al. 2006).
Fig. 2.
Mechanistic target of rapamycin (mTOR) and eIF2α control of transcription factors involved in translation initiation and elongation. Stress induces reduced mTOR signalling and subsequent supressed protein synthesis in order to preserve cellular resources
There are presently two known variants of mammalian S6K (S6K1 and S6K2), both of which exhibit comparable control mechanisms (Pende et al. 2004). It was previously assumed that S6K control of translation occurred through rapamycin sensitive phosphorylation of the 40S ribosomal protein S6 however this has been disputed. S6K1 knockout mice exhibit a smaller cell size phenotype than that seen in S6K2, suggesting a link between translational control and S6K1, but not S6K2 (Marke et al. 2005). Under normal cellular conditions, S6K1 is phosphorylated at residue THR389, through its association with mTOR. This generates a docking site for further phosphorylation by phosphoinositide-dependent kinase 1 (PDK1), within the activation loop at residue THR229, resulting in complete activation of S6K1 (Holz et al. 2005). Its two substrates, ribosomal protein (rp), a component of the 40S ribosomal subunit and mRNA helicase eIF4B are phosphorylated by its activation in preparation for translation initiation. eIF4B is an mRNA binding protein with no autonomous catalytic activity but it enhances the affinity of eIF4A to ATP and mRNA, which increases its helicase activity (Raught et al. 2004). These interactions are sensitive to rapamycin and environmental changes; thus, cellular stress induces mTOR1 inactivation and disassociation from S6K1, preventing its phosphorylation and subsequent activation of eIF4B and the 40S ribosomal subunit. S6K1 remains bound to pre-initiation complex eIF2α-GTP-tRNAimet and eIF3, which acts as a scaffold through which mTOR regulates S6K1 and 4E-BP activity. Previous studies have associated low eIF4A helicase activity, and decreased mRNA binding with the absence of eIF4B (Bordeleau et al. 2005). Therefore, it can be assumed that phosphorylated eIF4B and eIF4A have shared functions although this is yet to be fully investigated. S6K1 inactivity leads to inefficient translation of mRNA encoding proteins involved in ribosome biogenesis which is necessary for enhanced protein synthesis.
Mammalian cells also require eukaryotic elongation factors eEF1 (eEF1A and eEF1B) and eEF2 to facilitate the elongation phase of translation. Cellular activities of both proteins are subject to phosphorylation although eEF1 is phosphorylated by protein kinase C whilst eEF2 is controlled by downstream regulation of mTOR signalling (Browne and Proud 2002). eEF1A participates in translation elongation by binding to GTP and interacting with amino-acyl tRNA whilst eEF2 promotes translocation as the ribosome moves along the mRNA molecule by the equivalent of one codon (Frank et al. 2007). GTP-bound eEF1A transports aminoacyl-tRNA molecules to the site of peptide formation whilst eEF2 exposes the mRNA codon. Complementary base pairing between mRNA codons and tRNA anticodons causes disassociation of eEF1A, translocation from the A-site to the P-site and transportation of charged aminoacyl-tRNA molecules to the vacant A-site (Chefalo et al. 1998; Yan et al. 2002). When cellular stress occurs, eEF2 is deactivated by phosphorylation at threonine 56 causing decreased rates of translation elongation. This process is catalysed by a highly specific enzyme that does not belong to the main protein kinase super family but to another small group of enzymes with primary sequences that have no similarity to other protein kinases (Wang 2006). The regulation of eEF2 kinase (eEF2K) through mechanisms induced by cold stress is not fully understood but it is known to be involved in mTOR signalling through phosphorylation at SER366, SER78 and SER359 by PDK1-dependent protein kinases, p70 S6k and p90RSK (Wang et al. 2001).
mTOR activity is inhibited by a range of stress conditions; however, the underlying mechanisms are stress-specific and differ between cell types. When deprived of nutrients, S6K and 4E-BP undergo rapid dephosphorylation in mammalian cells, even with the addition of insulin, which normally results in phosphorylation of both proteins (Proud 2002). Sufficient nutrient availability therefore plays a crucial role in maintaining normal mTOR signalling and its ability to respond to hormonal changes. The link between amino acid and glucose starvation, and mTOR regulation is yet to be elucidated, but studies suggest that leucine is most effective in promoting mTOR signalling, although the absence of other essential amino acids from culture also impairs mTOR signalling, albeit to a lesser extent (Hara et al. 1998). The effects of nutrient deprivation are comparable to those of hypoxia because both are required for energy supply by ATP production. A lack of either of these resources interferes with mitochondrial function and decrease ATP synthesis and subsequently, ATP levels. This in turn activates AMP-sensor AMPK which directly regulates mTOR signalling and causes activation and dephosphorylation of S6K, thereby reducing the rates of protein synthesis and energy consumption. Oxidative stress also indirectly causes mTOR deactivation through AMPK signalling. Protein damage from ROS build up results in the accumulation of unfolded proteins which causes ER stress and subsequently PERK activation, and also AMPK activation via a mechanism involving a cytoplasmic form of the PI3K-like kinase, Ataxia-Telangiectasia Mutated (ATM)(Shiloh and Ziv 2013). In a similar manner, denaturation of proteins caused by elevated temperatures and subsequent build-up of unfolded proteins also lead to PERK and AMPK activation. One of the most unique features of the heat stress response is the sequestration of mTOR into SGs, which allows mTOR signalling to be controlled in order to achieve cell growth and recovery and to also reduce heat-induced mutations (Takahara and Maeda 2012). Ultimately, through stress-specific mechanisms, all stressors induce a series of signalling cascades that deactivate mTOR activity and lead to the dephosphorylation of S6K and 4E-BP which then attenuates translation initiation and elongation.
Concluding remarks and future prospects
Advance in the study of hypothermic stress in eukaryotic cells highlight the role of various phosphorylated kinases and enzymes in regulating cell survival processes through eIF2α phosphorylation, stress granule formation and mTOR regulation. Further study is however required in order to gain further insight into the changes that occur within the lipid bilayer and the mechanisms that govern these, other than the involvement of lipin 1. Additionally, identifying whether these responses are universal to all eukaryotic organisms or just a subset, could contribute to improved cryopreservation and cell and tissue handling solutions. It can therefore be concluded that eukaryotic response to hypothermia forms part of the integrated network of mechanisms that occurs in response to numerous stresses, which is unsurprising considering that damage caused by most stresses are comparable. Furthermore, it is clear that cells have overlapping needs during various stress conditions; for example, decreased membrane saturation accommodates accumulation of unfolded and unprocessed proteins in the ER, although these changes were previously associated with temperature changes only. Studies of response to stress in general are therefore useful in improving our knowledge of cellular response to hypothermia.
Abbreviations
- 4E-BP
eIF4E binding protein
- AMPK
AMP-activated protein kinase
- CIRP
Cold-inducible RNA-binding protein
- CSP
Cold shock protein
- EIF
Eukaryotic initiation factor
- ER
Endoplasmic reticulum
- GCN2
General Control Non-Derepressible 2
- HIF
Hypoxia-inducible factor
- HRE
Hypoxia-responsive elements
- HRI
Heme-regulated inhibitor
- HSE
Heat shock element
- HSF
Heat shock factor
- HSP
Heat shock protein
- ILK
Integrin-linked kinase
- mRNPs
Ribonucleoprotein particles
- mTOR
Mechanistic target of rapamycin
- PERK
PKR-like endoplasmic reticulum kinase
- PIKK
PI3K-related protein kinase family
- PIP2
Phosphatidylinositol (4,5)-biphosphate
- PIP3
Phosphatidylinositol (3,4,5)-triphosphate
- raptor
Regulatory-associated protein of mTOR
- RBM3
RNA-binding motif protein 3
- RBP
RNA-binding protein
- rictor
Rapamycin-insensitive companion of mTOR
- ROS
Reactive oxygen species
- S6K
Ribosomal S6 kinase
- SCD
Stearoyl-CoA desaturase
- SG
Stress granule
- SREBPs
Sterol regulatory element-binding proteins
Funding information
NAA is supported by funding from Genea Biomedx awarded to KEF and SCH.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguilar PS, Cronan JE, De Mendoza D (1998) A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase. J Bacteriol 180(8):2194–2200 http://jb.asm.org/content/180/8/2194.short. Accessed 31 Oct 2018 [DOI] [PMC free article] [PubMed]
- Aitken RJ, Gibb Z, Baker MA, Drevet JR. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. 2016;28:1–10. doi: 10.1071/RD15325. [DOI] [PubMed] [Google Scholar]
- Al-Fageeh MB, Smales CM. Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem J. 2006;397(2):247–259. doi: 10.1042/BJ20060166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Fageeh MB, Smales CM. Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem J. 2006;397(2):247–259. doi: 10.1042/BJ20060166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BW, Demchenko IT, Piantadosi CA. Two faces of nitric oxide: implications for cellular mechanisms of oxygen toxicity. J Appl Physiol. 2009;106(2):662–667. doi: 10.1152/japplphysiol.91109.2008. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N, Ivanov P (2015) Stress granules, P-bodies and cancer. Biochim Biophys Acta Gene Regul Mech. 10.1016/j.bbagrm.2014.11.009 [DOI] [PMC free article] [PubMed]
- Aulas A, Fay MM, Lyons SM, Achorn CA, Kedersha N, Anderson P, Ivanov P (2016) Journal of cell science • advance article. J Cell Sci. 10.1242/jcs.199240
- Aylett CHS, Sauer E, Imseng S, Boehringer D, Hall MN, Ban N, Maier T. Architecture of human mTOR complex 1. Science. 2016;351(6268):48–52. doi: 10.1126/science.aaa3870. [DOI] [PubMed] [Google Scholar]
- Bai X, Jiang Y. Key factors in mTOR regulation. Cell Mol Life Sci. 2010;67:239–253. doi: 10.1007/s00018-009-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balchin D, Hayer-Hartl M, Ulrich Hartl F. In vivo aspects of protein folding and quality control. Science. 2016;353:aac4354. doi: 10.1126/science.aac4354. [DOI] [PubMed] [Google Scholar]
- Barna J, Csermely P, Vellai T. Roles of heat shock factor 1 beyond the heat shock response. Cell Mol Life Sci. 2018;75:2897–2916. doi: 10.1007/s00018-018-2836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki R. Cellular stress. FEBS Lett. 2007;581(19):3581–3581. doi: 10.1016/j.febslet.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Basner RC. Continuous positive airway pressure for obstructive sleep apnea. N Engl J Med. 2007;356(17):1751–1758. doi: 10.1056/NEJMct066953. [DOI] [PubMed] [Google Scholar]
- Ben-Sahra I, Manning BD. mTORC1 signaling and the metabolic control of cell growth. Curr Opin Cell Biol. 2017;45:72–82. doi: 10.1016/j.ceb.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh A, Ollesoder O (2007) Studies of cryptorchidism in experimental animal models. Accessed October 31, 2018. 10.1111/j.1651-2227.2007.00295.x [DOI] [PubMed]
- Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, Scheuner D, Kaufman RJ, Bell J, Ron D, Wouters BG, Koumenis C. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24(19):3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordeleau M-E, Matthews J, Wojnar JM, Lindqvist L, Novac O, Jankowsky E, Sonenberg N, Northcote P, Teesdale-Spittle P, Pelletier J. Stimulation of mammalian translation initiation factor EIF4A activity by a small molecule inhibitor of eukaryotic translation. PNAS. 2005;102(30):10460–10465. doi: 10.1073/pnas.0504249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- Browne GJ, Finn SG, Chemistry CG, and Proud (2004) Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. ASBMB. http://www.jbc.org/content/early/2004/01/05/jbc.M309773200.full.pdf. Accessed 31 Oct 2018 [DOI] [PubMed]
- Buchan JR, Parker R (2009) Eukaryotic stress granules: the ins and outs of translation. Mol Cell Howard Hughes Med Inst. 10.1016/j.molcel.2009.11.020 [DOI] [PMC free article] [PubMed]
- Buchan JR, Muhlrad D, Parker R. P bodies promote stress granule assembly in Saccharomyces cerevisiae. J Cell Biol. 2008;183(3):441–455. doi: 10.1083/jcb.200807043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan KL (2000) Stress and the evolution of condition-dependent signals. Trends Ecol Evol Elsevier. 10.1016/S0169-5347(99)01812-1 [DOI] [PubMed]
- Chefalo PJ, Jihyun O, Rafie-Kolpin M, Kan B, Chen JJ. Heme-regulated EIF-2α kinase purifies as a hemoprotein. Eur J Biochem. 1998;258(2):820–830. doi: 10.1046/j.1432-1327.1998.2580820.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu B (2017) Relationships between stress granules, oxidative stress, and neurodegenerative diseases. 10.1155/2017/1809592 [DOI] [PMC free article] [PubMed]
- Cherkasov V, Hofmann S, Druffel-Augustin S, Mogk A, Tyedmers J, Stoecklin G, Bukau B. Coordination of translational control and protein homeostasis during severe heat stress. Curr Biol. 2013;23(24):2452–2462. doi: 10.1016/j.cub.2013.09.058. [DOI] [PubMed] [Google Scholar]
- Chip S, Zelmer A, Ogunshola OO, Felderhoff-Mueser U, Nitsch C, Bührer C, Wellmann S. The RNA-binding protein RBM3 is involved in hypothermia induced neuroprotection. Neurobiol Dis. 2011;43(2):388–396. doi: 10.1016/j.nbd.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Connolly E, Braunstein SF, Schneider RJ. Hypoxia inhibits protein synthesis through a 4E-BP1 and elongation factor 2 kinase pathway controlled by mTOR and uncoupled in breast cancer cells. Mol Cell Biol. 2006;26(10):3955–3965. doi: 10.4026/1303-2860.2013.0217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danno S, Itoh K, Matsuda T, Fujita J. Decreased expression of mouse Rbm3, a cold-shock protein, in Sertoli cells of cryptorchid testis. Am J Pathol. 2000;156(5):1685–1692. doi: 10.1016/S0002-9440(10)65039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkor J, Sariahmetoglu M, Dewald J, Brindley DN, Reue K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J Biol Chem. 2007;282(6):3450–3457. doi: 10.3390/e16094937. [DOI] [PubMed] [Google Scholar]
- Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, Mauro VP. Cold stress-induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci. 2005;102(6):1865–1870. doi: 10.1073/pnas.0409764102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara MM, Fujimura K, Sciaranghella D, Ivanova V, Ivanov P, Anderson P (2012) Hydrogen peroxide induces stress granule formation independent of EIF2α phosphorylation. Biochem Biophys Res Commun. 10.1016/j.bbrc.2012.06.033 [DOI] [PMC free article] [PubMed]
- Eskla KL, Porosk R, Reimets R, Visnapuu T, Vasar E, Hundahl CA, Luuk H. Hypothermia augments stress response in mammalian cells. Free Radic Biol Med. 2018;121(June):157–168. doi: 10.1016/j.freeradbiomed.2018.04.571. [DOI] [PubMed] [Google Scholar]
- Filomeni G, Desideri E, Cardaci S, Rotilio G, Ciriolo MR (2010) Under the ROS... thiol network is the principal suspect for autophagy commitment. Autophagy. 10.4161/auto.6.7.12754 [DOI] [PubMed]
- Frank J, Gao H, Sengupta J, Gao N, Taylor DJ. The process of MRNA-TRNA translocation. Proc Natl Acad Sci. 2007;104(50):19671–19678. doi: 10.1073/pnas.0708517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frink M, Flohé S, Van Griensven M, Mommsen P, Hildebrand F. Facts and fiction: the impact of hypothermia on molecular mechanisms following major challenge. Mediat Inflamm. 2012;2012:1–13. doi: 10.1155/2012/762840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Higashitsuji H, Higashitsuji H, Liu Y, Itoh K, Sakurai T, Kojima T (2017) et al, TRPV4-dependent induction of a novel mammalian cold-inducible protein SRSF5 as well as CIRP and RBM3 OPEN. Sci Rep. 10.1038/s41598-017-02473-x [DOI] [PMC free article] [PubMed]
- Fulda S, Gorman AM, Hori O, Samali A (2010) Cellular stress responses: cell survival and cell death. Int J Cell Biol. 10.1155/2010/214074 [DOI] [PMC free article] [PubMed]
- Gameiro PA, Struhl K. Nutrient deprivation elicits a transcriptional and translational inflammatory response coupled to decreased protein synthesis. Cell Rep. 2018;24(6):1415–1424. doi: 10.1016/j.celrep.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz J-F, Um SH, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24(21):9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh N, Das A, Chaffee S, Roy S, Sen CK (2017) Reactive oxygen species, oxidative damage and cell death. In Immunity and inflammation in health and disease: emerging roles of nutraceuticals and functional foods in immune support, 45–55. Elsevier. 10.1016/B978-0-12-805417-8.00004-4
- Giles FJ, Albitar M (2005) Mammalian target of rapamycin as a therapeutic target in leukemia. Curr Mol Med. Vol. 5. https://s3.amazonaws.com/academia.edu.documents/46310755/Mammalian_Target_of_Rapamycin_as_a_Thera20160607-1326-1k7ucid.pdf?AWSAccessKeyId=AKIAIWOWYYGZ2Y53UL3A&Expires=1541011667&Signature=rIL3m1hRU1P9IruNKHOmdfOO0vc%253D&response-content-disposition=inline%25. Accessed 31 Oct 2018 [DOI] [PubMed]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or MLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev Cell. 2017;11(6):859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Hannah VC, Ou J, Luong A et al (2001) Unsaturated fatty acids down-regulate Srebp isoforms 1a and 1c by two mechanisms in HEK-293 cells. ASBMB 276(6):4365–4372 http://www.jbc.org/content/276/6/4365.short. Accessed 31 Oct 2018 [DOI] [PubMed]
- Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate P70 S6 kinase and EIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273(23):14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman J, Rao Movva N, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253(5022):905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Hofmann S, Cherkasova V, Bankhead P, Bukau B, Stoecklin G (2012) Translation suppression promotes stress granule formation and cell survival in response to cold shock. 10.1091/mbc.E12-04-0296 [DOI] [PMC free article] [PubMed]
- Holland DB, Roberts SG, Wood EJ, Cunliffe WJ. Cold shock induces the synthesis of stress proteins in human keratinocytes. J Investig Dermatol. 1993;101(2):196–199. doi: 10.1111/1523-1747.ep12363791. [DOI] [PubMed] [Google Scholar]
- Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123(4):569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Homma T, Iwahashi H, Komatsu Y. Yeast gene expression during growth at low temperature. Cryobiology. 2003;46(3):230–237. doi: 10.1016/S0011-2240(03)00028-2. [DOI] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Investig. 2002;109(9):1125–1131. doi: 10.1172/JCI0215593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Davis RJ. Cell signaling and stress responses. Cold Spring Harb Perspect Biol. 2016;8(10):a006072. doi: 10.1101/cshperspect.a006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle NP, Castelli LM, Campbell SG, Holmes LEA, Ashe MP. Stress-dependent relocalization of translationally primed MRNPs to cytoplasmic granules that are kinetically and spatially distinct from P-bodies. J Cell Biol. 2007;179(1):65–74. doi: 10.1083/jcb.200707010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92(19):1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- Jones DP. Radical-free biology of oxidative stress. AJP Cell Physiol. 2008;295(4):C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror O, Bretschneider N, Kreydin E, Cavalieri D, Goldberg AL. Yeast adapt to near-freezing temperatures by STRE/Msn2,4-dependent induction of trehalose synthesis and certain molecular chaperones. Mol Cell. 2004;13(6):771–781. doi: 10.1016/S1097-2765(04)00148-0. [DOI] [PubMed] [Google Scholar]
- Kang S, Dong SM, Kim BR, Park MS, Trink B, Byun HJ, Rho SB. Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells. Apoptosis. 2012;17(9):989–997. doi: 10.1007/s10495-012-0717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN. Stress: common themes toward the next frontier. Front Neuroendocrinol. 2018;49:3–7. doi: 10.1016/j.yfrne.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Kedersha N, Anderson P (2009) Regulation of translation by stress granules and processing bodies. Chapter 4 Regulation of Translation by Stress Granules and Processing Bodies 10.1016/S1877-1173(09)90004-7 [DOI] [PMC free article] [PubMed]
- Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of EIF-2α to the assembly of mammalian stress granules. J Cell Biol. 1999;147(7):1431–1441. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fitzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of MRNP remodeling. J Cell Biol. 2005;169(6):871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koritzinsky M, Magagnin MG, Van Den Beucken T, Seigneuric R, Savelkouls K, Dostie J, Pyronnet S, et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 2006;25(5):1114–1125. doi: 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S, Queiroz R, Ellis L, Webb H, Hoheisel JD, Clayton C, Carrington M. Heat shock causes a decrease in polysomes and the appearance of stress granules in trypanosomes independently of EIF2 phosphorylation at Thr169. J Cell Sci. 2008;121(18):3002–3014. doi: 10.1242/jcs.031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuechler ER, Budzyńska PM, Bernardini JP, Gsponer J, Mayor T. Distinct features of stress granule proteins predict localization in Membraneless organelles. J Mol Biol. 2020;432(7):2349–2368. doi: 10.1016/j.jmb.2020.02.020. [DOI] [PubMed] [Google Scholar]
- Kumar EA, Giles D, Dalby K. AMPK can stimulate EEF2 phosphorylation without regulating its cognate kinase EEF2K. FASEB J. 2020;34(S1):1–1. doi: 10.1096/fasebj.2020.34.s1.09697. [DOI] [Google Scholar]
- Leprivier G, Remke M, Rotblat B, Dubuc A, ARF Mateo - Cell, and Undefined 2013 (2013) The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Elsevier. https://www.sciencedirect.com/science/article/pii/S0092867413005321. Accessed 13 Jul 2020 [DOI] [PMC free article] [PubMed]
- Li ZM, Xue CJ, Wang JH, Wang QM. Comparative study of the characteristics of typical mineral deposits in Xinjiang, China, and its neighboring countries and regions. Geol China. 2006;33(1):160–168. doi: 10.15252/embr.201642195. [DOI] [Google Scholar]
- Li GW, Burkhardt D, Gross C, Weissman JS. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell. 2014;157(3):624–635. doi: 10.1016/j.cell.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Qian SB. Translational reprogramming in cellular stress response. Wiley Interdisc Rev RNA NIH Public Access. 2014;5:301–305. doi: 10.1002/wrna.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan SM, Storey KB (2020) Cold-inducible RNA-binding protein Cirp, but not Rbm3, may regulate transcript processing and protection in tissues of the hibernating ground squirrel. Cell Stress Chaperones. 10.1007/s12192-020-01110-3 [DOI] [PMC free article] [PubMed]
- Los DA, Murata N. Membrane fluidity and its roles in the perception of environmental signals. Biochim Biophys Acta Biomembr. 2004;1666:142–157. doi: 10.1016/j.bbamem.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Luchetti F, Mannello F, Canonico B, Battistelli M, Burattini S, Falcieri E, Papa S. Integrin and cytoskeleton behaviour in human neuroblastoma cells during hyperthermia-related apoptosis. Apoptosis. 2004;9(5):635–648. doi: 10.1023/B:APPT.0000038043.03799.6f. [DOI] [PubMed] [Google Scholar]
- Mahat DB, Hans Salamanca H, Duarte FM, Danko CG, Lis JT. Mammalian heat shock response and mechanisms underlying its genome-wide transcriptional regulation. Mol Cell. 2016;62(1):63–78. doi: 10.1016/j.molcel.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch SB, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7(7):1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-{inducible} {factors} and the {response} to {hypoxic} {stress} Mol Cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marke JJ, Sloane R, Ryan LM (2005) Legal research and law library management. Biochem J 441. Portland Press Limited. 10.1042/BJ20110892
- Melanson G, Timpano S, Uniacke J (2017) The EIF4E2-directed hypoxic cap-dependent translation machinery reveals novel therapeutic potential for cancer treatment. 10.1155/2017/6098107 [DOI] [PMC free article] [PubMed]
- Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of Lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and independent mechanisms. J Biol Chem. 2004;279(24):25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- Murata N, Los DA. Membrane fluidity and temperature perception. Plant Physiol. 1997;115:875–879. doi: 10.1104/pp.115.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura MT, Nara TY. Gene regulation of mammalian desaturases. Biochem Soc Trans. 2002;30(Pt 6):1076–1079. doi: 10.1042/bst0301076. [DOI] [PubMed] [Google Scholar]
- Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: a master regulator of microRNA biogenesis and activity. Free Radic Biol Med. 2013;64:20–30. doi: 10.1016/j.freeradbiomed.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazi SA, Lewis JF. Profound hypothermia in man ’ report of a case * *. Annual Review of Surgery. 1958;147(2):264–266. doi: 10.1097/00000658-195802000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama H, Higashitsuji H, Yokoi H, Itoh K, Danno S, Matsuda T, Fujita J. Cloning and characterization of human CIRP (cold-inducible RNA-binding protein) CDNA and chromosomal assignment of the gene. Gene. 1997;204(1–2):115–120. doi: 10.1016/S0378-1119(97)00530-1. [DOI] [PubMed] [Google Scholar]
- Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM (2016) The integrated stress response. 10.15252/embr.201642195 [DOI] [PMC free article] [PubMed]
- Panas MD, Ivanov P, Anderson P. Mechanistic insights into mammalian stress granule dynamics. J Cell Biol. 2016;215:313–323. doi: 10.1083/jcb.201609081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende M, Um SH, Mieulet V, Sticker MV, Goss L, Mestan J, Mueller M, Fumagalli S, Kozma C, Thomas G. S6K1-/-/S6K2-/- mice exhibit perinatal lethality and rapamycin-sensitive 5’-terminal oligopyrimidine MRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24(8):3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira ER, Frudd K, Awad W, Hendershot LM. Endoplasmic reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (HIF-1) transcriptional activity on targets like vascular endothelial growth factor (VEGF) *. J Biol Chem. 2013;289(6):3352–3364. doi: 10.1074/jbc.M113.507194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péterfy M, Phan J, Xu P, Reue K. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet. 2001;27(1):121–124. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- Peterson TR, Sengupta SS, Harris TE, AE Carmack - Cell, and Undefined 2011 (2011) “mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway.” Elsevier. https://www.sciencedirect.com/science/article/pii/S0092867411007094. Accessed 31 Oct 2018 [DOI] [PMC free article] [PubMed]
- Proud CG (2002) Regulation of mammalian translation factors by nutrients. European Journal of Biochemistry. John Wiley & Sons, Ltd. 10.1046/j.1432-1033.2002.03292.x. [DOI] [PubMed]
- Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, Polakiewicz RD, Sonenberg N, Hershey JWB. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004;23(8):1761–1769. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- Reineke LC, Lloyd RE. The stress granule protein G3BP1 recruits protein kinase R to promote multiple innate immune antiviral responses. J Virol. 2015;89(5):2575–2589. doi: 10.1128/JVI.02791-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reue K, Zhang P. The lipin protein family: dual roles in lipid biosynthesis and gene expression. FEBS Lett. 2008;582:90–96. doi: 10.1016/j.febslet.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J. The {heat} {shock} {response}: {life} on the {verge} of {death} Mol Cell. 2010;40(2):253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia. 1962;18(12):571–573. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- Roobol A, Carden MJ, Newsam RJ, Mark Smales C. Biochemical insights into the mechanisms central to the response of mammalian cells to cold stress and subsequent rewarming. FEBS J. 2009;276(1):286–302. doi: 10.1111/j.1742-4658.2008.06781.x. [DOI] [PubMed] [Google Scholar]
- Saadeldin IM, Swelum AAA, Elsafadi M, Mahmood A, Osama A, Shikshaky H, Alfayez M, Alowaimer AN, Magdeldin S. Thermotolerance and plasticity of camel somatic cells exposed to acute and chronic heat stress. J Adv Res. 2020;22(March):105–118. doi: 10.1016/j.jare.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu Rev Nutr. 2005;25(1):317–340. doi: 10.1146/annurev.nutr.25.051804.101917. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of {Akt/PKB} by the rictor-{mTOR} complex. Science.Sciencemag.Org. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol. 2013;14:197–210. doi: 10.1038/nrm3546. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12(20):3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, MacLennan GT, Hartman DJ, Pingfu F, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121(7):1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- Smart F, Aschrafi A, Atkins A, Owens GC, Pilotte J, Cunningham BA, Vanderklish PW. Two isoforms of the cold-inducible mRNA-binding protein RBM3 localize to dendrites and promote translation. J Neurochem. 2007;101(5):1367–1379. doi: 10.1111/j.1471-4159.2007.04521.x. [DOI] [PubMed] [Google Scholar]
- Sonna LA, Fujita J, Gaffin SL, Lilly CM. Mammalian gene expression invited review: effects of heat and cold stress on downloaded from. J Appl Physiol. 2013;92:1725–1742. doi: 10.1152/japplphysiol.01143.2001. [DOI] [PubMed] [Google Scholar]
- Takahara T, Maeda T. Transient sequestration of TORC1 into stress granules during heat stress. Mol Cell. 2012;47(2):242–252. doi: 10.1016/j.molcel.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284(12):8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timalsina S, Arimoto-Matsuzaki K, Kitamura M, Xu X, Wenzhe Q, Ishigami-Yuasa M, Kagechika H, and Hata Y. 2018. “Chemical compounds that suppress hypoxia-induced stress granule formation enhance cancer drug sensitivity of human cervical cancer HeLa cells.” 10.1093/jb/mvy062 [DOI] [PubMed]
- Trinklein ND, Chen WC, Kingston RE, Myers RM. Transcriptional regulation and binding of heat shock factor 1 and heat shock factor 2 to 32 human heat shock genes during thermal stress and differentiation. Cell Stress Chaperones. 2004;9(1):21–28. doi: 10.1379/1466-1268(2004)009<0021:TRABOH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uniacke J, Kishan Perera J, Lachance G, Francisco CB, Lee S. Cancer cells exploit EIF4E2-directed synthesis of hypoxia response proteins to drive tumor progression. Cancer Res. 2014;74(5):1379–1389. doi: 10.1158/0008-5472.CAN-13-2278. [DOI] [PubMed] [Google Scholar]
- Wang X. The mTOR pathway in the control of protein synthesis. Physiology. 2006;21(5):362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by P90RSK1 and P70 S6 kinase. EMBO J. 2001;20(16):4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yu L, Schmidt RE, Chen S, Huang X, Gould K, Cao G. Characterization of HSCD5, a novel human Stearoyl-CoA desaturase unique to primates. Biochem Biophys Res Commun. 2005;332(3):735–742. doi: 10.1016/j.bbrc.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Weber C, Nover L, Fauth M. Plant stress granules and MRNA processing bodies are distinct from heat stress granules. Plant J. 2008;56(4):517–530. doi: 10.1111/j.1365-313X.2008.03623.x. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Suhan JP (1985) Morphological study of the mammalian stress response: characterization of changes in cytoplasmic organelles, cytoskeleton, and nucleoli, and appearance of. Jcb.Rupress.Org. http://jcb.rupress.org/content/101/4/1198.abstract. Accessed 13 Jul 2020 [DOI] [PMC free article] [PubMed]
- Wengrod JC, Gardner LB. Cellular adaptation to nutrient deprivation: crosstalk between the mTORC1 and EIF2α signaling pathways and implications for autophagy. Cell Cycle. 2015;14(16):2571–2577. doi: 10.1080/15384101.2015.1056947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Wang X, Proud CG. mTOR inhibitors in cancer therapy. F1000Research. 2016;5:2078. doi: 10.12688/f1000research.9207.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Frank CL, Korth MJ, Sopher BL, Novoa I, Ron D, Katze MG. Control of PERK EIF2 kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci. 2002;99(25):15920–15925. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Palm W, Peng M, King B, Lindsten T, Li MO, Koumenis C, Thompson CB. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 2015;29(22):2331–2336. doi: 10.1101/gad.269324.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RM, Ackerman D, Quinn ZL, Mancuso A, Gruber M, Liu L, Giannoukos DN. Dysregulated mTORC1 renders cells critically dependent on desaturated lipids for survival under tumor-like stress. Genes Dev. 2013;27(10):1115–1131. doi: 10.1101/gad.198630.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeshan HMA, Lee GH, Kim HR, Chae HJ (2016) Endoplasmic reticulum stress and associated ROS. Int J Mol Sci. 10.3390/ijms17030327 [DOI] [PMC free article] [PubMed]
- Zhu S, Henninger K, McGrath BC, Cavener DR. PERK regulates working memory and protein synthesis-dependent memory flexibility. PLoS One. 2016;11(9):e0162766. doi: 10.1371/journal.pone.0162766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Hrer CB, Wellmann S (2016b) Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. 10.1007/s00018-016-2253-7 [DOI] [PMC free article] [PubMed]