Abstract
RNA-binding proteins (RBPs) have important roles in transcription, pre-mRNA processing/transport, mRNA degradation, translation, and non-coding RNA processing, among others. RBPs that are expressed in response to cold stress, such as Cirp and Rbm3, could regulate RNA stability and translation in hibernating mammals that reduce their body temperatures from 37 °C to as low as 0–5 °C during torpor bouts. RBPs including Cirp, Rbm3, and stress-inducible HuR translocate from the nucleus to stabilize mRNAs in the cytoplasm, and thereby could regulate which mRNA transcripts are protected from degradation and are translated, versus stored, for future protein synthesis or degraded by nucleases during cell stress associated with metabolic rate depression. This is the first study to explore the transcriptional/translational regulation, and subcellular localization of cold-inducible RBPs in a model hibernator, the 13-lined ground squirrel (Ictidomys tridecemlineatus). Cirp protein levels were upregulated in liver, skeletal muscle, and brown adipose tissue throughout the torpor-arousal cycle whereas Rbm3 protein levels stayed constant or decreased, suggesting an important role for Cirp, but likely not Rbm3, in the hibernator stress response. Increased cytoplasmic localization of Cirp in liver and muscle and HuR in liver during torpor, but no changes in the relative levels of Rbm3 in the cytoplasm, emphasizes a role for Cirp and possibly HuR in regulating mRNA processing during torpor. This study informs our understanding of the natural adaptations that extreme animals use in the face of stress, and highlight natural stress response mediators that could be used to bolster cryoprotection of human organs donated for transplant.
Electronic supplementary material
The online version of this article (10.1007/s12192-020-01110-3) contains supplementary material, which is available to authorized users.
Keywords: RNA-binding motif 3, Cold-inducible RNA-binding protein, Human antigen R, Cold shock protein, Torpor-arousal cycle, Ictidomys tridecemlineatus
Introduction
Mammalian hibernation is an adaptation to uncompromising environments that is typically used for winter survival by species whose normally high metabolic rates cannot be sustained in the face of cold ambient temperatures and little or no food availability. This seasonal phenomenon is characterized by regulated long periods of deep torpor (days or weeks) in which body temperature (Tb) falls to near ambient (often to just 0–5 °C) that are interspersed with short arousal periods when animals rewarm to euthermia (often just a few hours). Hibernation is preceded by a period of hyperphagia (during late summer to autumn) to build the fat reserves that are the main fuel for the non-feeding winter months. During hibernation, metabolic rate may decrease by 95% or more (Storey and Storey 2000), core body temperature drops to near ambient temperature (but is regulated at about 0–5 °C to prevent freezing), and all basic physiological processes are reduced; e.g., oxygen consumption can drop to 1/100th and heart rate to 1/30th of euthermic levels (Wang and Lee 1996). Obligate hibernators arouse periodically, powered by thermogenesis in brown adipose tissue and muscle shivering, but often spend most of that time asleep while their bodies undergo metabolic recovery before descending again into the next torpor bout. During hibernation, animals switch from glucose metabolism to fatty acid oxidation and greatly suppress energy-expensive processes like transcript expression and protein translation (Frerichs et al. 1998; Knight et al. 2000; van Breukelen and Martin 2001; Morin and Storey 2006). However, to ensure tissue viability during torpor, select stress-responsive pathways that modulate cell survival and metabolic plasticity are upregulated. For instance, elevated antioxidants help manage oxidative stress (Rouble et al. 2014; Tessier et al. 2015; Wu et al. 2015), heat shock proteins and ATF transcription factors regulate proper protein folding (Mamady and Storey 2008; Grabek et al. 2011; Wu et al. 2015), and anti-apoptotic proteins prevent cell death (Rouble et al. 2013). Protein expression profiles change during torpor (with respect to spring-aroused, summer-active, post-reproductive, or pre-hibernation cold-room euthermic animals) and these changes in protein levels likely facilitate changes in energy metabolism as well as responses to hibernation-induced cell stress (e.g., low ATP abundance, changes in ion homeostasis, low-temperature stress, decreased O2 delivery by cold and viscous blood, changes in reactive oxygen species abundance) (Shao et al. 2010; Grabek et al. 2011).
Attempts to understand how hibernators regulate gene expression include studies that measure the relative abundance of (1) transcription and translation factors; (2) post-translational modifications of enzymes, histone proteins, and DNA; and (3) microRNAs and other non-coding RNAs capable of influencing mRNA transcript fate. In regard to how hibernators may regulate which pathways are “turned on” and which are “turned off,” the first two categories of study have helped identify, for example, which transcription factors may be responsible for various observed gene upregulation during torpor and how their activity is regulated, whereas the third category has helped elucidate which transcripts may be degraded or stored in stress granules until a later time. A majority of transcripts are stabilized during torpor as evidenced by studies that show that the poly(A) tails of mRNA transcripts are no shorter during torpor compared with non-hibernating ground squirrels, indicating that endonucleases are not more active (Knight et al. 2000). Indeed, the polyA tails of mRNAs may even be lengthened during torpor, suggesting an increase in the activity of polyA polymerases, and there is also no difference in mRNA abundance between euthermic and torpid ground squirrels (Frerichs et al. 1998; Knight et al. 2000; Grabek et al. 2015). Furthermore, several studies have shown increases in the levels of mRNA-sequestering proteins during torpor, indicating that hibernators utilize stress granules to store transcripts until they can be translated into protein during interbout arousals (Frerichs et al. 1998; Knight et al. 2000; Tessier et al. 2014). These studies imply a role for proteins involved in protecting mRNA stability and preventing their degradation, whether by physically inhibiting nucleases or sequestering the transcripts in another organelle.
For this reason, it is of interest to assess the regulation of stress-inducible RNA-binding proteins (RBPs) that control mRNA fate during torpor, and specifically those RBPs that are cold-inducible since temperature may regulate other aspects of mRNA processing (Lyons et al. 2013; Tessier and Storey 2014; Biggar and Storey 2017). We characterized three well-known cold-inducible proteins: the cold-inducible RNA-binding protein (Cirp or Cirbp), human antigen R (HuR), and RNA-binding motif protein 3 (Rbm3) over the torpor-arousal cycle in tissues of 13-lined ground squirrels. These RBPs are important because of their highly conserved mRNA and protein sequences across mammals, their stress-inducible expression, and their numerous roles in regulating transcript stability and translation. For instance, they are involved in (1) transporting select mRNA transcripts from the nucleus to the cytoplasm in order that they are translated immediately or stored in stress granules until they are required by the cell; (2) stabilizing the poly(A) tail and preventing its degradation by 3′ exonucleases, such that poly(A)-binding protein (PABP) is able to bind to the 3′-UTR and strengthen the interaction between eIF4E and the transcript; (3) regulating the enzymes that perform post-translational modifications to the translation machinery; and (4) mediating the formation of ribosomes (Yaman et al. 2002; Barreau et al. 2005; Lleonart 2010; Zhu et al. 2016). RBM3 was previously identified as being differentially expressed at the mRNA level in torpid and interbout aroused arctic ground squirrel liver, brain, heart, brown adipose, and skeletal muscle relative to a post-reproductive control group (Yan et al. 2008), in late torpid golden-mantled ground squirrel brain relative to a summer active control group (Williams et al. 2005), and in winter torpid black bear heart and liver relative to a summer active control group (Fedorov et al. 2011). Separate studies identified different CIRP splice variants in hamster heart (Sano et al. 2015). However, it was not until recently that the first study compared the expression of both RBM3 and CIRP transcript levels in muscle of active and hibernating bears (Chazarin et al. 2019). Additionally, no study to date has determined if Rbm3, Cirp, or HuR RNA-binding proteins are differentially translated or spatio-temporally regulated at the protein level in hibernating animals. Since we have no knowledge of how Cirp, HuR, or Rbm3 proteins are regulated in small mammalian hibernators capable of reducing their body temperatures far below non-hibernator capabilities, this study investigates the transcript levels, protein levels, and protein subcellular localization of cold-inducible RBPs in three tissues from hibernating 13-lined ground squirrels (Ictidomys tridecemlineatus). Brown adipose tissue (BAT) and skeletal muscle were selected as important tissues to investigate the regulation of cold shock proteins because of their roles in thermoregulation during hibernation. Non-shivering thermogenesis is initiated by BAT to maintain the hibernator’s thermogenic set point during deep torpor, and is strongly increased during the initial phases of arousal from a torpor bout to rapidly achieve rewarming (Ballinger and Andrews 2018). Skeletal muscle is less metabolically active than BAT and liver when a hibernator is in deep torpor, but shivering thermogenesis becomes important once body temperature has risen above a certain threshold (Hampton et al. 2010; Ballinger and Andrews 2018). Liver is a metabolically active tissue during torpor and has important roles in regulating whole-body energy metabolism (Hampton et al. 2010). Since liver is among the first organs to rewarm upon arousal without having direct roles in thermogenesis, it is crucial to include it in the investigation of cold shock RNA-binding proteins. Finally, tissues were sampled at 6 time points of the torpor-arousal cycle to attain novel information about the role of each RBP with respect to time and body temperature.
Materials and methods
Animals
Thirteen-lined ground squirrels (Ictidomys tridecemlineatus) weighing approximately 150–300 g were wild-captured by a United States Department of Agriculture-licensed trapper (TLS Research, Bloomingdale, IL) and transported to Dr. J.M. Hallenback’s laboratory at the Animal Hibernation Facility, National Institute of Neurological Disorders and Stroke (NIH, Bethesda, MD), where the hibernation experiments were performed. NINDS animal care and use committee (ACUC) animal housing and experimental procedures were followed. Each 13-lined ground squirrel was briefly anesthetized with 5% isofluorane and fitted with a sensor chip (IPTT-300; Bio Medic Data Systems) injected under the skin. Squirrels were housed individually in shoebox cages at 21 °C and fed a standard rodent diet with water ad libitum until they gained sufficient lipid stores (assessed by weight gain) to enter hibernation. To enable a natural transition into torpor, animals were transferred to an environmental chamber at ~ 5 °C in constant darkness. Once all animals had been through a series of torpor bouts, animals were sampled at several points of the torpor-arousal cycle as previously described and euthanized with 5% isofluorane anesthesia followed by decapitation (McMullen and Hallenbeck 2010). Squirrels from 6 different points in the torpor-arousal cycle were sampled: (a) euthermic (Tb ~ 37 °C) in the cold room (5 °C) (EC) animals have stable Tb and could enter torpor but did not for at least 3 days; (b) entrance (EN) animals have a decreasing Tb between 18 and 31 °C; (c) early torpor (ET) animals have a stable Tb of 5–8 °C for 1 day; (d) late torpor (LT) animals have Tb values between 5 and 8 °C for at least 5 days; (e) early arousal (EA) animals have an increased respiratory rate of more than 60 breaths/min and a rising Tb of 9–12 °C; and (f) interbout aroused (IA) animals have returned to Tb ~ 37 °C for approximately 18 h. Tissue samples were dissected, rinsed in ice-cold phosphate-buffered saline (PBS), and frozen immediately in 2-methylbutane chilled on dry ice (− 50 °C), and stored at − 80 °C. Tissue samples were then shipped to Carleton University on dry ice and stored at − 80 °C until use.
Total protein extraction
Frozen tissue samples (n = 4 independent biological replicates) of liver, skeletal muscle, and BAT tissue were prepared for the six time points (EC, EN, ET, LT, EA, and IA) collected over the torpor-arousal cycle. Frozen samples were quickly weighed and crushed into small pieces under liquid nitrogen. Each tissue sample was then homogenized 1:2 w:v in ice-cold homogenizing buffer adjusted to pH 7.5, containing 20 mM HEPES, 200 mM NaCl, 0.1 mM EDTA, 10 mM NaF, 1 mM Na3VO4, 10 mM β-glycerophosphate, a few crystals of phenylmethylsulfonyl fluoride (BioShop), and 1 μL of Sigma protease inhibitor (BioShop) using a Polytron P10 homogenizer. Following centrifugation of each sample at 10,000 rpm for 10 min at 4 °C, the supernatant containing soluble proteins was collected. Total protein concentration was determined using the Bio-Rad reagent (Bio-Rad; Cat no. 500-0005, Hercules, CA) with absorbance read at 595 nm on a MR500 microplate reader. The concentrations of all samples were adjusted to 10 μg/μL by adding appropriate amounts of homogenizing buffer and then 2× sodium dodecyl sulfate (SDS) loading buffer (100 mM Tris-base adjusted to pH 6.8, 4% w:v SDS, 20% v:v glycerol, 0.2% w:v bromophenol blue, 10% v:v 2-mercaptoethanol) was added to each sample in a 1:1 v:v ratio followed by mixing and boiling for 5 min. The final 5 μg/μL protein samples were stored at − 40 °C until use.
Cytoplasmic protein extraction
Cytoplasmic fractions were prepared using liver, muscle, and BAT of euthermic in the cold room (EC) and deeply hibernating (LT) 13-lined ground squirrels (n = 4 different individuals for each time point). Tissue samples weighing between 0.275 and 0.410 g were homogenized using 5–10 piston strokes of a Dounce homogenizer following the addition of 1 mL of homogenization buffer (100 mM HEPES pH 7.9, 100 mM KCl; 100 mM EDTA; 20 mM β-glycerophosphate), and 10 μL of both 100 mM DTT and protease inhibitor cocktail (BioShop). Samples were centrifuged at 4 °C for 10 min at 10,000 rpm. The supernatant containing the cytoplasmic fraction was separated from the pellet, and pellets were then re-suspended in 125 μL of extraction buffer (20 mM HEPES pH 7.9, 400 mM NaCl, 1 mM EDTA, 10% v/v glycerol, 20 mM β-glycerophosphate) with 1.5 μL of 100 mM DTT and 1.5 μL of protease inhibitor cocktail (BioShop). Protein concentration was determined for the cytoplasmic and nuclear fractions using the Bio-Rad reagent in the same manner as the total protein samples. Western blot analyses of the samples were conducted using a histone H3 antibody (Cell Signaling, Cat no. 9715) or a histone H3K4 monomethyl antibody (Abcam, Cat no. ab8895) as well as a beta-tubulin antibody (Cell Signaling, Cat no. 2146) (all diluted 1:1000 in TBST (20 mM Tris base, pH 7.6, 140 mM NaCl, 0.05% v:v Tween-20)) to evaluate extraction purity. Extracts were stored at − 80 °C until use or further processing with 2× SDS loading buffer for Western blotting (as per the method described under “Total protein extraction”).
Western blotting
Equal amounts of prepared protein homogenate and 4–5 μL of PiNK Plus pre-stained protein ladder (10.5–175 kDa, Froggabio, Cat no. PM005-0500) were loaded onto discontinuous SDS-PAGE gels with 5% stacking gels and 10–15% SDS-PAGE resolving gels. Electrophoresis was performed for 70–100 min at 180 V using the BioRad Mini Protean III system in Tris-glycine running buffer (0.25 M Tris-base, 2.45 M glycine, 0.035 M SDS). Depending on the molecular weight of the protein target, proteins were transferred onto 0.45 μm polyvinylidene difluoride (PVDF) paper, either at 160 mA for 70–90 min or at 30–50 V for 45–60 min in Tris-glycine transfer buffer (25 mM Tris pH 8.5, 192 mM glycine, and 10% v:v methanol). Blots were blocked with 2.0 or 5.0% (w:v) milk made up in TBST for 20–30 min, to prevent non-specific binding of antibodies. Membranes were then incubated overnight at 4 °C on a rocker with specific primary antibodies (diluted 1:1000 or 1:2000 v:v with TBST). Rabbit polyclonal primary antibodies were Cirp (Santa Cruz Biotechnology, Cat no. sc-133460), Rbm3 (Santa Cruz Biotechnology, Cat no. sc-367851), and the rabbit monoclonal antibody was HuR (Cell Signaling, Cat no. 12582). After the removal of primary antibody, the membranes were exposed to an HRP-linked anti-rabbit IgG secondary antibody (diluted with TBST to 1:8000–1:4000 v:v) for 30 min. The membranes were washed for 15–30 min in TBST before visualizing with enhanced chemiluminescence (ECL) reagents. The amount of protein in each lane was reassessed using Coomassie Blue staining (0.25% w:v Coomassie brilliant blue, 7.5% v:v acetic acid, 50% methanol) of the PVDF membranes.
Total RNA extraction
Briefly, approximately 50 mg (liver and muscle) or 100 mg (BAT) of frozen tissue was weighed and homogenized in 1 mL of Trizol (Invitrogen) using a Polytron PT1200 homogenizer. Four biological replicates were made for each tissue from individual animals, for each of the 5–6 experimental time points. Time points used for all tissues included EC, EN, ET, LT, and EA, whereas IA was also used for liver and BAT. Each sample was vortexed briefly following the addition of 200 μL of chloroform, before being centrifuged for 10 min at 10,000 rpm and 4 °C. The upper aqueous phase containing the RNA was separated from the organic layer. Then, 750 μL of isopropanol was added to the supernatant to precipitate the RNA. Each sample was centrifuged for 15 min at 12,000 rpm at 4 °C, washed with 1 mL of 70% ethanol, and then centrifuged again for 12,000 rpm for 5 min. The supernatant was removed and the pellets air-dried for 10–15 min. RNA purity was assessed for each biological replicate for all three tissues by determining the ratio of the absorbances detected at 260 and 280 nm. RNA integrity was assessed by visualizing 18S and 26S ribosomal bands on a 1% agarose gel with Sybr Green staining. To remove contaminating DNA from liver samples, the extracted liver RNA was treated with DNase I, RNase-free, HC (50 U/μL; Thermo Scientific, no. EN0523). The resulting RNA samples were visualized on a 1% agarose gel with Sybr Green staining to confirm the DNA contamination was eliminated before proceeding with cDNA synthesis.
cDNA synthesis and PCR amplification
RNA samples were normalized in a total volume of 10 μL DEPC-autoclaved ddH2O such that each biological replicate contained 1.65 μg RNA (liver), 2.5 μg RNA (muscle), or 4 μg (BAT). The normalized RNA samples were incubated with 1 μL Oligo-dT (200 ng/μL 5′-TTTTTTTTTTTTTTTTTTTTTTV-3′; where V = A, G, or C; Sigma Genosys) and placed in a thermocycler at 65 °C for 5 min before chilling on ice for 5 min. To each sample, 4 μL of 5× first-strand buffer (Invitrogen), 2 μL of 0.1 M DTT (Invitrogen), 1 μL of 10 mM dNTPs (BioShop), and 1 μL of MMLV Reverse transcriptase (Invitrogen) were added. Then, each sample was spun down and incubated at 42 °C for 45 min in the thermocycler to induce reverse transcription. Serial dilutions (10−1, 10−2, and 10−3) of the cDNA samples were prepared for relative quantification of select mRNAs and reference genes.
The 13-lined ground squirrel (Ictidomys tridecemlineatus) genomes from the NCBI webpage (http://www.ncbi.nlm.nih.gov/genome/?term=ictidomys) and Primer BLAST (http://www.ncbi. nlm.nih.gov/tools/primer-blast/) were used to create the PCR primers (Table 1). The primers were synthesized by Integrated DNA Technologies (Coralville, IA, USA). All PCR runs underwent melt-curve analysis and dilution curve testing to identify which primer set amplified more than one PCR product or amplified non-mRNA products, as indicated by more than one melt-curve or by no change in Ct values between serial dilutions, respectively. The PCR protocol was executed using a BioRad MyIQ2 Detection System (BioRad, Hercules, CA, USA) and began with 3 min of secondary structure denaturing at 94 °C, followed by 49 cycles of 94 °C for 10 s, 60 °C annealing temperature for 20 s, and elongation at 75 °C for 20 s. The protocol was concluded with one final step of 55 °C for 10 s and 75 °C for 20 s. The optimal cDNA dilution (10−1 or 10−2) and annealing temperature were determined for each gene prior to this procedure. The product from each working primer set was sent for sequencing (BioBasic, Markham, ON) to confirm that the correct message was amplified. A freeze-squeeze method was used to isolate cleaned PCR product for sequencing analysis (BioBasic, Markham, ON). The segment containing the PCR product was excised from the larger gel, frozen in liquid nitrogen for at least 5 min, then placed in 0.6-mL Eppendorf tubes with punctured bottoms and glass wool filters. The tubes containing gel and PCR product were placed in 1.5-mL Eppendorf tubes and spun at 12,000 rpm for 5 min. To the supernatant, 0.1 volume of 3 M sodium acetate and 1 volume of isopropanol were added before centrifuging again at 12,000 rpm for 15 min. The pellet was washed with 0.5 mL of 70% ethanol and allowed to air-dry for 10–15 min before resuspension in 30 μL water.
Table 1.
Forward and reverse primer sequences for RNA-binding proteins and target transcripts, purchased from Integrated DNA Technologies (IDT), Inc., and used for RT-PCR analysis, where gamma actin is the reference gene for both liver and muscle, and TATA-binding protein is the reference gene for brown adipose tissue
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| CIRP | TGCAGTCCAGTCCAGCATAG | CATCCACTCCCATCCAGACC |
| RBM3 | GGCAGCCTCCCTATTCTTCC | CCACGAACAGCTTCCCTTCT |
| HUR | AGAACAAAAACGTGGCGCTC | GGGAGAACCTGAACCTCTGC |
| ACTG2 | CTGTCTTCCCCTCCATCGTG | CCTCATCCCCAACGTAGCTG |
| TBP | AGAGTGTGCTGGGAATGCTC | CAGGCTGCTGTTCTGATCCA |
Quantification and statistical analysis
Western blot bands were imaged using the Chemi-Genius Bioimaging system (Syngene, Frederick, MD) and were quantified using GeneTools software, taking the background into account. For time-course Western blot quantification, not all protein extracts could be run on the same gel so one set of samples (e.g., all biological replicates for EN) was run on each gel and the band densities of the other samples were standardized to the same sample (e.g., EN1 on all three gels). Then, the average band density for each time point was normalized against the average EC band density, such that the relative band density of EC would be 1 for graphing purposes. Each PVDF membrane was subsequently stained using Coomassie Blue staining (0.25% w:v Coomassie brilliant blue, 7.5% v:v acetic acid, 50% methanol) to visualize the total amount of protein in each lane. Immunoblot band density in each lane was standardized against the summed intensity of a group of Coomassie-stained protein bands in the same lane; these bands were consistent in intensity across the entire gel and were well separated from the Western blot band of interest.
Raw Ct values obtained from each PCR run were converted to a linear form using the ΔΔCT method. If a one-way ANOVA with a Tukey post hoc test showed no change across the torpor-arousal cycle, signifying the gene’s expression was constant between the control and other sampling points, the gene was considered a candidate reference gene. Gamma actin (ACTG2) was used as the reference gene for both skeletal muscle and liver samples and TATA-binding protein (TBP) was used as the reference gene for BAT.
All data are expressed as means ± SEM; n = 4 independent samples from different animals for Western blot analysis. Differences between control and multiple torpor-arousal time points were analyzed using SigmaPlot software and considered statistically significant when the one-way ANOVA with a Tukey post hoc test yielded a result of p < 0.05. For subcellular distribution analysis, Student’s t test was used to compare relative cytoplasmic protein levels in EC and LT. Differences between control and hibernation conditions were considered statistically significant when Student’s t test yielded a result of p < 0.05.
Results
Analysis of RNA-binding protein levels over the course of the torpor-arousal cycle
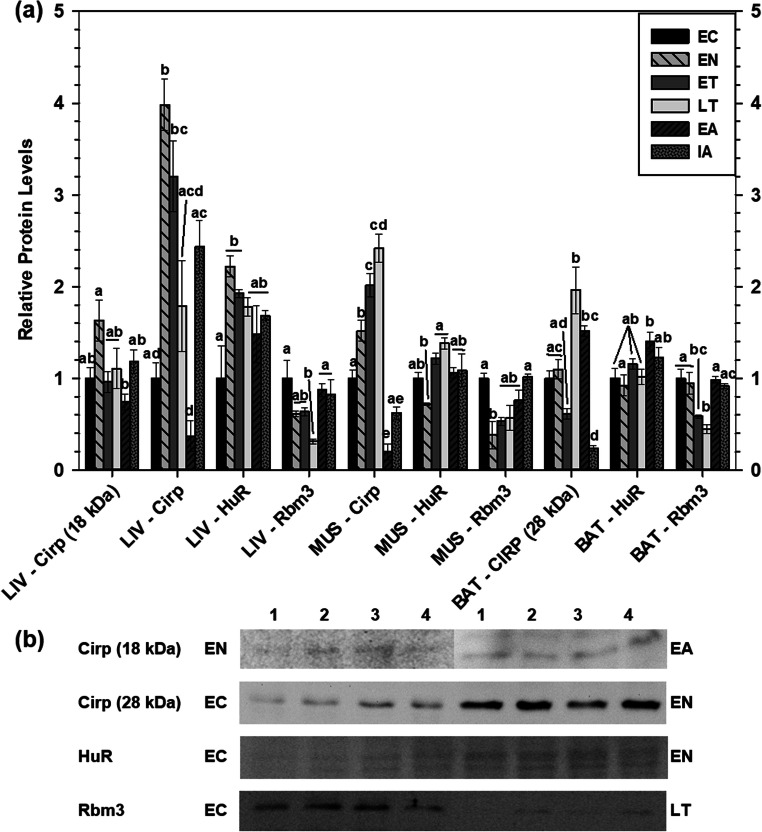
Relative total protein levels of Cirp, HuR, and Rbm3 were analyzed in liver, skeletal muscle, and BAT of 13-lined ground squirrels, comparing EC with EN, ET, LT, EA, and IA time points of the torpor-arousal cycle (Fig. 1a). Strong bands of Cirp protein were found at 28 kDa in each tissue, consistent with the reported molecular weight of the protein given by the antibody supplier (Santa Cruz Biotechnology, Inc.). In liver, but not skeletal muscle or BAT, a second less pronounced band cross-reacted with Cirp antibody at 18 kDa. Since this molecular weight matched the calculated molecular weight of Cirp (using the computationally predicted Ictidomys tridecemlineatus sequence available on the NCBI website, accession number XP_005332023), both bands were quantified to better estimate the relative abundance of Cirp over the torpor-arousal cycle.
Fig. 1.
Relative cold-inducible RNA-binding protein levels in liver (LIV), muscle (MUS), and brown adipose tissue (BAT) of 13-lined ground squirrels. a Histogram showing mean standardized expression levels of Cirp (18 kDa and 28 kDa), HuR, and Rbm3 (± S.E.M., n = 4 independent protein isolations from different animals). b Representative Western blots for certain torpor-arousal time points in liver. Data were analyzed using ANOVA with a Tukey post hoc test. Shared letters indicate data that are not significantly different from each other and different letters indicate statistically significant differences between sample points (p < 0.05). Flat lines across multiple histogram bars denote multiple time points that share the same letter(s)
In liver, Cirp (18 kDa) protein levels were constant across the torpor-arousal cycle with respect to the euthermic in the cold room (EC) condition but a significant decrease (over 50%) was seen in a comparison of the entrance phase (EN) with early arousal (EA) (Fig. 1a). Compared with EC, Cirp (28 kDa) levels increased nearly 4-fold during EN and remained high during early torpor (ET) at 3.2-fold greater than the EC value. Cirp (28 kDa) levels declined in late torpor (LT) to euthermic levels before strongly decreasing to just 40% of EC value during EA (significant with respect to EN, ET, and IA). Cirp (28 kDa) levels returned to EC levels during interbout arousal (IA). Relative Rbm3 levels were constant over most of the torpor-arousal cycle but decreased significantly during LT to just 31% of the EC value. Two protein bands that were very close in molecular weight cross-reacted with the primary antibody targeting HuR and were quantified together to estimate relative liver HuR levels over the torpor-arousal cycle. HuR total protein levels increased significantly during EN and ET to 2.2- and 1.9-fold higher than the EC value, respectively, before returning to EC levels during the subsequent phases of the torpor-arousal cycle (LT, EA, and IA). UniProt database sequence information was used to confirm the presence of HuR isoforms at 36 kDa and 38 kDa in humans (UniProt 2019). Clustal Omega (version 1.2.4) was used to align the computationally predicted 13-lined ground squirrel HuR sequence with the human HuR protein sequences from UniProt (Supplemental Fig. 1a). The ELAVL1 gene encodes the HuR protein. Finally, investigation of the 5′-UTR of Ictidomys tridecemlineatus ELAVL1 gene (NW_004936588.1: 4.9 M..5.0 M (32,842 nt)) using the annotated genome (accession: GCF_000236235.1) was done to investigate the possibility of alternative start sites upstream of the ELAVL1 transcription start site reported for the 36-kDa computationally predicted protein. An open reading frame was identified between bases 4,958,998 and 4,959,094, corresponding to the following amino acid sequence “N-MNLVISFAFIYCSVNIWNLLDLIFLYRFLRNT-C,” which differed from the human 38-kDa HuR sequence (Supplemental Fig. 1b). However, the 38-kDa human and ground squirrel proteins were still 94% similar in amino acid sequence.
Relative protein levels of Cirp (28 kDa), HuR, and Rbm3 were also quantified in skeletal muscle from 13-lined ground squirrel over the torpor-arousal cycle (Fig. 1a). Compared with EC, muscle Cirp (28 kDa) protein levels increased 1.5-fold during EN, before increasing again during ET and LT to values that were 2.0- and 2.4-fold higher than EC, respectively. Subsequently, during early arousal, Cirp (28 kDa) levels decreased significantly to just 20% of the euthermic controls but rose again during IA to about 60% of the EC level. Similar to liver, two bands representing HuR of similar molecular weight were quantified together to estimate relative HuR levels over the torpor-arousal cycle. HuR levels were largely unchanged but EN levels were significantly lower than ET and LT total protein levels. Rbm3 levels also remained relatively constant throughout the torpor-arousal cycle except for a significant decrease during EN to less than 40% of the EC level.
Similar trends were seen for BAT (Fig. 1a), where Cirp (28 kDa) total protein levels increased significantly during LT and EA to 2-fold and 1.5-fold higher than the EC level, respectively, before dropping to 23% of the EC value in IA. HuR protein levels were generally constant across the sampling points except for a significant difference between EN and EA (where total HuR levels increased 1.4-fold between these time points). Similar to liver and skeletal muscle, brown adipose Rbm3 protein levels decreased during ET and LT to 58% and 44% of EC, respectively. Rbm3 protein levels recovered during the arousal period to control levels, as in liver and muscle.
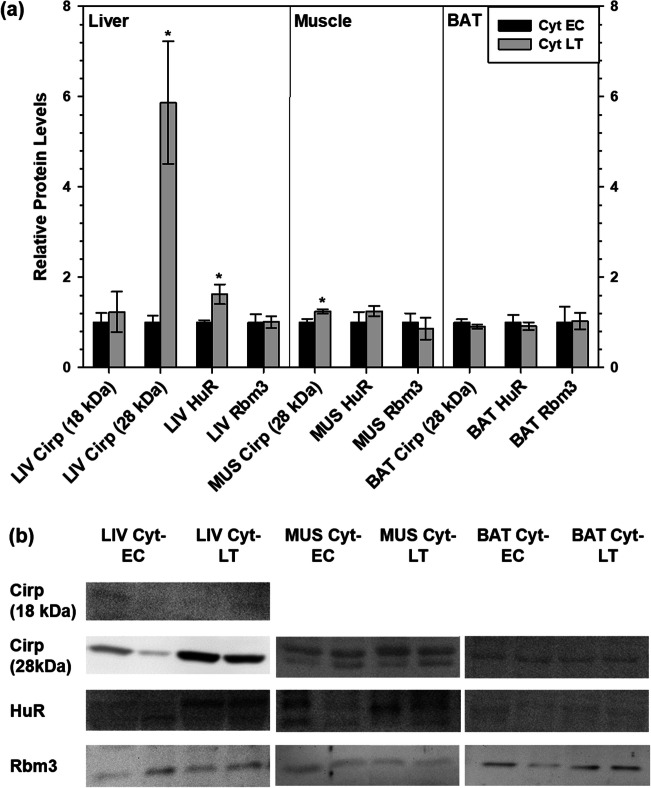
Cytoplasmic enrichment of RNA-binding proteins over the torpor-arousal cycle
Cirp, Rbm3, and HuR are known to translocate to the cytoplasm from the nucleus upon activation so immunoblotting was used to determine if these proteins had heightened presence in the cytoplasm during torpor relative to euthermia (Fig. 2). Interestingly, the relative levels of cytoplasmic Cirp (18 kDa) did not change during torpor but Cirp (28 kDa) protein content increased during LT by 5.9-fold in liver. Cirp (18 kDa) was not detected in muscle or BAT. Cirp (28 kDa) levels also increased in skeletal muscle cytoplasmic fractions during LT, by 1.24-fold the EC level, but did not change in BAT. Again, two bands representing HuR were quantified for all three tissues, and the results showed an increase in liver cytoplasmic HuR during LT compared with EC, but there were no changes in the cytoplasmic levels of HuR in either skeletal muscle or BAT. Cytoplasmic Rbm3 levels did not change in liver, skeletal muscle, or brown adipose tissue. The available nuclear levels of the RNA-binding proteins in EC and LT ground squirrels are presented in Supplemental Fig. 2.
Fig. 2.
Relative cold-inducible RNA-binding protein levels in cytoplasmic (cyt) fractions of liver (LIV), muscle (MUS), and brown adipose tissue (BAT), comparing euthermic (EC) and torpid (LT) 13-lined ground squirrels. a Histogram shows mean standardized expression levels of Cirp (18 kDa and 28 kDa), HuR, and Rbm3 (± S.E.M., n = 4 independent protein isolations from different animals). Cirp (18 kDa) was only detected in liver samples. b Representative Western blots show two different samples (n = 2) of the total n = 4 for each time point. Data were analyzed using Student’s t test. An asterisk above the LT time point indicates a significant difference from the EC value (p < 0.05)
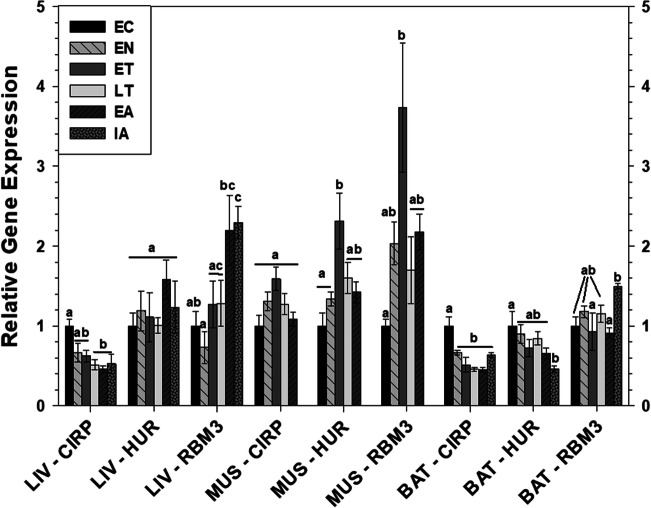
Gene expression of RNA-binding proteins and select targeted genes over the torpor-arousal cycle
RT-PCR was used to assess whether CIRP, RBM3, and HUR (ELAVL1) transcript expression was differentially regulated over the torpor-arousal cycle. The relative expressions of the gene transcripts of these three proteins were compared for EC, EN, ET, LT, EA, and IA time points of the torpor-arousal cycle. In liver, relative transcript levels of CIRP decreased during LT, EA, and IA to 51%, 46%, and 53% of EC, respectively (Fig. 3). By contrast, RBM3 transcript levels increased during EA and IA to between 2.2- and 2.3-fold the EC level. Transcript levels of HUR did not change in hibernating ground squirrels relative to the EC control. In muscle, there were no changes in the relative transcript levels of CIRP—but HUR and RBM3 transcript levels both increased during ET to 2.3-fold and 3.7-fold higher than EC, respectively, before returning to EC levels during LT and EA (Fig. 3). BAT CIRP levels decreased during EN and subsequently remained low at levels between 45 and 67% of the EC value, the lowest point being during EA (Fig. 3). By contrast, HUR and RBM3 transcript levels were relatively constant in BAT except for a decrease in HUR levels during IA to about 45% of EC, and an approximate 1.6-fold increase in RBM3 levels during IA relative to ET and EA.
Fig. 3.
Expression of mRNAs in liver (LIV), muscle (MUS), and brown adipose tissue (BAT) tissue samples evaluated by RT-qPCR for 6 time points over the torpor-arousal cycle. Relative expression of the indicated mRNAs was standardized to the expression of gamma actin mRNA (in liver and muscle) or TATA-binding protein (TBP) mRNA (in BAT) amplified from the same sample. Relative mRNA expression levels were then expressed relative to that of the EC value, which was set as 1.0. Data are mean ± SEM (n = 3–4 independent trials from different animals). Data were analyzed using a one-way ANOVA with a Tukey post hoc test. Shared letters indicate data that are not significantly different from each other and different letters indicate statistically significant differences between sample points (at least p < 0.05). Flat lines across multiple histogram bars denote multiple time points that share the same letter(s)
Discussion
Hibernating bears increase the expression of CIRP mRNA transcripts compared with summer-active animals (Chazarin et al. 2019), so an increase in CIRP mRNA levels was expected when hibernating ground squirrels entered torpor and reduced their core body temperature. Using primers specific for exon 7 of the CIRP gene from I. tridecemlineatus, the current study showed that relative total CIRP mRNA levels either decreased or were maintained in 13-lined ground squirrel skeletal muscle, liver, and brown adipose tissues across the torpor-arousal cycle, compared with the euthermic control (Fig. 3). Fascinating changes in the regulation of splice variants of CIRP mRNA have been identified in multiple tissues from hibernating Siberian hamsters, as a result of decreasing core body temperature, whether by natural torpor or by artificially induced hypothermia (Sano et al. 2015; Horii et al. 2019b). Briefly, when compared with euthermic hamsters housed at 22 °C, naturally hibernating and artificially induced hibernating hamsters expressed short and long CIRP mRNA transcripts at a ratio of 2:1 as opposed to 1:1 when hamsters were forced into hypothermic states and prevented from adapting to the change in environment (i.e., by increasing their body temperature through shivering thermogenesis). The long transcript that is produced less often in hypothermic animals contained an additional intron between exon 6 and exon 7 that contains a premature stop codon, so the resulting protein is C-terminally truncated and non-functional due to the removal of its arginine-glycine–rich region (Aoki et al. 2002; De Leeuw et al. 2007; Sano et al. 2015). CIRP mRNA is also alternatively spliced in non-hibernating organisms like the mouse (Horii et al. 2019a), such that the short transcript resulting in the full-length protein is more highly expressed when body temperature decreases. Therefore, the reason why there was either a decrease or no change in relative Cirp transcript levels as body temperature declined in torpid ground squirrels could be because there was a shift from long (inactive) CIRP mRNA expression to short (functional) CIRP mRNA expression and no change or a decrease in total CIRP mRNA. This would mirror the results found in hibernating hamster, where non-hibernating hamster hearts expressed all three isoforms of CIRP but hibernating hamster hearts showed a more dense banding pattern for short isoforms compared with long isoforms (Sano et al. 2015). Another explanation for these unexpected results could be that any CIRP expressed at the transcript level is actively being converted into protein when the organism decreases its body temperature from entry into torpor until early arousal. Indeed, a unique component of this study compared with other studies to date is that the analysis of Cirp protein levels provides meaning to changes detected at the mRNA level.
Low levels of 18-kDa Cirp were detected in liver tissue from ground squirrels at different points of the torpor-arousal cycle, but the relative protein levels did not change significantly across the time course (Fig. 1). A strong Cirp band at 28 kDa was observed in all three ground squirrel tissues where protein levels increased (a) during entry and early torpor (liver); or (b) during entry into torpor, early torpor, and late torpor (skeletal muscle); or (c) during late torpor and early arousal (BAT). These results were consistent with the expected molecular weights identified by the company who supplied the antibody against Cirp, and with the molecular weights of the computationally predicted Cirp splice variants (UniProtKB accession ID Q14011 (CIRBP_HUMAN)) (UniProt 2019). These data suggest that the reason for unchanged or reduced CIRP mRNA levels could indeed be active translation of CIRP mRNA pools into protein when body temperature decreased past a certain threshold. For this reason, it is possible that Cirp levels are relatively unchanged in BAT until an extended time in deep torpor (Fig. 1a) because BAT in hibernators has an important role in maintaining the thermogenic set point of the animal, and elevating core body temperature should Tb start to fall past this threshold (Geiser 2004).
Cirp post-translational modifications such as C-terminal arginine methylation are necessary for its activation (Aoki et al. 2002; De Leeuw et al. 2007). Specifically, cell line experiments identified Cirp arginine residues 94, 105, and 116 as being necessary for translocation from the nucleus, where Cirp typically resides under basal conditions, to the cytoplasm (De Leeuw et al. 2007). However, without validated methyl-Cirp antibodies, or antibodies against any post-translationally modified Cirp protein for that matter, alternative approaches were used to characterize Cirp activation status. Subcellular fractionation followed by Western blotting was used to quantify the cytoplasmic protein levels of Cirp as a marker of its activation, comparing EC and LT animals, since C-terminal methylation triggers the release of Cirp from the nucleus. If Cirp was highly expressed but not functional during torpor, we would expect no translocation from the nucleus to the cytoplasm, and therefore less cytoplasmic protein in tissues from LT animals compared with EC animals. Notably, increased nuclear levels during torpor could be possible if total protein levels also increased, but such a finding would not negate the interpretation of increased cytoplasmic protein levels as a translocation of the protein to the cytoplasm. As expected, the 28-kDa Cirp protein was more highly expressed in the cytoplasm in liver and muscle during torpor (Fig. 2). It is possible that BAT Cirp did not show increased cytoplasmic levels during torpor because there could be a delay in the expression of Cirp protein and its translocation to the cytoplasm. To elaborate, both tissues that showed increased cytoplasmic levels had heightened total Cirp protein expression at time points of the torpor-arousal cycle in advance of LT.
By contrast, RBM3 mRNA expression increased in hibernating bear skeletal muscle (Chazarin et al. 2019), as well as in the liver, heart, and brain tissue of both hibernating golden-mantled ground squirrels and hibernating 13-lined ground squirrels (Williams et al. 2005) relative to a summer control. These results suggested that the cold-inducible Rbm3 may also be more transcribed in LT ground squirrels relative to a euthermic in the cold room control (EC). As a result, it was surprising to find that RBM3 mRNA levels in hibernating 13-lined ground squirrels only increased during arousal (EA and IA) in liver and in early torpor of skeletal muscle (Fig. 3), but were otherwise fairly consistent at different points of the torpor-arousal cycle in all three tissues. This contradicts studies that suggest an important role for Rbm3 in hypothermia-induced tissue protection, including in species able to survive cold stress as a result of living at high altitudes (Williams et al. 2005; Zargar et al. 2015; Chazarin et al. 2019). In vitro studies using slices of murine hippocampal tissue and microglial cells of mouse origin show RBM3 mRNA and protein to be more highly expressed under conditions of moderate hypothermia (33.5 °C) relative to extreme hypothermia (17 °C) (Tong et al. 2013). Consistently, levels of Rbm3 were also elevated with moderate hypothermia compared with deep hypothermia in Pashmina goats, animals that can naturally tolerate lower ambient temperatures due to living at high altitudes (up to 6000 m above sea level) (Zargar et al. 2015). These results help highlight that in the current study, RBM3 mRNA transcripts were only significantly increased when body temperature was low but not for an extended period of time (ET), and as body temperature is increasing during arousal (9–35 °C). Importantly, these changes did not translate into increased Rbm3 protein levels, which was expected from the results of previous studies but never tested experimentally. Consistently, there were no changes in the relative cytoplasmic levels of Rbm3, suggesting there was no increase in the levels of active Rbm3, and also suggesting that Rbm3 may not have strong roles in stabilizing mRNA transcripts or translocating them to the cytoplasm during torpor. Furthermore, Rbm3 may have roles in enhancing global translation in hypothermic cells by interacting with ribosomal subunits or microRNAs that limit protein synthesis (Dresios et al. 2005; Smart et al. 2007). These functions conflict with what occurs during metabolic suppression: a significant downregulation of translation and changes in microRNA profiles to control a range of metabolic pathways (Frerichs et al. 1998; Wu and Storey 2012; Storey 2015; Logan et al. 2018).
Finally, HuR (ELAVL1) was investigated because it can be activated by cellular stresses that also activate Cirp and Rbm3 including low oxygen availability, UV irradiation, and heat shock, although it is not “cold-inducible.” HuR has similar functions as Rbm3 and Cirp in that it may transport mRNAs from the nucleus to the cytoplasm while also preventing endonuclease activity (Atasoy et al. 1998; Pang et al. 2013). Furthermore, HuR can associate with Cirp and Rbm3 during times of cell stress (Aoki et al. 2003; Guo et al. 2010), making it a relevant target to study in conjunction with the aforementioned cold-inducible proteins in the context of the descent into, and arousal from, natural torpor. Indeed, HUR mRNA levels were relatively constant with only skeletal muscle displaying a significant increase in expression during early torpor (Fig. 3). However, skeletal muscle HuR protein levels did not change significantly and neither did cytoplasmic levels, suggesting that the increase in HUR (ELAVL1) transcript expression could be due to cellular stress signals, but the transcripts were not being translated. By contrast, HUR mRNA levels in liver were maintained at the six time points of the torpor-arousal cycle but protein levels of HuR increased during EN and ET (Fig. 1a), and cytoplasmic localization increased during torpor (Fig. 2a), similarly to Cirp protein levels. These results suggest HuR may play an important role in facilitating mRNA association with other RBPs like Cirp during torpor and/or shuttling transcripts from the nucleus to the cytoplasm including to stress granules (Doller et al. 2010; Guo et al. 2010). Future studies could investigate if HuR and Cirp colocalize in the cytoplasm during torpor because they are interacting with one another, or if they both serve independent roles in regulating mRNA processing.
Notably, two HuR protein bands were quantified via Western blotting, which is consistent with the existence of an alternative splice variant of ELAVL1 that has been reported in humans (UniProt 2019). The molecular weights of the Western blot bands suggested that there are also 36-kDa and 38-kDa isoforms in ground squirrels. An alignment using Clustal Omega was performed to compare the amino acid sequences of the computationally predicted 13-lined ground squirrel HuR sequence (sourced from the NCBI database) and the sequences of the human alternative splice variants isoforms (sourced from UniProt) (Supplemental Fig. 1a) (UniProt 2019). This alignment revealed that an extra exon was incorporated as an additional string of amino acids in the N-terminal region of the 36-kDa human HuR protein to yield the 38-kDa human HuR protein, and that a similar exon incorporation may explain the second Western blot band residing between the 29- and 42-kDa protein markers of the molecular weight ladder following the use of the HuR antibody with ground squirrel tissue extracts. Bioinformatics analysis of the ELAVL1 gene (NW_004936588.1: 4.9 M..5.0 M (32,842 nt)) from the Ictidomys tridecemlineatus genome revealed a possible transcript variant with a sequence similar to the human alternative splice variant but unique to ground squirrel. Future human and ground squirrel experiments are warranted to fully characterize the function of the more massive HuR variant, since together with its lower molecular weight counterpart, HuR appears to be stress-inducible in selected tissues from hibernating 13-lined ground squirrels.
Overall, the results of this study suggest that Cirp, and possibly HuR, may be important in regulating mRNA processing at different points of the torpor-arousal cycle in three different hibernator tissues: liver, skeletal muscle, and BAT. Future experiments characterizing the identities and quantities of the mRNA binding partners of Cirp and its cofactor HuR would help elaborate on the experiments performed herein that highlighted the differences in the regulation of two highly conserved and homologous cold shock proteins, Cirp and Rbm3. Evidence at the protein level implies that Cirp is actively translated into protein and may be post-translationally modified, explaining its molecular weight (28 kDa) in all three hibernator tissues, as well as the increased Cirp protein levels in the cytoplasm during torpor in liver and skeletal muscle compared with euthermic ground squirrels. Furthermore, RBM3 transcript levels increased at different time points over torpor-arousal as was expected based on previous microarray, RT-qPCR, and transcriptome studies, but there was no correlation with Rbm3 protein levels or subcellular localization patterns that either decreased or remained constant. Therefore, we can conclude that hibernating 13-lined ground squirrels actively regulate cold-inducible proteins including Cirp, HuR, and Rbm3, but not in the way that was expected based on previous transcript studies. This study has important implications for those that wish to learn from the natural stress response of extreme organisms in order to promote cell viability in human transplantable organs, reduce muscle wasting in comatose patients, or reprogram the stress response to target disease.
Electronic supplementary material
HuR protein sequence of the experimentally validated human (Homo sapiens) HuR proteins (36 kDa and 38 kDa, sourced from UniProt database) and the computationally predicted 13-lined ground squirrel (Ictidomys tridecemlineatus) sequence from NCBI database. A) Sequence alignment of 36 kDa and 38 kDa human HuR proteins with 36 kDa I. tridecemlineatus HuR protein indicates the incorporation of an additional exon in the N-terminal end of the human HuR protein. B) Alignment of the human HuR protein (38 kDa) with the genome-predicted 38 kDa 13-lined ground squirrel HuR protein showing 94.05% sequence similarity between human and ground squirrel HuR proteins. An asterisk (*) indicates perfect alignment, a colon (:) indicates strongly similar amino acids and a period (.) indicates conservation between weakly similar groups. Lettering with the color red indicates small and hydrophobic amino acids, blue indicates acidic amino acids, magenta indicates basic amino acids, green indicates hydroxyl, sulfhydryl, amine and glycine. The region in yellow highlight signifies the N-terminal domain present in the 38 kDa HuR protein that is not present in the 36 kDa isoform. (PNG 1332 kb)
Relative cold-inducible RNA-binding protein levels in cytoplasmic (cyt) and nuclear (nuc) fractions of liver (LIV), muscle (MUS) and brown adipose tissue (BAT), comparing euthermic (EC) and torpid (LT) 13-lined ground squirrels. (a) Histogram shows mean standardized expression levels of Cirp (18 kDa and 28 kDa), HuR, and Rbm3 (± S.E.M., n = 4 independent protein isolations from different animals). (b) Representative nuclear Western blots show two different samples (n = 2) of the total n = 4 for each time point. Data were analyzed using a Student’s t test, where cytoplasmic and nuclear levels were analyzed separately. An asterisk above the LT time point indicates a significant difference from the EC value (p < 0.05) (PNG 3406 kb)
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aoki K, Ishii Y, Matsumoto K, Tsujimoto M. Methylation of Xenopus CIRP2 regulates its arginine- and glycine-rich region-mediated nucleocytoplasmic distribution. Nucleic Acids Res. 2002;30:5182–5192. doi: 10.1093/nar/gkf638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Matsumoto K, Tsujimoto M. Xenopus cold-inducible RNA-binding protein 2 interacts with ElrA, the Xenopus homolog of HuR, and inhibits deadenylation of specific mRNAs. J Biol Chem. 2003;278:48491–48497. doi: 10.1074/jbc.M308328200. [DOI] [PubMed] [Google Scholar]
- Atasoy U, Watson J, Patel D, Keene JD. ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J Cell Sci. 1998;111:3145–3156. doi: 10.1242/jcs.111.21.3145. [DOI] [PubMed] [Google Scholar]
- Ballinger MA, Andrews MT. Nature’s fat-burning machine: brown adipose tissue in a hibernating mammal. J Exp Biol. 2018;221:jeb162586. doi: 10.1242/jeb.162586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau C, Paillard L, Osborne HB. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 2005;33:7138–7150. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggar KK, Storey KB. Exploration of low temperature microRNA function in an anoxia tolerant vertebrate ectotherm, the red eared slider turtle (Trachemys scripta elegans) J Therm Biol. 2017;68:139–146. doi: 10.1016/j.jtherbio.2016.09.008. [DOI] [PubMed] [Google Scholar]
- van Breukelen F, Martin SL. Translational initiation is uncoupled from elongation at 18 degrees C during mammalian hibernation. Am J Phys Regul Integr Comp Phys. 2001;281:R1374–R1379. doi: 10.1152/ajpregu.2001.281.5.R1374. [DOI] [PubMed] [Google Scholar]
- Chazarin B, Ziemianin A, Evans AL et al (2019) Limited oxidative stress favors resistance to skeletal muscle atrophy in hibernating brown bears (Ursus arctos). Antioxidants 8. 10.3390/antiox8090334 [DOI] [PMC free article] [PubMed]
- De Leeuw F, Zhang T, Wauquier C, et al. The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor. Exp Cell Res. 2007;313:4130–4144. doi: 10.1016/j.yexcr.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Doller A, Schlepckow K, Schwalbe H, Pfeilschifter J, Eberhardt W. Tandem phosphorylation of serines 221 and 318 by protein kinase Cdelta coordinates mRNA binding and nucleocytoplasmic shuttling of HuR. Mol Cell Biol. 2010;30:1397–1410. doi: 10.1128/MCB.01373-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, Mauro VP. Cold stress-induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci U S A. 2005;102:1865–1870. doi: 10.1073/pnas.0409764102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov VB, Goropashnaya AV, Tøien O, Stewart NC, Chang C, Wang H, Yan J, Showe LC, Showe MK, Barnes BM. Modulation of gene expression in heart and liver of hibernating black bears (Ursus americanus) BMC Genomics. 2011;12:171. doi: 10.1186/1471-2164-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerichs KU, Smith CB, Brenner M, DeGracia D, Krause GS, Marrone L, Dever TE, Hallenbeck JM. Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc Natl Acad Sci U S A. 1998;95:14511–14516. doi: 10.1073/pnas.95.24.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- Grabek KR, Karimpour-Fard A, Epperson LE, Hindle A, Hunter LE, Martin SL. Multistate proteomics analysis reveals novel strategies used by a hibernator to precondition the heart and conserve ATP for winter heterothermy. Physiol Genomics. 2011;43:1263–1275. doi: 10.1152/physiolgenomics.00125.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabek KR, Behn CD, Barsh GS, et al. Enhanced stability and polyadenylation of select mRNAs support rapid thermogenesis in the brown fat of a hibernator. Elife. 2015;2015:1–19. doi: 10.7554/eLife.04517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wu Y, Hartley RS. Cold-inducible RNA-binding protein contributes to human antigen R and cyclin E1 deregulation in breast cancer. Mol Carcinog. 2010;49:130–140. doi: 10.1002/mc.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton M, Nelson BT, Andrews MT. Circulation and metabolic rates in a natural hibernator: an integrative physiological model. Am J Phys Regul Integr Comp Phys. 2010;299:R1478–R1488. doi: 10.1152/ajpregu.00273.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Y, Shiina T, Uehara S, Nomura K, Shimaoka H, Horii K, Shimizu Y. Hypothermia induces changes in the alternative splicing pattern of cold-inducible RNA-binding protein transcripts in a non-hibernator, the mouse. Biomed Res. 2019;40:153–161. doi: 10.2220/biomedres.40.153. [DOI] [PubMed] [Google Scholar]
- Horii Y, Shimaoka H, Horii K, Shiina T, Shimizu Y. Mild hypothermia causes a shift in the alternative splicing of cold-inducible RNA-binding protein transcripts in Syrian hamsters. Am J Phys Regul Integr Comp Phys. 2019;317:R240–R247. doi: 10.1152/ajpregu.00012.2019. [DOI] [PubMed] [Google Scholar]
- Knight JE, Narus EN, Martin SL, Jacobson A, Barnes BM, Boyer BB. mRNA stability and polysome loss in hibernating Arctic ground squirrels (Spermophilus parryii) Mol Cell Biol. 2000;20:6374–6379. doi: 10.1128/MCB.20.17.6374-6379.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleonart ME. A new generation of proto-oncogenes: cold-inducible RNA binding proteins. Biochim Biophys Acta - Rev Cancer. 2010;1805:43–52. doi: 10.1016/j.bbcan.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Logan SM, Wu C, Storey KB (2019) The squirrel with the lagging eIF2: global suppression of protein synthesis during torpor. Comp Biochem Physiol - A Mol Integr Physiol 227:161–171. 10.1016/j.cbpa.2018.10.014 [DOI] [PubMed]
- Lyons PJ, Lang-Ouellette D, Morin PJ. CryomiRs: towards the identification of a cold-associated family of microRNAs. Comp Biochem Physiol Part D Genomics Proteomics. 2013;8:358–364. doi: 10.1016/j.cbd.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Mamady H, Storey KB. Coping with the stress: expression of ATF4, ATF6, and downstream targets in organs of hibernating ground squirrels. Arch Biochem Biophys. 2008;477:77–85. doi: 10.1016/j.abb.2008.05.006. [DOI] [PubMed] [Google Scholar]
- McMullen DC, Hallenbeck JM. Regulation of Akt during torpor in the hibernating ground squirrel, Ictidomys tridecemlineatus. J Comp Physiol B Biochem Syst Environ Physiol. 2010;180:927–934. doi: 10.1007/s00360-010-0468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin P, Storey KB. Evidence for a reduced transcriptional state during hibernation in ground squirrels. Cryobiology. 2006;53:310–318. doi: 10.1016/j.cryobiol.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Pang L, Tian H, Chang N, Yi J, Xue L, Jiang B, Gorospe M, Zhang X, Wang W. Loss of CARM1 is linked to reduced HuR function in replicative senescence. BMC Mol Biol. 2013;14:15. doi: 10.1186/1471-2199-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouble AN, Hefler J, Mamady H, Storey KB, Tessier SN. Anti-apoptotic signaling as a cytoprotective mechanism in mammalian hibernation. PeerJ. 2013;1:e29. doi: 10.7717/peerj.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouble AN, Tessier SN, Storey KB. Characterization of adipocyte stress response pathways during hibernation in thirteen-lined ground squirrels. Mol Cell Biochem. 2014;393:271–282. doi: 10.1007/s11010-014-2070-y. [DOI] [PubMed] [Google Scholar]
- Sano Y, Shiina T, Naitou K, Nakamori H, Shimizu Y. Hibernation-specific alternative splicing of the mRNA encoding cold-inducible RNA-binding protein in the hearts of hamsters. Biochem Biophys Res Commun. 2015;462:322–325. doi: 10.1016/j.bbrc.2015.04.135. [DOI] [PubMed] [Google Scholar]
- Shao C, Liu Y, Ruan H, Li Y, Wang H, Kohl F, Goropashnaya AV, Fedorov VB, Zeng R, Barnes BM, Yan J. Shotgun proteomics analysis of hibernating arctic ground squirrels. Mol Cell Proteomics. 2010;9:313–326. doi: 10.1074/mcp.M900260-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart F, Aschrafi A, Atkins A, Owens GC, Pilotte J, Cunningham BA, Vanderklish PW. Two isoforms of the cold-inducible mRNA-binding protein RBM3 localize to dendrites and promote translation. J Neurochem. 2007;101:1367–1379. doi: 10.1111/j.1471-4159.2007.04521.x. [DOI] [PubMed] [Google Scholar]
- Storey KB. Regulation of hypometabolism: insights into epigenetic controls. J Exp Biol. 2015;218:150–159. doi: 10.1242/jeb.106369. [DOI] [PubMed] [Google Scholar]
- Storey KB, Storey JM (2000) Gene Expression and Protein Adaptations in Mammalian Hibernation. In: Heldmaier G., Klingenspor M. (eds) Life in the Cold. Springer, Berlin, Heidelberg, pp 303–313
- Tessier SN, Storey KB. To be or not to be: the regulation of mRNA fate as a survival strategy during mammalian hibernation. Cell Stress Chaperones. 2014;19:763–776. doi: 10.1007/s12192-014-0512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier SN, Audas TE, Wu CW, Lee S, Storey KB. The involvement of mRNA processing factors TIA-1, TIAR, and PABP-1 during mammalian hibernation. Cell Stress Chaperones. 2014;19:1–13. doi: 10.1007/s12192-014-0505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier SN, Katzenback BA, Pifferi F, et al. Cytokine and antioxidant regulation in the intestine of the gray mouse lemur (Microcebus murinus) during torpor. Genomics, Proteomics Bioinforma. 2015;13:127–135. doi: 10.1016/j.gpb.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Endersfelder S, Rosenthal LM, Wollersheim S, Sauer IM, Bührer C, Berger F, Schmitt KR. Effects of moderate and deep hypothermia on RNA-binding proteins RBM3 and CIRP expressions in murine hippocampal brain slices. Brain Res. 2013;1504:74–84. doi: 10.1016/j.brainres.2013.01.041. [DOI] [PubMed] [Google Scholar]
- UniProt UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LCH, Lee TF. Handbook of physiology - environmental physiology. 1996. Torpor and hibernation in mammals: metabolic, physiological, and biochemical adaptations; pp. 507–532. [Google Scholar]
- Williams DR, Epperson LE, Li W, Hughes MA, Taylor R, Rogers J, Martin SL, Cossins AR, Gracey AY. Seasonally hibernating phenotype assessed through transcript screening. Physiol Genomics. 2005;24:13–22. doi: 10.1152/physiolgenomics.00301.2004. [DOI] [PubMed] [Google Scholar]
- Wu C-W, Storey KB. Regulation of the mTOR signaling network in hibernating thirteen-lined ground squirrels. J Exp Biol. 2012;215:1720–1727. doi: 10.1242/jeb.066225. [DOI] [PubMed] [Google Scholar]
- Wu C-W, Biggar KK, Zhang J, Tessier SN, Pifferi F, Perret M, Storey KB. Induction of antioxidant and heat shock protein responses during torpor in the gray mouse lemur, Microcebus murinus. Genomics Proteomics Bioinformatics. 2015;13:119–126. doi: 10.1016/j.gpb.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaman I, Fernandez J, Sarkar B, Schneider RJ, Snider MD, Nagy LE, Hatzoglou M. Nutritional control of mRNA stability is mediated by a conserved AU-rich element that binds the cytoplasmic shuttling protein HuR. J Biol Chem. 2002;277:41539–41546. doi: 10.1097/WAD.0b013e3181aba588.MRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Barnes BM, Kohl F, Marr TG. Modulation of gene expression in hibernating arctic ground squirrels. Physiol Genomics. 2008;32:170–181. doi: 10.1152/physiolgenomics.00075.2007. [DOI] [PubMed] [Google Scholar]
- Zargar R, Urwat U, Malik F, Shah RA, Bhat MH, Naykoo NA, Khan F, Khan HM, Ahmed SM, Vijh RK, Ganai NA. Molecular characterization of RNA binding motif protein 3 (RBM3) gene from Pashmina goat. Res Vet Sci. 2015;98:51–58. doi: 10.1016/j.rvsc.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bührer C, Wellmann S. Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. Cell Mol Life Sci. 2016;73:3839–3859. doi: 10.1007/s00018-016-2253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HuR protein sequence of the experimentally validated human (Homo sapiens) HuR proteins (36 kDa and 38 kDa, sourced from UniProt database) and the computationally predicted 13-lined ground squirrel (Ictidomys tridecemlineatus) sequence from NCBI database. A) Sequence alignment of 36 kDa and 38 kDa human HuR proteins with 36 kDa I. tridecemlineatus HuR protein indicates the incorporation of an additional exon in the N-terminal end of the human HuR protein. B) Alignment of the human HuR protein (38 kDa) with the genome-predicted 38 kDa 13-lined ground squirrel HuR protein showing 94.05% sequence similarity between human and ground squirrel HuR proteins. An asterisk (*) indicates perfect alignment, a colon (:) indicates strongly similar amino acids and a period (.) indicates conservation between weakly similar groups. Lettering with the color red indicates small and hydrophobic amino acids, blue indicates acidic amino acids, magenta indicates basic amino acids, green indicates hydroxyl, sulfhydryl, amine and glycine. The region in yellow highlight signifies the N-terminal domain present in the 38 kDa HuR protein that is not present in the 36 kDa isoform. (PNG 1332 kb)
Relative cold-inducible RNA-binding protein levels in cytoplasmic (cyt) and nuclear (nuc) fractions of liver (LIV), muscle (MUS) and brown adipose tissue (BAT), comparing euthermic (EC) and torpid (LT) 13-lined ground squirrels. (a) Histogram shows mean standardized expression levels of Cirp (18 kDa and 28 kDa), HuR, and Rbm3 (± S.E.M., n = 4 independent protein isolations from different animals). (b) Representative nuclear Western blots show two different samples (n = 2) of the total n = 4 for each time point. Data were analyzed using a Student’s t test, where cytoplasmic and nuclear levels were analyzed separately. An asterisk above the LT time point indicates a significant difference from the EC value (p < 0.05) (PNG 3406 kb)