Abstract
Structures of cellular organelles are intertwined with their functions that undergo alterations once the organelles are stressed. Since organelle functions are dependent on each other, an organelle-specific stress possibly influences the structure and function of its associated organelles. In this perspective, our study demonstrated that endoplasmic reticulum (ER)-specific stress induced by tunicamycin in primary astroglial culture is associated with altered mitochondrial dynamics and matched with the changes as observed in the aging rat brain. However, the exogenous addition of biotin, a highly lipogenic and mitochondrial vitamin, ameliorates ER stress even though its direct targets are not known within ER. Alternatively, the increased biotinylation of mitochondrial carboxylases preserves its basal respiratory capacity by upregulating mitofusin 2 (Mfn2) and, possibly, its associated role on mitochondrial fusion. Furthermore, the Mfn2 increase by biotin augments physical interaction between ER and functional mitochondria to exchange biomolecules as a part of ER stress resolution. This suggests an increased demand for micronutrient biotin under ER stress resolves the same by undergoing appropriate structural and metabolic contacts between ER and mitochondria. These findings provide a paradigm to resolve stress in one organelle by sustaining the metabolic commitments of another interdependent organelle. The findings also highlight a novel role of biotin in inducing Mfn2 expression and localization under ER stress in addition to its known role as a co-enzyme.
Keywords: ER stress, Biotin, Mitochondrial dynamics, Mitochondrial respiration
Introduction
Cells display specialized structural changes within their organelles to favor their basal function and stress adaptations. Alterations in the execution of these structural changes result in disease pathology. For example, an imbalance in the regulation of mitochondrial structural dynamics is associated with a wide range of age-related pathologies such as Parkinson’s disease and Alzheimer’s disease (Reddy 2014). Mitochondrial morphology is shaped by ongoing events of fusion and fission of the outer and inner mitochondrial membranes. Uncontrolled fission is known to cause mitochondrial fragmentation and leads to metabolic dysfunction and disease. Uncontrolled fusion is known to result in a hyper fused network to counteract metabolic insults, preservation of cellular integrity, and escape from autophagic clearance (Wai and Langer 2016). In addition to the dynamic structural changes within themselves, mitochondria are also known to structurally interact with ER through nanoscale-sized contacts. These contacts allow the exchange of lipids, calcium, and reactive-oxygen species between both organelles which facilitates the stress adaptations of both the organelles toward achieving homeostasis (Csordás et al. 2018). Thus maintenance of a dynamic state of mitochondria structure could have direct implications in recovering stress in ER.
Accumulation of misfolded proteins in the ER, known as ER stress, triggers the unfolded protein response (UPR) to achieve proteostasis through translational attenuation, chaperone-assisted refolding of misfolded/unfolded proteins, and degradation of terminally misfolded proteins (Murao and Nishitoh 2017). Though the effects of ER stress response toward cellular signaling changes are deciphered, its effects on mitochondrial morphology and its intertwined metabolic changes are relatively unknown. Long-lived post-mitotic neurons demonstrate ER stress by progressive accumulation of misfolded proteins and its associated decline in quality control mechanisms during aging (Ghavami et al. 2014). Similarly, oligodendrocytes and Schwann cells of the nervous system are also susceptible to ER stress due to their active synthesis and trafficking of components of the plasma membrane (Lin and Popko 2009). However, a cell type-dependent mitochondrial dependence is present during neurogenesis in the adult brain between neurons and astrocytes. Specifically, in the presence of astroglial cells, neural progenitor cells are resistant to mitochondrial respiratory defects and its associated ER stress, mediated by cyanide and rotenone (Du et al. 2018). This cross-talk suggests the active role of astrocyte mitochondria and its metabolism in complementing neuronal ER stress adaptations. In this connection, our recent findings revealed the presence of ER stress and astroglia-specific deprivation of essential micronutrient, viz., biotin in the aging brain (Ganesan et al. 2020). In addition, the findings on the effect of exogenous supplementation with biotin in ameliorating ER stress in astrocytes (Ganesan et al. 2020) led us to question the specific effects of biotin on mitochondrial dynamics and function under ER stress.
Biotin is a co-enzyme for three mitochondrial carboxylases and a cytosolic acetyl-CoA carboxylase (ACC). The function of biotin-dependent carboxylases is associated with lipogenesis, amino acid-catabolism, and anaplerosis towards maintaining nutrient flux homeostasis (Tong 2013). In this regard, biotin deficiency is known to decrease the life span of Drosophila melanogaster (Landenberger et al. 2004). Moreover, proper induction of astroglia-specific ER stress response (Frakes et al. 2020) and mitochondrial dynamics (Sharma et al. 2019) is known to promote longevity in different animal models. These observations, along with our recent findings (Ganesan et al. 2020), led us to evaluate the changes in mitochondrial dynamics in the aging brain and determine the effects of exogenous biotin supplementation on mitochondrial dynamics and function in a primary culture model of astrocyte under ER stress. In this connection, we observed a significant reduction in mitochondrial fusion and fission in the aging brain which was recapitulated under ER stress induction using tunicamycin in astrocytes. The changes in ER stress-induced mitochondrial dynamics alterations, as well as respiratory function, were recoverable with biotin supplementation in astrocytes. The findings suggest that stress in one organelle can be alleviated by supplementing metabolic factors for another interdependent organelle even when the supplemented factor has no direct role in the stressed organelle. Moreover, the results suggest an additional effect of biotin on influencing mitochondrial dynamics, in addition to its known co-enzyme role.
Materials and methods
Animal maintenance and sample collection
All procedures were conducted in compliance with the institutional animal ethics committee guidelines (CBT/AU/IAEC/11/2012). A total of ten male albino Wistar rats including 4-month-old young rats (n = 5) and 24-month-old aged rats (n = 5), acquired from the central animal facility located in the Indian Institute of Science, Bangalore, India, were used. All the rats were barrier housed two-per cage at a temperature of 24 ± 2 °C in a light-controlled environment with a 12:12-h light-dark cycle and provided food and water ad libitum. After an acclimatization period of 2 weeks, the rats were subject to overnight fasting and anesthetized with 4.5% isofluorane in 100% oxygen (1.5 L/min) inhalation for approximately 1–2 min followed by cervical dislocation for killing. Loss of consciousness was confirmed by the lack of response to pedal reflex stimulation, before proceeding with the cervical dislocation. Death was verified by direct cardiac palpation and lack of pupillary response to light. This was followed by excision of cerebral cortex tissue from the brain which was immediately snap-frozen in liquid N2 and stored at − 80 °C until further experimentation. The tissue lysate was obtained by homogenization in a bead homogenizer (Minilys, Bertin) in the presence of cell lysis buffer (Cell Signaling Technology, Cat no. 9803) containing protease and phosphatase inhibitors (Sigma-Aldrich Cat no. PPC1010).
Primary astrocyte culture and treatments
The primary astrocyte culture was carried out using the cerebral cortex of male albino Wistar rats (2–3 months old) as described earlier (Ganesan et al. 2020). All the procedures were performed in compliance with the recommendations of the committee on institutional animal ethics (CBT/AU/IAEC/07/2017). Assessment of ER stress and the effects of exogenous biotin supplementation were performed on confluent astrocytes under following treatment groups: solvent control—dimethyl sulfoxide (SC), 0.5 μg/mL tunicamycin (0.5 T), 1 μg/mL tunicamycin (1 T), 2 μM biotin plus 1 μg/mL tunicamycin (1 T + B), and solvent control plus 2 μM biotin (SCB) for 24 h in serum-free Hank’s balanced salt solution (HBSS), and cell lysates were collected as described earlier (Ganesan et al. 2020).
Immunoblot analysis
Total protein concentrations of tissue/cell lysates were determined based on Lowry’s protocol, and equal concentration of proteins was resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to the nitrocellulose membrane and blocked with 5% skimmed milk powder. The membranes were subsequently probed with respective primary and secondary antibodies at appropriate dilutions and developed using SuperSignal West Femto Chemiluminescent Substrate (Thermo). Biotinylation status of carboxylases was revealed from immunoblot analysis using an anti-biotin-horseradish peroxidase-linked antibody based on the well-established molecular weight-based migration in SDS-PAGE (Bramwell 1987; Chandler and Ballard 1988; Pacheco-Alvarez et al. 2004). Our earlier study also validated the molecular weight-based migration and identification of biotinylated carboxylases using immunoprecipitation (Ganesan et al. 2020). The chemiluminescent signals were captured by LI-COR Odyssey Fc imager and analyzed using Image Studio version 5.2. The signals were normalized against respective Ponceau S stain intensity values. Immunoblot data were represented by dividing the value of each lane by the average values of the control group using bar graphs as percentage values.
Immunofluorescence analysis
To determine the effect of ER stress on mitochondrial dynamics in the presence and absence of biotin supplementation in primary astrocyte culture, immunofluorescence studies were conducted after p-formaldehyde-fixation as per the protocol recommended by Cell Signaling Technology, USA. Rabbit anti-Mfn2 (mitochondrial fusion marker) and Mouse Anti-Translocase of the inner membrane (Tim23) (mitochondrial marker) were used to detect the extent of mitochondrial fusion using their corresponding secondary antibodies, viz., Anti-Rabbit-Alexa Fluor-488 and Anti-Mouse Alexa Fluor-555, respectively. Similarly, Rabbit Anti-Mfn2 and Mouse Anti-Protein disulfide-isomerase (PDI) (ER marker) were used to determine the extent of mitochondrial fusion protein associating with ER under the treatment conditions using Anti-Rabbit-Alexa Fluor-555 and Anti-Mouse Alexa Fluor-488 as secondary antibodies, respectively. The nucleus was stained with DAPI, and imaging was carried out using a confocal laser scanning microscope (LSM 700, Zeiss) with laser settings corresponding to Alexa Fluor-488 and Alexa fluor-555 excitation/emission. Image processing was performed using Zen 2010 (Zeiss) software.
Measurement of oxygen consumption rate
A Seahorse Bioscience XFe24 extracellular flux analyzer was used to measure mitochondrial function in primary astrocytes subject to ER stress. All the steps including hydration, calibration, and preparation of mitochondrial inhibitors were done as per the manufacturer’s instructions. A total of 1.5 μM oligomycin, 4 μM carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP), 0.5 μM rotenone, and 0.5 μM antimycin were used after preliminary optimization. The cellular protein content of individual wells was quantified and was used to normalize the OCR data of each well. Each measurement cycle consisted of mixing time of 3 min and a data acquisition period of 3 min. Data from triplicate wells were collected for each treatment group.
Statistical analysis
GraphPad Prism version 7.01 (GraphPad Software) was used for statistical analysis. Results were reported as mean ± SEM, and p < 0.05 based on t test (unpaired) were considered significant. The sample size of n = 5 (animals per group) and n = 3 (three independent culture preparations) was used for in vivo and in vitro studies, respectively. No blinding was performed in this study. A normality test was done on the data using the Kolmogorov-Smirnov test, and all datasets did not deviate from a normal distribution.
Results and discussion
Aging brain is associated with altered mitochondrial dynamics
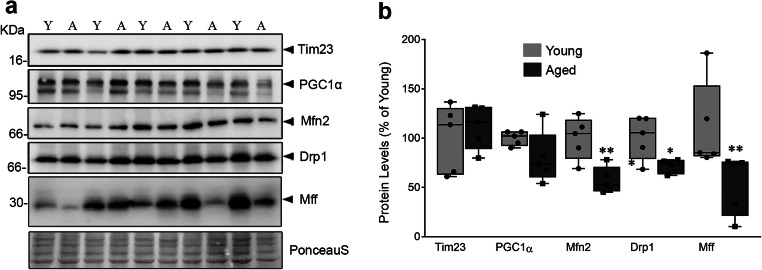
Effective mitochondrial metabolism is a top priority for highly oxidative organs like brain whose energy demand is higher than any other organ of the body, even when at rest (Castro et al. 2018). Mitochondrial fusion and fission replace aged or damaged mitochondria to maintain both its quality and quantity (Kowald and Kirkwood 2011). The accumulation of dysfunctional mitochondria with aberrant structures in aged tissues are associated with altered mitochondrial dynamics (Grimm and Eckert 2017; Sharma et al. 2019). In this perspective, we also assessed the status of mitochondrial dynamics by targeting fusion marker (Mfn2) and fission-specific markers like dynamin-related protein 1 (Drp 1) and mitochondrial fission factor (Mff) in young and aged rats. The levels of Mfn 2, Drp 1, and Mff were found to be decreased in aging brain (Fig. 1a, b) without affecting the levels of Tim23, the inner mitochondrial membrane protein, as well as peroxisomal proliferator-activated receptor gamma co-activator 1-alpha (PGC-1α), the central regulator of mitochondrial biogenesis. This suggests that altered mitochondrial dynamics, rather than content and its regulation, occur primarily during aging. Our recent study demonstrated the presence of ER stress and defective autophagy with astrocytes specific decrease in the levels of essential micronutrient, biotin. Notably, the addition of exogenous biotin ameliorates tunicamycin-induced ER stress in cultured primary astrocytes (Ganesan et al. 2020). Hence, we continued to elucidate the effect of ER specific stress, induced by tunicamycin, on mitochondrial dynamics and function, as well as the role of biotin on the same using primary astroglial cells in culture.
Fig. 1.
Mitochondrial dynamics is altered in aging rat brain. a Effect of aging on markers of mitochondrial content (Tim23), regulation (PGC1α), fusion (Mfn2), and fission (Drp1 and Mff) were determined using immunoblot. b The resulting band intensities were normalized using Ponceau S staining and quantified to represent the values as percentage change compared with young. The box represents the values between the 25th and 75th percentiles with median line. The whiskers represent the distances between the minimum and maximum values. The data from five animals in each group (n = 5) were used to obtain the box plots. The significance symbols * and ** indicates p < 0.05 and p < 0.01, respectively
Biotin influences mitochondrial dynamics in both ER stressed and unstressed astrocytes
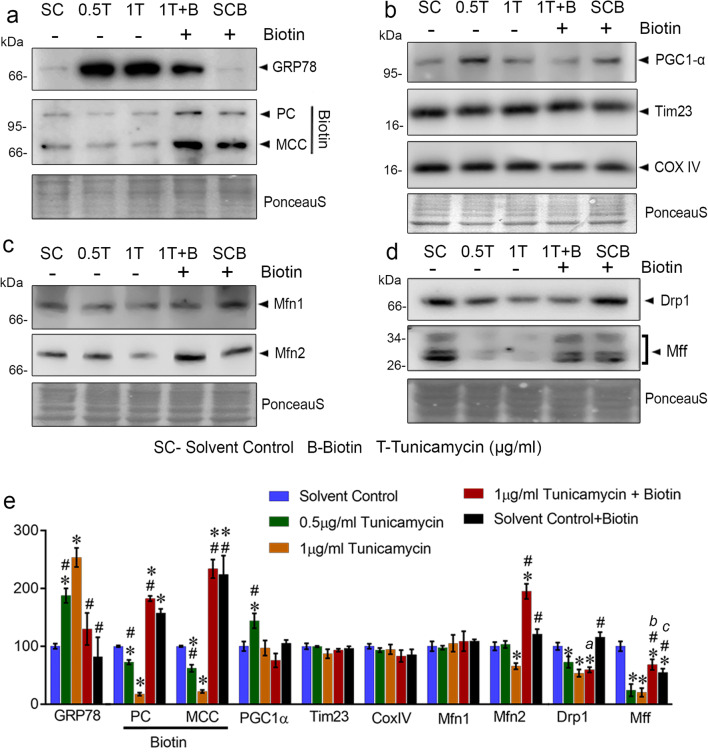
The effect of tunicamycin at maximal (1 μg/mL) and sub-maximal (0.5 μg/mL) concentrations on eliciting astroglial ER stress was ascertained by increased levels of ER resident chaperone, glucose-regulated protein 78 (GRP78) (Fig. 2a, e). The recruitment of GRP78 to misfolded proteins due to defective protein glycosylation by tunicamycin reverses its inhibitory role on unfolded protein response (UPR) pathway by binding to PERK, IRE1α, and ATF6 (Gardner et al. 2013). The resolution of ER stress demands its membrane expansion by activation of lipogenesis (Schuck et al. 2009). Because biotin is central to cellular lipid synthesis by acting as co-enzyme of rate-limiting cytosolic enzyme ACC as well as TCA cycle anaplerotic enzymes such as pyruvate carboxylase (PC), β-methylcrotonyl-CoA carboxylase (MCC), and propionyl-CoA carboxylase, the biotin demand is anticipated to be higher under ER stress. As predicted, in the absence of any exogenous biotin replenishment including serum, the levels of most prominent biotinylated proteins in mitochondria such as MCC and PC were significantly reduced under tunicamycin-induced ER stress and reversed by the addition of 2 μM biotin (Fig. 2a, e) thus signifying the role of biotin to cope with ER stress.
Fig. 2.
Biotin promotes Mfn2 levels in primary astrocytes under ER stress. Effect of tunicamycin-induced ER stress in the presence and absence of biotin supplementation on a ER stress marker (GRP78) and biotinylated carboxylase contents, b markers for mitochondrial content (Tim23, COX IV) and its regulation (PGC1α), c mitochondrial fusion (Mfn1 and Mfn2), and d mitochondrial fission markers (Drp1 and Mff) were determined using immunoblots. The percentage change in Ponceau S normalized values compared with control is indicated as e bar graph. Letters a, b, and c in the bar graph are provided for quick cross-referencing to indicate notable results described in “Results and discussion” section. Each bar represents the mean ± SEM of three independent cell culture preparations (n = 3). The significance symbols * and # indicates p < 0.05 compared with control and 1 μg/mL tunicamycin treatments, respectively. Respective group labeling are as follows: solvent control—dimethyl sulfoxide (SC), 0.5 μg/mL tunicamycin (0.5 T), 1 μg/mL tunicamycin (1 T), 2 μM biotin plus 1 μg/mL tunicamycin (1 T + B), and solvent control plus 2 μM biotin (SCB)
Though biotin has no known direct targets in the ER, the observed decrease in the levels of biotinylated carboxylases under ER stress suggests changes in its associated organelles, in particular mitochondria due to the localization of biotinylated carboxylases apart from ACC. Furthermore, the redirection of mitochondrial metabolic flux to cytosolic fatty acids possibly complements ER phospholipid synthesis required for membrane expansion and remodeling under ER stress. Hence, the effect of biotin supplementation on mitochondrial dynamics and function under ER specific stress in astroglia is envisaged. In the absence of exogenous biotin, tunicamycin (1 μg/mL) significantly decreased the levels of both fusion and fission markers (Fig. 2c–e). The addition of biotin distinctly increased the levels of Mfn2 in ER-stressed cells compared with all the groups (Fig. 2c, e) suggesting a stress-specific upregulation of mitochondrial fusion by biotin, whereas the fission markers were differentially regulated by biotin with neither of the markers increased compared with control (a, Fig. 2e) albeit the specific increase in the levels of Mff compared with ER stressed cells (b, Fig. 2e). This differential regulation of fission factors may be attributed to a temporal response as Mff is shown to be an essential factor for the recruitment of Drp1 into mitochondria (Otera et al. 2010). Moreover, the biotin supplementation to unstressed cells significantly reduced the levels of Mff (c, Fig. 2e) possibly to establish a delicate balance between mitochondrial fusion and fission in the absence of ER stress.
The markers of mitochondrial content such as Tim23 and cytochrome c oxidase subunit 4 (CoxIV) were not significantly altered between treatments (Fig. 2b, e) suggesting the possibility of mitochondrial membrane remodeling, rather than content, under ER stress adaptation. Moreover, the mitochondrial biogenesis marker, viz., PGC-1α was found to be higher only in 0.5 μg/mL tunicamycin treatment (Fig. 2b, e). Since PGC-1α is a transcriptional co-activator of multiple cellular events such as gluconeogenesis and antioxidant responses in addition to mitochondrial biogenesis (Austin and St-Pierre 2012), its expression could occur at different rates depending on the strength and duration of ER stress and possibly accounts for the non-linear response observed under astroglial ER stress.
Biotin enhances mitochondrial Mfn2 content as well as ER-mitochondria interaction as a part of ER stress resolution
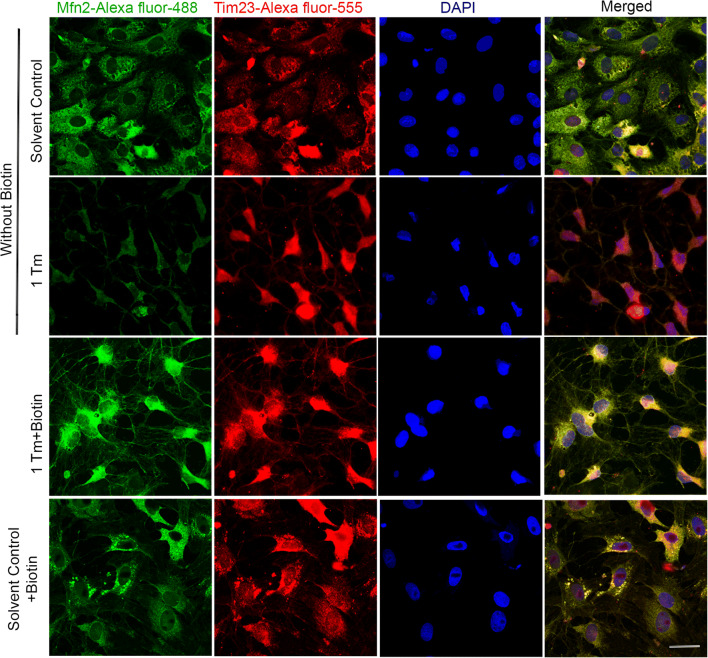
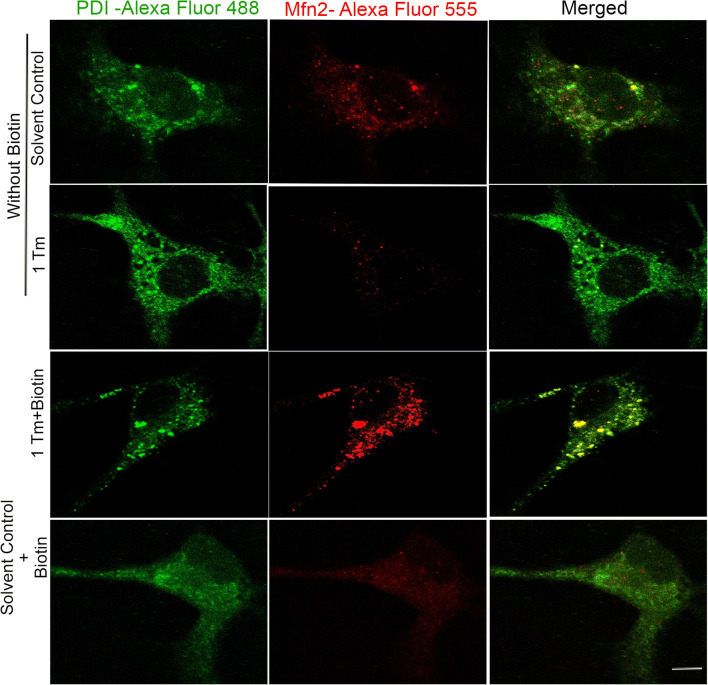
To correlate the observed differences in the levels of Mfn2 (Fig. 2c, e) on its cellular location, confocal co-localization study was performed by targeting Tim23 and Mfn2. The increase in co-localization (yellow) between Tim23 and Mfn2 confirms its accumulation on mitochondria, rather than mere cytosolic expression, by biotin under ER stress (Merged panel, Fig. 3). Since the interactions between mitochondria and ER are important to facilitate stress tolerance (Csordás et al. 2018) and Mfn2 is central to tether mitochondria with ER (De Brito and Scorrano 2008), the extent of ER-mitochondria association was evaluated. The increase in co-localization (yellow) of Mfn2 with an ER marker, protein disulfide isomerase (PDI), in the presence of biotin under ER stress (merged panel, Fig. 4) demonstrates the interaction between organelles possibly to sense each other’s status by undergoing appropriate structural remodeling to exchange molecules as a part of their stress resolution. Moreover, the lowering of Mfn2 content (Fig. 2c) and its ER localization (Fig. 4) under ER stress induction could be a strategy to decelerate the dysfunction in ER spreading to impacting mitochondrial function in the absence of essential micronutrients such as biotin.
Fig. 3.
Biotin-mediated increase in Mfn2 levels under ER stress are localized in mitochondria. Effect of tunicamycin (Tm)-induced ER stress in the presence and absence of biotin supplementation on the co-localization (yellow) between mitochondrial marker Tim23 (red) and mitochondrial fusion marker Mfn2 (green) were evaluated using confocal microscopy. The nucleus was stained with DAPI and the merged images are represented in the last column. The scale bar in the bottom merged image indicates a distance of 40 μm. The experiment was performed on three independent cell culture preparations (n = 3) with consistent outcomes and the outcome of one of the trials is represented
Fig. 4.
Biotin induced levels of Mfn2 containing mitochondria under ER stress are associated with ER. Effect of tunicamycin (Tm)-induced ER stress in the presence and absence of biotin supplementation on the co-localization (yellow) between mitochondrial fusion marker Mfn2 (red) and ER marker-PDI (green) were evaluated using confocal microscopy. The merged images obtained from the corresponding red and green channels are represented in the last column. The scale bar in the bottom merged image indicates a distance of 20 μm. The experiment was performed on three independent cell culture preparations (n = 3) with consistent outcomes and the outcome of one of the trials is represented
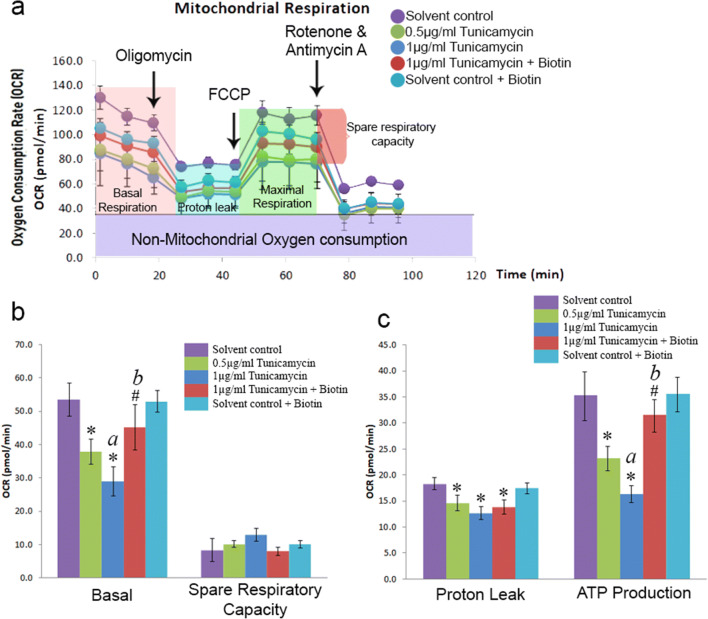
Biotin recovers ER stress-induced loss of mitochondrial respiration
Since ER has a direct regulation over mitochondrial homeostasis through ER-mitochondria contacts (Rieusset 2018), the induction of ER-specific stress is expected to impact the mitochondrial respiratory function. To determine the same, the status of basal, coupled (oligomycin), and uncoupled (FCCP) oxygen consumption rate (OCR) was measured in live cells using XFe24 extracellular flux analyzer. The difference between uncoupled and basal OCR indicates the spare respiratory capacity of cells which describes the maximum cellular ability to produce ATP on demand. In the absence of biotin, it has been observed that there was a significant reduction in the basal OCR and ATP production by ER stress (a, Fig. 5b, c), and biotin supplementation under ER stress recovered the same, similar to the control group (b, Fig. 5b, c). However, there were no significant changes in spare respiratory capacity indicating that the total mitochondrial content in astrocytes is not altered between treatments. Furthermore, the extent of proton leak was reduced by the induction of ER stress and was not recoverable by biotin supplementation possibly suggesting that the effects of biotin on rescuing mitochondrial respiration are independent or, at least, less dependent on mitochondrial proton leak management. Correlating the induction of Mfn2 (Fig. 2c, e) and the recovery of OCR and ATP production by biotin under ER stress (b, Fig. 5b, c) aligns with the findings that increased Mfn2 levels are associated with elevation in mitochondrial respiration (Yao et al. 2019).
Fig. 5.
Biotin supplementation restores mitochondrial respiration status under ER stress. Effect of ER stress induction using tunicamycin on a oxygen consumption rate response graph, b basal and spare respiratory capacity, and c proton leak and ATP production were determined using specific inhibitors of mitochondrial electron transport chain. Each data was determined in triplicate. The significance symbols * and # indicates p < 0.05 compared with control and 1 μg/mL tunicamycin treatments, respectively. Letters a and b in the bar graphs are provided for quick cross-referencing to indicate notable results described in “Results and discussion” section
Conclusion
In summary, ER-specific stress is associated with mitochondrial respiratory dysfunction. This negatively influences ER-mitochondria interaction possibly to protect each other by decelerating organelles dysfunction. The supplementation of key lipogenic as well as mitochondrial vitamin, biotin, resolves ER stress by recovering mitochondrial respiratory function to resume ER-mitochondria contact via upregulation of Mfn2. This sets a novel paradigm for exploring the alleviation of organelle stress from the perspective of micronutrient demands of other interconnected organelles under stress. Moreover, the findings establish the additional role of biotin in regulating the expression and localization of mitochondrial fusion protein Mfn2 in ER stress-dependent manner, which is a novel addition to its known co-enzymatic roles.
Acknowledgments
We thank the Department of Science and Technology, Govt. of India (DST)-FIST, DST-PURSE programs and Department of Biotechnology, Govt. of India (DBT)-BUILDER [BT/PR12153/INF/22/200/2014] program for extracellular flux analyzer, confocal microscope, and chemiluminescence instrumentation support. We thank the ICMR, Government of India for research fellowship awarded to A.R.S [IRIS-ID: 2015-25060] and S.R [IRIS-ID: 2017-3862/CMB-BMS]. We also thank UGC-RGNF, Government of India for Research Fellowship awarded to D.G (Award no.: F1-17.1/2014-15/RGNF-2014-15-SC-TAM-57123/(SAIII/Website)).
Author’s contributions
Anand Ramaian Santhaseela and Dhasarathan Ganesan contributed to conception, study design, data collection, analysis, and drafting of the article. Sudarshana Rajasekaran contributed to data collection and analysis. Tamilselvan Jayavelu contributed to conception, study design, data analysis and drafting of the article. All authors read and approved the final manuscript.
Funding information
This study was supported in part by grants-in-aid for research from Department of Biotechnology, Govt. of India (BT/PR15162/GBD/27/348/2011) and University Grants Commission, Govt. of India (41-1272/2012 (SR)).
Data availability
All data files associated with this manuscript are available upon request from the corresponding author.
Compliance with ethical standards
All procedures were conducted in compliance with the institutional animal ethics committee guidelines (CBT/AU/IAEC/11/2012)
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anand Ramaian Santhaseela and Dhasarathan Ganesan contributed equally to this work.
References
- Austin S, St-Pierre J. PGC1α and mitochondrial metabolism–emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. 2012;125:4963–4971. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- Bramwell ME. Characterization of biotinylated proteins in mammalian cells using 125I-streptavidin. J Biochem Biophys Methods. 1987;15:125–132. doi: 10.1016/0165-022x(87)90111-4. [DOI] [PubMed] [Google Scholar]
- Castro JP, Wardelmann K, Grune T, Kleinridders A. Mitochondrial chaperones in the brain: safeguarding brain health and metabolism? Front Endocrinol (Lausanne) 2018;9:196. doi: 10.3389/fendo.2018.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler CS, Ballard FJ. Regulation of the breakdown rates of biotin-containing proteins in Swiss 3T3-L1 cells. Biochem J. 1988;251:749–755. doi: 10.1042/bj2510749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G, Weaver D, Hajnóczky G. Endoplasmic reticulum–mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 2018;28:523–540. doi: 10.1016/j.tcb.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Du F, Yu Q, Chen A, et al. Astrocytes attenuate mitochondrial dysfunctions in human dopaminergic neurons derived from iPSC. Stem cell reports. 2018;10:366–374. doi: 10.1016/j.stemcr.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frakes AE, Metcalf MG, Tronnes SU, Bar-Ziv R, Durieux J, Gildea HK, Kandahari N, Monshietehadi S, Dillin A. Four glial cells regulate ER stress resistance and longevity via neuropeptide signaling in C. elegans. Science. 2020;367:436–440. doi: 10.1126/science.aaz6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan D, Santhaseela Ramaian A, Rajasekaran S, et al (2020) Astroglial biotin deprivation under endoplasmic reticulum stress uncouples BCAA-mTORC1 role in lipid synthesis to prolong autophagy inhibition in the aging brain. J Neurochem e14979. 10.1111/jnc.14979 [DOI] [PubMed]
- Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5:a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Łos MJ. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol. 2014;112:24–49. doi: 10.1016/j.pneurobio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Grimm A, Eckert A. Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. 2017;143:418–431. doi: 10.1111/jnc.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowald A, Kirkwood TBL. Evolution of the mitochondrial fusion–fission cycle and its role in aging. Proc Natl Acad Sci. 2011;108:10237–10242. doi: 10.1073/pnas.1101604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landenberger A, Kabil H, Harshman LG, Zempleni J. Biotin deficiency decreases life span and fertility but increases stress resistance in Drosophila melanogaster. J Nutr Biochem. 2004;15:591–600. doi: 10.1016/j.jnutbio.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Lin W, Popko B. Endoplasmic reticulum stress in disorders of myelinating cells. Nat Neurosci. 2009;12:379–385. doi: 10.1038/nn.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao N, Nishitoh H. Role of the unfolded protein response in the development of central nervous system. J Biochem. 2017;162:155–162. doi: 10.1093/jb/mvx047. [DOI] [PubMed] [Google Scholar]
- Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Alvarez D, Solorzano-Vargas RS, Gravel RA, et al. Paradoxical regulation of biotin utilization in brain and liver and implications for inherited multiple carboxylase deficiency. J Biol Chem. 2004;279:52312–52318. doi: 10.1074/jbc.M407056200. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Inhibitors of mitochondrial fission as a therapeutic strategy for diseases with oxidative stress and mitochondrial dysfunction. J Alzheimers Dis. 2014;40:245–256. doi: 10.3233/JAD-132060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieusset J. The role of endoplasmic reticulum-mitochondria contact sites in the control of glucose homeostasis: an update. Cell Death Dis. 2018;9:1–12. doi: 10.1038/s41419-018-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S, Prinz WA, Thorn KS, Voss C, Walter P. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol. 2009;187:525–536. doi: 10.1083/jcb.200907074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Smith HJ, Yao P, Mair WB (2019) Causal roles of mitochondrial dynamics in longevity and healthy aging. EMBO Rep 20: e48395. 10.15252/embr.201948395 [DOI] [PMC free article] [PubMed]
- Tong L. Structure and function of biotin-dependent carboxylases. Cell Mol Life Sci. 2013;70:863–891. doi: 10.1007/s00018-012-1096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27:105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Yao C-H, Wang R, Wang Y, Kung CP, Weber JD, Patti GJ. Mitochondrial fusion supports increased oxidative phosphorylation during cell proliferation. Elife. 2019;8:e41351. doi: 10.7554/eLife.41351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data files associated with this manuscript are available upon request from the corresponding author.