Abstract
Environmental toxicants such as phthalate have been involved in multiple health disorders including renal diseases. Oxidative damage is implicated in many alterations caused by phthalate especially the di(2-ethylhexyl) phthalate (DEHP), which is the most useful phthalate. However, information regarding its mechanism of renal damage is lacking. The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) regulates gene expression implicated in free radical scavenging and cytoprotection including the antioxidant glutathione (GSH) pathway. The aim of this study was to assess whether DEHP affects the Nrf2 pathway and the GSH concentration. Mice were divided into four groups: a control group and three groups treated with DEHP at different concentrations (5, 50, and 200 mg/kg body weight) for 30 days. Our results showed that DEHP altered the normal levels of serum biochemical parameters creatinine (CREA), urea, and lactate dehydrogenase (LDH). This phthalate caused oxidative damage through the induction of lipid peroxidation and protein oxidation as marked by increase of protein carbonyl (PC) and loss of protein-bound sulfhydryls (PSH). Simultaneously, DEHP treatment decreased the protein level of Nrf-2, HO-1, and GCLC (responsible of GSH synthesis) and decreased the GSH level. Inhibition of the Nrf2 pathway is related to the activation of the mitochondrial pathway of apoptosis. This apoptotic process is evidenced by an upregulation of p53 and Bax protein levels in addition to a downregulation of Bcl-2. Collectively, our data demonstrated that depletion of Nrf2 and GSH was associated with the elevation of oxidative stress and the activation of intrinsic apoptosis in mouse kidney treated with DEHP.
Keywords: Di(2-ethylhexyl) phthalate, Oxidative stress, Nrf2 antioxidant pathway, Glutathione homeostasis, Apoptosis
Introduction
Exposure to persistent environmental pollutants such as phthalate has been linked to the induction of multiple human pathologies including diabetes and cancer (Sun et al. 2015). Once utilized extensively as plasticizers to impart flexibility, transparency, and durability of plastic materials, the production of phthalate including di(2-ethylhexyl) phthalate (DEHP) was banned in the USA, Canada, and Europe. However, phthalate continues to impact ecosystems and human health due to their environmental prevalence and persistence (chemical stability). Additionally, the lipophilic nature of these plasticizers allows for their interaction with lipid membranes and lipid storage depots, leading to bioaccumulation and biomagnification through the food chain (Al-Saleh et al. 2017). More than 18 billion pounds of phthalate is used worldwide each year and humans are exposed through inhalation, ingestion, and dermal absorption on a daily basis (Latini 2005; Rael et al. 2009).
Human exposure to DEHP has been reported to range from 3 to 30 μg/kg/day (Doull et al. 1999), but can be exceeded in specific medical conditions reaching 1.5 mg/kg/day exposure in hemodialysis patients, or as high as 10–20 mg/kg/day during neonatal transfusion or parenteral nutrition (Kavlock et al. 2002).
There is cumulative evidence that DEHP is implicated in diverse health problems in animals and humans. DEHP was reported to be an endocrine disruptor and carcinogen (Kamrin 2009). Likewise, Takeshita et al. (2006) reported that in human cell line, DEHP can activate multidrug resistance gene expression by reacting with xenobiotic, steroid, and nuclear receptor. Other research studies evaluating DEHP toxicity in mammalian cells demonstrated that DEHP induced oxidative stress and DNA methylation (Lyu et al. 2016; Ma et al. 2018). In other lines of evidence, in vivo administration of DEHP revealed that kidney, liver, intestinal tissue, and the reproductive system are target organs to this plasticizer (Wang et al. 2017; Wang et al. 2015; Zhang et al. 2017). As a peroxisome proliferator–activated receptor, DEHP was reported by Dzhekova-Stojkova et al. (2001) to induce oxidative stress by increasing peroxidase expression and ROS generation.
To reduce ROS formation or to detoxify ROS, physiological systems have involved several adaptive mechanisms that implicated antioxidant enzymes or antioxidant compounds (Birben et al. 2012). The master cellular sensor for oxidative stress is the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (Han et al. 2012). Nrf2 is localized in the cytosol and regulated by its inhibitor Kelch-like ECH-associated protein 1 (Keap1), under basal conditions. Nrf2 is transcriptionally inactive until Keap1 is dissociated by compounds electrophiles, ROS, or bioactive phytochemicals found in healthful food (Kim et al. 2010; Baird and Dinkova-Kostova 2011; Higgins and Hayes 2011). This dissociation allows Nrf2 to move into the nucleus and mediate the induction of a battery of cytoprotective and antioxidant defense enzymes like heme oxygenase 1 (HO-1) (Rodrıguez -Ramiro et al. 2012). Under stressful conditions, HO-1 is expressed to protect cells against ROS generation. The role of this enzyme is to catalyze the conversion of hem to biliverdin, iron, and carbon monoxide, which can directly scavenge free radicals (Calabrese et al. 2006).
Nrf2 also regulates the expression of the subunit of glutamate-cysteine ligase (GCLC), the enzyme responsible for the synthesis of de novo of glutathione (GSH) (Wu et al. 2004). GSH is composed of glycine, cysteine, and glutamate, which under the combined effect of two enzymes, γ-glutamyl cysteine ligase and glutathione synthetase, is transformed into a tripeptide playing a key role in ROS detoxification (Brannan et al. 1980; Rahman et al. 1999). The oxidative stress can disrupt the level of GSH in the cell by altering its biosynthesis or deregulating the ratio of its reduced and oxidized forms (Harveya et al. 2009).
Since the main route of DEHP elimination and its metabolites is urinary excretion, so the kidney may be considered a target organ for this phthalate (Tanaka et al. 1975). In addition, high levels of DEHP were detected in rat kidneys, suggesting that this phthalate accumulates in this organ (Crocker et al. 1988). The accumulation of DEHP in the kidneys is favored in hemodialysis patients who use DEHP-based plastic tubes and they are regularly exposed to significant amounts of DEHP given that this phthalate leach out from tubes to dialysis liquid and then to blood circulation. In this context, Faouzi et al. (1999) showed that the plasma amount of DEHP retained by hemodialysis patients during a dialysis session varies between 3.6 and 59.6 mg.
Given that the DEHP can easily reach the blood circulation and accumulate in the kidneys, the present study has been designed to test the effect of an intraperitoneal injection on the renal function and to test the hypothesis if DEHP, by generating oxidative stress, can modulate the Nrf2-mediated GSH homeostasis in mouse kidney.
Then, we illustrated that DEHP-induced oxidative stress can be regulated by the Nrf2 antioxidant pathway. In addition, p53, Bax, and Bcl2 protein levels have been tested to evaluate whether DEHP induced apoptosis in mouse kidney, given that apoptosis is a result of oxidative stress.
Materials and methods
Chemicals
DEHP, thiobarbituric acid (TBA), and 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) were purchased from Sigma-Aldrich (St. Louis, MO). 2,4-Dinitrophenylhydrazine (2,4-DNPH) and guanidine were obtained from VWR International (Fontenaysous-Bois, France). The rest of used products were of an analytical grade.
Animals and experimental design
The experimental procedures were performed in accordance with the National Institute of Health Guidelines for Animal Care (Council of European Communities, 1986) and approved by the local Ethics Committee of Faculty of Pharmacy of Monastir (ethical number: FPHM/Pro, 201563). Twenty-four male mice of Balb/c strain (sexual, St. Doulchard, France) were used (weighing on overage 20 ± 0.3 g; 6 weeks old). These animals were fed with a standard granulated food and drinking water and were randomly grouped into four groups of six mice each and treated as follows:
Group 1: mice given corn oil intraperitoneally (negative controls)
Group 2: mice given DEHP at 5 mg/kg body weight (bw) intraperitoneally
Group 3: mice given DEHP at 50 mg/kg (bw) intraperitoneally
Group 4: mice given DEHP at 200 mg/kg (bw) intraperitoneally
The doses of DEHP (5, 50, and 200 mg/kg) represent respectively: a dose of the mean real daily exposure of human, the NOEL (no observed adverse effect level), and a relatively high dose but remains lower than the LD50 in mice (Kavlock et al. 2002; Thomas et al. 1978).
Thirty days after treatments, animals were euthanized by cervical dislocation and the kidneys were removed.
Preparation of kidney extracts
Kidneys were homogenized with a potter (glass Teflon) in the presence of Tris–HCl (10 mM, pH 7.4) at 4 °C and centrifuged at 12,000 rpm for 30 min at 4 °C. The clear supernatant was collected for analysis and for protein concentration determination using Bradford Colorimetric Protein Determination at 595 nm (Bradford 1976).
Evaluation of biochemical parameters
The biochemical parameters creatinine (CREA), urea, and lactate dehydrogenase (LDH) were assessed in the clear supernatant serum with the biochemical parameter counter (C × 9 PRO; Beckman, Switzerland). Results were expressed as micromoles per liter (μmol/L), millimoles per liter (mmol/L), or international units per liter (IU/L).
Evaluation of lipid peroxidation products
Malondialdehyde (MDA), as an index of lipoperoxydation, was quantified spectrophotometrically at 546 nm in kidney tissue (Ohkawa et al. 1979). By referring to a standard curve, the MDA concentration was calculated and the results were expressed as micromoles of malondialdehyde per milligram of proteins.
Evaluation of protein carbonyl contents
Protein carbonyl (PC) content was established referring to the method of Mercier et al. (2004). After protein precipitation with the trichloroacetic acid (20%) and carbonyl group derivatization with the 2,4-dinitrophenylhydrazine, a stable product was produced, the dinitrophenylhydrazone. Next, this product was suspended in guanidine hydrochloride (6 M) and its absorbance was detected at 370 nm. PC content was measured using the molar absorption coefficient of 22,000 M−1 cm−1.
Evaluation of protein-bound sulfhydryl contents
The method of Sedlck and Lindsay (Sedlack and Lindsay 1968) was used to determine protein-bound sulfhydryl (PSH) content. PSH concentration was calculated after the elimination of the nonprotein sulfhydryl (NPSH) concentration from the total sulfhydryl (TSH) concentration.
The content of TSH in the kidney homogenate is relative to the color intensity of yellow TNB derivative which absorbs at 421 nm. This derivative is the result of the reduction of 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) by thiols present in the samples (Sedlack and Lindsay 1968). Results were reported as micrograms of PSH per milligram of proteins.
Determination of the GSH and GSSG concentrations
The rate of GSH and GSSG in kidney tissues was quantified using Rahman et al.’s (2007) enzymatic method. Tissue homogenates were deproteinized using perchloric acid and the total glutathione and GSSG was analyzed in the supernatants. GSH was evaluated by measuring the reduction of DTNB by GSH at 412 nm. In parallel, GSSG was determined after GSH scavenging by 2 vinylpyridine which forms a pyridinium salt when complexed with thiol groups.
Protein extraction and western blotting
Cellular proteins were obtained after cell lysis with the lysis buffer containing HEPES 0.5 M, 0.5% Nonidet-P40, 1 mM PMSF, 1 mg/ml aprotinin, 2 mg/ml leupeptin, and pH 7.4. Cytoplasmic and nuclear fractions were separated and extracted using the NucBusterTM Protein Extraction Kit (Merck). Equal amounts of proteins were transferred onto membrane of nitrocellulose after SDS-PAGE separation. Membranes were incubated overnight at 4 °C with appropriate primary antibodies: anti-Nrf2 polyclonal (SC-13032, Santa Cruz Biotech), anti-HO-1 (SC-10789, Santa Cruz Biotech.Inc), anti-gamma-GCSc (D-4) (SC-166382, Santa Cruz Biotech.Inc), anti-Bax (SC-7480, Santa Cruz Biotech.Inc), anti-Bcl2 polyclonal (SC-7382, Santa Cruz Biotech.Inc), anti-P53 (SC-126, Santa Cruz Biotech.Inc), anti-β-actin (SC-1615 Santa Cruz Biotech.Inc), and anti-Lamin B1 (ab 65986, abcam). Membranes were then incubated with the secondary polyclonal antibody conjugated with horseradish peroxidase for 1 h at room temperature. Proteins were detected by Gel Logic 2200 PRO (Bioscience) after membrane incubation with Super Signal chemiluminescent detection system kit (Cod34080 Pierce Chemical Co., Rockford, IL, USA). ImageJ software was used to calculate the relative density (RD), and the intensity of each protein was normalized to β-actin. The data is then as a ratio between the intensity of the protein tested in the treated mice compared to that in the untreated mice.
Statistical analysis
Data were expressed as the mean ± SD of the means. Each experiment was done three times separately. To determine differences between groups and their control group, we used one-way ANOVA followed by Dunnett’s post hoc test. Differences were considered significant at P < 0.05.
Results
Assessment of DEHP effects on biochemical parameters in serum
The measurements of serum marker levels following DEHP treatment are indicated in Table 1. Compared to control, DEHP at different doses (5, 50, and 200 mg/kg) induced a significant increase in CREA, urea, and LDH. Therefore, DEHP disturbed significantly the homeostasis of kidney tissues by altering biochemical parameters in mice.
Table 1.
Effect of DEHP at different doses on biochemical serum markers in male Balb/c mice treated with DEHP by intraperitoneal route. Values are expressed as mean ± SD (six animals were treated per group)
| DEHP (mg/kg body weight) | ||||
|---|---|---|---|---|
| 0 | 5 | 50 | 200 | |
| Index | mean ± SD | mean ± SD | mean ± SD | mean ± SD |
| CREA (μmol/L) | 50.73 ± 0.41 | 52.33 ± 2.49 | 56.66 ± 3.68 | 65 ± 3.26* |
| Urea (mmol/L) | 2.83 ± 0.2 | 4.06 ± 0.44 | 5.23 ± 0.41* | 6.14 ± 0.28** |
| LDH (IU/L) | 590 ± 1 | 623.66 ± 19.95 | 694.33 ± 24.99* | 733.83 ± 8.83** |
*P < 0.05 and **P < 0.01, values are significantly different from control group
DEHP induced lipid peroxidation
The levels of MDA, a result product of polyunsaturated lipid degradation, were detected as the indicator of lipid peroxidation. Results in Fig. 1 showed that DEHP treatment (5, 50, and 200 mg/kg) increased MDA levels in mouse kidney tissues. This increase compared to the control indicated an enhancement in the lipid peroxidation in kidney tissues. It passed from 13.15 ± 1.19 nmol MDA/mg of proteins in the control group to reach 33.46 ± 2.74 nmol MDA/mg of proteins at 200 mg/kg bw of DEHP.
Fig. 1.
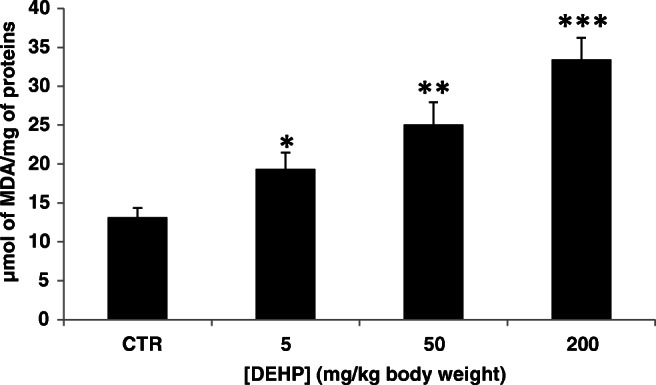
Effect of DEHP treatment on malondihaldéhyde (MDA) (μmol MDA/mg of proteins) in kidney tissues of experimental mice after treatment of 30 days. Values represent mean ± SD (six animals were treated per group); *P < 0.05, **P < 0.01, and ***P < 0.001, values are significantly different from the control group
DEHP induced protein oxidation
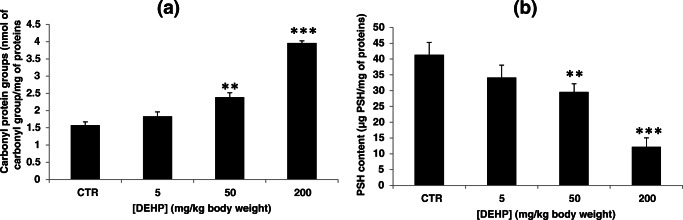
Assessment of protein carbonyl protein contents
DEHP exposure elevated significantly the PC level as compared with the control group (Fig. 2a). This elevation passed from 1.57 ± 0.09 nmol of carbonyl group/mg of proteins in the control group to 1.83 ± 0.12, 2.38 ± 0.13, and 3.95 ± 0.06 nmol of carbonyl group/mg of proteins in mice treated with DEHP at 5, 50, and 200 mg/kg bw respectively.
Fig. 2.
a Effect of DEHP treatment on carbonyl protein groups (nmol of carbonyl group/mg of protein) in kidney tissues of experimental mice after treatment of 30 days. Values represent mean ± SD (six animals were treated per group); **P < 0.01 and ***P < 0.001, values are significantly different from the control group. b Effect of DEHP treatment on protein-bound sulfhydryl (PSH) content (μg of PSH/mg of proteins) in kidney tissues of experimental mice after treatment of 30 days. Values represent mean ± SD (six animals were treated per group); **P < 0.01 and ***P < 0.001, values are significantly different from the control group
Protein-bound sulfhydryl contents
Kidney tissue PSH levels decreased significantly following DEHP treatment (Fig. 2b). This decrease passed from a basal value of 41.36 ± 3.96 μg PSH/mg of proteins in the control group to 34.10 ± 3.98, 29.62 ± 2.56, and 12.20 ± 2.85 μg PSH/mg of proteins in mice treated with DEHP at 5, 50, and 200 mg/kg bw respectively.
DEHP altered the Nrf2 pathway
Nrf2 has been recognized as a key transcription factor against oxidative damage. To test the hypothesis that oxidative stress occurred in renal tissues is the result of Nrf2-dependent antioxidant responses, Nrf2 (in the cytosol and the nucleus), HO-1 and GCLC protein levels were investigated by western blot analysis using β-actin and Lamin B1 as an internal reference and loading control for the cytosolic and nuclear fraction respectively. DEHP significantly inhibited the Nrf2 signaling pathway, as demonstrated by the decreased expression levels of Nrf2, both in the cytosol and in the nucleus, and its downstream effectors HO-1 and GCLC compared to the control group (Fig. 3).
Fig. 3.
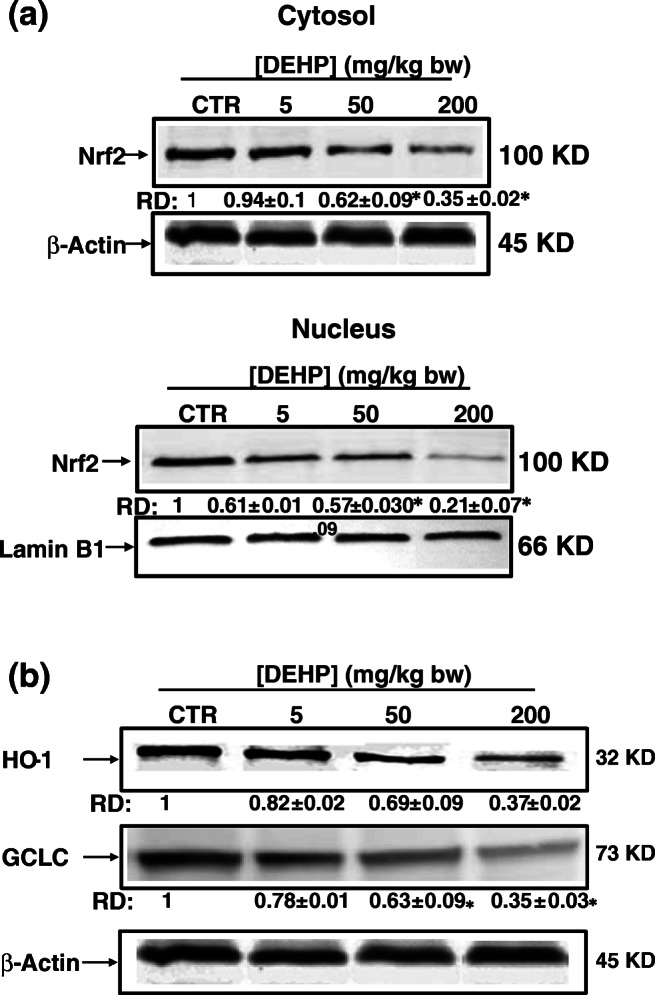
Inhibition of the Nrf2/HO-1/GCLC antioxidant pathway by DEHP in kidney tissues of experimental mice after treatment of 30 days. a Subcellular distribution of Nrf2 determined by Western blot analysis. β-Actin and Lamin B1 were served as loading controls for the cytosolic and nuclear fractions respectively. Data were collected from three independent experiments performed in replicate. b Protein levels of HO-1 and GCLC were analyzed by western blot. β-Actin was used as a loading control. RD: relative density as described in the “Material and methods” section. Values represent mean ± SD of three independent experiments. *P < 0.05 values are significantly different from the control group
Hence, this finding provides evidence that HO-1 and GSH synthesis are targets of DEHP-induced oxidative stress in renal tissues through the downregulation of Nrf2.
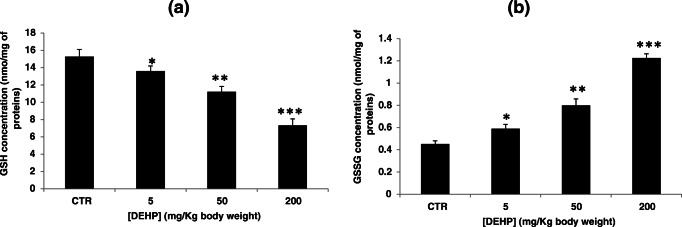
DEHP induces glutathione depletion
Reduced glutathione plays an important role in antioxidant defense thanks to its nucleophilic and reducing properties. Under normal conditions, the overproduction of ROS is neutralized by reduced glutathione which leads to its depletion inside the cell. Thus, we tested the effect of DEHP on glutathione modulation in mouse kidney tissues. Treatment with DEHP showed a decrease in the GSH content and an increase in the GSSG content as compared to the control group (Fig. 4). The GSH concentration changed from 15.25 ± 0.85 in the control group to 13.59 ± 0.59, 11.21 ± 0.59, and 7.32 ± 0.75 in mice treated with 5, 50, and 200 mg/kg bw respectively, while the GSSG concentration changed from 0.45 ± 0.02 in the control group to 0.58 ± 0.03, 0.79 ± 0.05, and 1.22 ± 0.03 in mice treated with 5, 50, and 200 mg/kg bw respectively.
Fig. 4.
Effect of DEHP treatment on GSH/GSSG ratio in kidney tissues of experimental mice after treatment of 30 days. Values represent mean ± SD (six animals were treated per group); *P < 0.05, **P < 0.01, and ***P < 0.001, values are significantly different from the control group
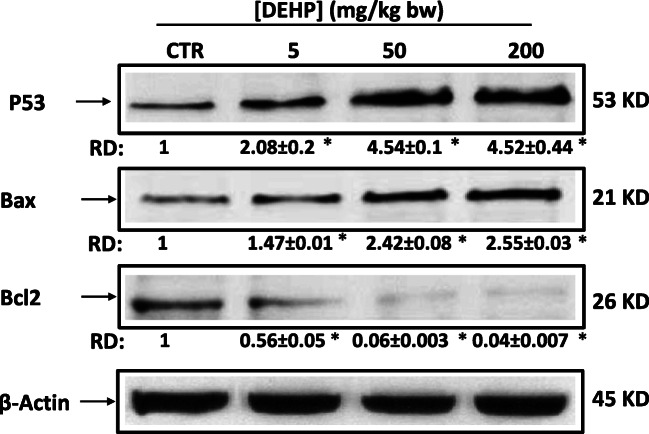
DEHP induced apoptosis in mouse kidney
To test whether DEHP was involved in apoptosis in mouse kidney tissues, western blotting and densitometric analysis were used to study the expression levels of characteristic proteins involved in apoptosis. The results in Fig. 5 showed that the expression of apoptosis biomarker, P53, increased after DEHP treatment. This induction was associated with the overexpression of proapoptotic protein, Bax, and the decrease in the antiapoptotic protein, Bcl2.
Fig. 5.
DEHP induces apoptosis in kidney of experimental mice after treatment of 30 days. Apoptosis markers P53, Bax, and Bcl2 were analyzed by western blot. β-Actin was used as a loading control. RD: relative density as described in the “Material and methods” section. Data are expressed as the mean ± SD of three separate experiments. *P < 0.05 values are significantly different from the control group
Discussion
Phthalates are not chemically bonded to the plastic matrix and leach out from the material over time, which prompts a growing concern about human exposure to these plasticizers. Although phthalate esters have long been regarded as substances of low acute toxicity, they possess potential toxic effects which may be exhibited when exposed to high doses or repeated low doses.
A great deal of research has provided evidence for the serious consequences of phthalates, including DEHP on animal health, such as liver, kidney, and testicular damage, as well as the under-development of reproductive organs in humans and animals (Rusyn et al. 2006; Miura et al. 2007; Takashima et al. 2008; Erkekoglu et al. 2011; Howdeshell et al. 2008, b; Miura et al. 2007). On the other hand, several studies described oxidative stress as a major pathway in the reproductive toxicity of DEHP (Kasahara et al. 2002; Erkekoglu et al. 2010). However, data on DEHP effects on kidney is very limited and the mechanism is not clear. Croker et al. (1988) reported that treatment of rats with the DEHP decrease kidney function and increase incidence of focal cysts. The current study was therefore undertaken to investigate the effects of DEHP exposure in kidney of Balb-c mice. We hereby present the effects of DEHP on the antioxidant status of the renal system.
We investigated the level of serum creatinine (CREA), urea, and lactate dehydrogenase (LDH) as an index of renal damage. In fact, the CREA and urea highlight the metabolism of nitrogenous compounds and the glomerular filtration function alteration (Atessahin et al. 2007). Our results showed that DEHP caused a significant increase of these biochemical parameters. Indeed, the increase in these levels reflects a defect in cell homeostasis caused by this phthalate. Noori and Mahboobc (2010) linked the increase of creatinine and urea content in serum to the decreased glomerular filtration rate or to increased ROS production in the kidney. In agreement with our studies, a significant increase in these serum parameters was observed in vivo study following DEHP treatment (Beltifa et al. 2018).
Our results showed that these changes in serum were associated with the increasing of renal MDA concentration, as evidence of lipid peroxydation which is a marker of oxidative stress.
Besides the lipids, also proteins are among the macromolecules affected by the oxidative stress. Protein damage may frequently be more important than lipid damage (Reznick et al. 1994). In fact, many cellular functions including proteins such as enzymes, transport systems, signal transduction mechanisms, and receptors may be affected by oxidative attacks (Samuel et al. 2005). Dalle-Donne et al. (2003) have reported that protein carbonyl (PC) and protein-bound sulfhydryls (PSH) are indicators of oxidative protein alteration that is reflected by an increase in levels of PC and decrease in levels of PSH (Dubey et al. 1996; Butterfield et al. 1998). For this purpose, the renal concentrations of these markers were determined in the current study. Our results showed that DEHP-induced renal damage was also associated with protein oxidative alteration as evidenced by the increase in the kidney PC and loss of PSH.
Oxidative damage to proteins and lipids might result from the alterations in the antioxidant defense system which includes non-antioxidant as well as antioxidant enzymes such as HO-1.
HO-1 is a target gene of Nrf2 which is a redox-sensitive transcription factor that can limit oxidative damage and maintain cellular redox homeostasis (Bryan et al. 2013). Indeed, HO-1 catalyzes the degradation of heme and produces carbon monoxide and bilirubin, which can directly scavenge free radicals and repair DNA damage caused by oxidative stress (Calabrese et al. 2006). In addition to HO-1, the Nrf2 regulates enzymes responsible of glutathione synthesis namely the GCLC. Because glutathione homeostasis is regulated by Nrf2, we tested if the DEHP alters this pathway. Our results showed that DEHP inhibited the Nrf2 signaling pathway in mouse kidney, as demonstrated by the decreased expression levels of Nrf2 in both the cytosol and the nucleus, and its downstream effectors HO-1 and GCLC which could be the cause of GSH depletion and oxidative stress generation. To confirm that, reduced (GSH) and oxidized (GSSG) forms of glutathione were quantified in mouse kidney treated with DEHP. We found a significant decrease in the GSH concentration and an increase of GSSG concentration. Changes in the redox state of glutathione were in agreement with the oxidative changes as well as the decrease in Nrf2 and GCLC protein levels already observed after treatment with DEHP. Our results are in line with Sun et al. (2015) who showed that DEHP decreased the amount of Nrf2 with a corresponding decrease in the transcription of the genes encoding antioxidant enzymes, such as Hmox-1, Cat, Gclc, Gclm, Nqo1, and GPx in rat INS-1 cells.
Among the factors regulating Nrf2 expression is mitochondrial ROS amount. In fact, it is becoming clear that small amount of ROS act as signaling molecules in the cells (Kasai et al. 2020). Imhoff et al. (2009) demonstrated that mitochondrial ROS activates Nfr2 expression. On the other hand, it was known that mitochondrial ROS can be responsible for Nrf2 repression in certain pathological conditions (Kasai et al. 2020). Accumulating evidence to date has demonstrated that Nrf2 activity is repressed in various tissues in diabetes patients (Cheng et al. 2013; Smith et al. 2010; Rabbani et al. 2019; Zuo et al. 2019). However, its precise mechanisms are not currently clear.
In our study, it could be possible that the amount of ROS generated by the DEHP in mouse kidney could, by a negative feedback, could cause a decrease in his level of expression.
Another factor that can regulate Nrf2 expression is the expression regulation of Keap1. Indeed, different reports have shown that Nrf2 repression during diabetes or chronic hyperglycemia often accompanies increased of Keap1 protein level. Thus, it is tempting to speculate that Keap1 upregulation might be an upstream event in Nrf2 repression, and Anzovino et al. demonstrated that Keap1 upregulation precedes Nrf2 downregulation in a frataxin-knockout mouse model (Anzovino et al. 2017). In our study, it is possible that the DEHP could upregulated the Keap1 expression. However, the mechanisms of DEHP-regulated expression of Keap1 need to be investigated. Additional studies are needed to investigate the role of DEHP in inhibition of Nrf2 expression.
A significant decrease in GSH content was observed in vivo and in vitro studies following DEHP treatment (She et al. 2017; Zhao et al. 2018). These results suggest that oxidative stress induced by DEHP could be result in a repression of the Nrf2 pathway leading to intracellular GSH depletion in mouse kidney.
Liu et al. (2017) indicated that the transcription factor Nrf2 have an important role in cell apoptosis process provoked by oxidative stress. At different levels, Nrf2 interfere with both intrinsic and extrinsic apoptosis pathways (Cao et al. 2015). Recently, Liu et al. (2017) demonstrated that Nrf2 repression in periodontal ligament stem cells decreased the antioxidant capacity and cell proliferation and upregulated the intrinsic apoptosis pathway.
Övey et al. (2015) have revealed that a drop in GSH content leads the cell to program its death. According to this, we supposed that the Nrf2 repression and the GSH depletion observed in mouse kidney treated with DEHP could lead to apoptosis.
It is well known that P53 is involved in the intrinsic apoptosis pathway regulation by controlling the expression of many target genes, namely the pro-apoptotic protein Bax (Mirzayans et al. 2012). The intrinsic apoptotic pathway is regulated by the Bcl-2 family proteins which consists of pro-apoptosis genes (Bax, Bim, Bid), anti-apoptosis genes (Bcl-2, Bcl-xl), and one of the components of the mitochondrial permeability transition pore (mPTP) (Chen and Lesnefsky, 2011). Thus, Bax and Bcl2 balance plays an important role in the apoptosis progression (Scorrano and Korsmeyer 2003).
In addition to P53 protein level, we also detected Bcl-2 and Bax levels using western blot to test DEHP-induced apoptosis in mouse kidney via the mitochondrial pathway.
Our results demonstrated that exposure to DEHP upregulated P53 and Bax, whereas it downregulated Bcl-2. It can be concluded that DEHP by decreasing the Nrf2 expression has led the kidney cells to trigger the intrinsic pathway of apoptosis.
Conclusion
In summary, the current study elucidated the in vivo effect of DEHP on mouse kidney and indicated that DEHP induced nephrotoxicity through the induction of oxidative stress. Furthermore, we demonstrated that Nrf2 repression caused oxidative stress and apoptosis in mouse kidney tissues. We showed also a decrease in GSH level, which is the final product of the Nrf2 signaling pathway. Hence, our work indicated that Nrf2, HO-1, and GCLC are molecular targets of DEHP in the generation of oxidative stress in mouse kidney.
Funding information
This research was supported by the Ministère Tunisien de l’Enseignement Superieur et de la Recherche Scientifique et de la Technologie (Laboratoire de Recherche sur les Substances Biologiquement Compatibles, LRSBC).
Compliance with ethical standards
The experimental procedures were performed in accordance with the National Institute of Health Guidelines for Animal Care (Council of European Communities, 1986) and approved by the local Ethics Committee of Faculty of Pharmacy of Monastir (ethical number: FPHM/Pro, 201563).
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Highlights
• DEHP induces oxidative stress in mouse kidney.
• DEHP inhibits Nrf2 antioxidant pathway in mouse kidney.
• DEHP decreases GSH content in mouse kidney.
• DEHP induces apoptosis through the mitochondrial pathway in mouse kidney.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Saleh I, Elkhatib R, Al-Rajoudi T, Al-Qudaihi G. Assessing the concentration of phthalate esters (PAEs) and bisphenol A (BPA) and the genotoxic potential of treated wastewater (final effluent) in Saudi Arabia. Sci Total Environ. 2017;578:440–451. doi: 10.1016/j.scitotenv.2016.10.207. [DOI] [PubMed] [Google Scholar]
- Anzovino A, Chiang S, Brown BE, Hawkins CL, Richardson DR, Huang ML. Molecular alterations in a mouse cardiac model of friedreich ataxia: an impaired Nrf2 response mediated via upregulation of Keap1 and activation of the Gsk3beta axis. Am J Pathol. 2017;187:2858–2875. doi: 10.1016/j.ajpath.2017.08.021. [DOI] [PubMed] [Google Scholar]
- Atessahin A, Ceribasi AO, Yuce A, Bulmus O, Cikim G. Role of ellagic acid against cisplatin-induced nephrotoxicity and oxidative stress in rats. Basic Clin Pharmacol Toxicol. 2007;100:121–126. doi: 10.1111/j.1742-7843.2006.00015.x. [DOI] [PubMed] [Google Scholar]
- Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- Beltifa A, Feriani A, Macherki M, Ghorbel A, Ghazouani L, Di Bella G, Sire O, Van Loco J, Reyns T, Mansour HB. Persistent plasticizers and bisphenol in the cheese of Tunisian markets induced biochemical and histopathological alterations in male BALB/c mice. Environ Sci Pollut Res Int. 2018;25:6545–6557. doi: 10.1007/s11356-017-0857-6. [DOI] [PubMed] [Google Scholar]
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19 [DOI] [PMC free article] [PubMed]
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed]
- Brannan TS, Maker HS, Weiss C, Cohen G. Regional distribution of glutathione peroxidase in the adult rat brain. J Neurochem. 1980;35:1013–1014. doi: 10.1111/j.1471-4159.1980.tb07102.x. [DOI] [PubMed] [Google Scholar]
- Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Koppal T, Howard B, Subramaniam R, Hall N, Hensley K, Yatin S, Allen K, Aksenova M, Carney J. Structural and functional changes in proteins induced by free radical mediated oxidative stress and protective action of the antioxidants N-tert-butyl-alpha-phenylnitrone and vitamin E. Ann N Y Acad Sci. 1998;854:448–462. doi: 10.1111/j.1749-6632.1998.tb09924.x. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Butterfield DA, Scapagnini G, Stella AM, Maines MD. Redox regulation of heat shock protein expression by signaling involving nitric oxide and carbon monoxide: relevance to brain aging, neurodegenerative disorders, and longevity. Antioxid Redox Signal. 2006;8:444–477. doi: 10.1089/ars.2006.8.444. [DOI] [PubMed] [Google Scholar]
- Cao Y, Liu Z, Xie Y, Hu J, Wang H, Fan Z, Zhang C, Wang J, Wu CT, Wang S. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res Ther. 2015;6:249. doi: 10.1186/s13287-015-0244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Lesnefsky EJ. Blockade of electron transport during ischemia preserves bcl-2 and inhibits opening of the mitochondrial permeability transition pore. FEBS Lett. 2011;585:921–926. doi: 10.1016/j.febslet.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Chapple SJ, Patel B, Puszyk W, Sugden D, Yin X, Mayr M, Siow RC, Mann GE. Gestational diabetes mellitus impairs Nrf2-mediated adaptive antioxidant defenses and redox signaling in fetal endothelial cells in utero. Diabetes. 2013;62:4088–4097. doi: 10.2337/db13-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council of European Communities Council instructions about the protection of living animals used in scientific investigations. Off JEur Communities (1986), pp. 1-18 (JO86/609/CEE) L358
- Crocker JF, Safe SH, Acott P. Effects of chronic phthalate exposure on the kidney. J Toxicol Environ Health. 1988;23:433–444. doi: 10.1080/15287398809531126. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Doull J, Cattley R, Elcombe C, Lake BG, Swenberg J, Wilkinson C, Williams G, van Gemert M. A cancer risk assessment of di(2-ethylhexyl)phthalate: application of the new U.S. EPA Risk Assessment Guidelines. Regul Toxicol Pharmacol. 1999;29:327–357. doi: 10.1006/rtph.1999.1296. [DOI] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and behavioral functions of the mouse. Arch Biochem Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Dzhekova-Stojkova S, Bogdanska J, Stojkova Z. Peroxisome proliferators: their biological and toxicological effects. Clin Chem Lab Med. 2001;39:468–474. doi: 10.1515/CCLM.2001.076. [DOI] [PubMed] [Google Scholar]
- Erkekoglu P, Rachidi W, Yuzugullu OG, Giray B, Favier A, Ozturk M, Hincal F. Evaluation of cytotoxicity and oxidative DNA damaging effects of di(2-ethylhexyl)-phthalate (DEHP) and mono(2-ethylhexyl)-phthalate (MEHP) on MA-10 Leydig cells and protection by selenium. Toxicol Appl Pharmacol. 2010;248:52–62. doi: 10.1016/j.taap.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Erkekoglu P, Rachidi W, Yüzügüllü OG, Giray B, Oztürk M, Favier A, et al. Induction of ROS, p53, p21 in DEHP- and MEHP-exposed LNCaP cells protection by selenium compounds. Food Chem Toxicol. 2011;49:1565–1571. doi: 10.1016/j.fct.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Faouzi MA, Dine T, Gressier B. Exposure of hemodialysis patients to di-2 ethylhexyl phthalate. Int J Pharm. 1999;180:113–121. doi: 10.1016/s0378-5173(98)00411-6. [DOI] [PubMed] [Google Scholar]
- Han SG, Han SS, Toborek M, Hennig B. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol Appl Pharmacol. 2012;261:181–188. doi: 10.1016/j.taap.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harveya CJ, Thimmulappaa RK, Singha A, Blakea DJ, Linga G, Wakabayashia NJ, Fujiib A, Myersc BS. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med. 2009;46:443–453. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins LG, Hayes JD. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev. 2011;43:92–137. doi: 10.3109/03602532.2011.567391. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, Gray LE., Jr A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague-Dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105:153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Imhoff BR, Hansen JM. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem J. 2009;424:491–500. doi: 10.1042/BJ20091286. [DOI] [PubMed] [Google Scholar]
- Kamrin MA. Phthalate risks, phthalate regulation, and public health: a review. J Toxicol Environ Health Part B. 2009;12:157–174. doi: 10.1080/10937400902729226. [DOI] [PubMed] [Google Scholar]
- Kasahara E, Sato EF, Miyoshi M, Konaka R, Hiramoto K, Sasaki J, Tokuda M, Nakano Y, Inoue M. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem J. 2002;365:849–856. doi: 10.1042/BJ20020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai S, Shimizu S, Tatara Y, Mimura J, Itoh K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules. 2020;17:10. doi: 10.3390/biom10020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, Zacharewski T. NTP Center for the evaluation of risks to human reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol. 2002;16:529–653. doi: 10.1016/s0890-6238(02)00032-1. [DOI] [PubMed] [Google Scholar]
- Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2010;690:12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Latini G. Monitoring phthalate exposure in humans. Clin Chim Acta. 2005;361:20–29. doi: 10.1016/j.cccn.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang H, Wen Y, Li B, Zhao Y, Xing J, Zhang M, Chen Y. Nrf2 inhibits periodontal ligament stem cell apoptosis under excessive oxidative stress. Int J Mol Sci. 2017;18:1076. doi: 10.3390/ijms18051076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu ZQ, Xie X, Ke YB. Effects of long term and low dose di-(2-ethylhexyl) phthalate exposure on global genome DNA methylation in HePG2 cells. Article in Chinese. 2016;34:346–351. doi: 10.3760/cma.j.issn.1001-9391.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Ma Y, Guo Y, Wu S, Lv Z, Zhang Q, Xie X. Analysis of toxicity effects of Di-(2-ethylhexyl) phthalate exposure on human bronchial epithelial 16HBE cells. Cytotechnology. 2018;70:119–128. doi: 10.1007/s10616-017-0111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier Y, Gatellier P, Renerre M. Lipid and protein oxidation in vitro, and antioxidant potential in meat from Charolais cows finished on pasture or mixed diet. Meat Sci. 2004;66:467–473. doi: 10.1016/S0309-1740(03)00135-9. [DOI] [PubMed] [Google Scholar]
- Mirzayans, R., Andrais, B., Scott, A., Murray, D. (2012). New insights into p53 signaling and cancer cell response to DNA damage: implications for cancer therapy. J Biomed Biotechnol 170325 [DOI] [PMC free article] [PubMed]
- Miura Y, Naito M, Ablake M, Terayama H, Yi SQ, Qu N, Cheng LX, Suna S, Jitsunari F, Itoh M. Short-term effects of di-(2-ethylhexyl) phthalate on testes, liver, kidneys and pancreas in mice. Asian J Androl. 2007;9:199–205. doi: 10.1111/j.1745-7262.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- Noori S, Mahboobc T. Antioxidant effect of carnosine pretreatment on cisplatin induced renal oxidative stress in rats. Indian J Clin Biochem. 2010;25:86–91. doi: 10.1007/s12291-010-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxide in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Övey IS, Naziroğlu M. Homocysteine and cytosolic GSH depletion induce apoptosis and oxidative toxicity through cytosolic calcium overload in the hippocampus of aged mice: involvement of TRPM2 and TRPV1 channels. Neuroscience. 2015;22:225–233. doi: 10.1016/j.neuroscience.2014.09.078. [DOI] [PubMed] [Google Scholar]
- Rabbani PS, Soares MA, Hameedi SG, Kadle RL, Mubasher A, Kowzun M, Ceradini DJ. Dysregulation of Nrf2/Keap1 redox pathway in diabetes affects multipotency of stromal cells. Diabetes. 2019;68:141–155. doi: 10.2337/db18-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rael LT, Bar-Or R, Ambruso DR, Mains CW, Slone DS, Craun ML, Bar-Or D. Phthalate esters used as plasticizers in packed red blood cell storage bags may lead to progressive toxin exposure and the release of pro-inflammatory cytokines. Oxidative Med Cell Longev. 2009;2:166–171. doi: 10.4161/oxim.2.3.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Antonicelli F, MacNee W. Molecular mechanism of the regulation of glutathione synthesis by tumor necrosis factor and dexamethasone in human alveolar epithelial cells. J Biol Chem. 1999;274:5088–5096. doi: 10.1074/jbc.274.8.5088. [DOI] [PubMed] [Google Scholar]
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2007;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- Rodrıguez -Ramiro I, Ramos S, Bravo L, Goya L, Martın MA. Procyanidin B2 induces NRF2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur J Nutr. 2012;51:881–892. doi: 10.1007/s00394-011-0269-1. [DOI] [PubMed] [Google Scholar]
- Rusyn I, Peters JM, Cunningham ML. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit Rev Toxicol. 2006;36:459–479. doi: 10.1080/10408440600779065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S, Kathirvel R, Jayavelu T, Chinnakkannu P. Protein oxidative damage in arsenic induced rat brain: influence of DL-alpha-lipoic acid. Toxicol Lett. 2005;155:27–34. doi: 10.1016/j.toxlet.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Korsmeyer S. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biophys Res Commun. 2003;304:437–444. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- Sedlack I, Lindsay RH. Estimation of total, protein-bound and non-protein bound sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- She Y, Jiang L, Zheng L, Zuo H, Chen M, Sun X, Li Q, Geng C, Yang G, Jiang L, Liu X. The role of oxidative stress in DNA damage in pancreatic β cells induced by di-(2-ethylhexyl) phthalate. Chem Biol Interact. 2017;1:8–15. doi: 10.1016/j.cbi.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- Sun X, Lin Y, Huang Q, Shi J, Qiu L, Kang M, Chen Y, Fang C, Ye T, Dong S. Di(2-ethylhexyl) phthalate-induced apoptosis in rat INS-1 cells is dependent on activation of endoplasmic reticulum stress and suppression of antioxidant protection. J Cell Mol Med. 2015;19:581–594. doi: 10.1111/jcmm.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima K, Ito Y, Gonzalez FJ, Nakajima T. Different mechanisms of DEHP-induced hepatocellular adenoma tumorigenesis in wild-type and Ppar alpha-null mice. J Occup Health. 2008;50:169–180. doi: 10.1539/joh.l7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita A, Inagaki K, Igarashi-Migitaka J, Ozawa Y, Koibuchi N. The endocrine disrupting chemical, diethylhexyl phthalate, activates MDR1 gene expression in human colon cancer LS174T cells. J Endocrinol. 2006;190:897–902. doi: 10.1677/joe.1.06664. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Adachi T, Takahashi T, Yamaha T (1975) Biochemical studies on phthalic esters I. Elimination, distribution and metabolism of di-(2-ethylhexyl)phthalate in rats. Toxicology 4:253–264 [DOI] [PubMed]
- Thomas JA, Darby TD, Wallin RF, Garvin PJ, Martis L. A review of the biological effects of di-(2-ethylhexyl) phthalate. Toxicol Appl Pharmacol. 1978;45:1–27. doi: 10.1016/0041-008x(78)90024-8. [DOI] [PubMed] [Google Scholar]
- Wang X, Jiang L, Ge L, Chen M, Yang G, Ji F, Zhong L, Guan Y, Liu X. Oxidative DNA damage induced by di-(2-ethylhexyl) phthalate in HEK-293 cell line. Environ Toxicol Pharmacol. 2015;39:1099–1106. doi: 10.1016/j.etap.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen B, Lin T, Wu S, Wei G. Protective effects of vitamin E against reproductive toxicity induced by di(2 ethylhexyl) phthalatevia PPAR-dependent mechanisms. Toxicol Mech Methods. 2017;27:551–559. doi: 10.1080/15376516.2017.1333556. [DOI] [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shen XY, Zhang WW, Chen H, Xu WP, Wei W. Di-(2-ethylhexyl) phthalate could disrupt the insulin signaling pathway in liver of SD rats and L02 cells via PPARγ. Toxicol Appl Pharmacol. 2017;316:17–26. doi: 10.1016/j.taap.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Zhao HM, Hu RW, Chen XX, Chen XB, Lü H, Li YW, Li H, Mo CH, Cai QY, Wong MH (2018) Biodegradation pathway of di-(2-ethylhexyl) phthalate by a novel Rhodococcus pyridinivorans XB and its bioaugmentation for remediation of DEHP contaminated soil. Sci Total Environ 640–641:1121–1131 [DOI] [PubMed]
- Zuo H, Wang S, Feng J, Liu X. BRD4 contributes to high-glucose-induced podocyte injury by modulating Keap1/Nrf2/ARE signaling. Biochimie. 2019;165:100–107. doi: 10.1016/j.biochi.2019.07.012. [DOI] [PubMed] [Google Scholar]