Abstract
Objective
To explore sexually transmitted infection (STI) clinic client attitudes and preferences towards STI vaccines and STI vaccine programming in an urban clinic setting.
Methods
A 31-item questionnaire was administered during check-in by clinic clerical staff at two STI clinics in Vancouver, Canada. Demographic characteristics and preferences were summarised descriptively. Multivariable logistic regression models to assess factors associated with STI vaccine interest (reported as ORs) were constructed using a priori clinically relevant variables and factors significant at p≤0.05 in bivariate analysis.
Results
293 surveys were included in analysis. 71.3% of respondents identified as male, 80.5% had college level education or higher and 52.9% identified as white/of European descent. The median age was 33. 86.5% of respondents reported they would be interested in receiving an STI vaccine, with a primary motivator to protect oneself. Bivariate analysis indicated several factors associated with vaccine interest, with differences for each infection. After adjusting for other variables, willingness to pay for an STI vaccine (OR=3.83, 95% CI 1.29 to 11.38, p=0.02) remained a significant factor for syphilis vaccine interest and intent to engage in future positive health behaviours remained a significant factor for chlamydia (OR=5.94, 95% CI 1.56 to 22.60, p=0.01) and gonorrhoea (OR=5.13, 95% CI 1.45 to 18.07, p=0.01) vaccine interest.
Conclusion
Respondents expressed a strong willingness to receive STI vaccines. These valuable findings will inform for eventual STI vaccine programme planning and implementation.
Keywords: vaccines, attitudes, infection control, bacterial infection, vaccination
Introduction
Sexually transmitted infections (STIs) continue to be a global health concern despite extensive prevention efforts that include risk-reduction counselling, sex education and condom promotion.1–3 Significant advancements in biomedical prevention include human papillomavirus (HPV) and hepatitis B virus vaccination and HIV pre-exposure prophylaxis (PrEP).1 4 Additionally, public health measures are used to control STI diagnosis, treatment and partner notification. In most settings, STI management is usually sought by those with symptoms requiring treatment.1
Antimicrobial treatments for bacterial STIs do not provide protection against repeat infections, and natural immunity does not develop for bacterial STIs leading to repeat and coinfections.5 Certain strains of Neisseria gonorrhoeae have become antimicrobial-resistant (AMR) to preferred agents, suggesting that pan-resistant, untreatable gonorrhoea is a possibility, even with dual-therapy antibiotic protocols in place.5 6 There is some evidence indicating that an immune response is seen after chlamydia infection which could support vaccine development for chlamydia; however, more research is needed.7
In British Columbia (BC), Canada, cases of chlamydia, gonorrhoea and syphilis have been increasing over the last decade. From 2006 to 2016, rates of chlamydia increased by 43%; gonorrhoea by 212% and syphilis by 113%. This trend is mirrored throughout Canada. Globally, rates of these bacterial infections also remain high, with an additional limitation on collected global data due to underreporting by some geographical regions.8 9 There are an estimated 376 million new cases of chlamydia, gonorrhoea, syphilis and trichomoniasis each year, which equates to approximately one million infections every day.
Reasons for increases in STI rates are multifactorial and may be attributed to decreased condom use, increased sexual contact,4 increasing AMR bacteria or an increase in the amount of people seeking STI testing, leading to an increase in reported rate of infections.8 10 However, barriers to accessing services still exist, including stigma associated with testing, inability to physically access a clinic (location or hours) or dismissal or unawareness of the need for routine testing.
One method to address increasing STI rates is the implementation of STI vaccines.2 8 11 Vaccines against several STIs are in varied stages of development, but are not yet available for syphilis, chlamydia or gonorrhoea.5 6 11–13 Vaccines offer primary prevention by preventing infection and minimising the health impacts and costs associated with STI infections and their sequelae. The World Health Organization (WHO) has developed a global roadmap for the development and implementation of STI vaccines,8 11 with one area indicating the importance of preparing in advance for STI vaccine programme implementation. This roadmap fuelled the development of this study.
With the rise of vaccine hesitancy, it is critical to understand whether vaccines designed to prevent STI infections will be acceptable to populations who would benefit. However, little evidence on perceptions and acceptability of bacterial STI vaccines currently exists. In this study, we assessed factors associated with bacterial STI vaccine interest to protect against syphilis, chlamydia and gonorrhoea infections. Our findings can ultimately help inform bacterial STI vaccine rollout by assessing factors related to preferred costs, information sources and reasons why people are motivated or hesitant to receive an STI vaccine.
Methods
Objectives
The primary objective of this study was to explore STI clinic client attitudes towards STI vaccines in Vancouver, Canada. The secondary objective was to assess STI clinic client perceptions about key programme implementation factors related to STI vaccines, such as cost and information sources.
Settings and population
The survey was available in English and administered from May to August 2018 in Vancouver, Canada in two large STI clinics. Both clinics are operated by the BC Centre for Disease Control (BCCDC), are free of charge, do not require identification or real names and do not require medical insurance coverage or a referral. Both clinics offer comprehensive testing and clinical services including: HIV counselling, testing and linkage to care; HIV PrEP and postexposure prophylaxis; STI testing, treatment, counselling and partner management and the provision of vaccines (eg, for hepatitis A and B and HPV).
Procedures
A 31-item, anonymous, paper-based questionnaire was developed and implemented at these clinics. Research questions were added to an existing survey that is routinely offered to all clinic clients. Questions were developed after reviewing the literature on vaccine perceptions, seeking feedback from experts in the field, and were grounded in the theoretical framework of the Health Belief Model.14–23 The Health Belief Model is used to explain and predict health behaviours of individuals. It is based on psychology and helps understand drivers and barriers to programme uptake, and the self-efficacy of those considering a new health intervention.23 The survey was pilot tested during the first week of data collection, and minor adjustments were made to improve clarity of some questions. Consent to participate in the study was determined by voluntary completion of the survey, which was outlined in the survey’s introduction.
Clinic clerical staff provided surveys to clients alongside appointment intake forms, who completed it in private, while waiting to see a clinician. The research team was not involved in administration or direct collection of the surveys. Once complete, respondents folded their surveys and placed them in a secure, lidded box near the administration desk. This box was emptied daily by administration staff who provided the surveys to the research team. Depending on the intent of the clinical visit, participants may not have been aware of their infection status at the time of survey completion.
Survey responses were entered into Microsoft Office Access Database (2010), exported and coded in binary in Excel 2010 (Microsoft Office) and analysed with SPSS Statistics software V.24 (IBM). Missing responses were not imputed with any value.
Survey items
Questions included demographic items, previous STI history, general interest in STI vaccines and motivators and barriers to STI vaccine interest. For full survey content, see online supplementary appendix 1.
sextrans-2019-054311supp001.pdf (213KB, pdf)
Analysis
Based on previous studies that primarily focused on viral STIs, we anticipated an average acceptance proportion of 82%.14–17 20–22 24 As such, we calculated the required sample size to detect an 80% acceptability proportion (related to objective 1) with a 5% margin of error (95% CI 77.5% to 82.5%) to be 246. We added 15% to this number to account for missing responses to the primary outcome question, for a total of 283. We felt this represented a conservative estimate based on the expected number of incomplete responses.
We assessed demographic characteristics and categorical data of overall trends using descriptive statistics. We performed bivariate analysis to assess the relationship between potential correlates and STI vaccine interest for three bacterial STIs (syphilis, chlamydia and gonorrhoea). Responses that were ‘neutral’ for STI vaccine interested were coded as ‘not interested’ for analysis. Factors deemed significant at a p≤0.05 level were included in a multivariable logistic regression (MLR) for each bacterial STI, as determined by Pearson’s χ² exact or Fisher’s exact test. A priori clinically relevant variables (age, sexual partners) and other variables deemed important from the literature (previous receipt of HPV and hepatitis B vaccines, willingness to pay out of pocket) were included in regression models. Participant gender is included with the reporting of gender of typical sex partner(s) (men who have sex with men (MSM), men who have sex with women (MSW), women who have sex with men (WSM) and women who have sex with women (WSM)). Participants who indicated they were gender variant were excluded from analysis due to small sample size.
MLR models were assessed for fit and significance using Hosmer-Lemeshow (p≥0.05) and Omnibus (p≤0.05) tests. Correlates of STI vaccine uptake were assessed using unadjusted and adjusted ORs with a 95% CI. Correlated responses were identified using Cronbach’s alpha at a 0.70 threshold.
Results
By 22 August 2018, 308 surveys were collected. Of these, three participants declined consent to participate and 12 did not complete the survey past the demographics section. Excluding these left 293 surveys for analysis (figure 1). The total number of responses for each question varied, as participants were able to skip questions. Descriptive results were analysed for demographic characteristics of respondents (table 1). Of all respondents, 71.3% (n=209) identified as male, 80.5% (n=235) had college level education or higher and 52.9% (n=144) identified as white/European descent. The median age was 33 years old. Half of respondents reported ever having an STI (49.8%), and just over half reported having access to a regular healthcare provider (52.1%). Most respondents had previously heard about two STI vaccines available, HPV (80.1%) and hepatitis B (90.6%) (table 1).
Figure 1.
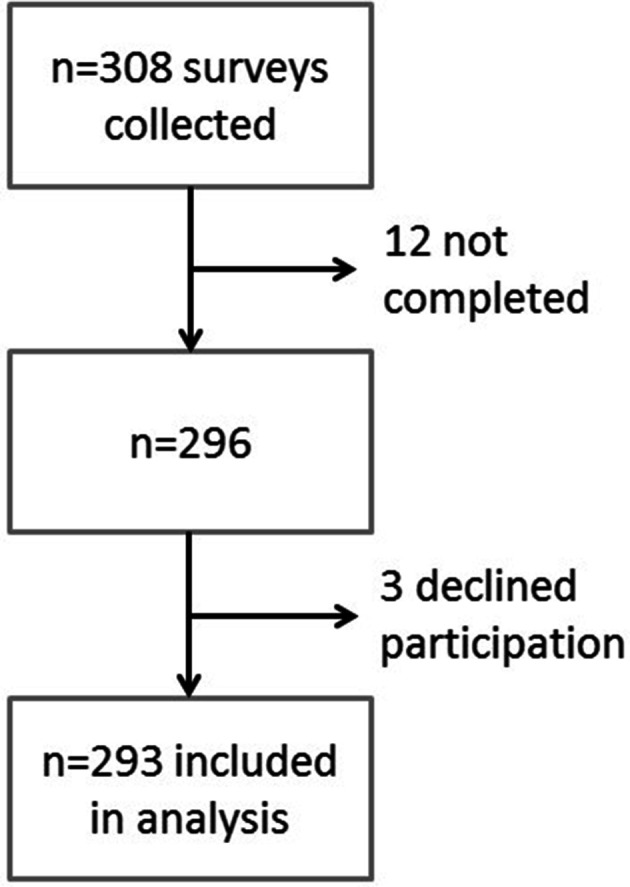
Survey response and analysis flowchart. Twelve surveys were incomplete past the initial demographic questions, and an additional three participants indicated they did not want their responses to be included in the study and thus were excluded.
Table 1.
Demographic characteristics of the survey participants
| Characteristic | N | Frequency (%) | |
| Age (median(range)) | 293 | 33 (18–71) | |
| Gender identity | Male | 293 | 209 (71.3) |
| Female | 80 (27.3) | ||
| Sexual partners—gender of typical sexual partner(s)* | MSM | 282 | 106 (37.6) |
| MSW | 96 (34.0) | ||
| WSM | 67 (23.8) | ||
| WSW | 13 (4.6) | ||
| Identify as two-spirit† | 280 | 8 (2.7) | |
| Education completed | College/ university/postgraduate | 292 | 235 (80.5) |
| High school degree | 51 (17.5) | ||
| Some high school | 6 (2.1) | ||
| Ethnicity | White, European descent | 272 | 144 (52.9) |
| Asian descent | 75 (27.6) | ||
| Central or South American | 18 (6.6) | ||
| Other, other mixed descent | 15 (5.5) | ||
| Aboriginal (including Aboriginal & White) | 11 (4.0) | ||
| African or Black | 9 (3.3) | ||
| Have had an STI ever | 289 | 144 (49.8) | |
| Number of partners in the last 6 months | 0 | 283 | 13 (4.6) |
| 1–2 | 115 (40.6) | ||
| 3–4 | 73 (25.8) | ||
| 5–9 | 48 (17.0) | ||
| 10–19 | 24 (8.5) | ||
| 20+ | 10 (3.5) | ||
| Have a regular healthcare provider or family doctor | 286 | 149 (52.1) | |
| Have heard of the HPV vaccine | 291 | 233 (80.1) | |
| Have previously received the HPV vaccine | 293 | 65 (22.2) | |
| Have heard of the hepatitis B vaccine | 288 | 261 (90.6) | |
| Have previously received the hepatitis B vaccine | 292 | 168 (57.5) | |
| Intend to engage in future in positive health behaviours‡ | 226 | 212 (93.8) | |
| Willing to pay out of pocket for an STI vaccine | 271 | 242 (89.3) | |
*Categorisation based on participant responses of their own gender identity and their typical partner(s) gender identity.
†Two spirit refers to a person who identifies as having both masculine and feminine spirit, used by some Indigenous populations in Canada.
‡Variable is aggregate of scaled agreeableness of future positive health behaviours.
HPV, human papillomavirus; MSM, men who have sex with men; MSW, men who have sex with women; STI, sexually transmitted infection; WSM, women who have sex with men; WSW, women who have sex with women.
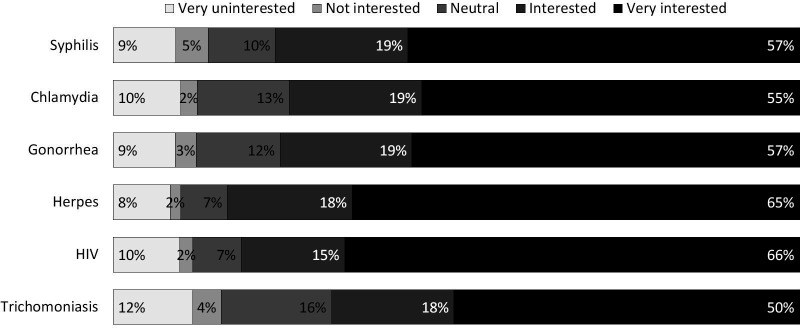
Overall, 86.5% of respondents agreed or strongly agreed they would be interested in receiving a vaccine against an STI if currently available. When asked about their interest in specific STI vaccines, 76%, 74% and 76% reported being interested or very interested in a vaccine for syphilis, chlamydia and gonorrhoea, respectively (figure 2). The most common motivating factor indicated for STI vaccine interest was the desire to protect oneself (86.3%). The most common reported barrier was cost if STI vaccines were not publicly funded (48.9%) (see online supplementary additional data). Participants were asked to indicate all reasons they would or would not be interested in an STI vaccine, where multiple answers could be selected. Overall, 97.9% indicated protecting oneself as part of any reason for being interested in an STI vaccine, and 65.0% indicated that cost would be a factor for not being interested in an STI vaccine. The second highest reported reason for being interested in STI vaccines was to protect a partner from an undiagnosed STI (78.8%), and the second most common reason for disinterest was uncertainty of the safety of a vaccine (25.1%).
Figure 2.
STI vaccine interest per infection based on a 5-item Likert scale from very uninterested to very interested. STI, sexually transmitted infection.
sextrans-2019-054311supp002.pdf (131.6KB, pdf)
Factors associated with bacterial STI vaccine interest
During the MLR model building process, seven highly correlated responses related to scaled agreement question were identified, with an acceptable inter-item reliability (Cronbach’s α=0.73). Because the introduction or removal of any of these variables shifted the magnitude and direction of coefficients in the MLR model (and therefore, the ORs), all were combined into one ‘intent to engage in future positive health behaviours’ variable for analysis. Components of this variable include continued condom use after STI vaccination, getting vaccinated to protect a future child, acceptance of others wishes to be vaccinated and continued STI screenings at the doctor’s office or STI clinic.
Independently, previous receipt of the HPV vaccine (OR=2.92, 95% CI 1.18 to 7.23, p=0.02), intent to engage in future positive health behaviours (OR=3.97, 95% CI 1.31 to 12.03, p=0.02) and willingness to pay out of pocket for an STI vaccine (OR=3.43, 95% CI 1.43 to 8.18, p=0.01) were significantly associated with an increased likelihood of syphilis vaccine interest (table 2).
Table 2.
Unadjusted and adjusted ORs for factors associated with bacterial STI vaccine interest
| Syphilis | Chlamydia | Gonorrhoea | |||||||||||||
| Unadjusted | Adjusted† (n=186) | Unadjusted | Adjusted† (n=188) | Unadjusted | Adjusted† (n=188) | ||||||||||
| N | OR (95% CI) |
P value | OR (95% CI) |
P value | N | OR (95% CI) |
P value | OR (95% CI) |
P value | N | OR (95% CI) |
P value | OR (95% CI) |
P value | |
| Age (continuous) | 251 | 0.98 (0.96 to 1.01) |
0.16 | 0.98 (0.94 to 1.02) |
0.33 | 252 | 0.97 (0.95 to 1.00) |
0.02* | 0.97 (0.94 to 1.01) |
0.19 | 253 | 0.98 (0.96 to 1.01) |
0.20 | 0.99 (0.95 to 1.03) |
0.60 |
| Gender and gender of typical sex partner (MSW—Ref) |
245 | 1.00 (1.00 to 1.00) |
0.23 | 1.00 (1.00 to 1.00) |
0.62 | 246 | 1.00 (1.00 to 1.00) |
0.03* | 1.00 (1.00 to 1.00) |
0.32 | 247 | 1.00 (1.00 to 1.00) |
0.08 | 1.00 (1.00 to 1.00) |
0.58 |
| MSM | 1.83 (0.92 to 3.65) |
0.09 | 1.38 (0.54 to 3.52) |
0.50 | 1.65 (0.86 to 3.19) |
0.13 | 1.70 (0.64 to 4.31) |
0.26 | 1.67 (0.86 to 3.24) |
0.13 | 1.74 (0.68 to 4.45) |
0.24 | |||
| WSM | 2.05 (0.90 to 4.71) |
0.09 | 1.02 (0.37 to 2.78) |
0.97 | 3.85 (1.55 to 9.59) |
0.00** | 2.53 (0.84 to 7.67) |
0.10 | 2.88 (1.20 to 6.95) |
0.02* | 1.88 (0.65 to 5.46) |
0.25 | |||
| WSW | 1.19 (0.29 to 4.87) |
0.81 | 0.45 (0.09 to 2.23) |
0.33 | 1.59 (0.40 to 6.33) |
0.51 | 0.89 (0.19 to 4.24) |
0.89 | 2.70 (0.56 to 13.05) |
0.22 | 1.58 (0.29 to 8.79) |
0.60 | |||
| Previously received HPV vaccine‡ | 252 | 2.92 (1.18 to 7.23) |
0.02* | 2.40 (0.74 to 7.78) |
0.14 | 253 | 2.02 (0.93 to 4.40) |
0.08 | 1.30 (0.42 to 4.01) |
0.65 | 254 | 1.81 (0.83 to 3.95) |
0.14 | 1.31 (0.43 to 3.97) |
0.64 |
| Previously received hepatitis B vaccine | 251 | 1.55 (0.86 to 2.78) |
0.15 | 1.15 (0.54 to 2.44) |
0.72 | 252 | 1.57 (0.89 to 2.78) |
0.12 | 1.48 (0.68 to 3.21) |
0.33 | 253 | 1.30 (0.73 to 2.31) |
0.38 | 1.19 (0.59 to 2.57) |
0.67 |
| Intent to engage in future positive health behaviours | 198 | 3.97 (1.31 to 12.03) |
0.02* | 2.82 (0.80 to 10.00) |
0.11 | 200 | 6.49 (2.01 to 20.98) |
0.00** | 5.94 (1.56 to 22.60) |
0.01** | 200 | 5.75 (1.88 to 17.64) |
0.00** | 5.13 (1.45 to 18.07) |
0.01** |
| Willingness to pay out of pocket | 236 | 3.43 (1.43 to 8.18) |
0.01** | 3.83 (1.29 to 11.38) |
0.02** | 237 | 2.98 (1.21 to 7.32) |
0.02* | 2.70 (0.80 to 9.06) |
0.11 | 238 | 2.99 (1.23 to 7.27) |
0.02* | 2.21 (0.69 to 7.14) |
0.18 |
*Significant at p≤0.05. **Significant at p≤0.01.
†Adjusted for all other variables listed.
‡In British Columbia, Canada, girls were eligible for publicly funded HPV vaccination in 2008 through the school-based immunisation programme, and boys became eligible for the same programme in 2017. The vaccine is also available for private purchase and may be covered by some insurance or subsidy programmes.
HPV, human papillomavirus; MSM, men who have sex with men; STI, sexually transmitted infection; WSM, women who have sex with men; WSW, women who have sex with women.
Being a WSM (OR=3.85, 95% CI 1.55 to 9.59, p=0.00), intent to engage in future positive health behaviours (OR=6.49, 95% CI 2.01 to 20.98, p=0.00) and willingness to pay out of pocket for an STI vaccine (OR=2.98, 95% CI 1.21 to 7.32, p=0.02) were significantly associated with an increased likelihood of chlamydia vaccine interest (table 2). The unadjusted results for age indicated that an increase in age was associated with a 3% decrease for interest in a chlamydia vaccine (OR=0.97, 95% CI 0.95 to 1.00, p=0.02).
Last, being a WSM (OR=2.88, 95% CI 1.20 to 6.95, p=0.02), intent to engage in future positive health behaviours (OR=5.75, 95% CI 1.88 to 17.64, p=0.00) and willingness to pay out of pocket for an STI vaccine (OR=2.99, 95% CI 1.23 to 7.27, p=0.02) were significantly associated with an increased likelihood of gonorrhoea vaccine interest (table 2).
After adjusting for other variables in the model, willingness to pay out of pocket for a vaccine remained a significant factor associated with syphilis vaccine interest (OR=3.83, 95% CI 1.29 to 11.38, p=0.02), whereas intent to engage in future positive health behaviours remained significant for chlamydia (OR=5.94, 95% CI 1.56 to 22.60, p=0.01) and gonorrhoea (OR=5.13, 95% CI 1.45 to 18.07, p=0.01) vaccine interest (table 2).
Discussion
The WHO has named vaccine hesitancy as one of the top ten threats to health,25 so understanding acceptability and factors associated with interest in a bacterial STI vaccines is critical for informing the future implementation of an STI vaccine programme. Results from this survey demonstrate that clients surveyed at two large STI clinics in Canada, individuals at greatest risk for STI acquisition, are receptive to STI vaccines to protect themselves from bacterial STIs including syphilis, chlamydia and gonorrhoea. These results of overall STI vaccine acceptance align with other previous research on bacterial STIs and reflect trends of acceptance in viral STI vaccines.14 15 17 19–22 24 Of those who indicated they would not be interested in receiving an STI vaccine, 76.3% agreed or strongly agreed that others should still be allowed to get vaccinated if they choose to. Based on our unadjusted and adjusted analyses, acceptability for these vaccines was comparable between categories of gender and gender of partners, except for the unadjusted model among WSM for chlamydia.
Based on previous knowledge and research, implementing an STI vaccine programme would be most effective at decreasing STI rates if it was targeted towards adolescents before sexual debut.15 24 The majority (62.8%) of respondents from this survey indicated that adolescence (ages 13–19) would be an acceptable age to first introduce STI vaccines. An additional 22.2% indicated that infancy (birth-2 years old) or childhood (3–12 years old) would be acceptable ages to first offer STI vaccines. One barrier to acceptance at these ages may be parental perceptions that an STI vaccine would increase their child’s sexual behaviour or that there is no need for an STI vaccine until closer to their child’s sexual debut.24 26 However, research findings have demonstrated that receipt of the HPV vaccine does not lead to increased sexual behaviour in adolescent girls.27 28
Some people will have concerns about STI vaccine safety, and questions about the benefits of the vaccines, as reflected by survey respondents who indicated these as possible barriers to STI vaccine interest. Vaccine hesitancy has been named a key health threat by the WHO,25 and experiences with hesitancy surrounding the HPV vaccine will likely persist for other STI vaccines. Appropriately communicating the protective benefits of STI vaccines may be useful in increasing acceptance and uptake among hesitant parents and patients.18 26 For example, presenting the HPV vaccine as a preventative tool cancer is better received than when it is presented solely for STI prevention.18 19 22 26 29 30 Comprehensive, multipronged, and well-timed communication strategies will be critical to facilitating acceptance for STI vaccines. Potential areas to explore would be to communicate benefits to sex partner(s), the potential to decrease medication use to treat recurrent infections and the risk of increasing AMR infections.
Another potential barrier to STI vaccine acceptance is the cost of STI vaccines if they are not publicly funded. While 42.7% of respondents who would be willing to pay out of pocket for a vaccine indicated they would pay more than $100 CAD, most respondents indicated cost as a barrier (65.0%). Given the early nature of current bacterial STI vaccine research, it is not yet known what administration schedules (one dose versus multiple doses, boosters) would be appropriate for STI vaccines, which will be important to consider when determining costs to patients. Administration schedules will impact how programmes plan to fund eventual STI vaccination rollout and how accepting the public would be for paying out of pocket to receive STI vaccines.
As a vehicle for STI vaccine delivery, healthcare professionals (HCPs) will be integral to successful vaccine rollout. Previous studies have shown that recommendations from a trusted HCP increases vaccine uptake.18 29 30 Similarly, 50.8% of respondents indicated that their main preference to receive information about STI vaccines would be from an HCP (physician, nurse or public health nurse). As the first line of contact for many seeking answers or treatment about sexual health, HCPs are well positioned to provide patients with information on STI vaccines once available. Conversations with HCPs will also allow for opportunities to counsel clients related to vaccine misinformation. Supporting the importance of HCPs in an STI vaccine programme, 91.8% of respondents indicated their main location preference to receive an STI vaccine would be within a clinical setting (STI clinic, 60.7%; a doctor’s clinic or practice, 19.3%; walk-in clinic, 11.9%).
Limitations
Due to the paper-based nature of this survey, respondents were able to unintentionally miss or intentionally skip questions which resulted in missing data across survey items. This led to a varied count in descriptive results, and for MLR results to have a lower sample size than the bivariate analyses. The small sample size and large CIs for some findings are limitations in the data that could be exaggerating current findings or obscuring other factors that correlate with bacterial STI vaccine interest.
This study used a convenience sample of respondents from two large STI clinics. These participants are already engaged in a certain level of health-seeking behaviour and may be more likely to be accepting of STI vaccines. Therefore, these results may not be generalisable to the overall public. The high proportion of STI vaccine acceptance among clinic clients could be due to a sampling or non-response selection bias, where clients that are unaccepting of STI vaccines (or vaccines in general) dismissed the survey as something not relevant to them. This may have biased the results to show a higher level of STI vaccine acceptance than is representative of all clinic clients.
In looking towards future implementation and marketing of STI vaccines, STI clinic clients would likely be a key target population; however, further research is required to obtain broader understandings of STI vaccine acceptance in a variety of groups including parents and adolescents, rural and suburban communities, and other key populations such as MSM.
Conclusion
To our knowledge, this is the first study that has investigated perceptions about bacterial STI vaccines within an STI clinic client population in Canada. Results from this study indicate that a majority of STI clinic clients are accepting of potentially forthcoming bacterial STI vaccines. Results also provide insight about prospective vaccination programme details such as preferred sources of information, preferred locations for vaccine administration and acceptable cost. These results can help inform further research on STI vaccine acceptance premarket, allowing for effective implementation and uptake. Areas of focus for further research must include gathering STI vaccine acceptability and perceptions from key groups and diverse populations, and further exploration of vaccine hesitancy as it relates to STI vaccines so that the positive impact of this novel biomedical prevention method for bacterial STIs is maximised.
Key messages.
The continued development of STI vaccines pose a unique prophylactic measure to address increasing STI rates in populations globally.
86% of STI clinic clients surveyed indicated they would be interested in receiving an STI vaccine for chlamydia, syphilis or gonorrhoea.
Results about preferred information sources, costs and identified barriers pose a unique opportunity to strategically plan STI vaccine programmes, prior to vaccine implementation.
Acknowledgments
We would like to thank the staff at both BCCDC STI clinics for their support and role in this study by providing the surveys to clients.
Footnotes
Handling editor: Alec Miners
Contributors: GSO and TG conceived the project. KMP, GSO, LS, HNP and TG created the survey. KMP entered and analysed data. GSO, LS and RD provided feedback and assistance with analysis. KP wrote the manuscript. GSO, LS, RD, HS and TG provided feedback on initial drafts. All authors reviewed the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study received ethics approval from the University of British Columbia Research Ethics Board (#H18-02772).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request.
References
- 1. Gottlieb SL, Low N, Newman LM, et al. . Toward global prevention of sexually transmitted infections (STIs): the need for STI vaccines. Vaccine 2014;32:1527–35. 10.1016/j.vaccine.2013.07.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization Global strategy for the prevention and control of sexually transmitted infections: 2006-2015. Geneva, Switzerland, 2007. [Google Scholar]
- 3. Centre for Communicable Diseases and Infection Control, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada Report on sexually transmitted infections in Canada: 2013-2014, 2017. [Google Scholar]
- 4. Wilton J, Kain T, Fowler S, et al. . Use of an HIV-risk screening tool to identify optimal candidates for PreP scale-up among men who have sex with men in Toronto, Canada: disconnect between objective and subjective HIV risk. J Int AIDS Soc 2016;19:20777. 10.7448/IAS.19.1.20777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wetzler LM, Feavers IM, Gray-Owen SD, et al. . Summary and Recommendations from the National Institute of Allergy and Infectious Diseases (NIAID) Workshop "Gonorrhea Vaccines: the Way Forward". Clin Vaccine Immunol 2016;23:656–63. 10.1128/CVI.00230-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rice PA, Shafer WM, Ram S, et al. . Neisseria gonorrhoeae: drug resistance, mouse models, and vaccine development. Annu Rev Microbiol 2017;71:665–86. 10.1146/annurev-micro-090816-093530 [DOI] [PubMed] [Google Scholar]
- 7. Omori R, Chemaitelly H, Althaus CL, et al. . Does infection with Chlamydia trachomatis induce long-lasting partial immunity? Insights from mathematical modelling. Sex Transm Infect 2019;95:115–21. 10.1136/sextrans-2018-053543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Broutet N, Fruth U, Deal C, et al. . Vaccines against sexually transmitted infections: the way forward. Vaccine 2014;32:1630–7. 10.1016/j.vaccine.2014.01.053 [DOI] [PubMed] [Google Scholar]
- 9. Rowley J, Vander Hoorn S, Korenromp E, et al. . Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 2019;97:548–62. 10.2471/BLT.18.228486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. BC Centre for Disease Control Sti in British Columbia: annual surveillance report 2016, 2018. [Google Scholar]
- 11. Gottlieb SL, Deal CD, Giersing B, et al. . The global roadmap for advancing development of vaccines against sexually transmitted infections: update and next steps. Vaccine 2016;34:2939–47. 10.1016/j.vaccine.2016.03.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bernstein DI, Wald A, Warren T, et al. . Therapeutic vaccine for genital herpes simplex virus-2 infection: findings from a randomized trial. J Infect Dis 2017;215:856–64. 10.1093/infdis/jix004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gottlieb SL, Johnston C. Future prospects for new vaccines against sexually transmitted infections. Curr Opin Infect Dis 2017;30:77–86. 10.1097/QCO.0000000000000343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Auslander BA, Rosenthal SL, Succop PA, et al. . Gender-Specific predictors of genital herpes vaccine acceptance in a College population. Int J STD AIDS 2005;16:27–30. 10.1258/0956462052932593 [DOI] [PubMed] [Google Scholar]
- 15. Boehner CW, Howe SR, Bernstein DI, et al. . Viral sexually transmitted disease vaccine acceptability among college students. Sex Transm Dis 2003;30:774–8. 10.1097/01.OLQ.0000078823.05041.9E [DOI] [PubMed] [Google Scholar]
- 16. Bonney LE, Rose JS, Clarke JG, et al. . Correlates of acceptance of a hypothetical gonorrhea vaccine by incarcerated women. Sex Transm Dis 2007;34:778–82. 10.1097/OLQ.0b013e31804b465b [DOI] [PubMed] [Google Scholar]
- 17. Liau A, Zimet GD. Undergraduates' perception of HIV immunization: attitudes and behaviours as determining factors. Int J STD AIDS 2000;11:445–50. 10.1258/0956462001916227 [DOI] [PubMed] [Google Scholar]
- 18. Rambout L, Tashkandi M, Hopkins L, et al. . Self-Reported barriers and facilitators to preventive human papillomavirus vaccination among adolescent girls and young women: a systematic review. Prev Med 2014;58:22–32. 10.1016/j.ypmed.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 19. Sundström K, Tran TN, Lundholm C, et al. . Acceptability of HPV vaccination among young adults aged 18-30 years--a population based survey in Sweden. Vaccine 2010;28:7492–500. 10.1016/j.vaccine.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 20. Zimet GD, Fortenberry JD, Fife KH, et al. . Acceptability of genital herpes immunization. The role of health beliefs and health behaviors. Sex Transm Dis 1997;24:555–60. 10.1097/00007435-199711000-00001 [DOI] [PubMed] [Google Scholar]
- 21. Zimet GD, Liau A, Fortenberry VD. Health beliefs and intention to get immunized for HIV. J Adolesc Health 1997;20:354–9. 10.1016/S1054-139X(97)00031-1 [DOI] [PubMed] [Google Scholar]
- 22. Zimet GD, Perkins SM, Sturm LA, et al. . Predictors of STI vaccine acceptability among parents and their adolescent children. J Adolesc Health 2005;37:179–86. 10.1016/j.jadohealth.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 23. Champion VL, Skinner CS. The Health Belief Model : Glanz K, Rimer BK, Viswanath K, Health behaviour and health education theory, research, and practice. 4th edn San Francisco, CA: John Wiley & Sons, Inc, 2008: 45–65. [Google Scholar]
- 24. Webb PM, Zimet GD, Mays R, et al. . Hiv immunization: acceptability and anticipated effects on sexual behavior among adolescents. J Adolesc Health 1999;25:320–2. 10.1016/S1054-139X(99)00066-X [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization Ten threats to global health in 2019, 2019. [Google Scholar]
- 26. Hawkes S, Kismödi E, Larson H, et al. . Vaccines to promote and protect sexual health: policy challenges and opportunities. Vaccine 2014;32:1610–5. 10.1016/j.vaccine.2013.09.039 [DOI] [PubMed] [Google Scholar]
- 27. Ogilvie GS, Phan F, Pedersen HN, et al. . Population-Level sexual behaviours in adolescent girls before and after introduction of the human papillomavirus vaccine (2003-2013). CMAJ 2018;190:E1221–6. 10.1503/cmaj.180628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donken R, Ogilvie GS, Bettinger JA, et al. . Effect of human papillomavirus vaccination on sexual behaviour among young females. Can Fam Physician 2018;64:509–13. [PMC free article] [PubMed] [Google Scholar]
- 29. Ogilvie GS, Remple VP, Marra F, et al. . Parental intention to have daughters receive the human papillomavirus vaccine. CMAJ 2007;177:1506–12. 10.1503/cmaj.071022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hofstetter AM, Rosenthal SL. Health care professional communication about STI vaccines with adolescents and parents. Vaccine 2014;32:1616–23. 10.1016/j.vaccine.2013.06.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
sextrans-2019-054311supp001.pdf (213KB, pdf)
sextrans-2019-054311supp002.pdf (131.6KB, pdf)