Abstract
The present study aimed to explore the early predictive marker of diabetic retinopathy (DR) and to elucidate the associated demographic, metabolic, and ocular factors. We enrolled 43 type 2 diabetic subjects with mild non-proliferative retinopathy (MNPDR), 30 diabetic subjects with no retinopathy (DNR), and 35 healthy controls (HC). The study groups showed no significant alteration in central macular thickness (CMT) and visual acuity (VA). The contrast sensitivity (CS) score was found to be significantly lower among DNR and MNPDR subjects compared to HCs (p < 0.0001). Between MNPDR and DNR subjects, the CS score was significantly lower in the former (p = 0.0036). CS score discriminated DNR subjects from HC, with 74% accuracy for the optimal threshold 0.71. The associated area under the ROC curve (AUC) is 0.82 (p < 0.0001) while the discrimination rule has 66% sensitivity and 80% specificity. The CS score also discriminated MNPDR subjects from DNR with 64% accuracy for the optimal threshold 0.53. The associated AUC is 0.65 (p < 0.023) and the rule has 86% sensitivity and 33% specificity. According to best subset regression analysis, not only glycaemic parameters but also lipid parameters [low-density lipoprotein cholesterol (LDL-C) (p = 0.045) and triglycerides (TG) (p = 0.0005)] were found to be significant predictors of CS. CMT (p = 0.058) was another marginally significant predictor of CS. CS may be used as an early predictive marker for DR. So, not only hyperglycemia, but also hyperlipidemia seems to significantly affect retinal CS function in diabetes.
Keywords: Ophthalmology, Visual acuity, Diabetic retinopathy, Hyperlipidemia, Central macular thickness, Contrast sensitivity
Ophthalmology; Visual acuity; Diabetic retinopathy; Hyperlipidemia; Central macular thickness; Contrast sensitivity
1. Introduction
Diabetic retinopathy (DR) is one of the most common microvascular complications in diabetics and is also a leading cause of non-traumatic visual impairment among the working-age adults in developed countries (Bourne et al., 2013). The prevalence of DR is 18% among the subjects with type 2 diabetes mellitus (T2DM) in the urban population of India and the prevalence is estimated to rise further particularly in areas that lack optimum care (Raman et al., 2009). Pathologically, DR is multifactorial with a duration of diabetes, hyperglycemia, hypertension, dyslipidemia, higher levels of urinary albumin to creatine ratio (UACR) being the most important and consistent risk factors in patients with T2DM (Lunetta et al., 1998; Lee et al., 2015).
DR can be clinically characterized into a non-proliferative (NPDR) stage and a proliferative (PDR) stage depending on the presence of ophthalmoscopically visible vascular lesions. Another important characterization in this regard is diabetic macular edema (DME) in which fluid accumulation in the retina leads to abnormal thickening and cystoid edema of the macula. DME could occur in both NPDR and PDR cases and represents the most common cause of vision loss among patients with DR (Rübsam et al., 2018).
Clinical evidence from different cross-sectional and observational studies have indicated a link between serum lipids and DR (Ucgun et al., 2007; Mathur and Mathur, 2013; Chew et al., 1996). Recent studies suggest that hyperglycemia and hyperlipidemia related biochemical derangements converge to inflammation and increased expression of vascular endothelial growth factor (VEGF), that result in retinal vascular endothelial damage by promoting vascular permeability and angiogenesis (Rübsam et al., 2018). Although the application of different anti-VEGF molecules (Pegaptanib, Ranibizumab, Bevacizumab) has provided a breakthrough in reducing DME and causing neovascular regression in combination with photocoagulation, still several limitations exist (Zhang et al., 2011). Anti-VEGF therapy requires repeated intraocular injections which may impair neuronal and vascular survival and most importantly, a good number of patients do not respond to an improvement in visual function and morphological changes (Nishijima et al., 2007; Singer et al., 2016). Therefore, investigators currently shifted their aims toward the early diagnosis of DR. Early detection may be helpful to implement effective strategies to prevent DR (Gartaganis et al., 2001). Numerous attempts have been undertaken to develop a test that predicts DR (Gartaganis et al., 2001; Ivers et al., 2001; Safi et al., 2018). With the advancement of diagnostic tools, clinicians observed that patients with DR exhibit changes in neuroretinal structure especially the thickness of the macula even before the appearance of microvascular lesions (Carpineto et al., 2016; Abrar et al., 2017). However, some studies did not find any significant alteration in macular structure between diabetic subjects and healthy individuals (Biallosterski et al., 2007; Massin et al., 2002). Recent researchers have also documented that functional neural changes begin soon after the onset of DM, which may also contribute to the pathogenic mechanism of DR (Gardner et al., 2011; Simó and Hernández, 2014). Subsequently, few studies have also reported that the changes in visual function among diabetic subjects occur before any structural abnormalities which can be detected by ophthalmoscopy or fluorescein angiography (Stavrou and Wood, 2003; Olafsdóttir and Stefánsson, 2007).
Visual function is usually routinely evaluated in the clinic in terms of visual acuity (VA). Recently researchers have shown that VA is not a sensitive measure to distinguish among the subjects with HC, DNR, and MNPDR (Stavrou and Wood, 2003). On the contrary, a group of vision researchers have shown that CS, a measure of the ability of an individual to discriminate among different shades of gray, is a more sensitive tool to detect the early retinal changes and distinguish different diabetic subgroups than VA (Dosso et al., 1998; Abrishami et al., 2007; McAnany et al., 2019). A study by Safi et al. (2017) showed that patients with diabetes (without any clinical signs of retinopathy) exhibited a uniform loss in CS at different special frequencies like 3, 6, 12, and 18 cycles/degree. Another report in this regard by Ismail and Whitaker (1998) deciphered that CS can differentiate between healthy control subjects and diabetic subjects but not within the different subgroups of DR. Due to the anomalous observations whether early neuro-retinal structural alterations precede functional anomalies and vice versa, the present study aimed to find out the early predictive marker of DR and to elucidate the demographic, metabolic and ocular factors associated with them.
2. Methods
2.1. Study population
43 subjects with mild non-proliferative diabetic retinopathy (MNPDR), 30 age and gender-matched diabetic subjects without clinically evident retinopathy (DNR), and 35 age and gender-matched healthy controls (HCs) were enrolled in the present cross-sectional study. Subjects with known coronary artery disease, hypertension, (systolic BP > 140 mm Hg and or diastolic BP > 90 mm Hg, or on antihypertensive medication), neuropathy (as evaluated by Michigan Neuropathy Screening Instrument), nephropathy (serum creatinine level >1.5 mg/dl and or urinary albumin creatinine ratio ≥300μg/mg), severe deficiency of B vitamins (such as thiamin, folic acid, cobalamin) any other ocular diseases (glaucoma, cataract, optic neuropathy, branch retinal vein occlusion, and Eales disease) were excluded from the present study.
The subjects were attendees of the ‘Retina Clinic’ of ‘Regional Institute of Ophthalmology’, Calcutta Medical College, Kolkata, India. Control subjects were chosen from amongst accompanied nondiabetic relatives of patients attending the ‘Retina Clinic’. The study was approved by the institutional ethical committee and informed consent was collected from all the patients according to the declaration of Helsinki.
Patients with type 2 DM were diagnosed based on the guideline of the American Diabetes Association (2010). Glycemic status was assessed by fasting plasma glucose (FPG), postprandial plasma glucose (PPG), and glycated hemoglobin (HbA1c %) level. None of the enrolled subjects in this study were taking insulin or lipid-lowering drugs during the period of study.
2.2. Ophthalmological examinations
All the study subjects who had undergone detailed ophthalmological examinations included slit-lamp biomicroscopy (by ± 90 diopters and Goldman 3 mirror lens), seven fields digital fundus photography with fluorescein angiography, and spectral-domain optical coherence tomography (SD-OCT). Visual functions were also evaluated by measuring VA and CS. The subjects with MNPDR were diagnosed according to the modified guideline of ‘Early Treatment of Diabetic Retinopathy Study’ (ETDRS), (1991).
2.2.1. Measurement of macular thickness
Macular thickness was measured by SD-OCT [Heidelberg, Germany; Model: Heidelberg Spectralis OCT/HRA + OCT/FA + OCT/OCT plus, Software: Heidelberg Eye Explorer, Version-1.6.4.0] using the 3D macular protocol. It consists of a raster scan, composed of a 20ᵒ×20ᵒ volume scan at high speed with a 6/frame rate and an axial resolution of 6μm covering an area of 6 × 6 mm in the macular region. It reconstitutes a topographic image and is displayed with a numeric average of thickness measurements for 9 map regions with a 6 × 6 mm area centered on the fovea as defined by ETDRS, (1991). According to the ETDRS map, the macula is divided into 9 regions with 3 concentric rings. The innermost ring of 1mm diameter representing the foveal region or central macular region and the other two rings (3mm and 6 mm diameter respectively) represent different sub-foveal regions. In the present study, we have used only the thickness of the central foveal region as CMT.
2.2.2. Measurement of VA
Best-corrected VA was recorded by a standard back-illuminated (luminance 85 cd/m2) multi-letter Snellen's chart at a viewing distance of 6m. The test was performed in a standardized low light condition. Each subject was asked to start reading with one eye occluded from the top of the chart, row-by-row up to down as far as possible. The score was recorded in Snellen fraction notation and then the fraction was transformed to the logarithmic unit (log MAR) for statistical analysis.
2.2.3. Measurement of CS
The visual CS of each subject was measured by the ‘Rabin Contrast Sensitivity Test’, using a standard illuminator cabinet [(luminance 170 cd/m2), (Precision Vision®, La Salle, IL, USA)] at a viewing distance of 4m. The letter size of the test chart was 20/50. Each line on the Rabin CS test was a lower contrast than the one before it, using Log CS steps. Log CS increased 0.25 steps per row (0.05 Log CS/letter). The chart included eight separate contrast levels, which help to identify the most precise and true CS of a subject.
This test was administered with the best optical correction in a dark room with only the illuminator box light turned on. The subject was asked to cover one eye with his hand and start reading letters from left to right and to continue row-by-row, down the chart as far as possible. The log CS score was recorded according to the last row which an individual was able to read correctly. The same procedure was repeated for another eye and the worse value between two eyes was considered as the CS score of the target person. This approach was followed uniformly for each subject in all study groups as we attempted to find out an early predictive marker for DR. A log CS value of less than 1.30 was below the normative threshold.
2.3. Sample collection and biochemical assessments
After overnight fast 6 ml of the venous blood sample was collected from each study subject in ethylenediaminetetraaceticacid (EDTA) vacutainers. 50 μl of whole blood was used for HbA1c estimation. The remaining blood sample was centrifuged at 3000 r.p.m for 10 min to separate plasma from cellular components. The plasma sample was collected in cryotubes and further used for the estimation of glucose and lipid profile components.
2.3.1. Measurement of plasma glucose level
Plasma glucose level was measured by the colorimetric endpoint test method using a commercially available kit (Labcare Diagnostics (India) Pvt. Ltd., Mumbai, India).
2.3.2. Measurement of glycosylated hemoglobin (HbA1c %)
HbA1c % was measured by an ion exchange resin method using a commercially available kit [Labcare Diagnostics (India) Pvt. Ltd., Mumbai, India].
2.3.3. Estimation of lipid profile components
The conventional lipid profile components like plasma total cholesterol (TCH), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) levels were estimated by CHOD/PAP, PEG/CHOD-PAP, and GPO/PAP methods respectively using commercially available kits (Coral Clinical Systems, Goa, India). The low-density lipoprotein cholesterol (LDL-C) was estimated using Fridewald's formula [LDL-C = TCH-(HDL-C)-TG/5] (Friedewald et al., 1972).
2.4. Statistical analysis
For the different study groups, observations were summarized by their means, associated standard errors, and using box-whisker plots. To compare all the study groups, one-way analysis of variance (ANOVA) is carried out followed by Tukey's multiple (group-wise) comparisons. The associated p-values are multiplicity-adjusted following Tukey's HSD test. Based on these findings, linear discrimination analysis (LDA) was performed with the CS score to discriminate DNR from HC subjects and subsequently MNPDR from DNR subjects. The sensitivity and specificity of the LDA were evaluated by ROC curve analysis. Taking the DNR and MNPDR subjects together, the important factors determining the CS score were identified by the best subset selection algorithm. A diagnostic test is performed to validate the results. The statistical analyses were carried out using R.
3. Results
As shown in Table 1, different study groups enrolled in the present study showed no statistically significant differences for gender distributions, age, duration of diabetes, body mass index (BMI), systolic and diastolic blood pressure. Fasting (FPG) and postprandial (PPG) plasma glucose levels were found to be increased significantly in the DNR group (p < 0.0001) and MNPDR group (p < 0.0001) compared to the HC group. The level of HbA1c (%) was also found to be higher among DNR (p < 0.0001) and MNPDR (p < 0.0001) subjects compared with those chosen as HC. Further, statistical analysis showed no significant differences in FPG, PPG levels, and HbA1c between DNR and MNPDR subjects.
Table 1.
Demographic and clinical characteristics of study subjects.
| Parameters | HC (N = 35) | DNR (N = 30) | MNPDR (43) | P value | |
|---|---|---|---|---|---|
| Gender | Male | 19 (54.28%) | 18 (60%) | 24 (55.81%) | 0.892 |
| Female | 16 (45.71%) | 12 (40%) | 19 (44.18%) | ||
| Age (Years) | 50.57 ± 1.24 | 52.67 ± 1.32 | 52.79 ± 1.28 | 0.402 | |
| Duration of DM (Years) | ------ | 10.50 ± 1.04 | 11.33 ± 0.74 | 0.510 | |
| BMI (kg/m2) | 23.74 ± 0.55 | 24.45 ± 0.56 | 24.14 ± 0.61 | 0.723 | |
| Blood pressure (mm Hg) | Systolic | 125.20 ± 0.84 | 126.80 ± 1.36 | 127.20 ± 1.00 | 0.303 |
| Diastolic | 79.66 ± 0.86 | 80.93 ± 0.97 | 81.16 ± 0.79 | 0.418 | |
| Plasma glucose level (mg/dl) | FPG | 87.94 ± 1.44 | 152.00 ± 2.80¥ | 157.5 ± 6.81† | <0.0001 |
| PPG | 119.8 ± 1.69 | 191.10 ± 7.16¥ | 202.50 ± 8.44† | <0.0001 | |
| HbA1c (%) | 4.84 ± 0.06 | 7.75 ± 0.21¥ | 8.13 ± 0.18† | <0.0001 | |
HC, healthy control; DNR, diabetic subjects without retinopathy; MNPDR, mild nonproliferative diabetic retinopathy; DM, diabetes mellitus; BMI, body mass index; FPG, fasting plasma glucose, PPG, postprandial plasma glucose; HbA1c, glycosylated hemoglobin.
Comparison of different study groups enrolled in the present study showed no statistically significant difference in gender distribution, age, duration of DM, BMI, systolic and diastolic blood pressure. The FPG, PPG and HbA1c were found to be higher among DNR and MNPDR subjects compared to HC subjects. There was no statistically significant difference observed in FPG, PPG and HbA1c level between DNR and MNPDR subjects. Data were presented as mean ± standard error of means. Yen Sign (¥) indicates p value < 0.0001 between HC vs. DNR and Dagger (†) indicates p value < 0.0001 between HC vs. MNPDR.
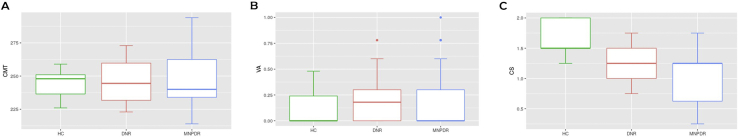
3.1. CMT, VA, and visual CS scores of different study groups
With regard to CMT level, no statistically significant difference was observed among HC (245 ± 1.47 μm), DNR (247.5 ± 2.97 μm), and MNPDR (248.6 ± 3.09 μm) subjects (p = 0.607) (Figure 1A). VA scores of HC (0.13 ± 0.02), DNR (0.19 ± 0.03) and MNPDR (0.21 ± 0.04) subjects also showed no statistically significant difference (p = 0.295) (Figure 1B). However, visual CS score was significantly lower in MNPDR (0.97 ± 0.06 vs. 1.65 ± 0.04; p < 0.0001) and DNR (1.25 ± 0.048 vs. 1.65 ± 0.04; p < 0.0001) subjects compared with HC individuals. Yet again, visual CS score was significantly lower in MNPDR subjects compared with DNR individuals (p = 0.0036) (Figure 1C).
Figure 1.
CMT, VA and visual CS score of different study group. CMT, central macular thickness; VA, visual acuity; CS, contrast sensitivity., HC, healthy control subjects; DNR, diabetic subjects without retinopathy; MNPDR, mild nonproliferative diabetic retinopathy. Box-and-whisker plots A, B, C represent the median and minimum to maximum range of CMT, VA and CS score respectively. (A) Comparison of CMT score among the study groups showed no statistically significant differences (p = 0.607) (B) Similarly, comparison of VA score among the study groups showed no statistically significant differences (p = 0.295). (C) The CS score was found to be significantly lower among DNR and MNPDR subjects compared to HC subjects (HC vs DNR at p < 0.0001, HC vs MNPDR at p < 0.0001) and MNPDR subjects than DNR subjects (p = 0.0036) respectively.
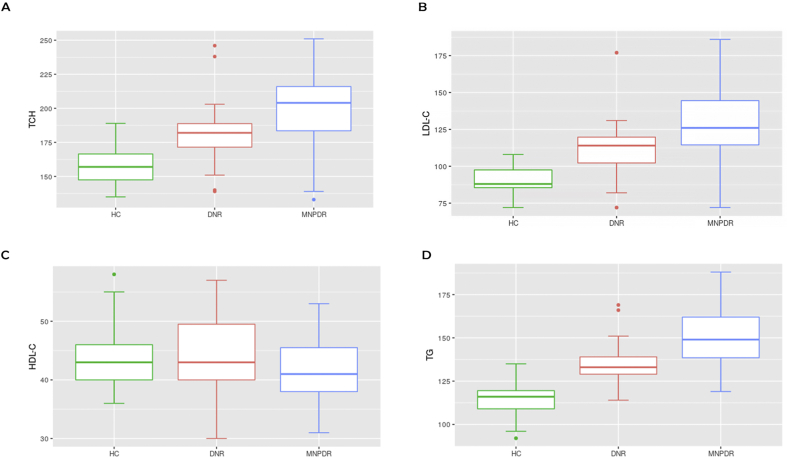
3.2. Plasma level of different lipid components of the study groups
Plasma TCH level was found to be increased significantly among MNPDR (199.7 ± 3.88 mg/dl, p < 0.0001) and DNR subjects (181.2 ± 4.24 mg/dl, p < 0.0001) compared to HC (157 ± 2.12 mg/dl). Moreover, MNPDR subjects showed significantly higher (p = 0.00128) TCH level compared to DNR subjects (Figure 2A). Plasma LDL-C level was found to be increased significantly among MNPDR (128.9 ± 3.40 mg/dl, p < 0.00001) and DNR (111.5 ± 3.42 mg/dl, p = 0.000015) subjects compared to HC (90.29 ± 1.36 mg/dl). Further, MNPDR subjects showed higher LDL-C compared to DNR subjects (p = 0.00022) (Figure 2B). The MNPDR (150.6 ± 2.57, p < 0.000001) and DNR (134.6 ± 2.29 mg/dl, p < 0.000001) subjects also had significantly elevated plasma TG level compared to HC (113.9 ± 1.52 mg/dl) subjects. The DNR subjects also showed a lower TG level compared to MNPDR subjects (p = 0.000008) (Fig: 2D). Plasma HDL-C level was not found to be altered significantly among HC (43.51 ± 0.83 mg/dl), DNR (43.40 ± 1.35 mg/dl) and MNPDR subjects (42.07 ± 0.95 mg/dl) (p = 0.5257) (Figure 2C).
Figure 2.
Plasma level of different lipid components among the study groups. TCH, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; HC, healthy control subjects; DNR, diabetic subjects without retinopathy; MNPDR, mild nonproliferative diabetic retinopathy. Box and Whisker plots of A,B,C,D represent median and minimum to maximum range of plasma TCH, LDL-C, HDL-C and TG levels of HC, DNR and MNPDR subjects respectively. (A) The TCH level was found to be higher significantly among DNR and MNPDR subjects than HC subjects (HC vs DNR at p < 0.0001, HC vs MNPDR at p < 0.0001) and MNPDR subjects than the DNR subjects (p = 0.00128). (B) The LDL-C level was also found to be higher significantly among DNR and MNPDR subjects than HC subjects (HC vs DNR at p = 000015, HC vs MNPDR at p < 0.000001) and MNPDR subjects than the DNR subjects (p = 0.00022) respectively. (C) Study showed no significant difference in HDL-C level among the groups (p = 0.5257). (D) Plasma TG level was found to be higher significantly among DNR and MNPDR subjects compared to HC subjects (HC vs DNR at p < 0.000001, HC vs MNPDR at p < 0.000001) and MNPDR subjects than the DNR subjects (p = 000008).
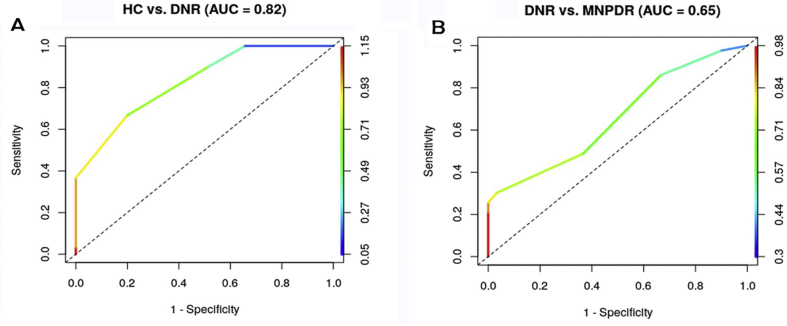
3.3. Discrimination analysis of CS
Linear discrimination analysis showed that CS score discriminated DNR subjects from HC, with 74% accuracy for the optimal threshold 0.71. The associated area under the ROC curve (AUC) is 0.82 [95% CI (0.73–0.92), p < 0.0001] (Figure 3A) while the discrimination rule has 66% sensitivity and 80% specificity. The CS score also discriminated MNPDR subjects from DNR with 64% accuracy for the optimal threshold 0.53. The associated AUC is 0.65 [95% CI (0.53–0.78), p < 0.023] and the rule has 86% sensitivity and 33% specificity (Figure 3B).
Figure 3.
ROC for CS. Linear discriminant analysis showed that CS score discriminated DNR subjects from HC, with 74% accuracy for the optimal threshold 0.71. The associated area under the ROC curve (AUC) is 0.82, while the discrimination rule has 66% sensitivity and 80% specificity (Fig 3A). The CS score also discriminated MNPDR subjects from DNR with 64% accuracy for the optimal threshold 0.53. The associated AUC is 0.65 and the rule has 86% sensitivity and 33% specificity (Fig 3B).
3.4. Best subset selection for predictors of CS
The important factors of CS score for the MNPDR and DNR subjects were identified by the best subset selection algorithm for multiple linear regression with 12 predictors. Table 2 shows 6 best predictors are explaining 58% (Adjusted R-square 54%) of the variability in the CS score. Among these 6 predictors, HbA1c and TG are highly significant; LDL-C is significant while CMT and PPG are only marginally significant. The study also showed that, for every 1% increase in HbA1c, there is a decrease in CS of the affected eye by 0.12 units (95% CI indicates a decrease by 0.18 to 0.065 units, p = 4.7 × 10−5). Similarly, for every 1 mg/dl increase in TG, there is a decrease in CS value by 0.008 units (95% CI indicates a decrease by 0.013 to 0.004 units, p = 0.000524). Next, for every 1 mg/dl increase in LDL-C level, there is a decrease in CS value by 0.003 units (95% CI indicates decrease by 0.006–7 × 10−5 units, p = 0.0454). Also, for every 1 μm increase in CMT, there is a decrease in CS by 0.003 units (95% CI indicates a decrease by 0.007 to 0.0001 units, p = 0.058). Further, for every 1 mg/dl increase in PPG, there is a decrease in CS value by 0.001 units (95% CI indicates a decrease by 0.003 to 0.0001 units, p = 0.0684). However, for every 1 Kg/m2 increase in BMI, CS decreases by 0.01 unit, though the variation in CS is not significant (p = 0.220). Shapiro-Wilk test for normality confirmed that the residuals do not deviate from normal distribution significantly (p = 0.3985).
Table 2.
Best subset selection for predictors of CS.
| Estimate | Std error | t value | p value | LCI | UCI | |
|---|---|---|---|---|---|---|
| Intercept | 5.0537094 | 0.5240068 | 9.644 | 3.16 × 10−14 | 4.0075 | 6.0999 |
| BMI | -0.0110452 | 0.0089314 | -1.237 | 0.220590 | -0.0289 | 0.0068 |
| PPG | -0.0013064 | 0.0007052 | -1.853 | 0.068425 | -0.0027 | 0.0001 |
| HbA1c | -0.1208589 | 0.0277353 | -4.358 | 4.70 × 10−5 | -0.1762 | -0.0655 |
| LDL-C | -0.0033050 | 0.0016208 | -2.039 | 0.045441 | -0.0065 | -6.9 × 10−5 |
| TG | -0.0082280 | 0.0022558 | -3.647 | 0.000524 | -0.0127 | -0.0037 |
| CMT | -0.0035583 | 0.0018447 | -1.929 | 0.058044 | -0.0072 | 0.0001 |
BMI, body mass index; PPG, postprandial plasma glucose, HbA1c, glycosylated hemoglobin; LDL-C, low density lipoprotein cholesterol, HDL-C, high density lipoprotein cholesterol; TG, triglycerides, CMT, central macular thickness.
In best subset selection algorithm for multiple linear regression, HbA1c and TG are highly significant, LDL-C is significant while CMT and PPG are only marginally significant predictors of contrast sensitivity (CS) function for MNPDR and DNR subjects.
4. Discussion
Over the last two decades, extensive evidence has shown that DM affects the entire neurovascular unit of the retina, not merely the microvasculature (Gardner et al., 2011; Gardner and Davila., 2017). The integrity of neuroretina is not readily determined by ophthalmoscopic examination in the absence of features of DR but can be evaluated through functional testing and imaging studies. To determine the effect of diabetes on inner and outer retinal function in persons with DNR or with NPDR, Jackson et al. (2012) assessed visual function by contrast sensitivity, frequency doubling technology (FDT) sensitivity, acuity, dark adaptation, light-adapted visual sensitivity and dark-adapted visual sensitivity. They reported that both inner and outer retinal functions exhibited impairment related to NPDR. Inner retinal function measured by FDT perimetry was the most impaired visual function for patients with NPDR, with 83 % of patients exhibiting clinically significant impairment. Their study concluded that FDT perimetry and other visual function tests can reveal an expanded range of diabetes-induced retinal damage even in patients with good visual acuity.
In a landmark study, Joltikov et al. (2017) demonstrated neuroretinal impairment in early diabetic retinopathy by multidimensional functional and structural evaluation with e-ETDRS acuity, the quick contrast sensitivity function (qCSF), short-wavelength automated perimetry (SWAP), standard automated perimetry (SAP), frequency doubling perimetry (FDP), rarebit perimetry (RBP) and spectral-domain optical coherence tomography (SD-OCT). Their group found the following results: ETDRS acuity and RBP were not sensitive for functional differences among subjects with diabetes, whereas qCSF was reduced in diabetics with moderate NPDR compared to mild NPDR, and in subjects with no DR compared to controls. SWAP and SAP mean deviation (MD) and foveal threshold (FT) were reduced in moderate NPDR compared to mild NPDR. FDP 10-2 showed reduced MD in moderate NPDR compared to mild NPDR whereas FDP 24-2 revealed reduced pattern standard deviation (PSD) in mild NPDR compared to no DR. Structural analysis revealed thinning of the ganglion cell layer and the inner plexiform layer of moderate NPDR subjects compared to controls.
Another important study by Stem et al. (2016) revealed the relation between glucose variability and inner retinal sensory neuropathy in persons with type 1 diabetes mellitus with the assessment of retinal function and structure by frequency doubling perimetry (FDP), contrast sensitivity, dark adaptation and optical coherence tomography (OCT). This group of investigators demonstrated reduced log CS in diabetic subjects compared to controls and thinning of the inner temporal inner nuclear layer in OCT imaging of patients with type 1 DM without retinopathy and with mild or moderate NPDR. This study highlighted those patients with type 1 DM with no DR to moderate DR exhibit alterations in inner retinal structure and function and increased correlation between increased glycemic variability and retinal thinning (Stem et al., 2016).
A very recent work of Joltikov et al. (2018) elucidated the relationship between disorganization of retinal inner layers (DRILs) and retinal function in diabetic patients without diabetic retinopathy (DR) and with nonproliferative DR, but without diabetic macular edema (DME), using ETDRS visual acuity, the quick contrast sensitivity function, short-wavelength automated perimetry (SWAP), standard automated perimetry (SAP), and frequency doubling perimetry (FDP). They identified that DRIL subjects had higher body mass index and longer diabetes duration compared to diabetic subjects without DRIL. Subjects with DRIL had reduced ETDRS visual acuity, contrast sensitivity function, and SAP performance compared to controls and diabetic subjects without DRIL. Structural analysis revealed inner retinal thinning, and some outer retinal thinning, associated with DRIL.
This cross-sectional comparative study observed that decreased CS function was associated with DNR and MNPDR without significant alteration of CMT and VA. Additionally, visual CS function was able to discriminate significantly the DNR subjects from HC, and MNPDR subjects from DNR. These findings are in line with a study by Massin et al. (2002) showing no appreciable alterations in CMT among HC, DNR, and MNPDR subjects. Similarly, another study by Stavrou and Wood (2003) reported changes in CS function among diabetics without alteration of VA. Impairment of visual function occurs earlier than morphological changes in the retina of diabetic patients (Abcouwer and Gardner, 2014). In diabetic patients, persistent hyperglycemia causes apoptosis of neural and vascular cells in the retina before the appearance of striking features of retinopathy in form of microaneurysms, hemorrhage, exudates, and macular edema (Simó and Hernández, 2014; Galvao et al., 2015). As the neurovascular cell loss in diabetic individuals is insidious, the consequences of ongoing cell death are very difficult to detect especially at this early stage. However, compromised neural functions may give some evidence in advance of apoptosis contributing to early deterioration of vision. Although VA is the most widely used indicator of visual function; it might not be sensitive enough to distinguish between diabetic subgroups (Stavrou and Wood, 2003). On the other hand, CS suggested to be produced from neural processing within the inner part of the retina, could be a more sensitive indicator of retinal function as demonstrated by several studies (Dosso et al., 1998; Abrishami et al., 2007; Nasralah et al., 2013).
Biochemical parameters analyzed in our study showed an increased level of FPG, PPG, and HbA1c among DNR and MNPDR subjects compared to HC individuals. Additionally, HbA1c (highly significant) and PPG (marginally significant) were found to be the predictors of CS change among diabetic subjects. A report by Misra et al. (2010) suggested that CS function decline in DR to the levels of HbA1c. In another report, Noticewala and Shastri (2017) demonstrated that CS function decreases as metabolic control of blood sugar levels fluctuate. Higher plasma glucose and HbA1c levels indicate that there might be some deregulation of glycolysis in retinal tissues. A prolonged and faster rate of glycolysis exhaust the supply of NAD+, resulting in a condition of pseudo hypoxia and increased concentration of lactate, which reversibly lowers cellular pH and cessation of activity of glycolytic enzymes. Less production of adenosine triphosphate (ATP) impairs the autoregulatory systems of retinal microcirculation and the function of highly metabolically active cone receptors. Insidious apoptosis of neural and microvascular cells on one side and dysregulated microcirculation on the other side, converge to result in retinal ischemia, which is liable to impair the most sensitive visual function namely, CS (Mondal et al., 2018).
Apart from the hyperglycemic event, hyperlipidemia has also been proposed to play an important role in the pathogenesis of DR. In hyperlipidemia, high lipid levels reduce the bioavailability of nitric oxide and result in endothelial dysfunction among diabetic patients (Cetin et al., 2013). Moreover, recent studies have shown that hyperlipidemia leads to increased production of advanced lipoxidation end products (ALEs) which result in dysfunction of the retinal neuronal cell (Curtis et al., 2011; Onorato et al., 2000). In the present study, increased levels of TCH, LDL-C, and TG were found among MNPDR and DNR compared to HC. Further higher levels of TCH, LDL-C, and TG were found in MNPDR patients compared to DNR. Our results are in conformity with a study by Choudhuri et al. (2017) showing the alteration of TCH, LDL-C, and TG levels among DNR and MNPDR subjects compared to HC. Besides, another study by Tolonen et al. (2013) showed a higher level of TCH, LDL-C, and TG in patients with mild or moderate to severe NPDR compared with those without DR. But the interesting finding of this study was to find out LDL-C and TG levels as significant predictors of CS among diabetic subjects. Previous studies suggested that increased levels of LDL-C pass through the blood-retinal barrier in the diabetic retina when compared to non-diabetic conditions (Du et al., 2013). Researchers also documented that in addition to increased LDL-C uptake through the retinal pigment epithelial (RPE) layer, increased permeability of retinal capillaries was found to cause extravasations of plasma lipoproteins (Wu et al., 2008; Wu and Lyons, 2011). Müller cells and the inner segments of photoreceptors have been shown to aid in the synthesis of local cholesterol (Zheng et al., 2012; Pikuleva and Curcio, 2014). Moreover, unlike other organs, the retina relies on active processes to remove cholesterol (Omarova et al., 2012). Obstruction of pathways that control cholesterol metabolism can cause retinal cholesterol accumulation which affects normal retinal function.
On the other hand, higher TG levels in diabetic patients have been reported to alter membrane fluidity and permeability that lead to hemorrhage and edema (Ucgun et al., 2007). We suspect that, at the early stage of DM, before severe vision loss occurs due to hard exudates or macular edema, lipid-derived increased formation of ALEs affects CS by alteration of neuroretinal function.
The relationship between macular thickness and contrast sensitivity score, the two crucial anatomical and functional parameters of vision, is very important. In the present study, though the change of CMT was found to be statistically not significant among the groups, it appeared to be a marginally significant predictor of CS change of the study population. This finding suggests that change of the macular structure at DNR or MNPDR with respect to HC is very minor but it affects the visual CS function at a greater level. It can be suggested from our findings that strict control of hyperglycemia and hyperlipidemia may prevent these early anatomical and functional deficits in T2 DM with or without retinopathy.
Apart from the valuable findings, our study had some limitations, which are important to mention. First, the sample size for each subgroup of the study was relatively small. Validation with a larger sample size about the loss of visual function of each sub-group, especially in DNR and MNPDR groups might be more informative. Secondly, in the study, the visual function was assessed in terms of VA and CS. There are some other psychophysiological tests, such as color vision, dark adaptation, which are recently used to investigate the status of visual function (Nasralah et al., 2013). So, further study with the inclusion of all potential parameters along with VA and CS is required to detect the early loss of visual function among diabetic subjects with or without clinically evident retinopathy.
5. Conclusions
Our study demonstrates an early loss of CS in diabetic subjects even before the clinical onset of retinopathy. Subsequently, CS was able to discriminate significantly DNR subjects from HC, and MNPDR from DNR subjects. Therefore, CS may be used for early monitoring of retinal function in diabetes and also can be used as an early predictive marker for DR. Besides, our study observed that not only chronic hyperglycemia but also hyperlipidemia significantly affected CS function. This may have therapeutic implications in disease management.
Declarations
Author contribution statement
Subhasish Pramanik: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Subhankar Chowdhury: Conceived and designed the experiments; Wrote the paper.
Upasana Ganguly, Anindita Banerjee: Analyzed and interpreted the data; Wrote the paper.
Basudev Bhattacharya: Conceived and designed the experiments.
Lakshmi Kanta Mondal: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to Kaustav Banerjee, Decision Sciences Area, Indian Institute of Management, Lucknow, UP, India, for assistance with revision and editing of the manuscript and correction of statistical analysis.
References
- Abcouwer S.F., Gardner T.W. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann. N. Y. Acad. Sci. 2014;1311:174–190. doi: 10.1111/nyas.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrar F., Rastogi P.S., Ansari M. Central macular thickness in diabetic retinopathy- A comprehensive study. Ann. Int. Med. Dent. Res. 2017;3(2):1–4. [Google Scholar]
- Abrishami M., Heravian J., Derakhshan A., Mousavi M., Banaee T., Daneshvar R. Abnormal Cambridge low-contrast grating sensitivity results associated with diabetic retinopathy as a potential screening tool. East. Mediterr. Health J. 2007;13(4):810–818. [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Supplement 1):S62–69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biallosterski C., van Velthoven M.E.J., Michelis R.P.J., deVries R.O.S.J.H., Verbraak F.D. Decreased optical coherence tomography-measured pericentral retinal thickness in patients with diabetes mellitus type 1 with minimal diabetic retinopathy. Br. J. Ophthalmol. 2007;91(9):1135–1138. doi: 10.1136/bjo.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne R.R., Stevens G.A., White R.A., Smith J.L., Flaxman S.R., Price H. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob. Health. 2013;1(6):339–349. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- Carpineto P., Toto L., Aloia R., Ciciarelli V., Borrelli E., Vitacolonna E. Neuroretinal alterations in the early stages of diabetic retinopathy in patients with type 2 diabetes mellitus. Eye. 2016;30(5):673–679. doi: 10.1038/eye.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin E.N., Balgu Y., Ozdemir S., Topsakal S., Akin F., Aybek H. Association of serum lipid levels with diabetic retinopathy. Int. J. Ophthalmol. 2013;6(3):346–349. doi: 10.3980/j.issn.2222-3959.2013.03.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew E.Y., Klein M.L., Ferris F.L., Remaley N.A., Murphy R.P., Chantry K. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) report 22. Arch. Ophthalmol. 1996;114(9):1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- Choudhuri S., Roy P.K., Mitra B., Sen S., Sarkar R. Hyperlipidemia- mediated increased advanced lipoxidation end products formation, an important factor associated with decreased erythrocyte glucose-6-phosphate dehydrogenase activity in mild nonproliferative diabetic retinopathy. Can. J. Diabetes. 2017;41(1):82–89. doi: 10.1016/j.jcjd.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Curtis T.M., Hamilton R., Yong P.H., McVicar C., Berner A., Pringle R. Müller glial dysfunction during diabetic retinopathy in rats is linked to accumulation of advanced glycation end-products and advanced lipoxidation end-products. Diabetologia. 2011;54(3):690–698. doi: 10.1007/s00125-010-1971-x. [DOI] [PubMed] [Google Scholar]
- Dosso A.A., Yenice-Ustun F., Sommerhalder J., Golay A., Morel Y., Leuenberger P.M. Contrast sensitivity in obese dyslipidemic patients with insulin resistance. Arch. Ophthalmol. 1998;116:1316–1320. doi: 10.1001/archopht.116.10.1316. [DOI] [PubMed] [Google Scholar]
- Du M., Wu M., Fu D., Yang S., Chen J. Effects of modified LDL and HDL on retinal pigment epithelial cells: a role in diabetic retinopathy? Diabetologia. 2013;56(10):2318–2328. doi: 10.1007/s00125-013-2986-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs– an extension of the modified Airlie House classification. Ophthalmology. 1991;98(Supplement 5):786–806. [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- Galvao J., Elvas F., Martins T., Cordeiro M.F., Ambrósio A.F., Santiago A.R. Adenosine A3 receptor activation is neuroprotective against retinal neurodegeneration. Exp. Eye Res. 2015;140:65–74. doi: 10.1016/j.exer.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Gardner T.W., Davila J.R. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017;255:1–6. doi: 10.1007/s00417-016-3548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner T.W., Abcouwer S.F., Barber A.J., Jackson G.R. An integrated approach to diabetic retinopathy research. Arch. Ophthalmol. 2011;129(2):230–235. doi: 10.1001/archophthalmol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartaganis S.P., Psyrojannis A.J., Koliopoulos X.J., Mela E.K. Contrast sensitivity function in patients with impaired oral glucose tolerance test. Optom. Vis. Sci. 2001;78(3):157–161. doi: 10.1097/00006324-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Ismail G.M., Whitaker D. Early detection of changes in visual function in diabetes mellitus. Ophthalmic Physiol. Optic. 1998;18(1):3–12. [PubMed] [Google Scholar]
- Ivers R.Q., Optom B., Macaskill P., Cumming R.G., Mitchell P. Sensitivity and specificity of tests to detect eye disease in an older population. Ophthalmology. 2001;108(5):968–975. doi: 10.1016/s0161-6420(00)00649-7. [DOI] [PubMed] [Google Scholar]
- Jackson G.R., Scott I.U., Quillen D.A., Walter L.E., Gardner T.W. Inner retinal visual dysfunction is a sensitive marker of non-proliferative diabetic retinopathy. Br. J. Ophthalmol. 2012;96(5):699–703. doi: 10.1136/bjophthalmol-2011-300467. [DOI] [PubMed] [Google Scholar]
- Joltikov K.A., de Castro V.M., Davila J.R., Anand R., Khan S.M. Multidimensional functional and structural evaluation reveals neuroretinal impairment in early diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2017;58(6):BIO277–BIO290. doi: 10.1167/iovs.17-21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joltikov K.A., Sesi C.A., de Castro V.M., Davila J.R., Anand R. Disorganization of retinal inner layers (DRIL) and neuroretinal dysfunction in early diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2018;59(13):5481–5486. doi: 10.1167/iovs.18-24955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R., Wong T.Y., Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vision. 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunetta M., Infantone L., Cologero A.E., Infantone E. Increased urinary albumin excretion is a marker of risk for retinopathy and coronary heart disease in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 1998;40(1):45–51. doi: 10.1016/s0168-8227(98)00024-2. [DOI] [PubMed] [Google Scholar]
- Massin P., Erginay A., Haouchine B., Mehidi A.B., Paques M., Gaudric A. Retinal Thickness in healthy and diabetic subjects measured using optical coherence tomography mapping software. Eur. J. Ophthalmol. 2002;12(2):102–108. doi: 10.1177/112067210201200205. [DOI] [PubMed] [Google Scholar]
- Mathur A., Mathur R. Study of association of serum lipids with diabetic retinopathy in type 2 diabetes mellitus. People's J. Sci. Res. 2013;6(1):25–28. [Google Scholar]
- McAnany J.J., Park J.C., Liu K., Liu M., Chen Y., Chau Y.F. Contrast sensitivity is associated with outer-retina thickness in early-stage diabetic retinopathy. Acta Ophthalmol. 2019:1–8. doi: 10.1111/aos.14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S., Saxena S., Kishore P., Bhaskar S.K., Mishra A., Meyer C.H. Association of contrast sensitivity with Log MAR visual acuity and glycosylated hemoglobin in non-insulin dependent diabetes mellitus. J. Ocul. Biol. Dis. Inf. 2010;3:60–63. doi: 10.1007/s12177-010-9056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal L.K., Bhaduri G., Bhattacharya B. Biochemical scenario behind initiation of diabetic retinopathy in type 2 diabetes mellitus. Indian J. Ophthalmol. 2018;66(4):535–540. doi: 10.4103/ijo.IJO_1121_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasralah Z., Robinson W., Jackson G., Barber A.J. Measuring visual function in diabetic retinopathy: progress in basic and clinical research. J. Clin. Exp. Ophthalmol. 2013;4(6):1–8. [Google Scholar]
- Nishijima K., Ng Y., Zhong L., Bradley J., Schubert W., Jo N. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 2007;171(1):53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noticewala V., Shastri M. A study of contrast sensitivity changes in normal individual and diabetic patients with and without diabetic retinopathy. Int. J. Res. Med. Sci. 2017;5(11):4840–4845. [Google Scholar]
- Olafsdóttir E., Stefánsson E. Biennial eye screening in patients with diabetes without retinopathy: 10-year experience. Br. J. Ophthalmol. 2007;91(12):1599–1601. doi: 10.1136/bjo.2007.123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omarova S., Charvet C.D., Reem R.E., Mast N., Zheng W. Abnormal vascularization in mouse retina with dysregulated retinal cholesterol homeostasis. J. Clin. Invest. 2012;122(8):3012–3023. doi: 10.1172/JCI63816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onorato J.M., Jenkins A.J., Thorpe S.R., Baynes J.W. Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions: mechanism of action of pyridoxamine. J. Biochem. 2000;275(28):21177–21184. doi: 10.1074/jbc.M003263200. [DOI] [PubMed] [Google Scholar]
- Pikuleva I.A., Curcio C.A. Cholesterol in the retina: the best is yet to come. Prog. Retin. Eye Res. 2014;41:64–89. doi: 10.1016/j.preteyeres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman R., Rani P.K., Reddi Rachepalle S., Gnanamoorthy P., Uthra S., Kumaramanickavel G. Prevalence of diabetic retinopathy in India: sankara nethralaya diabetic retinopathy epidemiology and molecular genetics study report 2. Ophthalmology. 2009;116(2):311–318. doi: 10.1016/j.ophtha.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Rübsam A., Parikh S., Fort P.E. Role of inflammation in diabetic retinopathy. Int. J. Mol. Sci. 2018;19(4):1–31. doi: 10.3390/ijms19040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi S., Rahimi A., Raeesi A., Safi H., Amiri M.A., Malek M. Contrast sensitivity to spatial gratings in moderate and dim light conditions in patients with diabetes in the absence of diabetic retinopathy. BMJ Open Diabetes Res. Care. 2017;5:1–9. doi: 10.1136/bmjdrc-2017-000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi H., Safi S., Hafezi-Moghadam A., Ahmadieh H. Early detection of diabetic retinopathy. Surv. Ophthalmol. 2018;63(5):601–608. doi: 10.1016/j.survophthal.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Simó R., Hernández C. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol. Metabol. 2014;25(1):23–33. doi: 10.1016/j.tem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Singer M.A., Kermany D.S., Waters J., Jansen M.E., Tyler L. Diabetic macular edema: it is more than just VEGF. F1000Research. 2016;5 doi: 10.12688/f1000research.8265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrou E.P., Wood J.M. Letter contrast sensitivity changes in early diabetic retinopathy. Clin. Exp. Optom. 2003;86(3):152–156. doi: 10.1111/j.1444-0938.2003.tb03097.x. [DOI] [PubMed] [Google Scholar]
- Stem M.S., Dunbar G.E., Jackson G.R., Farsiu S., Busui R.P. Glucose variability and inner retinal sensory neuropathy in persons with type 1 diabetes mellitus. Eye. 2016;30(6):825–832. doi: 10.1038/eye.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolonen N., Hietala K., Forsblom C., Harjutsalo V., Mäkinen V.P., Kytö J. Associations and interactions between lipid profiles, retinopathy and nephropathy in patients with type-1 diabetes: the FinnDiane Study. Journal of Internal Medicine. J. Intern. Med. 2013;274(5):469–479. doi: 10.1111/joim.12111. [DOI] [PubMed] [Google Scholar]
- Ucgun N.I., Yildirim Z., Kilic N., Gursel E. The importance of serum lipids in exudative diabetic macular edema in type 2 diabetic patients. Ann. N. Y. Acad. Sci. 2007;1100:213–220. doi: 10.1196/annals.1395.021. [DOI] [PubMed] [Google Scholar]
- Wu M., Lyons T.J. Treatment approaches for diabetes and dyslipidemia. Hormone Res. Paediatr. 2011;76(1):76–80. doi: 10.1159/000329180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Chen Y., Wilson K., Chirindel A., Ihnat M.A. Intraretinal leakage and oxidation of LDL in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2008;49(6):2679–2685. doi: 10.1167/iovs.07-1440. [DOI] [PubMed] [Google Scholar]
- Zhang W., Liu H., Rojas M., Caldwell R.W., Caldwell R.B. Anti- inflammatory therapy for diabetic retinopathy. Immunotherapy. 2011;3(5):609–628. doi: 10.2217/imt.11.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Reem R.E., Omarova S., Huang S., DiPatre P.L. Spatial distribution of the pathways of cholesterol homeostasis in human retina. PloS One. 2012;7(5) doi: 10.1371/journal.pone.0037926. [DOI] [PMC free article] [PubMed] [Google Scholar]