Abstract
Peritoneal tuberculosis (TB) is a rare extrapulmonary manifestation of TB with non-specific clinical characteristics which can produce test results mimicking malignancy and granulomatous peritonitis. This case describes a Filipino 59-year-old, nulliparous woman who was admitted with abdominal pain, ascites, and an elevated CA-125 level. Radiographically, peritoneal nodules were visualized and initial suspicion was high for malignancy. Following a bilateral salpingo-oophorectomy and peritoneal biopsy, histology was negative for malignancy but revealed non-caseating granulomas. She was discharged then readmitted with progressive abdominal pain, and a repeat laparoscopic biopsy yielded specimens with growth of acid-fast bacilli (AFB). A delay in diagnosis and treatment of tuberculous peritonitis increases mortality rates, making early diagnosis with laparoscopic biopsy of paramount importance in prompt diagnosis and initiation of therapy. This patient was initiated on standard anti-TB therapy and experienced no complications.
Keywords: Tuberculosis, Gynecology, Tumor markers, Peritoneal
Highlights
-
•
Tuberculosis can have many extrapulmonary manifestations.
-
•
Peritoneal tuberculosis is a rare but potentially rapidly fatal disease.
-
•
Peritoneal tuberculosis shares many identical characteristics with malignancy and granulomatous peritonitis.
-
•
Laparoscopic peritoneal biopsy is the gold standard for diagnosis.
-
•
Delay in treatment and unnecessary procedures or testing should be avoided.
1. Introduction
Abdominal tuberculosis (TB) is relatively rare [1]. One subtype of abdominal tuberculosis, peritoneal TB, is characterized by non-specific symptoms, laboratory tests, and imaging, making a high index of suspicion crucial for early diagnosis. Patients with peritoneal TB frequently have findings identical to those with underlying malignancy or recent abdominal surgery due to nonspecific inflammatory changes [2]. This makes differentiating various etiologies critical in the early stages of diagnostic work-up to minimize delay in treatment and unnecessary procedures or testing. The gold standard for diagnosis is laparoscopic biopsy. Here, the authors present a case of peritoneal TB in a postmenopausal woman originally from the Philippines presenting with abdominal pain, ascites, and an elevated CA-125 level.
2. Case
A 59-year-old nulliparous woman, a native of the Philippines currently residing in the United States, presented with reported left-sided abdominal pain, unintentional weight loss, nausea, and early satiety. Her medical history was significant for BCG vaccination, untreated latent Mycobacterium TB infection, colonic granuloma noted on colonoscopy biopsy, and recent acute perforated appendicitis with peri-appendiceal abscess requiring appendectomy. Initial laboratory evaluation was significant for CA-125 elevation to 479 U/mL. A CT scan of the abdomen with contrast demonstrated moderate ascites, enhancing peritoneal membranes, and multifocal, subcentimeter nodules (see Fig. 1). Due to these findings and her clinical presentation, she was admitted for diagnostic paracentesis and peritoneal biopsy. Unfortunately, the peritoneal nodules were not amenable to biopsy and analysis of the peritoneal fluid yielded only reactive appearing mesothelial cells in a background of chronic inflammation. No malignant cells were identified. The calculated serum ascitic albumin gradient (SAAG) was 1.2. She was subsequently discharged home with oncology and gynecologic oncology follow-up within the week.
Fig. 1.
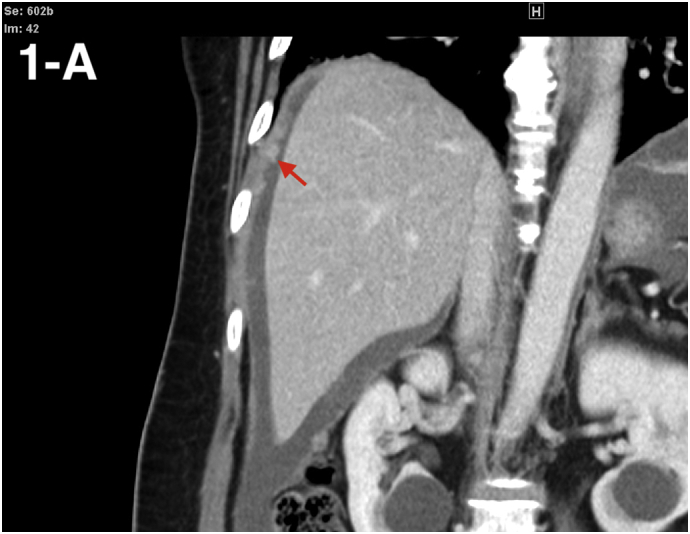
Coronal view showing peritoneal nodules (red arrows) and ascites. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
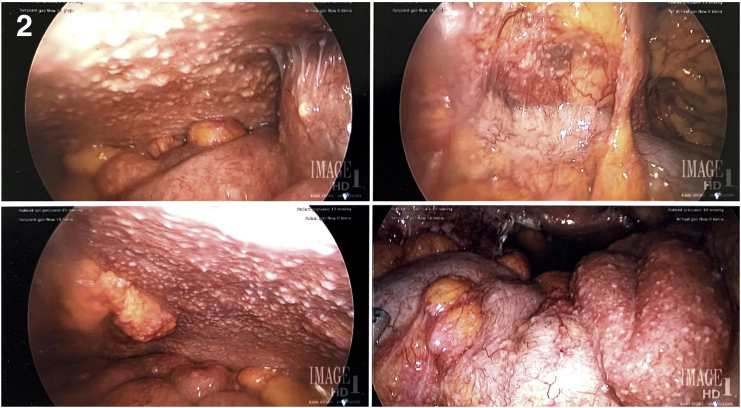
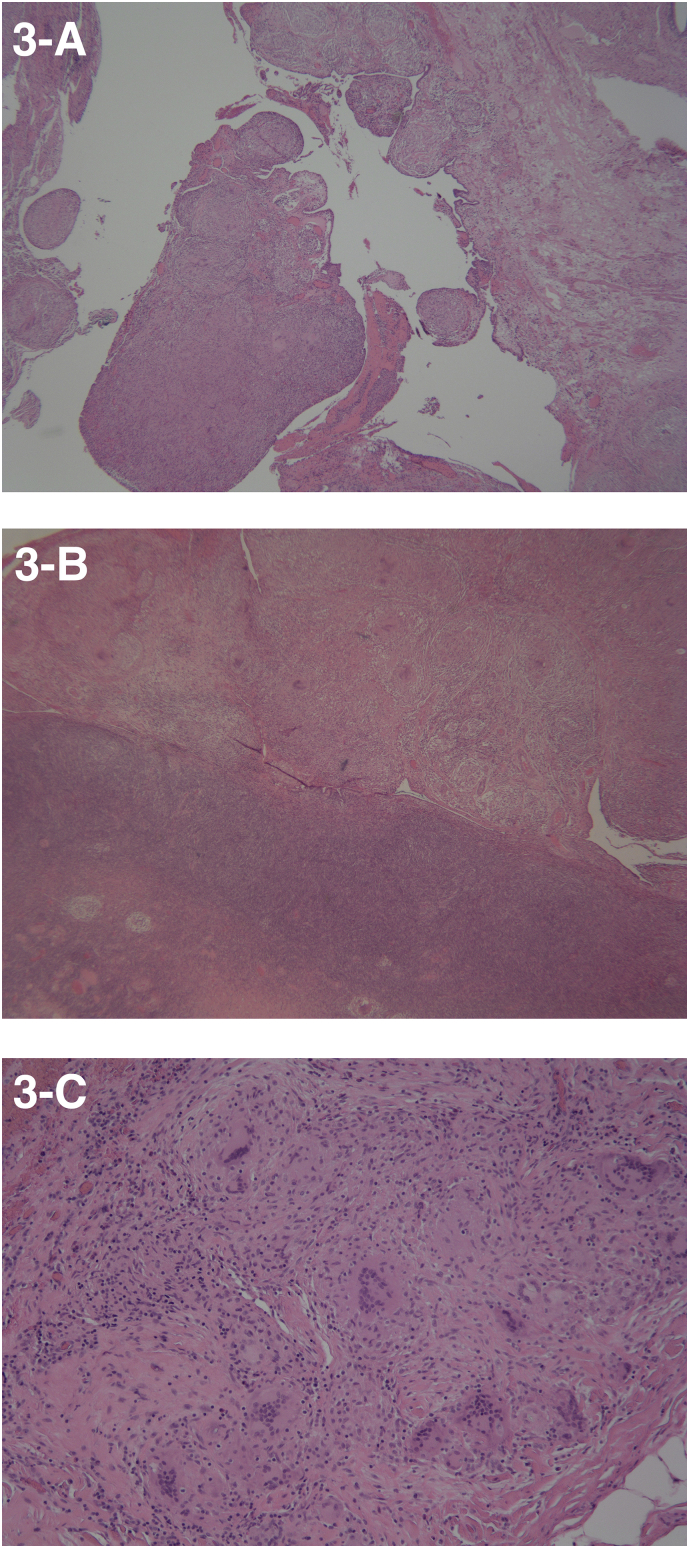
After additional consultation and continued concern for underlying malignancy, the patient consented to diagnostic laparoscopy with bilateral salpingo-oophorectomy, possible hysterectomy, omentectomy, and exploratory laparotomy. For staging purposes, she underwent CT of the chest which did not reveal evidence of metastatic disease, scarring, or granulomatous disease. The patient underwent an uncomplicated laparoscopic peritoneal biopsy and bilateral salpingo-oophorectomy. Findings were significant for yellow/white nodules diffusely covering the peritoneum (see Fig. 2), dark yellow-brown ascitic fluid in the abdomen and pelvis, and adhesions over the right fallopian tube and ovary to the right pelvic side wall and bowel. The peritoneal biopsy, pelvic washings, fallopian tubes, and ovaries were sent to pathology for analysis. She was discharged home the same day after an uncomplicated postoperative course. Pathology results showed non-caseating granuloma throughout all specimens and no malignant cells were identified (see Fig. 3). Additionally, tissue fixed in formalin was negative for TB polymerase chain reaction (PCR).
Fig. 2.
Small nodules covering the anterior peritoneal surface in the left and right lower quadrants. Adhesive disease and small bowel covered with small nodules.
Fig. 3.
(A-C): (A) Non-caseating granulomatous inflammation of the Fallopian tube (10×); (B) Normal ovary with surface nodule with non-caseating granulomatous inflammation (10×); (C) Peritoneal nodule with non-caseating granulomatous inflammation and abundant giant cells (40×).
The patient presented to the emergency department on postoperative day 2 and was readmitted due to progressive abdominal pain with concern for postoperative hematoma. Repeat CT scan demonstrated asymmetric infiltration of left lateral abdominal subcutaneous tissue and fluid within the muscular tissues, suspicious for a postoperative hematoma. Infectious disease physicians were consulted and they recommended expanded work-up to capture a more comprehensive differential including malignancy, sarcoidosis, tuberculosis, actinomycosis, and fungal etiologies. Recommendations included repeat Mycobacterium tuberculosis PCR along with tissue acid-fast bacilli (AFB) and fungal stains. Initial tissue samples had been placed in formalin which made the samples unsuitable for these tests. The patient consented to repeat laparoscopic biopsy along with cystoscopy and dilation and curettage for further characterization. Tissue samples were sent for AFB by Ziehl-Neelsen staining, culture, and PCR. The patient had an uneventful postoperative course following repeat laparoscopy.
On postoperative day 16, AFB cultures became positive. A standard 12-month regimen of rifampin, isoniazid, pyrazinamide, and ethambutol was initiated. Susceptibility testing was not performed prior to initiating therapy, and the effectiveness of the regimen was set to be determined by monitoring the patient's overall clinical and radiographic response. At the time of writing, the patient continued to have stable abdominal discomfort and remained compliant with medication without immediate adverse reaction.
3. Discussion
Abdominal TB is a rare disease that presents diagnostic challenges for clinicians due to the characteristics it shares with peritoneal malignancy and its relatively infrequent occurrence in developed countries [3]. With rapid globalization, physicians must consider TB as a potential etiology for intra-abdominal processes, especially in migrant populations from endemic regions [4]. Abdominal TB is classified by extent of tissue involvement, being peritoneal, nodal, luminal, or visceral. Peritoneal involvement is further classified into dry and wet types depending on presence or absence of ascites. The dry type is again divided into fibrotic-fixed and dry-plastic types [5]. Current theories for peritoneal TB development include tuberculous salpingitis, or hematogenous spread [6].
Peritoneal TB manifestations include abdominal pain, unintentional weight loss, night sweats, abdominal distention, nausea, and vomiting. Abdominal pain is usually involved to a lesser degree in the wet-ascitic type [7]. Unfortunately, given the wide spectrum of presenting symptoms, rarity of the syndrome and significant overlap with more sinister etiologies, clinical suspicion for peritoneal TB is typically low during diagnostic evaluation. Peritoneal TB typically occurs as an isolated event, making diagnosis even more elusive [8]. Laboratory testing can further confound analysis due to clinicians' anchoring bias towards malignancy as cancer antigens, in particular CA-125, are elevated in both peritoneal TB and malignancy [9].
Diagnosis of peritoneal TB includes fluid analysis from paracentesis. While fluid acid-fast bacilli (AFB) smear and culture have a relatively low yield (3%) in diagnostic paracentesis, yield may be increased with therapeutic paracentesis if volumes of greater than one liter are removed [5]. Disadvantages are that AFB smears have high false negative rates and AFB cultures may take up to 8 weeks for growth [10]. Cell counts in patients with peritoneal TB typically range from 150 to 4000 cells/mm3 with lymphocytic predominance, though neutrophilic pleocytosis is possible [4]. Further fluid analysis includes the calculation of the SAAG. Classically, patients with peritoneal TB have a SAAG <1.1. Perhaps the most useful single test is adenosine deaminase (ADA), an enzyme present in lymphocytes, which confers a sensitivity of 97% and specificity of 100% without surgical intervention [2,3]. However, diagnostic paracentesis in patients with ovarian cancer has been associated with an increased risk of preoperative tumor rupture or seeding, and may be avoided in cases with high clinical suspicion for malignancy [11].
Radiographic findings for peritoneal TB, carcinomatosis, and postoperative granulomatous peritonitis overlap. Findings that favor a diagnosis of peritoneal TB are a thin omental line covering the infiltrated omentum and splenic abnormalities [6]. Additionally, the presence of macronodules (≥ 5 mm) can be an uncommon CT finding in peritoneal carcinomatosis [6]. Of note, the diffuse mesenteric infiltration with macronodules apparent in this patient is a feature that has been reported to favor tubercular peritonitis over peritoneal carcinomatosis, whereas micronodules can be present in either disease [12]. Although the pathophysiology of postoperative granulomatous peritonitis has not been completely elucidated, it is proposed to be a hypersensitivity reaction to foreign bodies that may include talc or starch found in surgical gloves as well as body fluids that intraoperatively leak into the peritoneum. While data on imaging of postoperative granulomatous peritonitis is limited, shared features among reported cases include the nonspecific features of abundant ascites and a diffusely thickened omentum [13].
Laparoscopic peritoneal biopsy is the current gold standard for diagnosis [14]. The yellow/white nodules within the peritoneum observed in this patient's laparoscopic images are a typical feature of wet peritoneal TB (see Fig. 2). Although not depicted in this case, omental thickening and abdominal cocoon with matted small bowel are other classic laparoscopic findings for peritoneal TB [2]. Since granulomatous peritonitis and peritoneal TB share many identical clinical and histopathologic characteristics, postoperative granulomatous peritonitis remains a diagnosis of exclusion and the presence of Mycobacterium TB must first be excluded [1].
Early diagnosis of peritoneal TB is crucial to reduce the risk of morbidity and mortality [15]. This poses a significant dilemma given that AFB cultures can take up to 8 weeks for growth. A protracted course for diagnosis not only delays the initiation of anti-TB chemotherapy, but also introduces the risk of commencing inappropriate medical therapy that can worsen prognosis. Obtaining PCR assay of specimens may expedite diagnosis [16]. In cases without definitive diagnosis and deteriorating clinical status, empiric therapy for peritoneal TB may be justified [10]. While CA-125 has poor diagnostic potential, it is often used to monitor response to anti-TB treatment. In a study by Mas et al., changes in CA-125 level by the end of a four-month therapy course reflected clinical improvement [17]. If peritoneal TB continues to progress despite treatment, malignancy should be maintained as a differential diagnosis [18].
4. Conclusion
Diagnosis of peritoneal TB is challenging due to its nonspecific clinical presentation, the limitation of laboratory testing, and the similarities of radiographic and laparoscopic evaluation to other diseases. For this patient, a high index of suspicion for peritoneal TB was present due to her clinical symptoms, being a native of a TB endemic region, and having a history of untreated latent Mycobacterium TB infection. Our case illustrates peritoneal TB as a diagnostic conundrum and highlights the multidisciplinary approach necessary for its early and accurate diagnosis.
Acknowledgments
Contributors
Dilek Sen contributed to writing and discussion.
Joshua Brunton contributed to writing and the compiling manuscript.
Landon Melchior contributed to acquiring and reviewing the radiology images.
David Klein contributed to acquiring and reviewing the radiology images.
Gillian H Levy contributed to acquisition of pathology images and descriptions.
Booth Wainscoat contributed to review of the manuscript.
Linus Chuang contributed to review of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Funding
No funding from an external source supported the publication of this case report.
Patient Consent
Obtained.
Provenance and peer review
This case report was peer reviewed.
Contributor Information
Dilek Sen, Email: dileksenbc@gmail.com.
Joshua Brunton, Email: joshua.brunton@nuvancehealth.org.
Landon Melchior, Email: Landon.Melchior@nuvancehealth.org.
David Klein, Email: David.Klein@nuvancehealth.org.
Gillian H. Levy, Email: Gillian.Levy@nuvancehealth.org.
Booth Wainscoat, Email: Booth.Wainscoat@nuvancehealth.org.
Linus Chuang, Email: Linus.Chuang@nuvancehealth.org.
References
- 1.Kasper P., Pütz K., Fünger S. Postoperative granulomatous peritonitis mimicking abdominal tuberculosis. Clin. Case Rep. 2018;6(9):1810–1814. doi: 10.1002/ccr3.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Y.E., Tan K., Hendahewa R. Intra-abdominal tuberculosis masquerading as ovarian carcinoma. J. Surg. Case Rep. 2019;2019(12) doi: 10.1093/jscr/rjz361. rjz361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho J.-K., Choi Y.M., Lee S.S. Clinical features and outcomes of abdominal tuberculosis in southeastern Korea: 12 years of experience. BMC Infect. Dis. 2018;18(1):699. doi: 10.1186/s12879-018-3635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jehangir W., Khan R., Gil C. Abdominal tuberculosis: an Immigrant’s disease in the United States. North. Am. J. Med. Sci. 2015;7(6):247–252. doi: 10.4103/1947-2714.157484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts S., Newsholme W., Gibson T. Diagnosis and management of intra-abdominal tuberculosis. Br. J. Hosp. Med. 2018;79(6):C86–C89. doi: 10.12968/hmed.2018.79.6.C86. [DOI] [PubMed] [Google Scholar]
- 6.Ha H.K., Jung J.I., Lee M.S. CT differentiation of tuberculous peritonitis and peritoneal carcinomatosis. AJR Am. J. Roentgenol. 1996;167(3):743–748. doi: 10.2214/ajr.167.3.8751693. [DOI] [PubMed] [Google Scholar]
- 7.Zhu C., Liu S., Zhai J., Chen Z., Wu K., Li N. Clinical and pathological features of three types of peritoneal tuberculosis: a single Centre in China. Biomed. Res. 2016;27(4) https://www.alliedacademies.org/abstract/clinical-and-pathological-features-of-three-types-of-peritoneal-tuberculosis-a-single-centre-in-china-4697.html Accessed April 20, 2020. [Google Scholar]
- 8.Suárez I., Fünger S.M., Kröger S., Rademacher J., Fätkenheuer G., Rybniker J. The diagnosis and treatment of tuberculosis. Dtsch Arzteblatt Int. 2019;116(43):729–735. doi: 10.3238/arztebl.2019.0729. [DOI] [PubMed] [Google Scholar]
- 9.Dasgupta A., Wahed A., Dasgupta A. Chapter 13 - tumor markers. In: Wahed A., editor. Clinical Chemistry, Immunology and Laboratory Quality Control. Elsevier; 2014. pp. 229–247. [DOI] [Google Scholar]
- 10.WLA Chiew, JXJ Liu, Teo L.T. A rare case of peritoneal tuberculosis. J. Med. Cases. 2016;7(4) doi: 10.14740/jmc.v7i4.2449. 130–132-132. [DOI] [Google Scholar]
- 11.Wang H., Qu X., Liu X., Ding L., Yue Y. Female peritoneal tuberculosis with ascites, pelvic mass, or elevated CA 125 mimicking advanced ovarian cancer: a retrospective study of 26 cases. J. Coll. Physicians Surg. Pak. 2019;29(6):588–589. doi: 10.29271/jcpsp.2019.06.588. [DOI] [PubMed] [Google Scholar]
- 12.Arvind S., Raje S., Rao G., Chawla L. Laparoscopic diagnosis of peritoneal tuberculosis. J. Minim. Invasive Gynecol. 2019;26(2):346–347. doi: 10.1016/j.jmig.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Divjak E., Markovic M.V. Imaging findings of granulomatous peritonitis in a female patient presenting with erythema Nodosum- a case report. J. Clin. Case Rep. 2015;5(10) doi: 10.4172/2165-7920.1000616. [DOI] [Google Scholar]
- 14.Bhargava D.K., Null Shriniwas, Chopra P., Nijhawan S., Dasarathy S., Kushwaha A.K. Peritoneal tuberculosis: laparoscopic patterns and its diagnostic accuracy. Am. J. Gastroenterol. 1992;87(1):109–112. [PubMed] [Google Scholar]
- 15.Chow K.M., Chow V.C.Y., Hung L.C.T., Wong S.M., Szeto C.C. Tuberculous peritonitis–associated mortality is high among patients waiting for the results of mycobacterial cultures of ascitic fluid samples. Clin. Infect. Dis. 2002;35(4):409–413. doi: 10.1086/341898. [DOI] [PubMed] [Google Scholar]
- 16.Kosseifi S., Hoskere G., Roy T.M., Byrd R.P., Mehta J. Peritoneal tuberculosis: modern peril for an ancient disease. South. Med. J. 2009;102(1):57–59. doi: 10.1097/SMJ.0b013e31819007c8. [DOI] [PubMed] [Google Scholar]
- 17.Mas M.R., Cömert B., Sağlamkaya U. CA-125; a new marker for diagnosis and follow-up of patients with tuberculous peritonitis. Dig Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital Assoc. Study Liver. 2000;32(7):595–597. doi: 10.1016/s1590-8658(00)80841-5. [DOI] [PubMed] [Google Scholar]
- 18.Gupta P., Rana S., Agarwal P. Peritoneal tuberculosis or carcinomatosis: a diagnostic conundrum. Int. J. Mycobacteriol. 2018;7(2):198–199. doi: 10.4103/ijmy.ijmy_62_18. [DOI] [PubMed] [Google Scholar]