Abstract
Salinity is a major environmental stress that limits crop production worldwide. It is well-understood that environmental adaptations, physiological and biochemical traits adjust salinity tolerance in plants, but imparting the knowledge gained towards crop improvement remain arduous. Utilizing the potentially of beneficial microorganisms present in the rhizosphere is an alternative strategy to improve crop production under optimal or stress conditions. The current study aims at examining the ability of plant growth-promoting rhizobacteria (PGPR) in improving coriander growth under salt stress condition. Coriander seeds were inoculated via dual culture of Azospirillum brasiliense and Azotobacter chroococcum, and therefore subjected to four levels of salt stress (0, 40, 80 and 120 mM NaCl) with three replications in a research greenhouse. Seventy-five days after sowing, when leaves fully developed, leaf samples were collected and the traits were measured. The results indicated that the dual inoculation improved chlorophyll a and b content, in comparison to the un-inoculated plants. The dual inoculation increased grain yield, stem fresh and dry weights by 11.6, 11.3 and 17.2%, respectively; it also enhanced total plant fresh and dry weights by 6.1 and 10.2%, respectively, as compared to control. As a result, the dual inoculation significantly improved catalase (CAT), but decreased ascorbate peroxidase (APX) and guaiacol peroxidase (GPX) enzymes activities, as compared to control plants. Salt stress significantly increased (CAT) activity in the leaves, whereas it resulted in significant reduction in (APX) and (GPX) activity, especially in inoculated plants. Furthermore, dual inoculation decreased Na and subsequently increased K concentration in coriander leaves comparing with untreated plants. Overall, these results indicate that the PGPRs has improved coriander growth under control as well as salt stress conditions. Thus, PGPR can could significantly contribute to solve the coriander plant production problems caused by high salinity.
Keywords: Agricultural sciences, Plant biology, Microbiology, Bio-inoculant, Chlorophyll, Coriandrum sativum, Enzyme activity, PGPRs, Vegetative traits
Agricultural sciences, Plant biology, Microbiology, Bio-inoculant, Chlorophyll, Coriandrum sativum, Enzyme activity, PGPRs, Vegetative traits.
1. Introduction
Coriander (Coriandrum sativum L.), known as cilantro, is an annual herb belonging to Apiaceae family. It has also been used as an aromatic and medicinal plant for centuries. Coriander is native to southern Europe, northern Africa, and some parts of Asia (e.g., Iran), with extensive adaptation, well-growing power under different kind of soil and climate conditions. However, the yield and physiology of this plants is adversely affected by salinity (Fredj et al., 2013; Mishra et al., 2017; Al-Garni et al., 2019). Saline soils and saline irrigation water cause serious problems for medicinal plant production. Salinity hinders plant growth, especially in arid and semi-arid regions, where salt leaching is poor because of low rainfall rate (Younesi et al., 2013).
Salt induces osmotic stress by declining soil water potentials and water availability, which leads to dehydration at the cellular level; and is strongly linked to the production of reactive oxygen species (ROS) like (superoxide (O2–), hydrogen peroxide (H2O2) and hydroxyl radicals (•OH)) damaging the DNA, RNA, and proteins (Younesi et al., 2013; Stefan et al., 2013; Kang et al., 2014). The ROSs are highly reactive and cytotoxic; they can react with vital biomolecules, such as lipids, proteins and nucleic acid, triggering lipid peroxidation, protein denaturation and mutation, respectively (Zeid and Hassan, 2011). However, plants seem to exhibit a complex strategy to protect itself from the oxidative stress with a large scale of enzymatic and non-enzymatic antioxidants to scavenge ROS and to restore the redox homeostasis of the cells induced by several environmental stress conditions including salinity (Sharma et al., 2012). Maintenance of a high antioxidant capacity to scavenge the toxic ROS has been associated with increased plants tolerance to these environmental stress conditions (Sharma et al., 2012; Younesi et al., 2013; Maksimović et al., 2013). The major antioxidant enzymes are superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT) and glutathione peroxidase (GPX), while the most important non-enzymatic ROS-scavenging compounds are ascorbate and glutathione (Stefan et al., 2013; Maksimović et al., 2013). These enzymes pave the way for understanding the physiological and biochemical mechanisms of protection against salt-induced oxidative damage and ROS reduction by catalyzing the breakdown of H2O2 into H2O and O2 (Habib et al., 2016).
Plant growth-promoting rhizobacteria (PGPR) and symbiotic microorganisms play an important role in agricultural systems and proved to be useful in developing strategies to enhance growth and health of plants under saline conditions (Stefan et al., 2013). The PGPRs can also prevent the deleterious effects of phytopathogenic organisms and environmental stressors (Han and Lee, 2005). Moreover, some PGPR strains are able to produce cytokinins and antioxidant compounds, which result in abscisic acid (ABA) accumulation and degradation of reactive oxygen species (Grover et al., 2011). To date, many studies have demonstrated that inoculation with PGPRs improves plant growth under stressful conditions, such as salt stress (Han and Lee, 2005; Tank and Saraf, 2010; Younesi et al., 2013). Ionic regulation plays an important role in plant growth and stress resistance, and potassium is of ion's share over other elements. The ratio of potassium (K+) to sodium (Na+) is also important in stress conditions, maintenance higher K+/Na+ ratio prevents interfering of various enzymatic processes regulated by K+. a higher K+/Na+ ratio is usually maintained through proper expression of regulation of the K+ and Na+ onion transporter's activity and hydrogen pumps, thereby, leading to better stress adaptation (Assaha et al., 2017). Younesi et al. (2013) revealed that PGPR-inoculated alfalfa plants generally show augmented tolerance compared to salt stress as a result of increased proline production and K uptake (Younesi and Moradi, 2014). PGPRs contribute significantly to enhancing plant nutrient use efficiency and nutrient uptake (Sharma et al., 2014; Khatri et al., 2016).
The PGPR term was primary used for bacterial genus Pseudomonas spp, however, it now includes various other kinds of soil bacteria, such as Azospirillum, Azotobacter, Bacillus, and burkholderia (Yousefi et al., 2017). Among mentioned bacteria, Azospirillum spp and Azotobacter spp are reported to be the most vital ones, which affect the growth and yield of medicinal plants via various mechanisms such as producing hormones that regulate plant growth, solubilizing nutrients, and siderophore production, enhancing the activity of antioxidant enzymes, lowering the stress-induced ethylene and production of exopolysaccharides (Nadeem et al., 2014; Yousefi et al., 2017). These general mechanisms lead to plant growth promotions within different environments, also protect the plant from the deleterious effects of environmental stresses.
Salt stress has been found to generally reduce chlorophyll content (both a and b); due to salinity inhibited chlorophyll synthesis and/or accelerated chlorophyll degradation (Christen et al., 2007). The effects of PGPRs on chlorophyll have been previously examined in several crop species, such as runner bean (Phaseolus coccineus L.) (Stefan et al., 2013), lettuce (Lactuca sativa L.) (Han and Lee, 2005) and cucumber (Cucumis sativa L.) (Kang et al., 2014).
Studies have shown PGPR and salinity stress affects the growth, yield and physiology of coriander (Bashtanova and Flowers, 2012; Neffati et al., 2011; Warwate et al., 2017; Mishra et al., 2017). Furthermore, the initiation of salt stress tolerance using PGPR is an efficient and low-cost method. Nevertheless, there are very few reports on PGPR induced salinity tolerance in the coriander plant caused by changes in physiological and biochemical responses. Therefore, this study was designed to evaluate the effect of dual inoculation with PGPR on coriander grown under various levels of salt stress.
2. Materials and methods
2.1. Bacterial culture and inoculum preparation
Azospirillum brasiliense and Azotobacter chroococcum were used as dual inoculum. The bacteria were cultured in standard Luria Bertani (LB) medium and incubated on an orbital shaker at 120 rpm for 48 h at 28 °C, as described by Han and Lee (2005). The inoculum was prepared and then adjusted to an inoculation level of 107 Colony Forming Unit (CFU) mL−1.
2.2. Plant material and growth conditions
The factorial experiment was conducted in a completely randomized design with three replications. Coriander seeds were sterilized by gentle shaking in 70% ethanol for 3 min, followed by sterilization using 5% sodium hypochlorite solution for 1 min. Seeds were rinsed three times in sterile distilled water and dried overnight. Surface-sterilized coriander seeds were then soaked in the bacterial suspension for 4 h at 25 °C in a shaker at 100 rpm in incubator with shaker before being sown in plastic pots 20 × 20 × 15 cm (length × width × height). Non-inoculated seeds were soaked in nutrient broth without bacterial inoculation. The studied soil was sandy loam in texture, EC 1.2 dS/m, pH 7.8 and organic matter content 1.02%. The major available nutrients like N, P and K were quantified as 0.2 %, 20 and 402 mg/kg, respectively. Ten seeds were sown in each pot (each treatment had 4 pots per replicate) filled with 4 kg of unsterile soil. The pots were placed in a growth chamber maintaining at 22/28 °C day/night temperature, an 8/18 h light/dark photoperiod, and 70% relative humidity. Pots were irrigated using fresh tap water. Thinning was done to keep 4 plants per pot after seedling establishment. In order to keep 4 plants per pot, they thinned after emergence and seedling establishment.
2.3. Salt stress induction
Forty-five days after sowing, salt stress was induced by irrigating plants with different concentrations (0, 40, 80 and 120 mM) of NaCl solution. Field water capacity of pots was calculated by a suction plate, and pots were weighed regularly and watered to approximately 80 % of field capacity and placed in saucers so that any water that drained through was later recovered. The salt stress continued until the end of growth period. Seventy-five days after sowing, when leaves were fully developed, leaf samples collected and immediately frozen in liquid nitrogen (three samples for the determinations of each treatments); samples were kept frozen at 20 °C for further analyses.
2.4. Pigment analysis
Chlorophyll and carotenoids pigments in the frozen leaves were extracted with 80% methanol and the concentrations of chlorophyll a, b and carotenoids were measured by spectrophotometer (Analytik Jena, Spekol 1300, Germany) at 665.2, 652.0 and 470 nm respectively. Pigment concentrations in μg mL−1 then calculated according to Porra (2002) protocol (Eqs. (1), (2), and (3)). Also, the ratio of chlorophyll a to b was obtained by dividing chlorophyll a to b.
| (1) |
| (2) |
| (3) |
2.5. Enzyme activity
The activity of ascorbate peroxidase (APX) was determined via a modified procedure of Nakano and Asada (1981): leaf tissues were homogenized in 3 ml of 50 mM sodium phosphate buffer (pH 7.8) containing 1% (w/v) polyvinyl-pyrrolidone (PVP), 1 mM ascorbate, and 1 mM phenylmethyl sulfonyl fluoride (PMSF). The homogenate was centrifuged at 20,000 g for 15 min at 4 °C. The reaction mixture (1 ml) consisted of 50 mM sodium phosphate buffer (pH 7.0), 0.2 mM ethylene diamine tetra acetic acid (EDTA), 0.5 mM ascorbate, and 1 mM H2O2. The reaction rate was calculated according to the decrease in absorption attributable to the oxidation of ascorbate at 290 nm, and APX activity was determined using the molar absorption coefficient of 2.8 mM−1 cm−1.
Total catalase (CAT) activity was evaluated according to the method of Cakmak and Horst (1991): 0.5 g of frozen plant tissue in 3 ml of extraction buffer (25 mM sodium phosphate, pH 7.8) was powdered in a mortar. The homogenate was centrifuged at 18000 G for 30 min at 4 °C; the supernatant was used for enzyme assay. The reaction mixture contained 100 μL crude enzyme extract, 500 μL 10 mM H2O2 and 1.4 mL−1 25 mM sodium phosphate buffer. A reduction in absorbance at 240 nm was recorded for 1 min with a spectrophotometer (Analytik Jena Spekol 1300).
The activity of guaiacol peroxidase (GPX) was determined in a 3-mL reaction mixture containing 50 mM K3PO4, pH 7.0, 0.1 mM Na2 EDTA, 5 mM H2O2, and 30 mM guaiacol (Fielding and Hall 1978). The increase in absorbance, __attributable to tetraguaiacol formation__ was recorded at 470 nm hiring a spectrophotometer (Analytik Jena Spekol 1300).
The APX, CAT and GPX activity of the extract was expressed as enzyme unit mg−1 protein min−1. One unit of enzyme activity was defined as the amount necessary to decompose 1 μl mol of substrate per min.
2.6. Determination of potassium and sodium contents
Total potassium (K+) and sodium (Na+) concentration were measured via the Flame Photometric method (JENWAY PFP 7 Flame Photometer). This method is fully described in Hajiboland et al. (2010).
2.7. Measurement of growth parameters
Harvested plants were transferred to laboratory and some traits included plant height, leaves and stem fresh and dry weights were measured. Dry weights were measured after drying the plants at 70 °C for 72 h in oven.
2.8. Statistical analysis
The main and interaction effects of experimental factors were determined from analysis of variance (ANOVA) using the general linear model (GLM) procedure in Statistical Analysis System (SAS) software (version 9.1); and comparisons concerning treatment tools were made by recruiting the least significant difference (LSD) at the 0.05 and 0.01 probability level (SAS Institute 2004).
3. Results and discussion
3.1. Vegetative traits
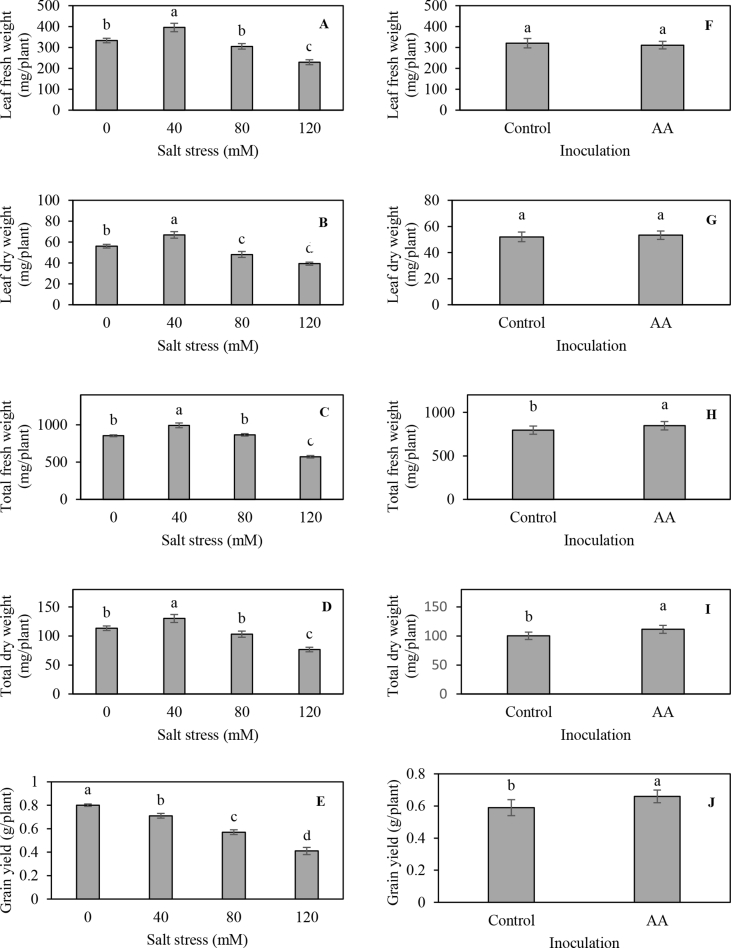
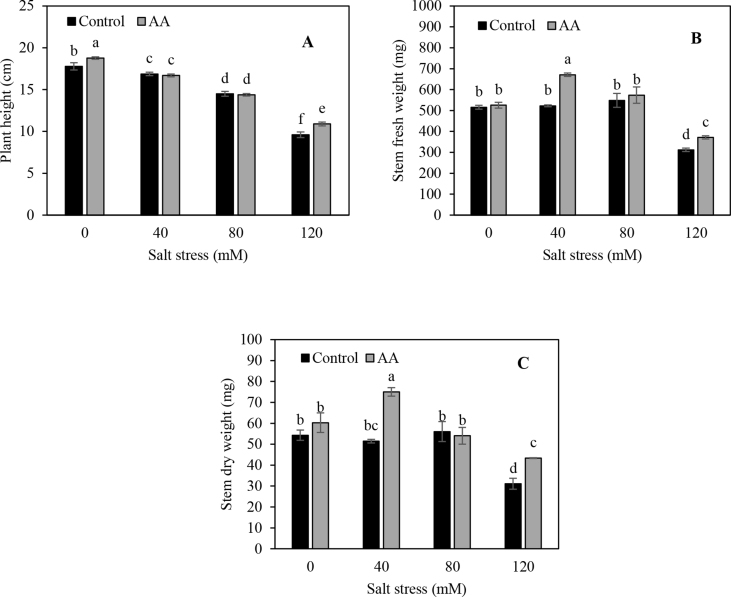
Statistical analysis showed that the effect of inoculation was significant, except for leaf fresh, dry weight and plant height (Table 1). Stem fresh weight and dry weight increased from 475 and 48 mg plant−1 in non-inoculated plants to 536 and 58 mg plant−1 in inoculated plants, respectively. Similarly, total plant fresh and dry weights increased by 6.1 and 10.2%, respectively in inoculated compared to non-inoculated plants (Figure 1). There were no significant differences between non-inoculated and inoculated plants for leaves fresh, dry weights and plant height. All the trait values significantly had decreased when salt stress levels increased from 0 to 120 mM (P ≤ 0.01). The reduction was more pronounced in non-inoculated plants. The interaction between inoculation and salt stress treatments was significant for stem fresh weight, stem dry weight and plant height (P ≤ 0.05 or P ≤ 0.01) (Table 1 and Figure 2, a, b and c). The results also indicated that the dual inoculation improved plant growth under salt stress conditions as compared to untreated control plants. Grain yield showed a falling trend with increasing salinity rate. In comparison with the control treatment, grain yield at 40, 80 and 120 mM salt solutions decreased by 10, 28 and 48%, respectively. Also, the yield under inoculated conditions was 11.6% higher than that under non-inoculated conditions. Several studies have demonstrated that inoculation with PGPRs promotes plant growth and development under saline conditions (Mayak et al., 2004a, b; Han and Lee, 2005; Stefan et al., 2013; Kang et al., 2014). Higher plant dry matter accumulation in pepper (Capsicum annuum) plants inoculated with Azospirillum brasilense and Pantoea dispersa under salinity condition was related to enhanced stomatal conductance and photosynthesis, but neither chlorophyll concentration nor photochemical efficiency of photosystem II was affected (del Amor and Cuadra-Crespo, 2012). The bacterial PGPR contains 1-aminocyclopropane-1-carboxylate (ACC) deaminase enzyme, which cleaves ACC, the precursor of ethylene in plants, to ammonia and α-ketobutyrate (Glick et al., 1998). In addition, some groups of PGPR may also synthesize and secrete indole-3-acetic acid (IAA), cytokinines and antioxidants in root and remove ABA, which can be absorbed by plant seeds or roots (Hong et al., 1991; Figueiredo et al., 2008). The PGPR with ACC-deaminase activity can lower ethylene production in plants and enable plant to growth under salt stress condition (Sarkara et al., 2018). The results of this study proposed that PGPR promotes the growth of coriander plants in control, as well as under salt stress conditions.
Table 1.
Analysis of variance (mean squares) for plant height, stem fresh weight, stem dry weight, leaf fresh weight, leaf dry weight, total fresh weight, total dry weight and grain yield of Coriandrum sativum L.
| Sources of variation | df | Plant height | Stem fresh weight | Stem dry weight | Leaf fresh weight | Leaf dry weight | Total fresh weight | Total dry weight | Grain yield |
|---|---|---|---|---|---|---|---|---|---|
| Inoculation (I) | 1 | 1.50ns | 22149.39∗∗ | 592.51∗∗ | 494.39ns | 10.55ns | 16025.34∗∗ | 761.23∗ | 0.028∗∗ |
| Salinity (S) | 3 | 73.43∗∗ | 76903.67∗∗ | 751.25∗∗ | 28579.02∗∗ | 823.32∗∗ | 189979.19∗∗ | 3017.91∗∗ | 0.168∗∗ |
| I x S | 3 | 0.86∗ | 5769.23∗ | 173.78∗∗ | 303.53ns | 7.75ns | 4103.32ns | 206.39ns | 0.002ns |
| Error | 16 | 0.20 | 1148.20 | 27.61 | 1441.52 | 40.96 | 1476.47 | 116.29 | 0.001 |
| CV (%) | - | 3.25 | 9.03 | 11.97 | 9.21 | 9.39 | 5.07 | 8.59 | 14.34 |
ns, ∗ and ∗∗: are non-significant and significant at 5 and 1% probability levels, respectively.
Figure 1.
Main effects of PGPR and salt stress on leaf fresh weight (A and F), leaf dry weight (B and G), total fresh weight (C and H), total dry weight (D and I) and grain yield (E and J) of Coriandrum sativum L.
Figure 2.
The plant height (A), stem fresh weight (B) and stem dry weight (C) of plant on thirty days after application of different slat stress levels on both AA (Azospirillum brasiliense and Azotobacter chroococcum) and non-inoculated treatment, means of each parameter were analyzed using PROC GLM method in SAS software to compare values between treatments.
3.2. Pigment content
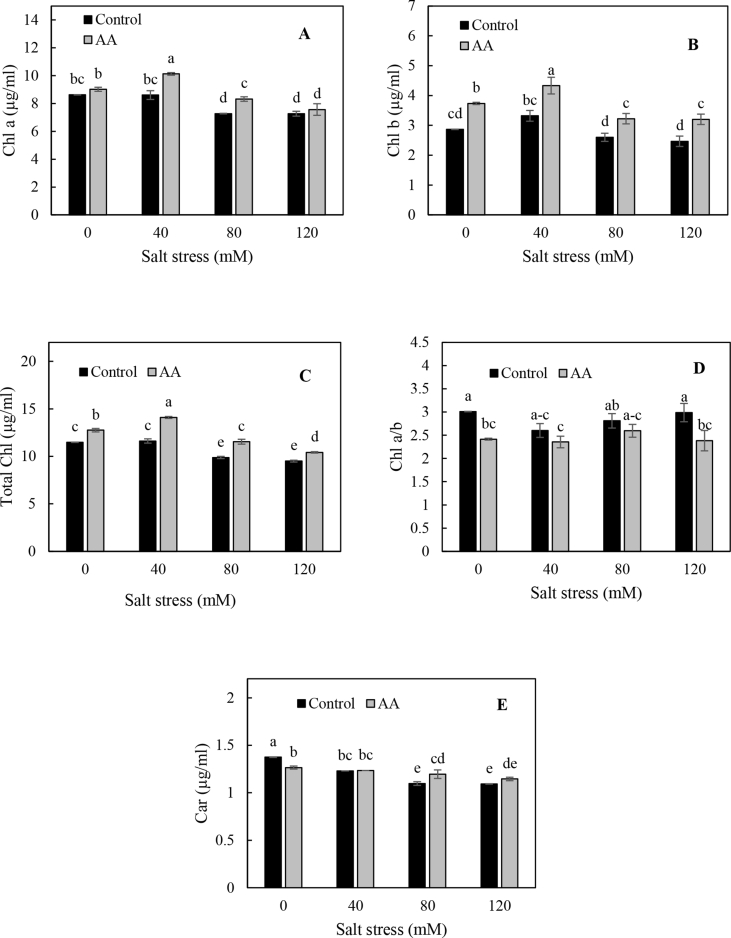
ANOVA showed that the interaction effects of salinity and inoculation were significant in all the photosynthetic pigments (Table 2). The highest (14.1 μg mL−1) and lowest (9.5 μg mL−1) total chlorophyll contents were observed in inoculated plants treated with 40 and 120 mM NaCl, respectively. In addition, chlorophyll a (10.1%), Chl b (22.2%) and total chlorophyll contents (13.1%) were found to be higher in inoculated plants than in non-inoculated ones (Figure 3 a, b and c). These results suggested that the dual inoculation significantly increases chlorophyll a, b and total chlorophyll, content. The chlorophyll a/b ratio was lower (18%) in inoculated plants comparing with non-inoculated plants (Figure 3 d). There were significant differences among salt stress levels for chlorophyll a, b, total chlorophyll, a/b and carotenoid in both inoculated and non-inoculated plants. Generally, chlorophyll a, b and total chlorophyll contents significantly decreased with increasing salt stress levels from 0 to 120 mM (Figure 3 a, b and c). The carotenoids were not significantly decreased by inoculation in comparison with non-inoculated plants. However, the increment in salt stress levels led to a significant reduction in carotenoids content (P < 0.01, Figure 3 e). Leaf chlorophyll concentration is an indicator of salt tolerance and also responsible for responding salinity increment (Shah et al., 2017). Generally, the results indicated that PGPR inoculation improved chlorophyll a, b and total chlorophyll contents under salt stress condition relative to control plants. The highest chlorophyll content was observed in inoculated plants under 40 mM salinity stress level (Figure 3). Accordingly, several studies have shown that inoculation with PGPR increases chlorophyll content under salt stress and drought stress conditions (Chang et al., 1997; Han and Lee, 2005; Heidari and Golpayegani, 2012; Stefan et al., 2013; Kang et al., 2014). Increased oxidative stress caused by salinity affects the chloroplast structure and decreases the content of chlorophyll (Li et al., 2015). The PGPR can enhance the photosynthetic pigments by increasing stomatal conductance (Vivas et al., 2003), photosynthetic potential (Marcelis and Hooijdonk 1999) and absorption of water and ions (Mahmoud et al., 2017). İpek and Eşitken (2017) reported iron uptake by microbial siderophores via increasing iron bioavailability in the soil. On the other hand, the inoculated salt stressed coriander plants exhibited higher chlorophyll content and dark green leaves owing to the possibility presence of ACC deaminase-containing PGPR isolates that maintain the photosynthetic efficiency of plants by reducing ethylene biosynthesis (Habib et al., 2016).
Table 2.
Analysis of variance (mean squares) for Chl a, Chl b, total Chl a, Chl a/b, Car, CAT, APX, GPX, K and Na traits of Coriandrum sativum L.
| Sources of variation | df | Chl a | Chl b | Total Chl | Chl a/b | car | CAT | APX | GPX | K | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inoculation (I) | 1 | 2.09∗∗ | 3.92∗∗ | 15.06∗∗ | 3.99∗∗ | 0.001ns | 0.000008∗ | 0.00104∗∗ | 0.00007∗∗ | 0.496∗∗ | 0.003∗ |
| Salinity (S) | 3 | 0.038∗∗ | 1.23∗∗ | 10.37∗∗ | 4.84∗∗ | 0.049∗∗ | 0.000102∗∗ | 0.00244∗∗ | 0.00012∗∗ | 0.278∗∗ | 0.036∗∗ |
| I x S | 3 | 0.89∗ | 0.04∗∗ | 0.68∗∗ | 0.49∗ | 0.012∗∗ | 0.000008∗ | 0.00016∗∗ | 0.00018∗∗ | 0.058∗∗ | 0.002∗ |
| Error | 16 | 0.19 | 0.08 | 0.07 | 0.13 | 0.001 | 0.000017 | 0.00001 | 0.000001 | 0.002 | 0.001 |
| CV (%) | - | 5.10 | 8.53 | 3.74 | 4.97 | 4.62 | 18.68 | 11.18 | 16.38 | 4.78 | 2.44 |
Chl a: Chlorophyll a, Chl b: Chlorophyll b, Total Chl: Total chlorophyll, Chl a/b: Chlorophyll a/b, car: Carotenoid, CAT: Catalase, APX: Ascorbate peroxidase, GPX: Guaiacol peroxidase. ns, ∗ and ∗∗: are non-significant and significant at 5 and 1% probability levels, respectively.
Figure 3.
Chlorophyll a (Chl a), (A), chlorophyll b (Chl b), (B), total chlorophyll (Total Chl), (C), chlorophyll a/b ratios (Chl a/b), (D), and carotenoid content (Car), (E), measured after thirty days under different salt stress levels and both AA (Azospirillum brasiliense and Azotobacter chroococcum) and non-inoculated treatment, means of each parameter were analyzed using PROC GLM method in SAS software to compare values between treatments.
3.3. Antioxidant enzyme activity
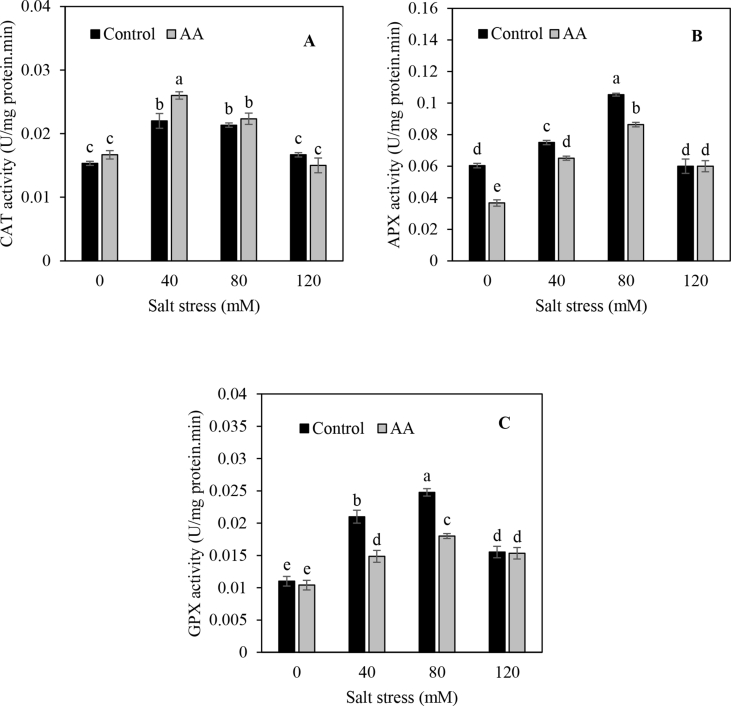
ANOVA showed the significant effect of salinity in all antioxidant enzymes activities (Table 2). In addition, APX, CAT and GPX enzyme activities were significantly affected by inoculation (Figure 4). Interactions between salinity and PGPR inoculation were also significant regarding the activity of all antioxidant enzymes (Figure 4 a, b and c). Under salinity condition, the plant response to PGPR is different, the PGPR have been able to influence the salinity stress on coriander, so that the variation in the activity of the enzymes is indicative of this claim. The CAT activity enhanced with increasing salt stress levels from 0 to 40 mM. Inoculation improved CAT activity with the maximum value at 40 mM (0.02 U mg protein−1 min−1, Figure 4 a). Correspondingly, previous reports have demonstrated that plant-associated microorganisms attenuate salt-induced lipid peroxidation as well has higher CAT activities resulting in enhanced salt tolerance (Bharti et al., 2016). The APX and GPX activities significantly decreased by 21 % and 23 %, respectively in inoculated plants relative to un-inoculated plants (Figure 4 b and c). The GPX activity increased with increase salt stress levels and the highest value was observed in non-inoculated plants treated with 40 and 80 mM (0.02 and 0.03 U mg protein−1 min−1, respectively) and 120 mM NaCl solutions (0.02 U mg protein−1 min−1). However, at 40 mM and 80 mM salinity levels, inoculation significantly decreased GPX activity by 29% and 28% relative to non-inoculated plants, respectively. We have observed that APX and GPX activities in leaves of coriander grown in saline water was decreased by PGPRs inoculation compared to untreated plants. It is widely known that salt stress increases the production and accumulation of reactive oxygen species (ROS) while PGPRs colonizing plant tissue reducing H2O2 synthesis may protect the membrane lipids from peroxidation (Egamberdieva and Lugtenberg, 2014).
Figure 4.
PGPR effect on the CAT (A), APX (B) and GPX (C) measured of coriander grown in thirty days under salt stress levels and both AA (Azospirillum brasiliense and Azotobacter chroococcum) and non-inoculated treatment. Means of each parameter were analyzed using PROC GLM method in SAS software to compare values between treatments.
Similarly, when salt stress levels increased from 0 to 80 mM, APX activity increased but then decreased at 120 mM NaCl treatment level (Figure 4 b). Inoculation significantly decreased APX activity. The APX activity at both 0 and 80 mM NaCl levels decreased from 0.06 and 0.11 in non-inoculated plants to 0.04 (33%) and 0.09 (18%), respectively in inoculated plants. In general, the results indicated that APX and GPX activity decreased when plants were inoculated. Similar results were reported (Han and Lee 2005) for lettuce, where the PGPRs Serratia sp. and Rhizobium sp. decreased APX and GPX activity under increasing salinity stress, however, CAT activity increased under the same treatment condition. Heidari and Golpayegani (2012) reported that CAT activity increased by increasing drought stress in basil plants. They also confirmed a rapid increase in CAT activity revealing the role of the major enzyme in eliminating hydrogen peroxide production under drought stress. In the present study, the activity of antioxidant enzymes APX and GPX in coriander leaves treated with PGPR strains was significantly reduced as compared to control plants growing under salinity stress. Many of the mechanisms that PGPR utilize to protect plants from salt stress are interconnected and affect one another. Moreover, a detailed description of the nature of these interconnections, for the most part, remains to be elaborated. In addition, while PGPR can provide some protection against the inhibitory effects of salt or drought stress (e.g., by promoting plant growth), they may also alter plant gene expression so that the plant is less likely to succumb to these stresses (Forni et al., 2017). For example, various PGPR have been shown to increase the activities of enzymes, such as SOD, CAT and GPX that can detoxify reactive oxygen species (Nautiyal et al., 2013).
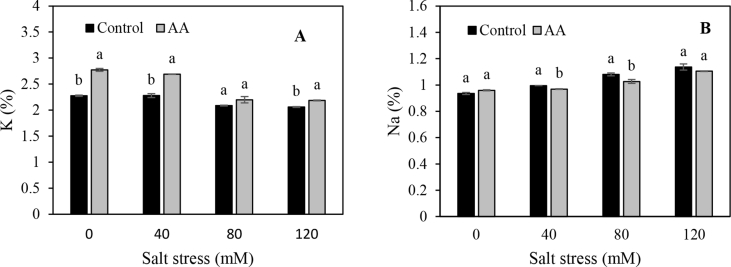
3.4. Mineral content
The effects of inoculation, salt stress and interaction between them were significant for mineral contents (Table 2). When salt concentration level increased from 0 to 120 mM, K+ content decreased, while Na+ content increased (Figure 5 a and b). As results showed, in all salt stress levels, PGPR inoculation increased the K+ content, but decreased Na+ content. The stimulated root system induced by endophytic bacteria could explain the enhanced capacity of the plant to acquire and utilize more nutrients. PGPR induced nutrient cycling (mineralization), rhizosphere pH changes (organic acids), and also contributed to facilitate k+ availability, and to increase plant uptake (Setiawati and Mutmainnah, 2016; Lugtenberg et al., 2013). The highest K+ absorption (2.7 mg kg−1) was observed in inoculated plants (2.2 mg kg−1) grown without salt stress treatment (Figure 5 a). Inversely, the highest Na+ absorption (1.1 mg kg−1) was obtained with 120 mM solution, and no significant difference was found between inoculated and non-inoculated plants (Figure 5 b). The lower Na+ and higher K+ uptake and maintenance of high K+/Na + ratio was observed in the dual-inoculated plants than the control in leaves during salinity stress. Similar results were reported for lettuce plants by Han and Lee (2005), who demonstrated antagonistic absorption between Na+ and K+ under salinity stress conditions. Ashraf (2004) observed that a variety of growth-promoting bacteria secrete exopolysaccharide compounds that bind with Na+ ion in the root, through which the plant's Na+ accumulation decreases. PGPR help ion homeostasis regulation and high K+/Na+ ratios in shoots by reducing Na+ accumulation in leaves, increasing Na+ exclusion via roots, and boosting the activity of high affinity K+ transporters (Ilangumaran and Smith, 2017). Researchers reported an increase in K+ ions uptake in the presence of PGPR inoculation in alfalfa (Younesi et al., 2013), cucumber (Kang et al. (2014)) and maize (Rojas-Tapias et al. (2004)). According to Sivritepe et al. (2013), an increase in the K+ content of plants grown under salt stress can reduce the negative effects of salinity on plant growth and yield.
Figure 5.
PGPR effect on K+ (A) and Na+ (B) uptake of coriander grown in thirty days under salt stress levels and both AA (Azospirillum brasiliense and Azotobacter chroococcum) and non-inoculated treatment. Means of each parameter were analyzed using PROC GLM method in SAS software to compare values between treatments.
4. Conclusion
The combination of Azospirillum brasiliense and Azotobacter chroococcum is of great potential to improve chlorophyll content and vegetative growth in coriander. Inoculation with bacterial isolates-maintained ion homeostasis and also enhanced antioxidant enzymes activities under salt-stress conditions. Inoculation with PGPR resulted in increasing K uptake but decreasing Na uptake. In general, the findings suggested that a combination of Azospirillum brasiliense and Azotobacter chroococcum may be used as PGPR complex to improve growth and health of coriander plants. Therefore, this PGPR bacteria seems to be a promising method and eco-friendly strategy could be used for reduce the harmful effects of salinity stress on coriander cultivation in areas where salinity is a major constraint. Since that our study was conducted in unsterile soil conditions, so more similar to field conditions, but further research is necessary for validating the effectiveness of PGPR in field conditions before recommending large scale coriander cultivation at the agricultural level.
Declarations
Author contribution statement
Z. Rabiei: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
S. J. Hosseini: Conceived and designed the experiments; Wrote the paper.
H. Pirdashti: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Contributed reagents, materials, analysis tools or data. S. Hazrati: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Faculty of Agriculture, Department of Agronomy, Sari Agricultural Sciences and Natural Resources University, Sari, Iran.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Al-Garni S.M., Khan M.M.A., Bahieldin A. Plant growth-promoting bacteria and silicon fertilizer enhance plant growth and salinity tolerance in Coriandrum sativum. J. Plant Interact. 2019;14(1):386–396. [Google Scholar]
- Ashraf M. Photosynthetic capacity and ion accumulation in a medicinal plant henbane (Hyoscyamus niger L.) under salt stress. J. Appl. Bot. Food Qual. 2004;78:91–96. [Google Scholar]
- Assaha D., Ueda A., Saneoka H., Al-Yahyai R., Yaish M. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017;8:509. doi: 10.3389/fphys.2017.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashtanova U., Flowers T. Effect of low salinity on ion accumulation, gas exchange and postharvest drought resistance and habit of Coriandrum sativum L. Plant Soil. 2012;355:199–214. [Google Scholar]
- Bharti N., Pandey S., Barnawal D., Patel V., Kalra A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016;6:34768. doi: 10.1038/srep34768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I., Horst W. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soybean (Glycine max) Physiol. Plantarum. 1991;83:463–468. [Google Scholar]
- Chang C., Locy R., Smeda R., Sahi S., Singh N. Photoautotrophic tobacco cells adapted to grow at high salinity. Plant Cell Rep. 1997;16:495–502. doi: 10.1007/BF01092773. [DOI] [PubMed] [Google Scholar]
- Christen D., Schönmann S., Jermini M., Strasser R., Défago G. Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environ. Exp. Bot. 2007;60:504–514. [Google Scholar]
- del Amor F., Cuadra-Crespo P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct. Plant Biol. 2012;39:82–90. doi: 10.1071/FP11173. [DOI] [PubMed] [Google Scholar]
- Egamberdieva D., Lugtenberg B. Use of plant growth-promoting rhizobacteria to alleviate salinity stress in plants. In: Miransari M., editor. Use of Microbes for the Alleviation of Soil Stresses Volume 1. 2014. pp. 73–96. [Google Scholar]
- Fielding J., Hall J. A biochemical and cytochemical study of peroxidase activity in roots of Pisum sativum. J. Exp. Bot. 1978;29:969–981. [Google Scholar]
- Figueiredo M., Martinez C., Burity H., Chanway C. Plant growth-promoting rhizobacteria for improving nodulation and nitrogen fixation in the common bean (Phaseolus vulgaris L.) World J. Microbiol. Biotechnol. 2008;24:1187–1193. [Google Scholar]
- Forni C., Ducak D., Glic B. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil. 2017;410:335–356. [Google Scholar]
- Fredj M., Zhani K., Hannachi C., Mehwachi T. Effect of NaCl priming on seed germination of four coriander cultivars (Coriandrum sativum) EurAsia J. BioSci. 2013;7:11–29. [Google Scholar]
- Glick B., Penrose D., Li J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998;190:63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- Grover M., Ali S., Sandhya V., Rasul A., Venkateswarlu B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011;27:1231–1240. [Google Scholar]
- Habib S., Kausar H., Saud H. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. BioMed Res. Int. 2016;10 doi: 10.1155/2016/6284547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajiboland R., Aliasgharzadeh N., Laiegh S., Poschenrieder C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil. 2010;331:313–327. [Google Scholar]
- Han H., Lee K. Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res. J. Agric. Biol. Sci. 2005;1:210–215. [Google Scholar]
- Heidari M., Golpayegani A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.) J. Saudi Soc. Agric. Sci. 2012;11:57–61. [Google Scholar]
- Hong Y., Glick B., Pasternak J. Plant-microbial interaction under gnotobiotic conditions: a scanning electron microscope study. Curr. Microbiol. 1991;23:111–114. [Google Scholar]
- Ilangumaran G., Smith D. Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front. Plant Sci. 2017;8:1768. doi: 10.3389/fpls.2017.01768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İpek M., Eşitken A. The Actions of PGPR on micronutrient availability in soil and plant under calcareous soil conditions: an evaluation over Fe nutrition. In: Singh D., Singh H., Prabha R., editors. Plant-Microbe Interactions in Agro-Ecological Perspectives. Springer; Singapore: 2017. (Plant-Microbe Interactions in Agro-Ecological Perspectives). [Google Scholar]
- Kang S., Khan A., Waqas M., You Y., Kim J., Kim J., Hamayun M., Lee I. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014;9:673–682. [Google Scholar]
- Khatri D., Durgapal A., Joshi P. Biofertilization enhances productivity and nutrient uptake of foxtail millet plants. J. Crop Improv. 2016;30:32–46. [Google Scholar]
- Li J., Hu L., Zhang L., Pan X., Hu X. Exogenous spermidine is enhancing tomato tolerance to salinity–alkalinity stress by regulating chloroplast antioxidant system and chlorophyll metabolism. BMC Plant Biol. 2015;15:303. doi: 10.1186/s12870-015-0699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Malfanova N., Kamilova F., Berg G. Plant growth promotion by microbes. In: de Bruijn Frans J., editor. first ed. Vol. 2. John Wiley & Sons, Inc.; 2013. pp. 559–573. (Molecular Microbial Ecology of the Rhizosphere). [Google Scholar]
- Mahmoud O., Slimene I., Zribi O., Abdelly C., Djébali N. Response to salt stress is modulated by growth-promoting rhizobacteria inoculation in two contrasting barley cultivars. Acta Physiol. Plant. 2017;39:1–13. [Google Scholar]
- Maksimović J., Zhang J., Zeng F., Živanović B., Shabala L., Zhou M., Shabala S. Linking oxidative and salinity stress tolerance in barley: can root antioxidant enzyme activity be used as a measure of stress tolerance? Plant Soil. 2013;365:141–155. [Google Scholar]
- Marcelis L., Hooijdonk J. Effect of salinity on growth, water use and nutrient use in radish (Raphanus sativus L.) Plant Soil. 1999;215:57–64. [Google Scholar]
- Mayak S., Tirosh T., Glick B. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004;42:565–572. doi: 10.1016/j.plaphy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Mayak S., Tirosh T., Glick B. Plant growth-promoting bacteria that confer resistance to water stress in tomato and pepper. Plant Sci. 2004;166:525–530. doi: 10.1016/j.plaphy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Mishra B., Dubey P., Aishwath O., Kant K., Sharma Y., Vishal M. Effect of plant growth promoting rhizobacteria on coriander (Coriandrum sativum) growth and yield under semi-arid condition of India. Indian J. Agric. Sci. 2017;87:607–612. [Google Scholar]
- Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nautiyal C., Srivastava S., Chauhan P., Seem K., Mishra A., Sopory S. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013;66:1–9. doi: 10.1016/j.plaphy.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Nadeem S.M., Ahmad M., Zahir Z.A., Javaid A., Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014;32:429–448. doi: 10.1016/j.biotechadv.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Neffati M., Sriti J., Hamdaoui G., Kchouk M., Marzouk B. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem. 2011;124:221–225. [Google Scholar]
- Porra R. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002;37:149–156. doi: 10.1023/A:1020470224740. [DOI] [PubMed] [Google Scholar]
- Rojas-Tapias D., Moreno-Galván A., Pardo-Díaz S., Obando M., Rivera D., Bonilla R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays) Appl. Soil Ecol. 2004;61:264–272. [Google Scholar]
- Sarkara A., Ghosh P., Pramanik K., Mitra S., Soren T., Pandeyd S., Mondal M., Maitia T. A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Res. Microbiol. 2018;1:20–32. doi: 10.1016/j.resmic.2017.08.005. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . Release 90. Statistical Analysis Systems Institute; Cary, NC, USA: 2004. The SAS system for windows. [Google Scholar]
- Setiawati C., Mutmainnah L. Solubilization of potassium containing mineral by microorganisms from sugarcane rhizosphere. Agric. Agric. Sci. Proc. 2016;9:108–117. [Google Scholar]
- Shah S.H., Houborg R., McCabe M.F. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.) Agron. 2017;7(3):61. [Google Scholar]
- Sharma P., Jha A., Dubey R., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012;1–26 [Google Scholar]
- Sharma A., Shankhdhar D., Sharma A., Shankhdhar S. Micronutrient enhancement and localization in rice grains under influence of plant growth promoting rhizobacteria. J. Crop. Improv. 2014;28:502–517. [Google Scholar]
- Sivritepe N., Sivritepe H., Eris A. The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci. Hortic. (Canterb.) 2013;97:229–237. [Google Scholar]
- Stefan M., Munteanu N., Stoleru V., Mihasan M. Effects of inoculation with plant growth promoting rhizobacteria on photosynthesis, antioxidant status and yield of runner bean. Rom. Biotechnol. Lett. 2013;18:8131–8143. [Google Scholar]
- Tank N., Saraf M. Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact. 2010;5:51–58. [Google Scholar]
- Vivas A., Marulanda A., Ruiz-Lozano J., Bareaand J., Azcón R. Influence of a Bacillus sp. on physiological activities of two arbuscular mycorrhizal fungi and on plant responses to PEG-induced drought stress. Mycorrhiza. 2003;13:249–256. doi: 10.1007/s00572-003-0223-z. [DOI] [PubMed] [Google Scholar]
- Warwate S., Kandoliya U., Bhadja N., Golakiya B. The effect of seed priming with plant growth promoting rhizobacteria (PGPR) on growth of coriander (Coriandrum sativum L.) seedling. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:1926–1934. [Google Scholar]
- Younesi O., Moradi A. Effects of plant growth-promoting rhizobacterium (PGPR) and arbuscular mycorrhizal fungus (AMF) on antioxidant enzyme activities in salt-stressed bean (phaseolus vulgaris L.) Agric. For. 2014;60:10–21. [Google Scholar]
- Younesi O., Chaichi M., Postini K. Salt tolerance in alfalfa following inoculation with Pseudomonas. Middle East J. Sci. Res. 2013;16:101–107. [Google Scholar]
- Yousefi S., Kartoolinejad D., Bahmani M., Naghdi R. Effect of Azospirillum lipoferum and Azotobacter chroococcum on germination and early growth of hopbush shrub (Dodonaea viscosa L.) under salinity stress. J. Sustain. For. 2017;36:107–120. [Google Scholar]
- Zeid H., Hassan I. Salinity ameliorating effect of aqueous solutions of Nigella sativa treatment on faba bean seedlings. Afr. J. Plant Sci. 2011;5:13–21. [Google Scholar]