Abstract
Despite increased malaria control efforts, school-aged children (5–14 years) have higher a malaria prevalence compared to children under-five. In high-transmission settings, up to 70% of school-aged children harbour malaria parasitaemia and therefore contribute significantly to the reservoir for transmission. A systematic review was performed to explore the correlation between the malaria parasite carriage in pregnant women and school-aged children living in similar endemic settings of sub Saharan Africa to inform strategies to improve targeted malaria control.
In order to obtain data on malaria prevalence in pregnant women and school-aged children living in the same endemic setting, we searched the Malaria in Pregnancy Library, PubMed, Cochrane library and Web of Science in December 2018. We fit a fixed effect model to obtain a pooled risk ratio (PRR) of malaria in school-aged children versus pregnant women and used Poisson regression to estimate risk ratios in school-aged children for every increase in prevalence in pregnant women. We used data from six (out of 1096) sources that included 10 data points. There was a strong linear relation between the prevalence of malaria infection in pregnant women and school-aged children (r = 0·93, p < 0·0001). School-aged children were nearly twice at risk to carry parasites compared to pregnant women (RR = 1.95, 95% CI: 1·69–2.25, p < 0.01). Poisson regression showed that a 1% increase in prevalence of malaria infection in pregnant women was significantly associated with increase in risk in school-aged children by 4%.
Malaria infection prevalence in school-aged children is strongly correlated with the prevalence in pregnant women living in the same community, and may be considered as alternative indicators to track temporal and spatial trends in malaria transmission intensity. Chemoprevention strategies targeting school-aged children should be explored to reduce malaria burden and transmission in school-aged children and its potential impact on communities.
Keywords: Malaria burden, School-aged children, Pregnant women, Correlation, Risk-ratio, Transmission intervention
Graphical abstract
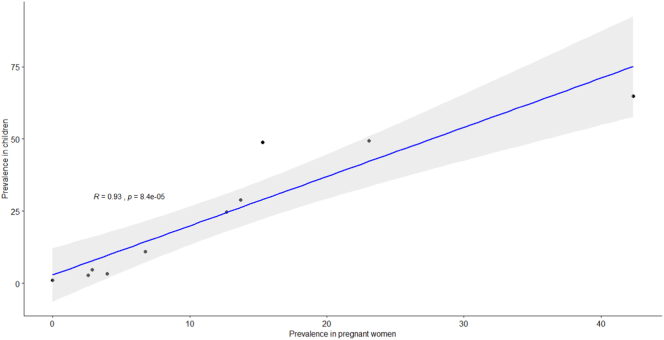
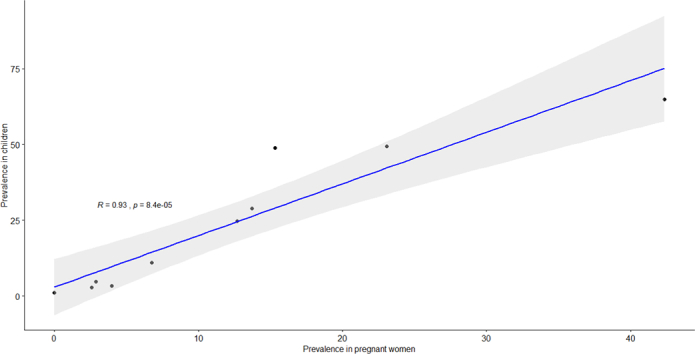
Fig. 5 Scatter plot with Pearson correlation coefficient of malaria parasitaemia in pregnant women and school-aged children living in a common setting, sub Saharan Africa
1. Introduction
Malaria remains a major public health threat despite considerable progress on control in the past decade. The World Health Organisation (WHO) 2019 malaria report estimated 228 million cases and 405,000 deaths related to malaria in the year 2018, with the African region bearing the brunt, with 93% of all cases (World Health Organization, 2019). Malaria control interventions often target vulnerable populations, pregnant women and children under-fives (Walldorf et al., 2015). However, the burden of malaria in terms of detectable parasitaemia, asymptomatic and submicroscopic infections in school-aged children (5–14 years) remain underappreciated (Staedke et al., 2018). A previous systematic review (Nankabirwa et al., 2014) reported, a prevalence of malaria parasitaemia among school-aged children ranging from 18 to 83% at different time points across sixteen high transmission settings in sub-Saharan countries. In endemic settings, among school-aged children, malaria accounts for about 13–50% of all school absenteeism (Bundy et al., 2000), causing anaemia and around 50% of the their age specific overall mortality (Snow et al., 2003), which in turn impairs the educational achievement of children (Clarke et al., 2017; Halliday et al., 2012; Clarke et al., 2008). Recent changes in malaria epidemiology puts school-aged children increasingly more at risk for malaria due to a delayed acquisition of protective immunity (Nankabirwa et al., 2014). Furthermore, school-aged children act as a malaria reservoir and hence contribute to ongoing malaria transmission, thus undermining the effectiveness of control programs in vulnerable populations (Walldorf et al., 2015; Ministry of health and Social Welfare, 2013; WHO, 2016; van Eijk et al., 2015; World Health Organization. Updated WHO Policy Recommendation (, 2012). Yet, limited interventions are targeting control malaria in school-aged children despite their high burden of malaria infection. Strategies targeting this population could impact malaria transmission and infection control in the community (Staedke et al., 2018).
A recent systematic review (van Eijk et al., 2015) reported a strong correlation between malaria prevalence in children aged 0–59 months and pregnant women (r:0.87, p < 0.0001), with a higher average prevalence observed in children than in pregnant women (pooled prevalence ratio (PPR):1.44, 95%CI:1.2–1.62). It has also been suggested that children above five and children of school age have a higher asymptomatic malaria parasitaemia prevalence than their under-five counterparts (Walldorf et al., 2015; Nankabirwa et al., 2014; Sultana et al., 2017). However, a relationship between malaria parasite carriage in pregnant women and school-aged children has not been established. In light of the diversity of epidemiological indices, such as, malaria endemicity, resistance to antimalarial drugs and insecticide, there is a need to better understand risk distribution in the communities to improve targeted malaria control strategy. We therefore, conducted a systematic review and a meta-analysis, to investigate the correlation between the malaria parasite carriage in pregnant women and school-aged children living in similar endemic settings.
2. Methods
2.1. Search strategy and selection criteria
In 2015 van Eijk et al (van Eijk et al., 2015) published a systematic review that compared prevalence of malaria infection in pregnant women with children under-five years in sub-Saharan Africa. From studies included in van Eijk's publication, we selected studies reporting on prevalence of malaria in children above 5 years. We then performed a complementary search on Malaria In Pregnancy (MIP) database (MIP Consortium, 2018; Van Eijk et al., 2012), PubMed, Cochrane library and Web of Science, to include studies indexed /published until 10th of December 2018. Combinations of the following search terms were used: children OR pregnant women AND malaria OR Plasmodium falciparum AND prevalence AND Africa. Studies obtained after a search were combined in Endnote before transfer to a web and mobile app for systematic reviews (rayyan.qcri.org) (Ouzzani et al., 2016), where eligibility screening was conducted.
Studies undertaken in sub-Saharan Africa recruiting both children and pregnant women were selected without language restriction. Two reviewers GM and SM screened the studies independently and discrepancies were subsequently resolved by consensus. The inclusion criteria were studies that screened for malaria parasitaemia by microscopy or rapid diagnostic test in asymptomatic pregnant women and children aged above 5 years. School- aged children were defined as children aged 5–14 years old. We excluded studies restricted to women or children with a history of fever or malaria, and or diagnosed malaria only at delivery. We extracted data on the source population, participant selection (community based, random sample, asymptomatic individuals), appropriate tests, age of children and completeness of test results. A quality assessment was undertaken for the eligible study population and a study was considered to be of good quality if it met both, the target population, as well as the participant selection. i.e. pregnant women and children selected from the same location, who were tested at about the same study period with the presentation of disaggregated results. A study was ranked moderate quality if it missed one of the criteria but reported on the prevalence of both women and children in the same setting. A study of poor quality was defined as one that did not fulfil the selection criteria.
Following a systematic assessment of studies obtained from search engines, data extracted included study location, study population, sample size, year of study, inclusion and exclusion criteria applied, rainy or dry season, endemicity (high, moderate, or low), malaria diagnostics tests deployed and test results. Whenever, necessary, authors of the included studies were contacted for additional information. Data on prevalence of malaria infection was extracted based on the availability of data on both pregnant women and children aged above 5 years within the same study.
2.2. Statistical analysis
Extracted data was regarded as a binary outcome (i.e. presence or absence of malaria parasite). This was analysed using R statistical software, where Metabin package was used for meta-analysis. The differences between prevalence estimates in pregnant women and children were expressed as pooled prevalence ratio (PPR). The proportion of total variability explained by heterogeneity rather than chance was measured using the I2, and was expressed as percentage, as previously reported (van Eijk et al., 2015; Higgins et al., 2003), 0–40% represented no or low heterogeneity, 30–60% moderate heterogeneity, 50–90%substantial heterogeneity, and 75–100% considerable heterogeneity (van Eijk et al., 2015; Deeks et al., 2008; O'Connor et al., 2008). Pooled prevalence ratio was obtained by meta-analysis using DerSimonian and Laird random effect models (DerSimonian and Kacker, 2007). Pearson correlation coefficient was used to determine the extent of correlation between the prevalence of malaria in pregnant women and that in children above 5 years. Scatter plot was made using “ggscatter” function that also showed Pearson correlation coefficient. We developed Meta plots using metabin function and reported in risk ratio “RR” summary measures, and Mantel-Haenszel (MH) and DerSimonian-Laird estimator (DL) methods. Forest plots were made to report both fixed effect model (FEM) and Random effect model (REM) in RR. Rank correlation test was used to test funnel plots asymmetry using Metabias function. Funnel plot trim and fill method was used to determine publication bias. We used a Poisson regression model to determine risk of malaria infection in school-aged children for every increase in prevalence of malaria infection in pregnant women, factoring seasonality and endemicity.
2.3. Role of the funding source
The funding institution had no role in the study design, data extraction, analysis and interpretation of the data, or preparation, review, or approval of the paper. GM had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Search results
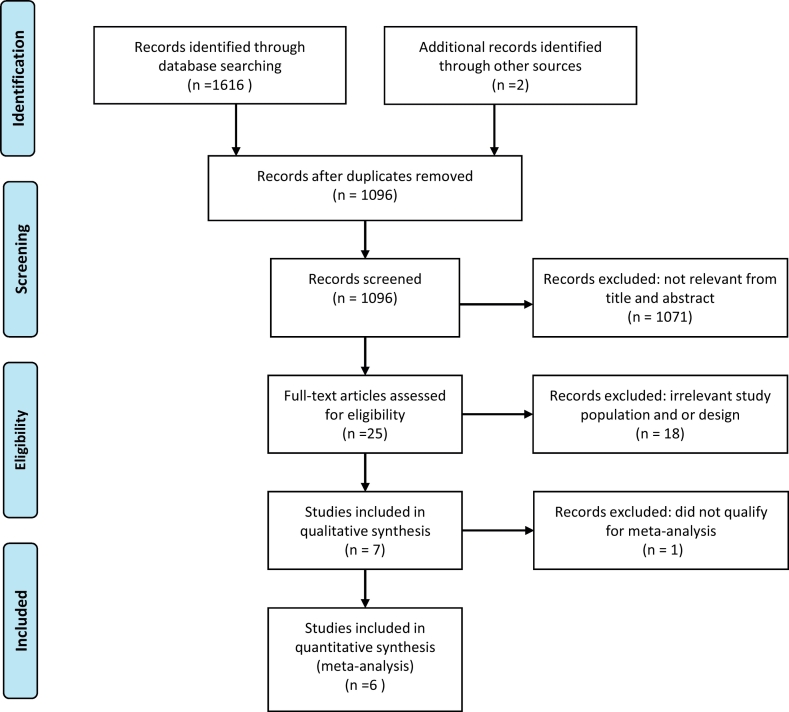
We identified a total of 1618 articles through database searching (n = 1616) and 2 were presented in an article by van Eijk et al. (van Eijk et al., 2015). After the removal of duplicates, 1096 articles were screened, of which 1071 were articles that did not explore the outcome of interest nor meet the inclusion criteria, e.g. with one population only i.e. studies that enrolled pregnant women or children only. The remaining 25 articles were accessed for further screening through full text reading. Eighteen of the articles were excluded either due to an outcome which was not relevant to our study or due to a study design which did not meet our inclusion criteria. The seven remaining studies were eligible for qualitative assessments. Finally, six studies of good quality (n = 6) were included for meta- analysis (Fig. 1). One study (Lamptey et al., 2018) had four surveys conducted at different time periods and another study consisted of two surveys carried out in different time periods (Dicko et al., 2005), these were considered independent surveys and hence made to 10 data points included in the meta-analysis.
Fig. 1.
PRISMA study flow diagram of asymptomatic malaria prevalence rate in pregnant women and school-aged children living in common settings in sub-Saharan Africa (Moher et al., 2009).
3.2. Description of studies
Table 1 provides information about characteristics of each study in the analysis. Studies took place between 1993 and 2014. All of the studies recruited participants from the community, except one that recruited from both, the community and the hospital outpatient department (Owusu et al., 2017). Malaria was tested using both microscopy (8 data points) (Dicko et al., 2005; Owusu et al., 2017; Sonko et al., 2014; Dicko et al., 2003) and rapid diagnostic tests (3 data points) (Diarra et al., 2017; Ministry of Health, 2008-2009). Five data points were studies conducted in hyperendemic regions and six sources were from low endemic regions. Six of the 11 data points were studies conducted during or immediately after the rainy season. All sources were considered of good quality again here except for one (Owusu et al., 2017), that was evaluated as moderate quality. However, most of these studies had varying primary objectives.
Table 1.
Studies included in the pooled analyses of asymptomatic malaria parasitaemia prevalence in pregnant women and school-aged children living in sub-Saharan Africa.
| Reference | Location | Endemicity | Season | Malaria test | Study year |
Pregnant women |
Children |
Quality | Risk Ratio | %Weight (FEM) | % Weight (REM) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # Tested | # Positive | # Tested | # Positive | Age group (years) | ||||||||||
| (Dicko et al., 2003) | Mali Bandiagara | Hyperendemic | Rainy season | Mx | 1993 | 111 | 47 | 1230 | 797 | 5 to 10 | Good | 1.53 (1.23–1.91) | 30.8 | 21.4 |
| (Dicko et al., 2005) | Mali Bandiagara | Hyperendemic | Dry season | Mx | 1994 | 124 | 19 | 1191 | 581 | 5 to 10 | Good | 3.18 (2.10–4.84) | 12.3 | 14.5 |
| (Ministry of Health, 2008-2009) | Equatorial Guinea Bioko | Low endemic | Rainy season | RDT | 2009 | 197 | 27 | 3112 | 896 | 5 to 14 | Good | 2.10 (1.47–3.00) | 18.1 | 16.6 |
| (Ministry of Health, 2008-2009) | Equatorial Guinea Bioko | Low endemic | Rainy season | RDT | 2008 | 284 | 36 | 2020 | 500 | 5 to 14 | Good | 1.95 (1.43–2.67) | 22.6 | 18.0 |
| (Sonko et al., 2014) | The Gambia | Hyperendemic | Dry season | Mx | 2010 | 148 | 10 | 1987 | 219 | 5 to 14 | Good | 1.63 (0.89–3.01) | 6.7 | 9.6 |
| (Lamptey et al., 2018) | Accra, Ghana | Low endemic | Dry season | Mx | 2013 | 126 | 5 | 184 | 6 | 5 to 11 | Good | 0.82 (0.26–2.63) | 2.1 | 3.6 |
| (Lamptey et al., 2018) | Accra, Ghana | Low endemic | Dry season | Mx | 2014 | 77 | 2 | 182 | 5 | 5 to 11 | Good | 1.06 (0.21–5.33) | 1.0 | 2.0 |
| (Lamptey et al., 2018) | Accra, Ghana | Low endemic | Rainy season | Mx | 2014 | 70 | 2 | 170 | 8 | 5 to 11 | Good | 1.65 (0.36–7.56) | 1.0 | 2.2 |
| (Lamptey et al., 2018) | Accra, Ghana | Low endemic | Dry season | Mx | 2014 | 54 | 0 | 180 | 2 | 5 to 11 | Good | 1.51 (0.07–30.97) | 0.3 | 0.6 |
| (Owusu et al., 2017) | Ghana | Hyperendemic | Rainy season | Mx | 2014 | 94 | 0 | 40 | 9 | 6 to 10 | Moderate | 44.33 (2.64–743.69) | 0.1 | 0.7 |
| (Diarra et al., 2017) | Chad | Hyperendemic | Rainy season | RDT | 2012 | 52 | 12 | 73 | 36 | 5 to 14 | Good | 2.14 (1.24–3.70) | 5.0 | 10.9 |
| Total | 1337 | 160 | 10,369 | 3059 | 2.00 (1.73–2.30) | 100.0 | 100.0 | |||||||
Mx: Microscopy, RDT: Malaria rapid diagnostic test, FEM: Fixed effect Model, REM: Random effect model.
3.3. Analysis results
There was a strong linear relation between the asymptomatic malaria prevalence rate in pregnant women and school-aged children (r = 0·93, 95% CI 0.73–0.98, p < 0·0001), (Fig. 2).
Fig. 2.
Scatter plot with Pearson correlation coefficient for asymptomatic malaria prevalence rate in school-aged children versus pregnant women living in a common setting, sub-Saharan Africa.
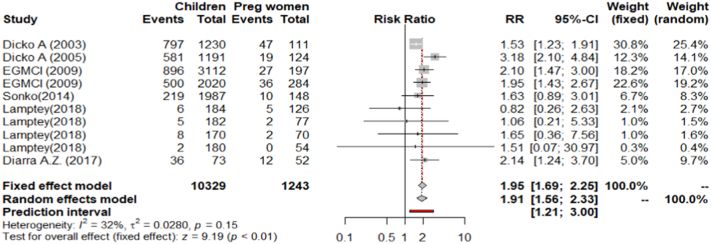
On average asymptomatic malaria prevalence rate in pregnant women was 12% (160/1337) and that of school-aged children 30% (3059/10369). The fixed effect model (FEM) showed risk was two-fold higher in school-aged children compared to pregnant women (PRR = 1.95, 95% CI 1·69–2.25, p < 0.001). There was a moderate non-significant heterogeneity between studies (I2 = 32.2%, CI 0.0%–67.6%, p = 0.15), (Fig. 3). When data from Owusu et al., 2017 (a study that had a moderate quality on evaluation) the heterogeneity increased to 46% (p = 0.05) and the PRR was 2.0, (95% CI 1.73–2.30, p < 0.01).
Fig. 3.
Forest plot showing relative risks of malaria infection in school-aged children compared to pregnant women in a common setting, sub-Saharan Africa.
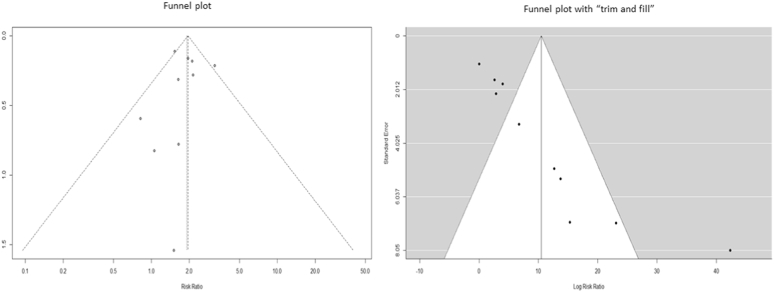
As funnel plots showed that most studies fell within the limits, with a rank correlation test not being statistically significant (p = 0.72, ks = −4.0000, se.ks = 11.18), there appears to be no evidence of bias in the included studies. The trim and fill method showed no pseudo studies could have changed the overall fixed effect. (Fig. 4).
Fig. 4.
Funnel plots showing distribution of studies involved in meta-analysis.
Poisson regression showed that a 1% increase in prevalence of malaria infection in pregnant women was associated with an increase in risk in children above 5 by 4% (p < 0.001). Factors such as rainy season and endemicity did not appear to exert a significant influence on the risk ratio for asymptomatic malaria prevalence rate in school-aged children compared to pregnant women.
4. Discussion
We conducted a meta-analysis to compare the asymptomatic malaria prevalence rate, as detected by microscopy or rapid diagnostic malaria tests, in pregnant women with the asymptomatic malaria prevalence in school-aged children using data from studies conducted in the sub-Saharan Africa testing both pregnant women and children all together in the same location or region and in the same calendar period. Characteristically, the included studies were conducted at different time period, factoring seasonality (rainy or dry), and endemicity, which are important in malaria transmission (Rek et al., 2016; Greenwood, 2010; Omondi et al., 2017). Though most of the studies included were from West African countries, the diversity of endemicity and seasonality mimic the setting in other sub-Saharan countries. Malaria infection prevalence comparisons drawn in this study for a population residing in the same community has generated meta-analysis results important for public health.
This review and analysis followed the basic protocol used by the MiP consortium in a similar comparison of malaria parasitaemia rate in pregnant women versus children under-five years old (van Eijk et al., 2015). We found that school-aged children were twice more at risk (RR: 1.95; 95% CI 1.69–2.25) than pregnant women. This was a higher risk compared to the risk reported for a comparison between pregnant women and children under-five (PPR 1.44 95%CI 1.29–1.62), (van Eijk et al., 2015). We also found a stronger correlation (r = 93%, p ≤0.0001) in malaria prevalence between school-aged children and pregnant women. This was also higher than the reported correlation between pregnant women and children under-five (r = 87%) (van Eijk et al., 2015). The analysis revealed the relationship on asymptomatic parasite carriage in school-aged children and pregnant women of the same community is fairly robust. Additionally, this analysis has demonstrated that school-aged children were likely to be asymptomatic carriers, and act as reservoir for perpetual malaria transmission in the community. Van Eijk et al., 2015 (van Eijk et al., 2015) also found the correlation in children under-five and pregnant women was more noticeable with increasing child's age. We therefore suggest, testing school-aged children may be convenient and an indicative approach for estimating the community parameter.
In most sub-Saharan African countries antenatal care (ANC) is limited in terms of availability of infrastructures such as a ‘nearby’ health facility, poverty and health awareness of the pregnant women (WHO, n.d.; Kyei-Nimakoh et al., 2017). In these countries, whilst more than 80% of pregnant women would make at least one ANC visit, their adherence to subsequent visits for the recommended IPTp 3+ policy (WHO, 2014) remains poor (WHO, 2015), with only 24% making their first ANC visit during the first trimester (Mgata and Maluka, 2019; Chimatiro et al., 2018). Several studies have suggested pregnant women attending their first ANC could provide data as proxy for malaria transmission or as sentinel population for malaria surveillance (van Eijk et al., 2015; Kitojo et al., 2019; Brunner et al., 2019). However, the same studies reports some shortfalls in using ANC data such as variation in sensitivity at different endemicities, suggesting ANC data was particularly useful for monitoring in areas approaching elimination (Kitojo et al., 2019). Moreover, ANC malaria test-positivity cannot be used to directly predict the prevalence in other population subgroups, where complementary community-level measurements remain highly relevant (Brunner et al., 2019). Other limitations are typically associated with routine data, such as imperfect reporting and difficult to quantify errors in data collection, transcription, entry, and collation at all levels (Kitojo et al., 2019). In addition, clinics may lack or have frequent stock outs of testing kits hence prioritize testing only patients presenting with symptoms (Kitojo et al., 2019). Likewise, focusing on pregnant women alone, though less expensive, has in some cases, shown that social mobility of pregnant women may make them non-representative of the referenced testing site or community due to various prevailing social cultural factors (Moller et al., 2017; Atuhaire and Mugisha, 2020; Madhi et al., 2018).
As suggested by (Simmons et al., 2017), we argue that ANC data might not show trends on malaria transmission and exposure (including effect of an intervention) in the respective community, since it is difficult to obtain longitudinal data from ANC owing to poor ANC attendance on successive visits (Atuhaire and Mugisha, 2020; Madhi et al., 2018; Nuamah et al., 2019). Furthermore, malaria infection risk among pregnant women themselves is influenced by gravidity where primigravidae (pregnant for the first time) are at greater risk than multigravidae (pregnant more than one time) (van Eijk et al., 2015; Kitojo et al., 2019),hence, poor documentation of parity at ANC may compromise interpretation and inference to the general population.
Likewise, given the dynamics on malaria infection and immunity (Langhorne et al., 2008), pregnant women being adults are likely to have a stronger acquired immunity to malaria, and that malarial parasites are likely to sequester in the placenta rendering it difficult to detect on peripheral smear (van Eijk et al., 2015; Cowman et al., 2016).Therefore, as noted elsewhere, ANC malaria test-positivity cannot be used to directly predict the prevalence in other population subgroups, prompting the need of complementary community-level measurements (Brunner et al., 2019). School-aged children are immunologically in transition i.e. have a partial immunity and are consequently more vulnerable to parasite infection than adults (Langhorne et al., 2008; Cowman et al., 2016; Rodriguez-barraquer et al., 2018). Studies have demonstrated a high asymptomatic malaria prevalence in this age group (Nankabirwa et al., 2014; Clarke et al., 2008; Sultana et al., 2017; Nankabirwa et al., 2013; Nankabirwa et al., 2015; Yeka et al., 2015; Ahorlu et al., 2009). Conversely, in high transmission areas, children under-five are more at risk of severe malaria due to lack of acquired immunity (World Health Organization, 2019; Langhorne et al., 2008; Cowman et al., 2016; World Health Organization, n.d.; Ndungu et al., 2012). Thus, testing children under-five cannot be a convenient and an indicative approach for estimating the community parameter, since they are likely to be symptomatic and it would be pragmatically challenging to gather children under-five, especially those post vaccination ages.
Our Poisson regression model (Table 2) predicted that a 1% increase in prevalence of malaria infection in pregnant women was significantly associated with an increase in risk in school-aged children by 4% (p < 0.0001) after adjusting for potential confounders. This suggests that malaria infection prevalence in school-aged children could offer an alternative approach to track temporal and spatial trends in malaria transmission intensity and would therefore be able to predict the risk in pregnant women in the same community. All these factors conspire to illustrate the benefits of school-based surveys performed longitudinally in the same community to tackle the shortfalls of relying solely on ANC data. Such surveys can be conducted twice in a year (matching malaria seasonality) after every one or two years, and can be used to assess the impact of any intervention introduced beforehand.
Table 2.
Risk of asymptomatic malaria infection in children factoring pregnant women, rainy season and endemicity.
| Variable | Coefficient estimate | Std. Error | t value | Pr(>|t|) |
|---|---|---|---|---|
| Intercept | −1.77917 | 0.07366 | −24.154 | <2e-16 |
| Prevalence in pregnant women | 0.04195 | 0.0042 | 9.986 | <2e-16⁎ |
| Rainy season | −0.16123 | 0.12303 | −1.31 | 0.19 |
| Hyperendemic | −0.15078 | 0.11582 | −1.302 | 0.193 |
p-value <0.0001.
Malaria indicator surveys(MIS) mostly focus on pregnant women and children under-five to assess malaria prevalence as a monitoring indicator for various malaria intervention being rolled out (WHO, 2005; The DHS, 2020; Partnership, 2018). These surveys are being conducted in a cross-sectional design during high transmission seasons, and since they exclude school-aged children, they fail to incorporate important elements on transmission trends and age-group shifts where school-aged children nowadays play an important role (Nankabirwa et al., 2014; Chacky et al., 2018). This underscores the importance of quantifying the impact of control strategies on exposure and infection to inform on improvement and or scale up of effective strategies (Simmons et al., 2017; Hay et al., 2010). School malaria surveys (Chacky et al., 2018), if well programmed and employ a longitudinal design, can be used to complement both ANC data and MIS (or can as well be incorporated on MIS). Pragmatically, school-aged children can be accessed throughout the year, hence making it more amenable to comparisons on malaria transmission at different seasons, and hence inform the impact of malaria intervention in the same community.
Intermittent preventive treatment of malaria in pregnant women (IPTp), and in infants (IPTi), and seasonal malaria chemoprevention (SMC) do not include school-aged children and that despite these efforts malaria burden in Africa is still high (World Health Organization, 2019; WHO, 2018). Recently, there have been efforts to expand the age limit for SMC which has yet to become a policy (Cissé et al., 2016). Malaria infection in school-aged children is mostly asymptomatic (Walldorf et al., 2015; Nankabirwa et al., 2014; Nankabirwa et al., 2013; Yeka et al., 2015) making them reservoirs for transmission within their communities, at the cost of increased malaria related morbidities such as anaemia and impaired cognitive development (Nankabirwa et al., 2014; Clarke et al., 2008; Nankabirwa et al., 2013; Maccario et al., 2017) in this age group. Given the high correlation shown (r = 93%), targeted interventions such as (IPT) need to be expanded to school-aged children (IPTsc) since they are more likely to harbour malaria parasites compared to under-fives and pregnant women (Walldorf et al., 2015; Staedke et al., 2018). This would likely reduce the parasite burden in children and boost the effectiveness of other control programs including IPTp and SMC (Staedke et al., 2018). Recent evidence in Uganda demonstrated that with high coverage, implementation of IPTsc using Dihydroartemisinin-Piperaquine (DP) was effective in reducing community parasite prevalence and entomological inoculation rates (Staedke et al., 2018). Implementation of IPT can reduce undetected asymptomatic and sub-microscopic infections that are hampering control efforts. However, further studies are needed to explore safe and effective antimalarial drugs for IPTsc.
We suggest that IPT be extended to school-aged children because other malaria control interventions e.g. bednets, are accessible to both pregnant women and school-aged children. However, targeted intervention such as IPT has not been extensively applied in this age group (WHO, 2015). Furthermore, given the fact that primary school enrolment in most African countries has significantly improved (UNICEF, 2017; Warady et al., 2014; World Bank, n.d.; Zerihun et al., n.d.; World Bank, 2018), school-aged children can be easily accessed for a seasonal surveys in longitudinal studies. Therefore, schools may be used to track malaria transmission trends and predict effectiveness of intervention on pregnant women even when access to IPTp is poor. As a corollary, schools could be established as a proxy to track hotspot areas for other malaria control measures.
School-aged children could benefit from long lasting insecticide treated nets (LLITN). However, studies have shown that bednet usage in this age group is challenging and complementary interventions are clearly needed (Noor et al., 2009; Olapeju et al., 2018; Buchwald et al., 2016). In Kenya after nationwide net distribution, increased parasite prevalence was observed in asymptomatic school-aged children (Zhou et al., 2011). This could have been due to decreased efficacy of the insecticide or inappropriate use of the bednets. Yet, this may further indicate that school-aged children would benefit much from IPT if added to complement existing control programme. Studies (Clarke et al., 2017; Nankabirwa et al., 2010; Trial et al., 2014; Matangila et al., 2017) on IPTsc have shown high impact on reducing malaria prevalence, anaemia and improving cognitive development in school children. Involving school children in health programs has been shown to be feasible (Cissé et al., 2016; Ayi et al., 2010; Afenyadu et al., 2005).
Our findings are subject to several limitations; firstly, there was a lack of studies adhering to our criteria from other regions of sub-Saharan Africa. Secondly, some studies or the corresponding authors could not be accessed. Thirdly, in most endemic countries, data relying on malaria indicator surveys was limited to pregnant women and children under-five, and the few studies obtained, did not document gravidity of pregnant women tested. All these reasons conspired to limit the number of data sources included in this review. However, we managed to screen as many articles (n = 1096) as possibly obtained on the five databases we searched. Use of sensitive versions of PCR would have been effective in parasite detection in sub-microscopic and asymptomatic individuals, but for a given setting in the sub-Saharan Africa where most countries rely on mRDT or microscopy for testing, generalisation of the findings to pragmatic implementation has been highly considered and our results have coincided with other reviews (van Eijk et al., 2015).
In conclusion, our meta-analysis suggests that school-aged children have a two-fold higher risk of carrying malaria parasitaemia compared to pregnant women. Due to the strong correlation and high school attendance rate, malaria infection prevalence in school-aged children may be considered as an alternative indicator to track temporal and spatial trends in malaria transmission intensity longitudinally. Extending IPT strategies to school-aged children as a complementary intervention could contribute to reducing malaria burden and transmission in the communities. Future studies are warranted to evaluate effectiveness of malaria chemoprevention approaches in school-aged children and its impact in reducing community transmission to support control and elimination efforts.
Acknowledgments
Acknowledgments
The Flemish Interuniversity Council (VLIR-UOS), Belgium is acknowledged for funding. The authors would like to thank the GHI-UA for training on Systematic review through the Epidemiology Biostatistics and Qualitative analysis course (EBQ). They also thank Dr. Bruno Mmbando from NIMR, Tanga Centre for helping on interpretation of the analysed data.
Contributors
GM, JPVg, conceived and designed the study. GM and SM did the literature search and acquired the data. GM, JPVg, SN, CDR, SM and FF analysed and interpreted the data. GM wrote the first draft of the paper. JPVg, VB, DTRM, CDR, SM and JPL, critically revised subsequent drafts of the paper. All authors approved the final version. JPVg and JPL obtained funding.
Funding
This review was funded by the Flemish Interuniversity Council (VLIR-UOS), Belgium, TEAM initiative, grant number TZ2017TEA451A102 received to the Global Health Institute, University of Antwerp (GHI-UA) and the National Institute for Medical Research (NIMR), Tanga Centre, Tanzania.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Afenyadu G.Y., Agyepong I.A., Barnish G., Adjei S. Improving access to early treatment of malaria: a trial with primary school teachers as care providers. Tropical Med. Int. Health. 2005;10:1065–1072. doi: 10.1111/j.1365-3156.2005.01484.x. [DOI] [PubMed] [Google Scholar]
- Ahorlu C.K., Koram K.A., Seakey A.K., Weiss M.G. Vol. 7. 2009. Effectiveness of combined intermittent preventive treatment for children and timely home treatment for malaria control; pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atuhaire S., Mugisha J.F. Vol. 11. 2020. Determinants of antenatal care visits and their impact on the choice of birthplace among mothers in Uganda : a systematic review; pp. 77–81. [DOI] [Google Scholar]
- Ayi I., Nonaka D., Adjovu J.K., Hanafusa S., Jimba M., Bosompem K.M. School-based participatory health education for malaria control in Ghana: engaging children as health messengers. Malar. J. 2010;9:1–12. doi: 10.1186/1475-2875-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner N.C., Chacky F., Mandike R., Mohamed A., Runge M., Thawer S.G. The potential of pregnant women as a sentinel population for malaria surveillance. Malar. J. 2019;18:1–10. doi: 10.1186/s12936-019-2999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald A.G., Walldorf J.A., Cohee L.M., Coalson J.E., Chimbiya N., Bauleni A. Bed net use among school-aged children after a universal bed net campaign in Malawi. Malar. J. 2016 doi: 10.1186/s12936-016-1178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy D.A.P., Lwin S., Osika J.S., McLaughlin J., Pannenborg C.O. What should schools do about malaria? Parasitol. Today. 2000;16:181–182. doi: 10.1016/s0169-4758(00)01658-6. [DOI] [PubMed] [Google Scholar]
- Chacky F., Runge M., Rumisha S.F., Machafuko P., Chaki P., Massaga J.J. Nationwide school malaria parasitaemia survey in public primary schools, the United Republic of Tanzania. Malar. J. 2018;17:1–16. doi: 10.1186/s12936-018-2601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimatiro C.S., Hajison P., Chipeta E., Muula A.S. Understanding barriers preventing pregnant women from starting antenatal clinic in the first trimester of pregnancy in Ntcheu District-Malawi. Reprod. Health. 2018;15:1–7. doi: 10.1186/s12978-018-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cissé B., Ba E.H., Sokhna C., NDiaye J.L., Gomis J.F., Dial Y. Effectiveness of seasonal malaria chemoprevention in children under ten years of age in Senegal: a stepped-wedge cluster-randomised Trial. PLoS Med. 2016;13:1–18. doi: 10.1371/journal.pmed.1002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S.E., Jukes M.C., Njagi J.K., Khasakhala L., Cundill B., Otido J. Effect of intermittent preventive treatment of malaria on health and education in school children: a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:127–138. doi: 10.1016/S0140-6736(08)61034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S.E., Rouhani S., Diarra S., Saye R., Bamadio M., Jones R. Impact of a malaria intervention package in schools on Plasmodium infection, anaemia and cognitive function in schoolchildren in Mali: a pragmatic cluster-randomised trial. BMJ Glob. Health. 2017;2 doi: 10.1136/bmjgh-2016-000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A.F., Healer J., Marapana D., Marsh K. Malaria: biology and disease. Cell. 2016;167:610–624. doi: 10.1016/j.cell.2016.07.055. [DOI] [PubMed] [Google Scholar]
- Deeks J.J., Higgins J.P., Altman D.G. Analysing data and undertaking meta-analyses. Cochrane Handb. Syst. Rev. Interv. Cochrane B. Ser. 2008 doi: 10.1002/9780470712184.ch9. [DOI] [Google Scholar]
- DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Diarra A.Z., Dabo A., Saye R., Coulibaly D., Guindo M.A., Sagara I. Entomological and parasitological parameters of malaria transmission in Douguia, Chad. Med Sante Trop. 2017;27:253–259. doi: 10.1684/mst.2017.0715. [DOI] [PubMed] [Google Scholar]
- Dicko A., Mantel C., Thera M.A., Doumbia S., Diallo M., Diakité M. Risk factors for malaria infection and anemia for pregnant women in the Sahel area of Bandiagara, Mali. Acta Trop. 2003;89:17–23. doi: 10.1016/j.actatropica.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Dicko A., Mantel C., Kouriba B., Sagara I., Thera M.A., Doumbia S. Season, fever prevalence and pyrogenic threshold for malaria disease definition in an endemic area of Mali. Tropical Med. Int. Health. 2005;10:550–556. doi: 10.1111/j.1365-3156.2005.01418.x. [DOI] [PubMed] [Google Scholar]
- van Eijk A.M., Hill J., Noor A.M., Snow R.W., ter Kuile F.O. Prevalence of malaria infection in pregnant women compared with children for tracking malaria transmission in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob. Health. 2015;3:e617–e628. doi: 10.1016/S2214-109X(15)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. Anti-malarial drugs and the prevention of malaria in the population of malaria endemic areas. Malar. J. 2010;9(Suppl. 3):S2. doi: 10.1186/1475-2875-9-S3-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday K.E., Karanja P., Turner E.L., Okello G., Njagi K., Dubeck M.M. Vol. 17. 2012. Plasmodium falciparum , anaemia and cognitive and educational performance among school children in an area of moderate malaria transmission : baseline results of a cluster randomized trial on the coast of Kenya; pp. 532–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay S.I., Okiro E.A., Gething P.W., Patil A.P., Tatem A.J., Guerra C.A. Estimating the global clinical burden of plasmodium falciparum malaria in 2007. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitojo C., Gutman J.R., Chacky F., Kigadye E., Mkude S., Mandike R. Estimating malaria burden among pregnant women using data from antenatal care centres in Tanzania: a population-based study. Lancet Glob. Health. 2019;7:e1695–e1705. doi: 10.1016/S2214-109X(19)30405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyei-Nimakoh M., Carolan-Olah M., McCann T.V. Access barriers to obstetric care at health facilities in sub-Saharan Africa-a systematic review. Syst Rev. 2017;6:1–16. doi: 10.1186/s13643-017-0503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamptey H., Ofori M.F., Kusi K.A., Adu B., Owusu-Yeboa E., Kyei-Baafour E. The prevalence of submicroscopic Plasmodium falciparum gametocyte carriage and multiplicity of infection in children, pregnant women and adults in a low malaria transmission area in southern Ghana 11 medical and health sciences 1108 medical microbiology 1. Malar. J. 2018;17:331. doi: 10.1186/s12936-018-2479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne J., Ndungu F.M., Sponaas A.M., Marsh K. Immunity to malaria: more questions than answers. Nat. Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- Maccario R., Rouhani S., Drake T., Nagy A., Bamadio M., Diarra S. Cost analysis of a school-based comprehensive malaria program in primary schools in Sikasso region. Mali. BMC Public Health. 2017;17:572. doi: 10.1186/s12889-017-4490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi S.A., Rivera L.M., Sáez-Llorens X., Menéndez C., Carrim-Ganey N., Cotton M.F. Factors influencing access of pregnant women and their infants to their local healthcare system: a prospective, multi-Centre, observational study. BMC Pregnancy Childbirth. 2018;18:1–11. doi: 10.1186/s12884-017-1655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matangila J.R., Doua J.Y., Mitashi P., Inocêncio R., Lutumba P., Pierre J. International journal of antimicrobial agents efficacy and safety of intermittent preventive treatment in schoolchildren with sulfadoxine / pyrimethamine (SP) and SP plus piperaquine in Democratic Republic of the Congo : a randomised controlled trial. Int. J. Antimicrob. Agents. 2017;49:339–347. doi: 10.1016/j.ijantimicag.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Mgata S., Maluka S.O. Factors for late initiation of antenatal care in Dar Es Salaam, Tanzania: A qualitative study. BMC Pregnancy Childbirth. 2019;19:415. doi: 10.1186/s12884-019-2576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health . 2008-2009. Bioko Island Malaria Control Project (BIMCP) Annual Malaria Indicator Survey Report; p. 2009. [Google Scholar]
- Ministry of health and Social Welfare . 2013. National Guidelines for Diagnosis and Treatment of Malaria. Tanzania. [Google Scholar]
- MIP Consortium Malaria in Pregnancy Library. 2018. http://library.mip-consortium.org/index.php?home (accessed March 20, 2018)
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Altman D., Antes G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller A.B., Petzold M., Chou D., Say L. Early antenatal care visit: a systematic analysis of regional and global levels and trends of coverage from 1990 to 2013. Lancet Glob. Health. 2017;5:e977–e983. doi: 10.1016/S2214-109X(17)30325-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankabirwa J., Cundill B., Clarke S., Kabatereine N., Rosenthal P.J., Brooker S. Efficacy, safety, and tolerability of three regimens for prevention of malaria: a randomized. Placebo- Controlled Trial in Ugandan Schoolchildren. 2010;5 doi: 10.1371/journal.pone.0013438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankabirwa J., Wandera B., Kiwanuka N., Staedke S.G., Kamya M.R., Brooker S.J. Vol. 88. 2013. Asymptomatic Plasmodium Infection and Cognition among Primary Schoolchildren in a High Malaria Transmission Setting in Uganda; pp. 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankabirwa J., Brooker S.J., Clarke S.E., Fernando D., Gitonga C.W., Schellenberg D. Malaria in school-age children in Africa: an increasingly important challenge. Tropical Med. Int. Health. 2014;19:1294–1309. doi: 10.1111/tmi.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankabirwa J.I., Yeka A., Arinaitwe E., Kigozi R., Drakeley C., Kamya M.R. Estimating malaria parasite prevalence from community surveys in Uganda : a comparison of microscopy , rapid diagnostic tests and polymerase chain reaction. Malar. J. 2015:1–11. doi: 10.1186/s12936-015-1056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndungu F.M., Olotu A., Mwacharo J., Nyonda M., Apfeld J., Mramba L.K. Memory B cells are a more reliable archive for historical antimalarial responses than plasma antibodies in no-longer exposed children. Proc. Natl. Acad. Sci. U. S. A. 2012;109:8247–8252. doi: 10.1073/pnas.1200472109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor A.M., Kirui V.C., Brooker S.J., Snow R.W. Vol. 8. 2009. The use of insecticide treated nets by age : implications for universal coverage in Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuamah G.B., Agyei-Baffour P., Mensah K.A., Boateng D., Quansah D.Y., Dobin D. Access and utilization of maternal healthcare in a rural district in the forest belt of Ghana. BMC Pregnancy Childbirth. 2019;19:1–11. doi: 10.1186/s12884-018-2159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor D., Green S., Higgins J.P. 2008. Defining the review question and developing criteria for including studies. [DOI] [Google Scholar]
- Olapeju B., Choiriyyah I., Lynch M., Acosta A., Blaufuss S., Filemyr E. Age and gender trends in insecticide-treated net use in sub-Saharan Africa: a multi-country analysis. Malar. J. 2018;17:1–12. doi: 10.1186/s12936-018-2575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omondi C.J., Onguru D., Kamau L., Nanyingi M., Ong’amo G., Estambale B. Perennial transmission of malaria in the low altitude areas of Baringo County. Kenya. Malar J. 2017;16:257. doi: 10.1186/s12936-017-1904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan---a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu E.D., Brown C.A., Grobusch M., Mens P. Prevalence of Plasmodium falciparum and non-P. falciparum infections in a highland district in Ghana, and the influence of HIV and sickle cell disease. Malar. J. 2017:16. doi: 10.1186/s12936-017-1823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R.B.M. Partnership Malaria Indicator Survey Access to Datasets. 2018. https://malariasurveys.org/ (accessed June 11, 2020)
- Rek J., Katrak S., Obasi H., Nayebare P., Katureebe A., Kakande E. Characterizing microscopic and submicroscopic malaria parasitaemia at three sites with varied transmission intensity in Uganda. Malar. J. 2016:1–8. doi: 10.1186/s12936-016-1519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-barraquer I., Arinaitwe E., Jagannathan P., Kamya M.R., Rosenthal P.J., Rek J. 2018. Quantification of anti-parasite and anti- disease immunity to malaria as a function of age and exposure; pp. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons R.A., Mboera L., Miranda M.L., Morris A., Stresman G., Turner E.L. A longitudinal cohort study of malaria exposure and changing serostatus in a malaria endemic area of rural Tanzania. Malar. J. 2017;16:1–13. doi: 10.1186/s12936-017-1945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R., Craig M., Newton C., Steketee R. The public health burden of Plasmodium falciparum malaria in Africa. Dis Control Priorities Proj. 2003 http://archives.who.int/prioritymeds/report/append/610snow_wp11.pdf [Google Scholar]
- Sonko S.T., Jaiteh M., Jafali J., Jarju L.B.S., D’Alessandro U., Camara A. Does socio-economic status explain the differentials in malaria parasite prevalence? Evidence from the Gambia. Malar. J. 2014;13 doi: 10.1186/1475-2875-13-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staedke S.G., Maiteki-Sebuguzi C., Rehman A.M., Kigozi S.P., Gonahasa S., Okiring J. Assessment of community-level effects of intermittent preventive treatment for malaria in schoolchildren in Jinja, Uganda (START-IPT trial): a cluster-randomised trial. Lancet Glob. Health. 2018;6:e668–e679. doi: 10.1016/S2214-109X(18)30126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana M., Sheikh N., Mahumud R.A., Jahir T., Islam Z., Sarker A.R. Prevalence and associated determinants of malaria parasites among Kenyan children. Trop Med Health. 2017;45:1–9. doi: 10.1186/s41182-017-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The DHS Program. Malaria Indicators Survey (MIS) 2020. https://dhsprogram.com/What-We-Do/Survey-Types/MIS.cfm (accessed June 11, 2020)
- Trial P., Nankabirwa J.I., Wandera B., Amuge P., Kiwanuka N., Dorsey G. Vol. 58. 2014. Impact of Intermittent Preventive Treatment With Dihydroartemisinin-Piperaquine on Malaria in Ugandan Schoolchildren : A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF Generation 2030 Africa 2.0. 2017. https://www.unicef.org/publications/files/Generation_2030_Africa_2.0.pdf
- Van Eijk A.M., Hill J., Povall S., Reynolds A., Wong H., Ter Kuile F.O. The malaria in pregnancy library: a bibliometric review. Malar. J. 2012;11:1–12. doi: 10.1186/1475-2875-11-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walldorf J.A., Cohee L.M., Coalson J.E., Bauleni A., Nkanaunena K., Kapito-Tembo A. School-age children are a reservoir of malaria infection in Malawi. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warady B.A., Neu A.M., Schaefer F. National education profile 2014 update: Congo. Am. J. Kidney Dis. 2014;64:128–142. doi: 10.1053/j.ajkd.2014.01.430. [DOI] [PubMed] [Google Scholar]
- WHO . 2005. Malaria indicator survey : basic documentation for survey design and implementation / Roll Back Malaria Monitoring and Evaluation Reference Group. doi:http://www.rbm.who.int/merg for. [Google Scholar]
- WHO . WHO Press; 2014. WHO policy brief for the Implementation of Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP) pp. 1–13. doi:WHO/HTM/GMP/2014.4. [Google Scholar]
- WHO . third edition. 2015. Guideline for treatment of malaria.https://www.who.int/malaria/publications/atoz/9789241549127/en/ [Google Scholar]
- WHO WHO: weekly epidemiological record: malaria vaccine: WHO position paper – January 2016. World Heal Organ Geneva. 2016;91:33–52. doi: 10.1186/1750-9378-2-15.Voir. [DOI] [Google Scholar]
- WHO . 2018. World Malaria Report 2018. ISBN 978 92 4 1564403. [Google Scholar]
- WHO Maternal Health 2017. https://www.afro.who.int/health-topics/maternal-health (accessed July 15, 2019)
- World Bank National Education Profile: Tanzania 2018 Update. 2018. https://www.epdc.org/sites/default/files/documents/EPDC_NEP_2018_Tanzania.pdf
- World Bank National Education Profile: Burkina Faso 2018 Update 2018. https://www.epdc.org/sites/default/files/documents/EPDC_NEP_2018_Burkinafaso.pdf
- World Health Organization World Malaria Report 2019. Geneva. 2019. https://apps.who.int/iris/handle/10665/330011
- World Health Organization. Malaria in Children under five 2018. https://www.who.int/malaria/areas/high_risk_groups/children/en/ (accessed June 13, 2020).
- World Health Organization. Updated WHO Policy Recommendation Intermittent preventive treatment of malaria in pregnancy using Sulfadoxine- Pyrimethamine ( PTp-SP) Glob Malar Program. October 2012;2012:3–4. http://www.who.int/malaria/mpac/sep2012/iptp_sp_erg_meeting_report_july2012.pdf [Google Scholar]
- Yeka A., Nankabirwa J., Mpimbaza A., Kigozi R. Factors associated with malaria Parasitemia. Anemia and Serological Responses in a Spectrum of Epidemiological Settings in. 2015:1–19. doi: 10.1371/journal.pone.0118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerihun AW, Kibret H, Wakiaga J. National Education Profile 2018 Update: Ethiopia 2018:15. doi:https://www.epdc.org/sites/default/files/documents/EPDC_NEP_2018_Ethiopia.pdf.
- Zhou G., Afrane Y.A., Vardo-Zalik A.M., Atieli H., Zhong D., Wamae P. Changing patterns of malaria epidemiology between 2002 and 2010 in western Kenya: the fall and rise of malaria. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020318. [DOI] [PMC free article] [PubMed] [Google Scholar]