Abstract
Background
Myelodysplastic syndromes and acute leukemias after allogeneic stem cell transplantation (allo-SCT) are mainly caused by recurrence of the primitive leukemic clones. More rarely, they originate from donor hematopoietic stem cells, developing the so-called donor cell leukemia (DCL) or myelodysplastic syndromes (DC-MDSs). DCL and DC-MDS can be considered as an in vivo model of leukemogenesis, and even if the pathogenetic mechanisms remain speculative, a genetic predisposition of donor progenitor cells, an altered host microenvironment, and the impairment of immune surveillance are considered the main causes.
Case Presentation
We report a case of DC-MDS diagnosed 5 years after an allo-SCT from a matched related donor (patient’s sister) in a patient with Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia (Ph+ B-ALL). The sex-mismatch allowed us to identify the donor cell origin. At the onset, the DC-MDS was characterized by chromosome seven monosomy and NRAS, RUNX1, and BCOR mutations. Because of a familiar history of colorectal neoplasia and the variant allele frequency (VAF) of NRAS mutation at the onset, this mutation was searched on germline DNA in both the donor and the recipient, but the result was negative. Moreover, after transplant (+4 months), the patient developed severe and long-lasting chronic graft-versus-host disease (cGVHD), requiring multiple lines of treatments. Because of the severe immunosuppression, recurrent infections occurred and, lately, the patient died due to septic shock.
Conclusion
This case report highlights the need, whenever possible, to evaluate the donor origin of the posttransplant myelodysplasia and acute leukemias. The potential key role of the impaired immune surveillance and of long-lasting immunosuppression appears to be emerging in the development of this case of DC-MDS. Finally, this case reminds the importance to investigate the familiar genetic predisposition in donors with a familiar history of neoplasia.
Keywords: donor cell myelodysplasia, transplant, leukemogenesis, immunosurveillance, immunosuppression, stem cells
Introduction
Allogeneic stem cell transplantation (allo-SCT) is the most effective treatment for many hematologic diseases, being a curative approach. Unfortunately, posttransplant relapse is the first cause of transplant failure, and it occurs in at least 30–50% of patients with acute leukemia (AL) or myelodysplastic syndrome (MDS) (1, 2).
Most cases originate from the regrowth of the primitive leukemic clones. More rarely, they originate de novo from donor progenitor cells. Being an extremely rare event, very few cases are described in the literature and most of them are ALs, “donor cell leukemia” (DCL), while sporadically MDSs [“donor cell MDS” (DC-MDS)] (3, 4).
DCL and DC-MDS can be considered as an in vivo model of leukemogenesis, and even if the pathogenetic mechanisms remain speculative, a genetic predisposition of donor progenitor cells, an altered host microenvironment, and the impairment of immune surveillance are considered the main causes.
Although the historic division of DCL/DC-MDS into two groups remains useful (5), (1) a malignant clone inadvertently transferred to the recipient at the time of transplant, and (2) a group in which donor cells became malignant in the new host environment, it clearly appears that the DLC etiology is a multistep process, in which these two mechanisms could interact. Moreover, another crucial aspect to be taken into consideration is the importance of an effective and competent immune system in controlling the growth of a leukemic clone, whether it derives from a primitive damage/“hit” in the donor cells or by the influence of an altered host environment.
Here, we present a case of DC-MDS diagnosed 5 years after matched related allo-SCT in a patient with Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia (Ph+ B-ALL).
Case Presentation
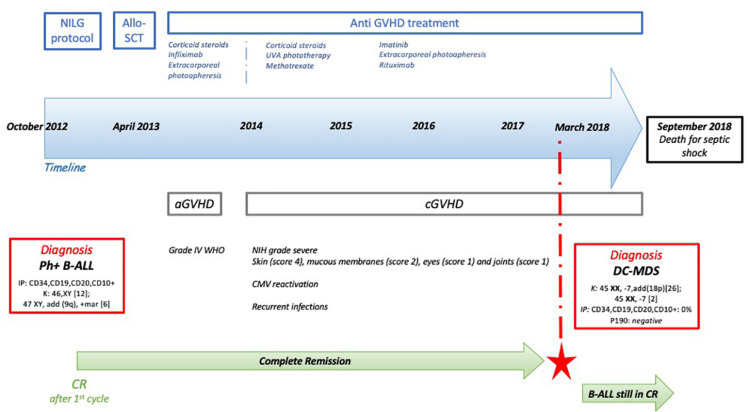
In October 2012, a 49-year-old man was diagnosed with Ph+ B-ALL. At diagnosis, the patient presented with hyperleukocytosis and lymphoblasts expressing B-ALL immunophenotype (CD34+, CD19+, CD20+, CD10+). The Ph+ t(9; 22) translocation (Figure 1) resulted in a p190 protein (e1a2 transcript type), determining a high risk (HR) of disease relapse. The patient did not present any significant comorbidities, and he was enrolled in the NILG ALL10/07 trial (6) (Figure 1 and Supplementary Material 2), combining chemotherapy (idarubicin, cyclophosphamide, 6-mercaptopurine, dexamethasone, and intrathecal chemotherapy) and imatinib (800 mg/day).
FIGURE 1.
Patient’s timeline. At the top, the main treatments that the patient has received, with a focus on the anti-graft-versus-host disease (GVHD) therapies. Then, the blue arrow represents the timeline. In the red rectangles, the main diagnosis that the patient has received and the biological characteristics [Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia (Ph+ B-ALL) and donor cell myelodysplastic syndrome (DC-MDS)]. In green, the persistence of the complete response (CR) over time, even after the DC-MDS onset (red star). K, karyotype; IP, immunophenotype.
The treatment was well tolerated, and the patient obtained a complete morphological, cytogenetic, and molecular response after induction therapy with no significant side effects. In April 2013, the patient underwent allo-SCT from a matched related donor (53-year-old patient’s sister, with no history of neoplasia or hematologic malignancies), considering the HR disease relapse. The conditioning regimen was based on cyclophosphamide 60 mg/kg for 2 days (4,800 mg total dose) and total body irradiation (total dose: 1,200 cGy), and he received methotrexate and cyclosporine-A (CyA) for graft-versus-host disease (GVHD) prophylaxis.
In the first 100 days after transplant, the patient developed a WHO grade 4 acute GVHD (aGVHD) (7) of the skin (stage III), gut (stage II), and liver (stage II). He was treated with steroids (6-methylprednisone 2 mg/kg per day), infliximab (four total doses), and extracorporeal photopheresis (ECP), obtaining complete remission.
Four months later, he developed severe chronic GVHD (cGVHD) (8) involving the skin (score 4), mucous membranes (score 2), eyes (score 1), and joints (score 1). The cGVHD was treated with UVA phototherapy, then with methotrexate, and lastly with imatinib, ECP, and rituximab. The posttransplant course was also characterized by several CMV reactivations and multiple infections of skin ulcers, complicated by recurrent sepsis and one episode of septic shock caused by Pseudomonas aeruginosa (July 2018).
During all the follow–up, the patient remained in continuous complete morphological/molecular remission with 100% CD34+ donor chimerism.
Five years after allo-SCT (January 2018), the patient presented with anemia [hemoglobin (Hb) 12.2 g/dl], thrombocytopenia [platelet (PLT): 71 × 109/L], and hyperleukocytosis [white blood cell count (WBC): 20.45 × 109/L] (Figure 1). MDS with excess blasts type 1 (MDS-EB1) was diagnosed according to the last WHO classification (9), with a very high International Prognostic Scoring System (IPSS)-revised score risk. The patient was still on cGVHD treatment with imatinib and tacrolimus. In the meantime, the primitive Ph+ B-ALL was still in complete response (CR) (p190 negative; IF: CD34+, CD19+, CD10+ = 0% of cells).
The cytogenetic analysis suggested the donor cell origin of MDS due to the female karyotype. Moreover, the karyotype was characterized by the presence of a monosomy of chromosome 7 (45 XX, −7, add(18p) [26]; 45 XX, −7q [2]). Conversely, no Ph+ metaphases were found. The chimerism on CD34+ cells (100% donor), together with the fluorescent in situ hybridization (FISH) for sex mismatch (361/361 XX), confirmed the donor cell origin (patient’s sister) of the MDS. At the time of DC-MDS, the patient presented severe lymphopenia (CD4+ : 80/μl; total lymphocytes: 500/μl). In parallel, the patient’s sister has been periodically evaluated, and her peripheral blood counts have always been in range.
A gene panel deep sequencing has been performed at DC-MDS diagnosis to analyze some genes known to be related to myeloid malignancies (Supplementary Figure 1). All the promoters and coding sequences of the genes were considered for the analysis. Next-generation sequencing (NGS) analysis revealed NRAS [variant allele frequency (VAF) 51%], RUNX1 (VAF 62.2%), and BCOR (VAF 44.7%) mutations. Notably, the variants detected were not known to be associated with malignancies or other diseases. Considering the VAF of NRAS and the familial history for neoplasia (mother and a sister, not the transplant donor, with colorectal cancer), we investigated the presence of a familiar genetic predisposition for the development of hematologic and solid malignancies. To this purpose, the patient and the donor were enrolled in the NEXT-FamlY clinical trial (NCT03058588) (10). This is a national multicentric study focused on the analysis of known and unknown mutations related to familiar predisposition to hematologic malignancies. The germline DNA of both the donor and the recipient resulted negative for the NRAS mutation.
Unfortunately, because of the patient’s conditions compromised by the recurrent infections and GVHD complications, any treatments for the MDS could not be given and the patient died of septic shock a few months after the onset of DC-MDS (September 2018).
Discussion
DCL and DC-MDS are very rare events that may occur in transplanted patients (3, 5, 11–18). In the literature, they usually onset after a median of 20 months (12, 17), with a maximum of 193 (12) months from transplant. They account for about 0.1% of the transplants, with an estimated incidence between 80.5 and 476 cases per 100,000 transplant recipients (11, 17, 18).
The first DCL case was described by Fialkow et al. (19) in 1971. Since then, few cases have been described (5, 11–18), even if they appear to be steadily increasing in the last years as result of more routine and sensitive post-allo-SCT chimerism tests or, possibly, of the increasing number of older donors who are more likely to carry clonal hematopoiesis (20–22).
The umbilical cord seems to bear a potential higher risk for DLC than other stem cell sources (23). Conversely, no differences have been described between bone marrow or peripheral blood sources. In addition, no relationship between DCL and the use of a related or unrelated donor has been observed. Therefore, in this reported case, since the patient received peripheral stem cells from his sister, he did not present a higher risk of DCL, considering the source of stem cells.
Cyclophosphamide with total body irradiation (Cy + TBI) is the conditioning regimen reported to be more frequently associated with DCL development (24). This is the case of our patient who received Cy + TBI before developing DC-MDS 60 months later.
In this presented case, the sex-mismatch easily allowed the recognition of the donor cell origin—thanks to the karyotype and the FISH analysis for gonosomal. Conversely, the cytogenetic analysis is not helpful for detecting the donor origin of MDS/leukemias in sex-matched transplants, for whom the DCL diagnosis is more difficult and often underestimated. In these patients, variable number of tandem repeats or short tandem repeat (STR) analysis should be used to detect DCL/DC-MDS, together with the study of chimerism on CD34+ cells in peripheral blood.
At DC-MDS onset, the patient presented with chromosome seven monosomy, which is the most common cytogenetic alteration observed in DC-MDS and DCL (12). Despite being the most frequently cytogenetic alteration, its impact on patients’ outcome is not yet very well established due to the low number of cases described. Recently, the presence of additional somatic mutations has been reported to influence the outcome of MDS patients with chromosome 7 alterations (25).
NRAS, RUNX1, and BCOR genes were mutated as shown by NGS analysis. NRAS and RUNX1 are among the most frequently mutated genes in patients with MDS and monosomy 7/deletion 7q (25), and they were associated with adverse prognosis in previous studies (26, 27). Interestingly, in our case, NRAS presented a VAF suspicious for being a germline mutation. In addition, the patient and the related donor presented a familiar history for neoplasia (colorectal cancer in the mother and the sister), and the so-called “blend pedigree” have been described in literature in the last years (10, 28).
Therefore, germline DNA from epithelial buccal cells of the donor and the patient was sequenced using traditional Sanger methods. However, neither the donor nor the recipient presented the variant of NRAS on germline DNA. Nevertheless, the evaluation of the donors’ clinical history investigating the presence of a possible pedigree suspicious for familiar neoplasms should always be strongly recommended. That is in order to avoid the use of donor cells that may already present a genetic familiar predisposition to develop hematological malignancies after allo-SCT.
As expected, DCL and DC-MDS prognosis is poor (11), with a median overall survival of 11 months (0–91) (11). In our case, the poor condition of the patient did not allow us to employ any treatment and the death of the patient occurred 8 months after the onset of DC-MDS due to septic shock.
Although several hypotheses have been made, the underlying pathogenetic mechanisms for DCL/DC-MDS developing remain unknown and speculative (12, 13). The main mechanisms proposed included occult leukemia in the donor, alteration of the niche, immune-mediated phenomenon, infections (29), toxicities of posttransplant therapies, and GVHD (30–35). The long latency, together with the fact that the donor remains free from malignancies, in addition to the absence of NRAS variants on germline DNA, supports the hypothesis that either the host bone marrow microenvironment or the immunosuppression could play a pathogenetic role in this presented case. On one hand, the primitive Ph+ ALL and the subsequent chemotherapies should have determined an altered microenvironment, which plays a crucial role in leukemogenesis (36–39). Nevertheless, the long story of cGVHD and the large use of different immunosuppressive therapies could possibly have favored the onset of DC-MDS, though an impaired immunosurveillance. Indeed, cGVHD (40, 41) and GVHD treatments have a profound suppressive effect on T-lymphocytes, which are the main responsible for the immune response against tumor-specific antigens (42). In fact, our patient presented severe lymphopenia at the time of DC-MDS diagnosis. Furthermore, the immunosuppressive effects of cGVHD and of the related treatments worsen the T-immunodeficiency caused by allo-SCT for itself (13, 43). In addition to this scenario, the recurrent infections, which required hospitalizations and, ultimately, led to the death of the patient, strongly support the hypothesis of an impaired immunosurveillance in this presented case.
Conclusion
Despite being rare events, DC-MDS and DCL may occur as late complications after allo-SCT. They are an intriguing phenomenon, and they were probably underdiagnosed for many years. Therefore, searching for the donor cell origin should be performed in all patients relapsing after allo-SCT. The new molecular technologies for chimerism monitoring made easier and more reliable the DCL diagnosis even when the donor origin could not be easily detected, such as in sex-matched cases.
Donor cell-myelodysplastic syndrome and DCL represent an in vivo leukemogenesis model to investigate the underlying pathogenetic mechanisms, which, so far, remain speculative. Beyond the hypothetical pathogenetic mechanisms, the setting of cGVHD and related immunosuppressive treatments may allow DCL development through an impaired immunosurveillance. Conventional and new interesting approaches are available and should be considered to monitor the immune reconstitution and the development of different competent lymphocyte populations (44–46).
Finally, considering that the DCL and DC-MDS may be a consequence of a genetic predisposition, the familiar history for neoplasia of the donors has to be investigated. When it is present, searching for DNA germline mutations both in the recipient and in the donor is mandatory to avoid the use of donor cells that may have a genetic predisposition to develop DCL/DC-MDS after allo-SCT.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Comitato Etico di Brescia. The patient provided their written informed consent to participate in this study and for the publication of this case report.
Author Contributions
MF, SB, MM, and DR designed the study. MF, SB, MM, TZ, LG, DG, EM, NP, AT, CZ, and BR collected and analyzed the data. SB and CZ performed the laboratory NGS analysis. MF, SB, MM, and DR wrote the manuscript. All authors gave their final approval before submission and contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- allo-SCT
allogeneic stem cell transplantation
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- DC-MDS
donor cell myelodysplastic syndrome
- DCL
donor cell leukemia
- FISH
fluorescent in situ hybridization
- cGVHD
chronic graft-versus-host disease
- aGVHD
acute graft-versus-host disease
- GVHD
graft-versus-host disease
- MDS
myelodysplastic syndrome
- Ph+ B-ALL
Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia
- STR
short tandem repeat
- VAF
variant allele frequency
- WBC
white blood cell count
- PLT
platelets
- Hb
hemoglobin.
Footnotes
Funding. The research was supported by funds of the Department of Clinical and Experimental Sciences of University of Brescia.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.564521/full#supplementary-material
References
- 1.Kahl C, Storer BE, Sandmaier BM, Mielcarek M, Maris MB, Blume KG, et al. Relapse risk in patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. (2007) 110:2744–8. 10.1182/blood-2007-03-078592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kröger N, Brand R, van Biezen A, Zander A, Dierlamm J, Niederwieser D, et al. Risk factors for therapy-related myelodysplastic syndrome and acute myeloid leukemia treated with allogeneic stem cell transplantation. Haematologica. (2009) 94:542–9. 10.3324/haematol.2008.000927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orciuolo E, Azzara A, Bandini G, Galimberti S, Bonifazi F, Fazzi R, et al. Contemporaneous appearance, 18 years after allogeneic bone marrow transplantation, of myelodysplastic syndrome in the patient and the donor. Bone Marrow Transplant. (2004) 33:859–61. 10.1038/sj.bmt.1704417 [DOI] [PubMed] [Google Scholar]
- 4.Shah NN, Bacher U, Fry T, Calvo KR, Stetler-Stevenson M, Arthur DC, et al. Myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation: Diagnostic and therapeutic challenges. Am J Hematol. (2012) 87:916–22. 10.1002/ajh.23174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torra OS, Loeb KR. Donor cell-derived leukemia and myelodysplastic neoplasm: Unique forms of leukemia. Am J Clin Pathol. (2011) 135:501–4. 10.1309/AJCPXW8DKEG5QMTB [DOI] [PubMed] [Google Scholar]
- 6.Bassan R, Masciulli A, Pharm D, Intermesoli T, Spinelli O, Tosi M, et al. Final results of northern italy leukemia group (NILG) trial 10/07 combining pediatric-type therapy with minimal residual disease study and risk-oriented hematopoietic cell transplantation in adult acute lymphoblastic leukemia (ALL). Blood. (2016) 128:176–176. 10.1182/blood.V128.22.176.176 [DOI] [Google Scholar]
- 7.Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the mount sinai acute gvhd international consortium. Biol. Blood Marrow Transplant. (2016) 22:4–10. 10.1016/j.bbmt.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. (2017) 129:30–7. 10.1182/blood-2016-07-686642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. (2016) 127:2391–405. 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 10.Bernardi S, Farina M, Zanaglio C, Cattina F, Polverelli N, Schieppati F, et al. ETV6: A candidate gene for predisposition to “Blend Pedigrees”? A case report from the NEXT-famly clinical trial. Case Rep Hematol. (2020) 2020:1–7. 10.1155/2020/2795656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel N, Rovo A, Badoglio M, Labopin M, Basak GW, Beguin Y, et al. European experience and risk factor analysis of donor cell-derived leukaemias/MDS following haematopoietic cell transplantation. Leukemia. (2019) 33:508–17. 10.1038/s41375-018-0218-6 [DOI] [PubMed] [Google Scholar]
- 12.Wang E, Hutchinson CB, Huang Q, Lu CM, Crow J, Wang FF, et al. Donor cell-derived leukemias/myelodysplastic neoplasms in allogeneic hematopoietic stem cell transplant recipients: a clinicopathologic study of 10 cases and a comprehensive review of the literature. Am J Clin Pathol. (2011) 135:525–40. 10.1309/AJCPPJUQ9DNR1GHP [DOI] [PubMed] [Google Scholar]
- 13.Wiseman DH. Donor cell leukemia: a review. Biol Blood Marr Transplant. (2011) 17:771–89. 10.1016/j.bbmt.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 14.Komrokji R, Ifthikharuddin JJ, Felgar RE, Abboud CN, Wedow LA, Connaughton A, et al. Donor cell myelodysplastic syndrome after allogeneic stem cell transplantation responding to donor lymphocyte infusion: case report and literature review. Am J Hematol. (2004) 76:389–94. 10.1002/ajh.20111 [DOI] [PubMed] [Google Scholar]
- 15.Dietz AC, Defor TE, Brunstein CG, Wagner JE. Donor-derived myelodysplastic syndrome and acute leukaemia after allogeneic haematopoietic stem cell transplantation: Incidence, natural history and treatment response. Br J Haematol. (2014) 166:209–12. 10.1111/bjh.12847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fialkow PJ, Gartler SM, Yoshida A. Clonal origin of chronic myelocytic leukemia in man. Proc Natl Acad Sci USA. (1967) 58:1468–71. 10.1073/pnas.58.4.1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hertenstein B, Hambach L, Bacigalupo A, Schmitz N, McCann S, Slavin S, et al. Development of leukemia in donor cells after allogeneic stem cell transplantation–a survey of the european group for blood and marrow transplantation (EBMT). Haematologica. (2005) 90:969–75. [PubMed] [Google Scholar]
- 18.Sala-Torra O, Hanna C, Loken MR, Flowers MED, Maris M, Ladne PA, et al. Evidence of donor-derived hematologic malignancies after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2006) 12:511–7. 10.1016/j.bbmt.2006.01.006 [DOI] [PubMed] [Google Scholar]
- 19.Fialkow PJ, Thomas ED, Bryant JI, Neiman PE. Leukaemic transformation of engrafted human marrow cells in vivo. Lancet (London England). (1971) 1:251–5. 10.1016/S0140-6736(71)90998-6 [DOI] [PubMed] [Google Scholar]
- 20.Gondek LP, Zheng G, Ghiaur G, DeZern AE, Matsui W, Yegnasubramanian S, et al. Donor cell leukemia arising from clonal hematopoiesis after bone marrow transplantation. Leukemia. (2016) 30:1916–20. 10.1038/leu.2016.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herold S, Kuhn M, Bonin MV, Stange T, Platzbecker U, Radke J, et al. Donor cell leukemia: evidence for multiple preleukemic clones and parallel long term clonal evolution in donor and recipient. Leukemia. (2017) 31:1637–40. 10.1038/leu.2017.104 [DOI] [PubMed] [Google Scholar]
- 22.Hasserjian RP, Steensma DP, Graubert TA, Ebert BL. Clonal hematopoiesis and measurable residual disease assessment in acute myeloid leukemia. Blood. (2020) 135:1729–38. 10.1182/blood.2019004770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greaves MF. Cord blood donor cell leukemia in recipients [9]. Leukemia. (2006) 20:1633–4. 10.1038/sj.leu.2404293 [DOI] [PubMed] [Google Scholar]
- 24.Majhail NS. Old and new cancers after hematopoietic-cell transplantation. Hematol Am Soc Hematol Educ Program. (2008) 8:142–9. 10.1182/asheducation-2008.1.142 [DOI] [PubMed] [Google Scholar]
- 25.Crisà E, Kulasekararaj AG, Adema V, Such E, Schanz J, Haase D, et al. Impact of somatic mutations in myelodysplastic patients with isolated partial or total loss of chromosome 7. Leukemia. (2020) 20:1–10. 10.1038/s41375-020-0728-x [DOI] [PubMed] [Google Scholar]
- 26.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. (2011) 364:2496–506. 10.1056/NEJMoa1013343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung SH, Kim Y-J, Yim S-H, Kim H-J, Kwon Y-R, Hur E-H, et al. Somatic mutations predict outcomes of hypomethylating therapy in patients with myelodysplastic syndrome. Oncotarget. (2016) 7:55264–75. 10.18632/oncotarget.10526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Churpek JE, Bresnick EH. Transcription factor mutations as a cause of familial myeloid neoplasms. J Clin Invest. (2019) 129:476–88. 10.1172/JCI120854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malagola M, Rambaldi B, Ravizzola G, Cattaneo C, Borlenghi E, Polverelli N, et al. Bacterial blood stream infections negatively impact on outcome of patients treated with allogeneic stem cell transplantation: 6 years single-centre experience. Mediterr J Hematol Infect Dis. (2017) 9:e2017036. 10.4084/mjhid.2017.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Socié G, Curtis RE, Deeg HJ, Sobocinski KA, Filipovich AH, Travis LB, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. (2000) 18:348–57. 10.1200/JCO.2000.18.2.348 [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Argüelles G, Ruiz-Argüelles A, Garcés-Eisele J. Donor cell leukemia: a critical review. Leukem Lymph. (2007) 48:25–38. 10.1080/10428190601003462 [DOI] [PubMed] [Google Scholar]
- 32.Boulton-Jones R, Parker A, Hepburn M, Bowen DT. Secondary myelodysplasia of donor cell origin following nonmyeloablative sibling allogeneic stem cell transplantation [8]. Bone Marrow Transplant. (2005) 36:471–2. 10.1038/sj.bmt.1705086 [DOI] [PubMed] [Google Scholar]
- 33.Brunstein CG, Hirsch BA, Hammerschmidt D, McGlennen RC, Nguyen PL, Verfaillie CM. Leukemia in donor cells after allogeneic hematopoietic stem cell transplant. Bone Marrow Transplant. (2002) 29:999–1003. 10.1038/sj.bmt.1703577 [DOI] [PubMed] [Google Scholar]
- 34.Aizawa S, Nakano M, Iwase O, Yaguchi M, Hiramoto M, Hoshi H, et al. Bone marrow stroma from refractory anemia of myelodysplastic syndrome is defective in its ability to support normal CD34-positive cell proliferation and differentiation in vitro. Leuk Res. (1999) 23:239–46. 10.1016/S0145-2126(98)00163-5 [DOI] [PubMed] [Google Scholar]
- 35.Raaijmakers MHGP, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. (2010) 464:852–7. 10.1038/nature08851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krause DS, Fulzele K, Catic A, Sun CC, Dombkowski D, Hurley MP, et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med. (2013) 19:1513–7. 10.1038/nm.3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernasconi P, Farina M, Boni M, Dambruoso I, Calvello C. Therapeutically targeting SELF-reinforcing leukemic niches in acute myeloid leukemia: a worthy endeavor? Am J Hematol. (2016) 91:507–17. 10.1002/ajh.24312 [DOI] [PubMed] [Google Scholar]
- 38.Flores-Figueroa E, Gratzinger D. Beyond the niche: myelodysplastic syndrome topobiology in the laboratory and in the clinic. Int J Mol Sci. (2016) 17:553. 10.3390/ijms17040553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perucca S, Palma AD, Piccaluga PP, Gemelli C, Zoratti E, Bassi G, et al. Mesenchymal stromal cells (MSCs) induce ex vivo proliferation and erythroid commitment of cord blood haematopoietic stem cells (CB-CD34+ cells). PLoS One. (2017) 12:e0172430. 10.1371/journal.pone.0172430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizzo JD, Curtis RE, Socié G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. (2009) 113:1175–83. 10.1182/blood-2008-05-158782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skert C, Fogli M, Garaffa E, Perucca S, Fiorentini S, Cancelli V, et al. A specific Toll-like receptor profile on T lymphocytes and values of monocytes correlate with bacterial, fungal, and cytomegalovirus infections in the early period of allogeneic stem cell transplantation. Transpl Infect Dis. (2014) 16:697–712. 10.1111/tid.12264 [DOI] [PubMed] [Google Scholar]
- 42.Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol. (2004) 22:1136–51. 10.1200/JCO.2004.10.041 [DOI] [PubMed] [Google Scholar]
- 43.Skert C, Perucca S, Chiarini M, Giustini V, Sottini A, Ghidini C, et al. Sequential monitoring of lymphocyte subsets and of T-and-B cell neogenesis indexes to identify time-varying immunologic profiles in relation to graft-versus-host disease and relapse after allogeneic stem cell transplantation. PLoS One. (2017) 12:e0175337. 10.1371/journal.pone.0175337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sottini A, Ghidini C, Zanotti C, Chiarini M, Caimi L, Lanfranchi A, et al. Simultaneous quantification of recent thymic T-cell and bone marrow B-cell emigrants in patients with primary immunodeficiency undergone to stem cell transplantation. Clin Immunol. (2010) 136:217–27. 10.1016/j.clim.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 45.Tessitore MV, Sottini A, Roccaro AM, Ghidini C, Bernardi S, Martellosio G, et al. Detection of newly produced T and B lymphocytes by digital PCR in blood stored dry on nylon flocked swabs. J Transl Med. (2017) 15:70. 10.1186/s12967-017-1169-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCusker C, Upton J, Warrington R. Primary immunodeficiency. Allergy Asthma Clin Immunol. (2018) 14:61. 10.1186/s13223-018-0290-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.