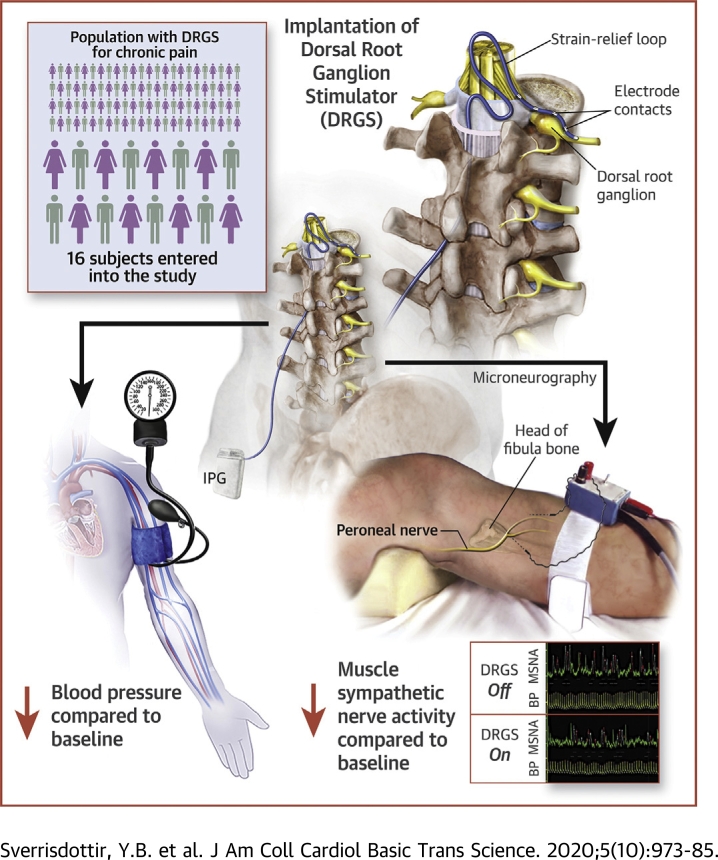
Visual Abstract
Key Words: blood pressure, DRG stimulation, hypertension, neuromodulation, sympathetic nerve activity
Abbreviations and Acronyms: BF, burst frequency; BI, burst incidence; BP, blood pressure; DBP, diastolic blood pressure; DRG, dorsal root ganglion; DRGS, dorsal root ganglion stimulation; HR, heart rate; MAP, mean arterial pressure; MME, morphine milligram equivalent; MRBA%, median relative burst amplitude; MSNA, muscle sympathetic nerve activity; SBP, systolic blood pressure; SCS, spinal cord stimulation; VAS, visual analogue score of pain
Highlights
-
•
DRGS at upper lumbar levels significantly reduces sympathetic nerve firing
-
•
Reduction in sympathetic activity appears to be independent to pain relief
-
•
DRGS significantly reduced BP at 6 months and 2 years
-
•
BP reduction was lateralized to DRGS on the left side
-
•
Three refractory hypertensives became normotensive after chronic stimulation.
Summary
This study hypothesized that dorsal root ganglion (DRG) stimulation would reduce sympathetic nerve activity and would alter hemodynamic variables. This study directly recorded muscle sympathetic nerve activity during ON and OFF stimulation of the DRG while measuring hemodynamic parameters. DRG stimulation significantly reduced the firing frequency of sympathetic nerves, as well as significantly reducing blood pressure, with greater reductions evident when stimulation was left-sided. Left-sided DRG stimulation lowers sympathetic nerve activity, leading to long-term phenotypic changes. This raises the potential of DRG stimulation being used to treat de novo autonomic disorders such as hypertension or heart failure.
Dorsal root ganglion stimulation (DRGS) is a relatively new but promising treatment for chronic pain (1). It involves the percutaneous placement of an electrode over the DRG via an epidural approach that in turn is connected to a subcutaneous pulse generator to provide continuous electrical current. The DRG is a nerve swelling just lateral to the spine that contains the axons and cell bodies of afferent nerves such as pain-mediating C-fibers and sympathetic neurons (Figure 1A). In chronic pain, DRG neurons are hyperexcitable (2,3), and this hyperexcitability is attenuated with DRGS (4), leading to effective pain relief. Both somatic and autonomic afferent pathways pass through the DRG to the dorsal horn of the spinal cord. However, autonomic efferent pathways differ from somatic ones in that, unlike the direct connections from the ventral horn to skeletal muscle, they involve a projection from the central neuron to a peripheral ganglion (followed by a second projection from that ganglion to an effector organ such as vascular smooth muscle). In the case of sympathetic efferents, the ganglia of note lie in the sympathetic chain (or paravertebral ganglia) that are interconnected between spinal levels (Figure 1A). These in turn project to pre-vertebral ganglia and onto effector organs. For example, L1 to L3 sympathetic efferents project to the lesser splanchnic nerves that innervate distal colon and blood vessels in skeletal muscle (5). The L1 (and lower thoracic) sympathetic efferents provide direct projections from the sympathetic chain to the adrenal glands (with no intervening pre-vertebral ganglia) and can influence blood pressure (BP) via circulating catecholamines (6). At the C8/T1 level, the sympathetic chain is enlarged into the stellate ganglion, which sends sympathetic efferents to the heart via the cardiac nerves (7). Thus, effects of DRGS may differ between levels, with some levels affecting cardiac activity directly but others (i.e., lumbar) affecting other aspects of the cardiovascular system such as vascular smooth muscle or the adrenal glands and acting via hormonal changes (see Figure 2).
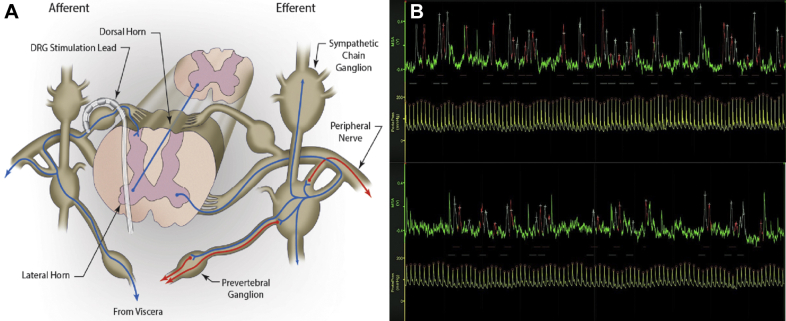
Figure 1.
Recording MSNA During DRGS
(A) Schematic showing the relationship of the dorsal root ganglion (DRG) to the sympathetic chain and spinal cord. Dorsal root ganglion stimulation (DRGS) via the implanted lead, lying over the dorsal DRG, may influence sympathetic neurons in the afferent pathways from the sympathetic ganglia. This in turn may either influence efferent pathways via the ventral horn at the same segmental level or travel via propriospinal pathways to higher levels. (B) Mean voltage neurograms of muscle sympathetic nerve activity (MSNA) (green traces) and spontaneous blood pressure (BP) recording (yellow traces) during left DRG L1 level OFF (upper) and ON (lower) stimulation phases. The OFF trace shows a period of 1 min with heart rate 90 beats/min; MSNA burst frequency 39 and burst incidence 43, median relative burst amplitude 40; systolic/diastolic BP 185/53 mm Hg. The ON trace shows a period of 1 min with heart rate 88 beats/min; MSNA burst frequency 32 and burst incidence 36, median relative burst amplitude 48; systolic/diastolic BP 161/71 mm Hg. Y = axis voltage and mm Hg for MSNA and BP, respectively.
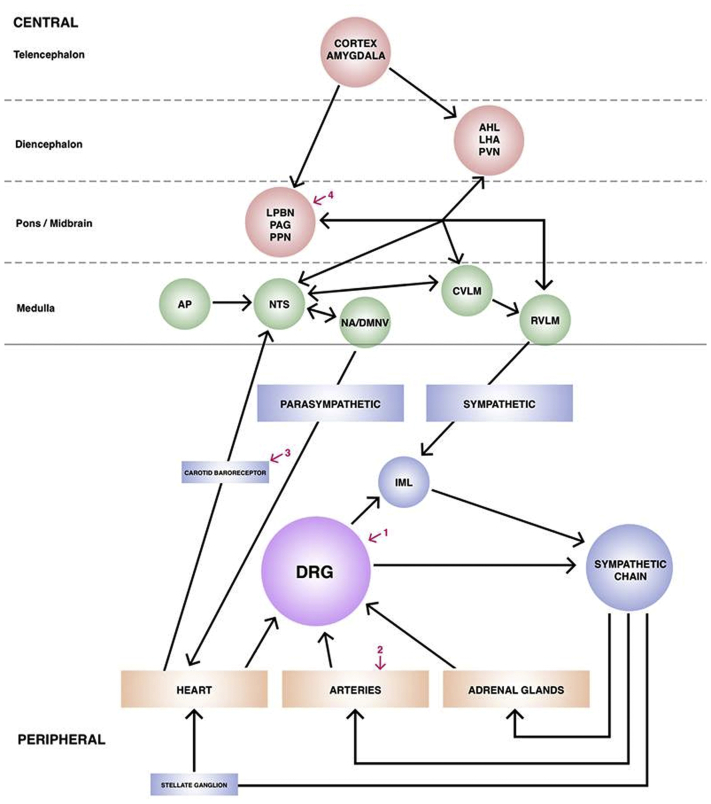
Figure 2.
Role of the DRG in the Neural Control of the Cardiovascular System and Surgical Targets for Modulation
Schematic showing the autonomic control of hemodynamic and cardiovascular physiology from higher brain centers to end organs and their feedback loops. This shows the location of the DRG in this network, and why disruption of autonomic neurons at this peripheral point may be helpful in lowering blood pressure. The red numbered arrows show other points at which surgical intervention may be helpful in modulating the system: 1) dorsal root ganglion (DRG) stimulation; 2) renal sympathetic denervation; 3) carotid sinus stimulation; 4) periaqueductal gray (PAG) or pedunculopontine nucleus (PPN) deep brain stimulation. Adapted with permission from Green and Paterson (38). AHL = anterior hypothalamus; AP = area postrema; CVLM = caudal ventrolateral medulla; DMNV = dorsal motor nucleus of the vagus; IML = intermediolateral cell column; LPBN = lateral parabrachial nucleus; LHA = lateral hypothalamic nucleus; NA = nucleus ambiguus; NTS = nucleus of the tractus solitarius; PVN = paraventricular nucleus of the hypothalamus; RVLM = rostral ventrolateral medulla.
The relationship between the sympathetic and pain fibers is complex. In a rat sciatic nerve injury model of pain, it has been shown that coupling between sympathetic efferent and sensory afferents is increased, such that augmented sympathetic efferent activity can increase (or decrease) sensory afferent activity (8). Complex regional pain syndrome is associated with a decrease in cutaneous sympathetic vasoconstrictor neurons, although, paradoxically, this may lead to increased sensitivity of nociceptors to sympathetic catecholamines (9) and a “sympathetic-maintained” pain (10). In other chronic pain syndromes, there is evidence that sympathetic activity is increased (11).
In our previous work, we demonstrated that electrical stimulation in autonomic pathways in the brain, using deep brain stimulation can be used to alter cardiovascular functions (12, 13, 14). Here, we wanted to investigate whether similar neuromodulation techniques accessing peripheral nervous system pathways could be used for a similar effect. Figure 2 shows a schematic of the possible interconnected network of autonomic nerves and points at which it can be surgically manipulated. We hypothesized that blocking DRG activity with electrical stimulation would lead to a reduction in sympathetic nerve activity, in addition to its analgesic effect. This was based on our previous findings as well as studies showing that reducing peripheral efferent sympathetic activity (such as after sympathectomy) attenuates the excitability of DRG neurons (15), leading us to hypothesize that the reverse is true. It has also been shown that blocking nociception-related protein channels such as TRPV1 in the T8-L3 DRGs increases sympathetic nerve activity and consequently BP (16). Based on these results, we also hypothesized that reduction in sympathetic nerve activity would be associated with changes in BP. To test these hypotheses, we measured muscle sympathetic nerve activity (MSNA) and hemodynamic parameters in a cohort of patients undergoing DRGS for the treatment of chronic neuropathic pain.
Methods
Demographics
Contiguous patients undergoing DRGS system implantation were selected for this study. The study conformed to the Declaration of Helsinki and was approved by the Oxford Research Ethics Committee C (Study reference 13SC0298). Sixteen patients were recruited at University of Oxford and successful MSNA recordings were obtained in 14. Patients were selected for DRGS if they had severe, refractory neuropathic pain of at least 2 years’ duration and had failed treatment with at least 3 classes of analgesics. They all had focal pain, defined as spanning no more than 3 dermatomes. The spinal level that the DRG electrode was placed was determined by the pre-operative pain area and determination of the appropriate dermatome(s). Contraindications for DRGS included opiate dependence, untreated depression or low mood, catastrophization, ongoing litigation, and unrealistic expectations, as these are all poor outcome prognosticators. Mean age was 41 years (range 23 to 59 years), and male-female ratio = 7:7. See Table 1 for details including spinal levels stimulated and stimulation parameters. Chronic BP measurements were made at baseline and 6 months and 2 years post-operatively (an average over 3 recordings on at least 2 occasions). Drug doses were also recorded at baseline and 2 years.
Table 1.
Study Demographics
| Subject # | Sex | Age (yrs) | Etiology | Duration (yrs) | Pre-/Post-Op VAS | DRGS Level/Side | Stimulation Parameters |
Pre-Op BP | Post-Op BP | Drugs | Opioid Dosage (as MME) |
Other Conditions | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current | Pulse Width | Frequency | Pre | Post | |||||||||||
| 1 | M | 53 | Orchidectomy | 12 | 10/4.5 | L1 left | 1.500 mA | 340 ms | 20 Hz | 157/92 | 113/67 | Gabapentin, amitriptyline, lisinopril | 0 | 76 | Hypertension, anxiety, depression |
| 2 | M | 54 | Herniorrhaphy | 2 | 7/0 | L1 left | 1.330 mA | 220 ms | 20 Hz | 118/70 | 118/60 | MST | 140 | 36 | Nil |
| 3 | F | 38 | Pelvic girdle pain | 9 | 8.5/4 | L1 left | 0.950 ms | 380 ms | 20 Hz | 140/92 | 140/80 | MST, sevredol, amitriptyline | 220 | 140 | Nil |
| 4 | M | 24 | Hip trauma | 2 | 9.7/0.7 | L2 left | 2.700 mA | 400 ms | 20 Hz | 148/93 | 140/90 | Duloxetine, tapentadol, gabapentin, meloxicam, MST | 240 | 200 | Hypertension, anxiety, depression |
| 5 | M | 40 | Herniorrhaphy | 3 | 9/2.5 | L1, L2 right | 2.300 mA | 200 ms | 20 Hz | 110/70 | 110/65 | Pregabalin, codeine | 36 | 0 | Nil |
| 6 | F | 59 | Hysterectomy | 5 | 7.6/0.5 | L1 right | 0.575 mA | 240 ms | 20 Hz | 110/56 | 105/60 | Oxycodone, topiramate, nortriptyline, sertraline | 180 | 36 | Breast cancer (remission) |
| 7 | F | 46 | Meralgia paresthetica | 9 | 10/5.0 | L2 right | 0.100 mA | 300 ms | 20 Hz | 118/59 | 121/62 | Gabapentin, MST, sertraline, paracetamol | 50 | 40 | Migraine |
| 8 | F | 42 | Shingles right foot | 5 | 7/2.8 | L4, L5 right | 0.900 mA | 220 ms | 20 Hz | 104/62 | 102/67 | Tramadol, amitriptyline | 45 | 20 | Nil |
| 9 | F | 34 | Foot trauma | 14 | 7.5/2.5 | L5 left | 0.525 mA | 300 ms | 20 Hz | 120/80 | 115/60 | Naproxen, tramadol | 20 | 0 | Nil |
| 10 | M | 74 | Diabetic neuropathy | 4 | 5/1 | L5 right and left | 0.850 mA | 300 ms | 20 Hz | 117/72 | 112/65 | Amitriptyline, duloxetine, gabapentin, dothiepin, losartan, topiramate, tramadol | 40 | 0 | NIDDM type 2, hypertension |
| 11 | F | 25 | Left hand CRPS | 4 | 9.5/2.0 | C6 left | 0.550 mA | 300 ms | 20 Hz | 101/67 | 95/54 | Pregabalin, paracetamol, nortriptyline, tramadol | 20 | 20 | Nil |
| 12 | M | 45 | Nerve compression | 4 | 6.3/2.2 | C7, C8 right | 0.300 mA | 300 ms | 20 Hz | 131/89 | 114/76 | Tramadol, paracetamol, gabapentin | 15 | 0 | Nil |
| 13 | F | 23 | Loin pain hematuria syndrome | 4 | 7.5/2.5 | T11 left | 2.500 mA | 190 ms | 20 Hz | 102/58 | 100/57 | Oxycodone, naproxen | 180 | 120 | Nil |
| 14 | M | 57 | Knee surgery | 3 | 7.5/1.5 | L3 left and right | 0.900 mA | 200 ms | 20 Hz | 125/81 | 119/77 | Ibuprofen, paracetamol, tramadol | 40 | 10 | Nil |
BP = blood pressure; CRPS = complex regional pain syndrome; DRGS = dorsal root ganglion stimulation; MME = morphine milligram equivalent (i.e., total opioid dosage expressed as equivalent morphine dosage); MST = Morphine Sulphate; NIDDM = non–insulin-dependent diabetes mellitus; VAS = visual analogue score of pain.
DRG implantation
The DRG stimulator was implanted under local anesthetic with light sedation (propofol) in the prone position. Using fluoroscopic guidance, the delivery sheath was used to enter the epidural space and a DRG Axium lead (Spinal Modulation, Menlo Park, California) was introduced, under x-ray guidance, into the appropriate nerve root exit foramen, so that the electrode contacts were positioned over the dorsum of the DRG in the dorsal part of the foramen (Figure 1A). Sedation was weaned and the leads were tested for efficacy prior to resedation. After anteroposterior and lateral x-rays had confirmed satisfactory position, a strain-relief loop was fashioned in the spinal canal, and the wires were tunneled to an implantable pulse generator that was placed subcutaneously remote from the spine.
Microneurography technique and protocol
Direct recordings of multiunit postganglionic sympathetic nerve activity to the muscle vascular bed (MSNA) were obtained with a tungsten microelectrode inserted into a muscle fascicle of the peroneal nerve posterior to the fibular head. Patients were tested between 3 and 6 months after internalization of the DRGS system for treatment of their chronic pain. The DRG system was set to ON (patient’s usual settings for pain relief if they used subparesthesia threshold or 90% of the amplitude required to produce paresthesias if they were used to cause paresthesias) or OFF at random, and 15 min were allowed to elapse prior to MSNA recording. The examiner (Y.B.S.) and the subject were blinded to the stimulator setting, with use of subparaesthesia stimulation amplitudes to aid blinding. MSNA was recorded for at least 15 min (maximum 30 min) for each ON and OFF stimulation phase with a 15-min washout period in between phases. A 15-min recording period was chosen as this allowed for natural variations in activity but prevented loss of data from subjects becoming intolerant of the procedure and increase in pain with stimulation off. Visual analogue score of pain (VAS) was measured during each recording period (values were taken at the beginning, end, and 15 min into each recording and were then averaged). Details of the nerve recording technique and criteria for MSNA has been reported previously (17). A low impedance reference electrode was inserted subcutaneously a few centimeters away from the tungsten microelectrode. When a muscle nerve fascicle was identified, small electrode adjustments were made until a site was found at which spontaneous, pulse-synchronous bursts of neural activity could be recorded. The original nerve signal was amplified with a gain of 50,000 and bandpass filtered (width 700 to 2,000 Hz) and then fed through an integrating network with a time constant of 0.1 s to obtain a mean voltage display of nerve activity. All recordings were performed with the subject in a supine position. Continuous arterial BP was measured noninvasively using a finger plethysmograph (Finapres 2300, Ohmeda, Louisville, Kentucky). Heart rate (HR) was monitored via electrocardiography chest electrodes. Signals were sampled at 200 Hz. The signals were analyzed with the aid of custom-made software computer scripts (using Matlab, MathWorks, Natick, Massachusetts) before the data were unblinded. VAS and noninvasive BP were also obtained at baseline (within 4 weeks pre-operatively) and at 6 months and 2 years post-operatively. Noninvasive BP measurements were an average of 3 recordings on at least 2 separate days.
Statistics and signals analysis
Analyses of sympathetic nerve activity
A 5-minute resting period during each ON-OFF stimulation phase was chosen for analyses. Bursts of neural activity were identified by analysis of the mean voltage neurogram, aided by custom-made software computer scripts (using Matlab) and quantified by their occurrence over time (bursts per minute), expressed as burst frequency (BF), or their incidence in association to heart rate (bursts per 100 heartbeats), expressed as burst incidence (BI). As a measure of the strength of a sympathetic burst, its mean voltage amplitude was also generated by the software. All of these measures depend on a semiautomatic procedure where a computer algorithm suggests bursts, which can be accepted or rejected during manual scrutiny. Additional bursts can be manually inserted. The agreement between automatic and manual assessment is normally 80% to 90%.
The software calculates the start, maximum, and endpoint for each individual sympathetic burst separately, leaving measures of burst strength unaffected by shifts in baseline of the neurogram. The amplitude of the largest burst in each analyzed period was set to 100% and the strength of all other burst amplitudes then expressed in proportion to this value. In this way, a relative burst amplitude spectrum was obtained and from it a median relative burst amplitude (MRBA%) value was extracted and used for statistical analysis (18).
Assessment of individual baroreflex sensitivity diagrams
The mean values for systolic blood pressure (SBP) and diastolic blood pressure (DBP) and cardiac interval were calculated by custom-made computer scripts (using Matlab) and the respective standard deviation was used as a measure of the variability of pressure and cardiac interval.
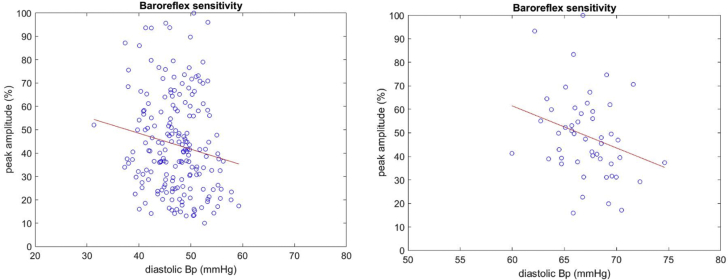
Baroreflex sensitivity for BP was evaluated in a linear regression analysis of individual sympathetic burst amplitude versus the diastolic pressure during the cardiac interval corresponding to the burst. The sensitivity was defined as the slope of regression line (Figure 3) (16).
Figure 3.
Baroreflex Sensitivity for BP ON and OFF Stimulation
Baroreflex sensitivity for blood pressure (BP) was evaluated in a linear regression analysis of individual sympathetic burst amplitude (x-axis) versus the diastolic pressure (y-axis) during the cardiac interval corresponding to the sympathetic burst. The sensitivity was defined as the slope of regression line. The left panel corresponds to the OFF trace (r = −0.159 ± 0.138 [95% confidence interval]; p = 0.027), and the right panel corresponds to the ON trace (r = −0.275 ± 0.261 [95% confidence interval]; p = 0.049).
Statistical analysis
Parametric or nonparametric analyses were performed depending on data attributes and assessment by histogram or Shapiro–Wilk testing. Analyses were adjusted to minimize the risk of bias and influence on the results. All analyses were undertaken in SPSS version 24 (IBM Corp., Armonk, New York). Any p values <0.05 were considered statistically significant. The following specific analysis methods were used.
Does sympathetic nerve activity change with DRGS?
Only the first measurement from the uppermost DRG level was used if more than 1 DRG lead was present. This reduced the influence of positive or negative selection bias from individuals who had differential responses at different levels or subsequent recordings. Only the lumbar DRG levels were included because there were only 2 subjects with cervical levels and the mechanisms are potentially different (see introduction).
Do any identified sympathetic nerve changes related to DRGS correlate with acute pain perception changes?
Any patient and all levels were included regardless of DRG level or side. Our rationale for including all levels in this analysis is that pain is multilevel and limiting it to a single level increases the risk of a false-negative result. Only the first MSNA measurement was taken for each DRG lead to reduce the risk of repeat bias. The differences between the sympathetic ON-OFF measures for each DRG level were taken and then correlated with ON-OFF VAS differences.
Is DRG on 1 side more effective at changing sympathetic measures than another?
Only those patients with a unilateral DRG lead were included and only the first MSNA measurement was taken. The 6-month and 2-year differences among SBP, diastolic blood pressure DBP, and mean arterial pressure (MAP) were compared.
Results
MSNA recordings were obtained in 14 of 16 subjects (see Table 1). The spinal level of DRG electrode placement was determined by the pre-operative pain area and determination of the appropriate dermatome(s). Two subjects (#10 and #14) had bilateral implants, and 1 of these had testing with unilateral stimulation and the other with bilateral stimulation. The variable duration of return of pain and/or pain relief with stimulator adjustment between patients allowed comparison of stimulation with and without pain relief. Figure 1B shows a representative MSNA recording.
MSNA and hemodynamic parameters
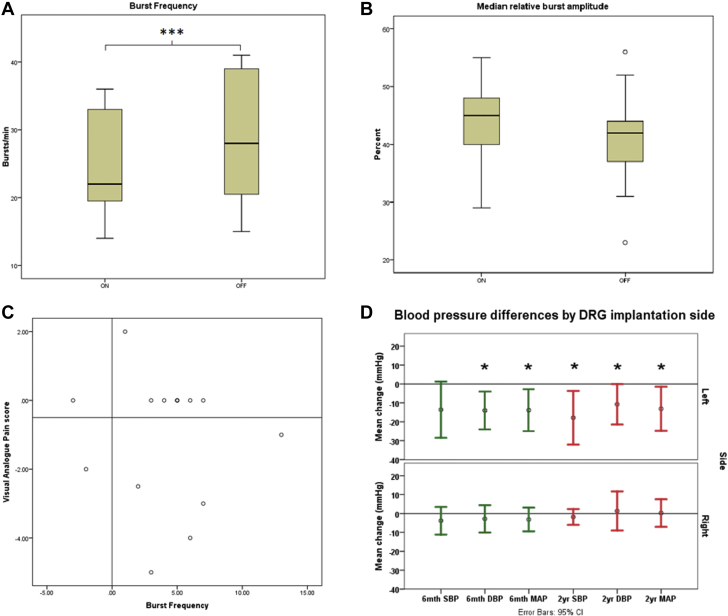
Mean MSNA BF was reduced by 13.3% with DRGS (p < 0.001) (Figure 4A, Table 2). The reduction in MSNA BF tended to be greater with left sided stimulation than right (16.3% left vs. 12.9% right), although the difference between left and right was not significantly different (Mann–Whitney U test; p > 0.05), possibly due to small sample size. MSNA BI was reduced by 11.8% (p = 0.011). The median burst amplitude of sympathetic outflow (MRBA%) in any given recording period showed no significant changes between OFF and ON stimulation phases (Figure 4B), although, on the right side, variability was markedly increased with stimulation (MRBA% change between OFF and ON stimulation ranged between −31.9% and +20% on the left and −41.7% and +104% on right). HR did not change between conditions (Wilcoxon signed rank test; p = 0.163).
Figure 4.
Effects of Stimulation on MSNA and BP
(A) Burst frequency (bursts/min) of MSNA ON and OFF DRG stimulation. Stimulation significantly reduced burst frequency indicating lower sympathetic activity. ∗∗∗p < 0.001. 2-tailed Student’s t-test, n = 14. (B) Median relative burst amplitude ON and OFF DRG stimulation. DRG stimulation did not significantly alter median relative burst amplitude (p = 0.119), 2-tailed Student’s t-test, n = 14. (C) Scatter plot of pain change (using visual analogue score of pain) and changes in MSNA burst frequency, with groupings depicting the 4 possible outcomes. No particular pain change was associated with either an increase or decrease in burst frequency. Note that these were visual analogue scores of pain during acute testing and do not represent long-term outcomes. (D) Changes in BP among baseline (pre-operative) and at 6 months (green) and at 2 years (red). There was a significant reduction in BP parameters at 6 months (except systolic blood pressure [SBP]), which was maintained at 2 years; this finding was seen in left-sided stimulation only. ∗p < 0.05. CI = confidence interval; DBP = diastolic blood pressure; MAP = mean arterial pressure; other abbreviations as in Figure 1.
Table 2.
MSNA Characteristics ON and OFF DRGS
| Subject # | Level/Side Tested | DRGS ON |
DRGS OFF |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | MSNA |
BRS/DBP | BP (mm Hg) | VAS | HR | MSNA |
BRS/DBP | BP (mm Hg) | VAS | ||||
| BF/BI | MRBA% | BF/BI | MRBA% | ||||||||||
| 1 | L1 left | 88 | 32/36 | 48 | −0.28 ± 0.261 | 161/71 | 0 | 90 | 39/43 | 40 | −0.16 ± 0.138 | 185/53 | 0 |
| 2 | L1 left | 65 | 34/53 | 48 | 0.07 ± 0.452 | 207/112 | 0 | 67 | 39/58 | 52 | — | — | 0 |
| 3 | L1 left | 62 | 21/34 | 40 | −0.02 ± 0.265 | 153/63 | 4 | 61 | 28/44 | 44 | −0.04 ± 0.249 | 142/59 | 7 |
| 4 | L2 left | 83 | 20/23 | 33 | −0.18 ± 0.295 | 150/65 | 1 | 92 | 31/34 | 47 | −0.25 ± 0.243 | 226/91 | 6 |
| 5 | L1 right | 65 | 34/52 | 46 | −0.05 ± 0.126 | 128/72 | 0 | 64 | 40/62 | 43 | −0.08 ± 0.123 | 117/66 | 0 |
| L2 right | 60 | 36/61 | 41 | −0.11 ± 0.124 | 129/70 | 0 | 62 | 41/66 | 42 | −0.17 ± 0.120 | 119/64 | 0 | |
| 6 | L1 right | 81 | 21/26 | 55 | 0.06 ± 0.299 | 204/135 | 0.5 | 82 | 23/29 | 50 | −0.05 ± 0.373 | 168/115 | 3 |
| 7 | L2 right | 56 | 17/30 | 36 | −0.023 ± 0.338 | 151/79 | 5 | 58 | 15/26 | 31 | −0.04 ± 0.411 | 154/77 | 7 |
| 8 | L4 right | 35 | 18/52 | 47 | — | — | 6 | 55 | 31/58 | 23 | — | — | 7 |
| L5 right | 41 | 14/34 | 29 | — | — | 0 | 37 | 17/47 | 32 | — | — | 5 | |
| 9 | L5 left | 78 | 29/37 | 44 | −0.23 ± 0.293 | 162/102 | 0 | 82 | 34/42 | 37 | −0.3 ± 0.296 | 164/102 | 0 |
| 10 | L5 right | 64 | 25/39 | 34 | −0.108 ± 0.284 | 120/62 | 0 | 62 | 28/45 | 36 | −0.077 ± 0.269 | 116/66 | 0 |
| 11 | C6 left | 80 | 34/42 | 36 | — | — | 2 | 80 | 40/50 | 37 | — | — | 6 |
| 12 | C7 right | 70 | 21/31 | 47 | — | — | 0 | 67 | 18/27 | 42 | — | — | 0 |
| C8 right | 74 | 22/30 | 42 | — | — | 0 | 69 | 26/38 | 44 | — | — | 0 | |
| 13 | T11 left | 64 | 17/26 | 53 | 0.06 ± 0.347 | 160/83 | 5 | 62 | 18/30 | 56 | −0.17 ± 0.24 | 125/64 | 7 |
| 14 | L3 left + right | 82 | 18/22 | 25 | — | 181/76 | 7.5 | 81 | 16/20 | 25 | — | 185/84 | 3 |
Results are presented as Spearman correlations with 95% confidence intervals. VAS is on a scale of 0 to 10.
BF = burst frequency; BI = burst incidence; BRS = baroreflex sensitivity; C = cervical; DBP = diastolic blood pressure; DRGS = dorsal root ganglion stimulation; HR = heart rate; L = lumbar; MRBA% = median relative burst amplitude; MSNA = muscle sympathetic nerve activity; T = thoracic; other abbreviations as in Table 1.
Baroreceptor sensitivity for BP was not altered by DRGS (Table 2). In general, for an individual subject, if baroreflex sensitivity was significant with DRG ON stimulation phase, it remained so for DRG OFF stimulation phase and vice versa (Figure 3).
Relationship between MSNA changes and pain relief
We observed 5 recordings in which MSNA changed but VAS remained constant between the ON and OFF stimulation phases, despite satisfactory analgesic effects of chronic stimulation (Figure 4C). However, the variation in VAS response allowed us to test the hypothesis that the 2 variables are independent. A Spearman correlation analysis was performed looking at the differences produced between the ON and OFF conditions correlating sympathetic parameters (BF, BI, MRBA%, HR) and VAS change. We found that not only were all correlations poor between VAS change and sympathetic parameters, but they were all nonsignificant.
Chronic BP changes
To look for phenotypic changes, we analyzed long-term BP at baseline and at 6-month and at 2-year follow-up. We found that BP was significantly reduced at 6 months, and that this reduction was maintained at 2 years following surgery, but this was only the case for left-sided DRG implantation (Figure 4D).
When comparing the change in 2-year BP from baseline, for left-sided versus right-sided DRGS, there was a significant difference in all 3 BP parameters (SBP: left-side: −17.9 ± 16.9 mm Hg, right-side: −1.8 ± 4.0 mm Hg; p = 0.033; DBP: left-side: −10.8 ± 12.8 mm Hg, right-side: 3.0 ± 8.5 mm Hg; p = 0.033; MAP: left-side: −13.1 ± 14.0 mm Hg, right-side: 1.4 ± 5.8 mm Hg; p = 0.025; all Student’s t-test), showing that left-sided DRGS affects BP more than right-sided DRGS.
The BP reduction achieved by left-sided DRGS is shown by comparison of baseline BP and 2-year BP: MAP was reduced by 13.1 ± 14.0 mm Hg; p = 0.033; SBP was reduced by 17.9 ± 17.0 mm Hg; p = 0.021; and DBP was reduced by 10.8 ± 12.8 mm Hg; p = 0.049 (all Student’s t-test). There was no significant change between 6-month BP and 2-year BP, showing that BP improvement is attained relatively early following surgery and is maintained long term. Right-sided DRGS did not show the same reduction in BP parameters: MAP was increased by 1.4 ± 5.8 mm Hg; p = 0.309; SBP was reduced by 1.8 ± 4.0 mm Hg; p = 0.309; and DBP was increased by 3.0 ± 8.5 mm Hg; p = 0.426 (all Student’s t-test).
Of particular note, 3 of our patients had a diagnosis of hypertension prior to DRGS. All of these patients underwent left-sided DRGS. All 3 achieved a reduction in BP such that their BP has now normalized, and 2 of the 3 are now no longer treated with antihypertensive medications: Patient #1: pre-operative BP: 157/92 mm Hg, 2-year follow-up BP: 126/71 mm Hg; Patient #2: pre-operative BP: 148/93 mm Hg, 2-year follow-up BP: 106/59 mm Hg; and Patient #3: pre-operative BP: 155/98 mm Hg, 2-year follow-up BP: 120/80 mm Hg.
Change in opioid usage
Opioid usage was standardized by expressing each patient’s pre-operative and long-term follow-up dosages as morphine milligram equivalent (MME) dose (Table 1). Mean MME reduced significantly from 87.6 mg pre-operatively to 49.9 mg post-operatively (p = 0.014, Wilcoxon signed rank test). The mean reduction in MME was a reduction in dosage of 61%: 4 patients came off opioids altogether, with only 1 patient having to start them, who was not on them before intervention. Correlation analysis shows that there is no correlation between the change in opioid dosage and change in long-term BP (Pearson product moment correlation: −0.257; 95% confidence interval: −0.71 to 0.34).
Discussion
Albeit previously a matter of debate in published reports, it is now well-recognized that both frequency and incidence of sympathetic outflow is increased in conditions such as hypertension, severe congestive heart failure, aging (19), and in chronic pain states such as rheumatoid arthritis (20), and sympathetic MRBA% has been shown to be a sensitive indicator of cardiac sympathetic abnormalities (19). In this study, using each patient as their own control subject, we have demonstrated that DRGS (at the upper lumbar levels L1 to L3) significantly reduces efferent sympathetic nerve firing frequency and incidence in a cohort of patients undergoing treatment for chronic neuropathic pain. Whereas 2 subjects with cervical stimulation had similar and concordant results, these were not included in the statistical analyses as it is likely that the mechanisms are different and the numbers are too small to analyze. The variability in MRBA% in our study is in line with previous findings showing that relative burst amplitude may increase or decrease in healthy subjects when given an acute pain stimulus (21).
The lateralization of effect on MSNA and BP was an unexpected and intriguing finding. Whether this is a random phenomenon in the small cohort studied or a real physiological phenomenon with important clinical implications for the possibility of DRGS to reduce BP remains to be determined. We found a significant reduction in chronic BP with left-sided stimulation only (despite pain relief with left- or right-sided stimulation), implying that the reduction is independent of pain relief.
It is a well-recognized fact that acute pain can increase HR and peripheral resistance, and hence BP, by activating the sympathetic nervous system through recruitment of segmental spinal reflexes. Whether such a relationship exists between chronic pain and hypertension, which is of potentially great pathophysiological and clinical interest, remains a matter of debate.
It is worthy of note that the overall mean reduction in SBP with left-sided stimulation is around 18 mm Hg, which is very similar in magnitude to that obtained in the Rheos study, a randomized controlled trial of carotid body stimulation to treat hypertension (22). However, it should be noted that in our current study, only 3 of 8 of these left-sided patients had a pre-existing diagnosis of hypertension. The mean reduction in SBP for the hypertensive patients was 36 mm Hg, which is nearly double that seen in the Rheos study, and is maintained at 2 years unlike the findings of the SYMPLICITY HTN-3 (SYMPLICITY HTN-3 Renal Denervation in Patients With Uncontrolled Hypertension) trial of renal sympathetic denervation (23).
The 3 patients with hypertension had a baseline BP >140/90 and all reduced to the normotensive range at 6 months and at 2 years following surgery. One of these patients remained on the same dose of lisinopril throughout the study, although arguably may not require it now, and the other 2 had ceased antihypertensive medication prior to the study (but after DRGS). Although this is an interesting finding, this is a very small sample, and further work is necessary to demonstrate that the reduction in BP is independent of pain and/or pain relief and would require examining the effects of DRGS in a de novo hypertensive model (or a patient with hypertension but not chronic pain).
It has been shown previously that epidural spinal cord stimulation (SCS) at the T5 to T6 level does not lead to significant changes in MAP or HR, but during sympathetic stress (a cold pressor test) stimulation significantly increases MAP (24). However, there is some controversy as other studies in canines and humans have shown a reduction in BP with T5 to T6 epidural stimulation (25,26) and more recently, a delayed reduction in MSNA has been demonstrated (27). Foreman et al. (28) proposed that intrinsic cardiac neuron activity is modulated by SCS. Phillips et al. (29) have shown that, in patients with spinal cord injury, transcutaneous SCS can reverse the orthostatic fall in BP and have proposed an “autonomic neuroprosthesis.” It thus appears that standard SCS may have a hypertensive effect that is beneficial in spinal cord injury, whereas DRGS has a hypotensive effect, and it is likely that the mechanisms of BP change are different for SCS and DRGS as they stimulate different neural pathways.
Regarding the asymmetry of BP effect for DRGS, injections into porcine inferior mesenteric ganglia (the main output of sympathetic activity at L2 to L3) show bilateral projections to DRG with no predominance of 1 side over the other (30). Similarly, C-fiber activation is, to our knowledge, symmetrical. However, some degree of asymmetry has been discovered at the T1 to T3 level, where microinjections of L-glutamate into the intermediolateral cell column causing increases in HR predominate on the right, whereas injections into the left are more likely to increase myocardial contractility (31). In humans, left stellate ganglion block (C8, T1) increases HR, whereas the right block does not (32).
Furthermore, our findings of a differentiated modulation of sympathetic outflow during deep brain stimulation of the periaqueductal gray (12), showed a reduction on MSNA BF and BP on the left side only, raising speculation that lateralization exists in the central nervous system, at least at the level of the midbrain.
Electrical stimulation of the DRG may potentially affect both autonomic fibers as well as C-fibers that encode pain, and it has been shown that there may be increased sympathetic-sensory coupling in patients with chronic neuropathic pain (8) (i.e., increased pain leads to increased sympathetic activity and vice versa). Autonomic involvement in the generation and propagation of pain pathways is not a new concept. It was experimentally shown in rats in the early 1990s that connections between autonomic and sensory pathways are generated in the DRG following peripheral nerve injury (33).
In our study, reduction in MSNA was poorly correlated with pain relief. It may be that MSNA changes are more dynamic than changes in pain perception when the stimulator is turned on, leading to more rapid changes in MSNA compared with those indicated by pain scores. It is unlikely that the difference is due to different latency of C-fibers and sympathetic fibers as both respond to stimulation in the millisecond range (4,34) and each of our ON and OFF stimulation phase nerve recordings lasted for a minimum of 15 min. Therefore, it would appear that at least some of the MSNA effects are independent of pain relief, indicating that stimulation is directly influencing sympathetic fibers within the DRG. As with BP effects, a nonpain model would be required to confirm this hypothesis. It is of note that, in our cohort, there was a significant reduction in opiate use after DRG implantation. Whereas opiate doses did not vary between ON and OFF stimulation phases during MSNA recording, it is possible that changes in MME could influence long-term BP changes. In general, opiates are associated with reduction in BP (35), although the effects of reduction in opiates on long-term BP (and whether it would be expected to increase) is not well researched, and there is even some suggestion that long-term opioid usage could be related to increased risk of cardiac-related adverse events (36). However, the fact that opiate use and BP were reduced in any case, adds credence to the hypothesis that the reduction in BP was not related to changes in opiate use.
To further elucidate spinal and/or supraspinal mechanisms causing MSNA reduction, we calculated baroreceptor sensitivity in both the ON and OFF stimulation phases (Table 2, Figure 3). Carotid baroreceptors are stretch receptors within the carotid arteries in the neck, distension of which (due to increased BP) causes a lowering of BP via a complex reflex loop that travels via the brain stem cardiovascular centers (37,38) and enables beat-to-beat control of BP. We found that DRGS did not influence baroreflex sensitivity, implying that the reduction in sympathetic output is not via “higher” centers (i.e., does not reach the medulla oblongata) and is likely either a “spinal” or local peripheral mechanism. The DRG (Figure 1A) is in the afferent part of the autonomic reflex loop, and therefore any alteration to efferent sympathetic activity is likely to include the spinal cord at least at that segmental level. We think it is unlikely that our electrodes are directly affecting efferent activity in the ventral root because the stimulation amplitude is generally <1 mA and current spread beyond 1 to 2 mm from DRG is highly unlikely. Whether altering activity at a specific level influences other levels (either via spinal cord pathways or via the sympathetic chain) is a matter for further investigation. The spinal cord provides sympathetic efferents via the intermediolateral cell column that runs between the first thoracic (T1) and second lumbar (L2) segments (39). At any given spinal level, sympathetic afferents from both the periphery and the white ramus communicans travel via the DRG into the dorsal horn of the spinal cord (Figure 1A). Reflexes may be segmental (at the same level) (40) or may travel to other levels via propriospinal pathways (41). Mid-thoracic spinal cord injury can lead to a debilitating (and even life-threatening) condition called autonomic dysreflexia. Rabchevsky (42), using a rat model of spinal cord injury, found that impeding C-fiber sprouting using the chemorepulsive agent semaphorin A mitigated the increased sympathetic activity associated with autonomic dysreflexia and that this effect is associated with an increase in ascending propriospinal neurons. If DRG electrical stimulation of C-fibers has a similar neurophysiological effect to impeding sprouting, it would suggest that our results may, at least in part, be related to C-fiber suppression and supports the notion that our findings are not segmental but involve higher levels of the spinal cord. However, this will require further studies.
In this study, we used the subject’s usual stimulation parameters (or 90% paresthesias threshold), with frequency of 20 Hz. We have not explored the effects of different parameters such as alternative frequencies and stimulation patterns. In SCS, “burst” stimulation has been shown to produce superior results pertaining to analgesia (43). If we are going to explore DRGS as a potential treatment for autonomic control of pain, exploration of parameters that optimally modulate the DRG (either up or down) and affect autonomic control of pain specifically (rather than analgesia) will be necessary.
Study limitations
This study is limited by sample size, dependent on availability of patients. Moreover, this is a heterogeneous group. We have assumed that MSNA recordings reflect bilateral changes in efferent sympathetic activity and all except 1 MSNA recording were unilateral. However, 1 recording was bilateral, revealing no differences in outflow between the right and left.
A further limitation is that the numbers are too small to compare the effects of stimulation of different spinal DRG levels. It is likely that the mechanisms differ depending on level (e.g., C7, C8–T1 affecting cardiac function directly, T11-L1 affecting renal function and other levels producing peripheral vasodilatation). Therefore, further work should include larger studies looking at each level with assessment of end organs such as cardiac activity, acute renal function, and peripheral blood flow.
Whereas it is not appropriate to perform a correction for multiple comparisons (we were comparing separate relationships between independent and dependent variables and were not looking at a universal H0), we acknowledge that there is a risk of false-positive results. This comes not from performing multiple tests for our study, but because we are looking at 3 separate relationships within the same small sample of which ideally a larger sample size would be better (e.g., 15 patients per relationship). However, simply the nature and complexity of data collection, along with the rarity of DRG implantation, would make attaining large sample sizes not feasible and not timely. However, within our data there is plenty of evidence of scientific causality: such as the temporal relationship between stimulation and BP reduction (BP only reduces after stimulation is turned ON); the size of the effect that is comparable with medications; the specificity to the left side suggesting a specific mechanism.
Conclusions
We have shown, in a cohort of patients with chronic pain, that DRGS reduces efferent sympathetic activity and also reduces long-term BP. Furthermore there appears to be a lateralizing phenomenon that warrants further investigation.
To study the effects of DRGS on BP, it is necessary to remove the confounding factor of chronic pain and study DRGS in the context of de novo hypertension, cardiac failure, and other autonomic conditions. Therefore, large animal studies may be necessary prior to human studies. These results, however, open up the possibility of using DRGS to treat cardiovascular conditions.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Hemodynamic variables can be affected by manipulation of the autonomic nervous system. There is complex interplay of the central autonomic nervous system in the telencephalon and brain stem, all the way down to the peripheral nervous system via the spinal cord and sympathetic chain. These reach end organs such as the heart, blood vessels, and adrenal glands, and so play a critical role in hemodynamic homeostasis.
TRANSLATIONAL OUTLOOK: The DRG relays autonomic fibers and so may be a target for surgical manipulation of otherwise treatment-resistant hypertension. This is likely to be by disruption of the sympathetic pathways to end organs, either directly, or via the intermediolateral cell column of the spinal cord. This type of surgical intervention is safe and reversible.
Author Relationship With Industry
This study was supported by an unrestricted research grant from Spinal Modulation Inc. and the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Kent was employed by Abbott at the time of the study and provided technical support for the study. Drs. FitzGerald and Green have received occasional honoraria from Abbott (primarily for teaching courses about dorsal root ganglion stimulation). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors acknowledge the contribution of Tomas Karlsson (Department of Neurophysiology, Sahlgrenska Academy, Gothenburg University) who wrote the original script for analysis of MSNA signals.
Footnotes
Kalyanam Shivkumar, MD, PhD, served as Guest Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Deer T.R., Levy R.M., Kramer J. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158:669–681. doi: 10.1097/j.pain.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rush A.M., Dib-Hajj S.D., Liu S., Cummins T.R., Black J.A., Waxman S.G. A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons. Proc Natl Acad Sci U S A. 2006;103:8245–8250. doi: 10.1073/pnas.0602813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogan Q.H. Labat lecture: the primary sensory neuron: where it is, what it does, and why it matters. Reg Anesth Pain Med. 2010;35:306–311. doi: 10.1097/AAP.0b013e3181d2375e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koopmeiners A.S., Mueller S., Kramer J., Hogan Q.H. Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation. 2013;16:304–311. doi: 10.1111/ner.12028. discussion 310–1. [DOI] [PubMed] [Google Scholar]
- 5.Janig W. Cambridge University Press; Cambridge, UK: 2006. The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis. [Google Scholar]
- 6.Kopp U.C. Morgan & Claypool Life Sciences; San Rafael, CA: 2011. Neural Control of Renal Function. [PubMed] [Google Scholar]
- 7.Mehrotra M., Reddy V., Singh P. StatPearls Publishing; Island, FL: 2020. Neuroanatomy, Stellate Ganglion. StatPearls. Treasure. [PubMed] [Google Scholar]
- 8.Devor M., Janig W., Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve-injured rats. J Neurophysiol. 1994;71:38–47. doi: 10.1152/jn.1994.71.1.38. [DOI] [PubMed] [Google Scholar]
- 9.Baron R., Levine J.D., Fields H.L. Causalgia and reflex sympathetic dystrophy: does the sympathetic nervous system contribute to the generation of pain? Muscle Nerve. 1999;22:678–695. doi: 10.1002/(sici)1097-4598(199906)22:6<678::aid-mus4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs G.F., Drummond P.D., Finch P.M., Phillips J.K. Unravelling the pathophysiology of complex regional pain syndrome: focus on sympathetically maintained pain. Clin Exp Pharmacol Physiol. 2008;35:717–724. doi: 10.1111/j.1440-1681.2007.04862.x. [DOI] [PubMed] [Google Scholar]
- 11.Perry F., Heller P.H., Kamiya J., Levine J.D. Altered autonomic function in patients with arthritis or with chronic myofascial pain. Pain. 1989;39:77–84. doi: 10.1016/0304-3959(89)90177-2. [DOI] [PubMed] [Google Scholar]
- 12.Sverrisdottir Y.B., Green A.L., Aziz T.Z. Differentiated baroreflex modulation of sympathetic nerve activity during deep brain stimulation in humans. Hypertension. 2014;63:1000–1010. doi: 10.1161/HYPERTENSIONAHA.113.02970. [DOI] [PubMed] [Google Scholar]
- 13.Green A.L., Wang S., Owen S.L. Deep brain stimulation can regulate arterial blood pressure in awake humans. Neuroreport. 2005;16:1741–1745. doi: 10.1097/01.wnr.0000183904.15773.47. [DOI] [PubMed] [Google Scholar]
- 14.Green A.L., Wang S., Owen S.L., Paterson D.J., Stein J.F., Aziz T.Z. Controlling the heart via the brain: a potential new therapy for orthostatic hypotension. Neurosurgery. 2006;58:1176–1183. doi: 10.1227/01.NEU.0000215943.78685.01. discussion 1176–83. [DOI] [PubMed] [Google Scholar]
- 15.Iwase T., Takebayashi T., Tanimoto K. Sympathectomy attenuates excitability of dorsal root ganglion neurons and pain behaviour in a lumbar radiculopathy model. Bone Joint Res. 2012;1:198–204. doi: 10.1302/2046-3758.19.2000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing J., Lu J., Li J. TRPA1 mediates amplified sympathetic responsiveness to activation of metabolically sensitive muscle afferents in rats with femoral artery occlusion. Front Physiol. 2015;6:249. doi: 10.3389/fphys.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallbo A.B., Hagbarth K.E., Torebjork H.E., Wallin B.G. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 18.Sverrisdottir Y.B., Rundqvist B., Elam M. Relative burst amplitude in human muscle sympathetic nerve activity: a sensitive indicator of altered sympathetic traffic. Clin Auton Res. 1998;8:95–100. doi: 10.1007/BF02267819. [DOI] [PubMed] [Google Scholar]
- 19.Sverrisdottir Y.B., Rundqvist B., Johannsson G., Elam M. Sympathetic neural burst amplitude distribution: a more specific indicator of sympathoexcitation in human heart failure. Circulation. 2000;102:2076–2081. doi: 10.1161/01.cir.102.17.2076. [DOI] [PubMed] [Google Scholar]
- 20.Adlan A.M., Paton J.F., Lip G.Y., Kitas G.D., Fisher J.P. Increased sympathetic nerve activity and reduced cardiac baroreflex sensitivity in rheumatoid arthritis. J Physiol (Lond) 2017;595:967–981. doi: 10.1113/JP272944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fazalbhoy A., Birznieks I., Macefield V.G. Individual differences in the cardiovascular responses to tonic muscle pain: parallel increases or decreases in muscle sympathetic nerve activity, blood pressure and heart rate. Exp Physiol. 2012;97:1084–1092. doi: 10.1113/expphysiol.2012.066191. [DOI] [PubMed] [Google Scholar]
- 22.Bisognano J.D., Bakris G., Nadim M.K. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos pivotal trial. J Am Coll Cardiol. 2011;58:765–773. doi: 10.1016/j.jacc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt D.L., Kandzari D.E., O'Neill W.W. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 24.Schultz D.M., Musley S., Beltrand P., Christensen J., Euler D., Warman E. Acute cardiovascular effects of epidural spinal cord stimulation. Pain Physician. 2007;10:677–685. [PubMed] [Google Scholar]
- 25.Issa Z.F., Zhou X., Ujhelyi M.R. Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a postinfarction heart failure canine model. Circulation. 2005;111:3217–3220. doi: 10.1161/CIRCULATIONAHA.104.507897. [DOI] [PubMed] [Google Scholar]
- 26.Levin B.E., Hubschmann O.R. Dorsal column stimulation: effect on human cerebrospinal fluid and plasma catecholamines. Neurology. 1980;30:65–71. doi: 10.1212/wnl.30.1.65. [DOI] [PubMed] [Google Scholar]
- 27.Holwerda S.W., Holland M.T., Reddy C.G., Pierce G.L. Femoral vascular conductance and peroneal muscle sympathetic nerve activity responses to acute epidural spinal cord stimulation in humans. Exp Physiol. 2018;103:905–915. doi: 10.1113/EP086945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foreman R.D., Linderoth B., Ardell J.L. Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res. 2000;47:367–375. doi: 10.1016/s0008-6363(00)00095-x. [DOI] [PubMed] [Google Scholar]
- 29.Phillips A.A., Squair J.W., Sayenko D.G., Edgerton V.R., Gerasimenko Y., Krassioukov A.V. An autonomic neuroprosthesis: noninvasive electrical spinal cord stimulation restores autonomic cardiovascular function in individuals with spinal cord injury. J Neurotrauma. 2018;35:446–451. doi: 10.1089/neu.2017.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bossowska A. Distribution of primary afferent neurons associated with the porcine inferior mesenteric ganglion (IMG) Folia Histochem Cytobiol. 2002;40:367–372. [PubMed] [Google Scholar]
- 31.Sundaram K., Sapru H. NMDA receptors in the intermediolateral column of the spinal cord mediate sympathoexcitatory cardiac responses elicited from the ventrolateral medullary pressor area. Brain Res. 1991;544:33–41. doi: 10.1016/0006-8993(91)90882-v. [DOI] [PubMed] [Google Scholar]
- 32.Wong C.W. Stimulation of left stellate ganglion prolongs Q-T interval in patients with palmar hyperhidrosis. Am J Physiol. 1997;273:H1696–H1698. doi: 10.1152/ajpheart.1997.273.4.H1696. [DOI] [PubMed] [Google Scholar]
- 33.McLachlan E.M., Janig W., Devor M., Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–546. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- 34.Orstavik K., Weidner C., Schmidt R. Pathological C-fibres in patients with a chronic painful condition. Brain. 2003;126:567–578. doi: 10.1093/brain/awg060. [DOI] [PubMed] [Google Scholar]
- 35.Shanazari A.A., Aslani Z., Ramshini E., Alaei H. Acute and chronic effects of morphine on cardiovascular system and the baroreflexes sensitivity during severe increase in blood pressure in rats. ARYA Atheroscler. 2011;7:111–117. [PMC free article] [PubMed] [Google Scholar]
- 36.Chen A., Ashburn M.A. Cardiac effects of opioid therapy. Pain Med. 2015;16(Suppl 1):S27–S31. doi: 10.1111/pme.12915. [DOI] [PubMed] [Google Scholar]
- 37.Spyer K.M. Organisation of baroreceptor pathways in the brain stem. Brain Res. 1975;87:221–226. doi: 10.1016/0006-8993(75)90419-9. [DOI] [PubMed] [Google Scholar]
- 38.Green A.L., Paterson D.J. Identification of neurocircuitry controlling cardiovascular function in humans using functional neurosurgery: implications for exercise control. Exp Physiol. 2008;93:1022–1028. doi: 10.1113/expphysiol.2007.039461. [DOI] [PubMed] [Google Scholar]
- 39.Gebber G.L., McCall R.B. Identification and discharge patterns of spinal sympathetic interneurons. Am J Physiol. 1976;231:722–733. doi: 10.1152/ajplegacy.1976.231.3.722. [DOI] [PubMed] [Google Scholar]
- 40.Janig W., McLachlan E. Neurobiology of the autonomic nervous system. In: Mathias C.J., Bannister R., editors. Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. 5th ed. Oxford University Press; New York, NY: 2013. pp. 3–15. [Google Scholar]
- 41.Duda P., Pavlasek J. Functional characteristics of the intraspinal spread of viscerosomatic activity. Physiol Bohemoslov. 1976;25:495–503. [PubMed] [Google Scholar]
- 42.Rabchevsky A.G. Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury. Prog Brain Res. 2006;152:265–274. doi: 10.1016/S0079-6123(05)52017-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deer T., Slavin K.V., Amirdelfan K. Success Using Neuromodulation With BURST (SUNBURST) Study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21:56–66. doi: 10.1111/ner.12698. [DOI] [PubMed] [Google Scholar]