Abstract
Background
Cardiac injury has been reported in up to 30% of coronavirus disease 2019 (COVID-19) patients. However, cardiac injury is defined mainly by troponin elevation without description of associated structural abnormalities and its time course has not been studied.
Research Question
What are the ECG and echocardiographic abnormalities as well as their time course in critically ill COVID-19 patients?
Study Design and Methods
The cardiac function of 43 consecutive COVID-19 patients admitted to two ICUs was assessed prospectively and repeatedly, combining ECG, cardiac biomarker, and transthoracic echocardiographic analyses from ICU admission to ICU discharge or death or to a maximum follow-up of 14 days. Cardiac injury was defined by troponin elevation and newly diagnosed ECG or echocardiographic abnormalities, or both.
Results
At baseline, 49% of patients demonstrated a cardiac injury, and 70% of patients experienced cardiac injury within the first 14 days of ICU stay, with a median time of occurrence of 3 days (range, 0-7 days). The most frequent abnormalities were ECG or echocardiographic signs, or both, of left ventricular (LV) abnormalities (87% of patients with cardiac injury), right ventricular (RV) systolic dysfunction (47%), pericardial effusion (43%), new-onset atrial arrhythmias (33%), LV relaxation impairment (33%), and LV systolic dysfunction (13%). Between baseline and day 14, the incidence of pericardial effusion and of new-onset atrial arrhythmias increased and the incidence of ECG or echocardiographic signs, or both, of LV abnormalities as well as the incidence of LV relaxation impairment remained stable, whereas the incidence of RV and LV systolic dysfunction decreased.
Interpretation
Cardiac injury is common and early in critically ill COVID-19 patients. ECG or echocardiographic signs, or both, of LV abnormalities were the most frequent abnormalities, and patients with cardiac injury experienced more RV than LV systolic dysfunction.
Key Words: cardiac injury, COVID-19, echocardiography, ECG, ICU
Abbreviations: COVID-19, coronavirus disease 2019; LV, left ventricular; RV, right ventricular; TTE, transthoracic echocardiography
Take-home Point.
In this French prospective cohort of critically ill patients with COVID-19, cardiac injury was common and occurred early within the first 14 days of ICU stay. The most frequent newly diagnosed abnormalities were ECG or echocardiographic signs, or both, of LV abnormalities, and patients with cardiac injury experienced more RV than LV systolic dysfunction.
FOR EDITORIAL COMMENT, SEE PAGE 1715
Beginning in December 2019, a worldwide pandemic with an emergent coronavirus, severe acute respiratory syndrome coronavirus 2, has been responsible for coronavirus disease 2019 (COVID-19).1 Among COVID-19 patients admitted to an ICU, from 12% to 30% of them experienced cardiac injury.2, 3, 4, 5 These patients were more likely to have underlying cardiovascular diseases, and cardiac injury was associated with in-hospital mortality.5 , 6
In most studies, cardiac injury was defined by troponin elevation, regardless of new abnormalities in ECG or echocardiography results.5 , 6 Moreover in studies also considering ECG or echocardiographic abnormalities, or both, to define cardiac injury, the latter were not described, were limited to assessment of left ventricular (LV) systolic function,2 , 4 , 7 or were described in non-critically ill patients.8 Finally, no study has described the time course of cardiac injury during COVID-19.
This study aimed to characterize cardiac injury and its time course in critically ill COVID-19 patients prospectively with multimodal assessment of cardiac function combining ECG, cardiac biomarkers, and transthoracic echocardiography (TTE) analyses.
Methods
This prospective, observational study was conducted in two ICUs of university hospitals. This study was approved by our institutional review board (Numéro d'Identification - Recherches et Collections Biologiques [IDRCB] Identifier: 2020-A01197-32), and all patients or next of kin were informed about the study and consented to participate.
Patients
We included all consecutive patients with COVID-19 confirmed by real-time reverse-transcriptase polymerase chain reaction assay of nasal swabs or pulmonary samples. Exclusion criteria were (1) patients younger than 18 years, (2) patients with a care-limitation decision, and (3) patients with poor echogenicity, defined as the inability to obtain reliable Doppler and accurate LV ejection fraction measurements.
ECG Analyses and Echocardiographic Measurements
Besides continuous ECG monitoring to look for arrhythmia, 12- or 18-lead ECGs in case of suspicion of circumflex occlusion and inferior or right ventricular (RV) infarction9 were performed at each TTE examination time. All ECG results were interpreted offline by the same experienced cardiologist blinded to patients’ identities. All TTE measurements were performed by experienced board-certified operators according to the current recommendations10 and were analyzed offline blinded to patients’ identities. All ECG and TTE measurements are detailed in e-Appendix 1.
Cardiac Injury Definition
Cardiac injury was defined by an increase in high-sensitivity troponin T or troponin I levels more than the 99th percentile upper reference limit9 and newly diagnosed ECG or TTE abnormalities, or both.2 , 7 The ECG and TTE abnormalities were considered newly diagnosed if they were unknown before ICU admission (patients with no or without available results of prior cardiac assessment in patients’ files held by the referring physicians) or if they emerged during ICU stay.
Newly diagnosed ECG abnormalities defining cardiac injury were the following: (1) ECG signs of LV abnormalities suggestive of coronary heart disease, Takotsubo syndrome, myocarditis or septic cardiomyopathy (repolarization abnormalities involving at least two contiguous leads of the same territory [T-wave inversion, ST-segment depression or elevation], pathologic Q waves [in at least two contiguous leads], newly diagnosed left bundle branch block, ventricular arrhythmia, and severe brady-arrhythmia])9 , 11, 12, 13, 14, 15 and (2) new-onset atrial arrhythmias (atrial fibrillation, atrial flutter, or atrial tachycardia).
Newly diagnosed echocardiographic abnormalities defining cardiac injury were the following: (1) echocardiographic signs of LV abnormalities suggestive of coronary heart disease, Takotsubo syndrome, myocarditis, or septic cardiomyopathy (mild, moderate, or severe LV systolic dysfunction or wall motion abnormalities)9 , 11, 12, 13, 14, 15; (2) LV relaxation impairment (ie, LV diastolic dysfunction) with at least two of the following abnormalities: abnormal e′-wave velocity (e′septal < 7 cm/s or e′lateral < 10 cm/s), E/e′averaged ratio > 14 in patients with sinus rhythm, E/e′septal ratio > 11 in patients with atrial arrhythmia, or left atrial dilation (volume > 34 mL/m2)16; (3) cor pulmonale (RV end-diastolic area-to-LV end-diastolic area > 0.6 and flattened interventricular septum); (4) RV systolic dysfunction (tricuspid annular plane systolic excursion < 16 mm, or systolic tricuspid annular velocity < 9.5 cm/s or RV fractional area change < 35%)10; (5) any new significant valvulopathy (aortic, mitral, tricuspid, and pulmonary valve disease)17; and (6) pericardial effusion.
Data Collection and Study Design
Demographic characteristics and comorbidities of patients, clinical and biological data, results of cardiac function assessment (ECG, TTE, and cardiac biomarkers, ie, high-sensitivity troponin T or troponin I and B-type or N-terminal pro-B-type natriuretic peptide), therapeutics, and ICU clinical outcomes (mortality, use of vasopressors, mechanical ventilation, and renal replacement therapy) were collected and analyzed. All biological, ECG, and echocardiographic analyses were performed at ICU admission (baseline), at day 3, and then weekly until ICU discharge or death, with a maximum follow-up of 14 days.
Statistical Analysis
Because this was a descriptive study, no statistical sample size calculation was performed a priori. The normality of the variables was tested with the Kolmogorov-Smirnov test. Continuous variables were summarized as mean ± SD or median (interquartile range) as appropriate, and categorical variables were summarized as counts and percentages. At baseline, categorical variables were compared with a Fisher exact test or a χ2 test, and continuous variables were compared with a Student t test or a Mann-Whitney U test. Longitudinal analyses were performed with a generalized linear mixed model. Because noninformative censoring could not be applied in ICU context (cardiac injury is a competing event with death without cardiac injury or discharge alive from ICU), we plotted cumulative incidence function to illustrate time-to-event analysis. Statistical analysis was performed with MedCalc version 11.6.0 software (MedCalc) and XLSTAT version 2020.1 software (Excel, Microsoft Corp.). A P value < .05 was considered statistically significant.
Results
Study Population
Between March and May 2020, 53 patients with COVID-19 were admitted in the two ICUs. Among them, 10 were excluded: six for poor echogenicity and four because of transfer in other French ICUs within the first 48 h of their ICU stay for the management of the COVID-19 epidemic. In the remaining 43 patients, 41 (95%) were mechanically ventilated in the volume assist-controlled mode, 14 (33%) demonstrated chronic hypertension, 23 (54%) received vasopressors, and 21 (49%) received specific treatments for COVID-19 (hydroxychloroquine, n = 14; lopinavir plus ritonavir, n = 5; Remdesivir [Gilead Sciences], n = 6; and corticosteroids, n = 17) at baseline. The other baseline characteristics of patients are shown in Table 1 . Twenty-one patients (49%) demonstrated a cardiac injury at baseline and 30 patients (70%) experienced cardiac injury within the first 14 days of ICU stay (e-Fig 1A). The day 14 mortality rate was 9%, and all deceased patients experienced cardiac injury. Causes of death were multiple-organ failure (n = 1), refractory hypoxemia (n = 2), and refractory septic shock (n = 1).
Table 1.
Baseline Characteristics of Patients With and Without Cardiac Injury
| Variable | Overall (N = 43) | Patients With Cardiac Injury (n = 30) | Patients Without Cardiac Injury (n = 13) | P Value |
|---|---|---|---|---|
| Age, y | 60 ± 13 | 61 ± 13 | 56 ± 12 | .17 |
| Sex, male / female | 36 (84) / 7 (16) | 25 (83) / 5 (17) | 11 (85) / 2 (15) | .92 |
| Simplified Acute Physiology Score II | 57 ± 21 | 61 ± 19 | 47 ± 23 | .04 |
| Mortality at day 14, % | 4 (9) | 4 (13) | 0 (0) | .17 |
| Comorbidities | . . . | . . . | . . . | . . . |
| Cardiovascular risk factors | 29 (67) | 19 (63) | 10 (77) | .38 |
| Hypertension | 14 (33) | 12 (40) | 2 (14) | .11 |
| Diabetes | 12 (28) | 9 (30) | 3 (23) | .64 |
| Smokers | 12 (28) | 7 (23) | 5 (39) | .31 |
| Dyslipidemia | 10 (23) | 6 (20) | 4 (31) | .44 |
| Atherosclerosis | 7 (16) | 6 (20) | 1 (8) | .44 |
| Coronary heart disease | 2 (5) | 2 (7) | 0 (0) | .34 |
| Othersa | 6 (14) | 6 (20) | 0 (0) | .08 |
| Chronic heart failure | 1 (2) | 1 (3) | 0 (0) | .51 |
| Chronic respiratory disease | 7 (16) | 3 (10.0) | 4 (31) | .09 |
| Chronic renal disease | 6 (14) | 6 (20) | 0 (0) | .08 |
| Chronic liver disease | 2 (5) | 1 (3) | 1 (8) | .53 |
| Malignancy | 6 (14) | 4 (13) | 2 (15) | .86 |
| Immunodepression | 8 (19) | 6 (20) | 2 (15) | .72 |
| Clinical parameters | . . . | . . . | . . . | . . . |
| BMI, kg/m2 | 29 ± 5 | 29 ± 5 | 29 ± 4 | .74 |
| Norepinephrine use | 23 (54) | 17 (59) | 6 (46) | .45 |
| Norepinephrine dosage, μg/kg/min | 0.05 (0.00-0.27) | 0.09 (0.00-0.28) | 0.00 (0.00-0.16) | .36 |
| Systolic arterial pressure, mm Hg | 118 ± 21 | 118 ± 22 | 118 ± 19 | .94 |
| Diastolic arterial pressure, mm Hg | 63 ± 14 | 61 ± 14 | 67 ± 11 | .21 |
| Mean arterial pressure, mm Hg | 82 ± 16 | 80 ± 17 | 85 ± 14 | .42 |
| Heart rate, beats per min | 85 ± 21 | 87 ± 24 | 82 ± 12 | .50 |
| Ventilation parameters | . . . | . . . | . . . | . . . |
| Invasive mechanical ventilation | 41 (95) | 30 (100) | 11 (85) | .03 |
| Pao2:Fio2 ratio | 187 ± 71 | 181 ± 70 | 202 ± 74 | .38 |
| PEEP, cm H2O | 13 ± 3 | 13 ± 3 | 13 ± 2 | .80 |
| Tidal volume, mL/kg of PBW | 5.9 ± 0.6 | 6.0 ± 0.5 | 5.7 ± 0.7 | .13 |
| Total respiratory rate, breaths/min | 27 ± 4 | 27 ± 4 | 27 ± 5 | .81 |
| Respiratory compliance, mL/cm H2O | 34 ± 10 | 33 ± 11 | 36 ± 8 | .47 |
| Biological parameters | . . . | . . . | . . . | . . . |
| HS troponin I (n = 17), ng/L | 29 (16-366) | 274 (27-491) | 16 (16-28) | .01 |
| HS troponin T (n = 26), ng/L | 23 (14-69) | 28 (17-88) | 13 (13-35) | .08 |
| BNP (n = 17), pg/mL | 72 (28-168) | 124 (67-187) | 25 (16-85) | .04 |
| NT-proBNP (n = 26), pg/mL | 245 (102-1227) | 277 (129-2540) | 107 (52-247) | .04 |
| Lactate, mM | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.0 ± 0.4 | .49 |
| IL-6, pg/mL | 158 (58-297) | 202 (54-1051) | 97 (66-216) | .32 |
| Creatinine clearance, mL/min | 81 ± 43 | 71 ± 40 | 105 ± 40 | .01 |
| Plasma creatinine, μM | 88 (67-138) | 92 (70-192) | 78 (57-115) | .19 |
| Plasma urea, mM | 6.8 (4.7-10.7) | 8.6 (5.1-12.1) | 5.6 (4.0-8.2) | .07 |
| Sodium bicarbonate, mM | 24 ± 4 | 23 ± 3 | 25 ± 4 | .03 |
| Potassium, mM | 4 ± 1 | 4 ± 1 | 4 ± 1 | .96 |
| SGOT, U/L | 61 ± 29 | 63 ± 32 | 58 ± 22 | .63 |
| SGPT, U/L | 47 ± 30 | 47 ± 35 | 48 ± 14 | .89 |
| Total bilirubin, mM | 11 ± 8 | 10 ± 7 | 13 ± 9 | .34 |
| Protein C reactive, mg/L | 181 ± 110 | 194 ± 115 | 152 ± 96 | .26 |
| Procalcitonin, ng/mL | 0.58 (0.27-1.67) | 0.68 (0.26-2.71) | 0.57 (0.37-1.06) | .61 |
| Lactate dehydrogenase, UI/L | 634 ± 264 | 627 ± 283 | 653 ± 220 | .79 |
| Prothrombin, % | 76 ± 15 | 75 ± 15 | 78 ± 13 | .47 |
| Fibrinogen, g/L | 6.4 ± 1.9 | 6.2 ± 1.8 | 6.8 ± 2.0 | .37 |
| D-dimer, μg/L | 2,231 (984-7762) | 1,921 (1081-6326) | 6,403 (767-10000) | .48 |
| WBC count, × 109/L | 9.19 ± 4.01 | 9.87 ± 4.32 | 7.63 ± 2.71 | .09 |
| Neutrophils, × 109/L | 7.83 ± 3.87 | 8.56 ± 4.18 | 6.13 ± 2.38 | .06 |
| Lymphocytes, × 109/L | 0.82 ± 0.52 | 0.76 ± 0.58 | 0.95 ± 0.33 | .27 |
| Hemoglobin, g/dL | 12.1 ± 1.9 | 11.9 ± 2.0 | 12.7 ± 1.8 | .18 |
| Platelet count, × 109/L | 239 ± 89 | 233 ± 81 | 254 ± 109 | .49 |
Data are presented as No. (%), mean ± SD, or median (interquartile range), unless otherwise indicated. BNP = B-type natriuretic peptide; HS = high-sensitivity; NT-proBNP = N-terminal pro B-type natriuretic peptide; PBW = predicted body weight; PEEP = positive end-expiratory pressure; SGOT = serum glutamo-oxalacetic transaminase; SGPT = serum glutamo-pyruvate transferase.
Presence of cerebrovascular disease, or aortic aneurysm, or extracranial carotid and vertebral artery disease, or lower extremity artery disease.
Characteristics of Patients With Cardiac Injury
At baseline, patients with cardiac injury showed a lower clearance of creatinine (71 ± 40 mL/min vs 105 ± 40 mL/min; P = .01), higher plasma levels of cardiac biomarkers, a lower tricuspid annular plane systolic excursion (19 ± 5 mm vs 23 ± 3mm; P = .01), and a lower systolic tricuspid annular velocity (13 ± 3 cm/s vs 15 ± 4 cm/s; P = .04) than patients without cardiac injury (Tables 1 and 2 ). The positive end-expiratory pressure level was similar between patients with cardiac injury and those without (13 ± 3 cm H2O vs 13 ± 2 cm H2O; P = .80), as well as the proportion of patients with LV hypertrophy (40% vs 30%; P = .50) and the proportion of patients receiving specific treatments for COVID-19 (47% vs 53%; P = .67).
Table 2.
Baseline ECG Abnormalities and Echocardiographic Parameters in Patients With and Without Cardiac Injury
| Variable | Overall (N = 43) | Patients With Cardiac Injury (n = 30) | Patients Without Cardiac Injury (n = 13) | P Value |
|---|---|---|---|---|
| ECG abnormalities | 11 (26) | 10 (33) | 1 (8) | .08 |
| ECG signs of left ventricular abnormalities | 11 (26) | 10 (33) | 1 (8) | .08 |
| Inverted T waves | 9 (21) | 8 (27) | 1 (8) | .16 |
| ST-segment elevation | 1 (3) | 1 (3) | 0 (0) | .51 |
| ST-segment depression | 4 (13) | 4 (13) | 0 (0) | .17 |
| Pathologic Q waves | 2 (7) | 2 (7) | 0 (0) | .34 |
| Localization of repolarization abnormalities and Q waves | . . . | . . . | . . . | . . . |
| Anterior | 4 (13) | 3 (10) | 1 (8) | .81 |
| Lateral | 2 (7) | 2 (7) | 0 (0) | .34 |
| Inferior | 2 (7) | 2 (7) | 0 (0) | .34 |
| Inferolateral | 2 (7) | 2 (7) | 0 (0) | .34 |
| Diffuse | 2 (7) | 2 (7) | 0 (0) | .34 |
| New left branch bundle block | 2 (7) | 2 (7) | 0 (0) | .34 |
| Life-threatening ventricular arrhythmia | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Severe bradyarrhythmia | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| New-onset atrial arrhythmias | . . . | . . . | . . . | . . . |
| Atrial fibrillation | 1 (3) | 1 (3) | 0 (0) | .50 |
| Atrial flutter | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Atrial tachycardia | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Signs of right ventricular strain | 5 (12) | 4 (13) | 1 (8) | .59 |
| Inverted T waves in leads V1-V4 | 3 (7) | 2 (7) | 1 (8) | .90 |
| QR pattern in V1a | 1 (3) | 1 (3) | 0 (0) | .51 |
| S1Q3T3 patternb | 1 (3) | 1 (3) | 0 (0) | .51 |
| Incomplete or complete right bundle branch | 1 (3) | 1 (3) | 0 (0) | .51 |
| Left ventricular hypertrophy | 4 (13) | 4 (13) | 0 (0) | .17 |
| Echocardiographic parameters | . . . | . . . | . . . | . . . |
| Left ventricular ejection fraction, % | 64 ± 10 | 62 ± 10 | 68 ± 7 | .09 |
| E/A ratio | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.0 ± 0.3 | .40 |
| e′lateral, cm/s | 10 ± 3 | 9 ± 3 | 10 ± 3 | .48 |
| e′septal, cm/s | 8 ± 2 | 8 ± 2 | 8 ± 2 | .43 |
| E/e′averaged ratio | 8 ± 3 | 8 ± 3 | 8 ± 2 | .62 |
| Indexed left atrial volume, mL/m2 | 24 ± 11 | 26 ± 12 | 21 ± 8 | .21 |
| Systolic pulmonary arterial pressure, mm Hg | 26 ± 10 | 27 ± 9 | 22 ± 11 | .33 |
| Tricuspid annular plane systolic excursion, mm | 20 ± 5 | 19 ± 5 | 23 ± 3 | .01 |
| Systolic tricuspid annular velocity, cm/s | 13 ± 3 | 13 ± 3 | 15 ± 4 | .04 |
| RV fractional area change, % | 50 ± 13 | 48 ± 13 | 53 ± 11 | .24 |
| RV:LV end-diastolic areas ratio | 0.51 ± 0.16 | 0.52 ± 0.15 | 0.46 ± 0.19 | .26 |
Data are expressed as No. (%) or mean ± SD. A = atrial peak velocity of the mitral flow with pulsed Doppler; E = early peak velocity of the mitral flow with pulsed Doppler; e′ = early diastolic peak velocity of the lateral and septal mitral annulus with tissue Doppler imaging; LV = left ventricular; RV = right ventricular.
The criteria for the diagnosis of QR pattern in V1 were the presence of a prominent Q wave of ≥0.2 mV and a ventricular depolarization <120 ms in lead V1.
The criteria for the diagnosis of S1Q3T3 pattern were the presence of an S wave in lead I, a Q wave in lead III and a T inversion in lead III.
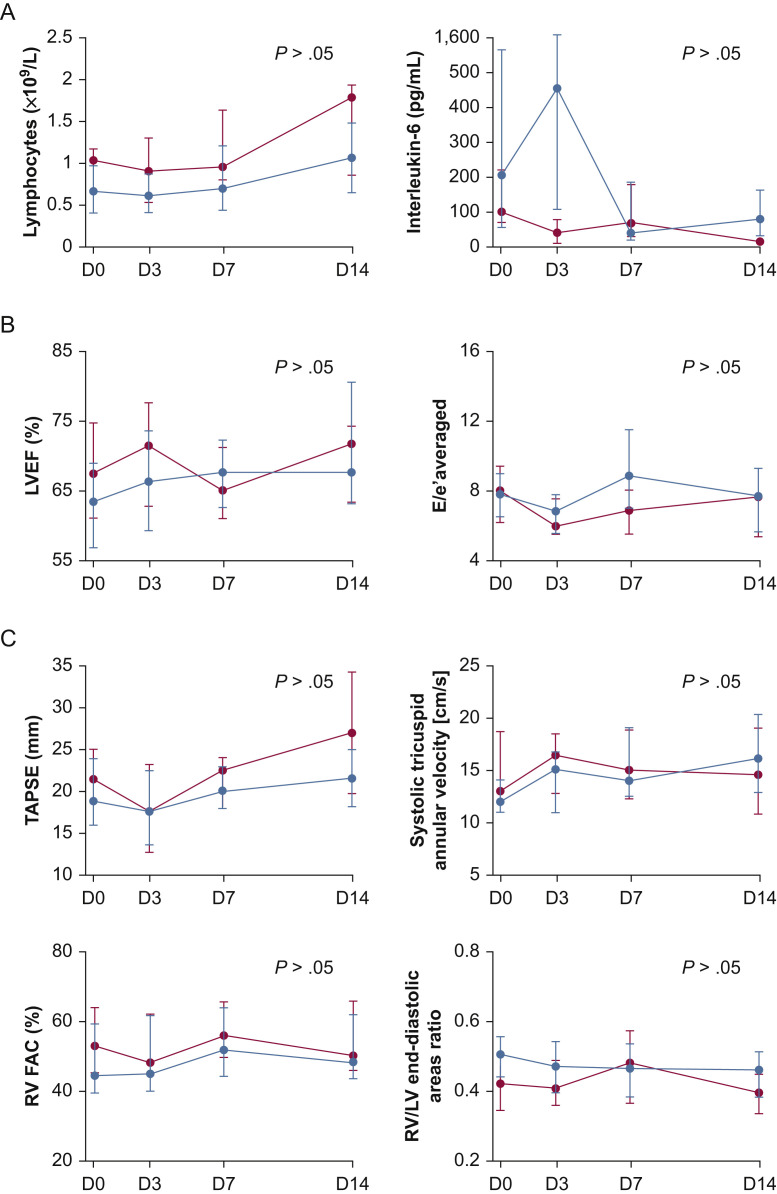
Within the first 14 days of ICU stay, patients with cardiac injury required more frequently vasopressors or inotropes administration, invasive mechanical ventilation, prone positioning, and renal replacement therapy than patients without cardiac injury (Table 3 ). Similarly, patients with new-onset atrial arrhythmias required more frequent vasopressor administration than those without (100% vs 66%; P = .02). The time course of inflammatory parameters levels (Fig 1 A), LV function parameters (Fig 1B), and RV systolic function parameters (Fig 1C) did not differ between patients with cardiac injury and those without.
Table 3.
Intensity of Supportive Care During ICU Stay in Patients With and Without Cardiac Injury
| Variable | Patients With Cardiac Injury(n = 30) | Patients Without Cardiac Injury(n = 13) | P Value |
|---|---|---|---|
| Vasopressor/inotrope use | 26 (87) | 7 (54) | .02 |
| Norepinephrine | 26 (87) | 7 (54) | .02 |
| Epinephrine | 1 (3) | 0 (0) | .54 |
| Maximum norepinephrine dosage, μg/kg/min | 0.50 (0.20-1.40) | 0.15 (0.00-0.70) | .09 |
| Ventilationa | . . . | . . . | . . . |
| Invasive mechanical ventilation | 30 (100) | 11 (85) | .03 |
| Noninvasive ventilation | 2 (7) | 2 (1) | .37 |
| High-flow oxygen therapy | 1 (3) | 2 (15) | .15 |
| Veno-venous extracorporeal membrane oxygenation | 3 (10) | 0 (0) | .28 |
| Prone positioning | 23 (77) | 5 (39) | .02 |
| Neuromuscular blocker use | 23 (77) | 7 (54) | .14 |
| Sedation use | 30 (100) | 11 (85) | .03 |
| Renal replacement therapy | 13 (43) | 0 (0) | < .01 |
Data are presented as No. (%) or median (interquartile range), unless otherwise indicated.
Noninvasive mechanical ventilation and high-flow oxygen therapy could have been performed before or after invasive mechanical ventilation.
Figure 1.
Line graphs showing the time course within the first 14 days of ICU stay of inflammatory parameters (A), of left ventricular function echocardiographic parameters (B), and of right ventricular systolic function echocardiographic parameters (C) in patients with and without cardiac injury. Data are expressed as median and interquartile range. Blue circles represent patients with cardiac injury (n = 30). Red circles represent patients without cardiac injury (n = 13). D = day; E = early peak velocity of the mitral flow with pulsed Doppler; e′ = early diastolic peak velocity of the mitral annulus with tissue Doppler imaging; FAC = fractional area change; LV = left ventricular; LVEF = left ventricular ejection fraction; RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion; TTE = transthoracic echocardiography.
Characteristics and Time Course of Cardiac Injury
Cardiac injury occurred in the 30 patients with a median delay of 3 days (interquartile range, 0-7 days) after ICU admission and 10 days (interquartile range, 5-14 days) after the onset of symptoms. The onset of cardiac injury was at baseline in 21 patients (70%), on day 3 in three patients (10%), on day 7 in five patients (17%), and on day 14 in one patient (3%).
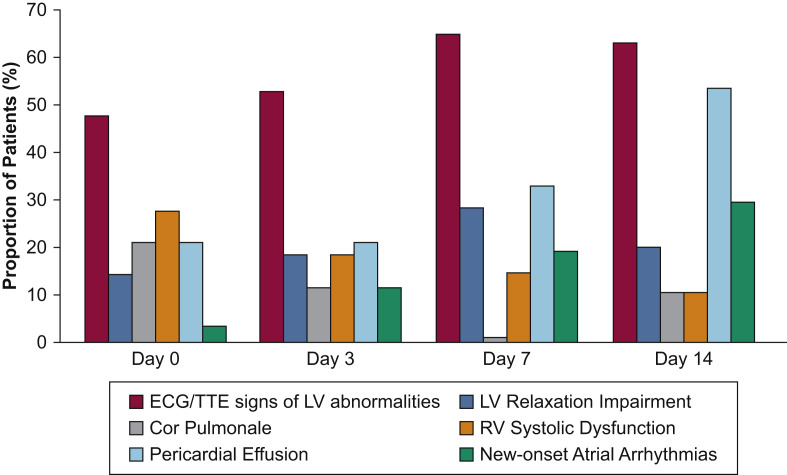
The time of occurrence and the time course of the different ECG and echocardiographic abnormalities involved in cardiac injury are summarized in Tables 4 and 5 , in Figure 2 , and in e-Figure 1B-G. The most frequent ECG and echocardiographic abnormalities were ECG or TTE signs, or both, of LV abnormalities (87% of patients with cardiac injury), RV systolic dysfunction (47%), pericardial effusion (43%), new-onset atrial arrhythmias (33%; ECG and TTE of patient 31) (e-Fig 2, Video 1), and LV relaxation impairment (33%) (Table 5, Fig 2). Eight patients (27%) with cardiac injury showed ECG signs of RV strain, 11 patients (37%) exhibited RV dilation or cor pulmonale (ECG and TTE of patient 8) (e-Fig 3, Video 2), and patients with cardiac injury experienced more RV (47%) than LV (13%) systolic dysfunction, with no patient experiencing LV systolic dysfunction after day 7 (Table 5). All pericardial effusions were mild (< 10 mm) without hemodynamic impairment, and no patient experienced tamponade. No new significant valvulopathy was found. Two patients experienced severe bradyarrhythmia: one second-degree and one third-degree atrioventricular block. No patient experienced life-threatening ventricular arrhythmia.
Table 4.
Time of Occurrence of the Different ECG and Echocardiographic Abnormalities in the 30 Patients With Cardiac Injury Within the First 14 Days of ICU Stay
| Variable | Cardiac Injury | ECG and/or TTE Signs of LV Abnormalities | LV Relaxation Impairment | Cor Pulmonale | RV Systolic Dysfunction | Pericardial Effusion | New-Onset Atrial Arrhythmias |
|---|---|---|---|---|---|---|---|
| Time of occurrence (d) | 3 (0-7) | 0 (0-7) | 3 (0-7) | 0 (0-10.5) | 0 (0-3) | 3 (0-14) | 7 (6-14) |
Data are presented as median (interquartile range). LV = left ventricular; RV = right ventricular; TTE = transthoracic echocardiography.
Table 5.
Time Course of the Different ECG and Echocardiographic Abnormalities in the 30 Patients With Cardiac Injury Within the First 14 Days of ICU Stay
| Variable | Overall (N = 30) | Day 0 (n = 21) | Day 3 (n = 20) | Day 7 (n = 17) | Day 14 (n = 18) | P Valuea |
|---|---|---|---|---|---|---|
| ECG abnormalities | ||||||
| ECG signs of LV abnormalities | 18 (60) | 10 (47) | 13 (65) | 10 (59) | 12 (67) | .03 |
| Inverted T waves | 16 (54) | 8 (38) | 11 (55) | 9 (53) | 10 (57) | .03 |
| ST-segment elevation | 2 (7) | 1 (5) | 2 (10) | 2 (12) | 1 (6) | .07 |
| ST-segment depression | 6 (20) | 4 (19) | 5 (25) | 3 (18) | 5 (28) | .75 |
| Pathologic Q waves | 2 (7) | 2 (10) | 1 (5) | 0 (0) | 0 (0) | .84 |
| New left branch bundle block | 2 (7) | 2 (10) | 2 (10) | 2 (12) | 2 (11) | .91 |
| Severe bradyarrhythmia | 2 (7) | 0 (0) | 0 (0) | 2 (12) | 0 (0) | .83 |
| New-onset of atrial arrhythmias | 10 (33) | 1 (5) | 3 (15) | 4 (24) | 6 (33) | .02 |
| Signs of right ventricular strain | 8 (27) | 5 (24) | 2 (10) | 2 (12) | 0 (0) | .04 |
| Echocardiographic abnormalities | ||||||
| Echocardiographic signs of LV abnormalities | 9 (30) | 7 (33) | 6 (30) | 6 (35) | 3 (17) | .82 |
| LV wall motion abnormalities | 9 (30) | 5 (24) | 5 (24) | 6 (35) | 3 (17) | .49 |
| LV systolic dysfunction | 4 (13) | 2 (10) | 2 (10) | 0 (0) | 0 (0) | .16 |
| LV relaxation impairment | 10 (33) | 4 (19) | 5 (25) | 6 (35) | 4 (22) | .35 |
| Cor pulmonale | 9 (30) | 6 (29) | 3 (15) | 0 (0) | 2 (11) | .22 |
| RV dilation or cor pulmonale | 11 (37) | 6 (29) | 4 (20) | 0 (0) | 3 (17) | .40 |
| RV systolic dysfunction | 14 (47) | 8 (38) | 5 (25) | 3 (18) | 2 (11) | .07 |
| Pericardial effusion | 13 (43) | 6 (29) | 6 (30) | 7 (41) | 11 (61) | .01 |
| ECG or echocardiographic signs of LV abnormalities | 26 (87) | 14 (67) | 13 (65) | 14 (82) | 12 (67) | .50 |
| ECG and echocardiographic signs of LV abnormalities | 3 (10) | 4 (19) | 4 (20) | 2 (12) | 2 (11) | .91 |
Data are presented as No. (%), unless otherwise indicated. LV = left ventricular; RV = right ventricular.
For the generalized linear mixed model.
Figure 2.
Bar graph showing the time course of ECG and echocardiographic abnormalities in the 30 patients with cardiac injury within the first 14 days of an ICU stay. LV = left ventricular; RV = right ventricular; TTE = transthoracic echocardiography.
Inverted T waves and ST-segment depression and wall motion abnormalities represented the most frequent ECG and TTE signs of LV abnormalities at baseline and within the first 14 days of ICU stay (Tables 2 and 5). Coronary angiography was performed in four patients (13%) and confirmed acute coronary syndrome in three of them: two patients showed a significant lesion of the left anterior descending artery and one patient showed a significant lesion of the circumflex artery (ECG and TTE of patient 9) (e-Fig 4, Video 3). Cardiac MRI was performed in one patient without coronary lesion on coronary angiography and confirmed the diagnosis of myocarditis.
Discussion
In our cohort of critically ill COVID-19 patients, 49% of patients demonstrated a cardiac injury at ICU admission and 70% of patients experienced cardiac injury within the first 14 days of ICU stay. The most frequent abnormalities were ECG or TTE signs, or both, of LV abnormalities, and patients with cardiac injury experienced more RV than LV systolic dysfunction. Four different patterns of cardiac injury were identified over time: between baseline and day 14, the incidence of pericardial effusion and of new-onset atrial arrhythmias increased, the incidence of ECG or TTE signs, or both, of LV abnormalities as well as the incidence of LV relaxation impairment remained stable, whereas the incidence of RV and LV dysfunction decreased, with no patient experiencing LV systolic dysfunction after day 7.
To our knowledge, this is the first study providing the incidence and a multimodal description of associated structural abnormalities, as well as the time course, of cardiac injury in critically ill COVID-19 patients, combining ECG, cardiac biomarkers, and TTE analyses. Most previous studies have defined cardiac injury by troponin elevation only.5 , 18 Although this latter seems to be an interesting tool for risk stratifying in COVID-19 patients,5 , 18 troponin elevation does not necessarily reflect cardiac injury and is likely to be multifactorial.19 Thus, it is crucial also to consider ECG and TTE to characterize cardiac injury related to COVID-19 adequately and more precisely. Using a multimodal assessment of cardiac function, we found that 49% of patients harbored a cardiac injury at ICU admission and that 70% of patients experienced cardiac injury within the first 14 days of ICU stay, which is higher than the incidence of between 12% and 30% found in previous studies.2, 3, 4, 5 , 7 , 18 This higher incidence could be explained either by the higher severity of COVID-19 of the patients included in our cohort, as attested by the higher proportion of patients requiring supportive care compared with previous studies2 , 3 , 5 or by the fact that RV systolic function was not taken into account to define cardiac injury,2 , 4 , 7 whereas we and others8 found that patients with cardiac injury experienced more RV than LV systolic dysfunction. Finally, the higher incidence of cardiac injury that we found could be explained by the potential ascertainment bias of prior cohort studies, related to a nonlongitudinal cardiac assessment. Thus, it cannot be excluded that the prevalence we found is more indicative of the true prevalence of cardiac injury in critically ill COVID-19 patients.
Patients with COVID-19 seemed to experience as much newly diagnosed cardiac abnormalities as non-COVID-19 critically ill patients,20 suggesting that these different cardiac abnormalities may not be totally specific to COVID-19, but also may reflect the severity of the disease and may be an epiphenomenon of critical illness.21, 22, 23, 24 The most frequent abnormalities at ICU admission and within the first 14 days of ICU stay were ECG or TTE signs, or both, of LV abnormalities. Inverted T waves and ST-segment depression represented the most frequent ECG signs of LV abnormalities, whereas ST-segment elevation was rare, confirming findings in noncritically ill COVID-19 patients.25 , 26 Similarly, wall motion abnormalities were the most frequent TTE signs of LV abnormalities, with an incidence of 30%, higher than the 12% incidence reported in non-COVID-19 patients.20 Considering ECG or TTE, or both, of LV abnormalities makes it possible to group together four different cardiomyopathies: coronary heart disease (chronic or acute), myocarditis, Takotsubo syndrome, and septic cardiomyopathy.9 , 11, 12, 13, 14, 15 Using this classification, Dweck and colleagues15 recently reported in a global echocardiographic survey a 39% incidence of LV abnormalities, including 8% of new myocardial infarction, myocarditis, and Takotsubo syndrome in a population of critically and noncritically ill COVID-19 patients, confirming that Takotsubo syndrome27, 28, 29 and myocarditis30 seemed to be rare in these patients as well as in non-COVID-19 patients.31 Mechanisms of cardiac injury in COVID-19 patients remain unclear and are probably multifactorial.32, 33, 34 First, inflammation could induce obstructive cardiac ischemia by plaque disruption or nonobstructive cardiac ischemia because of a mismatch between oxygen supply and demand.32 , 33 , 35 We performed coronary angiography in four patients and found a significant coronary artery lesion in three of them, confirming findings from Bangalore and colleagues.36 Second, myocardial inflammation could induce myocarditis or septic cardiomyopathy.12 , 32 , 33 , 35 Further cardiovascular explorations (coronary angiography or cardiac MRI) would be required to characterize ECG or TTE signs, or both, of LV abnormalities better in COVID-19 patients.
Patients with cardiac injury experienced more RV than LV systolic dysfunction, as evidenced by the higher proportion of patients with at least one impaired parameter of RV systolic function than with impaired LV ejection fraction within the first 14 days of ICU stay (47% vs 13%). Similar findings were found in patients with ARDS not related to COVID-19.22 Interestingly, previous studies showed that COVID-19 patients experienced RV systolic dysfunction without significant changes in LV systolic function8 , 37 and that RV systolic longitudinal strain37 as well as increased RV end-diastolic area8 , 38 were reliable prognosis factors in these patients. Overall, this highlights the importance of early and repeated assessment of RV systolic function in COVID-19 patients, which makes sense physiologically, given the high proportion of patients with ARDS, pulmonary embolism, or both.39 , 40
One-third of patients with cardiac injury experienced LV relaxation impairment, an incidence similar to that reported in critically ill patients with sepsis not related to COVID-19.24 , 41 It confirms that COVID-19 patients experienced more LV diastolic than systolic dysfunction.8 This could be explained by the high proportion of patients in our cohort with LV hypertrophy and with a medical history of arterial hypertension.16 , 42 Nevertheless, it must be kept in mind that echocardiographic parameters used to assess LV relaxation are dependent on loading conditions and that echocardiographic abnormalities suggestive of LV relaxation impairment may reflect fluid or vasopressor overload rather than an intrinsic LV diastolic dysfunction.
Besides these echocardiographic abnormalities, 33% of patients with cardiac injury also experienced arrhythmias. New-onset atrial arrhythmias were the most frequent arrhythmias, and no patient experienced sustained ventricular tachycardia, ventricular fibrillation, or cardiac arrest. Our incidence of arrhythmia is similar to that reported in the literature for COVID-197 , 32 , 43 and for previous viral outbreaks,44 as well as to that reported in non-COVID-19 critically ill patients with sepsis.45 Arrhythmia may be driven by myocardial damage, but also by nonmyocardial factors such as metabolic abnormalities, hypoxemia, or systemic inflammation,43 because some patients without cardiac injury also experienced arrhythmias such as atrial fibrillation during their ICU stay. Interestingly, patients with new-onset atrial arrhythmias required more frequent vasopressor administration than those without, suggesting that vasopressor use may be the trigger for atrial arrhythmias through the activation of β-receptors. Nevertheless, it cannot be excluded that the need for vasopressors is a consequence of the potential hemodynamic impairment induced by atrial arrhythmias.
We acknowledge some limitations to our study. First, we included a relatively small number of patients, but no new COVID-19 patients were admitted to our ICUs after May. Nevertheless, to our knowledge this is the first study providing the frequency and a multimodal description of cardiac injury over time in critically ill COVID-19 patients. Second, it was not possible for obvious reasons in the context of a pandemic to perform coronary angiography or cardiac MRI easily in all patients with ECG or TTE signs, or both, of LV abnormalities. Third, 11% of COVID-19 patients were not included because of poor echogenicity, mainly as a result of obesity and mechanical ventilation with high positive end-expiratory pressure level. However, this proportion is similar to that reported in non-COVID-19 critically ill patients.46 Fourth, because some patients did not have prior or had no available results of prior cardiac assessment, the potential chronicity of some newly diagnosed ECG or TTE abnormalities, or both, could not be ruled out with certainty. Fifth, we considered a maximum follow-up of 14 days to focus only on cardiac injury and to prevent the observed cardiac abnormalities from being related more to ICU stay complications than to COVID-19. Long-term follow-up with complete cardiovascular examination in COVID-19 patients deserves further study.
Interpretation
Cardiac injury was common and occurred early in critically ill COVID-19 patients. The most frequent newly diagnosed abnormalities were ECG or echocardiographic signs, or both, of LV abnormalities, and patients with cardiac injury experienced more RV than LV systolic dysfunction. Further studies are needed to characterize the potential chronic cardiac injury in COVID-19 patients.
Acknowledgments
Author contributions: D. D. and M. J. take responsibility for the content of the manuscript, including the data and analysis. D. D., G. B., A. C., J.-P. M., J. D., and M. J. conceived the study. D. D., P. D., E. F., and M. J. recorded the data. D. D., P. D., L. M., E. F., C. S., M. B., H. H., E. F., M. J., and M. J. analyzed data. All authors contributed to the interpretation of data. D. D. and M. J. drafted the first version of the manuscript. All authors contributed drafting the manuscript, and all authors approved the final version of the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figures, and Videos can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Supplementary Data
References
- 1.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S., Qin M., Cai Y. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ni W., Yang X., Liu J. Acute myocardial injury at hospital admission is associated with all-cause mortality in COVID-19. J Am Coll Cardiol. 2020;76(1):124–125. doi: 10.1016/j.jacc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szekely Y., Lichter Y., Taieb P. The spectrum of cardiac manifestations in coronavirus disease 2019 (COVID-19)—a systematic echocardiographic study. Circulation. 2020;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 10.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 11.Knuuti J., Wijns W., Saraste A. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 12.Martin L., Derwall M., Al Zoubi S. The septic heart: current understanding of molecular mechanisms and clinical implications. Chest. 2019;155(2):427–437. doi: 10.1016/j.chest.2018.08.1037. [DOI] [PubMed] [Google Scholar]
- 13.Sinagra G., Anzini M., Pereira N.L. Myocarditis in clinical practice. Mayo Clin Proc. 2016;91(9):1256–1266. doi: 10.1016/j.mayocp.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Ghadri J.R., Wittstein I.S., Prasad A. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032–2046. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dweck M.R., Bularga A., Hahn R.T. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21(9):949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagueh S.F. Left ventricular diastolic function: understanding pathophysiology, diagnosis, and prognosis with echocardiography. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):228–244. doi: 10.1016/j.jcmg.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 17.Falk V., Baumgartner H., Bax J.J. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2017;52(4):616–664. doi: 10.1093/ejcts/ezx324. [DOI] [PubMed] [Google Scholar]
- 18.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman A.R., Bularga A., Mills N.L. High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation. 2020;141(22):1733–1735. doi: 10.1161/CIRCULATIONAHA.120.047008. [DOI] [PubMed] [Google Scholar]
- 20.Bossone E., DiGiovine B., Watts S. Range and prevalence of cardiac abnormalities in patients hospitalized in a medical ICU. Chest. 2002;122(4):1370–1376. doi: 10.1378/chest.122.4.1370. [DOI] [PubMed] [Google Scholar]
- 21.Inciardi R.M., Adamo M., Lupi L., Metra M. Atrial fibrillation in the COVID-19 era: simple bystander or marker of increased risk? Eur Heart J. 2020;41(32):3094. doi: 10.1093/eurheartj/ehaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.See K.C., Ng J., Siow W.T., Ong V., Phua J. Frequency and prognostic impact of basic critical care echocardiography abnormalities in patients with acute respiratory distress syndrome. Ann Intensive Care. 2017;7(1):120. doi: 10.1186/s13613-017-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vignon P. Ventricular diastolic abnormalities in the critically ill. Curr Opin Crit Care. 2013;19(3):242–249. doi: 10.1097/MCC.0b013e32836091c3. [DOI] [PubMed] [Google Scholar]
- 24.Sanfilippo F., Scolletta S., Morelli A., Vieillard-Baron A. Practical approach to diastolic dysfunction in light of the new guidelines and clinical applications in the operating room and in the intensive care. Ann Intensive Care. 2018;8(1):100. doi: 10.1186/s13613-018-0447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCullough S.A., Goyal P., Krishnan U., Choi J.J., Safford M.M., Okin P.M. Electrocardiographic findings in coronavirus disease-19: insights on mortality and underlying myocardial processes. J Card Fail. 2020;26(7):626–632. doi: 10.1016/j.cardfail.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angeli F., Spanevello A., De Ponti R. Electrocardiographic features of patients with COVID-19 pneumonia. Eur J Intern Med. 2020;78:101–106. doi: 10.1016/j.ejim.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsao C.W., Strom J.B., Chang J.D., Manning W.J. COVID-19-associated stress (Takotsubo) cardiomyopathy. Circ Cardiovasc Imaging. 2020;13(7) doi: 10.1161/CIRCIMAGING.120.011222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giustino G., Croft L.B., Oates C.P. Takotsubo cardiomyopathy in males with Covid-19. J Am Coll Cardiol. 2020;76(5):628–629. doi: 10.1016/j.jacc.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer P., Degrauwe S., Van Delden C., Ghadri J.R., Templin C. Typical Takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. 2020;41(19):1860. doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395(10235):1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyen D., Moschietto S., Squara F. Incidence, clinical features and outcome of Takotsubo syndrome in the intensive care unit. Arch Cardiovasc Dis. 2020;113(3):176–188. doi: 10.1016/j.acvd.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Atri D., Siddiqi H.K., Lang J., Nauffal V., Morrow D.A., Bohula E.A. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5(5):518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Libby P. The heart in COVID19: primary target or secondary bystander? JACC Basic Transl Sci. 2020;5(5):537–542. doi: 10.1016/j.jacbts.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akhmerov A., Marban E. COVID-19 and the heart. Circ Res. 2020;126(10):1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5(7):751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 36.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with Covid-19—a case series. N Engl J Med. 2020;382(25):2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuman Li H.L., Shuangshuang Zhu, Xie Yuji. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13(11):2287–2299. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argulian E., Sud K., Vogel B. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging. 2020;13(11):2459–2461. doi: 10.1016/j.jcmg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creel-Bulos C., Hockstein M., Amin N., Melhem S., Truong A., Sharifpour M. Acute cor pulmonale in critically ill patients with Covid-19. N Engl J Med. 2020;382(21):e70. doi: 10.1056/NEJMc2010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pulido J.N., Afessa B., Masaki M. Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clin Proc. 2012;87(7):620–628. doi: 10.1016/j.mayocp.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams B., Mancia G., Spiering W. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 43.Lazzerini P.E., Boutjdir M., Capecchi P.L. COVID-19, arrhythmic risk and inflammation: mind the gap! Circulation. 2020;142(1):7–9. doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 44.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31(5):1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bosch N.A., Cimini J., Walkey A.J. Atrial fibrillation in the ICU. Chest. 2018;154(6):1424–1434. doi: 10.1016/j.chest.2018.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jozwiak M., Mercado P., Teboul J.L. What is the lowest change in cardiac output that transthoracic echocardiography can detect? Crit Care. 2019;23(1):116. doi: 10.1186/s13054-019-2413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.