Abstract
Fundamental restoration ecology and community ecology theories can help us better understand the underlying mechanisms of fecal microbiota transplantation (FMT) and to better design future microbial therapeutics for recurrent Clostridioides difficile infections (rCDI) and other dysbiosis-related conditions. In this study, stool samples were collected from donors and rCDI patients one week prior to FMT (pre-FMT), as well as from patients one week following FMT (post-FMT). Using metagenomic sequencing and machine learning, our results suggested that FMT outcome is not only dependent on the ecological structure of the recipients, but also the interactions between the donor and recipient microbiomes at the taxonomical and functional levels. We observed that the presence of specific bacteria in donors (Clostridioides spp., Desulfovibrio spp., Odoribacter spp. and Oscillibacter spp.) and the absence of fungi (Yarrowia spp.) and bacteria (Wigglesworthia spp.) in recipients prior to FMT could predict FMT success. Our results also suggested a series of interlocked mechanisms for FMT success, including the repair of the disturbed gut ecosystem by transient colonization of nexus species followed by secondary succession of bile acid metabolizers, sporulators, and short chain fatty acid producers.
Subject terms: Microbiology, Microbiome
Introduction
Antibiotics are the primary treatment method for Clostridioides difficile infections (CDI); however, the negative impact on the diversity, composition, and functionality of gut microbiota results in recurrent CDI (rCDI)1,2 requiring fecal microbiota transplantation (FMT). FMT is a strategy for the restoration of a disturbed microbial ecosystem and reinstatement of lost microbial functional networks. Although highly effective in the treatment of rCDI as well as promising in several other diseases2–8, FMT carries infectious and non-infectious risks9–12. In addition, under each specific disease scenario, it is crucial to understand how microbial ecosystems reassemble overtime after FMT and which microbial strains are the determining factors in this dynamic process. For rCDI treatment, the scientific lens in the past mainly had a uni-kingdom major focus on bacteria. It has been suggested that an ideal donor should have high Lachnospiraceae and Ruminococcaceae13, which are also positively associated with secondary bile acids that inhibit CDI germination1. Increased Clostridium scindens in donors has also shown a positive correlation with FMT efficacy and outcomes via the production of secondary bile acids14. Moreover, FMT restores short chain fatty acids (SCFAs) metabolism, with immune modulatory effects in rCDI patients15. SCFAs and butyrate producing bacteria have been found to decrease the induction of proinflammatory cytokines and promote the differentiation of colonic Treg cells, leading to the attenuation of colitis in mice and humans16,17. In addition, anaerobic, endospore-forming Firmicutes are dominant members of gut microbiota that can produce SCFAs18, which allow organisms to enter metabolically dormant states that aid in their survival and transmission to new hosts19. Thus, the oral delivery of SER-109, composed of sporulating bacteria, remains a promising therapeutic approach for rCDI treatment20,21. Furthermore, a critical consideration for FMT efficacy and durability is that the microbial consortium of the donors is not the only key player. The existing endogenous microbiome in recipients can also play a significant role in determining the colonization of those exogenous species. For example, focusing on bacterial engraftment, Smillie et al.22 suggested that selective forces in the patient’s gut (host control), rather than input dose dependence (bacterial abundance in the donor and patient), determines bacterial abundance after FMT and, subsequently, its efficacy. In contrast, a number of studies suggest that FMT success is only dependent on the bacterial diversity and composition of the stool donor, leading to the proposition of the existence of FMT super-donors3,23.
Beyond the gut bacterium, more recently, few studies have examined the role of gut mycobiome and virome on FMT efficacy. For example, Zuo and colleagues found a negative relationship between the abundance of fungi such as Candida albicans in donor stool and FMT efficacy24. Over the last decade, phages have gained increasing attention for therapeutic use due to their specificity25. The reduction in the abundance of Caudovirales bacteriophages and an increase in Microviridae abundance, specifically higher abundance of Eel River basin pequenovirus as a potential Proteobacteria predator, were shown to be related to FMT efficacy in CDI patients26,27. Using targeted refined phage therapy, Nale et al.28 used a cocktail of four C. difficile Myoviruses (CDHM1, 2, 5, and 6) to eradicate the CDI in a batch fermentation model, which suggests that a combination of bacteriophages may be needed to treat CDI. More recently, rCDI in five patients was prevented using sterile fecal filtrate, void of live bacteria29. Contrary to these, a study by Meader et al.30 showed that bacteriophages alone weren’t sufficient to eradicate CDI. These studies emphasize that in order to uncover mechanisms involved in FMT efficacy, it is fundamental to include the relative contribution of all domains and consider the microbiome-associated ecosystem heterogeneity in both donors and recipients. To this end, we specifically investigated whether FMT super-donors exists for rCDI treatment, or whether the donor-recipient compatibility and short-term fluctuations in the gut microbiomes (a combination of bacteria, fungi, archaea, and viruses) of both donors and recipients have profound implications in FMT success.
Materials and methods
Study design and sample collection
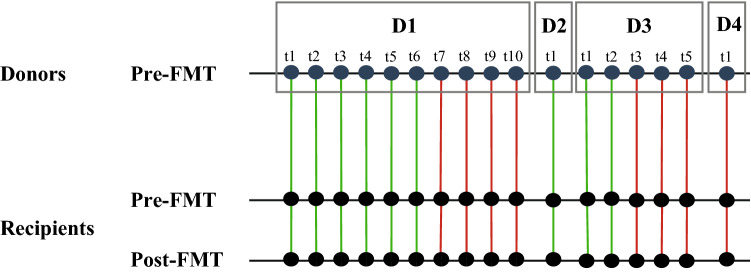
Seventeen adult male and female patients who received FMT for rCDI at the University of Alberta Hospital in Edmonton, Alberta, Canada, between October 2012 and November 2014 were included in this study31. All research methods were performed in accordance with the relevant guidelines and regulations. Criteria for receiving FMT were (1) at least 2 recurrent episodes of mild to moderate CDI, or (2) at least 1 recurrent episode of CDI requiring hospitalization. This study was approved by the University of Alberta Health Research Ethics Board, and all participants provided written informed consent. Patients aged 35–85 were included; however, individuals were excluded from participating if they had been exposed to any form of antibiotics, antifungals, antivirals, or antiparasitics within the previous six months31. More information pertaining to the patient characteristics, donor selection criteria, and screening processes have been described previously31 (see Supplementary Table S1). All participants received FMT by colonoscopy, with stool samples from unrelated donors registered with the Edmonton FMT program. After a failed FMT, each patient received FMT from the same donor or a different donor, depending on donor availability. Patients discontinued antibiotics for CDI 24 h prior to FMT and took 4 L of polyethylene glycol-based bowel preparation (GoLYTELY) one day prior to FMT. Stool samples were collected from donors and patients one week prior to FMT (pre-FMT) as well as from patients one week following FMT (post-FMT). Figure 1 shows the number of donors and recipients, as well as the FMT treatment outcomes. It’s important to note that although some donors had provided multiple stool samples, these samples were provided at different time points (minimum of a one-week gap), which were then administered to the recipients (Fig. 1). It has been perceived that the autocorrelation between microbiomes of stool samples of a given donor normally diminishes between 3 and 5 days32.
Figure 1.
The experimental data structure of stool samples collected. Samples were collected from 4 donors (D1–D4) and 17 patients one week prior to FMT (pre-FMT) and patients one week following FMT (post-FMT). For D1 and D3, multiple independent samples were taken for different patients. Green line indicates a successful FMT outcome and a red line indicates a failed FMT outcome.
Metagenomic data collection
DNA from stool samples were extracted using the Qiagen QIAamp DNA stool kit. Shotgun sequencing for metagenomics was applied using the Nextera XT DNA Sample Preparation Kit, and Illumina MiSeq platform was performed as previously described31. Host DNA was detected and reads were removed by mapping with the GEM program to the human genome with inclusive parameters33. A custom Kraken database was built of whole genomes of bacteria, viruses, archaea, eukaryotes, and viroids34. The Bayesian Reestimation of Abundance with KrakEN (Bracken) algorithm was used (kmer length of 30 and read length of 100 bp) to compute the abundance of species in DNA sequences originating from each metagenomic sample35. Singletons, as well as those taxa occurring in only one or two samples, were removed and abundances of different microbial genera were obtained by collapsing detected taxonomies to the genus level and summing features within the same genera. Subsequently, taxa abundances were normalized by the total number of reads sequenced in each sample.
Statistical analysis
The α-diversity (Shannon diversity index) of successful and failed FMT samples were compared for all organisms using the R package Vegan36. In addition, α-diversity of bacteria related to bile acid metabolizers37, SCFA producing genera38, and sporulators39 were compared for successful and failed FMT samples. The bacterial genera associated with these functions were extracted from previous studies37–39. Significant differences in α-diversity were determined using the non-parametric Kruskal–Wallis and Wilcoxon signed-rank test for unpaired and paired samples (pre- and post-FMT samples of recipients), respectively, using Bonferroni correction to adjust the probability. Differences among community structures across samples (β-diversity) were calculated using the Bray–Curtis dissimilarity metric using the R package Vegan and visualized via density plots using custom python scripts36. Significant differences in β-diversity across donors and recipients were evaluated using analysis of similarities (ANOSIM)40. Heatmap clustering graphs were constructed using the R pheatmap package to visualize the relative abundance of major bile acid producers in donors and recipients before and after FMT41.
To test whether donor and recipient microbial composition can predict FMT outcome, we trained a Random Forest (RF) model on pre-treatment samples of both donors and recipients at the genus level42. The microbial taxa of both donors and recipients constitute the feature space of the model and the following steps were performed using the Python library, Scikit-learn43. As the features’ count outnumbers that of the test samples, a dimensionality reduction method was implemented so that the trained model avoids overfitting and generalizes better on the test data44. Thus, the Principal Component Analysis (PCA) was used to exploit the features which describe the principal components the most. The top 20 features from this analysis were selected to be employed in the training process of the RF model. In order to assess how well the trained classifier generalizes in case of unseen data, the Leave One Out (LOO) cross-validation method was employed. In this method, each data point was used once as a test data, while the classifier was trained on the remaining data points. Subsequently, the cross-validation error value was calculated by averaging all the measured test errors. For each LOO data subset, the Receiver Operating Characteristic (ROC) curve was plotted. Next, the RF classifiers with the highest validation scores were compared by implementing a statistical significance test. Herein, McNemar’s test was used to determine the statistical significance of the difference between the predictive performance of the top RF candidates45. The RF model identified to be the most precise was then employed to find the most important features in the FMT treatment outcome task. After running the model 100 times, the average Mean Decrease in Impurity (MDI) of the most important features were also calculated46. Subsequently, the Kruskal–Wallis test with the Bonferroni correction to adjust the probability was utilized to compare the relative abundance of the top important features across the samples. Lastly, in an attempt to evaluate our model’s performance and its generalizability, another independent dataset was used47,48. This dataset consisted of DNA extracted from 5 fecal samples from 3 donors, and 5 fecal samples from each of 10 FMT recipients: collected at day 0 (pre-FMT) and days 2, 14, 42 and 84 after FMT.
Results
This study included seventeen adult male and female patients who received FMT for rCDI by colonoscopy from four unrelated donors. Stool samples were collected from donors and patients one week prior to FMT (pre-FMT), as well as from patients one week following FMT (post-FMT) (Fig. 1). Among recipients, 9/17 patients were successfully treated with a single FMT (53% successful FMT), while 8 patients failed the first FMT and required a second procedure. There was no difference between the two groups in factors of age, sex, or duration of CDI31 (Supplementary Table S1).
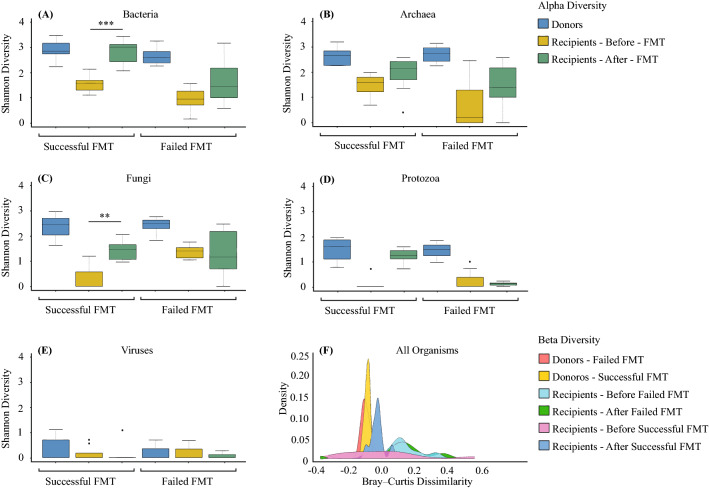
We found no significant difference in alpha diversities of different organisms in stool samples provided by donors used for all patients whether the treatment outcome was successful or not (Kruskal–Wallis test, p > 0.05, Fig. 2A–E). For the recipients, no significant differences in alpha diversities were observed between successful and failed pre-FMT samples (Kruskal–Wallis test, p > 0.05, Fig. 2A–E). There was a significant increase in the bacterial (Fig. 2A) and fungal (Fig. 2C) alpha diversities (Shannon diversity index) in post-FMT stool samples after successful FMT (Wilcoxon test, p value < 0.001 and p value < 0.01, respectively), but not failed ones. No significant changes in this index were seen post-FMT in archaeal, protozoan, and viral diversities (Fig. 2B,D,E). Results showed significant differences in beta diversities of all organisms in stool samples between recipients and donors pre-FMT (ANOSIM, R = 0.920), but no significant differences were detected between successful and failed donors (ANOSIM, R = 0.648), successful and failed recipients pre-FMT (ANOSIM, R = 0.098), and failed recipients pre-FMT and post-FMT (ANOSIM, R = 0.219) (Fig. 2F). After successful FMT, the recipients’ microbiome composition resembled their donors (ANOSIM, R = 0.595), while the composition of failed FMT recipients remained different compared to their donors (ANOSIM, R = 0.860) (Fig. 2F).
Figure 2.
Gut microbial diversity of FMT donors and recipients. The α-diversity (Shannon index) of (A) bacteria, (B) archaea, (C) fungi, (D) protozoa, and (E) viruses of donors, recipients pre- and post-FMT for successful and failed FMT outcomes of rCDI patients. Significant differences were determined using the Kruskal–Wallis and Wilcoxon signed-rank tests for unpaired and paired (i.e. when analysing pre- and post-FMT of recipients) samples, respectively, followed by Bonferroni post-hoc correction. Adjusted p values were defined at *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. The Beta diversity was calculated for all microorganisms (F) using the Bray–Curtis dissimilarity and analyzed using ANOSIM.
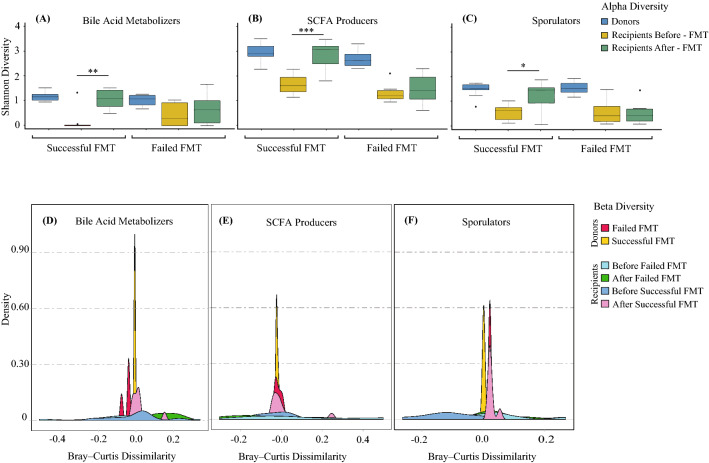
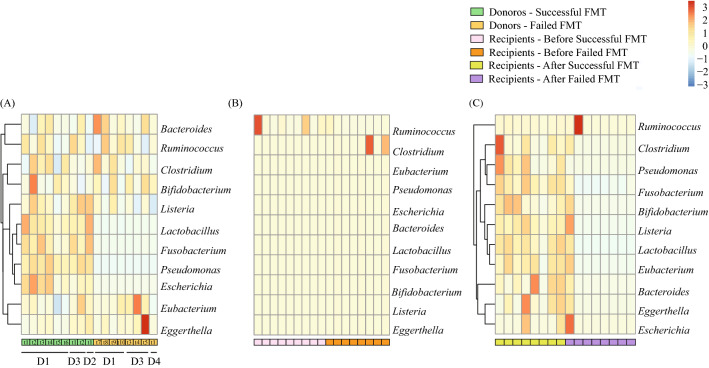
We then isolated bacterial genera associated with bile acid metabolism suggested by Gerard et al.37 and SCFA production suggested by Seekatz et al.38, as well as sporulating communities39, since it has been shown that they can impact FMT efficacy. Our results showed that donors had similar microbial alpha diversity (Kruskal–Wallis test, p > 0.05, Fig. 3A–C) and community structures for SCFA producers and sporulators (ANSIM, R = 0.524 and R = 0.582, respectively, Fig. 3E–F). However, the community structure of bile acid metabolizers was significantly different between successful and failed FMT donors (ANOSIM, R = 0.828) (Fig. 3D). Specifically, bacterial bile acid metabolizers including Lactobacillus (associated with deconjugation and esterification of bile salts), Fusobacterium (associated with desulfation of bile salts), Pseudomonas (desulfation of bile salts), and Escherichia (oxidation and epimerization of bile salts) were significantly less abundant in unsuccessful donor samples (Fig. 4A). Interestingly, intra-variability within donors pertaining to the abundance of bacterial bile acid metabolizers was observed (Fig. 4A), which shows that donor composition can vary over time and affect FMT outcome. Focusing on recipients, our results showed that successful FMT is associated with the colonization of bile acid metabolizers, SCFA producers, and sporulating bacterial genera, since the diversity (Wilcoxon signed-rank test, p = 0.003, p = 0.00014, and p = 0.015, respectively) and community structures of associated bacteria within each community significantly increased after successful FMT (ANOSIM, R = 0.670, R = 0.759, and R = 0.872, respectively) but not the failed ones (ANOSIM, R = 0.091, R = 0.117, and R = 0.134, respectively) (Fig. 3). Figure 4 also shows that no significant differences were detected between successful and failed recipients pre-FMT with the colonization of bile acid metabolizers, SCFA producers, and sporulating bacterial genera (Fig. 4B). However, after successful FMT, the recipients’ microbiome functionality resembled their donors, while failed FMT recipients remained different compared to their donors (Fig. 4C).
Figure 3.
Gut microbial diversity of bile acid metabolizers, SCFA producers, and sporulators. The α-diversity (Shannon index) of (A) bile acid metabolizers, (B) SCFA producers, and (C) sporulating bacteria of donors, recipients pre- and post-FMT for successful and failed FMT outcomes. Significant differences were determined using the Kruskal–Wallis and Wilcoxon signed-rank tests for unpaired and paired samples, respectively, followed by the Bonferroni post-hoc correction. Adjusted p-values were defined at *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. The Beta diversity was also calculated using the Bray–Curtis distance-based density plots (D–F) and analyzed using ANOSIM.
Figure 4.
Bile acid metabolizers of FMT donors and recipients. Heatmap of bile acid metabolizers of (A) donors (D1-D4), (B) recipients pre-FMT, and (C) recipients post-FMT for successful and failed FMT outcomes. The dendrogram shows clustering based on the relative abundances. The heatmap color (blue to red, corresponding to low to high) represents the row z-score of the relative abundance values. Each sub-block under heatmaps represent a different time point for the corresponding donor.
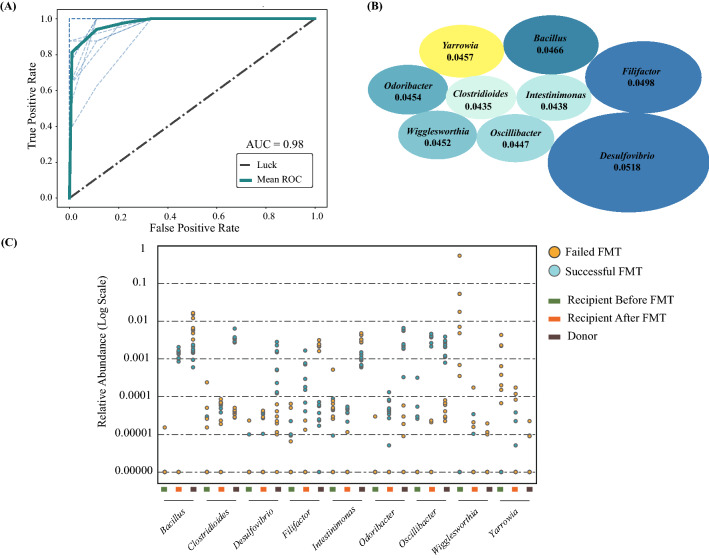
Finally, we investigated whether the gut microbiome of donor and recipients before FMT can predict the treatment outcomes. The top 20 features from PCA analysis were selected and employed in the subsequent training process of a classification model, using samples from both donor and recipient pre-FMT at the genus level. Using LOO cross validation, the prediction model was significant (p = 0.0099) (Fig. 5A), with the most important genera being Desulfovibrio, Filifactor, Bacillus, Yarrowia, Odoribacter, Wigglesworthia, Oscillibacter, Intestinimonas, and Clostridioides (Fig. 5B). Furthermore, in order to visualize the impact of the top features on FMT efficiency, the relative abundance of such features was plotted for donor and recipient samples pre- and post-FMT (Fig. 5C). Interestingly, the fungal genus of Yarrowia, as well as bacterial genus of Wigglesworthia, were significantly higher in pre-FMT failed recipients than pre-FMT successful recipients (Fig. 5C, Kruskal–Wallis, p = 0.001 and p = 0.002, respectively). The donor samples that contributed to a successful FMT outcome had a higher abundance of Clostridiodes (p = 0.002), Desulfovibrio (p = 0.004), Odoribacter (p = 0.002), and Oscillibacter (p = 0.003) compared to failed FMT donors (Fig. 5C), and intra-variability in the relative abundances of these genera for each donor was observed (Supplementary Fig. S1) when comparing successful and failed samples. It is important to note that, these genera were not detected in successful recipients post-FMT (Supplementary Fig. S1 and S2), indicating that long-term colonization of these genera in recipients may not be critical for FMT success.
Figure 5.
Machine learning model predicting FMT outcome. The random forest model utilized led to (A) the ROC curve displaying an AUC of 98%, (B) the top 9 most important microbes (blue ellipses represent bacteria; yellow ellipse represent fungi) involved in FMT success prediction where the size of the ellipses represents the feature importance magnitudes (average Mean Decrease in Impurity), and (C) the relative abundance (shown on logarithmic scale) for the top 9 features of donor, and recipient samples pre- and post-FMT.
We then evaluated our model’s performance against an independent dataset47,48. Interestingly, the model identified similar top features including Odoribacter and Clostrioides; albeit with no statistically significant discriminatory powers. This was expected due to technical variation between studies which overshadowed the biological variation, as well as the lack of full consistency between the two studies, rooted in the difference in the average age or ethnicity of the two cohorts.
Discussion
Despite long-term stability and plasticity of healthy and low to moderately disturbed gut systems49, severely damaged gut ecosystems are not self-renewing; therefore, FMT can help with restoring damaged systems through (a) the recreation of the original ecosystem (e.g., by autologous FMT) or (b) the construction of an entirely new and alternative ecosystem (e.g., by allogeneic FMT). In our study, we showed that the success of gut ecological recovery through FMT is dependent on several factors, including the donor gut microbiome (the presence of specific bacteria), as well as the pre-FMT recipient gut community structures and recovering habitat (the absence of specific bacteria and fungi) (Fig. 5). In addition, short-term fluctuations in the gut microbiome of both donors and recipients have profound implications in FMT success by producing temporary changes or loss of function (see Supplementary Fig. S1 and S2; Fig. 4). Therefore, the notion of the “super-donor” is oversimplified due to the observed short-term fluctuations, and a recipient’s microbiota may be just as important to consider when predicting treatment outcomes, especially in other dysbiotic conditions such as ulcerative colitis.
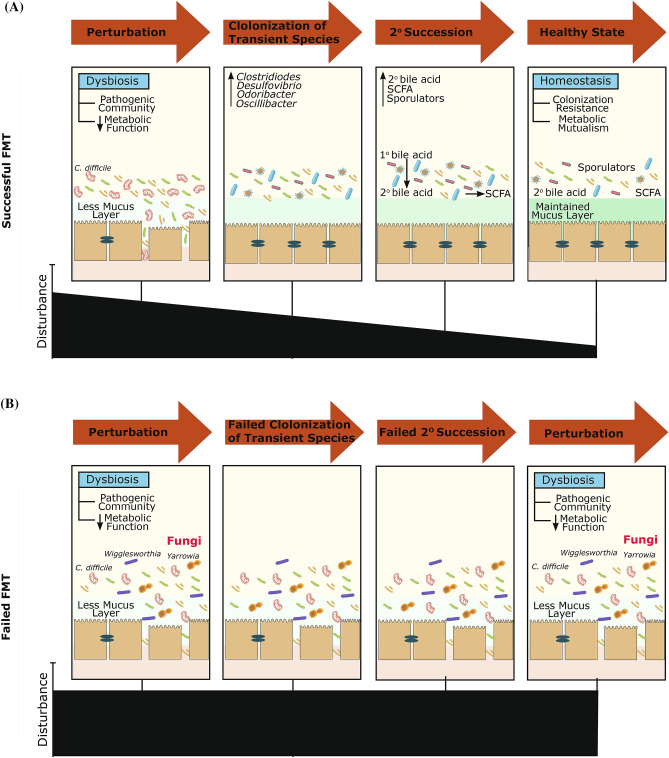
Our results also showed that a trans-kingdom interaction between bacteria and fungi may be important to consider in FMT outcomes. Considering ecological theories on community construction and recovery after disturbance, we hypothesize that the first step of a successful FMT is the colonization of “nexus species” including members of Desulfovibrio, Odoribacter, Oscillibacter, and Clostridioides genera, as identified in two independent datasets (Fig. 6). These are transient in the community development, but are ecosystem engineers that determine secondary succession trajectories of the ecosystem (Supplementary Figs. S1 and S2). For example, Odoribacter is a known SCFA producer50. Thus, its presence in the donor and the initial transfer to recipients may contribute to decreased inflammation51. In addition, the class Clostridia includes many endospore-forming organisms that have the capacity to produce SCFAs52,53, which can induce T regulatory cells and associated anti-inflammatory cytokines17. Following a successful repair, the secondary succession of endogenous or exogenous bile acid metabolizers can restore microbial diversity (lost commensals) and a variety of ecosystem functions54. Namely, when bile acid metabolizers colonize the repaired gut ecosystem, secondary bile acid concentrations, as pleiotropic signaling molecules in the gut, liver, and systemic circulation, increases55. This process entails the germination of endogenous or exogenous sporulators such as Clostridia and other putative endospore formers, which are considered stress-resistant and are particularly adaptive to cross-host dissemination19,56. Aligned with the above hypothesized mechanism, donors that led to a failed FMT had reduced Fusobacterium and Pseudomonas genera, which are both capable of desulfating primary bile acids. When these genera exist, sulfation can reduce primary bile acid toxicity and increase secondary bile acid excretion via urine and feces57. This reduced desulfation capacity in failed donor samples further perpetuates the already existing disturbed bile acid pool and inhibits successful secondary colonization for functional ecosystem restoration. Moreover, bacterial genera, which can dehydroxylate primary bile acids into secondary bile acids, are also known to produce SCFAs51. These gut microbiota associated metabolites, especially butyrate, are a main source of energy for colonocytes and can activate G-protein coupled receptors that regulate intestinal motility and inflammation51,58. Lack of such genera in donor samples may diminish the therapeutic potential of FMT.
Figure 6.
Multifaceted mechanisms affecting FMT treatment outcome. FMT treatment outcome of (A) successful FMT recipients, and (B) failed FMT recipients. A successful treatment outcome includes the repair of the disturbed gut microbial ecosystem by transient colonization of nexus species followed by secondary succession of bile acid metabolizers, sporulators, and short chain fatty acid producers. A failed treatment outcome may be due to the presence of fungal and bacterial genera including Yarrowia and Wigglesworthia in recipients, minimizing the establishment of repair or successful secondary colonization for functional ecosystem restoration.
However, interestingly, the presence of the Yarrowia and Wigglesworthia genera in pre-FMT recipients can act as a barrier for the establishment of repair or successful secondary colonization for functional ecosystem restoration (Fig. 5C). This can be due to nutrient cycling and carbon uptake elevation by fungal activity. Moreover, Yarrowia lipolytica has been vastly studied as a non-conventional yeast species capable of synthesizing a group of metabolites, in particular lipases and other hydrolytic enzymes59. These opportunistic fungal pathogens can cause infections in immunocompromised and critically ill patients60–62. To overcome this challenge, treatment targeted at these fungal elements prior to FMT may potentially enhance treatment efficacy.
In summary, there have been a number of studies focusing on understanding the underlying mechanisms in FMT treatment, which can accordingly be used for the optimization of future treatments. In the past, the scientific lens mainly had a uni-kingdom major focus on bacteria, leading to the proposition of the existence of FMT “super-donors”. However, our preliminary study along with a growing number of studies26,28,30,63,64 support the existence of complex trans-kingdom interactions. Our study here suggests that FMT is not necessarily a ‘one stool fits all’ approach and that donor-recipient cross-kingdom microbiota interactions, along with their short-term fluctuations in the gut, bring profound implications in FMT success. The results also conceptualize a series of interlocked mechanisms for FMT success, including first repairing the disturbed gut microbial ecosystem by transient species, followed by secondary succession of indigenous or exogenous bile acid metabolizers, sporulators, and SCFA producers. However, it should be noted that this study had limitations, including the small sample study cohort, as well as the lack of ethnic minorities within the sample population (88% Caucasian). This signifies the need for larger cohort studies that include patients with diverse demographic characteristics. Future studies with larger sample population can further assess the preliminary mechanisms suggested in this study and eventually optimize FMT treatment for rCDI.
Supplementary information
Author contributions
N.K. and S.P. wrote the first draft of the manuscript. SP assisted in reviewing the literature, guided the analysis, and provided intellectual input in the manuscript. M.R., A.S., and A.N. aided with the data analysis. N.K., M.R., A.N., G.K.-S.W., D.K., and S.P. reviewed and edited the manuscript. F.M.K. and A.H.Z.K. contributed to the metagenomic sequence handling and processing. N.K., M.R., A.N., G.K.-S.W., D.K., and S.P. actively contributed to the critical discussions. The authors read and approved the final manuscript.
Data availability
The normalized and non-normalized feature tables are available in supplementary data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dina Kao, Email: dkao@ualberta.ca.
Sepideh Pakpour, Email: sepideh.pakpour@ubc.ca.
Supplementary information
is available for this paper at 10.1038/s41598-020-75162-x.
References
- 1.Bajaj JS, et al. Antibiotic-associated disruption of microbiota composition and function in cirrhosis is restored by fecal transplant. Hepatology. 2018;68:1549–1558. doi: 10.1002/hep.30037. [DOI] [PubMed] [Google Scholar]
- 2.Pamer E. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science. 2016;352:535–538. doi: 10.1126/science.aad9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moayyedi P, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–109. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Johnsen PH, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. 2018;3:17–24. doi: 10.1016/S2468-1253(17)30338-2. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj JS, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727–1738. doi: 10.1002/hep.29306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang D, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome. 2017;5:10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Z, et al. Fecal microbiota transplantation cured epilepsy in a case with crohn's disease: The first report. World J. Gastroenterol. 2017;23:3565–3568. doi: 10.3748/wjg.v23.i19.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Nood E, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 9.DeFilipp Z, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N. Engl. J. Med. 2019;381:2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 10.Alang N, Kelly C. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis. 2015;2:ofv004. doi: 10.1093/ofid/ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am. J. Gastroenterol. 2013;108:1367. doi: 10.1038/ajg.2013.164. [DOI] [PubMed] [Google Scholar]
- 12.Quera R, Espinoza R, Estay C, Rivera D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with crohn's disease and recurrent Clostridium difficile infection. J. Crohn's Colitis. 2014;8:252–253. doi: 10.1016/j.crohns.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Taur Y, et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci. Transl. Med. 2018;10:eaap9489. doi: 10.1126/scitranslmed.aap9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buffie CG, et al. Precision microbiome restoration of bile acid-mediated resistance to Clostridium difficile. Nature. 2015;517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seekatz AM, et al. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe. 2018;53:64–73. doi: 10.1016/j.anaerobe.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crothers J, et al. Tu1893—A double-blind, randomized, placebo-control pilot trial of fecal microbiota transplantation capsules from rationally selected donors in active ulcerative colitis. Gastroenterology. 2018;154:S1050–S1051. doi: 10.1016/S0016-5085(18)33519-4. [DOI] [Google Scholar]
- 17.Atarashi K, et al. Treg induction by a rationally selected mixture of clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 18.Browne H, et al. Culturing of 'unculturable' human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533:543. doi: 10.1038/nature17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kearney SM, et al. Endospores and other lysis-resistant bacteria comprise a widely shared core community within the human microbiota. ISME J. 2018;12:2403–2416. doi: 10.1038/s41396-018-0192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khanna S, et al. A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent Clostridium difficile infection. J. Infect. 2016;214:173–181. doi: 10.1093/infdis/jiv766. [DOI] [PubMed] [Google Scholar]
- 21.Gerding DN, et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: A randomized clinical trial. JAMA. 2015;313:1719–1727. doi: 10.1001/jama.2015.3725. [DOI] [PubMed] [Google Scholar]
- 22.Smillie CS, et al. Strain tracking reveals the determinants of bacterial engraftment in the human gut following fecal microbiota transplantation. Cell Host Microbe. 2018;23:229–240. doi: 10.1016/j.chom.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paramsothy S, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet. 2017;389:1218–1228. doi: 10.1016/S0140-6736(17)30182-4. [DOI] [PubMed] [Google Scholar]
- 24.Zuo T, et al. Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nature. 2018;9:1–11. doi: 10.1038/s41467-018-06103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5:226–235. doi: 10.4161/viru.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo T, et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut. 2017;67:634–643. doi: 10.1136/gutjnl-2017-313952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryson SJ, Thurber AR, Correa AMS, Orphan VJ, VegaThurber R. A novel sister clade to the enterobacteria microviruses (family microviridae) identified in methane seep sediments. Environ. Microbiol. 2015;17:3708–3721. doi: 10.1111/1462-2920.12758. [DOI] [PubMed] [Google Scholar]
- 28.Nale JY, Redgwell TA, Millard A, Clokie MRJ. Efficacy of an optimised bacteriophage cocktail to clear Clostridium difficile in a batch fermentation model. Antibiotics. 2018;7:13. doi: 10.3390/antibiotics7010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ott SJ, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology. 2027;152:799–811. doi: 10.1053/j.gastro.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Meader E, Mayer MJ, Steverding D, Carding SR, Narbad A. Evaluation of bacteriophage therapy to control Clostridium difficile and toxin production in an in vitro human colon model system. Anaerobe. 2013;22:25–30. doi: 10.1016/j.anaerobe.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Millan B, et al. Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent Clostridium difficile infection. Clin. Infect. Dis. 2016;62:1479–1486. doi: 10.1093/cid/ciw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibbons S, Kearney S, Smillie C, Alm E. Two dynamic regimes in the human gut microbiome. PLoS Comput. Biol. 2017;13:e1005364. doi: 10.1371/journal.pcbi.1005364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Y, Mahony S, Gifford DK. High resolution genome wide binding event finding and motif discovery reveals transcription factor spatial binding constraints. PLoS Comput. Biol. 2012;8:e1002638–e1002638. doi: 10.1371/journal.pcbi.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood DE, Lu J, Langmead B. Improved metagenomic analysis with kraken 2. Genome Biol. 2019;20:257–213. doi: 10.1186/s13059-019-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu J, Breitwieser FP, Thielen P, Salzberg SL. Bracken: Estimating species abundance in metagenomics data. PeerJ Comput. Sci. 2017;3:e104. doi: 10.7717/peerj-cs.104. [DOI] [Google Scholar]
- 36.Oksanen, J. et al. Vegan: Community Ecology Package. R package version 2.4-6, https://cran.r-project.org/web/packages/vegan/index.html (2017).
- 37.Gérard P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens (Basel, Switzerland) 2013;3:13–24. doi: 10.3390/pathogens3010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seekatz AM, et al. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe. 2018;53:666. doi: 10.1016/j.anaerobe.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagier J, Cadoret F, Raoult D. Critical microbiological view of SER-109. J. Infect. 2017;215:161–162. doi: 10.1093/infdis/jiw489. [DOI] [PubMed] [Google Scholar]
- 40.Clarke KR. Non-parametric multivariate analyses of changes in community structure. Austral. Ecol. 1993;18:117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x. [DOI] [Google Scholar]
- 41.Kolde, R. pheatmap: Pretty Heatmaps.,https://cran.r-project.org/web/packages/pheatmap/index.html (2019).
- 42.Cutler DR, et al. Random forests for classification in ecology. Ecology. 2007;88:2783–2792. doi: 10.1890/07-0539.1. [DOI] [PubMed] [Google Scholar]
- 43.Pedregosa F, et al. Scikit-learn: Machine learning in python. J. Mach. Learn. Res. 2011;12:2825–2830. [Google Scholar]
- 44.Cao LJ, Chua KS, Chong WK, Lee HP, Gu QM. A comparison of PCA, KPCA and ICA for dimensionality reduction in support vector machine. Neurocomputing. 2003;55:321–336. doi: 10.1016/S0925-2312(03)00433-8. [DOI] [Google Scholar]
- 45.Dietterich TG. Approximate statistical tests for comparing supervised classification learning algorithms. Neural Comput. 1998;10:1895–1923. doi: 10.1162/089976698300017197. [DOI] [PubMed] [Google Scholar]
- 46.Louppe, G., Wehenkel, L., Sutera, A. & Geurts, P. in Neural Information Processing Systems 2013. (eds C. J. C. Burges et al.).
- 47.Li SS, et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2015;352:586–589. doi: 10.1126/science.aad8852. [DOI] [PubMed] [Google Scholar]
- 48.Vrieze A, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916.e917. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 49.David LA, et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15:R89–R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goker M, et al. Complete genome sequence of odoribacter splanchnicus type strain (1651/6(T)) Stand. Genomic Sci. 2011;4:200–209. doi: 10.4056/sigs.1714269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ríos-Covián D, et al. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van den Abbeele P, et al. Butyrate-producing clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013;7:949–961. doi: 10.1038/ismej.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paredes-Sabja D, Torres JA, Setlow P, Sarker MR. Clostridium perfringens spore germination: Characterization of germinants and their receptors. J. Bacteriol. 2008;190:1190–1201. doi: 10.1128/JB.01748-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whisenant SG. Repairing Damaged Wildlands: A Process-Oriented, Landscape-Scale Approach. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 55.Trauner M, Fickert P, Tilg H. Bile acids as modulators of gut microbiota linking dietary habits and inflammatory bowel disease: A potentially dangerous liaison. Gastroenterology. 2013;144:844–846. doi: 10.1053/j.gastro.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 56.Filippidou S, et al. Under-detection of endospore-forming firmicutes in metagenomic data. Comput. Struct. Biotech. 2015;13:299. doi: 10.1016/j.csbj.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alnouti Y. Bile acid sulfation: A pathway of bile acid elimination and detoxification. Toxicol. Sci. 2009;108:225–246. doi: 10.1093/toxsci/kfn268. [DOI] [PubMed] [Google Scholar]
- 58.Jung T, Park JH, Jeon W, Han K. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr. Res. Pract. 2015;9:343–349. doi: 10.4162/nrp.2015.9.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fabiszewska AU, Stolarzewicz IA, Zamojska WM, Białecka-Florjańczyk E. Carbon source impact on Yarrowia lipolytica KKP 379 lipase production. Appl. Biochem. Microbiol. 2014;50:404–410. doi: 10.1134/S000368381404005X. [DOI] [Google Scholar]
- 60.Gouba N, Drancourt M. Digestive tract mycobiota: A source of infection. Med. Mal. Infect. 2015;45:9–16. doi: 10.1016/j.medmal.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Boyd AS, Wheless L, Brady BG, Ellis D. Cutaneous Yarrowia lipolytica infection in an immunocompetent woman. JAAD Case Rep. 2017;3:219–221. doi: 10.1016/j.jdcr.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zieniuk B, Fabiszewska A. Yarrowia lipolytica: A beneficious yeast in biotechnology as a rare opportunistic fungal pathogen: A minireview. World J. Microb. Biot. 2019;35:1–8. doi: 10.1007/s11274-018-2583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ng SC, et al. Scientific frontiers in faecal microbiota transplantation: Joint document of asia-pacific association of gastroenterology (APAGE) and Asia-Pacific society for digestive endoscopy (APSDE) Gut. 2020;69:83–91. doi: 10.1136/gutjnl-2019-319407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lamendella R, et al. Antibiotic treatments for Clostridium difficile infection are associated with distinct bacterial and fungal community structures. Msphere. 2018;3:e00572–e517. doi: 10.1128/mSphere.00572-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The normalized and non-normalized feature tables are available in supplementary data.